Abstract
Immune stress exerts detrimental effects on growth performance and intestinal barrier function during intensive animal production with ensuing serious economic consequences. Chlorogenic acid (CGA) is used widely as a feed additive to improve the growth performance and intestinal health of poultry. However, the effects of dietary CGA supplementation on amelioration of the intestinal barrier impairment caused by immune stress in broilers are unknown. This study investigated the effects of CGA on growth performance, intestinal barrier function, and the inflammatory response in lipopolysaccharide (LPS) mediated immune-stressed broilers. Three hundred and twelve 1-day-old male Arbor Acres broilers were divided randomly into 4 groups with 6 replicates of thirteen broilers. The treatments included: i) saline group: broilers injected with saline and fed with basal diet; ii) LPS group: broilers injected with LPS and fed with basal diet; iii) CGA group: broilers injected with saline and feed supplemented with CGA; and iv) LPS+CGA group: broilers injected with LPS and feed supplemented with CGA. Animals in the LPS and LPS+CGA groups were injected intraperitoneally with an LPS solution prepared with saline from 14 d of age for 7 consecutive days, whereas broilers in the other groups were injected only with saline. LPS induced a decrease in feed intake of broilers during the stress period, but CGA effectively alleviated this decrease. Moreover, CGA inhibited the reduction of villus height and improved the ratio of villus height to crypt depth in the duodenum of broilers 24 and 72 h after LPS injection. In addition, dietary CGA supplementation significantly restored the expression of cation-selective and channel-forming Claudin2 protein 2 h after LPS injection in the ileum. LPS enhanced the expression of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in the small intestine, but this enhancement was blocked by CGA supplementation. The expression of interleukin-10 (IL-10) increased with LPS injection and CGA promoted the production of IL-10. CGA addition downregulated the expression of intestinal interleukin-6 (IL-6) of broilers under normal rearing conditions. However, CGA supplementation upregulated the expression of IL-6 of broilers 72 h after LPS injection. The data demonstrate that dietary supplementation with CGA alleviates intestinal barrier damage and intestinal inflammation induced by LPS injection during immune stress thereby improving growth performance of broilers.
Key words: lipopolysaccharide, chlorogenic acid, intestinal barrier function, inflammation, broilers
INTRODUCTION
The intestinal tract not only is the principal site of nutrient digestion and absorption, but also is the largest immune organ in poultry. Intestinal health is essential to poultry production efficiency and performance. With the development of modern intensive farming, high feeding density and high yield requirements make commercial broilers more susceptible to diverse stressors, including inappropriate environmental temperature and humidity, overcrowding, and catching practices (Gilani et al., 2021). Stress may damage the intestinal barrier and cause intestinal inflammation in poultry, thereby affecting production performance (Li et al., 2015; Tang et al., 2021; Zhou et al., 2021). The addition of antibiotics to diets alleviates the stress that results from poor feeding environments, but also may lead to antibiotic residue problems that endanger animal and human health. Moreover, the drive to limit antibiotic use in agriculture has encouraged the search to identify safe, efficient, and pollution-free dietary additives that alleviate animal stress without harm to animal or human well-being.
Numerous reports have demonstrated that Chinese herbal medicines and the active compounds within these medicines significantly enhance both animal immune function (Qiu et al., 2007) and intestinal mucosal integrity (Han et al., 2016), with the advantages of producing fewer side effects than other drugs, wide availability, and high efficacy (Chen et al., 2014). Chlorogenic acid (CGA) is present widely in diverse plants, including Eucommia ulmoides (Chinese rubber tree), honeysuckle and coffee (Upadhyay and Mohan Rao, 2013; Miao and Xiang, 2020). CGA has attracted increasing attention because of its extensive biological activities, including antioxidation (Zhao et al., 2019; Ji et al., 2021; Bai et al., 2022) and antibacterial properties (Wang et al., 2015a; Gong et al., 2018; Wu et al., 2018), and ability to regulate immune function (Liang and Kitts, 2015; Vukelic et al., 2018; Chen et al., 2021). Moreover, CGA exerts protective effects against intestinal-related inflammation in both cellular and animal models (Liang and Kitts, 2015). The compound also exerts anti-inflammatory effects in TNF-α and H2O2-induced human intestine epithelia Caco-2 cells by down regulating interleukin-8 (IL-8) production (Shin et al., 2015). In addition, CGA supplementation ameliorates intestinal inflammation, improves intestinal barrier integrity, and upregulates the expression of intestinal tight junction proteins in weaned rats challenged with lipopolysaccharide (LPS) (Ruan et al., 2014). Moreover, CGA as a feed additive improved growth performance, enhanced antioxidant capacity, promoted intestinal barrier function, and maintained the integrity of the intestinal mucosa morphology in weaned pigs (Chen et al., 2018a,b,c). Similarly, addition of CGA into broiler feed improved growth performance, inhibited small intestinal structural damage, and reduced the expression of inflammatory cytokines in both Clostridium perfringens-challenged broilers and coccidium-infected animals (Zhang et al., 2020; Liu et al., 2022a). CGA also alleviated acute heat stress-induced intestinal injury in young chickens by blocking inflammation and improving antioxidant capacity and cecal microbiota composition (Chen et al., 2021).
Intestinal diseases that result in altered immune function, lower productivity, higher mortality, and the contamination of food products pose significant threats to the poultry industry. Gram-negative bacterial LPS is the principal source of intestinal disease (Wang et al., 2015b), and intraperitoneal and intravenous LPS have been used widely to induce immune stress experimentally in poultry (Takahashi et al., 1997; Xie et al., 2000; Zhang et al., 2010; Li et al., 2015; Chen et al., 2018d). Although increasing numbers of studies have been conducted on the effects of CGA in poultry production, rarely data have been presented that examined the regulation by CGA of intestinal barrier function and the inflammatory response in broilers under LPS-induced immune stress. Therefore, in this study, an immune stress model in broilers was induced by intraperitoneal injection of LPS was established to investigate the protective effects of CGA on intestinal barrier function and inflammation of broilers. The results could provide reference and basis for the establishment of experimental models and future rational utilization of CGA in broiler feed.
MATERIALS AND METHODS
Broilers, Diets, and Experimental Treatments
Three hundred and twelve 1-day-old male AA broilers were purchased from a commercial broiler hatchery (Luoyang, China). All healthy broilers with similar average body weight (37 ± 0.5 g) were assigned randomly to 4 experimental treatments with 6 replicates per treatment for 23-day feeding trials. The 4 experimental treatments were: i) saline group (broilers injected with saline and fed with basal diet); ii) LPS group (broilers injected with LPS and fed with basal diet); iii) CGA group (broilers injected with saline and feed supplemented with CGA); and iv) LPS+CGA group (broilers injected with LPS and feed supplemented with CGA). From the age of 14 d, broilers in the LPS and LPS+CGA groups were injected intraperitoneally with LPS solution prepared with saline every morning according to the body weight of each chicken, whereas animals in other groups were injected with saline only. The injections were administered daily for 8 consecutive days. The dosage and route of administration of LPS were based on a previous study (Li et al., 2015). The supplemental CGA (purity 98%) was provided by Changsha Staherb Natural Ingredients Co. (Changsha, China). The level of CGA used here was selected based on the results of our previous unpublished studies. CGA was added throughout the trial period. The basal diet was formulated according to NRC (National Research Council, 1994) to meet the nutrient requirements of the broilers (Table 1). Broilers were placed in the terrace of a mechanically ventilated room under controlled temperature, humidity, and lighting conditions. Animals were raised in 3-layer ladder cages with free access daily to food and water. The feeding room temperature was maintained at 32°C to 34°C in the initial stage (d 1–5) and then gradually decreased by 2°C per wk to reach a final room temperature of 22°C to 24°C (d 5–23). The relative humidity was maintained between 40 and 60%, and natural ventilation was provided. Broilers received continuous light for the first 3 d, and were then maintained under 23L:1D for the remainder of the study. All procedures were approved by the Animal Care and Use Committee of Henan University of Science and Technology.
