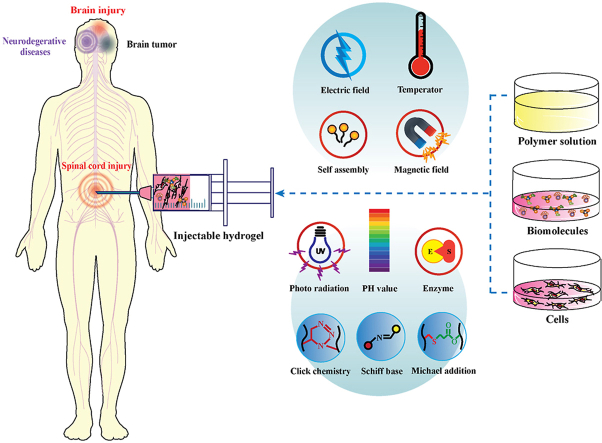
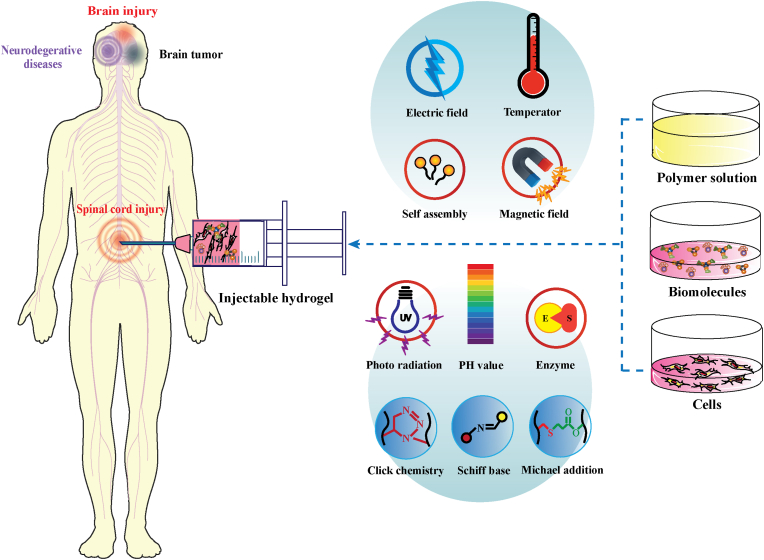
Abstract
Repairing central nervous system (CNS) is difficult due to the inability of neurons to recover after damage. A clinically acceptable treatment to promote CNS functional recovery and regeneration is currently unavailable. According to recent studies, injectable hydrogels as biodegradable scaffolds for CNS tissue engineering and regeneration have exceptionally desirable attributes. Hydrogel has a biomimetic structure similar to extracellular matrix, hence has been considered a 3D scaffold for CNS regeneration. An interesting new type of hydrogel, injectable hydrogels, can be injected into target areas with little invasiveness and imitate several aspects of CNS. Injectable hydrogels are being researched as therapeutic agents because they may imitate numerous properties of CNS tissues and hence reduce subsequent injury and regenerate neural tissue. Because of their less adverse effects and cost, easier use and implantation with less pain, and faster regeneration capacity, injectable hydrogels, are more desirable than non-injectable hydrogels. This article discusses the pathophysiology of CNS and the use of several kinds of injectable hydrogels for brain and spinal cord tissue engineering, paying particular emphasis to recent experimental studies.
Keywords: Injectable hydrogels, Tissue engineering, Biomaterials, Central nervous system, Spinal cord injuries, Brain injuries
Graphical abstract
Highlights
-
•
Will injectable hydrogels bring hope for regeneration of damage the central nervous system?
-
•
This paper reviews injectable hydrogels as novel platforms for neural tissue engineering.
-
•
Injectable hydrogels as unique materials for promoting ECM remodeling of central nervous system.
-
•
This review will discuss the CNS pathophysiology and production of hydrogels for CNS restoration.
Abbreviations
- aFGF
acidic fibroblast growth factor
- AHA/DTP
aldehyde-modified hyaluronic acid/3, 3′ -dithiobis (propionyl hydrazide)
- AOAA
O-(Carboxymethyl) hydroxylamine hemihydrochloride
- 4arm-PEG-diacerein
four-armed polyethylene glycol functionalized with an anti-inflammatory drug, diacerein
- BBB
blood-brain barrier
- BDNF
brain-derived neurotrophic factor
- BME
mesenchymal stem cell-derived exosomes
- CDAHs
O-carboxymethyl chitosan (CMC)/dialdehyde polyurethane (DAPU)/gold nanoparticles (Au-NPs) hydrogels
- CFL:
capillary force lithography
- CNFs
carbon nanofibers
- CNS
central nervous system
- CNTs
carbon nanotubes
- Col-I:
collagen type I
- CS/β-GP
chitosan/β-glycerophosphate
- CS–CNF
chitosan–cellulose nanofiber
- CS-HA
chitosan-hyaluronan
- CSPGs
chondroitin sulfate proteoglycans
- CST
critical solution temperature
- 3D
3-dimensional
- DA
dopamine
- ECM
extracellular matrix
- FPGEGa
poly (citrate-gallic acid)-based hybrid hydrogel
- GAGs
glycosaminoglycans
- GelMA-imid
imidazole groups-modified gelatin methacrylate
- GFAP
glial fibrillary acidic protein
- GO
graphene oxide
- H&E
hematoxylin and eosin
- HA
hyaluronic acid
- HA-PBA/Gel-Dopa
phenylboronic acid-grafted hyaluronic acid/and dopamine-grafted gelatin
- hAMSCs
human amniotic mesenchymal stromal cells
- hESCs
human embryonic stem cells
- HP
heparin-poloxamer
- HRP
horseradish peroxidase
- H2S
hydrogen sulfide
- HT/HGA
hyaluronic acid-tyramine combined with gallic acid-grafted hyaluronic acid
- I-5
imidazole-poly (organophosphazenes)
- ICH
intracerebral hemorrhage
- IF
immunofluorescence staining
- IKVAV
Isoleucine-lysine-valine–alanine–valine
- iPN
injectable peripheral nerve
- iPSCs
induced pluripotent stem cells
- LN-I:
laminin I
- M2 derived EVs
microglia derived extracellular vesicles
- MC
methylcellulose
- MR
magnetically-responsive
- MSCs
mesenchymal stromal cells
- MWM
Morris water maze
- Ncad-mRADA
amphiphilic peptide [(RADA)3-(RADG)] (mRADA)-tagged N-cadherin extracellular domain
- NGFs
nerve growth factors
- NPCs
neural progenitor/stem cells
- NSCs
neural stem cells
- 6-OHDA
6-hydroxydopamine
- OPF
oligo(polyethylene glycol) fumarate
- PA
peptide amphiphile
- PANI
polyaniline
- PCL:
polycaprolactone
- PD
Parkinson's disease
- PD/GO hydrogel
GO sheets with 4arm-PEG-diacerein, donated as PD hereafter
- PDA
polydopamine
- PEDOT
poly(ethylene dioxythiophene)
- PEG
poly(ethylene glycol)
- PLEL:
poly (d, l-lactide)-poly (ethylene glycol)-poly(d,l-lactide)
- PLGA
poly (lactic-co-glycolic acid)
- PNIPAAm
poly(N-isopropylacrylamide)
- PNS
peripheral nervous system
- Pol
poloxamer
- PPTases
phosphopantetheinyl transferase
- PPy
polypyrrole
- RADA
arginine (R, Arg), alanine (A, Ala) aspartic acid (D, Asp), alanine (A, Ala)
- RADA
16I [Ac-(AcN-ArgAlaAspAla)4-CONH2]
- RGD
arginine–glycine–aspartate
- ROS
reactive oxygen species
- SAP
self-assembling peptide
- SCI
spinal cord injury
- SDF-1α
stromal-cell-derived factor-1
- SF/PDA
silk fibroin/polydopamine
- SHED-Exo
stem cells from human exfoliated deciduous teeth-derived exosomes
- SHIELD
shear-thinning hydrogel for injectable encapsulation and long-term delivery
- SMOs
sulfamethazineoligomers
- SNpc
substantia nigra pars compacta
- T1AM
3-iodothyronamine;
- TBI
traumatic brain injury
- TNFα
tissue necrosis factor-alpha
- T2WI
T2-weighted imaging
- VEGF
vascular endothelial growth factor
- YIGSR
tyrosine–isoleucine–glycine–serine–arginine
1. Introduction
The repair of the human central nervous system (CNS), which includes the brain and spinal cord, is challenging. Acute ischemic injury or trauma to these structures results in complex pathophysiological changes in the nerve tissue that often lead to severe neurological impairment [1,2]. The key role of the brain is to process and merge the obtained information of neurons. As an essential component of the CNS, the spinal cord can control sensory and motor function, and it is primarily accountable for transmission among the brain and peripheral nerves [2,3].
Treatment of spinal cord injuries (SCI) and traumatic brain injuries (TBI) is complicated since the mature nervous system does not have the capacity to regenerate on its own and perform normal functions after being injured. This results in significant socioeconomic problems worldwide [2,4,5]. For example, only 577 TBI instances per 100,000 individuals are reported in the United States annually [6]. SCI is mostly linked with persistent impairment of motor function throughout the majority of cases [7,8]. Annually, 760,000 additional instances of catastrophic SCI are reported worldwide due to falls, traffic collisions, and other events [3].
A significant difficulty is repairing injured brain tissue due to its complex histological and anatomical structure and inadequate capacity to repair itself. In this regard, there are internal elements interfering with its rebuilding, making it complicated. For instance, scar tissue forms due to CNS traumas, including tumor formation, limiting the mechanism of regeneration. TBI typically results in lifelong impairment. TBI and SCI frequently result in mortality, and those who escape face lifelong impairment [[9], [10], [11], [12]].
There is currently no appropriate treatment for TBI and SCI [2,13]. The operation merely slowed the progression of malignancies, whereas pharmacological treatments have so far concentrated mainly on pain relief. Thus, the objective of CNS tissue engineering is to develop a biomaterial that enables neural stem cells) NSCs (to proliferate and differentiate effectively [14,15].
The constantly expanding area of biomaterials has produced new methods for developing therapies involving CNS regeneration over the last decade [1]. Multifunctional scaffolds, which carry therapeutic molecules, and cells, are suitable for the aforementioned challenges in developing neural tissue engineering [16]. Hydrogels, which are hydrophilic polymer systems effective in holding a significant quantity of water inside their architectures, may be utilized to transport cells, medicines, and proteins as well as to repair injured spinal cord and brain tissues [1,17].
The most significant challenge correlated with the implantation of premade polymeric scaffolds is the need to split and remove certain tissue from the damaged site to allow the insertion of the constructs. To enhance the regeneration capability of TBI and SCI by reducing the disadvantages of premade scaffolds, newly injectable hydrogels were assessed, including agarose [18], fibrin [19,20], the blend of methylcellulose and hyaluronan [21], and self-assembling peptide (SAP) nanofibers [22,23].
This review will discuss the pathophysiology of the CNS and the production of hydrogels for CNS restoration, with a concentration on the freshest and most sophisticated methods for CNS tissue engineering using injectable hydrogels.
1.1. CNS injury mechanisms
CNS injury varies from ischemic stroke to the damage of the tissue to the TBI or SCI, leading to diverse diseases and persistent symptomatic problems. More complex methods to repair the brain and spinal cord following damage are critically needed because treatments are not currently available for full or almost complete restoration and rehabilitation. Moreover, the capability of the CNS for healing by endogenous cells is extremely restricted, and there is no significant axonal restoration or functional recovery in the CNS due to its cellular and extracellular environment [2,[24], [25], [26]].
Additionally, the secondary injury phase intensifies dynamic alterations throughout the environment that result in postponed, chronic neurodegeneration in the setting of damage [27]. Despite different injury cascades and therapeutic results, a number of important biochemical and cellular markers of the secondary injury phase, including neuro-inflammation, glial scar formation, and neurodegeneration, may characterize CNS damages. As a rule, inflammatory processes, including macrophage infiltration, manifest themselves more gradually in the CNS than peripheral nervous system (PNS). This results in the accumulation of cellular debris in the region of the injury, which may interfere with the healing mechanism [28,29].
Furthermore, within indigenous CNS cells, especially glial cells, the neurogenic inflammation may also be increased in time to produce biochemical homeostasis disturbance in the manner of oxidative stress, excitotoxicity, and cellular activation [30].
An increasing number of cytokines, like interleukins and tissue necrosis factor-alpha (TNFα), and also reactive oxygen species (ROS), characterize the pro-inflammatory situations that inhibit regular cell function and sustain tissue reactivity. The ability to modulate the inflammatory environment following damage is critical for effective regeneration; however, it is very difficult from a clinical perspective since the subsequent reaction includes the synchronization of both resident and invading cells through a complicated series of occurrences [[30], [31], [32]]. When it comes to CNS damage, astrocyte reactivity is a key characteristic of the vast majority of the processes [33,34].
Astrocytosis or gliosis may be widely described as a multi-stage mechanism wherein astrocytes respond to the damage by modifying their phenotypic features. Enhanced proliferation, hypertrophy, and upregulation of glial fibrillary acidic protein (GFAP) comprise astrocyte responsiveness. An inherent protective process is the responsiveness of astrocytes; however, their development may contribute to persistent blood-brain barrier disturbances, chronic inflammation, neural toxicity, and cell death throughout the adjacent region [33,35].
At some point, activated astrocytes have a scarring characteristic. Although the glial scar has certain advantages, such as regulating immune responses and blood circulation and avoiding additional injury, it is a major therapy barrier during the repair of the CNS tissue [36,37].
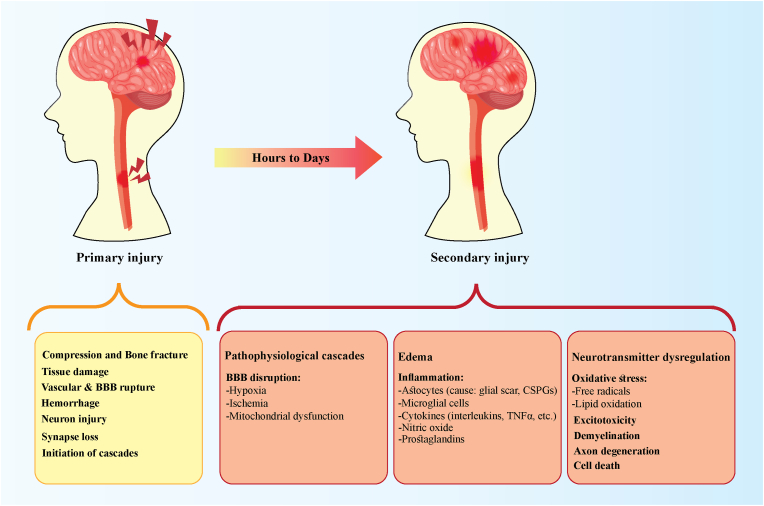
Deposit-inhibitory components of ECM, such as chondroitin sulfate proteoglycans (CSPGs), have been upregulated via astrocytes producing scar [35,38]. This scar tissue has a finer texture than normal tissues; it is also encompassed by a thick cell barrier. Such environmental alterations result in mechanical and biological hindrance to regular attachment pathways, making it impossible for axonal penetration and regeneration to occur in the damaged zone [37,39]. Demyelination, axonal degeneration, and neuronal death may occur as a consequence of the development of damage, inflammation, and cellular responsiveness (Fig. 1) [31,32].
Fig. 1.
Pathophysiological events of primary and secondary phases of central nervous system injuries.
BBB: blood-brain barrier; TNFα: tissue necrosis factor-alpha; CSPGs: chondroitin sulfate proteoglycans.
Neural degradation is both an acute and chronic mechanism of degeneration and is the main pathophysiological consequence to be prevented in the treatment of CNS damage. Treatment methods for CNS have faced difficulties due to restricted accessibility to injury locations, spatial intricacy and tissue structure, absence of regenerating property in indigenous cells, widely diverse damage pathways, and short remedial opportunities [26,40,41].
In order to properly manage the range of negative consequences that follow CNS damage, multifunctional, combinatorial approaches must be developed. Hydrogels are excellent candidates for such frameworks because of their delicate material characteristics and biochemical adjustability. Natural viscoelastic characteristics of hydrogels allow them to be engineered in such a way that they replicate the delicate mechanical characteristics of CNS tissue, which has compressed moduli on the scale of 2000 Pa [42].
Besides injected compositions, which are useful in situations where the injury location is impossible to reach, other engineering criteria for hydrogels utilized in the CNS are specialized functionalization methods that are unique to neuronal growth impulses involving both microarchitectural and biochemical indications. The subsequent methods show improvement in the functionalization of hydrogel to increase incorporation into CNS tissue as well as enhanced renewal of indigenous and transplanted cells throughout various contexts.
1.2. Neuroregenerative and neuroprotective therapeutic strategies
Apart from the challenges inherent in CNS self-renewal, the present treatment strategy for patients with CNS disorders is mostly neuroprotective. It is primarily designed to avoid subsequent cell death and reduce the extent of the damage. But, it has no effect on tissue healing or the re-establishment of axonal synapses that have been disrupted. On the other side, some methods are aimed solely at conquering one of the regenerating barriers, for example, targeting a blocker or stimulating the axonal regenerative process. However, the CNS has complex pathophysiology; hence combinatorial strategies addressing several aspects of the damage are likely to be more successful [[43], [44], [45], [46]].
During the treatment of CNS disorders, the fundamental objectives are to prevent cell death and decrease the extent of the damages (neuroprotection), even though the second aim is to promote the mechanism of neuroregeneration to regenerate the injured tissue. Because of the intricacy of the central nervous system, there is a significant number of active investigation efforts; however, there is no succinct and practical treatment at this time. Neuroprotective methods are already more progressed, with a few clinical trials now underway [47].
Recently, a potential neuroregenerative treatment strategy for CNS has emerged as cell transplantation. A number of scientific articles and a few current clinical trials have shown enhancements in neurological functioning after the administration of cells, including olfactory ensheathing cells, macrophages, Schwann cells, and adult stem cells, which include umbilical cord blood stem cells, NSCs, induced pluripotent stem cells (iPSCs) and mesenchymal stromal cells (MSCs) [[48], [49], [50], [51]].
Though cell transplantation studies have shown that no substantial restoration occurs outside the locations of damage, this is thought to be due to the need for bridge transplants, which serve to establish a physical substrate for guided axonal development over the defect space and to enable axons to penetrate the host region at the right place [45,52].
Researchers have successfully used fibrin matrices to fill the damage gap and enable cell transplantation. The transplantation of NSCs and two human stem cell lineages encapsulated in fibrin matrices carrying growth factor mixtures into patients with severe SCI. The transplanted cells developed into neurons and stretched a significant quantity of axons over a considerable space, thus restoring performance [53,54].
It was shown that when 8-week-old fetal cells were loaded in a fibrin scaffold with a growth factor cocktail and grafted into the primate CNS, they responded similarly to the findings seen in the human CNS [55]. To be more specific, neurological tissue engineering has received significant interest in recent years, with particular emphasis on the creation of injectable hydrogels that may be utilized as composites for CNS regeneration [56].
The combination of scaffolds with cells and biomolecules that positively repair the space may also alter the microenvironment at the damaged location, preventing the development of glial scar and accumulating neurotrophic growth factors while simultaneously stimulating axonal restoration resulting in improved outcomes. Based on these findings, a variety of scaffolds composed of natural or synthetic polymers were produced, and various structural functionality over the biomaterial, taking into consideration its rostral and caudal location in the damaged area [57,58].
