Abstract
Background
The prevalence of allergic rhinitis (AR) has been increasing steadily worldwide, especially in countries with increasing industrialization such as China. However, available evidence regarding AR prevalence among Chinese adults is scarce and limited to regional data collected in earlier years. We therefore aimed to provide a more recent and robust estimate of AR prevalence using a nationwide representative cross-sectional study in China.
Methods
Data of 184 326 participants aged 18 years or older were obtained from the China Chronic Disease and Risk Factor Surveillance conducted in 2018–2019. AR was determined by self-reported sneezing, nasal itching, obstruction, or rhinorrhea symptoms for at least 1 h in the absence of a cold or flu within the last 12 months. Multivariable logistic model was used to examine the risk factors of AR, and a possible non-linear relationship was further tested by restricted cubic spline. Potential additive interactions of risk factors with sex, residence, and geographic region were assessed by relative excess risk due to interaction (RERI).
Results
The weighted prevalence of AR was 8.1% (95% confidence interval [CI], 7.4%–8.7%), of whom 23.7% (95% CI, 21.3%–26.0%) were aware of their diagnosis. Increased odds of AR were associated with younger age, men, living in urban area or north region, more education, smoking, underweight, and higher income. Despite the nonsignificant linear trend, the spline regression demonstrated a non-linear association between AR and sleep duration, with higher odds at both ends. Additionally, the observed associations were generally stronger among men and people living in urban area and north region, with significant RERI ranging from 0.07 (95% CI, 0.00–0.14) to 0.40 (95% CI, 0.12–0.67).
Conclusions
AR is prevalent in China and the associated factors and interactions are helpful to design targeted preventive strategies towards certain subpopulations. The low awareness of AR calls for a national effort on AR screening.
Keywords: Allergic rhinitis, Chinese adults, Nationwide representative, Risk factors, Additive interaction
Introduction
Allergic rhinitis (AR) is a common chronic disease caused by immunoglobulin E (IgE)-mediated inflammatory response in the nasal mucosa.1 It not only adversely affects study and work performance but imposes considerable economic and health burdens on society. AR is estimated to affect 10%–30% of adults and 40% of children and its prevalence is still on the rise globally,2,3 particularly in developing countries.4 Such an increase is also observed in China, the largest developing country in the world with a population of 1.4 billion. Therefore, comprehensive and continuous monitoring is urgently needed to develop health policies aiming at reducing the burden of AR.
While numerous studies have studied the epidemic of AR in children,5, 6, 7 relatively fewer are available in adults among whom AR prevalence and relevant risk factors may be distinct from children in China. Existing results of AR prevalence in adults showed large variation. For example, Li et al reported 6.1% of adult participants in Guangzhou having AR,8 which was much lower than the prevalence in Beijing (13.5%) and Baoding (19.1%).9 Moreover, these 2 studies found opposite findings in terms of differences between urban and rural areas. Both discrepancies suggest that estimates based on samples restricted to certain regions may depend on local characteristics and cannot fully reflect the overall picture of China. To address this gap, 2 population-based nationwide studies had been performed in 11 and 18 large cities and demonstrated that the AR prevalence was 11.1% and 17.6%, respectively, in 2005 and 2011.10,11 However, these data neither were collected in recent years nor could be generalized to rural areas. In addition, although possible risk factors have been identified in previous studies, their impacts at extreme ends of distribution on AR are rarely assessed earlier mainly due to limited statistical power. Besides, no study has explored potential interactions between risk factors and sex, residence, and geographic region, which can facilitate more customized intervention measures.
In the current study, our primary aim was to provide a more recent estimate of AR prevalence in adults by using data from the China Chronic Disease and Risk Factor Surveillance (CCDRFS) conducted in 2018–2019, a nationally and provincially representative survey covering both urban and rural areas. Associations between risk factors and AR were examined and their dose-response relationships were also explored when appropriate. Finally, whether the above associations varied by sex, residence, and geographic region were evaluated.
Methods
Sampling and study participants
The CCDRFS is a consecutive nationwide cross-sectional study conducted every 3–5 years since 2004.12 The wave used in this study was carried out between August 2018 to June 2019 based on 298 national disease surveillance points (urban districts or rural counties) covering all 31 provinces, autonomous regions, and municipalities. A multistage stratified cluster sampling was used to select a nationally and provincially representative sample of the Chinese general population.13 The eligibility criteria included: aged 18 years or older; having lived in the selected region for at least 6 months in the past 12 months; not pregnant; and not having serious illness or cognitive and language disorders hindering participation. A total of 184 509 individuals completed the survey and the response rate was 94.9%. Among these, 183 participants were excluded due to missing data on AR or major risk factors (age, sex, urban-rural locality, geographic region, education, and smoking status), leaving 184 326 participants included in the main analysis.
Data collection
Through face-to-face interviews during a home visit, a standardized questionnaire was administered to all participants to collect data on sociodemographic characteristics, lifestyle factors, self-reported chronic diseases, and medication history by experienced investigators using portable pad.14 Both assisted interview system and information-management system were installed on each pad for better data quality control. Physical measurements including height and weight were also performed for each participant in accordance with standard protocols. Urban and rural areas were classified at committee/village level conforming to the definition of the National Bureau of Statistics of China. North and south regions were divided based on geographic locations relative to the line of Qinling Mountains and Huaihe River. Education was classified as below high school, high school, and college or above. People were categorized into never, former, and current smokers according to the definition of Global Adult Tobacco Survey.15 Men (women) who drank more than 25 g (15 g) pure alcohol per day were considered excessive drinkers.16 Sleep duration was divided into 4 categories, that were, less than 5 h, between 5 and 7 h, between 7 and 10 h, and more than 10 h. Annual household income per capita was categorized by tertiles with cutoff values of 7000 and 17 500 CNY. Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared (kg/m2) and 3 categories (<18.5, 18.5–23.9, ≥24 kg/m2) were generated following the recommendation proposed by the Working group on obesity in China.17
CCDRFS 2018 was the first survey containing AR-related questions.13 AR, the primary outcome of interest, was defined as self-reported symptoms including sneezing, nasal itching, obstruction, or rhinorrhea for at least 1 h in the absence of cold or flu within the last 12 months, which is consistent with previous studies.9,10 Participants were deemed as having physician-diagnosed AR if they answered yes to the question “Have you ever been told by a doctor that you have allergic rhinitis?”. Total AR was the sum of current AR and physician-diagnosed AR followed by subtracting the overlapping part. Awareness of AR was defined as the proportion of those who reported previous diagnosis of AR by a doctor among all participants with AR.