Table 1.
Composition and nutrient levels of the experimental basal diet.
| Ingredients, % | Content |
|---|---|
| Corn | 60.1 |
| Soybean meal | 33.07 |
| Soybean oil | 3.6 |
| Limestone-calcium carbonate | 1.1 |
| Calcium hydrogen phosphate | 1 |
| DL-Methionine (98%) | 0.2 |
| L-Lysine HCL (78%) | 0.2 |
| Sodium chloride | 0.3 |
| Vitamin premix1 | 0.03 |
| Mineral premix2 | 0.2 |
| Choline chloride (50%) | 0.15 |
| Ethoxyquin (33%) | 0.05 |
| Total | 100 |
| Calculated nutrient levels3 | |
| Metabolizable energy, kcal/kg | 2990 |
| Crude protein | 20.5 |
| Calcium | 0.99 |
| Available phosphorus | 0.44 |
| Lysine | 0.1 |
| Methionine | 0.47 |
Vitamin premix provided the following per kg of diet: vitamin A (retinylacetate), 12,500 IU; vitamin D3 (cholecalciferol), 2,500 IU; vitamin E (DL-a-tocopherol acetate), 18.75 mg; vitamin K3 (menadione sodium bisulfate), 2.65 mg; vitamin B1 2 mg; vitamin B2 6 mg; vitamin B6 6 mg; vitamin B12 (cyanocobalamin), 0.025 mg; biotin, 0.0325 mg; folic acid, 1.25 mg; pantothenic acid, 1.25 mg; nicotinic acid, 50 mg.
Mineral premix provided per kilogram of complete diet: Cu (as copper sulfate), 8 mg; Zn (as zinc sulfate), 75 mg; Fe (as ferrous sulfate), 80 mg; Mn (as manganese sulfate), 100 mg; Se (as sodium selenite), 0.15 mg; I (as potassium iodide), 0.35 mg.
Calculated value based on the analyzed data of experimental diets.
Determination of Growth Performance
Body weight and daily feed intake of birds were recorded on d 14, 15, 17, and 19 to calculate the average daily gain (ADG), average daily feed intake (ADFI), and feed conversion rate (FCR) of each group. Chicken mortality was recorded daily after which performance parameters were corrected for mortality.
Sample Collection
One bird from each replicate was randomly selected and sacrificed by decollation at 2 h, 4 h, 24 h, and 72 h after the first injection of LPS, and the whole intestinal tract was removed quickly. The tissues of the middle part of the duodenum, jejunum, and ileum were cut with sterile forceps and scissors and were rinsed gently with precooled PBS. One portion was placed in 4% paraformaldehyde tissue fixative and used to make hematoxylin and eosin (H&E)-stained sections to observe the effect of CGA addition on intestinal morphology in immune-stressed broiler chickens, and the other portion was quickly frozen in liquid nitrogen and stored at −80°C for mRNA expression analysis.
Intestinal Morphology
The histological measurement was done according to a method as previously described (Zhen et al., 2018). Intestinal samples were dehydrated in a graded ethanol series (50, 70, 80, 90, 95, and 100% ethanol), clarified with fresh xylene, and then embedded into paraffins. Serial sections (5 mm of thickness) were cut by pathology slicer (Leica Microsystems K. K., Tokyo, Japan), and stained with H&E. After staining, the sections were visualized using a scanner (Pannoramic MIDI, Budapest, Hungary) for better observation of intestinal morphology and measurement data. The villus height (VH; from the tip of the villus to the villus crypt opening) and crypt depth (CD; from the base of the crypt to the level of the crypt opening) of 20 well-oriented villi were measured per segment using CaseViewer software (version 2.0) and the mean values were calculated. The ratio of VH to CD then was determined.
Gene Expression Using Quantitative Real-Time PCR
Total RNA was extracted from duodenum, jejunum, and ileum tissue samples using the Trizol reagent (Thermo Fisher Scientific, Ottawa, Canada) according to the instructions of the manufacturer. The integrity of the RNA was assessed by visualization on agarose gels. RNA concentration and purity were determined using a Nanodrop ND-2000 spectrophotometer (Thermo Scientific, Ottawa, Canada). Synthesis of cDNA was performed according to the instructions of the kit manufacturer (Vazyme Biotech Co., Ltd., Nanjing, China) using primers based on the chicken genome sequence (Table 2) that were synthesized by Sagon Biotech (Shanghai, China). Reverse transcription was performed for 15 min at 37°C and terminated for 5 s at 85°C. The gene for GAPDH was used as a reference for normalization. Quantitative real-time PCR was performed on a CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA) using a SYBR Green PCR kit (Vazyme Biotech Co., Ltd.). The real-time PCR program began with denaturation at 95°C for 30 s, followed by 40 cycles at 95°C for 10 s and 60°C for 30 s. Dissociation analysis of the amplification products was performed after each PCR run to confirm that a single PCR product was amplified and detected. Results were calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Table 2.
Primers for RT-qPCR analysis.
| Gene1 | Primer sequence (5′–3′)2 | Length (nt) | GenBank number |
|---|---|---|---|
| OCLN | F: ACGGCAGCACCTACCTCAA R: GGGCGAAGAAGCAGATGAG |
123 | XM_025144247.2 |
| CLDN2 | F: CCTACATTGGTTCAAGCATCGTGA R: GATGTCGGGAGGCAGGTTGA |
131 | NM_001277622.1 |
| TNF-α | F: GAGCGTTGACTTGGCTGTC R: AAGCAACAACCAGCTA TGCAC |
176 | NM_214022.1 |
| IL-1β | F: ACTGGGCA TCAAGGGCTA R: GGTAGAAGA TGAAGCGGGTC |
154 | NM_214005.1 |
| IL-10 | F: AGAAATCCCTCCTCGCCAAT R: AAATAGCGAACGGCCCTCA |
121 | NM_001004414.2 |
| IL-6 | F: GCTGCGCTTCTACACAGA R: TCCCGTTCTCA TCCA TCTTCTC |
203 | NM_204628.1 |
| GAPDH | F: TGCTGCCCAGAACATCATCC R: ACGGCAGGTCAGGTCAACAA |
142 | NM_204305 |
OCLN = occludin; CLDN2 = claudin2; TNF-α = tumor necrosis factor-α; IL-1β = interleukin-1β; IL-10 = interleukin-10; IL-6 = interleukin-6; GAPDH = glyceraldehyde-3-phosphate.