Aside from that, scientists have also focused their interest on the scaffolds' fabrication techniques to better imitate the intricate 3-dimensional (3D) nanoenvironments that neural cells are subjected to. A variety of nanotopographical methods have been investigated to create specified cellular environments, including self-assembly, etching, capillary force lithography (CFL), hybrid composite nanomaterials, conductive hydrogels, 3D bioprinting, and electrospinning [2,54,59].
2. Biomaterials and their specifications to support CNS regeneration
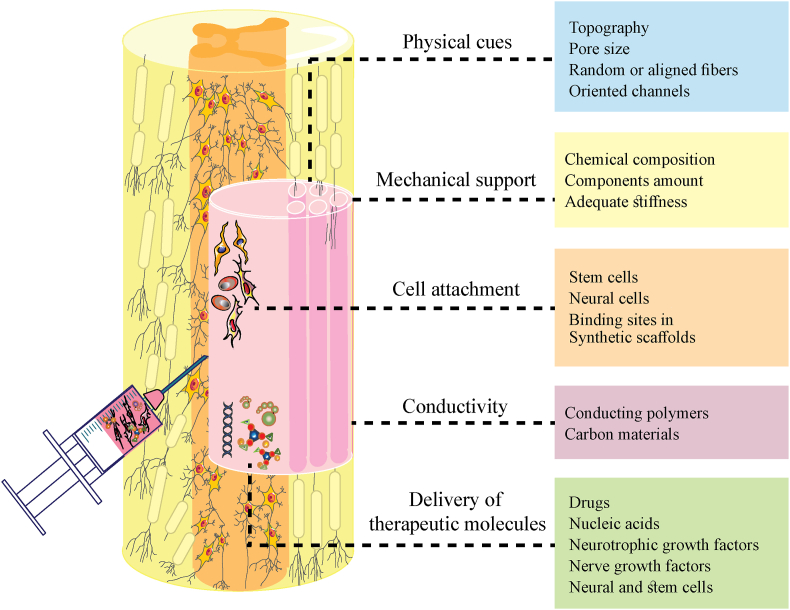
ECM, in addition to neural and glial cells, accounts almost for 20% of the CNS volume. The inductive qualities of ECM make it possible to restore soft tissue [60,61]. Scaffolds have several features that are significant in the regeneration of neural tissue (Fig. 2). In soft tissues like CNS after injury, the appropriate scaffold is required to simulate the structure and the chemical and mechanical properties of natural ECM. Also, scaffolds should not provide an immune response. Still, they must aid in the prevention of glial scarring-induced CNS damage, facilitate neurite regeneration, promote cell colonization, and repair injured blood vessels [8,11,62,63].
Fig. 2.
Biomaterial scaffolds in the regeneration of CNS. The physical, mechanical, and biochemical cues of the functionalized scaffolds can encourage neural regeneration and increase therapeutic outcomes. Furthermore, stem cell therapies for CNS benefit greatly from the use of biomaterials, which help retain cells at the site of injury, regulate cell fate, and promote cell proliferation.
The disturbance of the architecture is a common outcome of neurological diseases. Therefore, material for CNS regeneration should follow soft tissue architecture and topography to bridge the damaged area [64,65].
Pore size is one of the main features of scaffolds that could be modified. It is accountable for allowing proper cell attachment and migration, regulated diffusion of medium and cells' metabolites, and finally, cell survival, organization, and differentiation [66]. According to previous studies, the best-suited pore size and the scaffold porosity for neural cell growth range from 10 to 100 μm and 90%, respectively [67,68].
Natural biomaterials like ECM hydrogels and modified synthetic materials can efficiently regenerate CNS tissue after injury [60,69]. In general, neural ECM is a highly hydrated structure that consists of glycosaminoglycans (GAGs) such as hyaluronic acid, proteoglycans, and small amounts of several proteins like fibronectin and laminin. This structure could be mimicked by the appropriate biomaterials. In natural ECM, the specific and short peptide sequences in laminin (Isoleucine-lysine-valine–alanine–valine (IKVAV) and tyrosine-isoleucine-glycine-serine-arginine (YIGSR)) and fibronectin (i.e., arginine-glycine-aspartate (RGD), which can associate with integrin, are responsible for cell-ECM interaction. So, in synthetic scaffolds, we need some modifications to have such binding sites [70,71].
The chemical composition of ECM and the amount of its components determines its mechanical properties like stiffness [72,73]. For neural regeneration to succeed, the appropriate materials must have suitable mechanical properties compatible with natural CNS stiffness. An adequate stiffness can help cell attachment, migration, and differentiation, but high stiffness may inhibit axon elongation and neural cell regeneration [8]. According to the human brain and spinal cord stiffness, it has been shown that lower stiffness in biomaterials can promote neural cell activity, while hydrogels with higher stiffness may be helpful in TBI regeneration [74].
Another important property of scaffolds in neural tissue engineering is their conductivity and bioelectrical stimulation ability. The action potential is an electrical signal that is generated at the synapse and sent along axons. The electrical conductivity of the human spinal cord and brain are about 0.6–2.43 and 0.8–6.4 mS/cm, respectively [[75], [76], [77]]. Therefore, for CNS regeneration, many electrically conductive materials, including conducting polymers (i.e., polyaniline (PANI), polypyrrole (PPy), and polythiophene) and carbon materials (i.e., carbon nanofibers (CNFs), carbon nanotubes (CNTs), and graphene) have been studied [[78], [79], [80], [81], [82], [83]]. Their primary function is to facilitate neural cell communication and electrical signal transmission to simulate natural neural tissue [84,85]. Unlike the high electrical conductivity of mentioned materials, they have some limitations. For example, PPy scaffolds are difficult to work and process into soft 3D structures due to their stiff and brittle nature [86], Poly(ethylene dioxythiophene) (PEDOT) has a low water solubility [87], PANI may cause chronic inflammation because of its non-biodegradability [86], and carbon materials like CNTs may cause oxidative stress and have an adverse effect on axon elongation and neurite growth [78]. So, the production of conductive polymer hydrogels by combining the soft mechanical features of hydrogels (such as oligo(polyethylene glycol), fumarate (OPF), poly(ethylene glycol) (PEG), tannic acid, agarose, etc.) and the electrical properties of conductive biomaterials have been designed to overcome these limitations [[81], [82], [83],[88], [89], [90], [91]].
Various natural and synthetic polymers, in addition to drugs, neurotrophic growth factors, nerve growth factors (NGFs), and neural and stem cells, have been utilized to improve CNS regeneration. Hydrogels (non-injectable and injectable), nanofibers, and sponges are the primary types of biomaterials for CNS tissue engineering and regeneration that can fabricate with several techniques [71,92].
Hydrogels are 3D structures that are physically or chemically cross-linked by hydrophilic polymer chains to produce an interconnected network with high water retention [17,93]. Natural or synthetic hydrogels are now well suited for use in soft tissue engineering because of their high ability to mimic natural ECM, facile alteration of physicochemical characteristics, and efficient polymerization in situ at the injury site [17,[94], [95], [96]].
3. Injectable hydrogels
The concept behind injectable hydrogels is to inject a solution of a relatively low viscosity polymer into the injured area and allow it to gel. The injectable hydrogel's mechanical characteristics and degradation rate must match the desired soft tissue's regeneration rate. The rate of gelation is also critical since it must be both slow enough to allow for injection and rapid enough to prevent leakage. The type of polymers, their concentration, molecular weight, the type of crosslinking (chemical or physical), and the degree can influence the rate of gelation [17,71,97]. While non-injectable hydrogels require more complicated and lengthy operations, injectable materials can be introduced into the body much faster and have a better affinity for the host tissue [78]. On the other hand, they can be significantly affected by the complex inside environment of the body, which includes their mechanical characteristics and gelation rate. So, it must be remembered that injectable hydrogels act very differently in vivo and in vitro [98].
3.1. Optimal properties of injectable hydrogels for CNS repair
First of all, injectable hydrogels should be biocompatible, non-immunogenic, and not have any harmful effects on human cells or tissues to support CNS regeneration [99].
Additionally, injectable hydrogels should stimulate axonal growth and elongation while degrading over time to prevent the need for another surgery to remove the scaffold [53,58].
The mechanical features (stiffness) and surface topography of scaffolds are also crucial for designing biomaterials. Researchers have found that small variations in the mechanical and topographical features of scaffolds have a big impact on cell fate, attachment, polarity, proliferation, migration, differentiation, and survival [43,100]. In neural tissue engineering, scaffolds should be able to tolerate the stresses created by surrounding tissues [58,101]. So, injected hydrogels before injection must have a lower elastic modulus (G′) than their storage modulus (G″), while after injection, they must have a G″ more than G′ to form a soft and cohesive solid that avoids collapsing under the weight of the surrounding tissues [102].
There are a number of factors that influence the swelling ratio and degradation rate of biomaterials, including polymer type, biomaterial chemical and mechanical properties, and the presence of enzymes at the site of damage. In order for hydrogels to be effective during CNS regeneration, they must degrade slowly and swell minimally. The biomaterial's degradation rate should be a balance between its early degradation (which could affect glial scarring, additional inflammation, and inhibit axons elongation) and late degradation (which could cause new tissue generation and prevent long-distance axonal regeneration [102]. Before neural regeneration, a high rate of hydrogel degradation can produce swelling and a loss of mechanical integrity [103,104].
Another key metric to consider for proper nutrient and biological molecule diffusion, waste elimination, cell attachment, and tissue growth is the porosity of scaffolds and also the diameter and interconnectivity of the pores [105].
Hydrogel deformation, occlusion of the defect, and inhibition of neurite development may all be caused by a high swelling ratio [106,107]. When hydrophilic or electrostatically charged moieties are added to hydrogels, swelling increases, but greater network permeability and significant effects on hydrogel biomechanics will happen. It has been observed that maximal swelling may benefit the subdural injection of hydrogels because they could help remove excess fluid from the parenchyma after SCI and TBI, preventing further injury [108]. Some studies showed that before in vivo delivery, microparticle-based [109,110] and shape-memory [111] hydrogels could be pre-swollen, which may cause tissue damage similar to in situ swelling pressures. Furthermore, a higher macromer content or crosslinking density can reduce water uptake of hydrogels, but this comes at the expense of greater mechanical strength and lower permeability, both of which could be problematic and have negative consequences [112].
Another essential feature of injectable hydrogels is their solidification conditions to avoid further tissue and cell damage, and they should solidify in mild circumstances [95,102]. The initial viscosity of the hydrogel and other environmental parameters (i.e., crosslinker, pH, light, temperature, etc.) that influence solidification are very important. These parameters must be biocompatible and allow injectable hydrogels' gelation within seconds to minutes [112,113].
Injectable hydrogels' gelation time is a key consideration. This period of time must be long enough to ensure that the injection needle does not become clogged and that the spared spinal cord tissue has enough time to contact the hydrogel solution before its gelation. Also, it indicated that the hydrogel and any attached therapeutic agents (e.g., cells, medicines) should gel quickly enough to remain at the injection site [114]. More research is needed to determine optimal gelation times of various injectable hydrogels. For example, regardless of total macromere concentration, faster gelation is associated with lower swelling ratios in vitro [112].
3.2. Fabrication approaches for injectable hydrogels
When it comes to injectable materials, although some have developed as self-healing hydrogels, fabrication strategies must choose based on the aqueous CNS environment. In this field, gelation must occur after proper injection and penetration of hydrogel solution in the defect site. So, a close interface between the healthy tissue and injected materials will be created, which aids in cell survival and migration, and tissue regeneration [112]. So, injectable hydrogels can be made in a variety of ways, depending on the technique utilized, and they can be classified as physical or chemical hydrogels. Physical hydrogels spontaneously arise when temperature, pH, or ionic concentration changes cause weak secondary forces [[115], [116], [117]]. Furthermore, enzymatic, Schiff base, photo-crosslinking, Michael additions, and click chemistry are some of the chemical techniques used to create chemical hydrogels (Fig. 3) [[118], [119], [120], [121]]. Common mechanisms are employed in all of these approaches to controlling the maximal in-situ hydrogel swelling, degradation, gelation, etc. [112].
Fig. 3.
Schematic illustration of different approaches for injectable hydrogel production.
3.2.1. Physical methods for injectable hydrogels preparation
Network formation in hydrogels designed by non-covalent or physical gelation approaches happens by macromers' physical connection (i.e., electrostatic or hydrophilic-hydrophobic interactions) in physiological conditions. It has been observed that these kinds of hydrogels can swell about half of their original size, and the injection of them after intraspinal and intrathecal in vivo can be safe. The non-biological nature of chemical reagents has made them not biocompatible. So, such phase transition-based gelation technologies are extremely biocompatible since they do not require the use of chemical reagents to achieve gelation [112].
Besides investigating innovative methods for developing physical injectable hydrogels, the design of new hydrogels improves their mechanical characteristics, biodegradability, biocompatibility, and the maintenance of structural integrity in vivo is essential in tissue engineering [122].
3.2.1.1. Thermosensitive injectable hydrogels
The ability of thermosensitive injectable hydrogels to gel at physiological temperatures recently attracted significant attention for their applications in neural tissue engineering. What makes these injectable hydrogels so beneficial is their ability to phase transition without chemical stimulation. At ambient temperature, these hydrogels are liquid and have low viscosity that can be easily injected; but after injection into the target tissue, their viscosity in physiological temperature increases, and they will gel quickly [[123], [124], [125], [126]].
Actually, in these kinds of hydrogels, the critical solution temperature (CST) controls crosslinking; after temperature change, phase transition happens and leads to a change in the hydration state and finally alters the solubility of the hydrogel [9,127,128]. So, to make temperature-sensitive injectable hydrogels, synthetic (i.e., poly(N-isopropylacrylamide) (PNIPAAm), poly(ethylene glycol-b-[DL-lactic acid-co-glycolic acid]-b-ethylene glycol, etc.), poly(lactic-co-glycolic acid)–PEG, poly(N,N-diethylacrylamide) and natural (i.e., agarose, cellulose, fibrin, and other extracellular matrix (ECM) hydrogels) thermosensitive polymers are required. These hydrophilic polymers with temperature change and below or above CST miss a lot of their water and form a hydrogel network [9,123,124,[128], [129], [130]]. Actually, the most frequent type of physical hydrogel utilized in SCI repair is thermo gels [54].
3.2.1.2. pH-responsive injectable hydrogels
pH-sensitive hydrogels are made up of polymer chain networks that are sensitive to pH changes, and by altering the pH of the external environment, they can link to each other [124]. These kinds of hydrogels are encased in a salt solution, and a change in pH of the surrounding solution causes a physical swelling or deswelling of the gel. These hydrogels have great potential in regenerative medicine and neural tissue engineering and have attracted much attention in recent years [122,131].
pH-sensitive injectable hydrogels can be created by including a pH-sensitive moiety (i.e., polyacrylic acid, polyelectrolyte N-palmitoylchitosan, sulfamethazineoligomers (SMOs), and oligomeric sulfamethazine, into the hydrogel [[132], [133], [134], [135]].
3.2.1.3. Electrically responsive injectable hydrogels
Hydrogels can respond to external electric currents as an environmental cue and shrink, swell, or bend [136,137]. Electrically sensitive hydrogels under suitable physiological conditions of the body, like exposure to ion currents created by streaming potentials, respond quickly and deform. These hydrogel systems, which are typically composed of polyelectrolytes, could mimic many biological functions such as motility [[136], [137], [138]].
Because they may promote neuronal adhesion, proliferation, and differentiation, electrically responsive injectable hydrogels have great potential for CNS applications [139]. So far, numerous conductive materials have been studied for CNS regeneration to make it easier for neurons in the material to communicate with each other and send electrical signals like healthy neural tissue. Conductive and synthetic polymers such as polypyrrole, polyaniline, polythiophene, polyvinyl alcohol, acrylic acid/vinyl sulfonic acid, and sulfonated polystyrene have been utilized to generate electroactive injectable hydrogels [136,139,140]. Recently, these kinds of hydrogels are frequently made using the combination of bioactive materials (i.e., hydrogels produced from natural ECM) with conductive dopants (i.e., conductive polymers, gold nanoparticles, and carbon-based materials like graphene oxide and carbon nanotubes) [139]. However, there is still a lot of work to be done in this field, and further research is needed to improve the biocompatibility of electrically conductive materials [139].
3.2.1.4. Magnetic responsive injectable hydrogels
Magnetic field-sensitive hydrogels respond to tiny changes in external fields by changing their characteristics. These systems have been studied as swollen, shrinking, or bending hydrogel [136].
In some tissues, such as neural tissue, cell alignment is very important to provide the proper tissue 3D structure and mechanical properties for regeneration. To accomplish this, various ways of using external magnetic fields have been developed to align neural cells in situ, allowing them to grow in the right direction and become adequately differentiated [141].
By inserting exogenous paramagnetic or ferromagnetic compounds like superparamagnetic particles (i.e., CoFe2O4, Fe3O4, and -Fe2O3) within the polymeric matrix [142], magnetic-sensitive hydrogels offer several advantages, including fast reaction, attractive mechanical characteristics and biocompatibility [139,140].
However, due to restrictions such as the toxicity of magnetic materials and the low reproducibility of in vivo outcomes, we should optimize magnetic responsive injectable hydrogels for clinical usage [139].
3.2.1.5. Self-assembled injectable hydrogels
Self-assembled hydrogels made with amphiphilic polymers are another physical hydrogel that can frequently synthesize from designer polypeptides. Due to their user-specified amino acids, these designer polypeptides give a great degree of network assembly control [112].
Self-assembling peptides (SAPs) are synthetic peptides that can spontaneously assemble to form nanostructures under physiological environments by achieving suitable stability between various counteracting forces, including solvation, hydrogen bonding, hydrophobic, electrostatic repulsion, and electrostatic attraction [143,144].
SAPs are extremely distinctive for their simple and reproducible synthesis approach with high yield, flexibility for modification or engineering, characterization, and systematic enzymatic degradability capabilities. SAPs can fill the heterogeneous injury cavities, which leads to significant increases in cell migration, survival, differentiation, and axonal development [145]. Studies have shown that biomaterials made from the self-assembly of short peptides and peptide derivatives may have the potential to be outstanding novel regenerative medicine therapies. However, for the poor physical forces involved in self-assembly, the mechanical properties of SAPs are usually weak. Thus, many chemical cross-linking strategies have been developed to overcome this problem. But the cytocompatibility and post-linking purification of these compounds are still under investigation [144].
According to that, SAPs are usually designed to only focus on one or two desirable biomimetic features. Some studies proposed using complex combinatorial systems such as electroactive or chromophore functionalized self-assembling systems may be helpful for the improvement of neural regeneration [145].
3.2.1.6. Other physical injectable hydrogels
Several other types of physical injectable hydrogels with non-covalent crosslinking, including stress-sensitive, ion-sensitive, and ultrasound-sensitive have also been reported for CNS tissue engineering [112,122,139]. Ionic or electrostatic interactions generate hydrogel networks with strong and multivalent non-covalent connections [146].
Hydrogels that demonstrate a sol-gel transition in response to physical or chemical stimuli, such as stress-sensitive or stimuli-responsive, have drawn a lot of attention in recent years [147]. External environmental parameters such as electromagnetic radiation stimulate reversible transitory physical contact to produce these hydrogel networks [148,149].
In ionic hydrogels, ionic crosslinking can overcome the limitations of covalent crosslinking [150]. The inclusion of divalent or trivalent counter ions crosslinks ionic polymers [124]. In general, when two aqueous solutions containing oppositely charged polymers or polyelectrolytes are mixed, ion bridges between counter ionic polymers are made, and the crosslinking phenomenon occurs [151]. For example, sodium alginate that can form with physical crosslinking between the carboxylate anions of guluronate units in alginate and the calcium ions generates an ionic hydrogel [124].