Statistical analysis
The prevalence of AR was estimated after taking into account stratification, clusters, and sampling weights. The weighting coefficients were constructed on the basis of prespecified sampling design, non-response, and poststratification, and the differences in population distribution between the weighted sample and 2010 China population census were negligible (Supplemental figure 1). Also, the prevalence of AR was assessed across subgroups of age, educational level, smoking status, alcohol drinking, sleep duration, BMI, family size, and household income per capita, both overall and by sex, urban-rural locality, and geographic region, that would additionally serve as subgroup variables when the results were not stratified by them. The Taylor series linearisation method with finite population correction was employed to calculate standard errors and 95% confidence interval (CI). The Rao-Scott χ2 test was used to compare AR prevalence between groups of binary variables. The trend of AR prevalence across groups of ordinal variables was tested using orthogonal contrast matrices. Besides, the weighted prevalence of AR among all participants and awareness of AR among participants with AR were calculated by provinces and visualized with choropleth maps. Multivariable logistic regression was performed to simultaneously examine associations of relevant variables (age, sex, urban/rural residence, geographic region, educational level, cigarette smoking, excessive alcohol drinking, sleep duration, BMI, family size, and household income per capita) with odds of AR. If results of subgroup or regression analyses showed potential of nonlinearity for certain variables, their dose-response relationships with odds of AR would be formally tested using restricted cubic spline with 4 knots placed at 5th, 35th, 65th, and 95th percentiles and cluster-robust standard error. Logistic regression models were also done by sex, urban-rural locality, and geographic region. Relative excess risk due to interaction (RERI) was calculated to assess additive interaction, where RERI>0 indicates the combined effects are greater than expected based on the effects of each exposure alone. The standard error of RERI was estimated using the delta method.18
In the sensitivity analyses, logistic regression and nonlinear relationship were repeated among never smokers only to check the robustness to confounding effect of smoking. Moreover, a product term between variable and effect modifier of interest was constructed and added to the model, and a Wald test evaluating the joint significance of all associated cross-product terms was used to assess multiplicative interaction. False discovery rate was applied to account for multiple comparison. All analyses were done with R (version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria) and a P value less than 0.05 was considered statistically significant.
Results
Data are available for 184,326 adults aged 18 years or older. Of these participants, the mean age was 55.1 years and 102,498 (55.6%) were women. Table 1 presents the general characteristics of the study population. Compared with participants without AR, those with AR were more likely to be younger, men, residing in urban area and north region, highly educated, and wealthier.
Table 1.
Characteristics of Chinese adult participants, 2018–2019.
| Overall | Participants without allergic rhinitis | Participants with allergic rhinitis | |
|---|---|---|---|
| N | 184,326 | 171,936 | 12,390 |
| Age (years) | |||
| 18–29 | 9915 (5.4) | 9029 (5.3) | 886 (7.1) |
| 30–39 | 18,470 (10.0) | 16,839 (9.8) | 1631 (13.1) |
| 40–49 | 33,374 (18.1) | 31,026 (18.0) | 2348 (19.0) |
| 50–59 | 48,209 (26.2) | 45,117 (26.2) | 3092 (25.0) |
| 60–69 | 49,835 (27.0) | 46,769 (27.2) | 3066 (24.8) |
| 70+ | 24,523 (13.3) | 23,156 (13.5) | 1367 (11.0) |
| Sex | |||
| Men | 81,828 (44.4) | 75,877 (44.1) | 5951 (48.0) |
| Women | 102,498 (55.6) | 96,059 (55.9) | 6439 (52.0) |
| Residence | |||
| Urban | 75,117 (40.8) | 69,309 (40.3) | 5808 (46.9) |
| Rural | 109,209 (59.2) | 102,627 (59.7) | 6582 (53.1) |
| Region | |||
| North | 91,973 (49.9) | 85,359 (49.7) | 6614 (53.4) |
| South | 92,353 (50.1) | 86,577 (50.3) | 5776 (46.6) |
| Education | |||
| Less than high school | 147,399 (80.0) | 138,519 (80.5) | 8880 (71.7) |
| High school | 23,989 (13.0) | 21,945 (12.8) | 2044 (16.5) |
| College or above | 12,938 (7.0) | 11,472 (6.7) | 1466 (11.8) |
| Cigarette Smoking | |||
| Never | 127,752 (69.3) | 119,531 (69.5) | 8221 (66.3) |
| Former | 11,812 (6.4) | 10,788 (6.3) | 1024 (8.3) |
