Abstract
Objective
In recent years, the use of a ketogenic diet (KD) against obesity has gained popularity in KSA. This study was designed to determine the impact of KD on anthropometric indices and on the abnormal regulation of inflammatory activities in obese Saudi women. Moreover, we investigated the potential of beta-hydroxybutyrate (BHB) supplementation on the inhibition of pro-inflammatory activities.
Methods
We enrolled 31 Saudi women (aged, 35.3 ± 8.4 years) with an average BMI of 33.96 ± 4.44 kg/m2 underwent an 8-week KD (8KD) from January to March 2021. Changes in anthropometric measurements were collected at baseline and after 4–8 weeks of intervention. Compliance with the dietary regimen was monitored weekly by plasma BHB level.
Results
Twenty-nine females commenced the diets and 23 completed the study (a 79% completion rate). In comparison to pre-intervention, the 8KD resulted in a significant increase in the levels of plasma BHB (P < 0.001) throughout the duration of the trial. This was accompanied by a significant reduction in weight loss (7.7 kg ± 11.3; P < 0.001), BMI, waist circumference (P < 0.001), and levels of the inflammatory cytokine IL-1β (P < 0.001).
Conclusions
An 8-week KD was found to be useful in producing a positive impact on anthropometric indices, biochemical and inflammatory processes. This study indicated that the intake of a KD by obese Saudi women induced the release of BHB in the blood without stimulation of an overall starvation response. This may be useful to alleviate the severity of chronic inflammatory disorders associated with obesity.
Keywords: Inflammation, Ketogenic diet, Obesity, Saudi, Women
المخلص
أهداف البحث
في السنوات الأخيرة، اكتسب استخدام النظام الغذائي الكيتوني ضد السمنة شعبية في المملكة العربية السعودية. صممت هذه الدراسة لتحديد تأثير النظام الغذائي الكيتوني على مؤشرات قياس الجسم وعلى التنظيم غير الطبيعي للأنشطة الالتهابية لدى النساء السعوديات البدينات. علاوة على ذلك، بحثت الدراسة أيضا في إمكانات مكملات بيتا هيدروكسي بوتيرات في تثبيط الأنشطة المسببة للالتهابات.
طرق البحث
تم تسجيل 31 سيدة سعودية (بعمر 35.3 ± 8.4 سنة) بمتوسط مؤشر كتلة الجسم 33.96 ± 4.44 كجم / م 2 لمدة 8 أسابيع في النظام الغذائي الكيتوني من يناير إلى مارس 2021. تم جمع التعديلات في القياسات البشرية في الأساس وبعد 4-8 أسابيع من التدخل. تمت مراقبة الامتثال للنظام الغذائي أسبوعيا عن طريق مستوى بيتا هيدروكسي بوتيرات في البلازما.
النتائج
بدأت تسع وعشرون أنثى النظام الغذائي وأكملت 23 الدراسة (معدل إتمام 79٪). بالمقارنة مع ما قبل التدخل، أدى النظام الغذائي الكيتوني إلى زيادة كبيرة في مستويات البلازما بيتا هيدروكسي بوتيرات المكتشفة طوال مدة التجربة. من ناحية أخرى، أدى النظام الغذائي الكيتوني إلى انخفاض كبير في فقدان الوزن (7.7 كجم ± 11.3)، مؤشر كتلة الجسم ، محيط الخصر، مستويات السيتوكينات الالتهابية.
الاستنتاجات
وجد أن النظام الغذائي الكيتوني لمدة ثمانية أسابيع مفيد في إحداث تأثير إيجابي على مؤشرات القياسات البشرية والعمليات الكيميائية الحيوية والالتهابات. أشارت الدراسة إلى أن تناول النساء البدينات المصابات بمرض السكري يؤدي إلى إفراز بيتا هيدروكسي بوتيرات في الدم دون تحفيز استجابة الجوع الشاملة، مما قد يكون مفيدا في التخفيف من حدة الاضطرابات الالتهابية المزمنة المرتبطة بالسمنة.