3.2.2. Chemical methods for injectable hydrogels preparation
Photocrosslinking and non-photocrosslinking covalent techniques are two methods for making injectable chemical hydrogels. Photocrosslinking approaches provide greater control by the user because hydrogels can only develop when exposed to light from an external source. In this technique, the gelation time is usually relatively short and can avoid any negative consequences caused by the photo-initiators. But one of the best things about non-photocrosslinking methods is no requirement for cytotoxic photocrosslinking initiators [19,112,152,153].
3.2.2.1. Enzymatically cross-linked injectable hydrogels
For tissue engineering applications, several enzyme-mediated crosslinking systems have been used to synthesize injectable hydrogels. All enzymes like lysyl oxidase, transglutaminase, thermolysin, phosphopantetheinyl transferase (PPTases), tyrosinase, peroxidase, phosphatase, plasma amine oxidase, and β-lactamase have been used to make this kind of injectable hydrogels for tissue engineering [154]. Due to their capacity to work under physiological conditions, minimal cytotoxicity, high site-specificity, and quick gelation, the utilization of this approach for the synthesis of injectable hydrogels for neural tissue engineering has recently attracted interest [[154], [155], [156]].
One of the most common enzymatic techniques used in SCI regeneration is fibrin gel crosslinking with thrombin. Fibrin hydrogel has low swelling ratios and comparable compressive modulus to normal spinal cord tissue that utilizes the natural blood-clotting mechanism for gelation [53,110,153,157]. When it comes to injectable hydrogels, horseradish peroxidase (HRP) is another most widely utilized enzyme that can crosslink phenolic groups or other aminophenol compounds in natural and synthetic polymers [158,159].
3.2.2.2. Michael addition cross-linked injectable hydrogels
When creating injectable hydrogels, another widely utilized technique is known as the Michael addition reaction. This reaction may be time-controlled and occurs under physiological conditions. It is the nucleophilic addition of a carbanion or nucleophile to an α,β-unsaturated carbonyl molecule [160,161]. In tissue engineering, biomaterials, including hyaluronic acid, chitosan, and PEG, are often utilized in the Michael addition reaction to generate injectable hydrogels [122,162].
3.2.2.3. Schiff base cross-linked injectable hydrogels
The high reaction rate and ability to create imine bonds among aldehyde and amino groups under mild reaction and physiological conditions without external reagents make them an excellent choice for synthesizing injectable hydrogel for tissue engineering applications [163,164].
Several biomaterial-based injectable hydrogels with Schiff base cross-linking have also been studied that offer various advantages, including the ability to keep cells viable, enzyme-ready biodegradation, rapid room-temperature synthesis, and good self-healing [165]. For instance, due to a large number of amino groups on chitosans' backbone, it is a suitable biomaterial for generating injectable hydrogels by Schiff base crosslinking [166].
3.2.2.4. Click chemistry cross-linked injectable hydrogels
Because of limited reactivity with biological components and also step-growth and fast polymerization rates of “click” reactions, these processes hold considerable potential for the production of injectable hydrogels [112,122]. A wide range of reactions are included in the click chemistry concept, including Diels–Alder reactions, copper-catalyzed azide-alkyne cyclo-addition processes, tetrazine–norbornene chemistry, thiol-epoxy, thiol-ene reaction, and thiol-maleimide couplings [[167], [168], [169]]. For example, clear and transparent hydrogels can be made using the sequential thiol-ene “click” reaction [170].
3.2.2.5. Photo/light-responsive injectable hydrogels
The ability to adjust the time and position of crosslinking under physiological settings has made photo-crosslinking approaches popular in recent years to generate injectable hydrogels for CNS and other tissues engineering [[171], [172], [173]]. Considering that some injectable hydrogels have low mechanical strength, the use of photo-responsive hydrogels can overcome the conflict between hydrogel injectability and mechanical strength [174].
Photo-crosslinking approaches (such as chain-growth and step-growth polymerization) are a multi-step and complex process involving three stages: initiation, propagation, and termination. In these techniques, crosslinking triggers (such as light or heat) activate an initiator and create covalent bonds between polymer precursors [175,176]. In summary, first, the light in the initiation stage produces free radicals by excitation of photoinitiators. Then comes the propagation step, which involves propagating radicals across unreacted double bonds, and the termination step, which marks the end of crosslinking in the 3D structure [175].
There has been considerable research into chain-growth polymerization techniques, but in recent years, step-growth approaches have received attention because of their potential to establish better-developed structures with expected properties, high degrees of crosslinking, and less sensitivity to oxygen inhibition [177,178]. Because most light-responsive systems explored involve UV-sensitive groups, their biomedical applicability may be limited [136].
3.3. Injectable hydrogels for CNS repair and regeneration
The efficacy of hydrogels for CNS regeneration has been studied extensively in vitro and in vivo [26]. To imitate ECM features, including viscoelasticity, biodegradability, and biocompatibility, naturally generated hydrogels are particularly desirable for neural tissue engineering [179].
Collagen, laminin, fibrin, alginate, and other injectable polysaccharide-based hydrogels, such as chitosan and hyaluronic acid (HA), are among the natural materials and derivatives employed in neural applications. For instance, HA interacts effectively with cell receptors and can increase material and neural cell connections since it is a natural component of the ECM [71,107,180]. Because of batch-to-batch variation, low mechanical stiffness, and purification of natural hydrogels, synthetic and semi-synthetic materials such as PEG, SAP, and other polymers were developed for the synthesis of injectable hydrogels [181].
The brain and spinal cord are highly spatially complicated anatomical structures with various design needs for regeneration outcomes. The spatial architecture of axonal bundles differs significantly between the brain and spinal cord, even though their bulk mechanical characteristics are identical [182]. So, to promote proper cellular regeneration and axonal tract reconstruction in the brain and spinal cord, structural recapitulation of soft tissue organization is necessary [26].
Recent works using hydrogels in neural tissue engineering include developing brain tissue phantoms, bioprinting a 3D neurovascular unit, and examining cellular cascades linked with brain injury [[183], [184], [185]]. For example, it has been reported that methylcellulose (MC)/agarose composite thermosensitive hydrogel maintains the viability of neuronal cells so that it can be a promising hydrogel for neural tissue engineering [186]. Another thermosensitive hydrogel that can be an excellent example for CNS tissue engineering is PNIPAAm. It has been shown that the incorporation of this hydrogel with PEG could help excellent cell adhesion and sustain the release of growth factors in long-term CNS treatment [187].
So, biocompatible and biodegradable hydrogels that may stimulate axonal regeneration and reduce inflammation are ideal for CNS repair [188].
3.3.1. Injectable hydrogels for brain injury repair and regeneration
A variety of causes, such as stroke, aneurysms, and trauma can induce brain injury. Healthy cells in the injured brain need structural support (such as their natural ECM) to grow and regenerate brain tissue. The mentioned properties of hydrogels make them interesting for use in brain damage repair as a scaffold or structural support [26].
Table 1 highlights the application of synthetic (e.g., SAP and PEG) and natural (e.g., hyaluronan, alginate, and ECM hydrogels) hydrogels that have been used in preclinical models to repair and regenerate brain tissue injury.
Table 1.
Injectable hydrogels for brain tissue engineering.
|
Hydrogel composition, preparation |
Animal profile | In vivo model |
Loaded With |
Injection profile |
Results |
Year [ref] |
|
|
|
||||
| DHC, Self-assembled; Photo-responsive |
|
|
BME (2 mg/mL) |
|
After 4 weeks: Glial scar inhibition Axonal regrowth and remyelination occurred Brain structural remodeling was promoted in the lesions Neurogenesis and angiogenesis were achieved |
Liu-2023 [189] |
| HA-PBA/Gel-Dopa, PH-responsive |
|
|
None |
|
After 3 weeks: Reduced glial scar and closed the lesions Improved neural cell infiltration |
Hu-2023 [190] |
| Alginate/PEDOT, Ion-sensitive crosslinking; Electrostatic interaction; Hydrophobic interaction; Self-assembled |
|
|
None |
|
After 3 days: Reduces neuroinflammation in the brain upon injection relative to traditional Pt-based electrodes A suitable blend matches with the brain tissue An appropriate electrical stimulation characteristic The hydrogel is not cytotoxic to neural cells (7 days cytotoxicity test) |
Perkucin-2022 [191] |
| FPGEGa, Schiff base reaction; Electrostatic interaction; Thermosensitive |
|
|
SHED-Exo (100 μg Exo into100 μL hydrogel) |
|
After 3 weeks: Enhanced neuro-regeneration by restoring motor functions After 2 days: Reduced the formation of intracellular ROS |
Li-2022 [192] |
|
Hydrogel composition, preparation |
Animal profile | In vivo model |
Loaded With |
Injection profile |
Results |
Year [ref] |
|
|
|
||||
| Chitosan- DF-PEG hydrogel/GelMA-PCL nanofiber, Schiff base reaction |
|
|
BMSCs (5 × 105 cells/10 μL) |
|
After 2 weeks: Significant neurogenesis and angiogenesis The reduction of ischemic brain damage, infarct volume, microglial and astrocyte overactivation, and neurological deficits |
Pei-2023 [193] |
| Ncad-mRADA, Self-assembled |
|
|
None |
|
Neuroblast migration to the injured site in the striatum Neuronal regeneration Functional recovery improvement in neonates with cortical brain damage |
Ohno-2023 [194] |
| Fmoc-DDIKVAV, Self-assembled |
|
|
Myoglobin (1 mg/mL) and/or cortical neural stem cells, (5 × 104/μl cells) |
|
After 4 weeks: Assist engraftment by supply oxygen and stem cells to the brain simultaneously Established vascular network Significant improvement in neuronal differentiation Greater functional integration of stem cell-derived grafts |
Wang-2023 [195] |
| RADA16; RADA16-SVVYGLR, Self-assembled |
|
|
None |
|
After 4 weeks: Enhanced the migration and neurogenesis of NPCs Induction of angiogenesis via endothelial and pericyte stimulation Improved functional recovery by enhancing optomotor response |
Wang-2017 [196] |
|
Hydrogel composition, preparation |
Animal profile | In vivo model |
Loaded With |
Injection profile |
Results |
Year [ref] |
|
|
|
||||
| Fmoc-DDIKVAV, Self-assembled |
|
|
hESCs-derived cortical progenitors (1 × 104 cells) |
|
After 9 months: Improved the functional recovery and the electrical characteristics of fully developed and integrated neurons Neuronal differentiation at a higher level |
Somaa-2017 [197] |
| Self-healing composite of CS-CNF, Schiff base reaction |
|
|
NSCs (1 × 104 cells) |
|
After 1 week: Effected on NSC neuronal differentiation Restored neural-impaired zebrafish function |
Cheng-2019 [198] |
| CS-HA, Self-assembled |
|
|
None |
|
After 1 week: Promoted the healing of CNS damage and the functional recovery Prepared an adaptable environment that allows NSC migration, proliferation, and differentiation Created a microenvironment that encourages axonal development It had a healing impact on the brain-damaged cavity |
Liu-2020 [199] |
| SAP scaffold conjugated with IKVAV sequence, Self-assembled |
|
|
NSCs |
|
After 6 weeks: Allowed NSCs to differentiate into neurons Supported axon elongation |
Cheng-2013 [200] |
|
Hydrogel composition, preparation |
Animal profile | In vivo model |
Loaded With |
Injection profile |
Results |
Year [ref] |
|
|
|
||||
| Pluronic-CS/anilinepentamer, Electrically responsive |
|
|
VEGF (1 mg/mL) |
|
After 5 days: simulated the hippocampus' electrical, electrochemical, and mechanical properties Reduced infarction volume Improved the hippocampus-dependent learning and memory performance |
Nourbakhsh-2020 [201] |
| GelMA-imid, light -responsive, Click chemistry cross-linked |
|
|
PDA/SDF1α nanoparticles and hAMSCs |
|
After 2 weeks: Enhanced hAMSCs homing and neural differentiation Encouraged the growth of endogenous nerve cells Significant potential for TBI physiological recovery |
Zheng-2021 [202] |
| Pol and refrigerated hydrogel incorporated T1AM, Thermosensitive |
|
|
T1AM (50 mg/kg) |
|
After 26 days: Enhanced functional TBI recovery Maintained BBB integrity Stopped cell death Decreased brain inflammation and edema Useful for TBI local drug administration and cooling without major adverse effects |
Han-2020 [203] |
| HT, Enzymatically cross-linked |
|
|
BMSC and NGF |
|
After 2 weeks: Survival and proliferation of neural cells through the release of neurotrophic factors and the regulation of neuroinflammation |
Wang-2022 [204] |
| HAMC Physically cross-linked (Inverse thermal gelation) |
|
|
Cyclosporine and erythropoietin |
|
After 6 weeks: Cyclosporine improved plasticity in the striatum Erythropoietin stimulated endogenous NSPCs |
Tuladhar-2020 [205] |
| PNIPAAm-b-PLA-bPEG-b-PLA-b-PNIPAAm pentablock copolymer, Thermosensitive |
|
|
Hydrophobic molecules; riluzole |
|
After 1 week: Hydrophobic molecules/drugs can be efficiently loaded into the micelle cores of this cytocompatible hydrogel without any drug burst release |
Pertici-2018 [206] |
| IKVAV-functionalized PA, Self-assembled |
|
|
None |
|
After 1 week: Enhanced neurite outgrowth Acted as a non-cytotoxic microtubule stabilizer Increased crucial neural marker expression in mice cortical primary neurons led to significant neuroregeneration and rapid recovery of the sham injured mice brain Significantly led to the neural repair of the damaged brain by increasing reactive astrocytes in the hippocampal dentate gyrus region of the sham injured brain |
Pradhan-2018 [207] |
|
Hydrogel composition, preparation |
Animal profile | In vivo model |
Loaded With |
Injection profile |
Results |
Year [ref] |
|
|
|
||||
| Gelatin, Enzymatically cross-linked |
|
|
BMSC (5 × 104 cells/ml) |
|
After 5 weeks: Improved neurogenesis and functional reconstruction of TBI mice Significantly increased neural differentiation, cell viability, and neurotrophin secretion |
Li-2021 [208] |
| Gelatin Enzymatically cross-linked |
1. Rat/Sprague-Dawley 2. None 3. 250–300 g 4. None |
|
EGF (8 μg) |
|
After 4 weeks: Enhanced cell migration supplied sustaining release of EGF promoted tissue regeneration |
Lim-2020 [209] |
| TM/PC Self-assembled |
|
|
Curcumin |
|
After 3 weeks: Decreased reactive astrocytes and activated microglia had a strong anti-inflammatory effect promoted nerve regeneration |
Qian-2021 [210] |
| DCH Self-assembled |
|
|
Paclitaxel and hGBM (100,000 cells) | 1. Injected stereotactically into the center of the caudate-putamen nucleus 2. 2 μL 3. Pulled glass micropipettes ground to a beveled tip with 150–250 μm inner diameter 4. 2 5. None 6. 3 wt% 7. None |
After 5 weeks: Made local tumor control and enhanced survival. However, tumor cells could escape the area of treatment Produced minimal tissue reactivity Induced less cellular inflammation, reactive astrocytes, and tissue damage than cremaphor-taxol (typical taxol-carrier) or hydrogel alone |
Garrett-2020 [211] |
DHC: hyaluronan-collagen hydrogel; BME: bone marrow mesenchymal stem cell-derived exosomes; TBI: traumatic brain injury; PEDOT: Poly(3,4-ethylenedioxythiophene); HA-PBA/Gel-Dopa: penylboronic acid modified hyaluronic acid/dopamine modified gelatin, FPGEGa: poly (citrate-gallic acid)-based hybrid hydrogel; SHED-Exo: stem cells from human exfoliated deciduous teeth-derived exosomes; DF-PEG: dibenzaldehydeterminated polyethylene glycol; GelMA: gelatin methacryloyl); PCL: polycaprolactone; MCAO: middle cerebral artery occlusion; Ncad-mRADA: N-cadherin tagged with mRADA; DDIKVAV: aspartate-aspartate-isoleucine-lysine-valine-alanine-valine; Fmoc: Nfluorenylmethyloxycarbonyl; IKVAV: isoleucine-lysine-valine-alanine-valine; RADA: arginine (R, Arg), alanine (A, Ala) aspartic acid (D, Asp), alanine (A, Ala); SVVYGLR: Serin-Valanin-Valanin-Tyrosine-Glycine-Leusin-Arginine; CNS: the central nervous system; NPCs: neural progenitor cells; SAP: self-assembling injectable peptide; hESCs: human embryonic stem cells; CS–CNF: chitosan–cellulose nanofiber; NSCs: neural stem cells; VEGF: vascular endothelial growth factor; CS-HA: chitosan-hyaluronan; GelMA-imid: imidazole groups-modified gelatin methacrylate; PDA: polydopamine; SDF-1α: stromal-cell derived factor-1; hAMSCs: human amniotic mesenchymal stromal cells; ICH: intracerebral hemorrhage; Pol: poloxamer; T1AM: 3-iodothyronamine; BBB: blood-brain barrier; HA: hyaluronic acid; CNS: central nervous system; HT: tyramine-modified hyaluronic acid hydrogels; GalOx: galactose oxidase; HRP: horseradish peroxidase, BMSC: bone mesenchymal stem cells; NGF: nerve growth factors; HAMC: hyaluronan and methylcellulose; PEG: poly (ethylene glycol); CFGO: choline-functionalized injectable graphene oxide; SA: sodium alginate; ChOx: choline oxidase; EGF: epidermal growth factor; DCH: diblock copolypeptide hydrogel, hGBM: human glioblastoma.
Various injectable SAP hydrogels have recently been employed for brain tissue engineering [1]. These kinds of hydrogels that are spontaneously organized with non-covalent (physical) interactions are made up of self-assembling proteins that are incorporated into natural, synthetic, or hybrid hydrogels [212]. A variety of in vitro models for researching various brain disorders (e.g., TBI) have been built using hydrogels coupled with neural progenitor/stem cells (NPCs). In recent years, adjustable hydrogels have been improved to promote cell survival and tissue function in brain damage situations [26].
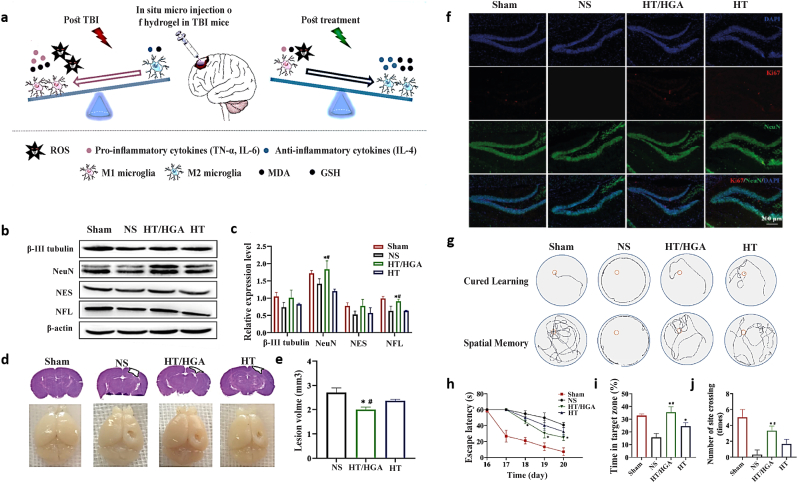
A TBI leaves brain tissues with extensive damage that cannot be repaired without glial scar formation. The poor differentiation capacity and survival of stem cells limit their effectiveness in treating TBI-induced brain tissue injury. So, several injectable hydrogels were developed to solve this issue and improve stem cell therapy efficacy [202]. In a recent study, an injectable hyaluronic acid hydrogel with reactive oxygen species scavenging activity was developed for TBI repair (Fig. 4) [213].