| Current | 44,762 (24.3) | 41,617 (24.2) | 3145 (25.4) |
| Excessive alcohol drinking | |||
| No | 168,172 (91.2) | 156,972 (91.3) | 11,200 (90.4) |
| Yes | 16,137 (8.8) | 14,949 (8.7) | 1188 (9.6) |
| Sleep duration (hours) | |||
| <5 | 8372 (4.5) | 7695 (4.5) | 677 (5.5) |
| 5–6.9 | 41,225 (22.4) | 38,233 (22.2) | 2992 (24.1) |
| 7–10 | 130,168 (70.6) | 121,743 (70.8) | 8425 (68.0) |
| >10 | 4560 (2.5) | 4264 (2.5) | 296 (2.4) |
| BMI (kg/m2) | |||
| <18.5 | 5318 (3.0) | 4935 (3.0) | 383 (3.2) |
| 18.5–23.9 | 78,146 (43.6) | 72,921 (43.7) | 5225 (43.2) |
| ≥24 | 95,537 (53.4) | 89,064 (53.3) | 6473 (53.6) |
| Family size | |||
| <4 | 112,961 (61.3) | 105,209 (61.2) | 7752 (62.6) |
| ≥4 | 71,365 (38.7) | 66,727 (38.8) | 4638 (37.4) |
| Annual household income per capita (CNY) | |||
| <7000 | 47,322 (25.7) | 44,368 (25.8) | 2954 (23.8) |
| 7000–17,499 | 46,824 (25.4) | 43,795 (25.5) | 3029 (24.5) |
| ≥17,500 | 47,479 (25.7) | 43,627 (25.4) | 3852 (31.1) |
| Don't know/refuse to answer | 42,701 (23.2) | 40,146 (23.3) | 2555 (20.6) |
Abbreviations: BMI, body mass index; CNY, Chinese Yuan; N, number. Note: Data are expressed as N (%). Some sums of numbers across groups did not equal to the expected total because there were missing values for that subgroup variable
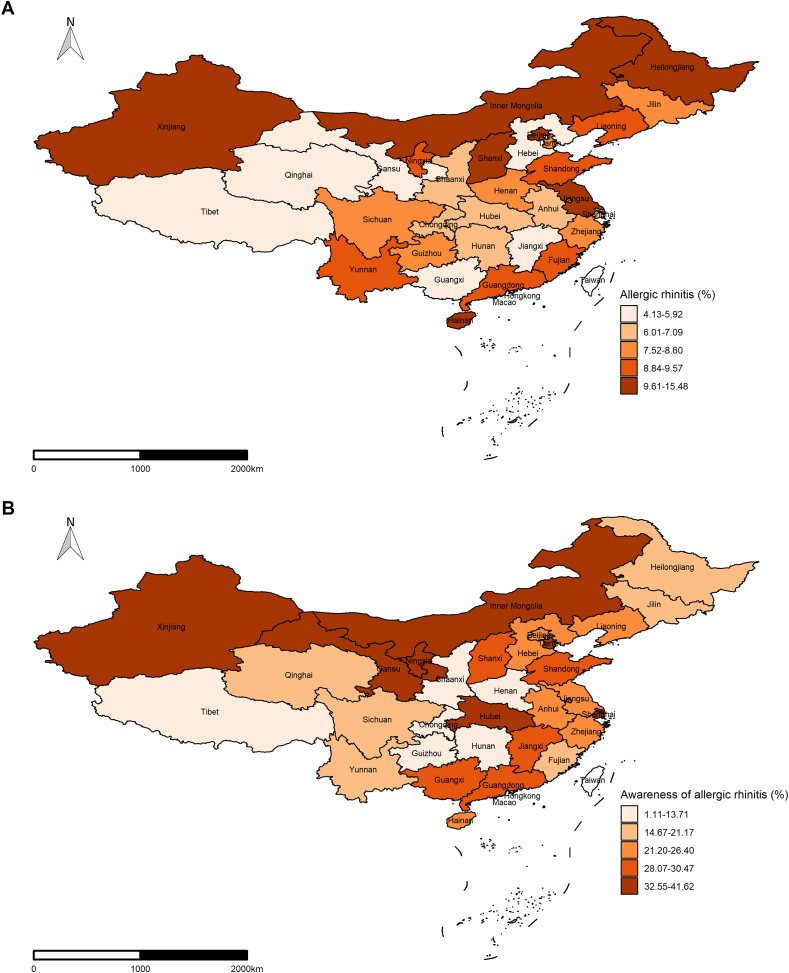
As shown in Table 2, the overall weighted prevalence of AR was estimated to be 8.1% (95% CI, 7.4%–8.7%). There was a clear gradient of AR prevalence across age groups, from 10.2% (95% CI, 9.0%–11.4%) in individuals aged 18–29 years to 5.4% (95% CI, 4.8%–6.0%) in those aged 70 years or older (p < 0.001). This decreasing trend with age was also found in both men and women. The weighted prevalence of AR was higher in men than women (9.0% vs. 7.1%, p < 0.001), and in urban area than in rural area (9.2% vs. 6.9%, p < 0.001). Although participants in north region displayed somewhat higher AR prevalence than those in south region, the difference was not statistically significant (8.4% vs. 7.7%, p = 0.261). However, considerable variations of AR prevalence were observed among different provinces (Fig. 1A). While Beijing, Heilong Jiang, and Hainan had AR prevalence >10% (12.9%, 11.9%, and 15.5%, respectively), Jiangxi, Guangxi, and Qinghai had AR <5% (4.1%, 4.3%, and 4.1%, respectively). For other subpopulations, the prevalence of AR was higher in participants who received more education (p < 0.001), ever smoked (p = 0.023), consumed excessive alcohol (p = 0.031), and were underweight (p = 0.002) or wealthier (p < 0.001) than their counterparts. In spite of the nonsignificant trend, AR prevalence seemed to be higher in participants with shorter or longer sleep. When further stratified by sex, residence, and region, most difference patterns remained except that significantly higher AR prevalence were only seen in ever smokers and excessive drinkers living in urban area or north region. In addition, fewer than one-quarter of the participants with AR were aware of their diagnosis (23.7% [95% CI, 21.3%–26.0%]). Apparent variations of awareness prevalence was seen across provinces, with potential cluster of higher prevalence in northwest region (Fig. 1B). The awareness prevalence of AR was highest in Shanghai (41.6%) and Gansu (40.3%) and lowest in Guizhou (1.1%).
Table 2.
Weighted prevalence of allergic rhinitis across groups of various characteristics, overall and by sex, residence and region.
| Overall | Sex |
Residence |
Region |
||||
|---|---|---|---|---|---|---|---|
| Men | Women | Urban | Rural | North | South | ||
| Total | 8.1 (7.4, 8.7) | 9.0 (8.3, 9.7) | 7.1 (6.5, 7.8) | 9.2 (8.3, 10.1) | 6.9 (6.3, 7.4) | 8.4 (7.4, 9.4) | 7.7 (7.0, 8.5) |
| Age (years) | |||||||
| 18–29 | 10.2 (9.0, 11.4) | 11.8 (10.0, 13.5) | 8.6 (7.3, 10.0) | 11.1 (9.5, 12.6) | 9.1 (7.5, 10.7) | 10.1 (8.5, 11.6) | 10.3 (8.6, 12.0) |
| 30–39 | 9.3 (8.4, 10.3) | 10.3 (9.1, 11.5) | 8.3 (7.3, 9.3) | 10.5 (9.2, 11.8) | 7.9 (6.8, 9.0) | 9.8 (8.2, 11.5) | 8.9 (7.9, 9.9) |
| 40–49 | 7.3 (6.7, 7.9) | 8.0 (7.2, 8.7) | 6.6 (6.0, 7.3) | 8.4 (7.5, 9.3) | 6.1 (5.5, 6.8) | 7.8 (6.9, 8.6) | 6.9 (6.1, 7.7) |
| 50–59 | 6.4 (5.8, 7.0) | 6.9 (6.1, 7.6) | 5.9 (5.3, 6.5) | 7.3 (6.4, 8.2) | 5.5 (4.9, 6.1) | 6.9 (6.0, 7.8) | 5.9 (5.1, 6.6) |
| 60–69 | 6.0 (5.4, 6.6) | 6.5 (5.8, 7.2) | 5.5 (5.0, 6.1) | 6.7 (5.9, 7.6) | 5.5 (4.7, 6.2) | 7.0 (6.0, 7.9) | 5.2 (4.5, 5.8) |
| 70+ | 5.4 (4.8, 6.0) | 6.2 (5.5, 7.0) | 4.7 (4.0, 5.4) | 6.3 (5.4, 7.2) | 4.8 (4.0, 5.5) | 6.2 (5.3, 7.2) | 4.8 (4.0, 5.5) |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Sex | |||||||
| Men | 9.0 (8.3, 9.7) | – | – | 10.0 (9.0, 11.0) | 7.9 (7.1, 8.8) | 9.3 (8.2, 10.4) | 8.7 (7.8, 9.7) |
