Abstract
Purpose
Adolescence is a period of transformation in humans with changes in the neural physiology at subcortical and cortical levels. However, its significance on auditory processing skills and working memory skills and their association is yet to be well understood. Hence, the current study was designed to evaluate and establish the association between auditory processing skills and working memory abilities in adolescents.
Method
A total of 125 adolescents within the age range of 10 to 15 years participated in the current study. All of them had normal hearing sensitivity and no associated obvious peripheral or central deficits. All the participants underwent auditory closure ability assessment using quick speech perception in noise test in Kannada, binaural integration ability assessment using dichotic CV test, and temporal processing assessment using gap detection test. Auditory working memory abilities were assessed using auditory digit span and digit sequencing.
Results
Spearman correlation was done to assess the correlation between auditory processing skills and working memory abilities. Results revealed a significant negative correlation between most of the central auditory processing abilities and all the working memory spans.
Conclusions
Findings of the current study indicate that individuals with poor working memory abilities have difficulty in auditory processing abilities.
Keywords: Adolescent, Auditory processing skills, Auditory working memory abilities
Introduction
Adolescence is a period of biological changes and transformation in humans. During adolescence, there is a decrease in gray matter volume and increase in white matter volume in the brain [1–4], which results in fine-tuning of neural circuits via synaptic pruning and increased axonal myelination. Neuronal proliferation, rewiring, dendritic pruning, and environmental exposure are essential components of brain plasticity during adolescence. A significant portion of brain growth and development occurring in adolescence is the construction and strengthening of regional neurocircuitry and pathways, particularly the brain stem, cerebellum, occipital lobe, parietal lobe, frontal lobe, and temporal lobe actively mature during adolescence. This developmental neuroplasticity and structural changes during adolescence are seen across various systems, including the auditory system [2, 3].
Studies have been done in the past to assess the maturation of various central auditory processing abilities [5–11]. These studies have demonstrated the developmental pattern and the variability of this maturation between different auditory processing skills. Among the various auditory processing skills, listening in noise skills shows developmental pattern till 11 years of age [6]. It has also been reported that SPIN scores in the right ear are adult-like by ten years of age; however, the left ear SPIN scores become adult-like between 13 and 14 years of age [9]. During binaural integration task, the right ear advantage for linguistically loaded stimuli reaches adult value by around 10–11 years [11]. The effect of age on dichotic listening may be different depending on the type of stimulus used. More linguistically loaded stimuli results in pronounced maturational effects in dichotic paradigm [5]. Development of skills such as masking level difference matures well into childhood through at least age 8 years [8]. It has been reported that the gap detection abilities improve with an increase in chronological age. However, adult-like performance is documented as early as ten years but observed as late as 16–18 years of age also [7]. Thus, it is evident that each auditory processing ability mature at different age, and the majority of the abilities are matured by adolescence.
Further, earlier researches have studied the association between auditory processing abilities and cognitive skills [12–15]. Tomlin et al. [14] reported that the cognitive abilities were affected in children with an auditory processing disorder. Kraus et al. [15] have also reported that working memory abilities play a crucial role in the neural processing of sound. However, Riccio et al. [12] reported that such an association exists between auditory processing and few cognitive skills. Thus, the characteristics and the extent of this association between these processes is yet to be well understood.
Moreover, the contradictory findings in the literature have also led to a variable representation of cognitive abilities in the definition of auditory processing abilities given by the various professional bodies in Audiology. As discussed earlier, age may be one of the factors that influence this association as the auditory processing skills, and cognitive skills show a developmental pattern. Adolescence is when biological transformation in the brain structure and connectivity in the brain interacts with increased experience, knowledge, and changing social demands to produce rapid cognitive growth. Hence, it would be worthwhile to study the relationship between auditory abilities and working memory.
Materials and Methods
Participants
A total of 125 participants (97 females and 28 males) were recruited for the study. Participants between 10 and 15 years were selected for the study. The upper age limit was limited to 15 years as maximum morphological changes in the central nervous system are completed by then. Participants with a history of delayed development, sensory issues, behavioral or neurological problems were excluded from the study. Further, none of the participants reported ill health during the testing, and none of them had any history or current condition of middle ear pathology. All the participants were native speakers of the Kannada language and were dominant right-handers. Written informed consent was taken from the participants/guardians of all the participants. Ethical clearance was obtained from the relevant ethics committee at the institute before the commencement of the study.
Procedure
A routine audiological evaluation was done to ensure normal hearing sensitivity. All the participants who had bilateral normal hearing sensitivity, i.e., pure tone average of less than 15 dBHL for air conduction thresholds at octave frequencies from 250 to 8000 Hz and bone conduction thresholds at octave frequencies from 250 to 4000 Hz, were selected for the study. Immittance evaluation was done to rule out middle ear pathology.
