Abstract
The new coronavirus infection (COVID-19) pandemic caused by SARS-CoV-2 has many times surpassed the epidemics caused by SARS-CoV and MERS-CoV. The reason for this was the presence of sites in the protein sequence of SARS-CoV-2 that provide interaction with a broader range of receptor proteins on the host cell surface. In this review, we consider both already known receptors common to SARS-CoV and SARS-CoV-2 and new receptors specific to SARS-CoV-2.
Keywords: SARS-CoV-2, coronavirus, receptors, furin, cathepsin L, ACE2, neuropilin, CD147, CD209, heparan sulfate, GRP78, ASGR1, KREMEN1, tyrosine protein kinase AXL
INTRODUCTION
Since the beginning of the 21st century, three coronaviruses have crossed the interspecies barrier and caused deadly pneumonia in humans: severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The first two of these viruses caused localized epidemics, while the emergence of SARS-CoV-2 led to a pandemic that has lasted for about three years. According to the World Health Organization (https://covid19.who.int), more than 570 million cases of coronavirus infection had been confirmed worldwide by early August 2022. About 6.4 million people have died. The mortality rate from COVID-19 has dropped significantly since mass vaccination began, but the presence of post-COVID complications, the so-called post-COVID syndrome, remains a serious problem. The emergence of more and more new variants of SARS-CoV-2 continues to cause great concern and attention worldwide. Accumulation of mutations in the virus genome is caused by continuous virus replication in the human body against the background of untimely virion elimination associated mostly with delayed clinical treatment and lack of specific and effective drugs. These mutations can lead to changes in epitopes and to a decrease in the affinity of the corresponding antibodies generated by vaccines. Therefore, it is important to develop universal, specific, and effective drugs against current and possible future variants of the coronavirus.
A way to protect against coronavirus infection can be to inhibit the transport of viral particles into cells. At the first stage of infection, the spike protein (S protein) of coronavirus binds to receptors on the target cell membrane, S protein splits into S1 and S2 fragments, and the viral membrane fuses with the cell plasma membrane or the viral particles are endocytosed [2–4]. SARS-CoV-2 mRNA then gets inside the cell. The canonical receptor for SARS-CoV-2 is angiotensin-converting enzyme 2 (ACE2) paired with the membrane protease TRMPSS2 [2]. The mechanism of cell invasion by ACE2 and TRMPSS2 was previously discovered for the SARS-CoV virus. An analysis of the pathogenesis of the new coronavirus infection revealed a paradoxical situation: SARS-CoV-2 lesions of cells and organs in many cases did not correlate with ACE2 expression in them [5]. This stimulated the search for SARS-CoV-2-specific receptor and accessory proteins. Studies over the past three years have confirmed the involvement of SARS-CoV target proteins in SARS-CoV-2 cell infection, but also discovered new receptors that account for the higher prevalence of COVID-19 (Fig. 1). This review briefly characterizes the targets of SARS-CoV coronavirus on the cell surface and the membrane proteases that activate the S protein. Their role in infecting different tissues and cells of the body is briefly discussed.
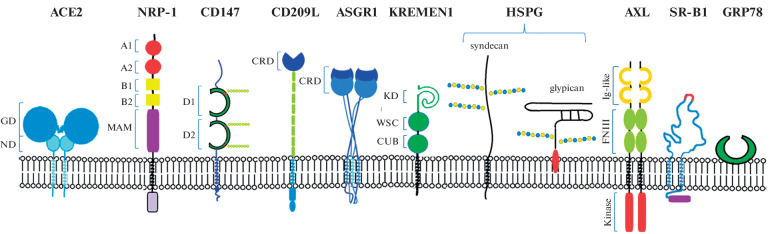
Fig. 1.
Receptors and co-receptors involved in cell infection with the SARS-CoV-2 coronavirus: angiotensin-converting enzyme 2 (ACE2), neuropilin-1 (NRP-1), CD147 or basigin, CD209L or L-SIGN, asialoglycoprotein receptor 1 (ASGR1), KREMEN1 (kringle containing transmembrane protein 1) protein, heparan sulfate proteoglycans (HSPG) syndecan and glypican, receptor tyrosine kinase AXL, high-density lipoprotein type 1 scavenger receptor (SR-B1), GRP78 (glucose-regulated protein 78). Receptor protein domains shown are GD (globular domain), ND (neck domain), MAM (meprin, A-5 protein, receptor protein-tyrosine phosphatase µ), immunoglobulin domains (D1 and D2), CRD (carbohydrate recognition domain), KD (kringle domain), WSC (cell wall integrity and stress response component), CUB (complement C1r/C1s, Uegf, Bmp1), Ig-like (immunoglobulin-like domain), FNIII (fibronectin type III domain), kinase (tyrosine kinase), glycosyl residues on molecule CD147 and heparan sulfates of syndecans and glypicans.
PRIMING OF CORONAVIRUS SPIKE PROTEIN AND ENTRY OF SARS-CoV-2 INTO CELLS
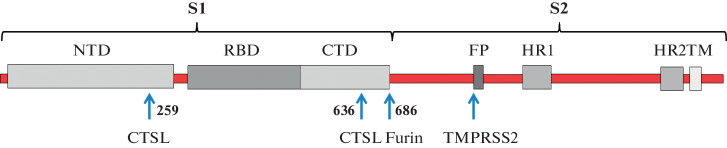
S protein belongs to the class I viral fusion proteins, which are characterized by a trimeric structure [6, 7]. The monomer mass is 180–200 kDa, and a large number of polysaccharides are attached to the protein [8]. The structure of S protein monomer is shown in Fig. 2. The peptide chain of SARS-CoV-2 is formed by 1273 amino acid residues (a.a.) and includes a signal peptide (a.a. 1–13) located at the N-end and fragments S1 (14–685 a.a.) and S2 (686–1273 a.a.). The S1 sequence has an N-terminal domain (14–305 a.a.) and a receptor binding domain (RBD, 319–541 a.a.) that interacts directly with ACE2. RBD can rotate as if on a hinge, moving from down to an up conformation and back, hiding or exposing the receptor binding motif (RBM), which binds to the receptor protein ACE2 [9]. On the surface of SARS-CoV-2 virion, the RBD of S protein spontaneously acquires any of the two conformations, and their ratio may vary [10]. Further, after the RBD domain, there is the C- terminal domain and a site connecting S1 and S2. The S2 fragment consists of the fusion peptide (FP) (788–806 a.a.), heptapeptide repeats HR1 (912–984 a.a.) and HR2 (1163–1213 a.a.), the transmembrane TM domain, and the cytoplasmic domain (1237–1273 a.a.) [8].
Fig. 2.