| Women | 7.1 (6.5, 7.8) | – | – | 8.4 (7.3, 9.4) | 5.8 (5.3, 6.3) | 7.6 (6.5, 8.7) | 6.7 (6.0, 7.4) |
| p for difference | <0.001 | – | – | 0.001 | <0.001 | 0.001 | <0.001 |
| Residence | |||||||
| Urban | 9.2 (8.3, 10.1) | 10.0 (9.0, 11.0) | 8.4 (7.3, 9.4) | – | – | 10.1 (8.6, 11.7) | 8.4 (7.4, 9.4) |
| Rural | 6.9 (6.3, 7.4) | 7.9 (7.1, 8.8) | 5.8 (5.3, 6.3) | – | – | 6.7 (5.9, 7.5) | 7.0 (6.1, 7.8) |
| p for difference | <0.001 | 0.001 | <0.001 | – | – | <0.001 | 0.015 |
| Region | |||||||
| North | 8.4 (7.4, 9.4) | 9.3 (8.2, 10.4) | 7.6 (6.5, 8.7) | 10.1 (8.6, 11.7) | 6.7 (5.9, 7.5) | – | – |
| South | 7.7 (7.0, 8.5) | 8.7 (7.8, 9.7) | 6.7 (6.0, 7.4) | 8.4 (7.4, 9.4) | 7.0 (6.1, 7.8) | – | – |
| p for difference | 0.261 | 0.435 | 0.190 | 0.062 | 0.714 | – | – |
| Education | |||||||
| Less than high school | 6.3 (5.8, 6.8) | 7.2 (6.5, 7.8) | 5.4 (4.9, 5.9) | 6.6 (5.9, 7.3) | 6.0 (5.4, 6.6) | 6.7 (6.0, 7.4) | 5.9 (5.2, 6.5) |
| High school | 9.9 (8.8, 11.1) | 10.3 (8.8, 11.7) | 9.5 (8.4, 10.6) | 10.2 (8.8, 11.5) | 9.5 (7.8, 11.1) | 10.8 (9.4, 12.2) | 9.2 (7.4, 10.9) |
| College or above | 12.8 (11.1, 14.4) | 14.1 (11.9, 16.3) | 11.4 (9.4, 13.5) | 13.0 (11.3, 14.7) | 11.7 (7.7, 15.8) | 12.5 (9.9, 15.2) | 13.0 (10.9, 15.0) |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| Cigarette Smoking | |||||||
| Never | 7.8 (7.1, 8.4) | 9.5 (8.3, 10.6) | 7.0 (6.4, 7.7) | 8.8 (7.8, 9.8) | 6.6 (5.9, 7.2) | 8.0 (6.9, 9.1) | 7.6 (6.8, 8.3) |
| Former | 9.2 (8.1, 10.2) | 9.2 (8.1, 10.3) | 8.7 (5.7, 11.7) | 10.7 (9.0, 12.5) | 7.4 (6.3, 8.5) | 10.3 (8.6, 12.1) | 8.1 (6.8, 9.3) |
| Current | 8.7 (7.8, 9.5) | 8.6 (7.8, 9.4) | 10.4 (4.5, 16.3) | 10.0 (8.8, 11.3) | 7.4 (6.5, 8.3) | 9.3 (8.2, 10.4) | 8.1 (6.9, 9.3) |
| p for trend | 0.023 | 0.163 | 0.195 | 0.034 | 0.093 | 0.019 | 0.329 |
| Excessive alcohol drinking | |||||||
| No | 8.0 (7.3, 8.6) | 9.0 (8.2, 9.7) | 7.1 (6.4, 7.7) | 8.9 (8.0, 9.9) | 6.9 (6.3, 7.5) | 8.2 (7.3, 9.2) | 7.7 (7.0, 8.5) |
| Yes | 9.1 (7.9, 10.3) | 9.1 (7.9, 10.3) | 9.0 (5.9, 12.1) | 12.2 (10.0, 14.4) | 6.5 (5.7, 7.4) | 10.4 (8.4, 12.3) | 7.8 (6.5, 9.2) |
| p for difference | 0.031 | 0.829 | 0.151 | <0.001 | 0.437 | 0.012 | 0.846 |
| Sleep duration (hours) | |||||||
| <5 | 8.5 (6.8, 10.3) | 8.2 (6.0, 10.3) | 8.7 (6.4, 11.0) | 10.6 (7.2, 14.0) | 7.3 (5.8, 8.8) | 8.3 (6.1, 10.4) | 8.8 (6.0, 11.5) |
| 5–6.9 | 8.0 (7.0, 8.9) | 9.0 (7.6, 10.4) | 6.9 (6.1, 7.7) | 9.5 (8.1, 11.0) | 6.1 (5.2, 7.0) | 8.8 (7.3, 10.4) | 7.2 (6.2, 8.2) |
| 7–10 | 8.0 (7.4, 8.7) | 9.0 (8.2, 9.7) | 7.1 (6.4, 7.8) | 9.1 (8.1, 10.0) | 6.9 (6.3, 7.5) | 8.3 (7.3, 9.3) | 7.8 (7.1, 8.6) |
| >10 | 9.3 (6.1, 12.5) | 10.9 (5.1, 16.7) | 7.8 (5.3, 10.3) | 9.6 (5.3, 13.8) | 9.2 (4.9, 13.4) | 11.3 (5.8, 16.7) | 7.3 (4.3, 10.2) |
| p for trend | 0.668 | 0.365 | 0.621 | 0.687 | 0.308 | 0.334 | 0.540 |
| BMI (kg/m2) | |||||||
| <18.5 | 11.6 (9.0, 14.2) | 17.2 (12.2, 22.2) | 6.9 (5.2, 8.6) | 11.4 (7.7, 15.0) | 11.9 (8.3, 15.4) | 12.9 (7.4, 18.4) | 11.0 (8.3, 13.7) |
| 18.5–23.9 | 7.9 (7.1, 8.7) | 8.1 (7.3, 9.0) | 7.8 (6.8, 8.7) | 9.4 (8.1, 10.6) | 6.5 (5.8, 7.1) | 8.6 (7.1, 10.1) | 7.4 (6.6, 8.3) |
| ≥24 | 8.0 (7.4, 8.7) | 9.3 (8.4, 10.2) | 6.5 (6.0, 7.1) | 8.9 (8.0, 9.8) | 7.0 (6.2, 7.8) | 8.2 (7.3, 9.1) | 7.8 (6.9, 8.7) |
| p for trend | 0.002 | <0.001 | 0.687 | 0.149 | 0.002 | 0.054 | 0.010 |
| Family size | |||||||
| <4 | 8.0 (7.2, 8.8) | 8.4 (7.5, 9.3) | 7.5 (6.7, 8.4) | 9.4 (8.2, 10.6) | 6.2 (5.6, 6.7) | 8.4 (7.2, 9.7) | 7.5 (6.6, 8.4) |
| ≥4 | 8.2 (7.4, 8.9) | 9.8 (8.7, 10.8) | 6.6 (5.9, 7.3) | 8.8 (7.7, 10.0) | 7.6 (6.7, 8.5) | 8.5 (7.5, 9.4) | 8.0 (6.9, 9.1) |
| p for difference | 0.715 | 0.038 | 0.052 | 0.448 | 0.003 | 0.960 | 0.483 |
| Annual household income per capita (CNY) | |||||||
| <7000 | 7.0 (6.2, 7.8) | 7.9 (6.9, 9.0) | 6.0 (5.1, 6.9) | 8.0 (6.6, 9.4) | 6.6 (5.8, 7.4) | 7.9 (6.8, 9.1) | 5.9 (5.0, 6.7) |
| 7000–17499 | 7.8 (7.0, 8.6) | 8.9 (7.5, 10.2) | 6.7 (5.9, 7.5) | 8.5 (7.3, 9.7) | 7.2 (6.1, 8.3) | 7.7 (6.8, 8.6) | 7.9 (6.5, 9.2) |
| ≥17,500 | 9.7 (8.6, 10.8) | 10.6 (9.4, 11.9) | 8.7 (7.6, 9.9) | 10.3 (9.1, 11.5) | 7.9 (6.6, 9.3) | 10.5 (8.7, 12.3) | 9.0 (7.8, 10.2) |
| Don't know/refuse to answera | 7.2 (6.3, 8.1) | 7.9 (6.8, 9.1) | 6.4 (5.4, 7.4) | 8.3 (7.1, 9.6) | 6.0 (4.9, 7.2) | 6.9 (5.6, 8.3) | 7.3 (6.2, 8.5) |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | 0.278 | <0.001 | 0.018 |
Abbreviations: BMI, body mass index; CNY, Chinese Yuan. Note: Data are expressed as weighted prevalence (95% CI). P values were derived from Rao-Scott chi-square test or logistic regression applicable to complex survey.