Assessment of Central Auditory Processing Skills
The participants were evaluated for all the major auditory processes to assess central auditory processing, viz. auditory closure, binaural integration, and temporal processing.
Test to Assess Auditory Closure
Auditory closure was assessed using a quick speech perception in noise test in Kannada [16]. The test has a total of seven lists, with each list containing seven sentences. The sentences are arranged in increasing levels of difficulty, i.e., the SNR starts from + 20 dB for the first sentence, and it reduces in 5 dB steps with each sentence SNR. For the present study, lists 1 and 2 were used. The sentences were presented with eight talker babble, and the participants were asked to repeat the target sentence heard by ignoring the babble. The participant was encouraged to repeat how many ever words he/she hears, and the total number of words correctly repeated was noted down. SNR 50 was calculated using the Spearman–Karber equation [17] as follows,
where i is the initial presentation level, d is the attenuation step size, w is the number of keywords per decrement, #correct is the total number of correct keywords.
Test to Assess Binaural Integration
Binaural integration was assessed using dichotic CV test [18]. The test consists of six syllables /pa/, /ta/, /ka/, /ba/, /da/ and /ga/ which were presented five times randomly to make it a total of 30 presentations. The two syllables were presented to both the ears simultaneously with a lag of 0 ms through a calibrated laptop connected to HDA 200 headphones. The participants were asked to note down the syllables heard from both ears. The scoring involved assessing for single correct scores for right and left ear and double correct scores.
Test to Assess Temporal Processing
Temporal processing was assessed using a gap detection test (GDT). GDT was done using the maximum likelihood parameter employed in MATLAB software. The participant's ability to perceive a temporal gap in the center of 500 ms broadband noise was measured for GDT [19]. GDT was measured using a three-block alternate forced-choice task, wherein two 500 ms broadband noise with no gap served as the standard stimulus, and the variable stimulus contained the gap. The participants were instructed to identify which among the three stimuli had the gap. GDT was measured for each ear separately.
Assessment of Working Memory
Working memory was measured using digit span and sequencing test through Smriti-Shravan software version 1.10 [20]. In this software to assess digit span and sequencing, stimuli consisted of digits from one to nine. The numbers were presented in random order with an increasing level of difficulty, with the minimum number of digits being two with an interstimulus interval of 250 ms. Forward and backward digit span tasks were done to assess digit span, and ascending and descending digit tasks were done to evaluate auditory digit sequencing.
In the forward digit span test, the participants were presented with clusters of numbers, and they were asked to repeat the numbers in the same order and repeat the digits in the reverse order for backward digit span. In ascending digit sequencing tasks, participants were presented with a cluster of numbers, and they were asked to arrange them in ascending order. In descending task, they were asked to arrange the numbers in descending order and then repeat them. All the working memory tests were done using one up one down procedure. The participants were given examples before the actual testing. Auditory working memory capacity was calculated as the number of digits that the participants can recall in sequencing and digit span.
Results
The data of the present study were subjected to statistical analyses using the Statistical Package for the Social Sciences (Version 20). Descriptive statistics were done to assess the mean and standard deviation (SD) of all the parameters. The data obtained were subjected to Shapiro–Wilk's test for normality. The results revealed a non-normal distribution of data (p < 0.05), and therefore non-parametric tests were administered. Table 1 shows the mean and standard deviation of all the central auditory processing tests and working memory in the adolescent group. A Spearman correlation was done to study the relationship between central auditory processing ability and working memory.
Table 1.
Mean, SD, median and range for central auditory processing tests and working memory tests
| Tests | Mean | SD | Median | Range | |
|---|---|---|---|---|---|
| QuickSIN (dB) | SNR 50 R | − 2.79 | 1.578 | − 2.50 | − 6.7 to 1.7 |
| SNR 50 L | − 3.49 | 1.948 | − 3.40 | − 6.7 to 2.9 | |
| DCV (No. of syllables repeated) | SCS R | 20.77 | 4.506 | 22.00 | 8 to 29 |
| SCS L | 17.90 | 4.506 | 19.00 | 4 to 29 | |
| DCS | 14.74 | 5.423 | 15.00 | 1 to 26 | |
| GDT R(ms) | 2.86 | 1.012 | 2.65 | 1.78 to 8.43 | |
| GDT L (ms) | 2.88 | 1.236 | 2.65 | 1.7 to 10.79 | |
| Working memory (digit spans) | Forward | 4.95 | 1.062 | 5.00 | 3 to 7 |
| Backward | 3.69 | 0.810 | 4 | 3 to 5 | |
| Ascending | 4.77 | 1.643 | 4 | 3 to 10 | |
| Descending | 4.66 | 1.283 | 4 | 3 to 7 |
A Spearman correlation was done to study the relationship between central auditory processing ability and working memory. Table 2 shows the correlation coefficients between central processing abilities and various working memory tasks. Figure 1 shows the scatter plots between various auditory processing skills and different working memory spans showing significant correlations.