Structure of the S protein of the SARS-CoV-2 coronavirus. NTD (N-terminal domain), RBD (receptor binding domain), CTD (C-terminal domain), FP (fusion peptide), TM (transmembrane domain), HR1 and HR2 (heptapeptide repeats 1 and 2), CTPL (cathepsin L), TMPRSS2 (transmembrane protease, serine 2). Arrows indicate the sites of S protein proteolysis.
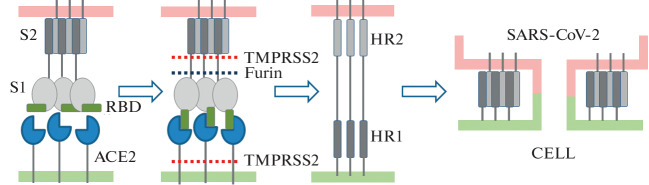
For the contents of the SARS-CoV-2 virus particle to enter the cell cytoplasm, activation of the S protein, the so-called priming, is required. Proteolytic cleavage results in the formation of subunits S1 and S2 [11]. The S1 subunit is responsible for binding to ACE2, while the S2 subunit is responsible for fusion with the cell membrane. After priming, the RBD domain acquires an open conformation (“up” position), at which it is able to bind the receptor [3]. The spatial limiting effect of S1 on S2 is also eliminated. The available data suggest that the priming process proceeds as follows (Fig. 3): the membrane preprotein convertase furin cleaves protein S into S1- and S2-fragments to the right of the site containing basic amino acids R-X-(K/R)-R↓ (proteolysis site Q677TNSPRAR-SV687, designated S1/S2). Furin is expressed ubiquitously in the cells of the body. The presence of a site accessible for proteolytic cleavage by furin is considered a major virulence factor of highly pathogenic strains of viruses such as HIV, respiratory syncytial virus, and measles virus. SARS-CoV-2 has this site, but it is absent in SARS-CoV [12]. This site appeared as a result of the insertion of a PRRA fragment into the polypeptide chain of the coronavirus [11]. S protein is further cleaved by TMPRSS2 protease at the S2' site (DPSKPSKR↓SFIED) at the N-terminus of the S2 subunit after furin acts on the S protein [11, 13]. TMPRSS2 cleaves S proteins of both SARS-CoV and SARS-CoV-2. TMPRSS2 has been shown to colocalize with ACE2 [14]. Interestingly, TMPRSS2 also cleaves the peptide chain of ACE2 and thereby leads to the separation of ACE2 from the membrane.
Fig. 3.
Priming of S protein of SARS-CoV-2 coronavirus and fusion of viral and cell membranes. HR1 and HR2 are heptapeptide repeats 1 and 2.
As a result of proteolysis, the N-terminal hydrophobic peptide FP (fusion peptide) of the S2 subunit unmasks at the S2' site. The FP attaches to the membrane of the infected cell, then the alpha-helical sites of HR1 and HR2 gluing occurs, the S2 subunit contracts and thus pulls the viral particle to the cell surface; the plasma membrane of the cell fuses with the membrane of the virus, contact is formed between the inner contents of the viral particle and the cytoplasm, and the RNA of the virus enters the cell (Fig. 3). Due to the trimeric structure, three S2 subunits are involved simultaneously in “pulling” the virus into the cell. A bundle of six alpha-helices is formed. The attachment of the virus to the cell probably involves not one but several S protein molecules of different coronavirus spikes.
S protein of SARS-CoV-2 can be cleaved into S1 and S2 subunits by the protease cathepsin L [15]. Zhao et al. [16] identified conserved sites for proteolysis of S protein by cathepsin L at positions 259 and 636 (Fig. 2) present in all variants of SARS-CoV-2 (including Omicron variant) and showed that proteolysis at these sites promotes transition of RBD to the activated conformation (up position), at which it attaches to ACE2 and infects cells. The SARS-CoV-2 pseudoviral construct was used in cell infection experiments. According to [17], priming of S protein by cathepsin L occurs after endocytosis of viral particles in contrast to activation of S protein by TMPRSS2 protease when viral and plasma membrane fusion occurs. SARS-CoV-2 is endocytosed via a clathrin-dependent mechanism [4]. It is extremely important in terms of possible treatment of COVID-19 that simultaneous exposure to the inhibitors of serine and cysteine proteases TMPRSS2 (camostat mesylate or naphamostat mesylate) and to the inhibitors of cathepsin L (hydroxychloroquine), which are on the list of known drugs, can synergistically suppress cell infection by SARS-CoV-2 by blocking both virion transport routes into cells [17]. Using genomic-guided gene set enrichment analysis (GSEA), estradiol and retinoic acid have also been identified as possible pharmacotherapies capable of inhibiting TMPRSS2 and cathepsin L activity and thereby reducing SARS-CoV-2 transport into cells [18]. Similarly, a network analysis of drug repurposing revealed that cyclosporine A, estradiol, and calcitriol can also be considered as potential drugs to inhibit both pathways of cell infection by coronavirus [19].
SARS-CoV-2 particles enter cells in two ways: as a result of endocytosis or fusion of the viral membrane with the cell plasma membrane and the release of viral mRNA into the cytoplasm [20]. The question of the role of each of these virion transport mechanisms has not yet been clearly answered. There is evidence that in case of SARS-CoV, infection of lung cells through membrane fusion is 2–3 orders of magnitude more efficient than through the endocytic pathway due to prior activation of S protein by extracellular proteases trypsin and thermolysin [21]. The transport of SARS-CoV-2 into cells depends on the expression of proteases on the plasma membrane [17].
ANGIOTENSIN CONVERTING ENZYME 2 (ACE2)
The role of ACE2 as a receptor for coronavirus S protein was originally shown for SARS-CoV [22, 23] and later also for SARS-CoV-2 [2, 24, 25]. The RBD domain of S1 subunit binds to the N-terminal peptidase domain of ACE2 [26]. ACE2 was discovered in 2000, its transcripts were found in the heart, kidneys, and testes, and histochemical analysis showed expression in the endothelium of coronary and renal vessels and in the tubular epithelium of the kidneys [27, 28]. According to the human protein atlas, ACE2 is expressed in the highest amounts in the intestine, kidneys, and testes and in lower concentrations, in the lungs and heart [29]. In the vascular system, the highest expression of ACE2 has been detected in the endothelium of lung microvessels and the lowest, in the endothelium of lymphatic vessels [30]. Although SARS-CoV-2 infection mainly manifests as symptoms of respiratory system lesions, sequencing of mRNA of individual cells shows low ACE2 expression in lung and bronchial tissues and, in contrast, high expression levels in cells of kidneys and digestive system [5, 31]. This suggests that in addition to ACE2, there are other SARS-CoV-2 targets in cells through which infection occurs. This view is also supported by data on the presence of neutralizing antibodies that bind to SARS-CoV-2 at a site or sites other than the RBD domain in COVID-19 survivors [32, 33].
