Abstract
Objective:
To analyze anti-SARS-CoV-2-S1-IgG levels, avidity, Omicron BA.2 variant neutralizing capacity, and SARS-CoV-2-specific T cells in anti-CD20-treated patients with multiple sclerosis (aCD20pwMS) after two, three, or four COVID-19 vaccinations.
Results:
Frequencies of aCD20pwMS with detectable SARS-CoV-2-S1-IgG increased moderately between two (31/61 (51%)), three (31/57 (54%)), and four (17/26 (65%)) vaccinations. However, among patients with detectable SARS-CoV-2-S1-IgG, frequencies of high avidity (6/31 (19%) vs 11/17 (65%)) and Omicron neutralizing antibodies (0/10 (0%) vs 6/10 (60%)) increased strongly between two and four vaccinations. SARS-CoV-2-specific T cells were detectable in >92% after two or more vaccinations
Conclusion:
Additional vaccinations qualitatively improve SARS-CoV-2 antibody responses.
Keywords: Multiple sclerosis, immunology, COVID, vaccine, disease-modifying therapies, SARS-CoV-2
Introduction
Anti-CD20 therapy is associated with worse courses of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections in patients with multiple sclerosis (pwMS). 1 While Coronavirus disease 2019 (COVID-19) vaccinations prevent severe disease, 2 anti-CD20-treated pwMS (aCD20pwMS) vaccinated twice have diminished SARS-CoV-2 antibody levels, avidity, and neutralizing capacity. 3 Guidelines therefore recommend additional SARS-CoV-2 vaccinations in aCD20pwMS. 4 Following dissemination of the SARS-CoV-2 Omicron variant, 5 it was shown that healthy individuals vaccinated thrice, but not twice, generate neutralizing antibodies against Omicron. 6 However, it remained unknown whether this holds true for aCD20pwMS. Here, we characterize immune responses in aCD20pwMS vaccinated two to four times.
Patients and methods
aCD20pwMS were recruited between March and June 2022 and participate in an ongoing, previously described, prospective study 3 (supplemental material). Ten healthy controls (HCs) vaccinated thrice were included for comparison. Anti-SARS-CoV-2 spike subunit 1 (S1, Non-VOC) immunoglobulin (Ig)G, maturation of SARS-CoV-2-S1 IgG avidity, neutralizing capacity against the Omicron BA.2 variant, and SARS-CoV-2 spike-specific T-cell responses were measured as described elsewhere (supplemental material). 3
Results
Demographic and clinical data of aCD20pwMS vaccinated two (n = 61), three (n = 57), or four times (n = 26) against COVID-19 are summarized in Supplementary Table 1. All aCD20pwMS had been treated with at least one infusion of anti-CD20 therapy (ocrelizumab or rituximab) a maximum of 18 months before their last COVID-19 vaccination and all pwMS were vaccinated after beginning anti-CD20 therapy.