F = forward primer; R = reverse primer.
Statistical Analysis
Normality of all the data was performed with SPSS statistical software (ver. 20.0 for Windows, SPSS Inc., Chicago, IL) by using 1-way ANOVA followed by Duncan multiple comparison tests. Significance (P value) was evaluated at 0.05. All results were represented by mean values and the standard error of the mean (SEM).
RESULTS
Growth Performance of Broilers During LPS-Induced Immune Stress
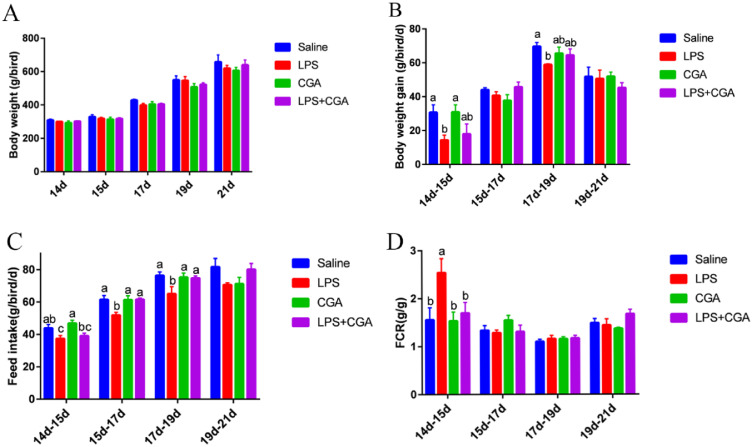
No significant difference in body weight during LPS-mediated immune stress was observed compared to the untreated control group. Similarly, CGA alone or in combination with LPS did not impact body weight (Figure 1A). In contrast, intraperitoneal injection of LPS significantly reduced (P < 0.05) the body weight gain of broilers at d 14 to 15 and 17 to 19 (Figure 1C), as well as the feed intake of broilers at d 14 to 15, 15 to 17, and 17 to 19 (P < 0.05) (Figure 1B) compared with the control group. CGA supplementation counteracted the LPS-mediated decrease in feed intake of broilers at d 15 to 17 and 17 to 19 (P < 0.05), but not the LPS-mediated reduction in body weight gain. Compared with the control group, intraperitoneal injection of LPS significantly increased (P < 0.05) the feed to meat ratio of broilers at d 14 to 15. This increase was not affected by CGA treatment (Figure 1D).
Figure 1.
Effects of dietary CGA supplementation on growth performance of LPS-challenged broilers (n = 6). (A) Body weight of broilers challenged with LPS. (B) Feed intake of broilers challenged with LPS. (C) Body weight gain of broilers challenged with LPS. (D) FCR of broilers challenged with LPS. Blue, saline; red, LPS; green, CGA; violet, LPS + CGA. Bars without the same letter differed significantly (P < 0.05).
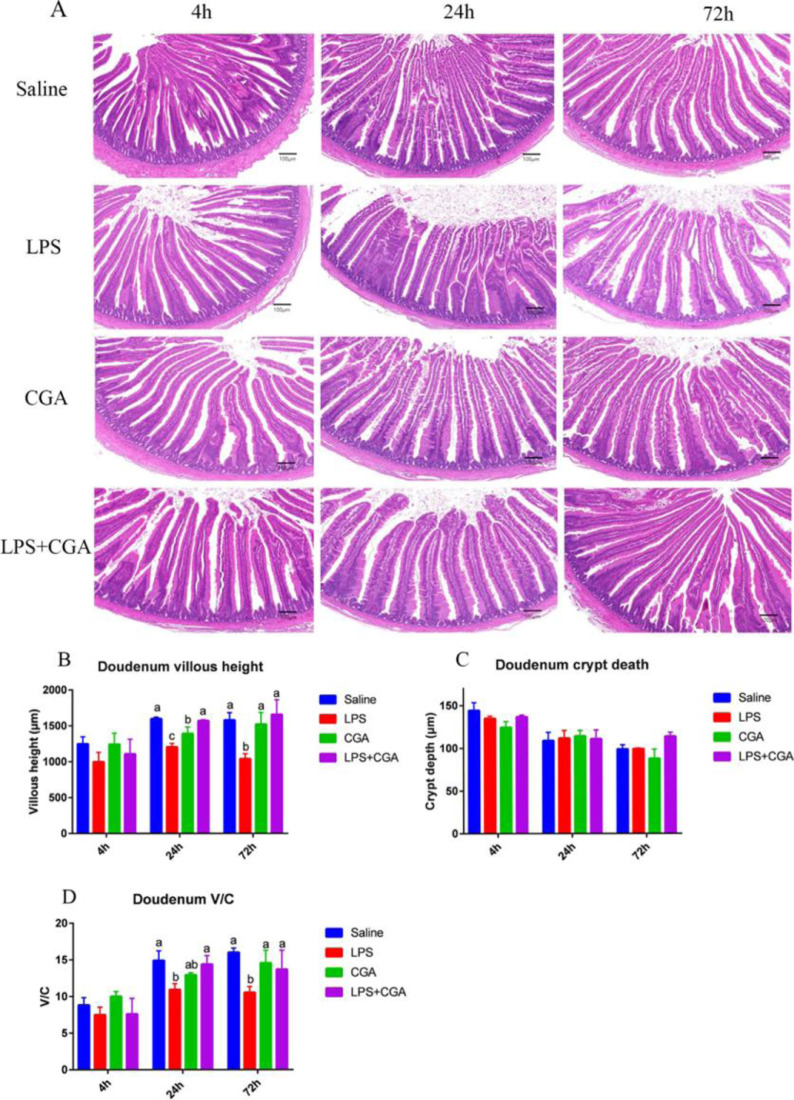
Effects of LPS and CGA on Intestinal Morphology in the Duodenum
Severe damage to the structure of duodenal villi was evident at 24 h and 72 h after intraperitoneal injection of LPS. This damage manifested both as VH shortening and morphological alterations (Figure 2A). Thus, the duodenal VH and the ratio of VH to CD at 24 h and 72 h after LPS injection were decreased in the LPS group (Figure 2B and D) compared with the saline-treated, control group (P < 0.05). No impact on duodenum CD was observed up to 72 h after LPS administration (P > 0.05) (Figure 2C). Addition of CGA alone to the broiler diet affected neither VH nor the VH:CD ratio. However, CGA fully inhibited the LPS-induced reduction in VH in the duodenum and also significantly ameliorated (P < 0.05) the impact of LPS on the VH:CD ratio (Figure 2B and D).
Figure 2.
Effects of dietary CGA supplementation on duodenum morphology of LPS-challenged broilers (n = 6). (A) Morphological structure of duodenum of broilers at 4 h, 24 h, and 72 h after LPS injection. (B) Villous height of duodenum intestine. (C) Crypt depth of duodenum intestine. (D) V/C (villous height/crypt depth) ratio of duodenum intestine. Blue, saline; red, LPS; green, CGA; violet, LPS + CGA. Bars without the same letter differed significantly (P < 0.05). Scale bar, 100 µm.