NEUROPILIN
The membrane protein neuropilin-1 (NRP1) was found to be involved in coronavirus entry into cells. It functions as a co-receptor (host factor) of ACE2 in SARS-CoV-2 infection [34]. NRP1 is a glycoprotein with a molecular weight of about 130 kDa that performs multiple functions in the nervous system and other organs and tissues. It was discovered as a factor necessary for formation and directed growth of nerve fiber bundles [35]. This is due to the binding to NRP1 of class 3 semaphorins, which are neuronal chemorepellants [36]. In addition, the role of NRP1 in cell adhesion through heterophilic molecular interaction has been established [37]. In endothelial cells, NRP1 acts as a receptor for vascular endothelial growth factor VEGF165, but not VEGF121, and regulates angiogenesis [38]. The extracellular part of NRP1 (Fig. 1) contains domains present in a number of proteins but forming a combination not typical of other membrane receptors [37]. In particular, a1/a2 have common sequences with complement components C1r and C1s, b1/b2-like domains are present in blood clotting factors V and VIII, domain MAM (Meprin, A-5 protein, receptor protein-tyrosine phosphatase µ) is contained in the metalloendopeptidases meprins and receptor protein-tyrosine phosphatase. Semaphorins have been shown to bind to the a1/a2 and b1/b2 domains, VEGF165 binds to b1/b2, and they also ensure cell adhesion [37].
Binding of VEGF165 and semaphorins 3 to the b1 domain is performed via pentabasic sequence Arg/Lys-X-X-Arg/Lys (C-end Rule motif – CendR) present in these proteins at the C-end. The viral particle of SARS-CoV-2 was found to attach to NRP1 at the same site [34]. In the case of SARS-CoV-2, furin cleaves S protein into S1 and S2 proteins, so that the Arg-Arg-Ala-Arg (RRAR) sequence is exposed at the C end of S1 protein to interact with NRP1. Binding to NRP1 facilitates the entry of viral particles into cells. This apparently occurs because of stabilization of the C-end terminus (residues 640–685) of the S1 molecule, which facilitates the release of the N-terminal fragment (686–700 a.a.) of the S2 subunit and subunit separation [39].
NRP1 initiates SARS-CoV-2 infection of olfactory epithelial cells despite the low level of ACE2 expression in these cells [40]. Experiments on rhesus macaques have shown that infection of the central nervous system (CNS) with SARS-CoV-2 involves a positive feedback mechanism: expression of NRP1 increases in the cerebral cortex, enhancing the immune response with release of inflammatory factors and the development of viral encephalitis [41]. In the Omicron variant, mutations that occurred next to the CendR motif in S1 made the binding of S1 to NRP1 more energetically advantageous [42]. This may account for an enhanced infectivity of Omicron. NRP1 is considered as a target for low-molecular weight anticoagulants acting on the CendR binding site of S1 protein [43].
CD147 (BASIGIN)
The transmembrane glycoprotein basigin (basic immunoglobulin; other names are CD147 and EMMPRIN, extracellular matrix metalloproteinase inducer) belongs to the immunoglobulin superfamily. It is represented by two isoforms that differ in the number of immunoglobulin domains in the extracellular part of the polypeptide chain. Basigin-1 has three domains; it is localized in the retina and serves as a receptor for the retinal rod trophic protein (RdCVF) secreted by cones [44]. The more common isoform, basigin-2, which is actually called basigin, or CD147, has two immunoglobulin domains [45]. CD147 acts as a receptor for cyclophilins, the S100A9 protein, and platelet glycoprotein VI. The immunoglobulin domains have glycosylated sites that bind CD147 to lectins such as E‑selectin and galectin [45, 46].
CD147 (basigin) is known as a protein that ensures the entry of malaria plasmodium into erythrocytes [47]. In addition, it acts as a receptor protein when cells are infected with human immunodeficiency virus type 1, hepatitis C and B viruses, Kaposi sarcoma-associated herpes virus, and SARS-CoV virus [48]. When cells become infected with HIV-1, it is due to the formation of a triple complex of the virus with cyclophilin A and CD147 [46].
There are studies indicating the participation of CD147 (basigin) in cellular reception of SARS-CoV-2. In the work of a large team of Chinese researchers [49] it was shown that the antibody against CD147 (basigin) meplazumab suppresses coronavirus amplification in cell lines Vero E6 and BEAS-2B. Viсe versa, expression of CD147 in BHK-21 cells resistant to SARS-CoV-2 promoted entry of the virus into these cells. Meplazumab suppressed infection of Vero E6 cells with the initial Wuhan variant of SARS-CoV-2 and its subsequent variants alpha, beta, gamma, and delta [50]. siRNA induced suppression of CD147 expression in CaLu3 cells (human epithelial cells from HTB-55™ lung adenocarcinoma) 2–3-fold reduced infection, as determined by SARS-CoV-2 mRNA synthesis [48]. Xu et al. [51] used pseudotyped SARS-CoV-2 virus grown in cells with serum-free medium and showed that it can infect A549 cells with low hACE2 levels with participation of CD147. In addition, mice hCD147KI-NSG, in which human CD147 was expressed, were more susceptible to infection with the coronavirus compared with wild-type WT-NSG littermates [52]. CD147 is classified as an oncomarker, which suggests that it may be an additional important target in the treatment of COVID-19 in cancer patients [53].
Despite strong evidence of an important role for CD147 in new coronavirus infection, it remains unclear how viral particles interact with CD147. Thus, the studies of Shilts et al. [54] did not reveal direct binding of recombinant peptide S1 and full trimeric S protein SARS-CoV-2 to CD147. In the experiments described in [55], no effect of polyclonal antibodies against CD147 on infection of Vero E6 cells with coronavirus was found. These researchers also found no direct interaction of the S protein and RBD-domain with CD147. It is possible that additional proteins are involved in the interaction between CD147 and viral particles. Thus, cyclophilin A is involved in the infection of cells with SARS-CoV, as in the case of HIV. Cyclophilin A is expressed in the cytoplasm and is also secreted into the extracellular environment. However, Fenizia et al. [48] showed that in contrast to SARS-CoV, CD147 complex with cyclophilin A is not involved in cell infection with SARS-CoV-2 coronavirus. According to these authors, suppression of CD147 expression by siRNA leads to a decrease in ACE2 levels, and this is considered as one of the possible mechanisms of CD147-mediated effect of SARS-CoV-2. In Vero E6 and Huh-7 cells infected with SARS-CoV-2 pseudo virus, S protein colocalizes with CD147 and Rab5a, suggesting CD147-mediated endocytosis of viral particles [56]. CD147-mediated endocytosis of SARS-CoV-2 occurs via an Arf6-dependent mechanism.