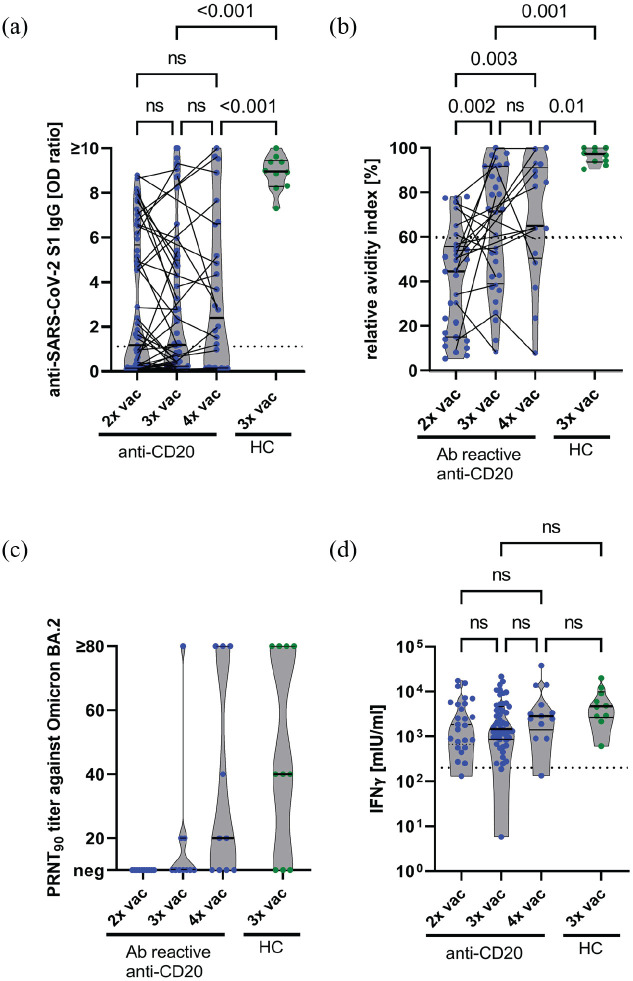
The proportion of aCD20pwMS with detectable SARS-CoV-2-S1 IgG increased only moderately between two (31/61, 50.8%, 95% confidence interval (CI): 38.6–62.9), three (31/57, 54.4%, 95% CI: 41.6–66.6), and four (17/26, 65.4%, 95% CI: 46.2–80.6) vaccinations (Figure 1(a)). Accordingly, SARS-CoV-2-S1 IgG levels were similar after two (median (IQR) 1.2 (0.1–5.7)), three (1.2 (0.2–5)), and four (2.4 (0.1–6.6)) vaccinations. In contrast, 10/10 (100%, 95% CI: 72.2–100) HC vaccinated thrice developed SARS-CoV-2-S1 IgG. Median (IQR) SARS-CoV-2-S1 IgG levels of HC (9.0 (8.3–9.5)) exceeded those of aCD20pwMS after three (p < 0.001) or four (p < 0.001) vaccinations (Figure 1(a)).
Figure 1.
(a) SARS-CoV-2-S1 IgG levels in two, three, and four times vaccinated anti-CD20-treated pwMS and three times vaccinated HC were measured by ELISA. pwMS sampled more than once are connected by lines. The dotted horizontal line indicates the manufacturer’s threshold of an SARS-CoV-2-S1 IgG OD ratio of 1.1, levels above which were considered positive. (b) Relative avidity indices in anti-S1 IgG reactive (Ab reactive) two, three, and four times vaccinated anti-CD20-treated pwMS and three times vaccinated HC. pwMS sampled more than once are connected by lines. The dotted horizontal line indicates a relative avidity index of 60, levels equal to or above which were considered high. (c) Serum neutralization titers against Omicron BA.2 were analyzed in two, three, and four times vaccinated pwMS and three times vaccinated HC. Serum dilutions with a plaque reduction of 90% are referred to as PRNT90 titers. PRNT90 titers ⩾ 20 were considered to indicate the presence of neutralizing antibodies. (d) Whole blood of pwMS vaccinated two, three, or four times and of HC vaccinated three times against SARS-CoV-2 was stimulated ex vivo with components of the S1 domain of the spike protein for 24 hours, and interferon (IFN)-γ concentration in the supernatant was measured by ELISA. The dotted horizontal line indicates IFN-γ concentrations of 200 mIU/mL, levels above which indicate the presence of SARS-CoV-2-specific T cells. The pwMS with a very low T-cell response following three vaccinations had an additional combined immunodeficiency syndrome. (a, b, d) p-values were calculated by a linear mixed model with Bonferroni correction.
HC: healthy controls; IFN-γ: interferon-γ; IgG: immunoglobulin G; IGRA: interferon-γ release assay; IU: international units; ns: not significant; OD: optical density; PRNT: plaque reduction neutralization test; S: SARS-CoV-2 spike protein S1 domain; vac: vaccination.