Fig. 4.
An in situ forming hyaluronic acid hydrogel with reactive oxygen species-scavenging activity by inhibiting oxidative stress and neuroinflammation in traumatic brain injury (TBI) repair. a) Schematic showing neural functional repair in TBI model by hyaluronic acid-tyramine combined with gallic acid-grafted hyaluronic acid (HT/HGA) hydrogel injection by removing free radicals and changing microglial polarization from M1 (pro-inflammatory phenotype) to M2 (anti-inflammatory phenotype). Neurogenesis and brain tissue remodeling were observed after 21 days of treatment by b) western blot, c) relative gene expression of NFL, NSE, NeuN, β-III tubulin, and d) imaging of TBI mice brain and hematoxylin and eosin stain (H&E) staining. e) Lesion volume of the TBI mice brain. f) Immunofluorescence staining (IF) of Ki67 and NeuN. The learning and memory abilities were assessed via the Morris water maze (MWM) behavior test, including g) swimming trails, h) escape latency, i) time in the target zone (I quadrant), j) the number of site crossings [213]. Copyright 2022 Elsevier.
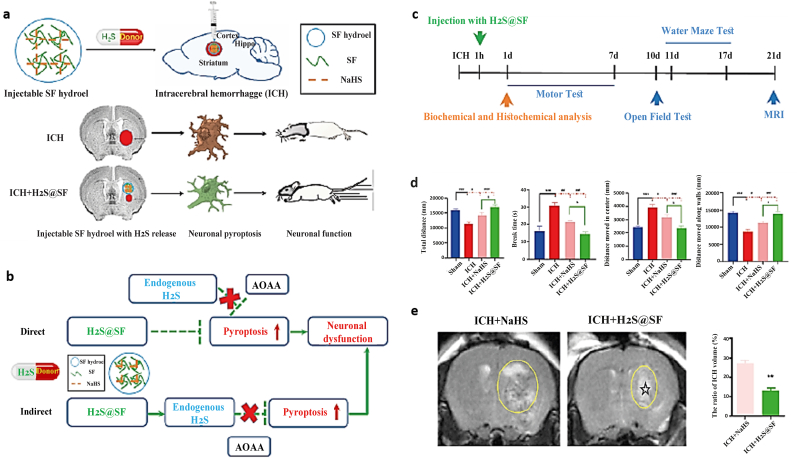
Also, using injectable hydrogels is one of the new ideas for developing stroke treatment over the last decade. They can promote the stem cells integration and delivery of biological factors or drugs to the post-stroke brain in a regulated manner [1]. A study showed the beneficial effect of sustained slow release of hydrogen sulfide (H2S) with silk-based injectable hydrogels for hemorrhagic stroke (Fig. 5) [214].
Fig. 5.
Silk fibroin (SF)-based hydrogel with sustained delivery of hydrogen sulfide decreases neuronal pyroptosis and improves functional retrieval for severe intracerebral hemorrhage. a) Schematic demonstrates the development of SF injectable hydrogel for intracerebral hemorrhage (ICH) treatment and shows the effective role of hydrogen sulfide sustained release in improving neuronal functionality. b) A hypothetical illustration proposes two possible ways (direct or indirect effect) for H2S@SF. In this study, significant improvement in neurological functional outcome was achieved with the H2S@SF hydrogel by the endogenous H2S synthesis pathway as an indirect effect (because adding H2S synthase inhibitor O-(Carboxymethyl) hydroxylamine hemihydrochloride (AOAA) significantly increased the expression of pyroptosis-related proteins in the striatum). c) The timeline of all experiments is shown at a glance. d) The retrieval of ICH-induced motor function, mental function, and cognitive function was improved by injecting H2S@SF hydrogel into the ICH. e) The ratio of ICH volume was evaluated after 21 days by T2-weighted imaging (T2WI), indicating a decrease in mice brains injected with H2S@SF hydrogel. The zone with irregular T2WI signals and its related zone is specified (yellow) on the ipsilateral side. Stars (☆), injected hydrogel (∗∗P < 0.01 vs. ICH group) [214]. Copyright 2022 Elsevier.
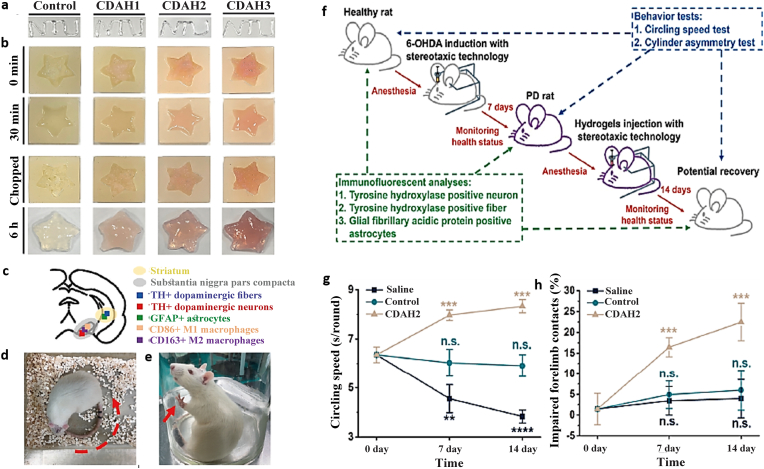
ECM hydrogels are a form of thermosensitive natural hydrogels that can be liquid at ambient temperature but solidify at 37 °C [60,215]. According to the findings of studies, such materials, with their ECM composition, can mimic natural tissue and promote brain tissue regeneration following a stroke in rats by helping to induce effective and rapid host cells (e.g., NPCs, microglia, and macrophages) infiltration [71]. The effective role of an anti-inflammatory electroconductive injectable hydrogel with self-healing properties for Parkinson's disease (PD) has also been reported in a recent study (Fig. 6) [216].
Fig. 6.
Anti-inflammatory electroconductive hydrogel for Parkinson's disease with self-healing property. a) The self-healing O-carboxymethyl chitosan (CMC)/DAPU/gold nanoparticles (Au-NPs) hydrogels (CDAHs) hydrogels have the capability to be injected with a 30G syringe needle to write letters (internal diameter = 152.4 μm). b) These hydrogels can be used to fill star-shaped molds. c) The atlas of the rat brain indicates the locations of the striatum, substantia nigra pars compacta (SNpc), and immunostaining types. The behavior tests of the PD rats after hydrogel injection for 7 and 14 days; d) the spontaneous circling speed (red arrow: circling direction) and e) the cylinder asymmetry (red arrow: forelimb contact). f) The schematic illustration presents the treatment of the Parkinson's disease rat model by injecting CDAH hydrogels into the damaged regions for 7 and 14 days. The PD was inducted via injecting 6-hydroxydopamine (6-OHDA) into the brain of rats. The efficacy of the hydrogels was assessed with immunofluorescent analyses and behavior tests. g, h) Functional recovery was quantitatively evaluated for the circling speeds and the impaired forelimb contact [216]. Copyright 2022 Elsevier.
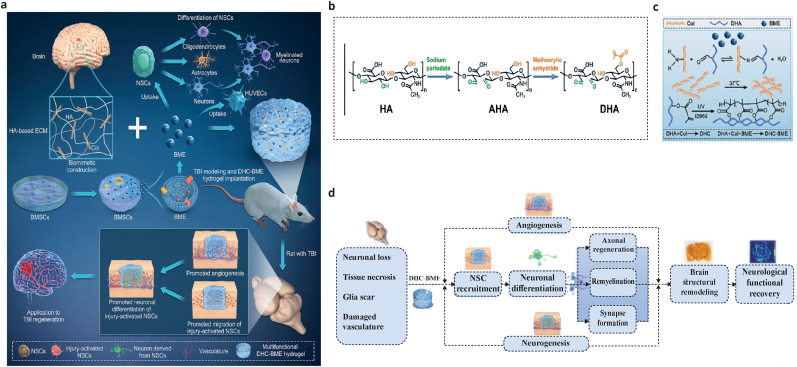
A study recently proposed a novel method of adding bone marrow mesenchymal stem cell-derived exosomes (BME) into hyaluronan-collagen hydrogel (DHC-BME) to produce both brain matrix mimicry and steady release of exosomes, therefore achieving TBI healing. The findings demonstrated that DHC-BME triggered neurogenesis and angiogenesis by recruiting endogenous NSCs. Moreover, remyelination, axonal regeneration, synapse formation, the inhibition of glial scar, brain structural remodeling, and remarkable functional recovery (e.g., motor, sensory, reflex, balance, and spatial learning and memory) were achieved in the TBI rat model (Fig. 7) [189].
Fig. 7.
Fig. Incorporating bone marrow mesenchymal stem cell-derived exosomes (BME) into hyaluronan-collagen hydrogel (DHC-BME) for traumatic brain injury (TBI) repair. a) The schematic image shows the different stages and objectives of the hybrid injectable hydrogel for TBI restoration. b) Synthesis of DHA hydrogel c) Crosslinking steps of DHC-BME. d) The implantation of hydrogel containing BMSC-exosomes enhanced functional recovery following TBI. Summary of DHA-BME for treating TBI by boosting angiogenesis and neurogenesis [189]. Copyright 2023 Elsevier.
It has been mentioned that some of the challenges raised in injectable hydrogels occur due to the imbalance of stiffness, composition, and viscoelasticity of hydrogels compared to the brain parenchyma. In this regard, a study reported phenylboronic acid-grafted hyaluronic acid/and dopamine-grafted gelatin (HA-PBA/Gel-Dopa) as a multi-functional injectable hydrogel mimics these features and can boost neural cell infiltration, reduce glial scarring, and close the lesions in a TBI mouse model [190].
Chitosan (CS)/β-glycerophosphate (β-GP) hydrogels have undergone significant research and demonstrated regeneration potential in various tissues, including cartilage and bone tissue engineering. However, not many studies have investigated the potential of CS/β-GP as injectable hydrogels for nerve tissue engineering. Within this context, a step was taken in a recent in vitro study to optimize the best percentage of CS/β-GP as an injectable hydrogel for nerve tissue engineering applications. Based on their biocompatibility, gelation time, and physiochemical properties, the 0.5%:3% and 0.75%:3% CS/β-GP hydrogels were proposed as suitable concentrations [217].
Some studies believe that local inflammation and excessive oxidative stress at the site of injury are two important determinants in treating TBI. In line with this, antioxidant poly (citrate-gallic acid)-based hybrid hydrogel (FPGEGa) that stem cells from human exfoliated deciduous teeth-derived exosomes (SHED-Exo) was used for a TBI rat model. Significant anti-inflammatory properties of FPGEGa@SHED-Exo were reported due to M1 (pro-inflammatory) inhibition and M2 (anti-inflammatory) promotion. This thermosensitive injectable hydrogel dramatically reduced the formation of intracellular ROS (caused by microglia) and enhanced neuro-regeneration by restoring motor functions [192].
A study indicated a novel composite scaffold containing a self-healing Schiff base injectable hydrogel and GelMA/Polycaprolactone (PCL) nanofibers loaded with BMSCs (hydrogel/nanofiber/BMSCs) significantly increased neurogenesis and angiogenesis and reduced ischemic brain damage, infarct volume, microglial and astrocyte overactivation, and neurological deficits. PCL was the shell polymer, and GelMA was the core polymer of the nanofibers [193].
Using biomaterials to boost the migration of neuroblasts derived from endogenous NSCs is a novel and promising strategy to improve brain repair after injury. However, there are so few scaffolds that lead to neuroblast migration. Thus, sending enough neuroblasts to far-distant damaged locations is challenging. Recently, an amphiphilic peptide [(RADA)3-(RADG)] (mRADA)-tagged N-cadherin extracellular domain (Ncad-mRADA) was designed to address this problem. Ncad-mRADA peptide was injected into the striatum (a deep brain region), promoting neuroblast migration, neuronal regeneration, and functional recovery in neonates with cortical brain damage. These self-assembling peptides were proposed as a new approach to CNS regenerative medicine [194].
Brain NPCs respond to the electric field by cathodal migration, differentiation, and proliferation. Recruiting the potential of NPC is a promising approach to speed up neural repair after TBI or brain disorders. Current electrode designs, which are typically made of stiff conductive metals that can cause neuroinflammation after implantation in part due to the mechanical mismatch between implanted materials and physiological conditions, limit the use of electric fields to activate NPCs. In a study, alginate/PEDOT, a novel injectable biobased soft electrode with appropriate electrical stimulation characteristics, indicated a suitable blend matches with the brain tissue and negligible activation of inflammatory cells compared to traditionally used platinum-based electrodes [191].
Along with the positive results of many studies, some hydrogels have disadvantages, such as hypoxia, which can lead to poor cell differentiation, survival, and functional integration due to the absence of a vascular network. Hybrid myoglobin: peptide was recently presented as a hydrogel that could supply oxygen and stem cells to the brain simultaneously to assist engraftment until spontaneous vascularization occurs, resulting in a considerable improvement in neuronal differentiation. Moreover, including myoglobin in the hydrogel resulted in greater innervation of the host tissue by the grafted cells, which is required for neuronal replacement procedures to ensure adequate synaptic formation. It was mentioned that this strategy could improve the functional integration of stem cell-derived grafts for treating central and peripheral nervous system injuries and disorders [195].
3.3.2. Injectable hydrogels for spinal cord applications
Ischemia and inflammation after SCI cause changes in biochemical pathways and subsequently create secondary responses such as demyelination, axon degradation, the pro-apoptotic activity of neural cells, glial scar, and loss of spinal cord function [181]. In this case, the technology of injectable hydrogels is particularly important because of their properties, such as the ability to provide the specific anisotropic microenvironments of the spinal cord and the ability to control cell behavior and therapeutic release of biomolecules with chemical modification (Table 2) [26].
Table 2.
Injectable hydrogels for spinal cord tissue engineering.