الكلمات المفتاحية: النظام الغذائي الكيتوني, بدانة, التهاب, سعودي, نحيف
Introduction
Obesity is one of the most significant challenges as its prevalence is rising globally, including the Kingdom of Saudi Arabia (KSA).1,2 According to the KSA statistics in the late 1980s to the mid-1990s, the national obesity rate was about 20%, varying from 13.1% among men to 26.6% among women. However, all prevalence estimates from 1995 and onwards are greater than 35%.3 Obesity is also linked to the development of a persistent low-grade inflammation caused by adipocyte growth and fat mass expansion which allows macrophages to penetrate the adipose tissue.4 In recent years, the management of calories with a ketogenic diet (KD) has been proposed and has gained significant attention of obese people as it has been proven to be an effective way to reduce weight.5,6 Several reports showed that the applicability of KD in a normal diet has no harmful effects on the management of total cholesterol, low-density lipoprotein (LDL) or the levels of triglycerides (TG).5 Moreover, it is also documented that the regular usage of KD also insulin levels, improves physical performance, and reduces the chances of obesity onset.7, 8, 9 It is now well established that the KD dietary approach has a positive impact on the obese population in both physiological and biochemical terms and is also helpful in triggering successful weight loss and reducing the chances of a number of human disorders, including diabetes and cardiovascular diseases.10, 11, 12 It is well documented that ketosis is one of the key factors in the effectiveness of very low-energy diets (VLED) and importantly that KD is reported to suppress the drive to eat, thus leading to improvements in Body Mass Index (BMI) and a reduced chance of excess weight and obesity.13, 14, 15 An important observation by Davidson and colleagues, suggested that ketones may protect against the cognitive impairment induced by weight gain and obesity.16 Elevated levels of ketones were shown to have therapeutic neuroprotective effects in the hippocampus, the specific brain region associated with cognitive performance.16,17 The precise mechanism(s) by which the KD affects the body and reduces seizures is currently unknown, although it is known that the diet mimics the same response to starving, where the brain uses ketone bodies as its fuel instead of glucose.18 It has now been well established that a KD increases the production of beta-hydroxybutyrate (BHB), which is known for promoting weight and fat loss.19 BHB is a metabolic product synthesized by the body and known to provide energy under starvation conditions.20 Due to high demands, BHB has been synthesized artificially in the laboratory and is commercially available in the market as a supplement for the management of obesity or weight loss.19, 20, 21 BHB seems to improve brain and nerve function and is also known to provide energy to muscles and to increase the ability to perform physical activities. In addition, BHB is also a good treatment for dry eye, migraine, Alzheimer's disease, Parkinson's disease and many human disorders, although solid scientific evidence is still needed to support these potential applications of BHB.19, 20, 21, 22 This study was undertaken to investigate the impact of KD on various anthropometric indices and on the abnormal regulation of inflammatory activities in obese Saudi women. Moreover, we also determined the potential of BHB as a supplement against the onset or progression of pro-inflammatory activities in obese women.
Materials and Methods
Participants, study design and ketogenic diet
Saudi women (aged range: 20–50 years) with a BMI >30 kg/m2 were recruited from the Buraidah city of KSA via advertisements on WhatsApp. All participating women were obese (BMI >30 kg/m2) but free from any disabilities and chronic diseases. All participants were medically examined at the Nutrition Clinic in Buraidah Central Hospital. All women with cardiovascular diseases, uncontrolled hypertension, impaired kidney function, liver failure, and pregnant or breastfeeding women were excluded from the study. Written consent was taken from all participating women. Ethical approval of the study (Ethical Approval No: 1442-849454) was obtained from the Regional Research Ethics Committee registered at the National Committee of Bio and Medical Ethics (NCBE) Registration No. H-04-Q-001.
The study was conducted at Buraidah Central Hospital from January to March 2021. This was an uncontrolled intervention study with repeated measurements. Potential participants attended a lecture on KD that showed the specific diet plan, the importance of nutritional structure and the daily time of eating. The recommended KD plan for all participants for each day is summarized in the Supplementary Table. All potential participants were required to consume a KD for 8 weeks and were requested to maintain their usual levels of physical activity. Before written consent, the study protocol and the potential risk were fully explained to the participants. All participants gave their consent prior to their participation in the study. During the intervention period, they were asked to attend six sessions, at baseline (pre-intervention), weeks 1, 2, and 4 (mid-intervention), and weeks 6 and 8 (post-intervention), to assess body weight, height, BMI, waist circumference, and BHB levels. The participants were instructed against drug or antioxidant use to eliminate potential confounding effects. Compliance with the dietary regimen was monitored weekly by measuring plasma BHB level.
