Summary
Major depressive disorder is one of the most disabling mental disorders worldwide. Increasing preclinical and clinical studies have highlighted that compositional and functional (e.g., metabolite) changes in gut microbiota, known as dysbiosis, are associated with the onset and progression of depression via regulating the gut-brain axis. However, the gut microbiota and their metabolites present a double-edged sword in depression. Dysbiosis is involved in the pathogenesis of depression while, at the same time, offering a novel therapeutic target. In this review, we describe the association between dysbiosis and depression, drug–microbiota interactions in antidepressant treatment, and the potential health benefits of microbial-targeted therapeutics in depression, including dietary interventions, fecal microbiota transplantation, probiotics, prebiotics, synbiotics, and postbiotics. With the emergence of microbial research, we describe a new direction for future research and clinical treatment of depression.
Keywords: Gut microbiota, Metabolites, Depression, Pathogenesis, Microbial-targeted therapeutics
Introduction
Major depressive disorder (MDD) is one of the most disabling mental disorders, affecting over 35 million people worldwide. MDD is characterized by significant and persistent low mood and is driven by a combination of genetic and environmental factors. The recent pandemic has added a marked depressive burden to this situation; the 2020 Global Burden of Disease (GBD) data from 204 countries indicates that, throughout 2020, the COVID-19 pandemic and associated lockdowns led to a 27.6% increase in MDD cases.1
The trillions of microorganisms that inhabit our guts, including bacteria, viruses, archaea, and fungi, play roles in human health. Recent studies have demonstrated the effects of the gut microbiome on distal organs, such as the brain. A pivotal role of gut microbiota in the pathogenesis of MDD has long been postulated.2 However, a clear cause-effect relationship has not been definitively shown. MDD is associated with microbial dysbiosis, which is defined as an alteration in microbial diversity owing to the disrupted balance of microbiota and corresponding functional changes.3 Circumstantial evidence comes from an observed increased risk of severe mental disorders (e.g., depression, anxiety, and psychosis) following antibiotic use, even 5–10 years after use.4, 5, 6 A recent systematic review indicated the association between antibiotics use and subsequent development of depression.7 This is largely because antibiotics reduce the diversity of gut microbiota.8 Moreover, an unhealthy diet and environmental exposures that influence the gut microbial composition were proved to be highly associated with the increased incidence of depression in recent years.9,10
Although increasing evidence has suggested a role of gut microbiota in MDD pathogenesis, the definitive role of dysbiosis remains unclear. So far, it is unclear whether microbial dysbiosis is intrinsically causal or merely a consequence of depression-related pathological changes. In addition, over decades of observational research, the gut microbiome field is shifting from association to modulation.11 The ultimate goal of researches is to promote the development of microbiota-based interventions in depression. However, there is still a long way to go to realize the clinical application of microbial therapy. Before this, antidepressants are still the therapeutic mainstays in depression. So, in the current stage, we should not only pay attention to the development of microbiota-targeted interventions, but also to the influence of gut microbiome on efficacy of antidepressant drugs. Thus, in this review, we fully describe the association between dysbiosis and MDD, drug–microbiota interactions in antidepressant treatment, and the potential clinical transformations of microbial-targeted therapeutics for MDD.
Gut microbiota composition and depression
Gut bacterial microbiota dysbiosis and depression
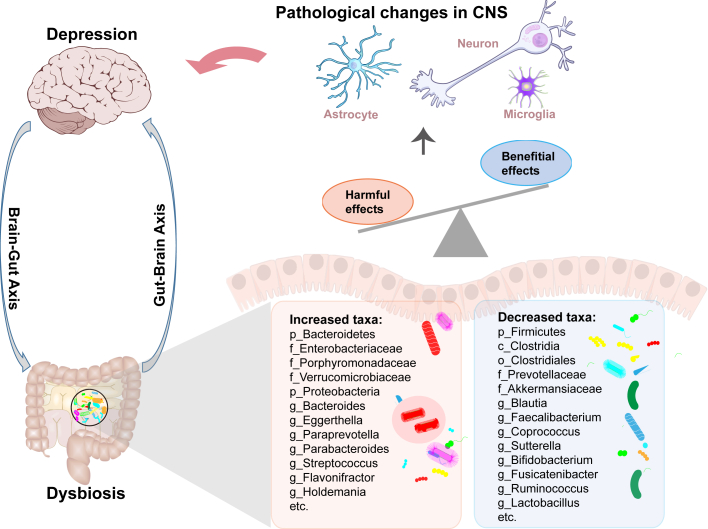
Accumulating studies have shown an association between dysbiosis and depression (Fig. 1) by reporting changes in the gut microbial composition of MDD patients compared to healthy individuals, particularly in terms of microbial diversity and the relative abundance of specific bacterial taxa.3,12,13 Although the findings between studies have been inconsistent, enriched pro-inflammatory bacteria and depletion of anti-inflammatory bacteria have been the most consistent findings3,13; this is similar to other disease conditions which are linked with systemic and gut inflammation, further strengthening the case for the inflammatory hypothesis of depression. In depression, Firmicutes, Actinobacteria, and Bacteroidetes are the most affected phyla2,14; especially an increase in the Bacteroidetes/Firmicutes ratio in MDD patients, characterized by an enrichment of the genus Bacteroides and a depletion of the genera Blautia, Faecalibacterium, and Coprococcus.3,12,13,15 An increase in Eggerthella and decrease in Sutterella were also consistently demonstrated in patients with MDD.3,12,13,16 Driven by succession and within-host evolution throughout life, gut microbiota have become increasingly pathogenic,17,18 leading to possible distinct microbial characteristics in MDD patients of different ages.19 There are also regional differences in microbial compositions in patients with MDD. For example, several taxa were primarily identified in patients from China, such as increased Eggerthella and decreased Coprococcus, and Fusicatenibacter.3 However, simple association studies do not provide information about causality. Transplanting of fecal microbiota from MDD patients to induce depressive-like behaviors in rodents suggests that microbial dysbiosis precedes MDD onset and might play a causal role in MDD.2,20 Meanwhile, there is also evidence supporting a pathological vicious loop in which depression-associated pathological changes also cause and exacerbate the dysbiosis.21 Thus, while microbiome shifts can occur early in MDD and perhaps contribute to the onset of MDD, over time pathological changes in MDD further contribute to dysbiosis by altering the gut environment.