This group was excluded from the trend test.
Fig. 1.
Weighted prevalence of allergic rhinitis (A) and awareness of allergic rhinitis (B) by province among Chinese adult participants, 2018–2019.
In the multivariable logistic models, higher educational level, ever smoking, extreme sleep duration, being underweight, and having income above the top tertile were associated with increased odds of AR, whereas being older, women, living in rural area or south region were associated with decreased odds (Table 3). It should be noted that former smoking showed higher odds ratio (OR) than current smoking (1.40 vs. 1.06), so did <5 h sleeping (OR = 1.51) than >10 h sleeping (OR = 1.16). The spline regression corroborated the non-liner association between odds of AR and sleep duration, with higher OR for both short and long sleep (Supplemental Fig. 2A, p < 0.001).
Table 3.
Associations between various characteristics and their additive interactions with sex, residence, and geographic region and odds of allergic rhinitis
| All | Sex |
Residence |
Geographic region |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | RERI (95% CI) | Urban | Rural | RERI (95% CI) | North | South | RERI (95% CI) | ||
| Age (10 year increment) | 0.9 | 0.9 | 0.9 | 0.01 | 0.9 | 0.91 | 0.03 | 0.93 | 0.87 | −0.06 |
| (0.89, 0.92) | (0.88, 0.92) | (0.88, 0.92) | (−0.02, 0.04) | (0.88, 0.92) | (0.89, 0.93) | (−0.01, 0.07) | (0.91, 0.96) | (0.85, 0.90) | (−0.10, −0.02) | |
| Women | 0.92 | – | – | – | 0.87 | 0.96 | 0.12 | 0.91 | 0.93 | 0.03 |
| (0.87, 0.97) | (0.83, 0.92) | (0.90, 1.02) | (0.05, 0.19) | (0.86, 0.97) | (0.87, 0.99) | (−0.05, 0.10) | ||||
| Rural | 0.86 | 0.82 | 0.9 | 0.12 | – | – | – | 0.77 | 0.99 | 0.29 |
| (0.80, 0.93) | (0.76, 0.89) | (0.83, 0.98) | (0.05, 0.19) | (0.69, 0.85) | (0.87, 1.12) | (0.12, 0.46) | ||||
| South | 0.89 | 0.88 | 0.9 | 0.03 | 0.78 | 1 | 0.29 | – | – | – |
| (0.80, 0.99) | (0.79, 0.99) | (0.81, 1.00) | (−0.05, 0.10) | (0.69, 0.88) | (0.87, 1.16) | (0.12, 0.46) | ||||
| Education (ref: Below high school) | ||||||||||
| High school | 1.29 | 1.26 | 1.32 | −0.04 | 1.39 | 1.16 | 0.27 | 1.37 | 1.19 | 0.23 |
| (1.22, 1.37) | (1.18, 1.35) | (1.23, 1.43) | (−0.15, 0.07) | (1.29, 1.49) | (1.07, 1.26) | (0.14, 0.40) | (1.27, 1.49) | (1.08, 1.30) | (0.07, 0.39) | |
| College or above | 1.58 | 1.62 | 1.54 | 0.13 | 1.68 | 1.35 | 0.4 | 1.53 | 1.65 | −0.06 |
| (1.45, 1.71) | (1.47, 1.79) | (1.40, 1.70) | (-0.04, 0.30) | (1.52, 1.85) | (1.15, 1.59) | (0.12, 0.67) | (1.35, 1.73) | (1.46, 1.86) | (−0.35, 0.23) | |
| Cigarette Smoking (ref: Never) | ||||||||||
| Former | 1.4 | 1.37 | 1.53 | −0.12 | 1.45 | 1.37 | 0.13 | 1.45 | 1.35 | 0.15 |
| (1.30, 1.51) | (1.27, 1.48) | (1.18, 2.00) | (−0.54, 0.30) | (1.33, 1.58) | (1.23, 1.52) | (−0.04, 0.31) | (1.33, 1.59) | (1.21, 1.50) | (−0.03, 0.33) | |
| Current | 1.06 | 1.02 | 1.28 | −0.26 | 1.1 | 1.02 | 0.09 | 1.09 | 1.02 | 0.08 |
| (1.00, 1.12) | (0.96, 1.08) | (1.13, 1.46) | (−0.44, −0.08) | (1.02, 1.19) | (0.96, 1.09) | (0.00, 0.18) | (1.03, 1.16) | (0.94, 1.10) | (−0.01, 0.17) | |
| Excessive alcohol drinking | 1.06 | 1.05 | 1.13 | −0.08 | 1.17 | 0.99 | 0.2 | 1.24 | 0.89 | 0.37 |
| (0.99, 1.14) | (0.98, 1.14) | (0.94, 1.37) | (−0.31, 0.15) | (1.06, 1.29) | (0.90, 1.08) | (0.06, 0.35) | (1.13, 1.36) | (0.80, 0.98) | (0.23, 0.51) | |
| Sleep duration (ref: 7–10 h) | ||||||||||
| <5 | 1.51 | 1.34 | 1.61 | −0.25 | 1.44 | 1.54 | −0.03 | 1.52 | 1.49 | 0.07 |
| (1.38, 1.64) | (1.18, 1.52) | (1.45, 1.79) | (−0.49, −0.01) | (1.26, 1.66) | (1.39, 1.71) | (−0.30, 0.23) | (1.35, 1.73) | (1.32, 1.68) | (−0.21, 0.35) | |
| 5−6.9 | 1.18 | 1.19 | 1.18 | 0.03 | 1.24 | 1.13 | 0.14 | 1.29 | 1.07 | 0.24 |
| (1.13, 1.24) | (1.13, 1.26) | (1.11, 1.26) | (−0.06, 0.11) | (1.17, 1.32) | (1.05, 1.21) | (0.03, 0.26) | (1.21, 1.37) | (1.00, 1.15) | (0.13, 0.35) | |
| >10 | 1.16 | 1.2 | 1.11 | 0.12 | 1.17 | 1.15 | 0.05 | 1.2 | 1.11 | 0.11 |
| (1.03, 1.31) | (1.04, 1.40) | (0.94, 1.31) | (−0.12, 0.36) | (0.95, 1.43) | (0.99, 1.33) | (−0.27, 0.37) | (1.01, 1.43) | (0.95, 1.31) | (−0.17, 0.39) | |
| BMI (ref: 18.5–23.9 kg/m2) | ||||||||||
| <18.5 | 1.11 | 1.29 | 0.96 | 0.34 | 1.05 | 1.14 | −0.09 | 1.1 | 1.11 | −0.01 |
| (1.00, 1.22) | (1.11, 1.50) | (0.84, 1.10) | (0.10, 0.59) | (0.88, 1.24) | (1.00, 1.30) | (−0.34, 0.17) | (0.92, 1.31) | (0.99, 1.25) | (−0.26, 0.25) | |
| ≥24 | 0.99 | 0.99 | 0.99 | 0 | 1 | 0.97 | 0.03 | 0.99 | 0.98 | −0.01 |
| (0.95, 1.02) | (0.94, 1.04) | (0.94, 1.03) | (−0.07, 0.07) | (0.95, 1.05) | (0.92, 1.03) | (−0.06, 0.11) | (0.94, 1.05) | (0.94, 1.03) | (−0.09, 0.07) | |
| Family size ≥4 | 0.97 | 1 | 0.94 | 0.07 | 0.91 | 1.02 | 0.13 | 0.9 | 1.03 | 0.14 |
| (0.91, 1.02) | (0.94, 1.07) | (0.88, 1.00) | (0.00, 0.14) | (0.84, 0.98) | (0.94, 1.10) | (0.02, 0.23) | (0.84, 0.97) | (0.95, 1.13) | (0.03, 0.25) | |
| Household income per capita (ref: <7000 CNY) | ||||||||||
| 7000–17,499 | 0.98 | 1.04 | 0.92 | 0.11 | 1.1 | 0.94 | 0.17 | 1.04 | 0.91 | 0.13 |
| (0.92, 1.04) | (0.95, 1.13) | (0.85, 0.99) | (0.01, 0.21) | (0.98, 1.23) | (0.87, 1.01) | (0.04, 0.30) | (0.95, 1.13) | (0.83, 0.99) | (0.01, 0.26) | |
| ≥17,500 | 1.12 | 1.17 | 1.07 | 0.1 | 1.28 | 1 | 0.27 | 1.24 | 0.99 | 0.25 |
| (1.03, 1.21) | (1.08, 1.28) | (0.97, 1.17) | (0.01, 0.19) | (1.13, 1.46) | (0.91, 1.10) | (0.12, 0.43) | (1.10, 1.40) | (0.88, 1.10) | (0.07, 0.43) | |
| Don't know/refuse to answer | 0.93 | 0.99 | 0.89 | − | 1.01 | 0.93 | − | 0.9 | 0.94 | − |
| (0.84, 1.04) | (0.87, 1.12) | (0.80, 0.99) | (0.89, 1.14) | (0.80, 1.08) | (0.82, 1.00) | (0.79, 1.12) | ||||
Abbreviations: BMI, body mass index; CNY, Chinese Yuan; RERI, relative excess risk due to interaction.