Anthropometric measures
At baseline and after 4–8 weeks of intervention, we determined the weight, height, BMI, and waist circumference of each participant. BMI (kg/m2) was calculated in accordance with the equation described by Mohorko et al.6
Lipid profile assessment
Total cholesterol, triglycerides, LDL-C, and HDL-C levels were measured before and at the end of the study by a Chemistry Analyzer (Beckman Coulter UniCel DxC 600), as described previously.23
Determining the levels of ketone bodies
Levels of beta-hydroxybutyrate (BHB) in the capillaries was identified by a portable meter (FreeStyle Optium Neo Blood Glucose and Ketone Monitoring meters). As with anthropometric assessments, all identifications of capillary ketonemia were made after an overnight fast of 8–10 h. During the entire KD diet, these measurements were performed in five sessions (1, 2, 4, 6, 8 weeks) by each participant, and the corresponding values were reviewed on the system's memory to control adherence.24
Quantification of serum IL-1β levels
Serum IL-1β levels were quantified by enzyme-linked immunosorbent assay (ELISA) kits specific for IL-1β according to the manufacturer's instructions (Cloud-Clone Corp., USA).25
Statistical analysis
The Statistical Package for Social Science (SPSS) version 23 (IBM, Chicago, IL, USA) was used for data entry and analysis. Statistical tests were used to analyze quantitative data: (1) basic frequencies and percentages to describe and compare responses to individuals, (2) mean, standard deviation, and a paired t-test were used to compare the means at baseline and each experimental time point. Analysis of variance (repeated measures) was used to examine the effects of the KD on changes in anthropometric measurements at different times. The statistical significance was set at P <0.05.23
Results
Participant characteristics
Thirty-one female volunteers out of forty were found to meet the study's eligibility criteria. All 31 females passed all the initial tests, except for two who showed unwillingness to adhere to the prescribed diet. During the study, three women withdrew due to their inability to continue the diet, and another three were excluded after week 6 due to carbohydrate consumption, which was confirmed by a drop in BHB levels. Twenty-three obese participants completed the study.
Demographic data of the participants are presented in Table 1. The mean age of the participants was 35.3 ± 8.4 years and most of were overweight, with an average weight of 84.7 ± 12.26 kg with a mean BMI of 33.96 ± 4.44 kg/m2. The subjects were in good health and did not have any food allergies that may have interfered with the diet.
Table 1.
Demographic data of participants.
| Parameters | Mean ± S.D. (n = 23) |
|---|---|
| Age | 35.3 ± 8.4 |
| Height (cm) | 156.8 ± 5.6 |
| Weight (kg) | 84.7 ± 12.3 |
| Waist circumference (cm) | 99.04 ± 11.62 |
| BMI (kg/m2) | 33.9 ± 4.4 |
| Marital status | |
| Single | 6 (26.1%) |
| Married | 15 (65.2%) |
| Divorced | 2 (8.7%) |
| Educational qualifications | |
| High school | 1 (4.3%) |
| University level | 20 (87.0%) |
| Postgraduate studies | 2 (8.7%) |
| Financial status | |
| High | 4 (17.4%) |
| Average | 19 (82.6%) |
| Medical condition | |
| Diabetes | 1 (4.3%) |
| Irregular blood pressure | 1 (4.3%) |
| Healthy | 21 (91.3%) |
| Food allergy | |
| No | 21 (91.3%) |
| Yes | 2 (8.7%) |
Alterations in anthropometric indices
Nutritional intervention resulted in a significant reduction in body weight, BMI and waist circumference (p < 0.001). After the first month, body weight decreased progressively. The BMI and waist circumference also decreased progressively throughout the period of study (p < 0.001) (Table 2).
Table 2.
Changes in body weight and anthropometric indices.
| Parameters | 0-day | 8-week | P value |
|---|---|---|---|
| Weight | 84.7 ± 12.3 | 77.0 ± 11.3 | <0.001 |
| Waist circumference | 99.0 ± 11.6 | 84.8 ± 7.6 | <0.001 |
| BMI | 33.9 ± 4.4 | 30.9 ± 4.2 | <0.001 |
N = 23; data presented as mean ± SD and paired t-test was used to compare the changes in anthropometric measurement (Weight, Waist and BMI). Statistical significance was set at (P < 0.0001).