We studied intraindividual courses of SARS-CoV-2 antibody responses in longitudinal blood samples collected after the second and third SARS-CoV-2 vaccinations from 35 aCD20pwMS (Figure 1(a)). Seventeen of 35 (48.6%, 95% CI: 33–64.4) aCD20pwMS had detectable and 18 of 35 (51.4%, 95% CI: 35.6–67) had undetectable SARS-CoV-2-S1 IgG after two vaccinations. Of the 18 aCD20pwMS without SARS-CoV-2-S1 IgG after 2 SARS-CoV-2 vaccinations, 4 (22.2%, 95% CI: 9.0–45.2) showed detectable antibodies after the third vaccination, while 14 remained negative (77.8%, 95% CI: 54.8–91). In 5 of 17 (29.4%, 95% CI: 13.3–53.1) aCD20pwMS with SARS-CoV-2-S1 IgG after the second vaccination, SARS-CoV-2-S1 IgG was not detectable after the third vaccination. In the remaining 12 of 17 (70.6, 95% CI: 46.9–86.7) pwMS, SARS-CoV-2-S1 IgG remained detectable after the third vaccination.
Maturation of IgG avidity was analyzed in all aCD20pwMS with detectable SARS-CoV-2-S1 IgG. After the second vaccination, only 6 of 31 pwMS (19.4%, 95% CI: 9.2–36.3) exhibited high (⩾60) SARS-CoV-2-S1 IgG avidity. However, after the third and fourth vaccinations, the proportion of pwMS with high SARS-CoV-2-S1 IgG avidity increased to 17 of 31 (54.8%, 95% CI: 37.8–70.8) and 11 of 17 (64.7%, 95% CI: 41.3–82.7), respectively (Figure 1(b)). Accordingly, the median (interquartile range, IQR) avidity indices increased between the second (44.4 (IQR, 15.1–55.7)), third (71.4 (39.0–91.8), p = 0.002), and fourth (65.0 (50.5–91.91), p = 0.003) vaccinations. Ten of 10 (100%) HCs had high avidity indices (97.7 (IQR, 93.6–100)).
Using an authentic SARS-CoV-2 plaque reduction neutralization test, the capacity of SARS-CoV-2 antibodies to neutralize Omicron (BA.2) was investigated in the 10 aCD20pwMS with highest SARS-CoV-2-S1 IgG levels after the second, third, and fourth vaccinations (Figure 1(c)). None (0/10, 0%, 95% CI: 0–27.8) of the aCD20pwMS vaccinated twice, 3 of 10 (30%, 95% CI: 10.8–60.3, p = 0.21 vs two vaccinations) pwMS vaccinated thrice, and 6 of 10 pwMS (60%, 95% CI: 31.3–89.2, p = 0.01 vs two vaccinations) vaccinated four times generated Omicron neutralizing antibodies. Likewise, 7 of 10 HCs (70%, 95% CI: 39.7–89.2) vaccinated thrice had Omicron neutralizing antibodies, typically higher (median (IQR), 40 (0–80)) than those of pwMS vaccinated three (0 (0–20)) or four (20 (0–80)) times. Intervals between anti-CD20 infusions and SARS-CoV-2 vaccinations in pwMS with or without neutralizing antibodies against Omicron after three or four vaccinations did not significantly differ (Supplementary Tables 2 and 3).
The frequencies of aCD20pwMS with SARS-CoV-2-specific T-cell responses were high and similar in aCD20pwMS vaccinated two (24/25, 96%, 95% CI: 80.5–99.8), three (49/51, 96.1%, 95% CI: 86.8–99.3), or four times (12/13, 92.3%, 95% CI: 66.7–99.6), and in thrice vaccinated HC (10/10, 100%, 95% CI: 72.3–100) (Figure 1(d)).