Note: Data are expressed as odds ratio (95% CI). RERI is significant if 0 is not included in corresponding CI.
Some of the above associations were stronger among men and people living in urban area and north region (Table 3). Specifically, positive additive interactions of urban area and men, underweight and men, higher income and men, north region and urban area, higher education and urban area, current smoking and urban area, excessive drinking and urban area, small family size and urban area, higher income and urban area, higher education and north region, excessive drinking and north region, small family size and north region, and higher income and north region were found, with RERI ranging from 0.07 (95% CI, 0.00–0.14) to 0.40 (95% CI, 0.12–0.67).
The weighted prevalence of physician-diagnosed and total AR was 2.8% (95% CI, 2.5%–3.2%) and 9.0% (95% CI, 8.3%–9.7%), respectively (Supplemental table 1). While total AR displayed similar results to AR with respect to subgroup analyses, no significant difference was found by sex and BMI categories and the patterns by smoking and drinking were even reverse for physician-diagnosed AR. In the sensitivity analyses confining to never smokers, no substantial changes were observed for regression results and the dose-response curve, although the impacts of geographic region, long sleep, and underweight were attenuated (Supplemental table 2 and Supplemental Fig. 2B). Most of the aforementioned interactions were also significant at multiplicative scale (Supplemental table 3).
Discussion
In this large nationally representative survey, we estimated that 8.1% of Chinese adults had AR in 2018–2019. Applying this weighted prevalence to 2018 Chinese population produces an approximate projection of 89.6 million adults. Our estimate was modestly lower than those reported by 2 earlier multicenter studies in China. In 2005, Zhang et al surveyed 38 203 subjects in 11 major cities located at central and eastern China and 4253 (11.1%) of them reported having AR.10 In 2011, the same group expanded the study by further including 7 major cities in western China and found the standardized prevalence of AR was 17.6% among 36 577 subjects from 18 major cities.11 Several reasons may help explain these disparities. First, we included participants from both urban and rural areas, whereas they only recruited participants from large cities. Second, our inclusion of subjects was not affected by telephone coverage rate and the response rate was relatively higher in our study (94.9%) than theirs (less than 80%). Third, our sample accounted for adults from all age groups instead of limiting to those aged 16–65 years as with their studies, and this has important implications in the context of China's rapidly aging population. Taken together, the current study is subject to less selection bias and thus provides more accurate and representative estimation of AR prevalence.
Our estimated prevalence of AR was generally lower than those in United States (30.2%) or Europe (19.0%–29.8%) regardless of definition.19, 20, 21 Nevertheless, there appears to be a rising trend of AR prevalence in China,11 suggesting potential influence of environmental factors on AR. Despite dramatic differences in outdoor and indoor environment between urban and rural settings, prior results regarding urban-rural difference in AR prevalence were mixed. One cross-sectional study in Guangzhou found significantly higher prevalence of AR in urban area (8.3%) than rural area (3.4%).8 In contrast, inverse finding was observed when comparing AR prevalence in a community in Beijing (13.5%) with a village in Baoding (19.1%).9 Based on a large dataset comprising of 727 communities and 1061 villages, our result added strong evidence indicating higher AR prevalence in urban area of China. Urbanization is usually accompanied by higher ambient levels of air pollutants, such as particulate matter, sulfur dioxide (SO2), and nitrogen dioxide, which can increase the permeability of nasal mucosa and subsequently facilitate entrance of allergens.22 Positive correlations between AR prevalence and atmospheric SO2 concentration have been reported previously.10 Another explanation is the hygiene hypothesis that early exposure to reduced microbes occurring in urban area may favor development of allergic diseases in later life.23 The more common carpet use, home renovation, and pet adoption seen in cities may also contribute to AR via introducing allergy-provoking substances into living environment. On the other hand, we found AR prevalence was higher in north region than south region, and results of regression analysis confirmed this observation. It is well established that air in northern China is generally more polluted than southern China.24 Moreover, the relatively drier air and higher wind speed in the north region may facilitate the suspension and spread of many allergens.25 A recent time-series study in China has demonstrated that the relative risk of AR showed a negative association with relative humidity but a positive association with wind speed.26 To our surprise, the awareness of AR was also better in northern China, with particularly higher prevalence in provinces located at relatively underdeveloped northwest region. This is conflicting with the notion that provinces with higher economic and developmental level often show higher awareness prevalence of chronic diseases. Since northwest China are highly affected by land desertification, we speculate that the special vegetation used to control desertification such as Kobresia species and consequent high pollen concentration may lead to longer duration and more severe symptoms of AR. Our speculation seems to be supported by previous result that the proportion of persistent AR was highest in northern cities and lowest in southern cities.10
We explored several individual-level conventional risk factors associated with AR among Chinese adults. The higher AR prevalence in younger age groups and men is in line with epidemiological investigations mainly involving adults but in contrast with those studying AR among children and adolescents.27, 28, 29, 30 As a result, consideration should be given to these distinct patterns in the case of screening high-risk subpopulation and designing intervention policies. In regard to education and income, we uncovered similar relation to other studies with AR prevalence, with educated and wealthier individuals being more prone to have AR.29,30 Apart from sociodemographic factors, both cigarette smoking and alcohol consumption may play crucial roles in the development of AR. Herein, ever smokers displayed higher AR prevalence than non-smokers. As with air pollutants, smoking is capable of enhancing allergic sensitization and exacerbating AR symptoms. A randomized, placebo-controlled crossover study showed that environmental tobacco smoke could promote the production of IgE and induce a shift to T-helper 2 immune response.31 Interestingly, our multivariable regression further found larger magnitude of association with AR for former smokers relative to current smokers, which was partially attributable to the fact that people suffering from annoying symptoms of AR may be more likely to quit smoking. Also, more participants who drank excessive alcohol were found to have AR. A cross-sectional study in Pomerania proved positive association between biomarker of alcohol exposure and serum total IgE level.32 Moreover, a prospective cohort study in Copenhagen provided additional support by showing higher adjusted OR for perennial AR associated with more alcohol intake.33
Previous studies examining associations of sleep duration and BMI categories with AR almost focused on insufficient sleep and overweight/obesity. To our knowledge, this is the first study reporting that there was a U-shaped association between AR and sleep duration and that increased odds of AR were associated with underweight. Using a nationally representative sample of US adults, Grandner et al demonstrated that participants with very short or very long sleep duration tended to exhibit elevated levels of C-reactive protein, a biomarker of systemic inflammation.34 The latter is beneficial to development of AR from the perspectives of inflammatory etiology. Given the nature of cross-sectional study, the possibility of reverse causation cannot be fully excluded. Put it another way, insomnia as well as obstructive sleep apnea and more frequent use of sleep medication, as a consequence of AR, may result in extreme sleep duration at either end.35 As for BMI categories, our result was consistent with a recent meta-analysis that no significant association between overweight/obesity and AR was found in adults.36 Underweight has been previously associated with increased risk of asthma in Anqing, China.37 The underlying mechanism for its association with AR is not clear yet, although stronger allergic sensitization had been linked with underweight.38,39 More work is warranted to confirm these associations and find the causes.