Levels of blood β–hydroxy butyrate (BHB)
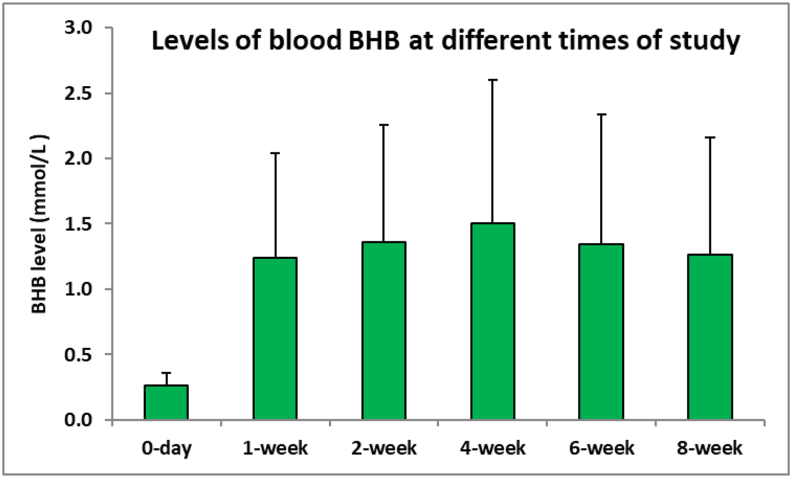
Analysis showed that there were significant differences in the serum level of BHB between baseline and the fourth week, indicating that all participants were committed to the KD (p < 0.001). Participants showed ketosis at the first session where the blood BHB level was 1.24 ± 0.956 mmol/L (P < 0.0001); the extent of ketosis increased up to week 4 (1.5 ± 1.129 mmol/L). After the sixth week, a slight decrease in BHB was observed; however, the level of BHB increased again in week 8 and participants still had ketosis (1.26 ± 0.894 mmol/L of BHB) (Figure 1). There was a significant increase in the levels of blood BHB at the study intervals as compared with the levels on day 0.
Figure 1.
Levels of blood BHB at different times.
Lipid profiles
There was no significant change in total cholesterol when compared before and after intervention. TG changed slightly but not significantly (1.04 ± 0.71 vs. 0.91 ± 0.63). During the intervention period, no significant changes in HDL-C were found, and a minor but non-significant reduction in serum was observed (1.35 ± 0.23 vs. 1.33 ± 0.27). In addition, a significant increase in serum LDL-C was observed (P < 0.05). High levels of LDL-C were observed in the post intervention period, most probably due to the sensitivity of LDL-C levels in the blood to high fat intake, but this would most likely decrease in the following weeks because the diet was only for 2 months, which is normal (Table 3).
Table 3.
Alterations in blood lipids.
| Parameters | 0-day | 30-day | p-value |
|---|---|---|---|
| Total cholesterol (mg/dl) | 4.76 ± 0.87 | 4.99 ± 1.07 | 0.151 |
| Triglycerides (mg/dl) | 1.04 ± 0.71 | 0.91 ± 0.63 | 0.198 |
| HDL-C (mg/dl) | 1.35 ± 0.23 | 1.33 ± 0.27 | 0.710 |
| LDL-C (mg/dl) | 2.93 ± 0.71 | 3.26 ± 0.94 | 0.024 |
Data presented as mean ± SD and paired t-test was used to compare the changes in concentrations of serum cholesterol, Triglycerides, HDL, and LDL. No significant differences between before and after intervention. Statistical significance was set at ∗(P < 0.05).
Effect of KD on the serum level of IL-1β
As shown in Table 4, the level of inflammatory cytokine IL-1b was significantly decreased at the end of the study when compared to that at the beginning of the study. Results indicated that the levels of proinflammatory cytokine decreased due to an increase in BHB.
Table 4.