Table 2.
Result of Spearman rank correlation between central auditory processing skills and auditory working memory measures
| Tests | SNR 50 R | SNR 50 L | SCS R | SCS L | DCS | GDT R | GDT L |
|---|---|---|---|---|---|---|---|
| Working memory (digit spans) | |||||||
| Forward | − 0.462** | − 0.424** | − 0.011 | 0.155 | 0.115 | − 0.213** | − 0.201* |
| Backward | − 0.421** | − 0.284** | − 0.029 | 0.140 | 0.088 | − 0.184* | − 0.195* |
| Ascending | − 0.372** | − 0.404** | 0.106 | 0.207* | 0.197* | − 0.227** | − 0.244** |
| Descending | − 0.390** | − 0.409** | 0.040 | 0.204* | 0.145 | − 0.280** | − 0.284** |
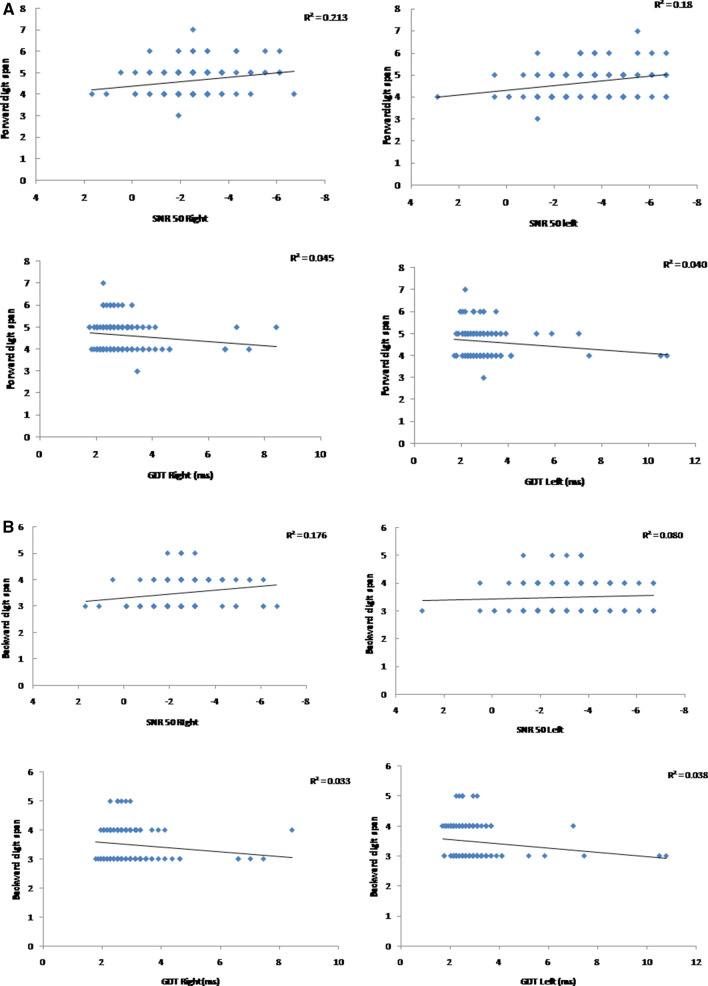
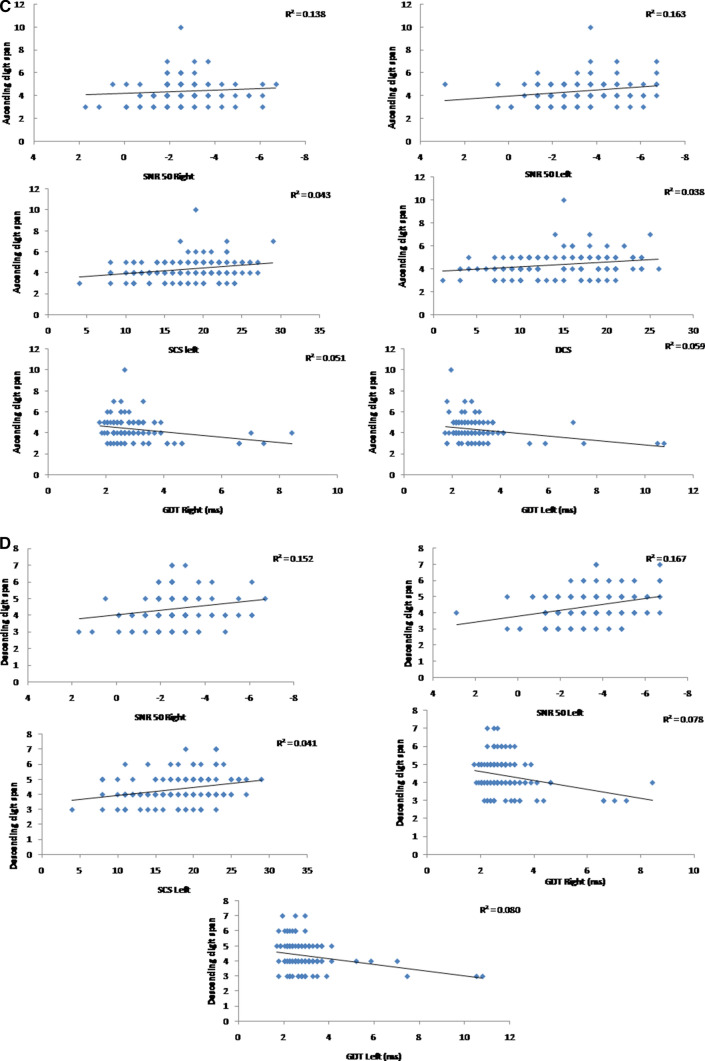
Fig. 1.
a Scatter plots of SNR 50 and GDT for right and left ear as a function of forward digit span. R square values are represented on the top right corner of the scatter plot. b Scatter plots of SNR 50 and GDT for right and left ear as a function of backward digit span. R square values are represented on the top right corner of the scatter plot. c Scatter plots of SNR 50 and GDT for right and left ear and single correct score of the left ear (SCS—left) and double correct score (DCS) as a function of ascending digit span. R square values are represented on the top right corner of the scatter plot. d Scatter plots of SNR 50 and GDT for right and left ear and single correct score of the left ear (SCS—left) as a function of descending digit span. R square values are represented on the top right corner of the scatter plot
Discussion
The present study aimed to study the relationship between auditory processing abilities and working memory during adolescence. This study showed a significant negative correlation between various auditory processing abilities with working memory. This indicates that participants with poor working memory also have poor auditory processing abilities. The correlation is seen for SNR 50 and GDT with the entire working memory task. For binaural integration abilities, the single correct score left and the double correct score showed a negative correlation with the ascending digit span. In contrast, single correct score-left alone showed a negative correlation with descending digit span.