|
Hydrogel composition, Preparation |
Animal profile | In vivo model |
Loaded With |
Injection profile |
Results |
Year [ref] |
|
|
|
||||
| IKVAV-functionalized PA, Self-assembled |
|
|
BDNF |
|
After 6 weeks: Minimal inflammation Appropriate for presenting axons |
Hassanejad-2019 [23] |
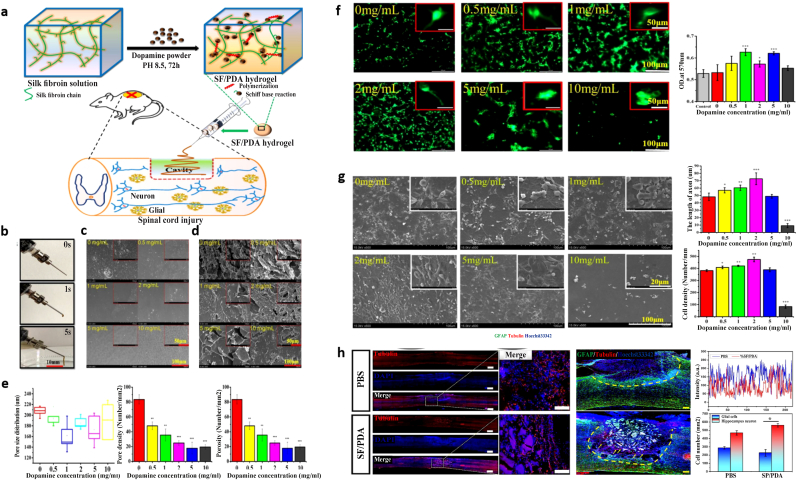
| SF/PDA, Schiff base reaction; Self-polymerization |
|
|
None |
|
After 2 weeks: Enhanced the development of neurons Possessed a healing impact on spinal cord injury |
Chen-2020 [218] |
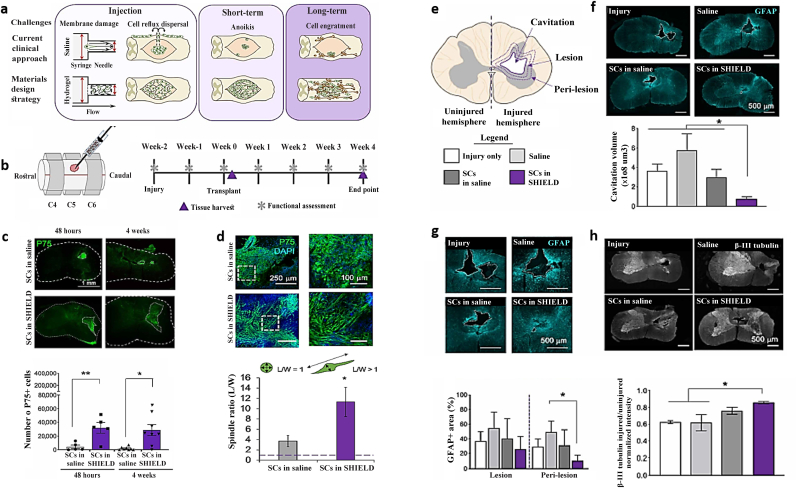
| SHIELD Michael addition reaction; Thermosensitive; Self-assembled |
|
|
Schwann cell (4.5 × 105 cells) |
|
After 4 weeks: Decreased neuronal damage, secondary injury, and cystic cavitation Contributed to cell transplantation outcomes Improve functional efficiency as a result of increased forelimb strength and coordination |
Marquardt-2020 [219] |
| Acellular peripheral nerve graft, Thermosensitive |
|
|
None |
|
After 8 weeks: Less invasive and more efficacious substrate for combined SCI therapy Enhanced neuronal regeneration and the fraction of distal spinal cord axons covered After 1 week, Reduced M1:M2 macrophage ratio in subacute SCI microenvironment |
Cornelison-2018 [220] |
| PD/GO, Non-covalent crosslinking |
|
|
Diacerein |
|
After 4 weeks: Regulated the hyperactivity and inflammation of the brain's astrocytes in vitro and induced the SCI repairing in vivo |
Zhang-2020 [221] |
|
Hydrogel composition, preparation |
Animal profile | In vivo model |
Loaded With |
Injection profile |
Results |
Year [ref] |
|
|
5. Time 6. Concentration 7. pH |
||||
| HA/PEG, Click chemistry cross-linked |
|
|
BDNF PLGA nanoparticles |
|
After 4 weeks: Biocompatible, safe and low-swell The spinal cord was not harmed as a result of the intrathecal injection |
Furhrman-2015 [152] |
| HP hydrogel, Thermosensitive |
|
|
aFGF |
|
After 1 week: Decrease apoptosis of neurons and astrogliosis After 4 weeks: Improved neurite growth, axonal rehabilitation, and the healing of SCI |
Wang-2017 [222] |
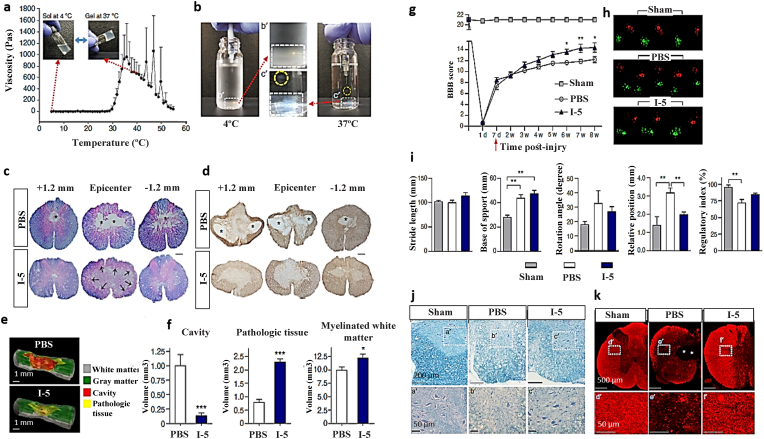
| I-5, Thermosensitive |
|
|
Taxol (1 μg/μl) or siRNA nanoparticles |
|
After 8 weeks of injury (7 weeks after injection): Improved coordinated locomotion Induced beneficial extracellular matrix remodeling that can stimulate tissue repair |
Hong-2017 [223] |
| Alginate, Ion-sensitive crosslinking |
|
1. Lateral hemisection-2. Left of the spinal cord (made with a surgical blade). A gap at the length of 2–4 mm alongside the rostral-caudal axis that was extended to the midline/T8-T10 | MH (50 μg) and paclitaxel (7.168 μg) |
|
After 4 weeks: Dual-drug treatment reduced inflammation and scar tissue. Increased neuronal regeneration |
Nazemi-2020 [224] |
| Modified gelatin matrix integrated with shape-memory polymer fibers, UV-sensitive crosslinking |
|
|
Motor neurons derived from ESCs (1 × 105 cells) |
|
After 4 weeks: Increased tissue regeneration and motor function recovery in mice |
Wang-2018 [225] |
|
Hydrogel composition, preparation |
Animal profile | In vivo model |
Loaded With |
Injection profile |
Results |
Year [ref] |
|
1. Type 2. Other details |
1. Site 2. Volume 3. Needle grade 4. Repetition 5. Time 6. Concentration 7. pH |
||||
| CHA gel, Schiff base reaction |
|
|
None |
|
After 1 week: Leads to neuroinflammatory inhibition |
Han-2020 [226] |
| HA-PH-RGD/F, Enzymatically cross-linked |
|
|
hWJ-MSCs |
|
After 8 weeks: Bridged the lesion cavity, helped vascularization, and enhanced axonal sprouting into the lesion No locomotor recovery or blood vessel ingrowth and density of glial scar around the lesion |
Zaviskova-2018 [227] |
| BC/FB, Thermosensitive |
|
|
Decorin |
|
Functional recovery was assessed at baseline, 2 days, and weekly for 6 weeks. After 6 weeks: Cavitation was fully inhibited, and lesion sites became filled with extracellular matrix materials and injury-responsive cells Up-regulated regeneration-associated genes (RAGs) of dorsal root ganglion neurons (DRGN), increased local axon regeneration/sprouting Improved electrophysiological, locomotor, and sensory function recovery |
Matthews-2021 [228] |
| GelMA, UV-sensitive crosslinking |
|
|
Bone MSCs-derived exosomes |
|
After 6 weeks: Led to neurological functional recovery After 7 days of cell culture: Induced Tuj-1-positive neurons differentiation, decreased astrocyte scars, and increased axonal elongation |
Cheng-2021 [229] |
| RADA-16I, Self-assembled |
|
|
human MSCs |
|
After 2 weeks: Led to axon elongation at the location of a spinal cord lesion Providing topological cues as guidance for axon alignment |
Tran-2022 [230] |
|
Hydrogel composition, preparation |
Animal profile | In vivo model |
Loaded With |
Injection profile |
Results |
Year [ref] |
|
|
|
||||
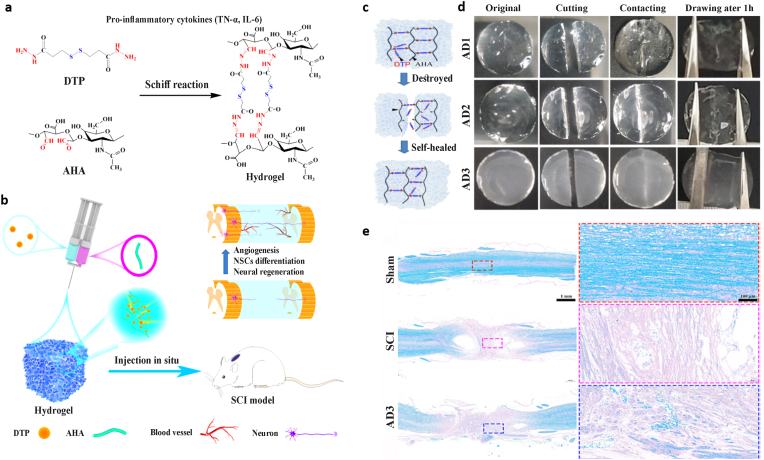
| AHA/DTP, Schiff base cross-linked |
|
|
NSCs |
|
After 1 week: Can help neural stem cells differentiate into neurons Bridged the lesion cavity, and promoted angiogenesis, remyelination, and neural regeneration Leads to promote motor functional recovery |
Yu-2023, Li-2022 [231,232] |
| BOCPG, Schiff base cross-linked and non-covalent crosslinking |
|
|
NSCs |
|
Enhanced neuronal differentiation, promoted axon outgrowth, and inhibited astrocyte differentiation After 6 weeks: Activated endogenous NSCs neurogenesis and induced myelinated axon regeneration into the injury site in vivo Leads to promote locomotor functional recovery |
Luo-2022 [233] |
| Ibuprofen-KYIGSRK, Michael addition reaction |
|
|
None |
|
Enhanced DRG neurons adhesion and neurite extension After 4 weeks: Decreased glial scars and increased neurites Leads to promote motor functional recovery. Remarkably suppress inflammatory responses and enhance nerve regeneration |
Zhang-2022 [234] |
| PLEL, Thermosensitive |
|
|
EVs |
|
After 4 weeks: Can reduce the local inflammation and the apoptosis of neural cells Leads to promote motor functional recovery. Providing topological cues as guidance for axon alignment |
Zhang-2022 [235] |
| cfGel, Thermosensitive |
|
|
NSCs Cetuximab FTY720 |
|
Enhanced neuronal differentiation, and inhibited astrocyte differentiation After 10 weeks: Promoted integration and neuronal differentiation of NSCs Enhanced axonal regeneration and inhibited glial scar formation Exhibited functional improvement |
Qi-2022 [236] |
PA: peptide amphiphile; SCI: spinal cord injury; BDNF: brain-derived neurotrophic factor; SF/PDA: silk fibroin/polydopamine; SHIELD: shear-thinning hydrogel for injectable encapsulation and long-term delivery; Col-I: collagen type I; LN-I: laminin I; HA: hyaluronic acid; GO: graphene oxide; PD/GO hydrogel: GO sheets with 4arm-PEG-diacerein, donated as PD hereafter; 4arm-PEG-diacerein: four-armed polyethylene glycol functionalized with an anti-inflammatory drug, diacerein; PLGA: poly (lactic-co-glycolic acid); HP: heparin-poloxamer; aFGF: acidic fibroblast growth factor; I-5: imidazole-poly(organophosphazenes); MH: minocycline hydrochloride; ESCs: embryonic stem cells; CHA gel: glycol chitosan cross-linked with an oxidized hyaluronate hydrogel; HA-PH-RGD/F: a hydroxyphenyl derivative of hyaluronic acid modified with the integrin-binding peptide RGD and combined with fibrinogen; hWJ-MSCs: human Wharton's jelly-derived mesenchymal stem cells; BC/FB: bovine collagen and fibrinogen; GelMA: gelatin methacrylate; MSCs: mesenchymal stem cells; RADA: arginine (R, Arg), alanine (A, Ala) aspartic acid (D, Asp), alanine (A, Ala); RADA-16I: [Ac-(AcN-ArgAlaAspAla)4-CONH2]; AHA/DTP: aldehyde-modified hyaluronic acid/3, 3′ -dithiobis (propionyl hydrazide); NSCs: neural stem cells; BOCPG: BOC-doped polypyrrole with gelatin; BOC: borax-functionalized oxidized chondroitin sulfate; Gel: gelatin; KYIGSRK: lysine-tyrosine-isoleucine-glycine-serine-arginine-lysine;; DRG: dorsal root ganglion; PLEL: poly (d, l-lactide)-poly (ethylene glycol)-poly(d,l-lactide); EVs: M2 microglia derived extracellular vesicles; cfGel: dual-drug (Cetuximab-FTY720) enhanced hydrogel; Cetuximab: an epidermal growth factor receptor signaling antagonist, FTY720: fingolimod, a first-in-class sphingosine 1-phosphate receptor modulator.
SAPs, as previously stated, are effective injectable hydrogels for CNS regeneration due to their chemical properties and stimulus-sensitive formation [23]. The use of dopamine (DA)-based injectable hydrogels have recently shown promise in an increasing number of soft tissue engineering applications such as spinal cord regeneration. In a study, an injectable silk fibroin/polydopamine (SF/PDA) composite hydrogel was created via a Schiff base reaction followed by self-polymerization and was employed in a rat model of SCI. The data showed that using this hydrogel as an SCI implant could promote the spinal cord's functional recovery (Fig. 8). DA plays the role of cross-linker in these kinds of hydrogels. As well as DA-hyaluronic acid composite hydrogels that have antioxidant activity and pH responsibility, researchers produced the self-healing polyallylamine polymer hydrogels based on DA for SCI tissue engineering [218]. To significantly improve a cervical contusion model of SCI, A shear-thinning has been developed, injectable hydrogel that quickly self-heals to dramatically increase the survival and therapeutic effectiveness of transplanted SCs as a combined therapy for the treatment of SCI (Fig. 9) [219].
Fig. 8.
Preparation and injection of silk fibroin/polydopamine (SF/PDA) composite hydrogel in a rat model with lateral hemisection spinal cord injury. a) Illustration of SF/PDA hydrogel fabrication. b) Hydrogel injection procedure. c) Surface topography of composite hydrogels as shown by SEM. d) SEM of hydrogels in cross-section. e) Inner pore size, pore density, and porosity distribution. f) In vitro cell culture employing immunofluorescence labeling of hippocampus neurons in different SF/DOPA hydrogels and cytotoxicity testing utilizing MTT assay. g) SEM images of hippocampal neurons morphology cultured in vitro, the axon length, and the cell density. h) Initial assessment of spinal cord regeneration two weeks after injection; Immunofluorescence staining of neurons (β-tubulin III, red), glial cells (GFAP), and cell nuclei (Hoechst33342, blue), immunofluorescence intensity of GFAP around the cavity, and statistical analysis of hippocampus neurons and glial cells [218]. Copyright 2022 Elsevier.
Fig. 9.
Shear-thinning Hydrogel for Injectable Encapsulation and Long-term Delivery (SHIELD) in a rat model of cervical SCI. a) Schematic illustrating three major problems that SCs have during the transplantation procedure. b) SCI model schematic. Fischer rats are subjected to a right 75-kdyne contusion injury following a C5 laminectomy. SCs are injected directly at the damage site two weeks later (subacute phase) utilizing an automated syringe needle equipment. c) Fluorescent pictures and assessment of the number of P75+ cells in spinal cord explants transplanted with SCs in SHIELD or saline (at 2 days and 4 weeks following transplantation). d) Fluorescent pictures and measurement of P75+ SCs morphology 2 days after transplantation show remarkable morphological changes between cells given in SHIELD and saline. e) Diagram of secondary injury characterization in spinal cord slices showing cystic cavitation, adjacent lesion and peri-lesion zones, and uninjured and injured hemispheres. f) Representative whole-scan fluorescence pictures and assessment of cavitation volume of spinal cord slices show cavity areas in all groups. g) GFAP staining to demonstrate the development of glial scars and to quantify the GFAP-positive region around the cystic cavity. h) Fluorescent images of β III-tubulin–stained spinal cord explants [219]. Copyright 2020 Science.
Moreover, Scientists used magnetically-responsive (MR) self-assembling peptide hydrogels to make injectable and patternable scaffolds. They introduced a mixture of RADA-16I [Ac-(AcN-ArgAlaAspAla)4-CONH2] self-assembling hydrogels and human MSCs into a rat model of spinal cord damage to see whether or not the cells aligned magnetically. The findings confirm that MR hydrogels can facilitate the axon elongation and alignment at the site of SCI, thereby offering a practical method for regulating the topology of injectable scaffolds [230].
Furthermore, researchers developed an injectable self-healing hyaluronic acid-based hydrogel with higher hydrolytic stability for nerve restoration. AHA/DTP (aldehyde-modified hyaluronic acid/3, 3′ -dithiobis (propionyl hydrazide)) hydrogels were produced by the Schiff reaction between AHA and DTP using a dual syringe injector system. By bridging the spinal cord lesion cavity and creating an optimal microenvironment for cellular activity, such hydrogels can assist in SCI repair by encouraging angiogenesis, remyelination, and neuronal regeneration. In conclusion, their data showed that the hydrogels had the potential to aid in the recovery of locomotor function in rats with SCI (Fig. 10) [231,232]. In a work, scientists combine electroconductive and biocompatible ECM-based hydrogels to design spinal cord tissue engineering materials for traumatic SCI. To create injectable, electroconductive, and self-healing, ECM-based hydrogels, they mixed borax-functionalized oxidized chondroitin sulfate (BOC), BOC-doped polypyrrole (BOCP), and gelatin (Gel) at physiological conditions. Noncovalent electrostatic interactions and dynamic covalent chemistry (borate-diol ester bonds and Schiff-base) combine to generate the hydrogel. The data indicated that this electroconductive extracellular matrix hydrogel could increase axon elongation, and enhance neuronal differentiation, but suppress astrocyte differentiation in vitro. In rats with spinal cord damage, this kind of hydrogel stimulated endogenous NSCs neurogenesis, myelinated axon regeneration at the damaged site, and finally restored locomotor function [233].
Fig. 10.
Preparation and injection of aldehyde-modified hyaluronic acid 3, 3′ -dithiobis (propionyl hydrazide) (AHA/DTP) hydrogel in a rat model with spinal cord injury. Schematic illustrations of injectable AHA/DTP hydrogels a) synthesis and, b) injection into the injury site. The self-healing c) mechanism and, d) photographs of hydrogels. e) LFB staining of longitudinal sections of the spinal cord at 56 days post-injury to demonstrate the remyelination capability of the AHA/DTP hydrogel [232]. Copyright 2023 Elsevier.
A recent study provides a practical in situ synthesis approach of injectable peptide functionalized hydrogels for spinal cord injury treatment. They reported ibuprofen-KYIGSRK conjugated soft hydrogels prepared by in situ Michael addition reaction. This soft injectable hydrogel promoted DRG neurons adhesion and neurite extension in vitro, and also could drastically reduce inflammatory reactions, fill up unusual cavities to prevent glial scar creation, enhance nerve regeneration, and improve motor functional recovery in vivo [234].
The therapeutic application of acellular nerve grafts for peripheral nerve healing has just emerged [221]. These kinds of nerve grafts have recently been used experimentally with some success in the spinal cord and suggested that they may also have the translational potential [224].
Actually, decellularization eliminates the risk of immunological rejection [237] but integrating an intact graft into the spinal cord may necessitate the unwanted removal of healthy tissue. So, tissue engineering is increasingly using injectable acellular hydrogel formulations to provide an efficient and practically applicable scaffold for spinal cord regeneration and healing. Therefore, since ECM-mimicking injectable hydrogels can promote the efficacy of cellular therapy [238], finding an injectable hydrogel scaffold to help different cell therapies in SCI clinical trials better might have significant clinical implications [220]. It has been showed that the imidazole-poly (organophosphazenes) (I-5) hydrogel with thermosensitive sol-gel transition behavior in a clinically relevant rat SCI model could almost completely eliminate cystic cavities (Fig. 11) [223].
Fig. 11.
Imidazole-poly (organophosphazenes) (I-5) injectable hydrogel for SCI repair in a rat with spinal contusion injury. a) A viscometer's measurement of temperature-dependent sol-gel transition and viscosity changes. b) Demonstration of the rapid gelation of an I-5 polymer solution at 4 °C and 37 °C. c, d) After four weeks of I-5 injection, there are no cystic cavity areas; (sections of spinal cord stained with (c) eriochrome cyanine and eosin a (d) GFAP antibodies). e) Three-dimensional regeneration of animal spinal cord tissues. f) Quantification diagrams that display the cavity volumes, pathological tissue, and myelinated white matter. g) Four weeks after injury, animals that had received an I-5 injection displayed improved BBB locomotor scores. h) Catwalk software analysis of representative footprints. i) After seven weeks of injury parameters such as stride length, the base of support, rotation angle, relative position, and regularity index were used to measure the quality of locomotion. j, k) The prevention of motor neurons and myelinated white matter after I-5 injection; (The samples were stained with (j) eriochrome cyanine and cresyl violet and (k) myelin basic protein immunostaining) [223]. Copyright 2017 Nature.
Also, a thermosensitive injectable decellularized tissue scaffold from rat peripheral nerve tissue called injectable peripheral nerve (iPN) was produced in a study as a potential minimally invasive treatment to fill spinal cord lesion gaps after injury. The findings indicate that iPN hydrogels can promote neural cell survival. So, this kind of hydrogel can use in combination therapy for neural tissue healing after SCI [239]. Some researchers encapsulated M2 derived EVs (microglia derived extracellular vesicles) into poly (d, l-lactide)-poly (ethylene glycol)-poly(d,l-lactide) PLEL hydrogel to fabricate an injectable, thermosensitive, anti-apoptotic, and anti-inflammatory hydrogel system. The results indicate that this EVs-loaded thermosensitive hydrogel can achieve local and sustainable release, and therefore can suppress local inflammation and neuronal apoptosis in the SCI site because of its anti-inflammatory and neural protective feature [235].
Normally in a healthy body, neurons use electrical signals to communicate with one another and other cell types, but in SCI, blood cells migrate to the damage site, inhibiting neuronal cells' migration and proliferation and disrupting signal transduction [221]. Also, one of the new and promising ways to treat spinal cord injuries is the development of optimum biomaterials with an aligned topology that allows increased neuronal regeneration. As a result, given the widespread use of hydrogels in central nervous system tissue engineering, the generation and use of injectable hydrogels with conductivity, anti-inflammatory characteristics, and aligned topography inside 3D amorphous hydrogels to improve treatment outcomes in SCI is urgently needed [221,240].
4. Conclusions, and future directions
In soft tissue engineering, injectable hydrogels offer potential scaffolds with their capacity to match uneven flaws and low invasive qualities. The criteria of the best biomaterials for supporting CNS regeneration, types of injectable hydrogels made from different fabrication methods, and biomaterials for brain and spinal cord tissue engineering applications were discussed in this review.
Injectable hydrogels can be prepared in a variety of ways, including physically and chemically processed. Physical hydrogels are easy to make because they can respond to pH, temperature, stress, and ion concentration as environmental cues. These kinds of hydrogels have low cytotoxicity but are often unstable in physiological environments and slow to respond. On the other hand, injectable chemical hydrogels have outstanding mechanical properties and good stability but have negative impacts in vivo as a result of chemical interactions.
Many types of injectable hydrogels have been developed from natural and synthetic biomaterials for CNS repair using tissue engineering strategy. In general, hydrogels based on synthetic biomaterials are stable and have good mechanical properties but low biological activity and biocompatibility. So natural biomaterials, on the other hand, are extensively employed in the creation of injectable hydrogels due to their excellent biocompatibility, low cytotoxicity, and biodegradable nature. But, typically, the lack of mechanical strength limits their potential in medical application. Therefore, composite hydrogels synthesized from natural and synthetic materials and associated with both components' advantages can be used in many studies. Finally, developing a mechanism to combine the benefits of diverse biomaterials and production methods for injectable hydrogels will be critical for clinical applications in CNS regeneration and engineering. So, the future direction may be a combination of synthetic and biological material.
Injectable hydrogels can overcome some of the restrictions of CNS treatment. They can fill irregular spaces in the injured CNS cavity. This provides an axonal growth-permissive environment and appropriate structural support to the surrounding tissue, which is mandatory in case of large trauma or surgical removal of tumors. Many studies have recently concentrated on developing innovative injectable hydrogels for the healing of the central nervous system.