Serum levels of IL-1β.
| Parameter | 0-day | 30-day | P value |
|---|---|---|---|
| IL-1β (Pg/ml) | 121.4 ± 96.1 | 20.7 ± 40.8 | <0.001 |
Data presented as mean ± SD and paired t-test was used to compare the changes in Serum levels of IL-1β. A significant reduction between before and after is observed. Statistical significance was set at (P < 0.0001).
Discussion
This study investigated the benefits of the KD on anthropometric indices and levels of the inflammatory cytokine IL-1β in obese Saudi women. Our data showed that the KD intervention resulted in considerable weight loss, a reduced BMI, and a reduced waist circumference in the subjects, as well as a decrease in IL-1β levels. Our finding is consistent with most studies that investigated such effects in that this method of weight loss has not been found to cause skeletal muscle loss. In obese people, low-carbohydrate and ketogenic diet therapy can effectively reduce body fat mass while significantly increasing skeletal muscle mass.26,27 All individuals in the present study lost weight, had a lower BMI, and had a smaller waist circumference. Due to the mechanism of ketone bodies resulting from the decomposition of stored fats to be produced as energy for the body, a recent study found that there is a correlation in weight loss and waist circumference with an increase in ketones bodies in the body during the ketogenic diet.28 Also, our findings agree with the previous findings of Mohorko et al.6 who revealed that the KD caused significant reduction in the weight, BMI, and waist circumference. The KD has long been recommended for its ability to cause numerous changes in the body. The results of a previous study found that the KD reduced body weight and fat mass in all subjects, while skeletal muscle mass remained unaffected in the keto group, thus indicating that the KD reduces body weight and waist circumference without affecting the muscles.23,29 Our results showed that total cholesterol, TG and HDL levels were ameliorated after a KD diet; however, the levels of LDL were elevated at the end of the study. This may be due to the sensitivity of blood LDL levels to higher fat intake, but this is likely to reduce in subsequent weeks because the diet used in this study only lasted for 2 months, which is the usual period used for KD diets. However, the KD resulted in beneficial changes in blood lipid subgroups but had no effect on total LDL cholesterol, except that it shifted it from small dense LDL to large buoyant LDL, which could reduce the risk of cardiovascular disease.30 The findings of a previous study concurred with our present findings regarding higher levels of LDL, as their findings showed that people who ate a high-fat and low-carbohydrate diet had unique cholesterol features, such as a continuous rise in LDL and HDL when compared to subjects who ate low fat and more carbohydrates. The high LDL was distinguished by its differing distributions in which the total concentration of large and floating LDL particles was much higher than that of small and dense LDL particles, which are defined by their propensity to cause damage. In addition, the lipoprotein profiles were consistent with a high level of insulin sensitivity.31 On the other hand, previous studies demonstrated that ketosis was related to lower levels of total cholesterol, triglycerides, and LDL, in addition to a significant increase in high-density lipoprotein cholesterol.32 Endogenous cholesterol is produced when insulin levels rise because of high blood sugar levels. The KD has been found to have the opposite effect by reducing carbohydrate consumption and lowering cholesterol synthesis due to the lack of insulin.33 High-fat meals have been demonstrated to have a considerable favorable effect on TG when compared to low-fat diets, reducing total cholesterol and LDL cholesterol while raising HDL cholesterol.34 Our data showed that the increased BHB levels in our participants resulted in several positive outcomes, including weight loss, reduced waist circumference, and relief from a variety of symptoms. Notably, the intervention reduced levels of IL-1β, a cytokine that activates the immune system causing a variety of inflammatory disorders when its levels are elevated. A previous study concurred with our current findings in that they found that increased levels of BHB inhibited the secretion of IL-1β from neutrophils in elderly adults and that increased BHB levels did not exacerbate the disease but rather reduced their bacterial burden. This means that BHB has a high potential to inhibit inflammatory attacks such as gout.35 Furthermore, a previous study also found that BHB is an essential mediator of amino acid and fatty acid catabolism that has been shown