Of note, the modifications of sex, residence, and geographic region on associations with AR have not been studied previously. It is plausible that being men and living in urban area or north region would act synergistically with some factors on AR as seen in this study. One explanation is that corresponding subpopulations are more likely to be exposed to allergens. Generally, men tend to take more outdoor exercise than women, which is also supported by the higher prevalence of usual exercise among men (17.0% vs. 14.6%) in this study. Considering the chronic nature of indoor allergen exposure, increased exposure to the outdoor allergen may outweigh corresponding reduced exposure to the indoor allergen. Similarly, the modern lifestyle in urban area and certain meteorological factors in north region, as above mentioned, can increase the possibility of allergen exposure. Besides, the air pollution related to urban area and north region and more stress related to men and fast-paced urban life may lead to a higher intrinsic inflammatory level that increase nasal vascularity and then exacerbate the responses to allergens.40,41 Yet, it would be interesting to study the explicit mechanisms underlying the additive interactions in future work.
Our study has some limitations. First, the question asked may merely lead to a diagnosis of rhinitis. However, the definition used in this study is consistent with the epidemiological criteria proposed by Allergic rhinitis and its impact on asthma, which excludes rhinitis caused by infection and short-term irritation by declaring symptoms in the absence of cold and with a duration of ≥1 h. Additionally, the association pattern of the resulting AR with increasing age is distinct from that of non-allergic rhinitis,42 and participants with AR were found to be much more likely than those without to have chronic urticaria (19.5% vs. 0.77%) and doctor-diagnosed AR (10.7% vs. 2.8%). Also, our estimate was generally comparable to those based on the combination of symptoms and skin prick test (7.2% and 6.2% in Beijing and Baoding, respectively) or allergen-specific IgE (9.1% in several northern regions of China).9,43 Taken together, although we acknowledge the possibility of including types of rhinitis other than AR cannot be completely excluded, the observed outcome mainly reflects AR. Second, since this survey is not exclusively designed for allergic diseases, the information on duration, severity, allergic causes, and concurrent diseases related to AR was not obtained, impeding a more detailed and thorough analysis. Third, the unavailability of some important covariates such as family history of AR and pet ownership increases the chance of residual confounding. Fourth, the results of interaction tests should be interpreted cautiously due to multiple comparison.
In conclusion, the present study provides robust and the most recent evidence on epidemic of AR and associated risk factors among Chinese adults. Our novel findings of significant additive interactions suggest that men and people living in urban area and north region may generally benefit more from targeted intervention measures. The overall low awareness calls for campaigns to improve detection of AR in China. Future studies are necessary to understand the long-term trend of AR prevalence and its longitudinal associations with potential determinants.
Abbreviations
AR, allergic rhinitis; CCDRFS, China Chronic Disease and Risk Factor Surveillance; CI, confidence interval; IgE, immunoglobulin E; OR, odds ratio; RERI, relative excess risk due to interaction; SO2, sulfur dioxide.
Funding
This study was supported by the Chinese central government (Key Project of Public Health Program) and the National Key Research and Development Program of China (2018YFC1311700).
Author contributions
Jing Wu, Zuotao Zhao, and Limin Wang contributed to conceptualization and critical revision of the manuscript. Mei Zhang and Xiao Zhang collected and interpreted the data. Xiao Zhang conducted data analysis and drafted the manuscript. All authors contributed to the article and approved the submitted version.
Ethics statement
The study protocol was approved by the ethical review committee of the Chinese Center for Disease Control and Prevention. All participants provided written informed consent.
Consent for publication
The authors provide their consent for the publication of the study results.