Furthermore, there are still a number of issues that need to be resolved before injectable hydrogels may be used to achieve optimal CNS regeneration. Designing injectable hydrogels for brain and spinal cord tissue engineering with optimal biodegradability, stability, biocompatibility, and mechanical qualities for supporting neurite outgrowth and 3D cell culture is difficult. While several injectable biomaterials have shown experimental efficacy in CNS regeneration in vivo in addition to other therapies, further research is needed to treat CNS injuries.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to express our thanks to Mazandaran University of Medical Sciences for the research grants (Project number: 14455).
Contributor Information
Elham Hasanzadeh, Email: el.hasanzadeh@mazums.ac.ir.
Narges Mahmoodi, Email: mahmoodi_n@alumnus.tums.ac.ir.
Data availability
Data will be made available on request.
References
- 1.Hong A., Aguilar M.-I., Del Borgo M.P., Sobey C.G., Broughton B.R., Forsythe J.S. Self-assembling injectable peptide hydrogels for emerging treatment of ischemic stroke. J. Mater. Chem. B. 2019;7:3927–3943. [Google Scholar]
- 2.Saremi J., Mahmoodi N., Rasouli M., Ranjbar F.E., Mazaheri E.L., Akbari M., et al. Advanced approaches to regenerate spinal cord injury: the development of cell and tissue engineering therapy and combinational treatments. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112529. [DOI] [PubMed] [Google Scholar]
- 3.Whalin M.K., Arora S.S. Springer; 2018. Anatomy of the Brain and Spinal Cord. Basic Sciences in Anesthesia; pp. 41–59. [Google Scholar]
- 4.Taylor C.A., Bell J.M., Breiding M.J., Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikbakht-Nasrabadi A., Mohammadi N. Toward Overcoming Physical Disability in Spinal Cord Injury: a Qualitative Inquiry of the Experiences of Injured Individuals and Their Families. BMC Neurol. 2019:171. doi: 10.1186/s12883-019-1391-6. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adrian H., Mårten K., Salla N., Lasse V. Biomarkers of traumatic brain injury: temporal changes in body fluids. Eneuro. 2016;3 doi: 10.1523/ENEURO.0294-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwab M.E., Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 8.Lis A., Szarek D., Laska J. [Biomaterials engineering strategies for spinal cord regeneration: state of the art] Polim. Med. 2013;43:59–80. [PubMed] [Google Scholar]
- 9.Stabenfeldt S.E., García A.J., LaPlaca M.C. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J. Biomed. Mater. Res. 2006;77:718–725. doi: 10.1002/jbm.a.30638. [DOI] [PubMed] [Google Scholar]
- 10.Delcroix G.J., Schiller P.C., Benoit J.P., Montero-Menei C.N. Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials. 2010;31:2105–2120. doi: 10.1016/j.biomaterials.2009.11.084. [DOI] [PubMed] [Google Scholar]
- 11.Lis A., Szarek D., Laska J. [The outlook for the use of polymeric scaffolds in the reconstruction and the regeneration stimulation of traumatic brain injuries] Polim. Med. 2013;43:302–312. [PubMed] [Google Scholar]
- 12.Park J.H., Han J.B., Kim S.K., Park J.H., Go D.H., Sun B., et al. Spinal GABA receptors mediate the suppressive effect of electroacupuncture on cold allodynia in rats. Brain Res. 2010;1322:24–29. doi: 10.1016/j.brainres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Silva P.A., Pereira P., Pinto R., Silveira F., Vaz R. Bilateral sciatic neuropathy due to fibrous bands in a patient with severe traumatic brain injury. Clin. Neurol. Neurosurg. 2014;120:93–95. doi: 10.1016/j.clineuro.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Moshayedi P., Nih L.R., Llorente I.L., Berg A.R., Cinkornpumin J., Lowry W.E., et al. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 2016;105:145–155. doi: 10.1016/j.biomaterials.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han D., Cheung K.C. Biodegradable cell-seeded nanofiber scaffolds for neural repair. Polymers. 2011;3:1684–1733. [Google Scholar]
- 16.Yu Z., Li H., Xia P., Kong W., Chang Y., Fu C., et al. Application of fibrin-based hydrogels for nerve protection and regeneration after spinal cord injury. J. Biol. Eng. 2020;14:22. doi: 10.1186/s13036-020-00244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellati A., Hasanzadeh E., Gholipourmalekabadi M., Enderami S.E. Injectable nanocomposite hydrogels as an emerging platform for biomedical applications: a review. Mater Sci Eng C Mater Biol Appl. 2021;131 doi: 10.1016/j.msec.2021.112489. [DOI] [PubMed] [Google Scholar]
- 18.Jain A., Kim Y.T., McKeon R.J., Bellamkonda R.V. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27:497–504. doi: 10.1016/j.biomaterials.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 19.King V.R., Alovskaya A., Wei D.Y., Brown R.A., Priestley J.V. The use of injectable forms of fibrin and fibronectin to support axonal ingrowth after spinal cord injury. Biomaterials. 2010;31:4447–4456. doi: 10.1016/j.biomaterials.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Petter-Puchner A.H., Froetscher W., Krametter-Froetscher R., Lorinson D., Redl H., van Griensven M. The long-term neurocompatibility of human fibrin sealant and equine collagen as biomatrices in experimental spinal cord injury. Exp. Toxicol. Pathol. 2007;58:237–245. doi: 10.1016/j.etp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Gupta D., Tator C.H., Shoichet M.S. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27:2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Cigognini D., Satta A., Colleoni B., Silva D., Donegà M., Antonini S., et al. Evaluation of early and late effects into the acute spinal cord injury of an injectable functionalized self-assembling scaffold. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassannejad Z., Zadegan S.A., Vaccaro A.R., Rahimi-Movaghar V., Sabzevari O. Biofunctionalized peptide-based hydrogel as an injectable scaffold for BDNF delivery can improve regeneration after spinal cord injury. Injury. 2019;50:278–285. doi: 10.1016/j.injury.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 24.Chitnis T., Weiner H.L. CNS inflammation and neurodegeneration. J. Clin. Invest. 2017;127:3577–3587. doi: 10.1172/JCI90609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quraishe S., Forbes L.H., Andrews M.R. The Extracellular Environment of the CNS: Influence on Plasticity, Sprouting, and Axonal Regeneration after Spinal Cord Injury. Neural Plast. 2018 doi: 10.1155/2018/2952386. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hlavac N., Kasper M., Schmidt C. Progress toward finding the perfect match: hydrogels for treatment of central nervous system injury. Mater. Today Adv. 2020;6 [Google Scholar]
- 27.Zuidema J.M., Gilbert R.J., Gottipati M.K. Biomaterial approaches to modulate reactive astroglial response. Cells Tissues Organs. 2018;205:372–395. doi: 10.1159/000494667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong Y., Mahmood A., Chopp M. Current understanding of neuroinflammation after traumatic brain injury and cell-based therapeutic opportunities. Chin. J. Traumatol. 2018;21:137–151. doi: 10.1016/j.cjtee.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrigan F., Mander K.A., Leonard A.V., Vink R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J. Neuroinflammation. 2016;13:264. doi: 10.1186/s12974-016-0738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bramlett H.M., Dietrich W.D. Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J. Neurotrauma. 2015;32:1834–1848. doi: 10.1089/neu.2014.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oyinbo C.A. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol. Exp. 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 33.Burda J.E., Bernstein A.M., Sofroniew M.V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016;275 Pt 3:305–315. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada S., Hara M., Kobayakawa K., Matsumoto Y., Nakashima Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2018;126:39–43. doi: 10.1016/j.neures.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson M.A., Burda J.E., Ren Y., Ao Y., O'Shea T.M., Kawaguchi R., et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver J., Miller J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 38.Wang H., Song G., Chuang H., Chiu C., Abdelmaksoud A., Ye Y., et al. Portrait of glial scar in neurological diseases. Int. J. Immunopathol. Pharmacol. 2018;31 doi: 10.1177/2058738418801406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moeendarbary E., Weber I.P., Sheridan G.K., Koser D.E. The soft mechanical signature of glial scars in the central nervous system. 2017;8 doi: 10.1038/ncomms14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blesch A., Tuszynski M.H. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–47. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Loane D.J., Faden A.I. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khaing Z.Z., Thomas R.C., Geissler S.A., Schmidt C.E. Advanced biomaterials for repairing the nervous system: what can hydrogels do for the brain? Mater. Today. 2014;17:332–340. [Google Scholar]
- 43.Führmann T., Anandakumaran P.N., Shoichet M.S. Combinatorial therapies after spinal cord injury: how can biomaterials help? Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201601130. [DOI] [PubMed] [Google Scholar]
- 44.Kabu S., Gao Y., Kwon B.K., Labhasetwar V. Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. J. Contr. Release. 2015;219:141–154. doi: 10.1016/j.jconrel.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novikova L.N., Pettersson J., Brohlin M., Wiberg M., Novikov L.N. Biodegradable poly-beta-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials. 2008;29:1198–1206. doi: 10.1016/j.biomaterials.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 46.Tam R.Y., Fuehrmann T., Mitrousis N., Shoichet M.S. Regenerative therapies for central nervous system diseases: a biomaterials approach. Neuropsychopharmacology. 2014;39:169–188. doi: 10.1038/npp.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsintou M., Dalamagkas K., Seifalian A.M. Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural Regen Res. 2015;10:726–742. doi: 10.4103/1673-5374.156966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa Y., Sawamoto K., Miyata T., Miyao S., Watanabe M., Nakamura M., et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. Res. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- 49.Park H.C., Shim Y.S., Ha Y., Yoon S.H., Park S.R., Choi B.H., et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11:913–922. doi: 10.1089/ten.2005.11.913. [DOI] [PubMed] [Google Scholar]
- 50.Park S.I., Lim J.Y., Jeong C.H., Kim S.M., Jun J.A., Jeun S.S., et al. Human umbilical cord blood-derived mesenchymal stem cell therapy promotes functional recovery of contused rat spinal cord through enhancement of endogenous cell proliferation and oligogenesis. J. Biomed. Biotechnol. 2012 doi: 10.1155/2012/362473. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabesh H., Amoabediny G., Nik N.S., Heydari M., Yosefifard M., Siadat S.O., et al. The role of biodegradable engineered scaffolds seeded with Schwann cells for spinal cord regeneration. Neurochem. Int. 2009;54:73–83. doi: 10.1016/j.neuint.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Davies J.E., Huang C., Proschel C., Noble M., Mayer-Proschel M., Davies S.J. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J. Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu P., Wang Y., Graham L., McHale K., Gao M., Wu D., et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgado P.I., Palacios M., Larrain J. In situ injectable hydrogels for spinal cord regeneration: advances from the last 10 years. Biomed Phys Eng Express. 2019;6 doi: 10.1088/2057-1976/ab52e8. [DOI] [PubMed] [Google Scholar]
- 55.Rosenzweig E.S., Brock J.H., Lu P., Kumamaru H., Salegio E.A., Kadoya K., et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat. Med. 2018;24:484–490. doi: 10.1038/nm.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wen X., Luo Z., Yang H., Wang W., Yang L. Elsevier; 2019. Biomaterials and Scaffolds for the Treatment of Spinal Cord Injury. Biomaterials in Translational Medicine; pp. 117–139. [Google Scholar]
- 57.Geller H.M., Fawcett J.W. Building a bridge: engineering spinal cord repair. Exp. Neurol. 2002;174:125–136. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 58.Shrestha B., Coykendall K., Li Y., Moon A., Priyadarshani P., Yao L. Repair of injured spinal cord using biomaterial scaffolds and stem cells. Stem Cell Res. Ther. 2014;5:91. doi: 10.1186/scrt480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S., Kim D., Park S., Kim S., Lee D., Kim W., et al. Nanopatterned scaffolds for neural tissue engineering and regenerative medicine. Adv. Exp. Med. Biol. 2018;1078:421–443. doi: 10.1007/978-981-13-0950-2_22. [DOI] [PubMed] [Google Scholar]
- 60.Ghuman H., Massensini A.R., Donnelly J., Kim S.M., Medberry C.J., Badylak S.F., et al. ECM hydrogel for the treatment of stroke: characterization of the host cell infiltrate. Biomaterials. 2016;91:166–181. doi: 10.1016/j.biomaterials.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bignami A., Hosley M., Dahl D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat. Embryol. 1993;188:419–433. doi: 10.1007/BF00190136. [DOI] [PubMed] [Google Scholar]
- 62.Cregg J.M., DePaul M.A., Filous A.R., Lang B.T., Tran A., Silver J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asghari F., Faradonbeh D.R., Malekshahi Z.V., Nekounam H., Ghaemi B., Yousefpoor Y., et al. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydrate Polym. 2022;278 doi: 10.1016/j.carbpol.2021.118926. [DOI] [PubMed] [Google Scholar]
- 64.Harris G.M., Madigan N.N., Lancaster K.Z., Enquist L.W., Windebank A.J., Schwartz J., et al. Nerve guidance by a decellularized fibroblast extracellular matrix. Matrix Biol. 2017;60–61:176–189. doi: 10.1016/j.matbio.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sensharma P., Madhumathi G., Jayant R.D., Jaiswal A.K. Biomaterials and cells for neural tissue engineering: current choices. Mater Sci Eng C Mater Biol Appl. 2017;77:1302–1315. doi: 10.1016/j.msec.2017.03.264. [DOI] [PubMed] [Google Scholar]
- 66.Alessandri M., Lizzo G., Gualandi C., Mangano C., Giuliani A., Focarete M.L., et al. Influence of biological matrix and artificial electrospun scaffolds on proliferation, differentiation and trophic factor synthesis of rat embryonic stem cells. Matrix Biol. 2014;33:68–76. doi: 10.1016/j.matbio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Dahlin R.L., Kasper F.K., Mikos A.G. Polymeric nanofibers in tissue engineering. Tissue Eng. B Rev. 2011;17:349–364. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang F., Murugan R., Ramakrishna S., Wang X., Ma Y.X., Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 69.Freudenberg U., Hermann A., Welzel P.B., Stirl K., Schwarz S.C., Grimmer M., et al. A star-PEG-heparin hydrogel platform to aid cell replacement therapies for neurodegenerative diseases. Biomaterials. 2009;30:5049–5060. doi: 10.1016/j.biomaterials.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Bignami A., Hosley M., Dahl D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat. Embryol. 1993;188:419–433. doi: 10.1007/BF00190136. [DOI] [PubMed] [Google Scholar]
- 71.Niemczyk B., Sajkiewicz P., Kolbuk D. Injectable hydrogels as novel materials for central nervous system regeneration. J. Neural. Eng. 2018;15 doi: 10.1088/1741-2552/aacbab. [DOI] [PubMed] [Google Scholar]
- 72.Buzańska L., Zychowicz M., Sarnowska A., Domańska-Janik K. [Bioengineering of neural stem cell niche] Postepy Biochem. 2013;59:175–186. [PubMed] [Google Scholar]
- 73.Farzin A., Hassan S., Ebrahimi-Barough S., Ai A., Hasanzadeh E., Goodarzi A., et al. A facile two step heat treatment strategy for development of bioceramic scaffolds for hard tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2019;105 doi: 10.1016/j.msec.2019.110009. [DOI] [PubMed] [Google Scholar]
- 74.Lins L.C., Wianny F., Livi S., Hidalgo I.A., Dehay C., Duchet-Rumeau J., et al. Development of bioresorbable hydrophilic-hydrophobic electrospun scaffolds for neural tissue engineering. Biomacromolecules. 2016;17:3172–3187. doi: 10.1021/acs.biomac.6b00820. [DOI] [PubMed] [Google Scholar]
- 75.Akhtari M., Emin D., Ellingson B., Woodworth D., Frew A., Mathern G. Measuring the local electrical conductivity of human brain tissue. J. Appl. Phys. 2016;119 [Google Scholar]
- 76.Scivill I., Barker A., Freeston I. IEEE; 1996. Finite Element Modelling of Magnetic Stimulation of the Spine. Proceedings of 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; pp. 393–394. [Google Scholar]
- 77.Hernández-Labrado G.R., Polo J.L., López-Dolado E., Collazos-Castro J.E. Spinal cord direct current stimulation: finite element analysis of the electric field and current density. Med. Biol. Eng. Comput. 2011;49:417–429. doi: 10.1007/s11517-011-0756-9. [DOI] [PubMed] [Google Scholar]
- 78.Wu D., Pak E.S., Wingard C.J., Murashov A.K. Multi-walled carbon nanotubes inhibit regenerative axon growth of dorsal root ganglia neurons of mice. Neurosci. Lett. 2012;507:72–77. doi: 10.1016/j.neulet.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fraczek-Szczypta A. Carbon nanomaterials for nerve tissue stimulation and regeneration. Mater Sci Eng C Mater Biol Appl. 2014;34:35–49. doi: 10.1016/j.msec.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 80.Mawad D., Gilmore K., Molino P., Wagner K., Wagner P., Officer D.L., et al. An erodible polythiophene-based composite for biomedical applications. J. Mater. Chem. 2011;21:5555–5560. [Google Scholar]
- 81.Liu X., Miller A.L., 2nd, Park S., Waletzki B.E., Zhou Z., Terzic A., et al. Functionalized carbon nanotube and graphene oxide embedded electrically conductive hydrogel synergistically stimulates nerve cell differentiation. ACS Appl. Mater. Interfaces. 2017;9:14677–14690. doi: 10.1021/acsami.7b02072. [DOI] [PubMed] [Google Scholar]
- 82.Liu X., Kim J.C., Miller A.L., Waletzki B.E., Lu L. Electrically conductive nanocomposite hydrogels embedded with functionalized carbon nanotubes for spinal cord injury. New J. Chem. 2018;42:17671–17681. [Google Scholar]
- 83.Zhou L., Fan L., Yi X., Zhou Z., Liu C., Fu R., et al. Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano. 2018;12:10957–10967. doi: 10.1021/acsnano.8b04609. [DOI] [PubMed] [Google Scholar]