to be beneficial in treating several inflammatory and autoimmune diseases, including colitis and experimental allergic encephalitis.36 The effect of BHB on the migration of C6 glioma cells in vitro has been previously investigated, as well as the activation of the inflammatory mechanism. During therapy, BHB ketone bodies reduced C6 cell motility, caspase-1 activation, and the formation of mature IL-1β; these authors showed that BHB inhibits the response of human glioma cells to IL-1β.37 Evidence showed that pro-inflammatory cytokines, including IL-1β, play an important role in the pathophysiology of major depressive disorder (MDD), and when rats with this disorder were treated with BHB, the ketogenic bodies attenuated depressive behavior as well as the elevation of IL-1β and TNF-α in the hippocampus of rodents.36,37 As previously indicated, other macronutrients in KD can cause hormonal changes and alterations in the plasma lipid profiles of patients. Furthermore, during KD, restricted glucose availability can activate autophagy, which inhibits the inflammasome at the cellular level.38 Previous studies also identified several complications associated with KD; the most common complications were constipation and increased levels of cholesterol and triglycerides. Moreover, women often experienced amenorrhea or other complications associated with the menstrual cycle.39,40 The obese females investigated in our present study also experienced similar complications as the levels of total cholesterol and LDL were slightly increased after 30 days of KD intake although these increases were statistically insignificant. The strength of this study is that we show the positive impact of KD on anthropometric indices and on the negative regulation of inflammatory activities in the obese population. In addition, these findings also shed light on the immunological activities of metabolic signals such as BHB, implying that dietary or pharmacological treatments to raise the levels of BHB without generating the overall starvation response could help to alleviate the severity of chronic inflammatory disorders. The importance of KD intake strongly supports our hypothesis in obese females and also supports the use of BHB as a supplement for health improvement. However, this study has some limitations; the most obvious limitation of this study is the sample size and region from which participants were selected. It would have been better to include around one hundred obese females from several different regions. In addition, the inclusion of a diverse range of participants, including both females and males from different regions associated with obesity would further strengthen our findings.
Conclusions
This study shows that KD intervention resulted in a considerable loss of body weight, improved BMI, reduced waist circumference, and also reduced the levels of inflammatory activities in obese Saudi women. Specifically, over a period of 8 weeks, the KD was useful for detecting the positive impact of the KD on anthropometric indices, along with biochemical and inflammatory processes. The study also provides support to the belief that the intake of a KD diet by obese women induces the release of BHB in the blood without stimulation of an overall starvation response. This may be useful to alleviate the severity of chronic inflammatory disorders associated with obesity.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This study was ethically approved by the Regional Research Ethics Committee (Ethical Approval No: 1442-849454) registered at the National Committee of Bio and Medical Ethics (NCBE) Registration No. H-04-Q-001 and was performed in accordance with the principles outlined in the Declaration of Helsinki.
Consent
Written consent was obtained from all participants.
Authors contributions
NA, FTA, ZR, SAA, ASMA, YANA, TS, AMA, AAABW, FSA, ASA and WAA initiated the project and performed data collection. NA performed data analysis. FTA, ZR and WAA worked on data interpretation and manuscript drafting. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