Fig. 1.
The association between dysbiosis and central pathological changes during the development of depression. CNS, Central nervous system.
Potential causative pathobionts in depression
Although global shifts in the gut microbiota have been reported in patients with MDD, the specific pathobionts remain to be fully elucidated. Previous studies suggested that several species belonging to the Bacteroidaceae family are specific to MDD,14 and that species from Bacteroides (e.g., B. thetaiotaomicron, B. fragilis and B. uniformis) significantly increase the susceptibility to depression.15,22 Additionally, clinical and preclinical studies have reported that segmented filamentous bacteria increase the susceptibility to depression by promoting T helper 17 (Th17) cell production.23 Moreover, a large-scale population-based cohort found that, even though Morganella was not specifically highlighted, the increasing abundances of Morganella predicted to have a causal effect on MDD.24 Furthermore, Liu et al.25 isolated Mycobacterium neoaurum, which can degrade testosterone by expressing the 3β-Hydroxysteroid dehydrogenase enzyme, from the feces of male MDD patients, and found that administering this strain to rats via oral gavage, induced depressive-like behaviors. However, in the context of a complex gut microbial community, these specific pathobionts probably act synergistically with others to cause depression, rather than merely as individual infectious agents. The interactions between microbial species are likely quite complex. Understanding these so-called higher-order interactions, that generate new effector molecules, may improve our understanding of the mechanisms underlying microbiome–host interactions that contribute to depression.26
Gut nonbacterial microbiota dysbiosis and depression
In addition to the intestinal bacteria, nonbacterial microbiota also participates in the pathogenesis of depression. As beneficial partners, bacteriophages influence the function of bacterial microbiota by promoting the diversity of the intestinal bacteria genotypes and phenotypes.27 Shifts in the composition of bacteriophages in MDD patients have been documented, particularly changes in Caudovirales bacteriophages.15 Similar changes were also identified in a non-human primate model of depression, indicating that Caudovirales alteration is a hallmark of depression.28 Further, gut fungal dysbiosis was documented in depressed patients, and a disrupted fungi-bacteria network appears to be involved in the onset of depression.29 The role of Candida albicans in the pathophysiology of psychiatric diseases such as depression was reviewed previously.30 The prevalent interactions between bacterial and nonbacterial microbiota in the gut are complex, suggesting that studying the properties of bacteria in vitro or in vivo without considering other nonbacterial microbiota might provide a distorted view of the role of functional networks that, in reality, involve multiple individual microorganisms and microbial communities.
Gut microbiota-derived metabolites and depression
There are multiple important mediators involved in gut microbiota-host interactions, such as microbial metabolites, derived from the fermentation of dietary substances by bacteria, and bacterially-modified host molecules, such as bile acids (BAs), or products produced by bacteria directly.31 Shifts in the composition of gut microbiota in MDD lead to changes in the microbial metabolome, which plays a role in the pathogenesis of MDD. Gut microbiota-derived metabolites present a double-edged sword in depression. Short-chain fatty acids (SCFAs: acetate, butyrate, and propionate) have been strongly implicated in depression. SCFAs were reported to be depleted in patients with MDD,2,32 while their administration resulted in antidepressant effects by ameliorating depression-related intestinal permeability and hypothalamic–pituitary–adrenal (HPA) axis reactivity, in particular butyrate.33,34 Neurotransmitters (e.g., serotonin and gamma amino butyric acid), produced by the gut microbiota directly or indirectly, can influence emotional behavior by modulating local gut physiology and by regulating the function of distal organs when absorbed into the bloodstream.34 In addition, the actions of BAs through binding to their dedicated receptors, farnesoid X receptor and Takeda G-protein-coupled receptor 5, regulate the physiological metabolic responses of the body. Disturbances in BA metabolism have been identified in MDD35; BA levels, especially of the secondary BAs modulated by gut microbiota, are negatively correlated with the severity of depressive symptoms,35 suggesting a protective role of higher BA levels for depression. Turicibacterales, Turicibacteraceae, and Turicibacter were related to the increased production of BAs.35 And this taxa has been reported to reduce in depression state.3 Furthermore, microbiota-derived trimethylamine-N-oxide (TMAO) from choline metabolism can cross the blood–brain barrier to influence brain pathology and the development of neuropsychiatric disorders.36 A positive association between serum TMAO levels and depression severity has been reported in individuals.37 Other microbiota-derived metabolites such as lipopolysaccharide (LPS), lactate, and B-vitamins are also associated with the pathogenesis of MDD.38, 39, 40 The levels of these metabolites reflect, at least in part, the metabolic capacity of the gut microbiota. Beyond the composition of gut microbiota, the microbial function (e.g., metabolism, metabolite) is equally important, and future diseases studies should take into account both the microbial composition and functional changes.