- 84.Landers J., Turner J.T., Heden G., Carlson A.L., Bennett N.K., Moghe P.V., et al. Carbon nanotube composites as multifunctional substrates for in situ actuation of differentiation of human neural stem cells. Adv Healthc Mater. 2014;3:1745–1752. doi: 10.1002/adhm.201400042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goganau I., Sandner B., Weidner N., Fouad K., Blesch A. Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury. Exp. Neurol. 2018;300:247–258. doi: 10.1016/j.expneurol.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balint R., Cassidy N.J., Cartmell S.H. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10:2341–2353. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 87.Xiao Y., Cui X., Hancock J.M., Bouguettaya M., Reynolds J.R., Martin D.C. Electrochemical polymerization of poly (hydroxymethylated-3, 4-ethylenedioxythiophene)(PEDOT-MeOH) on multichannel neural probes. Sensor. Actuator. B Chem. 2004;99:437–443. [Google Scholar]
- 88.Guiseppi-Elie A. Electroconductive hydrogels: synthesis, characterization and biomedical applications. Biomaterials. 2010;31:2701–2716. doi: 10.1016/j.biomaterials.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 89.Abidian M.R., Daneshvar E.D., Egeland B.M., Kipke D.R., Cederna P.S., Urbanchek M.G. Hybrid conducting polymer-hydrogel conduits for axonal growth and neural tissue engineering. Adv Healthc Mater. 2012;1:762–767. doi: 10.1002/adhm.201200182. [DOI] [PubMed] [Google Scholar]
- 90.Jiang L., Wang Y., Liu Z., Ma C., Yan H., Xu N., et al. Three-dimensional printing and injectable conductive hydrogels for tissue engineering application. Tissue Eng. B Rev. 2019;25:398–411. doi: 10.1089/ten.TEB.2019.0100. [DOI] [PubMed] [Google Scholar]
- 91.Runge M.B., Dadsetan M., Baltrusaitis J., Ruesink T., Lu L., Windebank A.J., et al. Development of electrically conductive oligo(polyethylene glycol) fumarate-polypyrrole hydrogels for nerve regeneration. Biomacromolecules. 2010;11:2845–2853. doi: 10.1021/bm100526a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sulejczak D., Andrychowski J., Kowalczyk T., Nakielski P., Frontczak-Baniewicz M., Kowalewski T. Electrospun nanofiber mat as a protector against the consequences of brain injury. Folia Neuropathol. 2014;52:56–69. doi: 10.5114/fn.2014.41744. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y.S., Khademhosseini A. 2017. Advances in Engineering Hydrogels; p. 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guan X., Avci-Adali M., Alarçin E., Cheng H., Kashaf S.S., Li Y., et al. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017;12 doi: 10.1002/biot.201600394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao Y.Z., Jiang X., Xiao J., Lin Q., Yu W.Z., Tian F.R., et al. Using NGF heparin-poloxamer thermosensitive hydrogels to enhance the nerve regeneration for spinal cord injury. Acta Biomater. 2016;29:71–80. doi: 10.1016/j.actbio.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu Z., Li H., Xia P., Kong W., Chang Y., Fu C., et al. Application of fibrin-based hydrogels for nerve protection and regeneration after spinal cord injury. J. Biol. Eng. 2020;14:22. doi: 10.1186/s13036-020-00244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mellati A., Fan C.M., Tamayol A., Annabi N., Dai S., Bi J., et al. Microengineered 3D cell-laden thermoresponsive hydrogels for mimicking cell morphology and orientation in cartilage tissue engineering. Biotechnol. Bioeng. 2017;114:217–231. doi: 10.1002/bit.26061. [DOI] [PubMed] [Google Scholar]
- 98.Grover G.N., Braden R.L., Christman K.L. Oxime cross-linked injectable hydrogels for catheter delivery. Adv. Mater. 2013;25:2937–2942. doi: 10.1002/adma.201205234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Williams D.F. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 100.Musah S., Morin S.A., Wrighton P.J., Zwick D.B., Jin S., Kiessling L.L. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano. 2012;6:10168–10177. doi: 10.1021/nn3039148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Straley K.S., Foo C.W., Heilshorn S.C. Biomaterial design strategies for the treatment of spinal cord injuries. J. Neurotrauma. 2010;27:1–19. doi: 10.1089/neu.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Macaya D., Spector M. Injectable hydrogel materials for spinal cord regeneration: a review. Biomed. Mater. 2012;7 doi: 10.1088/1748-6041/7/1/012001. [DOI] [PubMed] [Google Scholar]
- 103.Ji H., Song X., He C., Tang C., Xiong L., Zhao W., et al. Root-soil structure inspired hydrogel microspheres with high dimensional stability and anion-exchange capacity. J. Colloid Interface Sci. 2018;532:680–688. doi: 10.1016/j.jcis.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 104.Khyati D. 2012. Characterization of Rheological Properties and Degradation of Genipin Crosslinked Fibrin Hydrogel for Annulus Repair. [Google Scholar]
- 105.O'Connor S.M., Stenger D.A., Shaffer K.M., Ma W. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci. Lett. 2001;304:189–193. doi: 10.1016/s0304-3940(01)01769-4. [DOI] [PubMed] [Google Scholar]
- 106.Hasanzadeh E., Mahmoodi N., Basiri A., Esmaeili Ranjbar F., Hassannejad Z., Ebrahimi-Barough S., et al. Proanthocyanidin as a crosslinking agent for fibrin, collagen hydrogels and their composites with decellularized Wharton’s-jelly-extract for tissue engineering applications. J. Bioact. Compat Polym. 2020;35:554–571. [Google Scholar]
- 107.Mahmoodi N., Ai J., Hassannejad Z., Ebrahimi-Barough S., Hasanzadeh E., Nekounam H., et al. Improving motor neuron-like cell differentiation of hEnSCs by the combination of epothilone B loaded PCL microspheres in optimized 3D collagen hydrogel. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-01071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McBride D.W., Szu J.I., Hale C., Hsu M.S., Rodgers V.G., Binder D.K. Reduction of cerebral edema after traumatic brain injury using an osmotic transport device. J. Neurotrauma. 2014;31:1948–1954. doi: 10.1089/neu.2014.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jia X., Yeo Y., Clifton R.J., Jiao T., Kohane D.S., Kobler J.B., et al. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 110.Griffin D.R., Weaver W.M., Scumpia P.O., Di Carlo D., Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015;14:737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koshy S.T., Ferrante T.C., Lewin S.A., Mooney D.J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials. 2014;35:2477–2487. doi: 10.1016/j.biomaterials.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khaing Z.Z., Ehsanipour A., Hofstetter C.P., Seidlits S.K. Injectable hydrogels for spinal cord repair: a focus on swelling and intraspinal pressure. Cells Tissues Organs. 2016;202:67–84. doi: 10.1159/000446697. [DOI] [PubMed] [Google Scholar]
- 113.Macaya D.J., Hayakawa K., Arai K., Spector M. Astrocyte infiltration into injectable collagen-based hydrogels containing FGF-2 to treat spinal cord injury. Biomaterials. 2013;34:3591–3602. doi: 10.1016/j.biomaterials.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 114.Tam R.Y., Cooke M.J., Shoichet M.S. A covalently modified hydrogel blend of hyaluronan–methyl cellulose with peptides and growth factors influences neural stem/progenitor cell fate. J. Mater. Chem. 2012;22:19402–19411. [Google Scholar]
- 115.Shinde U.P., Yeon B., Jeong B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013;38:672–701. [Google Scholar]
- 116.Liu H., Liu J., Qi C., Fang Y., Zhang L., Zhuo R., et al. Thermosensitive injectable in-situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. Acta Biomater. 2016;35:228–237. doi: 10.1016/j.actbio.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 117.Kim H.K., Shim W.S., Kim S.E., Lee K.H., Kang E., Kim J.H., et al. Injectable in situ-forming pH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng Part A. 2009;15:923–933. doi: 10.1089/ten.tea.2007.0407. [DOI] [PubMed] [Google Scholar]
- 118.Yang J.-A., Yeom J., Hwang B.W., Hoffman A.S., Hahn S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014;39:1973–1986. [Google Scholar]
- 119.Hoffman A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 120.Tan H., Marra K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials. 2010;3:1746–1767. [Google Scholar]
- 121.Shinde U.P., Yeon B., Jeong B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013;38:672–701. [Google Scholar]
- 122.Liu M., Zeng X., Ma C., Yi H., Ali Z., Mou X., et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5 doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nagahama K., Takahashi A., Ohya Y. Biodegradable polymers exhibiting temperature-responsive sol–gel transition as injectable biomedical materials. React. Funct. Polym. 2013;73:979–985. [Google Scholar]
- 124.Sood N., Bhardwaj A., Mehta S., Mehta A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016;23:758–780. doi: 10.3109/10717544.2014.940091. [DOI] [PubMed] [Google Scholar]
- 125.Kim M.S., Park S.J., Chun H.J., Kim C.-H. Thermosensitive hydrogels for tissue engineering. Tissue Eng. Regen. Med. 2011;8:117–123. [Google Scholar]
- 126.Xu Y., Li Z., Li X., Fan Z., Liu Z., Xie X., et al. Regulating myogenic differentiation of mesenchymal stem cells using thermosensitive hydrogels. Acta Biomater. 2015;26:23–33. doi: 10.1016/j.actbio.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 127.Yu R., Zheng S. Poly(acrylic acid)-grafted poly(N-isopropyl acrylamide) networks: preparation, characterization and hydrogel behavior. J. Biomater. Sci. Polym. Ed. 2011;22:2305–2324. doi: 10.1163/092050610X538722. [DOI] [PubMed] [Google Scholar]
- 128.Ashraf S., Park H.-K., Park H., Lee S.-H. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: role in drug delivery and tissue engineering. Macromol. Res. 2016;24:297–304. [Google Scholar]
- 129.Lee P.Y., Cobain E., Huard J., Huang L. Thermosensitive hydrogel PEG-PLGA-PEG enhances engraftment of muscle-derived stem cells and promotes healing in diabetic wound. Mol. Ther. 2007;15:1189–1194. doi: 10.1038/sj.mt.6300156. [DOI] [PubMed] [Google Scholar]
- 130.Ayar Z., Shafieian M., Mahmoodi N., Sabzevari O., Hassannejad Z. A rechargeable drug delivery system based on pNIPAM hydrogel for the local release of curcumin. J. Appl. Polym. Sci. 2021;138 [Google Scholar]
- 131.De S.K., Aluru N.R. A chemo-electro-mechanical mathematical model for simulation of pH sensitive hydrogels. Mech. Mater. 2004;36:395–410. [Google Scholar]
- 132.Huynh C.T., Nguyen M.K., Jeong I.K., Kim S.W., Lee D.S. Synthesis, characteristics and potential application of poly(β-amino ester urethane)-based multiblock Co-polymers as an injectable, biodegradable and pH/temperature-sensitive hydrogel system. J. Biomater. Sci. Polym. Ed. 2012;23:1091–1106. doi: 10.1163/092050611X575423. [DOI] [PubMed] [Google Scholar]
- 133.Shim W.S., Yoo J.S., Bae Y.H., Lee D.S. Novel injectable pH and temperature sensitive block copolymer hydrogel. Biomacromolecules. 2005;6:2930–2934. doi: 10.1021/bm050521k. [DOI] [PubMed] [Google Scholar]
- 134.de Lima G.G., Campos L., Junqueira A., Devine D.M., Nugent M.J. A novel pH-sensitive ceramic-hydrogel for biomedical applications. Polym. Adv. Technol. 2015;26:1439–1446. [Google Scholar]
- 135.Chiu Y.L., Chen S.C., Su C.J., Hsiao C.W., Chen Y.M., Chen H.L., et al. Corrigendum to "pH-triggered injectable hydrogels prepared from aqueous N-palmitoyl chitosan: in vitro characteristics and in vivo biocompatibility. Biomaterials. 2021;264 doi: 10.1016/j.biomaterials.2020.120374. [biomaterials 30 (2009) 4877-4888] [DOI] [PubMed] [Google Scholar]
- 136.Mantha S., Pillai S., Khayambashi P., Upadhyay A., Zhang Y., Tao O., et al. 2019. Smart Hydrogels in Tissue Engineering and Regenerative Medicine; p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ebara M., Kotsuchibashi Y., Uto K., Aoyagi T., Kim Y.-J., Narain R., et al. Springer; 2014. Smart Hydrogels. Smart Biomaterials; pp. 9–65. [Google Scholar]
- 138.Lim H.L., Hwang Y., Kar M., Varghese S. Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2014;2:603–618. doi: 10.1039/c3bm60288e. [DOI] [PubMed] [Google Scholar]
- 139.Grimaudo M.A., Krishnakumar G.S., Giusto E., Furlani F., Bassi G., Rossi A., et al. Bioactive injectable hydrogels for on demand molecule/cell delivery and for tissue regeneration in the central nervous system. Acta Biomater. 2022;140:88–101. doi: 10.1016/j.actbio.2021.11.038. [DOI] [PubMed] [Google Scholar]
- 140.Mo C., Luo R., Chen Y. 2022. Advances in the Stimuli-Responsive Injectable Hydrogel for Controlled Release of Drugs. [DOI] [PubMed] [Google Scholar]
- 141.Guo C., Kaufman L.J. Flow and magnetic field induced collagen alignment. Biomaterials. 2007;28:1105–1114. doi: 10.1016/j.biomaterials.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 142.Liu Z., Liu J., Cui X., Wang X., Zhang L., Tang P. Recent advances on magnetic sensitive hydrogels in tissue engineering. Front. Chem. 2020;8:124. doi: 10.3389/fchem.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mojtabavi K., Gholami M., Ghodsi Z., Mahmoodi N., Shool S., Kargar-Soleimanabad S., et al. Comparing natural hydrogels to self-assembling peptides in spinal cord injury treatment: a systematic review. F1000Research. 2022;11:16. [Google Scholar]
- 144.Mukherjee N., Adak A., Ghosh S. Recent trends in the development of peptide and protein-based hydrogel therapeutics for the healing of CNS injury. Soft Matter. 2020;16:10046–10064. doi: 10.1039/d0sm00885k. [DOI] [PubMed] [Google Scholar]
- 145.Peressotti S., Koehl G.E., Goding J.A., Green R.A. Self-assembling hydrogel structures for neural tissue repair. ACS Biomater. Sci. Eng. 2021;7:4136–4163. doi: 10.1021/acsbiomaterials.1c00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Singh Y.P., Moses J.C., Bhardwaj N., Mandal B.B. Injectable hydrogels: a new paradigm for osteochondral tissue engineering. J. Mater. Chem. B. 2018;6:5499–5529. doi: 10.1039/c8tb01430b. [DOI] [PubMed] [Google Scholar]
- 147.He C., Kim S.W., Lee D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Contr. Release. 2008;127:189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 148.Van Tomme S.R., van Steenbergen M.J., De Smedt S.C., van Nostrum C.F., Hennink W.E. Self-gelling hydrogels based on oppositely charged dextran microspheres. Biomaterials. 2005;26:2129–2135. doi: 10.1016/j.biomaterials.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 149.Guisasola E., Vallet-Regí M., Baeza A. Magnetically responsive polymers for drug delivery applications. Stimuli Respons. Polym. Nanocarriers Drug Delivery Appl. 2018;ume 1:143–168. Elsevier. [Google Scholar]
- 150.de la Torre P.M., Enobakhare Y., Torrado G., Torrado S. Release of amoxicillin from polyionic complexes of chitosan and poly(acrylic acid). Study of polymer/polymer and polymer/drug interactions within the network structure. Biomaterials. 2003;24:1499–1506. doi: 10.1016/s0142-9612(02)00512-4. [DOI] [PubMed] [Google Scholar]
- 151.Meka V.S., Sing M.K.G., Pichika M.R., Nali S.R., Kolapalli V.R.M., Kesharwani P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today. 2017;22:1697–1706. doi: 10.1016/j.drudis.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 152.Führmann T., Obermeyer J., Tator C.H., Shoichet M.S. Click-crosslinked injectable hyaluronic acid hydrogel is safe and biocompatible in the intrathecal space for ultimate use in regenerative strategies of the injured spinal cord. Methods. 2015;84:60–69. doi: 10.1016/j.ymeth.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 153.Johnson P.J., Parker S.R., Sakiyama-Elbert S.E. Fibrin-based tissue engineering scaffolds enhance neural fiber sprouting and delay the accumulation of reactive astrocytes at the lesion in a subacute model of spinal cord injury. J. Biomed. Mater. Res. 2010;92:152–163. doi: 10.1002/jbm.a.32343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Teixeira L.S., Feijen J., van Blitterswijk C.A., Dijkstra P.J., Karperien M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials. 2012;33:1281–1290. doi: 10.1016/j.biomaterials.2011.10.067. [DOI] [PubMed] [Google Scholar]
- 155.Kuo K.C., Lin R.Z., Tien H.W., Wu P.Y., Li Y.C., Melero-Martin J.M., et al. Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix. Acta Biomater. 2015;27:151–166. doi: 10.1016/j.actbio.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Park K.M., Lee Y., Son J.Y., Bae J.W., Park K.D. In situ SVVYGLR peptide conjugation into injectable gelatin-poly(ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for enhancement of endothelial cell activity and neo-vascularization. Bioconjugate Chem. 2012;23:2042–2050. doi: 10.1021/bc300110b. [DOI] [PubMed] [Google Scholar]
- 157.Sharp K.G., Dickson A.R., Marchenko S.A., Yee K.M., Emery P.N., Laidmåe I., et al. Salmon fibrin treatment of spinal cord injury promotes functional recovery and density of serotonergic innervation. Exp. Neurol. 2012;235:345–356. doi: 10.1016/j.expneurol.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gohil S.V., Brittain S.B., Kan H.M., Drissi H., Rowe D.W., Nair L.S. Evaluation of enzymatically crosslinked injectable glycol chitosan hydrogel. J. Mater. Chem. B. 2015;3:5511–5522. doi: 10.1039/c5tb00663e. [DOI] [PubMed] [Google Scholar]
- 159.Furtmüller P.G., Zederbauer M., Jantschko W., Helm J., Bogner M., Jakopitsch C., et al. Active site structure and catalytic mechanisms of human peroxidases. Arch. Biochem. Biophys. 2006;445:199–213. doi: 10.1016/j.abb.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 160.Sepantafar M., Maheronnaghsh R., Mohammadi H., Rajabi-Zeleti S., Annabi N., Aghdami N., et al. Stem cells and injectable hydrogels: synergistic therapeutics in myocardial repair. Biotechnol. Adv. 2016;34:362–379. doi: 10.1016/j.biotechadv.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 161.Radhakrishnan J., Krishnan U.M., Sethuraman S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol. Adv. 2014;32:449–461. doi: 10.1016/j.biotechadv.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 162.Rodell C.B., MacArthur J.W., Dorsey S.M., Wade R.J., Wang L.L., Woo Y.J., et al. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater. 2015;25:636–644. doi: 10.1002/adfm.201403550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jia Y., Li J. Molecular assembly of Schiff Base interactions: construction and application. Chem. Rev. 2015;115:1597–1621. doi: 10.1021/cr400559g. [DOI] [PubMed] [Google Scholar]
- 164.Tan H., Chu C.R., Payne K.A., Marra K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma Y.-H., Yang J., Li B., Jiang Y.-W., Lu X., Chen Z. Biodegradable and injectable polymer–liposome hydrogel: a promising cell carrier. Polym. Chem. 2016;7:2037–2044. [Google Scholar]
- 166.Cheng Y., Nada A.A., Valmikinathan C.M., Lee P., Liang D., Yu X., et al. In situ gelling polysaccharide-based hydrogel for cell and drug delivery in tissue engineering. J. Appl. Polym. Sci. 2014;131 [Google Scholar]
- 167.Yang T., Long H., Malkoch M., Kristofer Gamstedt E., Berglund L., Hult A. Characterization of well-defined poly (ethylene glycol) hydrogels prepared by thiol-ene chemistry. J. Polym. Sci. Polym. Chem. 2011;49:4044–4054. [Google Scholar]