Studies have been done in the past to assess the relationship between auditory processing skills and working memory [12–15]. The relationship among peripheral, central auditory processing skills and cognitive skills, explicitly working memory, has also been studied [21–24]. Collectively their results show that cognition contributes substantially to speech understanding. It was also reported that the relationship between cognition and speech understanding was stronger in adverse listening conditions and for amplified speech. However, these relationships have been established in the aging population, as there is a deterioration of cognitive abilities. In the present study, we found an association between auditory processing abilities and working memory tests among adolescents. This indicates that working memory plays a vital role in auditory processing abilities. Further, as mentioned earlier, adolescence is a time of rapid cognitive development, and biological changes in brain structure and connectivity in the brain interact with increased experience, knowledge, and changing social demands to produce rapid cognitive growth. This could be the reason to see an association between working memory and auditory processing abilities.
Conclusion
The current study was planned to evaluate auditory processing skills and working memory abilities in adolescents and study the association between them. Auditory closure, binaural integration, and temporal processing abilities were assessed using standardized test procedures and were correlated with the auditory working memory ability. Non-parametric statistics were run, and the results revealed that there exists a negative correlation between most of these abilities being assessed. This suggests that poor working memory and poor auditory processing skills are related to each other. This finding is in agreement with the available literature which focused on this association in older adults. The results of the current study indicate the importance of long felt research in this expanse and its implications in the evaluation and management of auditory processing deficits in adolescents.
Declarations
Conflict of interest
Authors declare no conflict of interest.