HEPARAN SULFATE
Heparan sulfate (HS) is a linear polysaccharide whose structural unit is sulfated D-glucuronic or L‑iduronic acid coupled to N-acetylglucosamine. HS is attached to the core protein and forms heparan sulfate proteoglycans expressed on the surface of almost all mammalian cells and in the extracellular matrix. There are two types of membrane heparan sulfate proteoglycans, syndicans and glypicans [57]. Syndicans are bound to the membrane by a transmembrane C-end fragment, whereas glypicans are covalently attached to the phospholipid glycosylphosphatidylinositol (Fig. 1). HS acts as an ACE2 coreceptor in the binding of SARS-CoV-2 viral particles to the cell membrane. Clausen et al. [58] showed that HS binds to S protein in the RBD domain located near the ACE2 binding site. According to the molecular docking data, a triple complex is formed, with heparan sulfate promoting the unfolding of the ACE2 binding site. Sulfated polysaccharides competitively inhibit binding of S protein to heparan sulfate. Heparin and enoxaparin (low molecular weight heparin, mol. mass about 4500 Da) have been shown to suppress entry of pseudotyped SARS-CoV-2 virus into HEK293T cells with IC50 of 5.99 and 1.77 μg/L, respectively [59].
CD209L (L-SIGN) and CD209/DC-SIGN
CD209L glycoproteins, also referred to as L-SIGN (Liver/Lymph node-Specific ICAM-3 Grabbing Non-integrin) and CD209/DC-SIGN (Dendritic Cell-Specific Intrcellular Adhesion molecules (ICAM)-3 Grabbing Non-integrin), are C-type lectins. They directly recognize a wide range of microorganisms – HIV-1, Ebola, hepatitis C viruses, Mycobacterium tuberculosis, the parasitic microorganism Leishmania pifanoi, and several others (see review [60]). The role of CD209L and CD209 in cellular infection by coronaviruses was initially established for SARS-CoV [61] and subsequently confirmed for SARS-CoV-2 [62, 63]. CD209L and CD209 act as ACE2 co-receptors in S protein binding. Thepaut et al. [64] showed that lectin CD209L by itself does not induce infection of Vero E6 cells that do not express ACE2 but promotes virus transfer into ACE2+ Vero E6 cells after virus capture. CD209L acts as an ACE2 co-receptor, forming a heterodimer with it [63]. The same authors showed that SARS-CoV-2 can infect human vascular endothelial cells (ECs), with CD209L knockdown or addition of soluble CD209L reducing infection. The contribution of CD209L is particularly pronounced in cells in which the level of ACE2 expression is low and CD209L expression, in contrast, is high. This refers to human liver sinus ECs and lymph node endothelial cells [30, 65]. By confocal microscopy, viral particles were detected in the liver sinus ECs in autopsy material from COVID patients [65]. The liver sinus ECs are the main sources of Willebrand factor (vWF) and clotting factor VIII (FVIII). Infection of these ECs with coronavirus seems to be one of the causes of increased blood clotting and thrombosis during coronavirus infection. In myeloid cells, SARS-CoV-2 enhances proinflammatory responses through CD209 and other C-type lectins—secretion of interleukins 1b and 8, cytokines CCL2/3, and chemokine CXLC10 [66]. The specific antagonists of CD209L, PolyMan26 and other mannoside glycomimetics, inhibit S protein binding and cell infection [67]. There is evidence of involvement of CD209L and other C-type lectins in SARS-CoV-2-induced activation of proinflammatory cytokine and chemokine secretion by myeloid cells [66].
GRP78 (GLUCOSE-REGULATED PROTEIN 78)
GRP78 (Glucose-Regulated Protein 78) is a heat shock protein, a chaperone that in normal cells performs protein refolding in the endoplasmic reticulum or directs unfolded proteins to cellular degradation systems [68]. Endoplasmic reticulum stress results in overexpression and translocation of GRP78 to the cell membrane [69], where it also handles a large number of unfolded proteins. However, GRP78, exposed on the plasma membrane, in addition to its restorative work on protein refolding, promotes the entry of pathogens, such as viruses, bacteria, and fungi, into the cell. Hyperexpression of GRP78 is observed on the membranes of various cancer cells, which increases the aggressiveness of cancer. Molecular docking revealed a putative interaction between GRP78 and the receptor-binding domain (RBD) of S protein of SARS-CoV-2 [70]. GRP78 can form a complex with S protein and ACE2 on the surface and in the perinuclear region characteristic of the endoplasmic reticulum in VeroE6-ACE2 cells; the substrate-binding domain of GRP78 was shown to be crucial for this interaction [71]. To investigate the role of GRP78, the authors of the cited work performed a GRP78 knockdown in VeroE6-ACE2 cells. Loss of GRP78 markedly reduced ACE2 expression on the cell surface. It was concluded that GRP78 is an accessory factor for SARS-CoV-2 entry into cells [71].
RECEPTOR TYROSINE KINASE AXL
According to Wang et al. [5], the receptor tyrosine kinase AXL specifically interacts with the N-terminal domain of the S1 subunit of S protein of the coronavirus SARS-CoV-2. Overexpression of AXL in HEK293T cells promoted SARS-CoV-2 entry as efficiently as overexpression of ACE2. In H1299 lung cells and primary human lung epithelial cells, where ACE2 levels are low and AXL levels are high, knockout of the latter significantly reduced SARS-CoV-2 infection. The soluble human recombinant tyrosine kinase AXL blocked SARS-CoV-2 infection in cells expressing high levels of AXL. The authors of this article showed that AXL expression levels correlated well with the SARS-CoV-2 S protein levels in bronchoalveolar lavage fluid cells from patients with COVID-19. Based on these data, it was concluded that AXL is a novel receptor for SARS-CoV-2, which may play an important role in the spread of coronavirus infection of the human respiratory system. The ligand of AXL is the GAS6 protein, which serves as a bridge connecting the phosphatidylserine of the viral membrane to AXL [72]. A number of viruses, in particular Dengue, West Nile, and Ebola, infect cells in this way. In [5], the authors based on their own data, suggest that in the case of SARS-CoV-2, an alternative mechanism is involved.
ASGR1 and KREMEN1
Two new receptors that specifically bind S protein of SARS-CoV-2 have recently been identified [73]. These proteins are ASGR1 (asialoglycoprotein receptor-1) and KREMEN1 (Kringle Containing Transmembrane Protein 1). To identify receptors, 5054 human membrane proteins (91.6% of the total number of putative membrane proteins) were tested for their ability to bind S protein. Each of these proteins was individually expressed in HEK293E cells with ACE2 knockout, and the binding of the extracellular S protein fragment of SARS-CoV-2, SARS-CoV, and MERS viruses to these cells was determined. This screening identified 12 proteins capable of specifically binding the S protein of SARS-CoV-2 with high affinity (Kdis < 525 nM); among these proteins were the already known receptors ACE2 and CD209L (L-SIGN). The dissociation constants Kdis of the SARS-CoV-2 S protein for ASGR1 and KREMEN1 were 94.8 and 19.3 nM respectively, which is comparable to the Kdis for ACE2 (12.4 nM). The authors showed that KREMEN1 binds to the RBD, NTD, and S2 domains of the extracellular portion of the S protein with the highest affinity for RBD, while ASGR1 binds efficiently to the RBD domain and with lower affinity, to the NTD domain. The S proteins of SARS-CoV and MERS viruses did not bind to ASGR1 and KREMEN1.