- 168.Dong Y., Saeed A.O., Hassan W., Keigher C., Zheng Y., Tai H., et al. One-step" preparation of thiol-ene clickable PEG-based thermoresponsive hyperbranched copolymer for in situ crosslinking hybrid hydrogel. Macromol. Rapid Commun. 2012;33:120–126. doi: 10.1002/marc.201100534. [DOI] [PubMed] [Google Scholar]
- 169.Alge D.L., Azagarsamy M.A., Donohue D.F., Anseth K.S. Synthetically tractable click hydrogels for three-dimensional cell culture formed using tetrazine-norbornene chemistry. Biomacromolecules. 2013;14:949–953. doi: 10.1021/bm4000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kaga S., Gevrek T.N., Sanyal A., Sanyal R. Synthesis and functionalization of dendron-polymer conjugate based hydrogels via sequential thiol-ene “click” reactions. J. Polym. Sci. Polym. Chem. 2016;54:926–934. [Google Scholar]
- 171.Cho I.S., Cho M.O., Li Z., Nurunnabi M., Park S.Y., Kang S.W., et al. Synthesis and characterization of a new photo-crosslinkable glycol chitosan thermogel for biomedical applications. Carbohydr. Polym. 2016;144:59–67. doi: 10.1016/j.carbpol.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 172.Kim H.D., Heo J., Hwang Y., Kwak S.Y., Park O.K., Kim H., et al. Extracellular-matrix-based and Arg-Gly-Asp-modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Eng Part A. 2015;21:757–766. doi: 10.1089/ten.tea.2014.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li C., Zhang W., Wang R., Du X.-F., Jiang D., Liu B., et al. Nanocomposite multifunctional hydrogel for suppressing osteosarcoma recurrence and enhancing bone regeneration. J. Chem. Eng. 2022;435 [Google Scholar]
- 174.Qian Y., Zheng Y., Jin J., Wu X., Xu K., Dai M., et al. Immunoregulation in diabetic wound repair with a photoenhanced glycyrrhizic acid hydrogel scaffold. Adv. Mater. 2022;34 doi: 10.1002/adma.202200521. [DOI] [PubMed] [Google Scholar]
- 175.Ifkovits J.L., Burdick J.A. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 176.Jeon O., Bouhadir K.H., Mansour J.M., Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30:2724–2734. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 177.Azagarsamy M.A., Anseth K.S. Bioorthogonal click chemistry: an indispensable tool to create multifaceted cell culture scaffolds. ACS Macro Lett. 2013;2:5–9. doi: 10.1021/mz300585q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tibbitt M.W., Kloxin A.M., Sawicki L., Anseth K.S. Mechanical properties and degradation of chain and step polymerized photodegradable hydrogels. Macromolecules. 2013;46:2785–2792. doi: 10.1021/ma302522x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Drury J.L., Mooney D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 180.Seidlits S.K., Liang J., Bierman R.D., Sohrabi A., Karam J., Holley S.M., et al. Peptide-modified, hyaluronic acid-based hydrogels as a 3D culture platform for neural stem/progenitor cell engineering. J. Biomed. Mater. Res. 2019;107:704–718. doi: 10.1002/jbm.a.36603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ahuja C.S., Nori S., Tetreault L., Wilson J., Kwon B., Harrop J., et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9–s22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 182.Spearman B.S., Agrawal N.K., Rubiano A., Simmons C.S. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. 2020;108:279–291. doi: 10.1002/jbm.a.36814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stewart D.C., Rubiano A., Dyson K., Simmons C.S. Mechanical characterization of human brain tumors from patients and comparison to potential surgical phantoms. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Potjewyd G., Moxon S., Wang T., Domingos M., Hooper N.M. Tissue engineering 3D neurovascular units: a biomaterials and bioprinting perspective. Trends Biotechnol. 2018;36:457–472. doi: 10.1016/j.tibtech.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 185.Maclean F.L., Ims G.M., Horne M.K., Williams R.J., Nisbet D.R. A programmed anti-inflammatory nanoscaffold (PAIN) as a 3D tool to understand the brain injury response. Adv. Mater. 2018;30 doi: 10.1002/adma.201805209. [DOI] [PubMed] [Google Scholar]
- 186.Martin B.C., Minner E.J., Wiseman S.L., Klank R.L., Gilbert R.J. Agarose and methylcellulose hydrogel blends for nerve regeneration applications. J. Neural. Eng. 2008;5:221–231. doi: 10.1088/1741-2560/5/2/013. [DOI] [PubMed] [Google Scholar]
- 187.Comolli N., Neuhuber B., Fischer I., Lowman A. In vitro analysis of PNIPAAm-PEG, a novel, injectable scaffold for spinal cord repair. Acta Biomater. 2009;5:1046–1055. doi: 10.1016/j.actbio.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zimmermann D.R., Dours-Zimmermann M.T. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem. Cell Biol. 2008;130:635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- 189.Liu X., Wu C., Zhang Y., Chen S., Ding J., Chen Z., et al. Hyaluronan-based hydrogel integrating exosomes for traumatic brain injury repair by promoting angiogenesis and neurogenesis. Carbohydr. Polym. 2023;306 doi: 10.1016/j.carbpol.2023.120578. [DOI] [PubMed] [Google Scholar]
- 190.Hu Y., Jia Y., Wang S., Ma Y., Huang G., Ding T., et al. An ECM-Mimicking, Injectable, Viscoelastic Hydrogel for Treatment of Brain Lesions. 2023;12 doi: 10.1002/adhm.202201594. [DOI] [PubMed] [Google Scholar]
- 191.Perkucin I., Lau K.S.K., Chen T., Iwasa S.N., Naguib H.E. Facile Fabrication of Injectable Alginate and Poly(3,4-ethylenedioxythiophene)-Based Soft Electrodes toward the Goal of Neuro-Regenerative Applications. Adv. Healthc. Mater. 2022;11 doi: 10.1002/adhm.202201164. [DOI] [PubMed] [Google Scholar]
- 192.Li Y., Wang M., Sun M., Wang X., Pei D., Lei B., et al. Engineering antioxidant poly (citrate-gallic acid)-exosome hybrid hydrogel with microglia immunoregulation for traumatic Brain Injury-post neuro-restoration. Compos. B Eng. 2022;242 [Google Scholar]
- 193.Pei Y., Huang L., Wang T., Yao Q., Sun Y., Zhang Y., et al. Bone marrow mesenchymal stem cells loaded into hydrogel/nanofiber composite scaffolds ameliorate ischemic brain injury. Mater. Today Adv. 2023;17 [Google Scholar]
- 194.Ohno Y., Nakajima C., Ajioka I., Muraoka T., Yaguchi A., Fujioka T., et al. Amphiphilic peptide-tagged N-cadherin forms radial glial-like fibers that enhance neuronal migration in injured brain and promote sensorimotor recovery. Biomaterials. 2023;294 doi: 10.1016/j.biomaterials.2023.122003. [DOI] [PubMed] [Google Scholar]
- 195.Wang Y., Zoneff E.R., Thomas J.W., Hong N., Tan L.L., McGillivray D.J., et al. Hydrogel oxygen reservoirs increase functional integration of neural stem cell grafts by meeting metabolic demands. Nat. Commun. 2023;14:457. doi: 10.1038/s41467-023-36133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang Q., He Y., Zhao Y., Xie H., Lin Q., He Z., et al. A thermosensitive heparin-poloxamer hydrogel bridges aFGF to treat spinal cord injury. ACS Appl. Mater. Interfaces. 2017;9:6725–6745. doi: 10.1021/acsami.6b13155. [DOI] [PubMed] [Google Scholar]
- 197.Somaa F.A., Wang T.Y., Niclis J.C., Bruggeman K.F., Kauhausen J.A., Guo H., et al. Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Rep. 2017;20:1964–1977. doi: 10.1016/j.celrep.2017.07.069. [DOI] [PubMed] [Google Scholar]
- 198.Cheng K.-C., Huang C.-F., Wei Y., Hsu S-h. Novel chitosan–cellulose nanofiber self-healing hydrogels to correlate self-healing properties of hydrogels with neural regeneration effects. NPG Asia Mater. 2019;11:1–17. [Google Scholar]
- 199.Liu Y., Hsu Y.H., Huang A.P., Hsu S.H. Semi-interpenetrating polymer network of hyaluronan and chitosan self-healing hydrogels for central nervous system repair. ACS Appl. Mater. Interfaces. 2020;12:40108–40120. doi: 10.1021/acsami.0c11433. [DOI] [PubMed] [Google Scholar]
- 200.Cheng T.Y., Chen M.H., Chang W.H., Huang M.Y., Wang T.W. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials. 2013;34:2005–2016. doi: 10.1016/j.biomaterials.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 201.Nourbakhsh M., Zarrintaj P., Jafari S.H., Hosseini S.M., Aliakbari S., Pourbadie H.G., et al. Fabricating an electroactive injectable hydrogel based on pluronic-chitosan/aniline-pentamer containing angiogenic factor for functional repair of the hippocampus ischemia rat model. Mater Sci Eng C Mater Biol Appl. 2020;117 doi: 10.1016/j.msec.2020.111328. [DOI] [PubMed] [Google Scholar]
- 202.Zheng Y., Wu G., Chen L., Zhang Y., Luo Y., Zheng Y., et al. Neuro-regenerative imidazole-functionalized GelMA hydrogel loaded with hAMSC and SDF-1α promote stem cell differentiation and repair focal brain injury. Bioact. Mater. 2021;6:627–637. doi: 10.1016/j.bioactmat.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Han Z., Huang X., Qian F., Jia J., Zhang D., Zhang L., et al. 2020. An Injectable Refrigerated Hydrogel for Inducing Local Hypothermia and Neuroprotection against Traumatic Brain Injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang L., Zhang D., Ren Y., Guo S., Li J., Ma S., et al. Injectable hyaluronic acid hydrogel loaded with BMSC and NGF for traumatic brain injury treatment. Materials Today Bio. 2022;13 doi: 10.1016/j.mtbio.2021.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tuladhar A., Obermeyer J.M., Payne S.L., Siu R.C., Zand S., Morshead C.M., et al. Injectable hydrogel enables local and sustained co-delivery to the brain: two clinically approved biomolecules, cyclosporine and erythropoietin, accelerate functional recovery in rat model of stroke. Biomaterials. 2020;235 doi: 10.1016/j.biomaterials.2020.119794. [DOI] [PubMed] [Google Scholar]
- 206.Pertici V., Pin-Barre C., Rivera C., Pellegrino C., Laurin J., Gigmes D., et al. Degradable and injectable hydrogel for drug delivery in soft tissues. Biomacromolecules. 2018;20:149–163. doi: 10.1021/acs.biomac.8b01242. [DOI] [PubMed] [Google Scholar]
- 207.Pradhan K., Das G., Khan J., Gupta V., Barman S., Adak A., et al. Neuro-Regenerative choline-functionalized injectable graphene oxide hydrogel repairs focal brain injury. ACS Chem. Neurosci. 2018;10:1535–1543. doi: 10.1021/acschemneuro.8b00514. [DOI] [PubMed] [Google Scholar]
- 208.Li J., Zhang D., Guo S., Zhao C., Wang L., Ma S., et al. Dual-enzymatically cross-linked gelatin hydrogel promotes neural differentiation and neurotrophin secretion of bone marrow-derived mesenchymal stem cells for treatment of moderate traumatic brain injury. Int. J. Biol. Macromol. 2021;187:200–213. doi: 10.1016/j.ijbiomac.2021.07.111. [DOI] [PubMed] [Google Scholar]
- 209.Lim T.C., Mandeville E., Weng D., Wang L.-S., Kurisawa M., Leite-Morris K., et al. Hydrogel-based therapy for brain repair after intracerebral hemorrhage. Translational Stroke Res. 2020;11:412–417. doi: 10.1007/s12975-019-00721-y. [DOI] [PubMed] [Google Scholar]
- 210.Qian F., Han Y., Han Z., Zhang D., Zhang L., Zhao G., et al. In Situ implantable, post-trauma microenvironment-responsive, ROS Depletion Hydrogels for the treatment of Traumatic brain injury. Biomaterials. 2021;270 doi: 10.1016/j.biomaterials.2021.120675. [DOI] [PubMed] [Google Scholar]
- 211.Garrett M.C., O'Shea T.M., Wollenberg A.L., Bernstein A.M., Hung D., Staarman B., et al. Injectable diblock copolypeptide hydrogel provides platform to deliver effective concentrations of paclitaxel to an intracranial xenograft model of glioblastoma. PLoS One. 2020;15 doi: 10.1371/journal.pone.0219632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xu C., Kopeček J. Self-assembling hydrogels. Polym. Bull. 2007;58:53–63. [Google Scholar]
- 213.Zhang D., Ren Y., He Y., Chang R., Guo S., Ma S., et al. In situ forming and biocompatible hyaluronic acid hydrogel with reactive oxygen species-scavenging activity to improve traumatic brain injury repair by suppressing oxidative stress and neuroinflammation. Mater Today Bio. 2022;15 doi: 10.1016/j.mtbio.2022.100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang J., Li S., Yang Z., Liu C., Chen X., Zhang Y., et al. Implantation of injectable SF hydrogel with sustained hydrogen sulfide delivery reduces neuronal pyroptosis and enhances functional recovery after severe intracerebral hemorrhage. Biomater Adv. 2022;135 doi: 10.1016/j.bioadv.2022.212743. [DOI] [PubMed] [Google Scholar]
- 215.Massensini A.R., Ghuman H., Saldin L.T., Medberry C.J., Keane T.J., Nicholls F.J., et al. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater. 2015;27:116–130. doi: 10.1016/j.actbio.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Xu J., Tai C.-H., Chen T.-Y., Hsu S-h. An anti-inflammatory electroconductive hydrogel with self-healing property for the treatment of Parkinson's disease. Chem. Eng. J. 2022 [Google Scholar]
- 217.Bhuiyan M.H., Clarkson A.N., Ali M.A. Optimization of thermoresponsive chitosan/β-glycerophosphate hydrogels for injectable neural tissue engineering application. Colloids Surf. B Biointerfaces. 2023;224 doi: 10.1016/j.colsurfb.2023.113193. [DOI] [PubMed] [Google Scholar]
- 218.Chen S., Liu S., Zhang L., Han Q., Liu H., Shen J., et al. Construction of injectable silk fibroin/polydopamine hydrogel for treatment of spinal cord injury. Chem. Eng. J. 2020;399 [Google Scholar]
- 219.Marquardt L.M., Doulames V.M., Wang A.T., Dubbin K., Suhar R.A., Kratochvil M.J., et al. Designer, injectable gels to prevent transplanted Schwann cell loss during spinal cord injury therapy. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aaz1039. eaaz1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cornelison R.C., Gonzalez-Rothi E.J., Porvasnik S.L., Wellman S.M., Park J.H., Fuller D.D., et al. Injectable hydrogels of optimized acellular nerve for injection in the injured spinal cord. Biomed. Mater. 2018;13 doi: 10.1088/1748-605X/aaab82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang K., Li J., Jin J., Dong J., Li L., Xue B., et al. Injectable, anti-inflammatory and conductive hydrogels based on graphene oxide and diacerein-terminated four-armed polyethylene glycol for spinal cord injury repair. Mater. Des. 2020;196 [Google Scholar]
- 222.Wang Q., He Y., Zhao Y., Xie H. A thermosensitive heparin-poloxamer hydrogel bridges. aFGF to Treat Spinal Cord Injury. 2017;9:6725–6745. doi: 10.1021/acsami.6b13155. [DOI] [PubMed] [Google Scholar]
- 223.Hong L.T.A., Kim Y.-M., Park H.H., Hwang D.H., Cui Y., Lee E.M., et al. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017;8:1–14. doi: 10.1038/s41467-017-00583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nazemi Z., Nourbakhsh M.S., Kiani S., Heydari Y., Ashtiani M.K., Daemi H., et al. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J. Contr. Release. 2020;321:145–158. doi: 10.1016/j.jconrel.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 225.Wang C., Yue H., Feng Q., Xu B., Bian L., Shi P. Injectable nanoreinforced shape-memory hydrogel system for regenerating spinal cord tissue from traumatic injury. ACS Appl. Mater. Interfaces. 2018;10:29299–29307. doi: 10.1021/acsami.8b08929. [DOI] [PubMed] [Google Scholar]
- 226.Han G.H., Kim S.J., Ko W.K., Lee D., Lee J.S., Nah H., et al. Injectable Hydrogel Containing Tauroursodeoxycholic Acid for Anti-neuroinflammatory Therapy After Spinal Cord Injury in Rats. Mol. Neurobiol. 2020;57:4007–4017. doi: 10.1007/s12035-020-02010-4. [DOI] [PubMed] [Google Scholar]
- 227.Zaviskova K., Tukmachev D., Dubisova J., Vackova I., Hejcl A., Bystronova J., et al. Injectable hydroxyphenyl derivative of hyaluronic acid hydrogel modified with RGD as scaffold for spinal cord injury repair. J. Biomed. Mater. Res. 2018;106:1129–1140. doi: 10.1002/jbm.a.36311. [DOI] [PubMed] [Google Scholar]
- 228.Matthews J., Surey S., Grover L.M., Logan A., Ahmed Z. Thermosensitive collagen/fibrinogen gels loaded with decorin suppress lesion site cavitation and promote functional recovery after spinal cord injury. Sci. Rep. 2021;11:1–20. doi: 10.1038/s41598-021-97604-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cheng J., Chen Z., Liu C., Zhong M., Wang S., Sun Y., et al. Bone mesenchymal stem cell-derived exosome-loaded injectable hydrogel for minimally invasive treatment of spinal cord injury. Nanomedicine. 2021;16:1567–1579. doi: 10.2217/nnm-2021-0025. [DOI] [PubMed] [Google Scholar]
- 230.Tran K.A., Jin Y., Bouyer J., DeOre B.J., Suprewicz Ł., Figel A., et al. Magnetic alignment of injectable hydrogel scaffolds for spinal cord injury repair. Biomater. Sci. 2022;10:2237–2247. doi: 10.1039/d1bm01590g. [DOI] [PubMed] [Google Scholar]
- 231.Yu Q., Jin S., Wang S., Xiao H., Zhao Y. Injectable, adhesive, self-healing and conductive hydrogels based on MXene nanosheets for spinal cord injury repair. Chem. Eng. J. 2023;452 [Google Scholar]
- 232.Li S., Ke Z., Peng X., Fan P., Chao J., Wu P., et al. Injectable and fast gelling hyaluronate hydrogels with rapid self-healing ability for spinal cord injury repair. Carbohydr. Polym. 2022;298 doi: 10.1016/j.carbpol.2022.120081. [DOI] [PubMed] [Google Scholar]
- 233.Luo Y., Fan L., Liu C., Wen H., Wang S., Guan P., et al. An injectable, self-healing, electroconductive extracellular matrix-based hydrogel for enhancing tissue repair after traumatic spinal cord injury. Bioact. Mater. 2022;7:98–111. doi: 10.1016/j.bioactmat.2021.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang L., Yao K., Wei J., Li G., Lin Y., Zhou Y.L., et al. Convenient in situ synthesis of injectable lysine-contained peptide functionalized hydrogels for spinal cord regeneration. Appl. Mater. Today. 2022;27 [Google Scholar]
- 235.Zhang Z., Zhang X., Wang C., Teng W., Xing H., Wang F., et al. Enhancement of motor functional recovery using immunomodulatory extracellular vesicles-loaded injectable thermosensitive hydrogel post spinal cord injury. Chem. Eng. J. 2022;433 [Google Scholar]
- 236.Qi Z., Zhang T., Kong W., Fu C., Chang Y., Li H., et al. A dual-drug enhanced injectable hydrogel incorporated with neural stem cells for combination therapy in spinal cord injury. Chem. Eng. J. 2022;427 [Google Scholar]
- 237.Carriel V., Alaminos M., Garzón I., Campos A., Cornelissen M. Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 2014;14:301–318. doi: 10.1586/14737175.2014.887444. [DOI] [PubMed] [Google Scholar]
- 238.Patel V., Joseph G., Patel A., Patel S., Bustin D., Mawson D., et al. Suspension matrices for improved Schwann-cell survival after implantation into the injured rat spinal cord. J. Neurotrauma. 2010;27:789–801. doi: 10.1089/neu.2008.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hlavac N., Bousalis D., Pallack E., Li Y., Manousiouthakis E., Ahmad R.N., et al. Injectable neural hydrogel as in vivo therapeutic delivery vehicle. Regen. Eng. Transl. Med. 2023:1–7. doi: 10.1007/s40883-022-00292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Severs L.J., Cates L.N., Dewees D.M., Hoagland R.T., Horner P.J., Hofstetter C.H., et al. 2019. Biomimetic injectable 3D hydrogels with aligned topography for neural tissue engineering; pp. 1–35. 1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.