Informed Consent
Written informed consent was taken from the participants/guardians of all the participants for their willingness to participate in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 2.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paus TSAA. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 4.Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 2007;28:228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellis TJ. Assessment and management of central auditory processing disorders in the educational setting: from science to practice. 2. San Diego: Plural Publisher; 2011. [Google Scholar]
- 6.Elliott LL. Performance of children aged 9 to 17 years on a test of speech intelligibility in noise using sentence material with controlled word predictability. J Acoust Soc Am. 1979;66:651–653. doi: 10.1121/1.383691. [DOI] [PubMed] [Google Scholar]
- 7.Fischer B, Hartnegg K. On the development of low-level auditory discrimination and deficits in dyslexia. Dyslexia. 2004;10:105–118. doi: 10.1002/dys.268. [DOI] [PubMed] [Google Scholar]
- 8.Hall JW, Buss E, Grose JH, Dev MB. Developmental effects in the masking-level difference. J Speech Lang Hear Res. 2004;47:13–20. doi: 10.1044/1092-4388(2004/002). [DOI] [PubMed] [Google Scholar]
- 9.Chandni J, Vipin Ghosh PG, Chetak KB, Aishwarya L. Maturation of speech perception in noise abilities during adolescence. Int J Pediatr Otorhinolaryngol. 2020;139:110459. doi: 10.1016/j.ijporl.2020.110459. [DOI] [PubMed] [Google Scholar]
- 10.Tierney AT, Krizman J, Kraus N. Music training alters the course of adolescent auditory development. Proc Natl Acad Sci. 2015;112:10062–10067. doi: 10.1073/pnas.1505114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaikh MA, Fox-Thomas L, Tucker D. Maturational changes in ear advantage for monaural word recognition in noise among listeners with central auditory processing disorders. Audiol Res [Internet]. 2017. https://audiologyresearch.org/index.php/audio/article/view/157. Accessed 12 Feb 2021 [DOI] [PMC free article] [PubMed]
- 12.Riccio CA, Cohen MJ, Garrison T, Smith B. Auditory processing measures: correlation with neuropsychological measures of attention, memory, and behavior. Child Neuropsychol. 2005;11:363–372. doi: 10.1080/09297040490916956. [DOI] [PubMed] [Google Scholar]
- 13.Magimairaj BM, Nagaraj NK. Working memory and auditory processing in school-age children. Lang Speech Hear Serv Sch. 2018;49:409–423. doi: 10.1044/2018_LSHSS-17-0099. [DOI] [PubMed] [Google Scholar]
- 14.Tomlin D, Dillon H, Sharma M, Rance G. The impact of auditory processing and cognitive abilities in children. Ear Hear. 2015;36:527–542. doi: 10.1097/AUD.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 15.Kraus N, Strait DL, Parbery-Clark A. Cognitive factors shape brain networks for auditory skills: spotlight on auditory working memory: Kraus et al. Ann N Y Acad Sci. 2012;1252:100–107. doi: 10.1111/j.1749-6632.2012.06463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avinash MC, Meti R, Kumar U. Development of sentences for quick speech-in-noise (QuickSIN) test in Kannada. J Indian Speech Hear Assoc. 2010;2010(24):59–65. [Google Scholar]
- 17.Grimm H. FINNEY, D. J.: Statistical Method in Biological Assay. 3. ed. Charles Griffin & Co., London and High Wycombe 1978. VII, 508 S., £ 19 net. Biom J. 1979;21:689–90.
- 18.Yathiraj, A. The Dichotic CV Test. Department of Audiology, All India Institute of Speech and Hearing, Mysore, India. Mysore; 1999.
- 19.Harris KC, Eckert MA, Ahlstrom JB, Dubno JR. Age-related differences in gap detection: effects of task difficulty and cognitive ability. Hear Res. 2010;264:21–29. doi: 10.1016/j.heares.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar AU, Sandeep M. Auditory cognitive training module. Mysore: All India Institute of Speech and Hearing; 2013. [Google Scholar]
- 21.Humes LE, Coughlin M. Aided speech-identification performance in single-talker competition by older adults with impaired hearing. Scand J Psychol. 2009;50:485–494. doi: 10.1111/j.1467-9450.2009.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humes LE, Burk MH, Coughlin MP, Busey TA, Strauser LE. Auditory speech recognition and visual text recognition in younger and older adults: similarities and differences between modalities and the effects of presentation rate. J Speech Lang Hear Res. 2007;50:283–303. doi: 10.1044/1092-4388(2007/021). [DOI] [PubMed] [Google Scholar]
- 23.Humes LE. Factors underlying the speech-recognition performance of elderly hearing-aid wearers. J Acoust Soc Am. 2002;112:1112–1132. doi: 10.1121/1.1499132. [DOI] [PubMed] [Google Scholar]
- 24.Humes LE, Christopherson L. Speech identification difficulties of hearing-impaired elderly persons: the contributions of auditory processing deficits. J Speech Lang Hear Res. 1991;34:686–693. doi: 10.1044/jshr.3403.686. [DOI] [PubMed] [Google Scholar]