Abstract
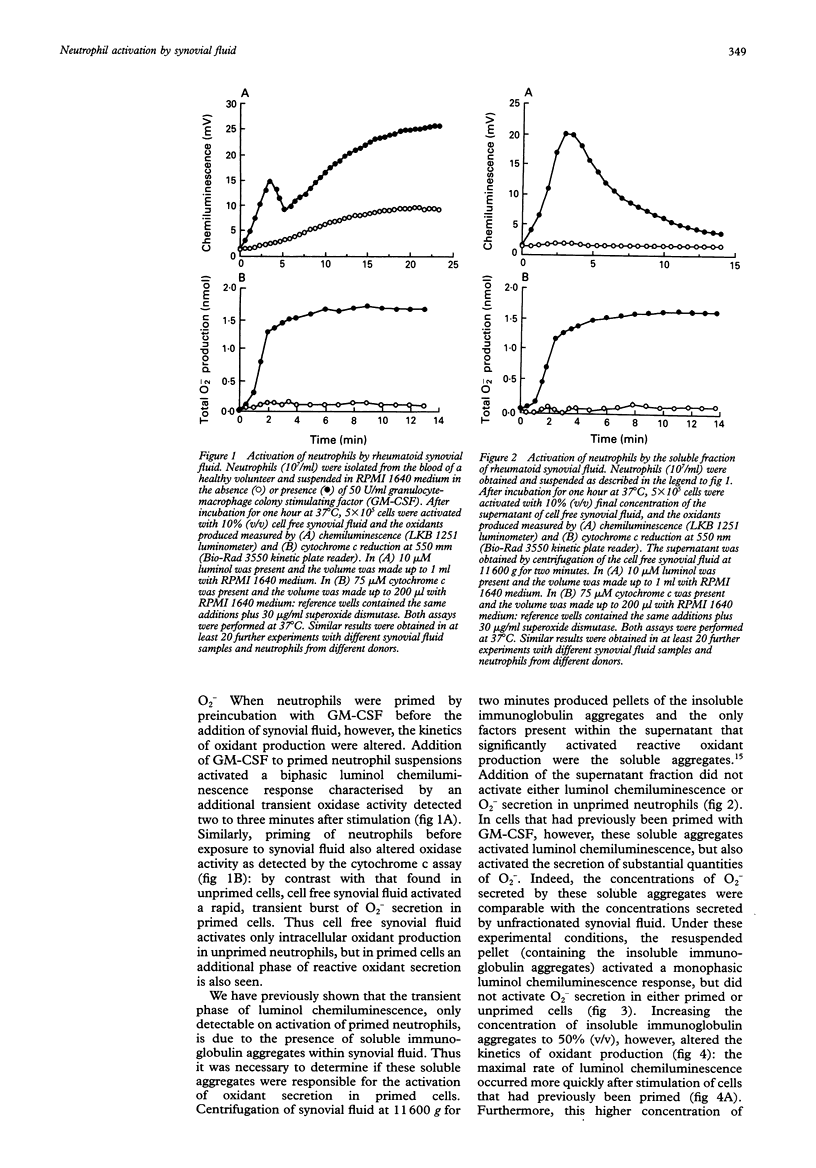
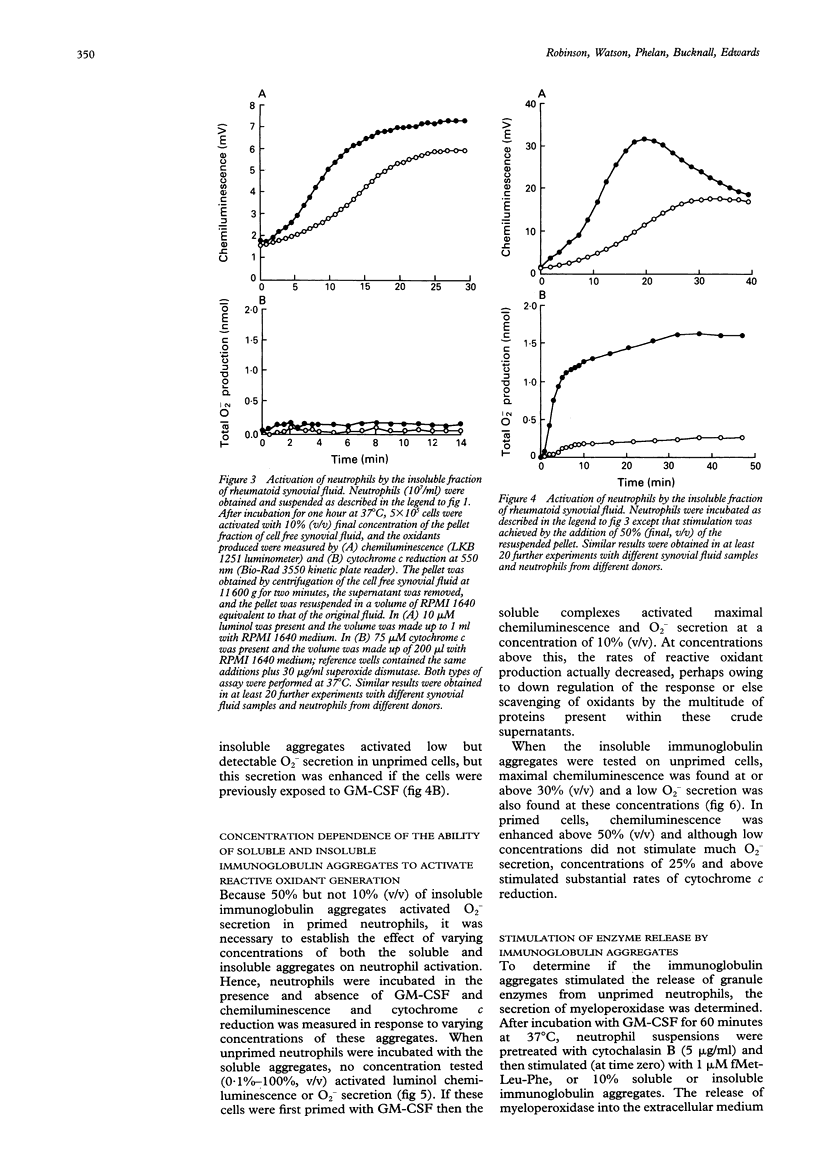
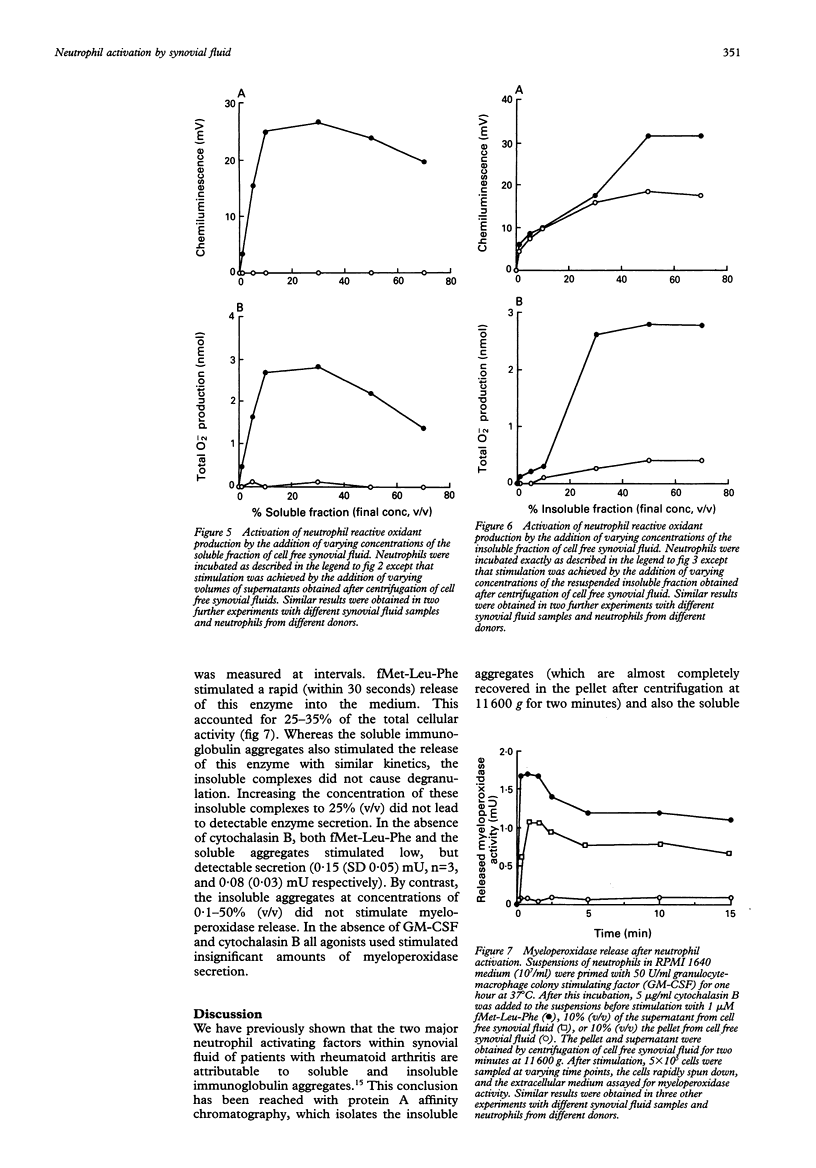
OBJECTIVES--Previous work has shown that synovial fluid isolated from patients with active rheumatoid arthritis contains soluble (not sedimented by centrifugation at 11,600 g for two minutes) and insoluble (sedimented by centrifugation at 11,600 g for two minutes) immunoglobulin aggregates that are capable of activating reactive oxidant production by bloodstream neutrophils. The purpose of this study was to determine which of these types of immunoglobulin aggregates activated the secretion of reactive oxygen metabolites and granule enzymes from neutrophils. METHODS--Cell free synovial fluid (from patients with rheumatoid arthritis) was added to neutrophils isolated from blood of healthy controls that had been incubated in the presence and absence of granulocyte-macrophage colony stimulating factor (GM-CSF). Reactive oxidant production was measured by luminol chemiluminescence (which detects both intracellular and extracellular oxidant production) and by cytochrome c reduction (which measures superoxide secretion). RESULTS--The soluble aggregates only activated neutrophils that were previously primed, and activated a rapid and transient burst of reactive oxidant secretion. On the other hand, the insoluble aggregates activated primed and unprimed neutrophils with similar efficacy and most of the oxidants generated (especially in unprimed cells) were intracellular. The soluble aggregates, but not the insoluble aggregates, also activated the secretion of myeloperoxidase from neutrophils that had either been pretreated with cytochalasin B or primed with GM-CSF. CONCLUSION--It is thus proposed that these soluble immunoglobulin aggregates are responsible for activation of the release of tissue damaging granule enzymes and reactive oxidants from primed neutrophils within the rheumatoid joint.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn W. D., Jr, Koopman W. J., Schrohenloher R. E., Heck L. W. Induction of neutrophil enzyme release by rheumatoid factors: evidence for differences based on molecular characteristics. Clin Immunol Immunopathol. 1986 Aug;40(2):347–355. doi: 10.1016/0090-1229(86)90039-5. [DOI] [PubMed] [Google Scholar]