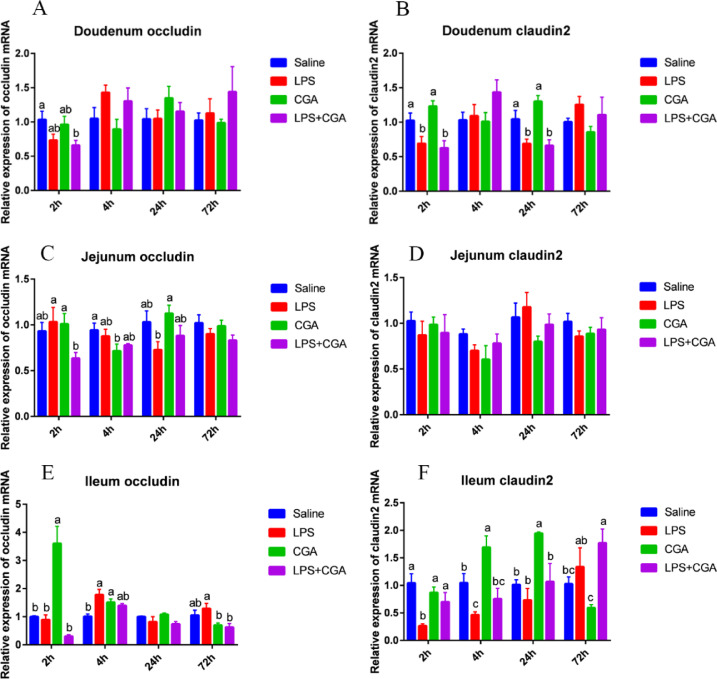
Effects of LPS and CGA on Expression of Genes Involved in Tight Junction Formation
The expression of CLDN2 in the duodenum decreased significantly (P < 0.05) in the LPS group compared with the control group 2 h and 24 h after injection, but not after 4 h or 72 h. The LPS-induced decrease in expression of CLDN2 in the duodenum after 2 h and 24 h was not relieved by inclusion of CGA in the broiler diet (Figure 3B). Similarly, the expression of CLDN2 in the ileum of the LPS group decreased significantly (P < 0.05) 2 h and 4 h after LPS injection, but not after 24 h or 72 h. However, dietary CGA supplementation restored CLDN2 expression at the 2 h timepoint (P < 0.05) (Figure 3F). Moreover, expression of ileum CLDN2 mRNA increased significantly (P < 0.05) in the CGA group after 4 h and 24 h compared with the saline, control group (Figure 3F). Intraperitoneal injection of LPS and/or dietary CGA supplementation did not significantly affect jejunal CLDN2 mRNA expression (Figure 3D). No major changes in expression of the OCLN mRNA expression were observed in the duodenum (Figure 3A) or jejunum (Figure 3C) following LPS and/or CGA treatment. However, expression of this gene spiked significantly (P < 0.05) after 2 h in ileum tissue of broilers fed a CGA-supplemented diet (Figure 3E).
Figure 3.
Effects of dietary CGA supplementation on mRNA levels of tight junctions of LPS-challenged broilers (n = 6). Relative expression of the gene for duodenum occluding (A) and claudin2 (B). Relative expression of the gene for jejunum occluding (C) and claudin2 (D). Relative expression of the gene for ileum occluding (E) and claudin2 (F). The gene for GAPDH was used as a reference for normalization. Blue, saline; red, LPS; green, CGA; violet, LPS+CGA. Bars without the same letter differed significantly (P < 0.05).
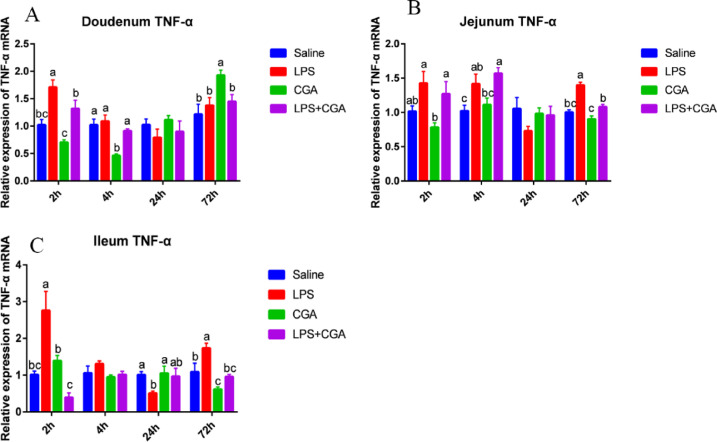
Effects of LPS and CGA on Expression of Genes Involved in the Inflammatory Response
Compared with the saline alone group, expression of the TNF-α mRNA decreased significantly (P < 0.05) in the duodenum at 4 h (Figure 4A) and in the ileum at 72 h (Figure 4C) of broilers that were fed a CGA supplement. In contrast, CGA induced an increase (P < 0.05) in TNF-α expression at 72 h in the duodenum (Figure 4A). Intraperitoneal injection of LPS caused a significant increase (P < 0.05) in TNF-α mRNA expression in the duodenum after 2 h (Figure 4A), in the jejunum after 4 h and 72 h (Figure 4B), and in the ileum after 2 h and 72 h (Figure 4C). However, dietary CGA supplementation significantly decreased (P < 0.05) the levels of TNF-α mRNA in the duodenum after 2 h (Figure 4A), in the jejunum after 72 h (Figure 4B), and in the ileum after 2 h and 72 h (Figure 4C) following LPS injection compared with the LPS alone group.
Figure 4.
Effects of dietary CGA supplementation on mRNA levels of TNF-α in the broiler intestine after LPS injection (n = 6). Relative expression of the gene for TNF-α in the duodenum (A), jejunum (B), and ileum (C) after LPS injection. TNF-α = tumor necrosis factor-α. The gene for GAPDH was used as a reference for normalization. Blue, saline; red, LPS; green, CGA; violet, LPS + CGA. Bars without the same letter differed significantly (P < 0.05).
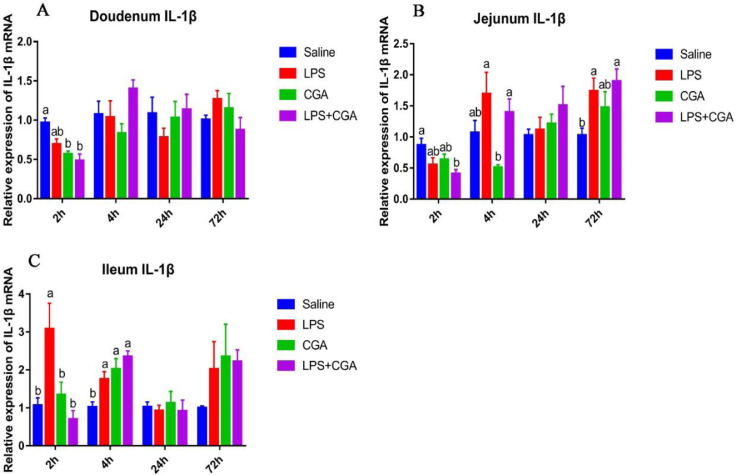
The changes in IL-1β mRNA expression in each intestinal segment are illustrated in Figure 5. The most notable changes were that CGA significantly decreased (P < 0.05) IL-1β expression in the duodenum at 2 h (Figure 5A). Intraperitoneal injection of LPS significantly increased (P < 0.05) the expression of IL-1β mRNA both in the jejunum 72 h after injection (Figure 5B), and in the ileum 2 h and 4 h after injection (Figure 5C). Dietary CGA significantly reduced (P < 0.05) IL-1β mRNA expression in the ileum 2 h after LPS administration compared with the LPS alone group (Figure 5C).