The participation of all Saudi women is gratefully acknowledged.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Gjermeni E., Kirstein A.S., Kolbig F., et al. Obesity-an update on the basic pathophysiology and review of recent therapeutic advances. Biomolecules. 2021;11(10):1426. doi: 10.3390/biom11101426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salem V., AlHusseini N., Abdul Razack H.I., Naoum A., Sims O.T., Alqahtani S.A. Prevalence, risk factors, and interventions for obesity in Saudi Arabia: a systematic review. Obes Rev. 2022;23(7) doi: 10.1111/obr.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aljaafari D., Ishaque N., Al-Shabeeb G., Alalwi S., Albakr A., Basheir O., et al. Body mass index and its association with various features of migraine: a cross-sectional study from Saudi Arabia. Ann Afr Med. 2021;20(4):288–292. doi: 10.4103/aam.aam_63_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y., Wang X., Huan J., Zhang L., Lin L., Li Y., Li Y. Effect of dietary inflammatory potential on the aging acceleration for cardiometabolic disease: a population-based study. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.1048448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenig S., Petelin A., Poklar Vatovec T., Mohorko N., Jenko-Pražnikar Z. Assessment of micronutrients in a 12-wk ketogenic diet in obese adults. Nutrition. 2019;67–68 doi: 10.1016/j.nut.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Mohorko N., Černelič-Bizjak M., Poklar-Vatovec T., et al. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr Res. 2019;62:64–77. doi: 10.1016/j.nutres.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Kuchkuntla A.R., Shah M., Velapati S., Gershuni V.M., Rajjo T., Nanda S., et al. Ketogenic diet: an endocrinologist perspective. Curr Nutr Rep. 2019;8(4):402–410. doi: 10.1007/s13668-019-00297-x. [DOI] [PubMed] [Google Scholar]
- 8.Ting R., Dugré N., Allan G.M., Lindblad A.J. Ketogenic diet for weight loss. Can Fam Physician. 2018;64(12):906. [PMC free article] [PubMed] [Google Scholar]
- 9.Roekenes J., Martins C. Ketogenic diets and appetite regulation. Curr Opin Clin Nutr Metab Care. 2021;24(4):359–363. doi: 10.1097/MCO.0000000000000760. [DOI] [PubMed] [Google Scholar]
- 10.Abbasi J. Interest in the ketogenic diet grows for weight loss and type 2 diabetes. JAMA. 2018;319(3):215–217. doi: 10.1001/jama.2017.20639. [DOI] [PubMed] [Google Scholar]
- 11.Crosby L., Davis B., Joshi S., et al. Ketogenic diets and chronic disease: weighing the benefits against the risks. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.702802. Published 2021 Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosinski C., Jornayvaz F.R. Effects of ketogenic diets on cardiovascular risk factors: evidence from animal and human studies. Nutrients. 2017;9(5):517. doi: 10.3390/nu9050517. Published 2017 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Espinoza M.Á., Chacón-Moscoso S., Sanduvete-Chaves S., Ortega-Maureira M.J., Barrientos-Bravo T. Effect of a ketogenic diet on the nutritional parameters of obese patients: a systematic review and meta-analysis. Nutrients. 2021;13(9):2946. doi: 10.3390/nu13092946. Published 2021 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellana M., Conte E., Cignarelli A., et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21(1):5–16. doi: 10.1007/s11154-019-09514-y. [DOI] [PubMed] [Google Scholar]
- 15.Muscogiuri G., El Ghoch M., Colao A., et al. European guidelines for obesity management in adults with a very low-calorie ketogenic diet: a systematic review and meta-analysis. Obes Facts. 2021;14(2):222–245. doi: 10.1159/000515381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holton K.F., Hargrave S.L., Davidson T.L. Differential effects of dietary MSG on hippocampal dependent memory are mediated by diet. Front Neurosci. 2019;13:968. doi: 10.3389/fnins.2019.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogarasi A., Gonzalez K., Dalamaga M., Magkos F. The impact of the rate of weight loss on body composition and metabolism. Curr Obes Rep. 2022;11(2):33–44. doi: 10.1007/s13679-022-00470-4. [DOI] [PubMed] [Google Scholar]
- 18.Dyńka D., Kowalcze K., Paziewska A. The role of ketogenic diet in the treatment of neurological diseases. Nutrients. 2022;14(23):5003. doi: 10.3390/nu14235003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman J.C., Verdin E. β-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr. 2017;37:51–76. doi: 10.1146/annurev-nutr-071816-064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poff A., Koutnik A., Moss S., Mandala S., D'Agostino D. Exploring the viability of exogenous ketones as weight loss supplements (P21-017-19) Curr Dev Nutr. 2019;3(Suppl 1) doi: 10.1093/cdn/nzz041.P21-017-19. nzz041.P21-017-19. Published 2019 Jun 13. [DOI] [Google Scholar]