It was further investigated, the expression of which proteins among the identified receptors of the extracellular S protein fragment of the coronavirus made cells not expressing ACE2 susceptible to infection with SARS-CoV-2 virus. It appeared that ACE2-independent infection occurs only with ASGR1 and KREMEN1. This result does not contradict previous findings that CD209L (L-SIGN) is involved in coronavirus transport into cells together with ACE2 [64]. It is possible that other S protein binding membrane proteins from the list given in [73], including other C‑type lectin related to CD209L, CD207, also function as coronavirus receptors in conjunction with ACE2. Although ASGR1 and KREMEN1 can function as independent transporters of SARS-CoV-2, their co-expression with ACE2 synergistically increased the ability of SARS-CoV-2 to infect cells [73].
The structures of ASGR1 and KREMEN1 are schematically represented in Fig. 1. There are three domains in the extracellular part of KREMEN1: Krm (Kringle domain), WSC, and CUB [74]. The CUB domain in KREMEN1 plays the main role in S protein binding. In ASGR1 binding occurs to the C-type lectin domain. KREMEN1 in the organism functions as a high-affinity receptor of DKK1, an antagonist of Wnt-signaling pathway [74, 75] and, in addition, is a carrier/transporter of some enteroviruses into cells [76]. ASGR1 is expressed predominantly in hepatocytes and regulates glycoprotein concentration in blood plasma by endocytosis of desialated glycoproteins [77]. ASGR1 is known to act as a receptor for hepatitis C virus [78].
SCAVENGER RECEPTOR CLASS B TYPE 1, HIGH-DENSITY LIPOPROTEIN RECEPTOR
The work of Wei et al. [79] showed that the high-density lipoprotein (HDL) scavenger receptor B type 1 (SR-B1) promotes ACE2-dependent penetration of SARS-CoV-2 into cells. SR-B1 facilitates SARS-CoV-2 entry into cells by enhancing virus attachment to the membrane. The authors show that the S protein is able to bind cholesterol and HDL, which possibly enables coronavirus attachment to SR-B1 receptors.
Another known pathogenetic role of SR-B1 is that it carries hepatitis C and Dengue viruses into hepatocytes. [80]. SR-B1 is mostly expressed in the liver and in steroidogenic cells, where it provides cholesterol entry for the synthesis of bile acids and steroid hormones, respectively [81].
NONMUSCLE MYOSIN HEAVY CHAIN IIA
Nonmuscle myosin heavy chain IIA (NTG-IIA) has been identified as another protein contributing to the infection of human lung cells by SARS-CoV-2 coronavirus [82]. The NTG domain of the S1 subunit and the S2 subunit have been shown to interact directly with the C-end domain of the heavy chain. Genetic deletion of NTCM IIA significantly reduced SARS-CoV-2 pseudovirus infection in wild-type (WT) A549 and Calu-3 cells, while overexpression of NTCM IIA enhanced it.
CONCLUSIONS
Intensive studies of SARS-CoV-2 coronavirus has shown that its mechanisms of action on human cells are more diverse and complex than originally imagined. Data have been obtained on the presence in cells of additional receptors for the S-protein in addition to ACE2, the concept of proteolytic priming of the coronavirus has been expanded, and new information has appeared on its effect on cell signaling systems. The study of these issues is of crucial importance since the course of new coronavirus infection in people of different ages, particularly in adults and children, and in people with different comorbidities exhibits essential features which may be due to the nature of expression and activity of certain SARS-CoV-2 coronavirus receptors and accessory proteins involved in cell infection. The study of molecular mechanisms of virus–cell interactions should also contribute to understanding why the existing and new emerging strains of coronavirus may differ in cell and organ specificity and in the severity of their impact on the human body.
FUNDING
The work was supported by the Russian Science Foundation (project no. 21-15-00441).
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflict of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Footnotes
Translated by A. Dunina-Barkovskaya
REFERENCES
- 1.Peeri N.C., Shrestha N., Rahman M.S., Zaki R., Tan Z., Bibi S., Baghbanzadeh M., Aghamohammadi N., Zhang W., Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: What lessons have we learned? Int. J. Epidemiol. 2020;49:717–726. doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell.181, 271–280 e278. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed]
- 3.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2020;117:11 727–11 734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayati A., Kumar R., Francis V., McPherson P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021;296:100306. doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S., Qiu Z., Hou Y., Deng X., Xu W., Zheng T., Wu P., Xie S., Bian W., Zhang C., Sun Z., Liu K., Shan C., Lin A., Jiang S., Xie Y., Zhou Q., Lu L., Huang J., Li X. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021;31:126–140. doi: 10.1038/s41422-020-00460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: Structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/jvi.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schibli D.J., Weissenhorn W. Class I and class II viral fusion protein structures reveal similar principles in membrane fusion. Mol. Membr. Biol. 2004;21:361–371. doi: 10.1080/09687860400017784. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y., Yang C., Xu X.F., Xu W., Liu S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Y., Zhang J., Xiao T., Peng H., Sterling S.M., Walsh R.M., Rawson S., Rits-Volloch S., Chen B. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020;369:1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. 2020. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell.181, 281–292 e286. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed]