Figure 5.
Effects of dietary CGA supplementation on mRNA levels of IL-1β in the broiler intestine after LPS injection (n = 6). Relative expression of the gene for IL-1β in the duodenum (A), jejunum (B), and ileum (C) after LPS injection. IL-1β = interleukin-1β. The gene for GAPDH was used as a reference for normalization. Blue, saline; red, LPS; green, CGA; violet, LPS + CGA. Bars without the same letter differed significantly (P < 0.05).
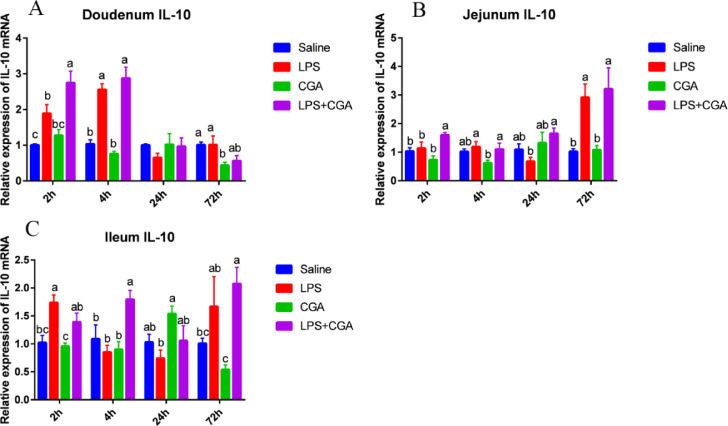
The expression of IL-10 mRNA decreased significantly (P < 0.05) in the duodenum of broilers fed with CGA at 72 h compared with the saline group (Figure 6A). In contrast, intraperitoneal injection of LPS significantly increased (P < 0.05) IL-10 expression in the duodenum 2 h and 4 h after injection (Figure 6A), in the jejunum 72 h after injection (Figure 6B), and in the ileum 2 h after injection (Figure 6C). Compared with the LPS alone group, dietary CGA supplementation significantly increased (P < 0.05) IL-10 mRNA expression in the duodenum 2 h after LPS injection (Figure 6A), in the jejunum 2 h and 24 h after injection (Figure 6B), and in the ileum 4 h after LPS administration (Figure 6C).
Figure 6.
Effects of dietary CGA supplementation on mRNA levels of IL-10 in the broiler intestine after LPS injection (n = 6). Relative expression of the gene for IL-10 in the duodenum (A), jejunum (B), and ileum (C) after LPS injection. IL-10 = interleukin-10. The gene for GAPDH was used as a reference for normalization. Blue, saline; red, LPS; green, CGA; violet, LPS + CGA. Bars without the same letter differed significantly (P < 0.05).
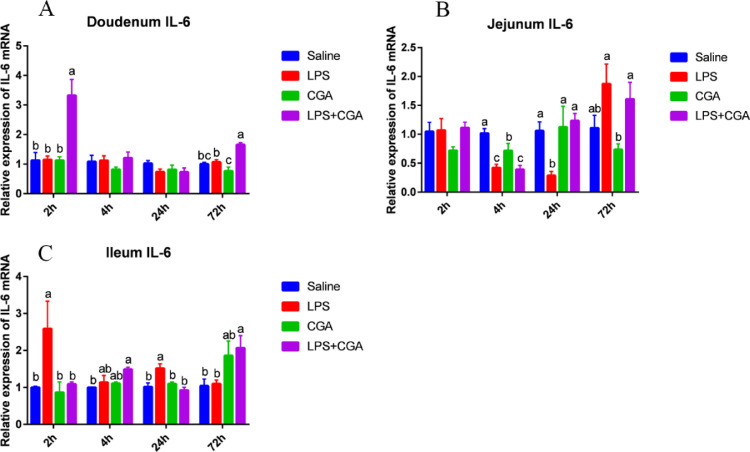
Expression of IL-6 mRNA decreased significantly (P < 0.05) after 4 h in the jejunum of broilers fed with CGA compared with the saline group (Figure 7B). Intraperitoneal injection of LPS reduced (P < 0.05) IL-6 mRNA levels in the jejunum 4 h and 24 h after injection (Figure 7B), but IL-6 expression in the ileum was enhanced (P < 0.05) after 2 h and 24 h of LPS treatment (Figure 7C). Coadministration of dietary CGA and LPS significantly increased (P < 0.05) the expression of IL-6 in the duodenum 2 h and 72 after LPS injection (Figure 7A), in the jejunum 24 h after injection (Figure 7B), and in the ileum 72 h after injection (Figure 7C). In contrast, dietary CGA supplementation reduced the expression of IL-6 mRNA in ileum 2 h and 24 h after LPS injection (Figure 7C).
Figure 7.
Effects of dietary CGA supplementation on mRNA levels of IL-6 in the broiler intestine after LPS injection (n = 6). Relative expression of the gene for IL-6 in the duodenum (A), jejunum (B), and ileum (C) after LPS injection. IL-6 = interleukin-6. The gene for GAPDH was used as a reference for normalization. Blue, saline; red, LPS; green, CGA; violet, LPS/CGA. Bars without the same letter differed significantly (P < 0.05).
DISCUSSION
LPS, a major component of the cell wall of gram-negative bacteria, is a highly potent activator of the innate immune system (Freudenberg et al., 2008; Rossol et al., 2011). Exposure to LPS alters the intrinsic barrier function and the permeability of the small intestine which results in immune stress and poor growth performance in poultry (Wang et al., 2015b). For example, intraperitoneal injection of LPS (500 μg/kg body weight) at d 16, 18, and 20 reduced the ADG and ADFI and increased FCR of Arbor Acre broiler chickens from d 16 to 21 (Li et al., 2015). Similarly, broilers that received intraperitoneal injections of LPS (1 mg/kg body weight) at d 17, 19, and 21 exhibited reduced ADG and ADFI, and increased FCR at d 17 to 21 (Chen et al., 2018d). In this study, we obtained equivalent experimental results: LPS injection at 500 μg/kg body weight significantly reduced both the body weight gain of broilers at d 14 to 15 and 17 to 19 and the feed intake during d 14 to 15, 15 to 17, and 17 to 19, but increased the FCR of broilers on d 14 to 15. These initial results indicated that the immune stress model was established successfully and was suitable for subsequent investigation of the effects of CGA on broiler health. The impaired growth performance of LPS-challenged broilers is attributable to reduced appetite and repartitioning of nutrients to improve immune system development (Spurlock, 1997) which consequently releases fewer nutrients for chicken growth. It was reported that dietary supplementation with CGA increased the ADG and decreased the feed: gain ratio in broilers challenged with the synthetic glucocorticoid DEX (Liu et al., 2022b). Dietary CGA supplementation also may improve the performance of broilers infected with C. perfringens type A by increasing ADG and reducing FCR (Zhang et al., 2020). Analogously, our data demonstrate that providing a CGA-supplemented diet restored the feed intake of 15 to 17-day and 17 to 19-day broilers treated with LPS.