Discussion
This study shows that the frequency of aCD20pwMS with detectable SARS-CoV-2-S1 IgG antibodies after two to four vaccinations increases only moderately. About 20% of anti-CD20-treated pwMS with no SARS-CoV-2-S1 IgG after the second vaccination developed SARS-CoV-2-S1 IgG after a third vaccination, matching findings in aCD20pwMS, who did not seroconvert after the second vaccination.7,8 In some previously SARS-CoV-2 IgG-positive pwMS, SARS-CoV-2 IgG became undetectable after the third vaccination, likely due to waning antibody levels. 9 Altogether, these findings indicate a persistently impaired quantitative SARS-CoV-2-specific humoral immune response in aCD20pwMS even after two booster vaccinations.
However, in anti-CD20-treated pwMS who developed SARS-CoV-2 antibodies following SARS-CoV-2 vaccinations, avidity and neutralizing capacity of SARS-CoV-2 antibodies improved after third or fourth vaccinations. This suggests that in some anti-CD20-treated pwMS, the physiological mechanisms of antibody maturation and diversification are preserved, though at an attenuated level.
The high proportion of aCD20pwMS with SARS-CoV-2-specific T-cell responses after three or four vaccinations is consistent with previous findings after two vaccinations. 3 The preserved T-cell responses appear relevant as T cells cross-recognize SARS-CoV-2 variants and are unlikely to be affected by antibody escape variants. 10
Limitations of this study are the rather small number of aCD20pwMS vaccinated four times and the lack of absolute B cell counts.
In summary, we show that humoral responses to SARS-CoV-2 remain quantitatively impaired in aCD20pwMS after three or four vaccinations. Still, ~20% of patients who did not develop antibodies after two vaccinations benefited from a third vaccination by developing SARS-CoV-2 antibodies. Furthermore, SARS-CoV-2 antibody functionality improves after three or four vaccinations in aCD20pwMS with detectable SARS-CoV-2 antibodies. These results support current recommendations for additional booster vaccinations in aCD20pwMS.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585231161253 for Humoral immune responses remain quantitatively impaired but improve qualitatively in anti-CD20-treated patients with multiple sclerosis after three or four COVID-19 vaccinations by Carolin Otto, Tatjana Schwarz, Lara M Jeworowski, Marie L Schmidt, Felix Walper, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Andi Krumbholz, Ruben Rose, Christian Drosten, Klemens Ruprecht and Victor M Corman in Multiple Sclerosis Journal
Acknowledgments
We thank Patricia Tscheak and Petra Mackeldanz for their excellent assistance. We thank Ulrike Grittner for statistical advice.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.O., T.S., L.M.J., M.L.S., F.W., F.P., P.S., M.N., A.K., R.R., and C.D. declare no disclosures relevant to the manuscript. V.M.C. is named together with Euroimmun GmbH on a patent application filed recently regarding the diagnostic of SARS-CoV-2 by antibody testing. K.R. is site principal investigator in clinical trials sponsored by Roche, the manufacturer of ocrelizumab and rituximab, and received research support from Novartis Pharma, Merck Serono, German Ministry of Education and Research, European Union (821283-2), Stiftung Charité, and Arthur Arnstein Foundation, and travel grants from Guthy Jackson Charitable Foundation.
Ethical Approval: The study was approved by the ethical committee of Charité—Universitätsmedizin Berlin (EA2/152/21 and EA1/068/20).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Parts of this work were supported by grants from the Berlin Institute of Health (BIH) and Berlin University Alliance to C.D. and V.M.C. This study was further supported by the German Ministry of Education and Research through Forschungsnetzwerk der Universitätsmedizin zu COVID-19, COVIM, FKZ: 01KX2021 to C.D. and V.M.C., and projects VARIPath (01KI2021) to V.M.C. F.P. and V.M.C. are participants in the BIH-Charité Clinician Scientist Program funded by Charité—Universitätsmedizin Berlin and the Berlin Institute of Health. K.R. is a participant in the BIH Clinical Fellow Program funded by Stiftung Charité.