- 12.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral. Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q., Xiang R., Huo S., Zhou Y., Jiang S., Wang Q., Yu F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Target Ther. 2021;6:233. doi: 10.1038/s41392-021-00653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guney C., Akar F. Epithelial and endothelial expressions of ACE2: SARS-CoV-2 entry routes. J. Pharm. Pharm. Sci. 2021;24:84–93. doi: 10.18433/jpps31455. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M.M., Yang W.L., Yang F.Y., Zhang L., Huang W.J., Hou W., Fan C.F., Jin R.H., Feng Y.M., Wang Y.C., Yang J.K. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target Ther. 2021;6:134. doi: 10.1038/s41392-021-00558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M.M., Zhu Y., Zhang L., Zhong G., Tai L., Liu S., Yin G., Lu J., He Q., Li M.J., Zhao R.X., Wang H., Huang W., Fan C., Shuai L., Wen Z., Wang C., He X., Chen Q., Liu B., Xiong X., Bu Z., Wang Y., Sun F., Yang J.K. Novel cleavage sites identified in SARS-CoV-2 spike protein reveal mechanism for cathepsin L-facilitated viral infection and treatment strategies. Cell Discov. 2022;8:53. doi: 10.1038/s41421-022-00419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padmanabhan P., Desikan R., Dixit N.M. Targeting TMPRSS2 and Cathepsin B/L together may be synergistic against SARS-CoV-2 infection. PLoS Comput. Biol. 2020;16:e1008461. doi: 10.1371/journal.pcbi.1008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad K., Al Omar S.Y., Almuqri E.A., Rudayni H.A., Kumar V. Genomics-guided identification of potential modulators of SARS-CoV-2 entry proteases, TMPR-SS2 and cathepsins B/L. PLoS One. 2021;16:e0256141. doi: 10.1371/journal.pone.0256141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad K., Ahamad S., Kanipakam H., Gupta D., Kumar V. Simultaneous inhibition of SARS-CoV-2 entry pathways by cyclosporine. ACS Chem. Neurosci. 2021;12:930–944. doi: 10.1021/acschemneuro.1c00019. [DOI] [PubMed] [Google Scholar]
- 20.Evans J.P., Liu S.L. Role of host factors in SARS-CoV-2 entry. J. Biol. Chem. 2021;297:100847. doi: 10.1016/j.jbc.2021.100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuyama S., Ujike M., Morikawa S., Tashiro M., Taguchi F. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc. Natl. Acad. Sci. USA. 2005;102:12543–12547. doi: 10.1073/pnas.0503203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin C.R., Bahmed K., Simborio H., Hayek H., Bolla S., Marchetti N., Criner G.J., Kosmider B. 2021. Expression of SARS-CoV-2 entry factors in human alveolar type II cells in aging and emphysema. Biomedicines.9. 10.3390/biomedicines9070779 [DOI] [PMC free article] [PubMed]
- 25.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 28.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 29.Hikmet F., Mear L., Edvinsson A., Micke P., Uhlen M., Lindskog C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020;16:e9610. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J., Gao J., Xu Y.P., Zhou T.L., Jin Y.Y., Lou J.N. Expression of severe acute respiratory syndrome coronavirus receptors, ACE2 and CD209L in different organ derived microvascular endothelial cells. Zhonghua Yi Xue Za Zhi. 2007;87:833–837. [PubMed] [Google Scholar]
- 31.Han X., Zhou Z., Fei L., Sun H., Wang R., Chen Y., Chen H., Wang J., Tang H., Ge W., Zhou Y., Ye F., Jia-ng M., Wu J., Xiao Y., Jia X., Zhang T., Ma X., Zhang Q., Bai X., Lai S., Yu C., Zhu L., Lin R., Gao Y., Wang M., Wu Y., Zhang J., Zhan R., Zhu S., Hu H., Wang C., Chen M., Huang H., Liang T., Chen J., Wang W., Zhang D., Guo G. Construction of a human cell landscape at single-cell level. Nature. 2020;581:303–309. doi: 10.1038/s41586-020-2157-4. [DOI] [PubMed] [Google Scholar]
- 32.Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Hao M., Zhang Z., Fan P., Dong Y., Yang Y., Chen Z., Guo Y., Zhang J., Li Y., Song X., Chen Y., Xia L., Fu L., Hou L., Xu J., Yu C., Li J., Zhou Q., Chen W. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020;369:650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouwer P.J.M., Caniels T.G., van der Straten K., Snitsela-ar J.L., Aldon Y., Bangaru S., Torres J.L., Okba N.M.A., Claireaux M., Kerster G., Bentlage A.E.H., van Haaren M.M., Guerra D., Burger J.A., Schermer E.E., Verheul K.D., van der Velde N., van der Kooi A., van Schooten J., van Breemen M.J., Bijl T.P.L., Sliepen K., Aartse A., Derking R., Bontjer I., Kootstra N.A., Wiersinga W.J., Vidarsson G., Haagmans B.L., Ward A.B., de Bree G.J., Sanders R.W., van Gils M.J. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daly J.L., Simonetti B., Klein K., Chen K.E., Williamson M.K., Anton-Plagaro C., Shoemark D.K., Simon-Gracia L., Bauer M., Hollandi R., Greber U.F., Horvath P., Sessions R.B., Helenius A., Hiscox J.A., Teesalu T., Matthews D.A., Davidson A.D., Collins B.M., Cullen P.J., Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujisawa H., Kitsukawa T., Kawakami A., Takagi S., Shimizu M., Hirata T. Roles of a neuronal cell-surface molecule, neuropilin, in nerve fiber fasciculation and guidance. Cell Tissue Res. 1997;290:465–470. doi: 10.1007/s004410050954. [DOI] [PubMed] [Google Scholar]
- 36.Telley L., Cadilhac C., Cioni J.M., Saywell V., Jahannault-Talignani C., Huettl R.E., Sarrailh-Faivre C., Dayer A., Huber A.B., Ango F. Dual function of NRP1 in axon guidance and subcellular target recognition in cerebellum. Neuron. 2016;91:1276–1291. doi: 10.1016/j.neuron.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu M., Murakami Y., Suto F., Fujisawa H. Determination of cell adhesion sites of neuropilin-1. J. Cell Biol. 2000;148:1283–1293. doi: 10.1083/jcb.148.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soker S., Takashima S., Miao H.Q., Neufeld G., Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 39.Li Z.L., Buck M. Neuropilin-1 assists SARS-CoV-2 infection by stimulating the separation of Spike protein S1 and S2. Biophys. J. 2021;120:2828–2837. doi: 10.1016/j.bpj.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Osterlund P., Joensuu M., Meunier F.A., Butcher S.J., Winkler M.S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao C.C., Li M., Deng W., Ma C.H., Chen Y.S., Sun Y.Q., Du T., Liu Q.L., Li W.J., Zhang B., Sun L., Liu S.M., Li F., Qi F., Qu Y., Ge X., Liu J., Wang P., Niu Y., Liang Z., Zhao Y.L., Huang B., Peng X.Z., Yang Y., Qin C., Tong W.M., Yang Y.G. 2022. Differential transcriptomic landscapes of multiple organs from SARS-CoV-2 early infected rhesus macaques. Protein Cell.10.1007/s13238-022-00915-5 [DOI] [PMC free article] [PubMed]