The development of gastrointestinal organs impacts the subsequent digestive and absorptive capacities of broilers. The small intestine is a key site for digestion and small intestinal mucosa with normal structure is necessary for nutrient digestion and absorption as well as for optimal growth (Gao et al., 2018). VH, CD, and VH:CD values reflect the integrity, development status, and nutrient absorption capacity of the animal intestine (Xu et al., 2003). Lower VH leads to reductions in the villus surface area, thereby resulting in a decreased absorptive ability, whereas decreased intestinal VH:CD is accompanied by fewer mature and functional enterocytes (Cairo et al., 2018). Immune stress induced by LPS in livestock and poultry causes damage to the morphology of intestinal villi which mainly manifests as villus necrosis, reduction of VH, and increase of CD (Pi et al., 2014; Li et al., 2015; Zheng et al., 2020). Similarly, we found here that intraperitoneal LPS injection severely injured villus morphology and reduced the VH and VH:CD in the broiler duodenum, but that dietary CGA supplementation significantly ameliorated this morphological damage. Dietary supplementation with CGA increased VH in coccidia-infected broilers (Liu et al., 2022a). CGA supplementation also exerted a positive influence on intestinal morphology in weaned piglets, including an increase in duodenal and jejunal VH and the VH:CD ratio, and a decrease in duodenal CD (Zhang et al., 2018). These observations suggest an improvement in the digestive and absorption functions of the intestine as a result of CGA intake and imply that CGA contributes to nutrient absorption through positive intestinal histological changes with consequent improved growth performance.
Maintaining and optimizing intestinal barrier function has important implications for the health and performance of broilers (Gilani et al., 2021). Tight junctions form dynamic intestinal epithelial barriers (Wang et al., 2014). These multiprotein, transmembrane complexes that include claudins, occludin, junctional adhesion molecule, and tricellulin proteins, interact with cytosolic scaffold proteins, including zonula occludens proteins, and mediate intestinal barrier functions and cell permeability (Suzuki, 2013). It is reported that broilers treated with LPS (1 mg/kg of body weight) in which the expression of the genes for zonula occludens-2 and occludin proteins were downregulated in the jejunum (Wang et al., 2014). Decreased zonula occludens-1 expression also was noted in the ileal mucosa of LPS-treated broilers (Chen et al., 2018d). Here, intraperitoneal injection of LPS decreased expression of the mRNA for CLDN2 in the duodenum and ileum at different timepoints. Under stress situation, dietary supplementation with CGA (0.5 g/kg) increased expression of OCLN in the ileum of DEX-challenged broilers (Liu et al., 2022b). Conversely, dietary CGA supplementation had no effect on OCLN expression in the duodenum of heat-stressed broilers (Chen et al., 2021) which may reflect differences in the species of chicken, the type of stress, and the CGA dosage. In the current research, downregulation of CLDN2 by LPS challenge was prevented by dietary CGA supplementation which supports the hypothesis that CGA elevates the expression of genes involved in tight junction formation and thereby protects intestinal barrier integrity from the detrimental effects of LPS. The upregulation of the OCLN and CLDN2 genes in the ileum of unchallenged broilers indicates that CGA may promote an improved intestinal barrier under general physiological conditions. In summary, we propose that CGA supplementation at 0.5 g/kg may enhance the expression of tight junctions in the small intestine and improve the intestinal barrier function of LPS-stressed broiler chickens.
The intestinal barrier impairment induced by pathogens or toxins frequently is accompanied by inflammation (Camilleri et al., 2012). LPS is the ligand of Toll-like receptor 4 (TLR4). LPS initiates a cascade of intracellular signal transduction after binding to the extracellular domain of TLR4 which promotes the secretion of numerous cytokines, including TNF-α and IL-1β (Angrisano et al., 2010). Accordingly, LPS-treated broilers exhibited higher expression of the proinflammatory cytokine in the jejunum and ileal mucosa (Wang et al., 2014; Chen et al., 2018d). In our study, intraperitoneal injection of LPS significantly increased the expression of TNF-α and IL-1β mRNAs in each intestinal segment at different points which confirms LPS-mediated inflammation in these broilers. Interestingly, IL-1β and TNF-α produced by the inflammatory response rearrange tight junction proteins and disrupt intestinal barrier function (Dai et al., 2022) which is consistent with the poorer expression of tight junction genes following LPS treatment that was described above. IL-10 plays important roles in the immune and inflammation responses which directly affect T and B lymphocytes and inhibit the synthesis of proinflammatory cytokines (Rothwell et al., 2004; Prasad et al., 2011). In this study, LPS treatment of broilers increased the expression of the IL-10 anti-inflammatory cytokine in each intestinal segment at different timepoints after injection. The imbalance of proinflammatory and anti-inflammatory factors is important during intestinal barrier damage and changes in cytokine profiles during inflammation are complex. IL-6 is a pleiotropic cytokine with both proinflammatory and anti-inflammatory effects (Suzuki et al., 2011). Here, intraperitoneal injection of LPS reduced the expression of IL-6 in the jejunum of broilers, but increased the expression of IL-6 mRNA in the ileum which confirms that the broilers were experiencing stress.
CGA has shown promising anti-inflammatory effects both in vitro and in vivo (Hwang et al., 2014; Ruan et al., 2014; Palócz et al., 2016; Chen et al., 2021). For example, dietary CGA inhibited the expression of proinflammatory cytokines in ileal epithelia of broilers after infection with C. perfringens type A (Zhang et al., 2020). Moreover, acute heat stress caused significant duodenal damage and CGA supplementation decreased the expression of IL-1β and TNF-α in the duodenum of young hens (Chen et al., 2021). Here, dietary CGA supplementation after LPS injection decreased the expression of TNF-α in each intestinal segment at different points, decreased the expression of IL-1β in the ileum after 2 h, increased IL-10 expression in each intestinal segment at different timepoints, and increased the expression of IL-6 mRNA in the ileum at the later stage of LPS injection. In addition, compared with the saline group, we found that chlorogenic acid addition decreased the mRNA expression of TNF-α, IL-1β, and IL-10 in the duodenum at different timepoints and decreased the expression of IL-6 in the jejunum of broilers under normal rearing condition. These results further demonstrate the potential of chlorogenic acid to relieve intestinal inflammation. Thus, CGA supplementation may attenuate the intestinal inflammatory response, thereby enhancing the integrity of the intestinal barrier and improving the performance of LPS-treated broilers.
In conclusion, we demonstrated that CGA has a promising protective effect on the intestinal morphology and growth performance of LPS-treated broilers. Furthermore, dietary supplementation with CGA attenuated intestinal inflammation and improved intestinal barrier function. Our findings provide new insights into the ability of CGA to promote intestinal health in broilers and suggest that further studies on the impact of CGA on broiler fitness will be highly informative.
ACKNOWLEDGMENTS
The authors are grateful to the College of Animal Science and Technology, Henan University of Science and Technology for the use of experimental facilities, and particularly acknowledge the International Joint Lab for Animal Welfare and Health Breeding of Henan Province and Expat Scientist Studio for Animal Stress and Health Breeding of Henan Province and Longmen Laboratory for the supportive academic advice provided during these studies.