- 21.Stefan M., Sharp M., Gheith R., Lowery R., Wilson J. The effect of exogenous beta-hydroxybutyrate salt supplementation on metrics of safety and health in adolescents. Nutrients. 2021;13(3):854. doi: 10.3390/nu13030854. Published 2021 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y.M., Ramprasath T., Zou M.H. β-hydroxybutyrate and its metabolic effects on age-associated pathology. Exp Mol Med. 2020;52(4):548–555. doi: 10.1038/s12276-020-0415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi H.R., Kim J., Lim H., Park Y.K. Two-week exclusive supplementation of modified ketogenic nutrition drink reserves lean body mass and improves blood lipid profile in obese adults: a randomized clinical trial. Nutrients. 2018;10(12):1895. doi: 10.3390/nu10121895. Published 2018 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro A.I., Gomez-Arbelaez D., Crujeiras A.B., et al. Effect of a very low-calorie ketogenic diet on food and alcohol cravings, physical and sexual activity, sleep disturbances, and quality of life in obese patients. Nutrients. 2018;10(10):1348. doi: 10.3390/nu10101348. Published 2018 Sep 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg E.L., Asher J.L., Molony R.D., et al. β-Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 2017;18(9):2077–2087. doi: 10.1016/j.celrep.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rupasinghe W.A.W.S., Perera T.S.H., Silva K.D.R.R., Samita S., Wickramaratne M.N. Nutritional intake of sport undergraduates in Sabaragamuwa University of Sri Lanka. BMC Nutr. 2023;9(1):2. doi: 10.1186/s40795-022-00662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rondanelli M., Faliva M.A., Gasparri C., Peroni G., Spadaccini D., Maugeri R., et al. Current opinion on dietary advice in order to preserve fat-free mass during a low-calorie diet. Nutrition. 2020;72 doi: 10.1016/j.nut.2019.110667. [DOI] [PubMed] [Google Scholar]
- 28.Friedman M.I., Appel S. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men: a secondary analysis of energy expenditure and physical activity. PLoS One. 2019;14(12) doi: 10.1371/journal.pone.0222971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaFountain R.A., Miller V.J., Barnhart E.C., et al. Extended ketogenic diet and physical training intervention in military personnel. Mil Med. 2019;184(9–10):e538–e547. doi: 10.1093/milmed/usz046. [DOI] [PubMed] [Google Scholar]
- 30.Luo W., Zhang J., Xu D., Zhou Y., Qu Z., Yang Q., et al. Low carbohydrate ketogenic diets reduce cardiovascular risk factor levels in obese or overweight patients with T2DM: a meta-analysis of randomized controlled trials. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.1092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creighton B.C., Hyde P.N., Maresh C.M., Kraemer W.J., Phinney S.D., Volek J.S. Paradox of hypercholesterolaemia in highly trained, keto-adapted athletes. BMJ Open Sport Exerc Med. 2018;4(1) doi: 10.1136/bmjsem-2018-000429. Published 2018 Oct 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong T., Guo M., Zhang P., Sun G., Chen B. The effects of low-carbohydrate diets on cardiovascular risk factors: a meta-analysis. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0225348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosseini F., Jayedi A., Khan T.A., Shab-Bidar S. Dietary carbohydrate and the risk of type 2 diabetes: an updated systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2022;12(1):2491. doi: 10.1038/s41598-022-06212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu M., Wan Y., Yang B., Huggins C.E., Li D. Effects of low-fat compared with high-fat diet on cardiometabolic indicators in people with overweight and obesity without overt metabolic disturbance: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. 2018;119(1):96–108. doi: 10.1017/S0007114517002902. [DOI] [PubMed] [Google Scholar]
- 35.Hooper L., Martin N., Jimoh O.F., Kirk C., Foster E., Abdelhamid A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2020;8(8):CD011737. doi: 10.1002/14651858.CD011737.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bishehsari F., Engen P.A., Voigt R.M., Swanson G., Shaikh M., Wilber S., et al. Abnormal eating patterns cause circadian disruption and promote alcohol-associated colon carcinogenesis. Cell Mol Gastroenterol Hepatol. 2020;9(2):219–237. doi: 10.1016/j.jcmgh.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamanashi T., Iwata M., Kamiya N., et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci Rep. 2017;7(1):7677. doi: 10.1038/s41598-017-08055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J., Zhu X., Hui R., Xing Y., Wang J., Shi S., et al. Associations of metal exposure with hyperuricemia and gout in general adults. Front Endocrinol (Lausanne) 2022;13 doi: 10.3389/fendo.2022.1052784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batch J.T., Lamsal S.P., Adkins M., Sultan S., Ramirez M.N. Advantages and disadvantages of the ketogenic diet: a review article. Cureus. 2020 Aug 10;12(8) doi: 10.7759/cureus.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crosby L., Davis B., Joshi S., Jardine M., Paul J., Neola M., et al. Ketogenic diets and chronic disease: weighing the benefits against the risks. Front Nutr. 2021 Jul 16;8 doi: 10.3389/fnut.2021.702802. [DOI] [PMC free article] [PubMed] [Google Scholar]