- 42.Baindara P., Roy D., Mandal S.M., Schrum A.G. Conservation and enhanced binding of SARS-CoV-2 Omicron spike protein to coreceptor neuropilin-1 predicted by docking analysis. Infect. Dis. Rep. 2022;14:243–249. doi: 10.3390/idr14020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolaric A., Jukic M., Bren U. Novel small-molecule inhibitors of the SARS-CoV-2 spike protein binding to neuropilin 1. Pharmaceuticals (Basel) 2022;15:165. doi: 10.3390/ph15020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ait-Ali N., Fridlich R., Millet-Puel G., Clerin E., Delalande F., Jaillard C., Blond F., Perrocheau L., Reichman S., Byrne L.C., Olivier-Bandini A., Bellalou J., Moyse E., Bouillaud F., Nicol X., Dalkara D., van Dorsselaer A., Sahel J.A., Leveillard T. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015;161:817–832. doi: 10.1016/j.cell.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 45.Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 2016;159:481–490. doi: 10.1093/jb/mvv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pushkarsky T., Zybarth G., Dubrovsky L., Yurchenko V., Tang H., Guo H., Toole B., Sherry B., Bukrinsky M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc. Natl. Acad. Sci. USA. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crosnier C., Bustamante L.Y., Bartholdson S.J., Bei A.K., Theron M., Uchikawa M., Mboup S., Ndir O., Kwiatkowski D.P., Duraisingh M.T., Rayner J.C., Wright G.J. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fenizia C., Galbiati S., Vanetti C., Vago R., Clerici M., Tacchetti C., Daniele T. SARS-CoV-2 entry: At the crossroads of CD147 and ACE2. Cells. 2021;10:1434. doi: 10.3390/cells10061434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., Wei D., Zhang Y., Sun X.X., Gong L., Yang X., He L., Zhang L., Yang Z., Geng J.J., Chen R., Zhang H., Wang B., Zhu Y.M., Nan G., Jiang J.L., Li L., Wu J., Lin P., Huang W., Xie L., Zheng Z.H., Zhang K., Miao J.L., Cui H.Y., Huang M., Zhang J., Fu L., Yang X.M., Zhao Z., Sun S., Gu H., Wang Z., Wang C.F., Lu Y., Liu Y.Y., Wang Q.Y., Bian H., Zhu P., Chen Z.N. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geng J., Chen L., Yuan Y., Wang K., Wang Y., Qin C., Wu G., Chen R., Zhang Z., Wei D., Du P., Zhang J., Lin P., Zhang K., Deng Y., Xu K., Liu J., Sun X., Guo T., Yang X., Wu J., Jiang J., Li L., Zhang K., Wang Z., Zhang J., Yan Q., Zhu H., Zheng Z., Miao J., Fu X., Yang F., Chen X., Tang H., Zhang Y., Shi Y., Zhu Y., Pei Z., Huo F., Liang X., Wang Y., Wang Q., Xie W., Li Y., Shi M., Bian H., Zhu P., Chen Z.N. CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma. Signal Transduct. Target Ther. 2021;6:347. doi: 10.1038/s41392-021-00760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu C., Wang A., Geng K., Honnen W., Wang X., Bruiners N., Singh S., Ferrara F., D’Angelo S., Bradbury A.R.M., Gennaro M.L., Liu D., Pinter A., Chang T.L. 2021. Human immunodeficiency viruses pseudotyped with SARS-CoV-2 spike proteins infect a broad spectrum of human cell lines through multiple entry mechanisms. Viruses.13. 10.3390/v13060953 [DOI] [PMC free article] [PubMed]
- 52.Badeti S., Jiang Q., Naghizadeh A., Tseng H.C., Bushkin Y., Marras S.A.E., Nisa A., Tyagi S., Chen F., Romanienko P., Yehia G., Evans D., Lopez-Gonzalez M., Alland D., Russo R., Gause W., Shi L., Liu D. 2022. Development of a novel human CD147 knock-in NSG mouse model to test SARS-CoV-2 viral infection. Res. Sq. rs.3.rs-1431484. 10.21203/rs.3.rs-1431484/v1 [DOI] [PMC free article] [PubMed]
- 53.Xia P., Dubrovska A. Tumor markers as an entry for SARS-CoV-2 infection? FEBS J. 2020;287:3677–3680. doi: 10.1111/febs.15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shilts J., Crozier T.W.M., Greenwood E.J.D., Lehner P.J., Wright G.J. No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor. Sci. Rep. 2021;11:413. doi: 10.1038/s41598-020-80464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ragotte R.J., Pulido D., Donnellan F.R., Hill M.L., Gorini G., Davies H., Brun J., McHugh K., King L.D.W., Skinner K., Miura K., Long C.A., Zitzmann N., Draper S.J. Human basigin (CD147) does not directly interact with SARS-CoV-2 spike glycoprotein. mSphere. 2021;6:e0064721. doi: 10.1128/mSphere.00647-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou Y.Q., Wang K., Wang X.Y., Cui H.Y., Zhao Y., Zhu P., Chen Z.N. SARS-CoV-2 pseudovirus enters the host cells through spike protein-CD147 in an Arf6-dependent manner. Emerg. Microbes Infect. 2022;11:1135–1144. doi: 10.1080/22221751.2022.2059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Pasquale V., Quiccione M.S., Tafuri S., Avallone L., Pavone L.M. 2021. Heparan sulfate proteoglycans in viral infection and treatment: A special focus on SARS-CoV-2. Int. J. Mol. Sci.22. 10.3390/ijms22126574 [DOI] [PMC free article] [PubMed]
- 58.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., Thacker B.E., Glass C.A., Yang Z., Torres J.L., Golden G.J., Bartels P.L., Porell R.N., Garretson A.F., Laubach L., Feldman J., Yin X., Pu Y., Hauser B.M., Caradonna T.M., Kellman B.P., Martino C., Gordts P., Chanda S.K., Schmidt A.G., Godula K., Leibel S.L., Jose J., Corbett K.D., Ward A.B., Carlin A.F., Esko J.D. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043–1057. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tandon R., Sharp J.S., Zhang F., Pomin V.H., Ashpole N.M., Mitra D., McCandless M.G., Jin W., Liu H., Sharma P., Linhardt R.J. 2021. Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives. J. Virol.95. 10.1128/JVI.01987-20 [DOI] [PMC free article] [PubMed]
- 60.Khoo U.S., Chan K.Y., Chan V.S., Lin C.L. DC-SIGN and L-SIGN: The SIGNs for infection. J. Mol. Med. (Berl.) 2008;86:861–874. doi: 10.1007/s00109-008-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E., Babcock G.J., Thomas W.D., Thackray L.B., Young M.D., Mason R.J., Ambrosino D.M., Wentworth D.E., Demartini J.C., Holmes K.V. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amraie R., Napoleon M.A., Yin W., Berrigan J., Suder E., Zhao G., Olejnik J., Gummuluru S., Muhlberger E., Chitalia V., Rahimi N. 2020. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2 and are differentially expressed in lung and kidney epithelial and endothelial cells. bioRxiv.10.1101/2020.06.22.165803