Author Contributions: Investigation, H. T. (Haiqiu Tan); methodology, K. L. and X. H.; software, H. T. and Y. L. (Yanhao Liu); data curation, Y. L. (Yuqian Li) and X. H.; writing-original draft preparation, H. T. and W. Z.; result interpretation, H. T. and Y. Z.; writing-review and editing, H. T., W. Z., D. B., K. I., and B. Z.; supervision, Y. M.; project administration, Y. M. All authors have read and agreed to the published version of the manuscript.
Funding: This work was partly supported by National Key Research and Development Program of China (Grant Number 2022YFE0111100, Grant Number 2017YFE0129900), and the Key Scientific Research Foundation of the Higher Education Institutions of He-nan Province (Grant Number 22A230001), and the Science Foundation for Expat Scientist Studio for Animal Stress and Health Breeding of Henan Province (Grant Number GZS2021006).
Institutional Review Board Statement: The animal study protocol was approved by the Animal Care and Use Committee of Henan University of Science and Technology, and the management and experimental procedures of the experiment animals complied with the regulations of the Institutional Animal Care and Use Committee.
DISCLOSURES
The authors declare that there are no conflicts of interest exit in the submission of this manuscript, and manuscript was approved by all authors for publication. I would like to declare on behalf of my coauthors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere.
REFERENCES
- Angrisano T., Pero R., Peluso S., Keller S., Sacchetti S., Bruni C.B., Chiariotti L., Lembo F. LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol. 2010;10:172. doi: 10.1186/1471-2180-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D., Liu K., He X., Tan H., Liu Y., Li Y., Zhang Y., Zhen W., Zhang C., Ma Y. Effect of dietary chlorogenic acid on growth performance, antioxidant function, and immune response of broiler breeders under immune stress and stocking density stress. Vet. Sci. 2022;9:582. doi: 10.3390/vetsci9100582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo P.L.G., Gois F.D., Sbardella M., Silveira H., de Oliveira R.M., Allaman I.B., Cantarelli V.S., Costa L.B. Effects of dietary supplementation of red pepper (Schinus terebinthifolius Raddi) essential oil on performance, small intestinal morphology and microbial counts of weanling pigs. J. Sci. Food Agric. 2018;98:541–548. doi: 10.1002/jsfa.8494. [DOI] [PubMed] [Google Scholar]
- Camilleri M., Madsen K., Spiller R., Greenwood-Van Meerveld B., Verne G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Chen X., Qiu S., Hu Y., Jiang C., Wang D., Fan Q., Zhang C., Huang Y., Yu Y., Yang H., Liu C., Gao Z., Hou R., Li X. Effects of epimedium polysaccharide-propolis flavone oral liquid on mucosal immunity in chickens. Int. J. Biol. Macromol. 2014;64:6–10. doi: 10.1016/j.ijbiomac.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Chen J., Li Y., Yu B., Chen D., Mao X., Zheng P., Luo J., He J. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 2018;96:1108–1118. doi: 10.1093/jas/skx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Xie H., Chen D., Yu B., Mao X., Zheng P., Yu J., Luo Y., Luo J., He J. Chlorogenic acid improves intestinal development via suppressing mucosa inflammation and cell apoptosis in weaned pigs. ACS Omega. 2018;3:2211–2219. doi: 10.1021/acsomega.7b01971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Yu B., Chen D., Huang Z., Mao X., Zheng P., Yu J., Luo J., He J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018;59:84–92. doi: 10.1016/j.jnutbio.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Cheng Y., Li Y., Wen C., Zhou Y. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 2018;119:1254–1262. doi: 10.1017/S0007114518000740. [DOI] [PubMed] [Google Scholar]
- Chen F., Zhang H., Zhao N., Yang X., Du E., Huang S., Guo W., Zhang W., Wei J. Effect of chlorogenic acid on intestinal inflammation, antioxidant status, and microbial community of young hens challenged with acute heat stress. Anim. Sci. J. 2021;92:e13619. doi: 10.1111/asj.13619. [DOI] [PubMed] [Google Scholar]
- Dai D., Qi G., Wang J., Zhang H., Qiu K., Han Y., Wu Y., Wu S. Dietary organic acids ameliorate high stocking density stress-induced intestinal inflammation through the restoration of intestinal microbiota in broilers. J. Anim. Sci. Biotechnol. 2022;13:124. doi: 10.1186/s40104-022-00776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M.A., Tchaptchet S., Keck S., Fejer G., Huber M., Schütze N., Beutler B., Galanos C. Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: benefits and hazards of LPS hypersensitivity. Immunobiology. 2008;213:193–203. doi: 10.1016/j.imbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Gao T., Zhao M.M., Li Y.J., Zhang L., Li J.L., Yu L.L., Gao F., Zhou G.H. Effects of in ovo feeding of L-arginine on the development of digestive organs, intestinal function and post-hatch performance of broiler embryos and hatchlings. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018;102:e166–e175. doi: 10.1111/jpn.12724. [DOI] [PubMed] [Google Scholar]
- Gilani S., Chrystal P.V., Barekatain R. Current experimental models, assessment and dietary modulations of intestinal permeability in broiler chickens. Anim. Nutr. 2021;7:801–811. doi: 10.1016/j.aninu.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X.X., Su X.S., Zhan K., Zhao G.Q. The protective effect of chlorogenic acid on bovine mammary epithelial cells and neutrophil function. J. Dairy Sci. 2018;101:10089–10097. doi: 10.3168/jds.2017-14328. [DOI] [PubMed] [Google Scholar]
- Han M., Song P., Huang C., Rezaei A., Farrar S., Brown M.A., Ma X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. OncoTargets Ther. 2016;7:80313–80326. doi: 10.18632/oncotarget.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.J., Kim Y.W., Park Y., Lee H.J., Kim K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014;63:81–90. doi: 10.1007/s00011-013-0674-4. [DOI] [PubMed] [Google Scholar]
- Ji X., Wang B., Paudel Y.N., Li Z., Zhang S., Mou L., Liu K., Jin M. Protective effect of chlorogenic acid and its analogues on lead-induced developmental neurotoxicity through modulating oxidative stress and autophagy. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.655549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang H., Chen Y.P., Yang M.X., Zhang L.L., Lu Z.X., Zhou Y.M., Wang T. Bacillus amyloliquefaciens supplementation alleviates immunological stress in lipopolysaccharide-challenged broilers at early age. Poult. Sci. 2015;94:1504–1511. doi: 10.3382/ps/pev124. [DOI] [PubMed] [Google Scholar]