- Breedveld F. C., Lafeber G. J., van den Barselaar M. T., van Dissel J. T., Leijh P. C. Phagocytosis and intracellular killing of Staphylococcus aureus by polymorphonuclear cells from synovial fluid of patients with rheumatoid arthritis. Arthritis Rheum. 1986 Feb;29(2):166–173. doi: 10.1002/art.1780290203. [DOI] [PubMed] [Google Scholar]
- Briheim G., Stendahl O., Dahlgren C. Intra- and extracellular events in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1984 Jul;45(1):1–5. doi: 10.1128/iai.45.1.1-5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. A. The polymorphonuclear cell in rheumatoid arthritis. Br J Rheumatol. 1988 Apr;27(2):150–155. doi: 10.1093/rheumatology/27.2.150. [DOI] [PubMed] [Google Scholar]
- Dahlgren C., Aniansson H., Magnusson K. E. Pattern of formylmethionyl-leucyl-phenylalanine-induced luminol- and lucigenin-dependent chemiluminescence in human neutrophils. Infect Immun. 1985 Jan;47(1):326–328. doi: 10.1128/iai.47.1.326-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dularay B., Badesha J. S., Dieppe P. A., Elson C. J. Oxidative response of polymorphonuclear leucocytes to synovial fluids from patients with rheumatoid arthritis. Ann Rheum Dis. 1990 Sep;49(9):661–664. doi: 10.1136/ard.49.9.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dularay B., Elson C. J., Dieppe P. A. Enhanced oxidative response of polymorphonuclear leukocytes from synovial fluids of patients with rheumatoid arthritis. Autoimmunity. 1988;1(3):159–169. doi: 10.3109/08916938808997161. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Holden C. S., Humphreys J. M., Hart C. A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) primes the respiratory burst and stimulates protein biosynthesis in human neutrophils. FEBS Lett. 1989 Oct 9;256(1-2):62–66. doi: 10.1016/0014-5793(89)81718-1. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Hughes V., Barlow J., Bucknall R. Immunological detection of myeloperoxidase in synovial fluid from patients with rheumatoid arthritis. Biochem J. 1988 Feb 15;250(1):81–85. doi: 10.1042/bj2500081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W. Luminol- and lucigenin-dependent chemiluminescence of neutrophils: role of degranulation. J Clin Lab Immunol. 1987 Jan;22(1):35–39. [PubMed] [Google Scholar]
- Edwards S. W., Nurcombe H. L., Hart C. A. Oxidative inactivation of myeloperoxidase released from human neutrophils. Biochem J. 1987 Aug 1;245(3):925–928. doi: 10.1042/bj2450925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Say J. E., Hart C. A. Oxygen-dependent killing of Staphylococcus aureus by human neutrophils. J Gen Microbiol. 1987 Dec;133(12):3591–3597. doi: 10.1099/00221287-133-12-3591. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Watson F., MacLeod R., Davies J. Receptor expression and oxidase activity in human neutrophils: regulation by granulocyte-macrophage colony-stimulating factor and dependence upon protein biosynthesis. Biosci Rep. 1990 Aug;10(4):393–401. doi: 10.1007/BF01117239. [DOI] [PubMed] [Google Scholar]
- Emery P., Lopez A. F., Burns G. F., Vadas M. A. Synovial fluid neutrophils of patients with rheumatoid arthritis have membrane antigen changes that reflect activation. Ann Rheum Dis. 1988 Jan;47(1):34–39. doi: 10.1136/ard.47.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Chantry D., Haworth C., Turner M., Abney E., Buchan G., Barrett K., Barkley D., Chu A. Cytokine production in the rheumatoid joint: implications for treatment. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):480–486. [PubMed] [Google Scholar]
- Gale R., Bertouch J. V., Bradley J., Roberts-Thomson P. J. Direct activation of neutrophil chemiluminescence by rheumatoid sera and synovial fluid. Ann Rheum Dis. 1983 Apr;42(2):158–162. doi: 10.1136/ard.42.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry P., Winyard P. G., Morris C. J., Grootveld M., Blake D. R. Oxygen free radicals, inflammation, and synovitis: and synovitis: the current status. Ann Rheum Dis. 1989 Oct;48(10):864–870. doi: 10.1136/ard.48.