- 63.Amraei R., Yin W., Napoleon M.A., Suder E.L., Berrigan J., Zhao Q., Olejnik J., Chandler K.B., Xia C., Feldman J., Hauser B.M., Caradonna T.M., Schmidt A.G., Gummuluru S., Muhlberger E., Chitalia V., Costello C.E., Rahimi N. CD209L/L-SIGN and CD209/DC-SIGN Act as receptors for SARS-CoV-2. ACS Cent Sci. 2021;7:1156–1165. doi: 10.1021/acscentsci.0c01537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thepaut M., Luczkowiak J., Vives C., Labiod N., Bally I., Lasala F., Grimoire Y., Fenel D., Sattin S., Thielens N., Schoehn G., Bernardi A., Delgado R., Fieschi F. DC/L-SIGN recognition of spike glycoprotein promotes SARS-CoV-2 trans-infection and can be inhibited by a glycomimetic antagonist. PLoS Pathog. 2021;17:e1009576. doi: 10.1371/journal.ppat.1009576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kondo Y., Larabee J.L., Gao L., Shi H., Shao B., Hoover C.M., McDaniel J.M., Ho Y.C., Silasi-Mansat R., Archer-Hartmann S.A., Azadi P., Srinivasan R.S., Rezaie A.R., Borczuk A., Laurence J.C., Lupu F., Ahamed J., McEver R.P., Papin J.F., Yu Z., Xia L. 2021. L-SIGN is a receptor on liver sinusoidal endothelial cells for SARS-CoV-2 virus. JCI Insight.6. 10.1172/jci.insight.148999 [DOI] [PMC free article] [PubMed]
- 66.Lu Q., Liu J., Zhao S., Gomez Castro M.F., Laurent-Rolle M., Dong J., Ran X., Damani-Yokota P., Tang H., Karakousi T., Son J., Kaczmarek M.E., Zhang Z., Yeung S.T., McCune B.T., Chen R.E., Tang F., Ren X., Chen X., Hsu J.C.C., Teplova M., Huang B., Deng H., Long Z., Mudianto T., Jin S., Lin P., Du J., Zang R., Su T.T., Herrera A., Zhou M., Yan R., Cui J., Zhu J., Zhou Q., Wang T., Ma J., Koralov S.B., Zhang Z., Aifantis I., Segal L.N., Diamond M.S., Khanna K.M., Stapleford K.A., Cresswell P., Liu Y., Ding S., Xie Q., Wang J. 2021. SARS-CoV-2 exacerbates proinflammatory responses in myeloid cells through C-type lectin receptors and Tweety family member 2. Immunity.54, 1304–1319 e1309. 10.1016/j.immuni.2021.05.006 [DOI] [PMC free article] [PubMed]
- 67.Pollastri S., Delaunay C., Thepaut M., Fieschi F., Bernardi A. Glycomimetic ligands block the interaction of SARS-CoV-2 spike protein with C-type lectin co-receptors. Chem. Commun. (Camb.) 2022;58:5136–5139. doi: 10.1039/d2cc00121g. [DOI] [PubMed] [Google Scholar]
- 68.Ibrahim I.M., Abdelmalek D.H., Elfiky A.A. GRP78: A cell’s response to stress. Life Sci. 2019;226:156–163. doi: 10.1016/j.lfs.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elfiky A.A., Baghdady A.M., Ali S.A., Ahmed M.I. GRP78 targeting: Hitting two birds with a stone. Life Sci. 2020;260:118317. doi: 10.1016/j.lfs.2020.118317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ibrahim I.M., Abdelmalek D.H., Elshahat M.E., Elfiky A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. J. Infect. 2020;80:554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carlos A.J., Ha D.P., Yeh D.W., Van Krieken R., Tseng C.C., Zhang P., Gill P., Machida K., Lee A.S. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J. Biol. Chem. 2021;296:100759. doi: 10.1016/j.jbc.2021.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morizono K., Chen I.S. Role of phosphatidylserine receptors in enveloped virus infection. J. Virol. 2014;88:4275–4290. doi: 10.1128/JVI.03287-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gu Y., Cao J., Zhang X., Gao H., Wang Y., Wang J., He J., Jiang X., Zhang J., Shen G., Yang J., Zheng X., Hu G., Zhu Y., Du S., Zhu Y., Zhang R., Xu J., Lan F., Qu D., Xu G., Zhao Y., Gao D., Xie Y., Luo M., Lu Z. Receptome profiling identifies KREMEN1 and ASGR1 as alternative functional receptors of SARS-CoV-2. Cell Res. 2022;32:24–37. doi: 10.1038/s41422-021-00595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakamura T., Nakamura T., Matsumoto K. The functions and possible significance of Kremen as the gatekeeper of Wnt signalling in development and pathology. J. Cell Mol. Med. 2008;12:391–408. doi: 10.1111/j.1582-4934.2007.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B.M., Delius H., Hoppe D., Stannek P., Walter C., Glinka A., Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 76.Staring J., van den Hengel L.G., Raaben M., Blomen V.A., Carette J.E., Brummelkamp T.R. 2018. KREMEN1 Is a host entry receptor for a major group of enteroviruses. Cell Host Microbe.23, 636–643 e635. 10.1016/j.chom.2018.03.019 [DOI] [PubMed]
- 77.Hoober J.K. ASGR1 and its enigmatic relative, CLEC10A. Int. J. Mol. Sci. 2020;21:48818. doi: 10.3390/ijms21144818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saunier B., Triyatni M., Ulianich L., Maruvada P., Yen P., Kohn L.D. Role of the asialoglycoprotein receptor in binding and entry of hepatitis C virus structural proteins in cultured human hepatocytes. J. Virol. 2003;77:546–559. doi: 10.1128/jvi.77.1.546-559.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei C., Wan L., Yan Q., Wang X., Zhang J., Yang X., Zhang Y., Fan C., Li D., Deng Y., Sun J., Gong J., Yang X., Wang Y., Wang X., Li J., Yang H., Li H., Zhang Z., Wang R., Du P., Zong Y., Yin F., Zhang W., Wang N., Peng Y., Lin H., Feng J., Qin C., Chen W., Gao Q., Zhang R., Cao Y., Zhong H. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat. Metab. 2020;2:1391–1400. doi: 10.1038/s42255-020-00324-0. [DOI] [PubMed] [Google Scholar]
- 80.Kluck G.E.G., Yoo J.A., Sakarya E.H., Trigatti B.L. 2021. Good cholesterol gone bad? HDL and COVID-19. Int. J. Mol. Sci.22. 10.3390/ijms221910182 [DOI] [PMC free article] [PubMed]
- 81.Shen W.J., Asthana S., Kraemer F.B., Azhar S. Scavenger receptor B type 1: Expression, molecular regulation, and cholesterol transport function. J. Lipid Res. 2018;59:1114–1131. doi: 10.1194/jlr.R083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J., Fan J., Chen Z., Zhang M., Peng H., Liu J., Ding L., Liu M., Zhao C., Zhao P., Zhang S., Zhang X., Xu J. 2021. Nonmuscle myosin heavy chain IIA facilitates SARS-CoV-2 infection in human pulmonary cells. Proc. Natl. Acad. Sci. USA.118. 10.1073/pnas.2111011118 [DOI] [PMC free article] [PubMed]