ORCID iDs: Marie L Schmidt  https://orcid.org/0000-0002-8512-7531
https://orcid.org/0000-0002-8512-7531
Andi Krumbholz  https://orcid.org/0000-0002-1094-350X
https://orcid.org/0000-0002-1094-350X
Ruben Rose  https://orcid.org/0000-0003-0657-2993
https://orcid.org/0000-0003-0657-2993
Victor M Corman  https://orcid.org/0000-0002-3605-0136
https://orcid.org/0000-0002-3605-0136
Data Availability Statement: Data are available upon reasonable request.
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Carolin Otto, Department of Neurology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Tatjana Schwarz, Institute of Virology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany/German Centre for Infection Research (DZIF), Berlin, Germany.
Lara M Jeworowski, Institute of Virology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany/German Centre for Infection Research (DZIF), Berlin, Germany.
Marie L Schmidt, Institute of Virology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany/German Centre for Infection Research (DZIF), Berlin, Germany.
Felix Walper, Institute of Virology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany/German Centre for Infection Research (DZIF), Berlin, Germany.
Florence Pache, Department of Neurology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Patrick Schindler, Department of Neurology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Moritz Niederschweiberer, Department of Neurology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Andi Krumbholz, Institute for Infection Medicine, Christian-Albrechts-Universität zu Kiel and University Medical Center Schleswig-Holstein, Kiel, Germany/Labor Dr. Krause und Kollegen MVZ GmbH, Kiel, Germany.
Ruben Rose, Institute for Infection Medicine, Christian-Albrechts-Universität zu Kiel and University Medical Center Schleswig-Holstein, Kiel, Germany.
Christian Drosten, Institute of Virology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany/German Centre for Infection Research (DZIF), Berlin, Germany.
Klemens Ruprecht, Department of Neurology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Victor M Corman, Institute of Virology, Charité—Universitätsmedizin Berlin, Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany/German Centre for Infection Research (DZIF), Berlin, Germany/Labor Berlin-Charité Vivantes GmbH, Berlin, Germany.
References
- 1.Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American Registry of patients with multiple sclerosis. JAMA Neurol 2021; 78: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Self WH, Tenforde MW, Rhoads JP, et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March-August 2021. Morb Mortal Wkly Rep 2021; 70: 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarz T, Otto C, Jones TC, et al. Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis. Mult Scler 2022; 28(7): 1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.STIKO. Ständige Impfkommission: Beschluss der STIKO zur 20. Aktualisierung der COVID-19-Impfempfehlung. Epid Bull 2022; 21: 3–19. [Google Scholar]
- 5.ECDC. Threat assessment brief: Implications of the further emergence and spread of the SARS-CoV-2 B.1.1.529 variant of concern (Omicron) for the EU/EEA—first update, 2021, https://www.ecdc.europa.eu/sites/default/files/documents/threat-assessment-covid-19-emergence-sars-cov-2-variant-omicron-december-2021.pdf
- 6.Carreño JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature 2022; 602(7898): 682–688. [DOI] [PubMed] [Google Scholar]
- 7.Sidler D, Born A, Schietzel S, et al. Trajectories of humoral and cellular immunity and responses to a third dose of mRNA vaccines against SARS-CoV-2 in patients with a history of anti-CD20 therapy. RMD Open 2022; 8(1): e002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konig M, Torgauten HM, Tran TT, et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol 2022; 79: 307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med 2021; 385: e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madelon N, Heikkila N, Sabater Royo I, et al. Omicron-specific cytotoxic T-cell responses after a third dose of mRNA COVID-19 vaccine among patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol 2022; 79: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585231161253 for Humoral immune responses remain quantitatively impaired but improve qualitatively in anti-CD20-treated patients with multiple sclerosis after three or four COVID-19 vaccinations by Carolin Otto, Tatjana Schwarz, Lara M Jeworowski, Marie L Schmidt, Felix Walper, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Andi Krumbholz, Ruben Rose, Christian Drosten, Klemens Ruprecht and Victor M Corman in Multiple Sclerosis Journal