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe H. L., Bucknall R. C., Edwards S. W. Activation of the neutrophil myeloperoxidase-H2O2 system by synovial fluid isolated from patients with rheumatoid arthritis. Ann Rheum Dis. 1991 Apr;50(4):237–242. doi: 10.1136/ard.50.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe H. L., Bucknall R. C., Edwards S. W. Neutrophils isolated from the synovial fluid of patients with rheumatoid arthritis: priming and activation in vivo. Ann Rheum Dis. 1991 Mar;50(3):147–153. doi: 10.1136/ard.50.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe H. L., Edwards S. W. Role of myeloperoxidase in intracellular and extracellular chemiluminescence of neutrophils. Ann Rheum Dis. 1989 Jan;48(1):56–62. doi: 10.1136/ard.48.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. J., Watson F., Bucknall R. C., Edwards S. W. Stimulation of neutrophils by insoluble immunoglobulin aggregates from synovial fluid of patients with rheumatoid arthritis. Eur J Clin Invest. 1992 May;22(5):314–318. doi: 10.1111/j.1365-2362.1992.tb01468.x. [DOI] [PubMed] [Google Scholar]
- Robinson J., Watson F., Bucknall R. C., Edwards S. W. Activation of neutrophil reactive-oxidant production by synovial fluid from patients with inflammatory joint disease. Soluble and insoluble immunoglobulin aggregates activate different pathways in primed and unprimed cells. Biochem J. 1992 Sep 1;286(Pt 2):345–351. doi: 10.1042/bj2860345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. R., Williams R. B., Farrell A. J., Blake D. R. Hypoxia and inflammatory synovitis: observations and speculation. Ann Rheum Dis. 1991 Feb;50(2):124–132. doi: 10.1136/ard.50.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson F., Robinson J., Edwards S. W. Protein kinase C-dependent and -independent activation of the NADPH oxidase of human neutrophils. J Biol Chem. 1991 Apr 25;266(12):7432–7439. [PubMed] [Google Scholar]
- Westacott C. I., Whicher J. T., Barnes I. C., Thompson D., Swan A. J., Dieppe P. A. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis. 1990 Sep;49(9):676–681. doi: 10.1136/ard.49.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]