- Liang N., Kitts D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. 2015;8:16. doi: 10.3390/nu8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Chen P., Lv X., Zhou Y., Li X., Ma S., Zhao J. Effects of chlorogenic acid on performance, anticoccidial indicators, immunity, antioxidant status, and intestinal barrier function in coccidia-infected broilers. Animals (Basel) 2022;12:963. doi: 10.3390/ani12080963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Li X., Shi S., Zhou Y., Zhang K., Wang Y., Zhao J. Chlorogenic acid improves growth performance and intestinal health through autophagy-mediated nuclear factor erythroid 2-related factor 2 pathway in oxidatively stressed broilers induced by dexamethasone. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Miao M., Xiang L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020;87:71–88. doi: 10.1016/bs.apha.2019.12.002. [DOI] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry. 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. [Google Scholar]
- Palócz O., Pászti-Gere E., Gálfi P., Farkas O. Chlorogenic acid combined with Lactobacillus plantarum 2142 reduced LPS-induced intestinal inflammation and oxidative stress in IPEC-J2 cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi D., Liu Y., Shi H., Li S., Odle J., Lin X., Zhu H., Chen F., Hou Y., Leng W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014;25:456–462. doi: 10.1016/j.jnutbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Prasad A.S., Bao B., Beck F.W., Sarkar F.H. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 2011;27:816–823. doi: 10.1016/j.nut.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Hu Y.L., Cui B.A., Zhang H.Y., Kong X.F., Wang D.Y., Wang Y.G. Immunopotentiating effects of four Chinese herbal polysaccharides administered at vaccination in chickens. Poult. Sci. 2007;86:2530–2535. doi: 10.3382/ps.2007-00076. [DOI] [PubMed] [Google Scholar]
- Rossol M., Heine H., Meusch U., Quandt D., Klein C., Sweet M.J., Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- Rothwell L., Young J.R., Zoorob R., Whittaker C.A., Hesketh P., Archer A., Smith A.L., Kaiser P. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 2004;173:2675–2682. doi: 10.4049/jimmunol.173.4.2675. [DOI] [PubMed] [Google Scholar]
- Ruan Z., Liu S., Zhou Y., Mi S., Liu G., Wu X., Yao K., Assaad H., Deng Z., Hou Y., Wu G., Yin Y. Chlorogenic acid decreases intestinal permeability and increases expression of intestinal tight junction proteins in weaned rats challenged with LPS. PLoS One. 2014;9:e97815. doi: 10.1371/journal.pone.0097815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.S., Satsu H., Bae M.J., Zhao Z., Ogiwara H., Totsuka M., Shimizu M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015;168:167–175. doi: 10.1016/j.foodchem.2014.06.100. [DOI] [PubMed] [Google Scholar]
- Spurlock M.E. Regulation of metabolism and growth during immune challenge: an overview of cytokine function. J. Anim. Sci. 1997;75:1773–1783. doi: 10.2527/1997.7571773x. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Yoshinaga N., Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011;286:31263–31271. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Ohta N., Akiba Y. Influences of dietary methionine and cysteine on metabolic responses to immunological stress by Escherichia coli lipopolysaccharide injection, and mitogenic response in broiler chickens. Br. J. Nutr. 1997;78:815–821. doi: 10.1079/bjn19970197. [DOI] [PubMed] [Google Scholar]
- Tang L.P., Li W.H., Liu Y.L., Lun J.C., He Y.M. Heat stress aggravates intestinal inflammation through TLR4-NF-κB signaling pathway in Ma chickens infected with Escherichia coli O157:H7. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay R., Mohan Rao L.J. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013;53:968–984. doi: 10.1080/10408398.2011.576319. [DOI] [PubMed] [Google Scholar]
- Vukelic I., Detel D., Pucar L.B., Potocnjak I., Buljevic S., Domitrovic R. Chlorogenic acid ameliorates experimental colitis in mice by suppressing signaling pathways involved in inflammatory response and apoptosis. Food Chem. Toxicol. 2018;121:140–150. doi: 10.1016/j.fct.2018.08.061. [DOI] [PubMed] [Google Scholar]
- Wang L., Bi C., Cai H., Liu B., Zhong X., Deng X., Wang T., Xiang H., Niu X., Wang D. The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase A inhibition. Front. Microbiol. 2015;6:1031. doi: 10.3389/fmicb.2015.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li Y., Shen J., Wang S., Yao J., Yang X. Effect of Astragalus polysaccharide and its sulfated derivative on growth performance and immune condition of lipopolysaccharide-treated broilers. Int. J. Biol. Macromol. 2015;76:188–194. doi: 10.1016/j.ijbiomac.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Wang X., Shen J., Li S., Zhi L., Yang X., Yao J. Sulfated Astragalus polysaccharide regulates the inflammatory reaction in LPS-infected broiler chicks. Int. J. Biol. Macromol. 2014;69:146–150. doi: 10.1016/j.ijbiomac.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Wu Y., Liu W., Li Q., Li Y., Yan Y., Huang F., Wu X., Zhou Q., Shu X., Ruan Z. Dietary chlorogenic acid regulates gut microbiota, serum-free amino acids and colonic serotonin levels in growing pigs. Int. J. Food Sci. Nutr. 2018;69:566–573. doi: 10.1080/09637486.2017.1394449. [DOI] [PubMed] [Google Scholar]
- Xie H., Rath N.C., Huff G.R., Huff W.E., Balog J.M. Effects of Salmonella typhimurium lipopolysaccharide on broiler chickens. Poult. Sci. 2000;79:33–40. doi: 10.1093/ps/79.1.33. [DOI] [PubMed] [Google Scholar]
- Xu Z.R., Hu C.H., Xia M.S., Zhan X.A., Wang M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Y., Chen D., Yu B., Zheng P., Mao X., Luo Y., Li Y., He J. Dietary chlorogenic acid supplementation affects gut morphology, antioxidant capacity and intestinal selected bacterial populations in weaned piglets. Food Funct. 2018;9:4968–4978. doi: 10.1039/c8fo01126e. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhao Q., Ci X., Chen S., Xie Z., Li H., Zhang H., Chen F., Xie Q. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type A. Poult. Sci. 2020;99:6606–6618. doi: 10.1016/j.psj.2020.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhong X., Zhou Y., Wang G., Du H., Wang T. Dietary RRR-α-tocopherol succinate attenuates lipopolysaccharide-induced inflammatory cytokines secretion in broiler chicks. Br. J. Nutr. 2010;104:1796–1805. doi: 10.1017/S0007114510002801. [DOI] [PubMed] [Google Scholar]
- Zhao J.S., Deng W., Liu H.W. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019;98:3040–3049. doi: 10.3382/ps/pez081. [DOI] [PubMed] [Google Scholar]
- Zhen W., Shao Y., Gong X., Wu Y., Geng Y., Wang Z., Guo Y. Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella Enteritidis. Poult. Sci. 2018;97:2654–2666. doi: 10.3382/ps/pey119. [DOI] [PubMed] [Google Scholar]
- Zheng C., Song B., Duan Y., Zhong Y., Yan Z., Zhang S., Li F. Dietary β-hydroxy-β-methylbutyrate improves intestinal function in weaned piglets after lipopolysaccharide challenge. Nutrition. 2020;78 doi: 10.1016/j.nut.2020.110839. [DOI] [PubMed] [Google Scholar]
- Zhou H.J., Kong L.L., Zhu L.X., Hu X.Y., Busye J., Song Z.G. Effects of cold stress on growth performance, serum biochemistry, intestinal barrier molecules, and adenosine monophosphate-activated protein kinase in broilers. Animal. 2021;15 doi: 10.1016/j.animal.2020.100138. [DOI] [PubMed] [Google Scholar]