Data availability statement
The data analyzed during the current study are available from the corresponding author upon reasonable request.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2023.100744.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Bousquet J., Khaltaev N., Cruz A.A., et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 2.Nathan R.A. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28:3–9. doi: 10.2500/aap.2007.28.2934. [DOI] [PubMed] [Google Scholar]
- 3.Berger W.E. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233–250. doi: 10.2165/00148581-200406040-00003. [DOI] [PubMed] [Google Scholar]
- 4.Katelaris C.H., Lee B.W., Potter P.C., et al. Prevalence and diversity of allergic rhinitis in regions of the world beyond Europe and North America. Clin Exp Allergy. 2012;42:186–207. doi: 10.1111/j.1365-2222.2011.03891.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J., Bai J., Shen K., et al. Self-reported prevalence of childhood allergic diseases in three cities of China: a multicenter study. BMC Publ Health. 2010;10:551. doi: 10.1186/1471-2458-10-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li F., Zhou Y., Li S., et al. Prevalence and risk factors of childhood allergic diseases in eight metropolitan cities in China: a multicenter study. BMC Publ Health. 2011;11:437. doi: 10.1186/1471-2458-11-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Liu W., Hu Y., et al. Updated prevalences of asthma, allergy, and airway symptoms, and a systematic review of trends over time for childhood asthma in Shanghai, China. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C.W., Chen D.D., Zhong J.T., et al. Epidemiological characterization and risk factors of allergic rhinitis in the general population in Guangzhou City in China. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng M., Wang X., Bo M., et al. Prevalence of allergic rhinitis among adults in urban and rural areas of China: a population-based cross-sectional survey. Allergy Asthma Immunol Res. 2015;7:148–157. doi: 10.4168/aair.2015.7.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L., Han D., Huang D., et al. Prevalence of self-reported allergic rhinitis in eleven major cities in China. Int Arch Allergy Immunol. 2009;149:47–57. doi: 10.1159/000176306. [DOI] [PubMed] [Google Scholar]
- 11.Wang X.D., Zheng M., Lou H.F., et al. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71:1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Gao P., Zhang M., et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X., Song X., Zhang M., et al. Prevalence and risk factors of chronic urticaria in China: a nationwide cross-sectional study. Allergy. 2022;77:2233–2236. doi: 10.1111/all.15287. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M., Wang L., Wu J., et al. Data resource profile: China chronic disease and risk factor surveillance (CCDRFS) Int J Epidemiol. 2022;51:e1–e8. doi: 10.1093/ije/dyab255. [DOI] [PubMed] [Google Scholar]
- 15.Giovino G.A., Mirza S.A., Samet J.M., et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–679. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 16.Li Y., Jiang Y., Zhang M., Yin P., Wu F., Zhao W. Drinking behaviour among men and women in China: the 2007 China chronic disease and risk factor surveillance. Addiction. 2011;106:1946–1956. doi: 10.1111/j.1360-0443.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B., Cooperative Meta-Analysis Group Of China Obesity Task Force Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Chin J Epidemiol. 2002;23:5–10. [PubMed] [Google Scholar]
- 18.Hosmer D.W., Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Nathan R.A., Meltzer E.O., Derebery J., et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29:600–608. doi: 10.2500/aap.2008.29.3179. [DOI] [PubMed] [Google Scholar]
- 20.Bauchau V., Durham S.R. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24:758–764. doi: 10.1183/09031936.04.00013904. [DOI] [PubMed] [Google Scholar]
- 21.Bachert C., van Cauwenberge P., Olbrecht J., van Schoor J. Prevalence, classification and perception of allergic and nonallergic rhinitis in Belgium. Allergy. 2006;61:693–698. doi: 10.1111/j.1398-9995.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 22.London N.R., Jr., Lina I., Ramanathan M., Jr. Aeroallergens, air pollutants, and chronic rhinitis and rhinosinusitis. World J Otorhinolaryngol Head Neck Surg. 2018;4:209–215. doi: 10.1016/j.wjorl.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada H., Kuhn C., Feillet H., Bach J.F. The 'hygiene hypothesis' for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y., Ebenstein A., Greenstone M., Li H. Evidence on the impact of sustained exposure to air pollution on life expectancy from China's Huai River policy. Proc Natl Acad Sci USA. 2013;110:12936–12941. doi: 10.1073/pnas.1300018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F., Sun F., Liu W., et al. On wind speed pattern and energy potential in China. Appl Energy. 2019;236:867–876. [Google Scholar]
- 26.Luo X., Hong H., Lu Y., et al. Impact of Air Pollution and Meteorological Factors on Incidence of Allergic Rhinitis: A Low-Latitude Multi-City Study in China. Allergy. 2022 doi: 10.1111/all.15469. [DOI] [PubMed] [Google Scholar]
- 27.Lim F.L., Hashim Z., Than L.T., Md Said S., Hisham Hashim J., Norback D. Asthma, airway symptoms and rhinitis in office workers in Malaysia: associations with house dust mite (HDM) allergy, cat allergy and levels of house dust mite allergens in office dust. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X.Y., Ma T.T., Wang X.Y., et al. Prevalence of pollen-induced allergic rhinitis with high pollen exposure in grasslands of northern China. Allergy. 2018;73:1232–1243. doi: 10.1111/all.13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y., Jiang Y., Li S., Shen X., Liu J., Jiang F. Pre- and postnatal risk factors in relation to allergic rhinitis in school-aged children in China. PLoS One. 2015;10 doi: 10.1371/journal.pone.0114022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsowaidi S., Abdulle A., Shehab A., Zuberbier T., Bernsen R. Allergic rhinitis: prevalence and possible risk factors in a Gulf Arab population. Allergy. 2010;65:208–212. doi: 10.1111/j.1398-9995.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Sanchez D., Rumold R., Gong H., Jr. Challenge with environmental tobacco smoke exacerbates allergic airway disease in human beings. J Allergy Clin Immunol. 2006;118:441–446. doi: 10.1016/j.jaci.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich N., Husemoen L.L., Petersmann A., Nauck M., Volzke H., Linneberg A. The association between alcohol consumption and biomarkers of alcohol exposure with total serum immunoglobulin E levels. Alcohol Clin Exp Res. 2008;32:983–990. doi: 10.1111/j.1530-0277.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 33.Bendtsen P., Gronbaek M., Kjaer S.K., Munk C., Linneberg A., Tolstrup J.S. Alcohol consumption and the risk of self-reported perennial and seasonal allergic rhinitis in young adult women in a population-based cohort study. Clin Exp Allergy. 2008;38:1179–1185. doi: 10.1111/j.1365-2222.2008.02945.x. [DOI] [PubMed] [Google Scholar]
- 34.Grandner M.A., Buxton O.M., Jackson N., Sands-Lincoln M., Pandey A., Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36:769–779E. doi: 10.5665/sleep.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J., Zhang X., Zhao Y., Wang Y. The association between allergic rhinitis and sleep: a systematic review and meta-analysis of observational studies. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J., Luo F., Han Y., Lou H., Tang X., Zhang L. Obesity/overweight and risk of allergic rhinitis: a meta-analysis of observational studies. Allergy. 2020;75:1272–1275. doi: 10.1111/all.14143. [DOI] [PubMed] [Google Scholar]
- 37.Celedon J.C., Palmer L.J., Litonjua A.A., et al. Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med. 2001;164:1835–1840. doi: 10.1164/ajrccm.164.10.2105033. [DOI] [PubMed] [Google Scholar]
- 38.Stemeseder T., Klinglmayr E., Moser S., et al. Influence of intrinsic and lifestyle factors on the development of IgE sensitization. Int Arch Allergy Immunol. 2017;173:99–104. doi: 10.1159/000475499. [DOI] [PubMed] [Google Scholar]
- 39.Van Gysel D., Govaere E., Verhamme K., Doli E., De Baets F. Body mass index in Belgian schoolchildren and its relationship with sensitization and allergic symptoms. Pediatr Allergy Immunol. 2009;20:246–253. doi: 10.1111/j.1399-3038.2008.00774.x. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y.Z., Wang Y.X., Jiang C.L. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316. doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori S., Fujieda S., Sunaga H., Fox S.B., Saito H. Expression of platelet-derived endothelial cell growth factor and vascularity in the nasal mucosa from allergic rhinitis. Clin Exp Allergy. 2000;30:1637–1644. doi: 10.1046/j.1365-2222.2000.00903.x. [DOI] [PubMed] [Google Scholar]
- 42.Baptist A.P., Nyenhuis S. Rhinitis in the elderly. Immunol Allergy Clin North Am. 2016;36:343–357. doi: 10.1016/j.iac.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z.H., Lin W.S., Li S.Y., et al. Analysis of the correlation of prevalence in allergic rhinitis and other allergic diseases. Chin J Otorhinolaryngol Head Neck Surg. 2012;47:379–382. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed during the current study are available from the corresponding author upon reasonable request.