Microbiota-gut-brain (MGB) axis in depression
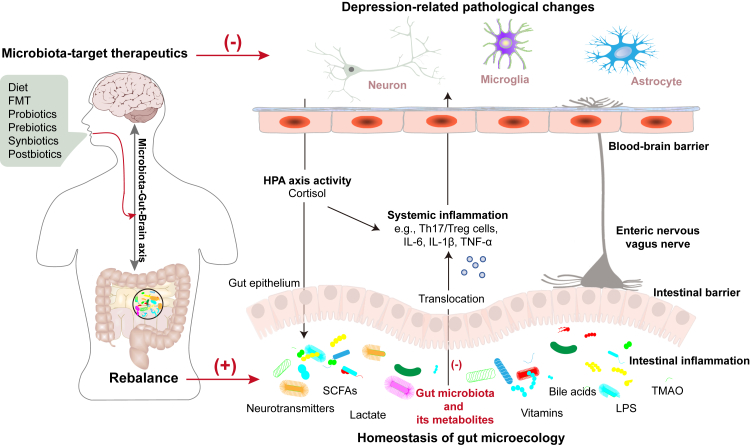
Communication between the gut and the brain is bidirectional and involves various routes including neural, endocrine, and immune pathways.41 The microbiota and their derived metabolites act as key modulators in gut-brain signaling, leading to the concept of an MGB axis.42 The role of the routes involved in the MGB axis in depression has been well delineated (Fig. 2). The microbial composition and metabolites change in MDD patients results in a disrupted homeostasis of the gut microenvironment, affecting the function of the gut epithelium and causing intestinal barrier dysfunction and inflammatory responses.43,44 The increased systemic translocation of gut metabolites, microbial cell components, or even the microbiota via the damaged intestinal barrier (the “leaky gut”) heightens systemic inflammatory responses (e.g., Th17/regulatory T cell (Th17/Treg) imbalance, interleukin [IL]-6, IL-1β, and tumor necrosis factor-alpha (TNF-α)) that have been implicated in the pathogenesis of depression.45,46 The enteric nervous system (ENS), referred to as the “second brain”, has been reported to be involved in the development of brain disorders. Abnormal ENS activity arising from intestinal pathology aggravates depression-related pathological changes by altering gut secretion, immune defenses, motility, and permeability.47 Besides the ENS, the vagus nerve also plays an important role in transmitting microbial signals from the gut to the brain in depression.48 Preclinical studies have verified that subdiaphragmatic vagotomy blocked the development of depression-like behaviors in rodents after LPS injection or fecal microbiota transplantation (FMT) from Chrna7 knock-out mice with depression-like behaviors.48,49 In clinical, vagus nerve stimulation has long been approved for treatment-resistant depression.50 The microbial cell components, e.g., LPS produced by gram-negative bacteria, and peripheral inflammatory signals reach the brain by crossing the blood–brain barrier resulting in neuroinflammation,38 subsequently induce neuropathological changes through chronic activating specific cells, including synaptic defects, demyelination, abnormal neurogenesis and neurotransmitters release,22,51, 52, 53 that are involved in the pathogenesis of depression. Microbial signals, pathological neurobiological changes, and depressive emotions can activate the HPA axis, increasing the synthesis and release of cortisol. As a part of the brain-gut axis, excessive levels of cortisol promote gut pathology by modulating intestinal barrier function and inflammatory responses, resulting in a leaky gut54; this process is a key component of the MGB axis in depression. Beyond these routes, various signal transduction systems and metabolic pathways are also implicated in the MGB-based pathogenesis of depression, such as the endocannabinoid system, CAMKII-CREB and MAPK signaling, and glycerophospholipid metabolism.55, 56, 57, 58 In addition, mitochondria are reported to be potential key mediators of the gut microbiota dysbiosis and depression relationship.56,59 These routes in gut-brain bidirectional communication form a complex network of mechanisms; their interactions complicate investigations of mechanisms involved in gut microbiota regulation of depression.
Fig. 2.
The potential role of the microbiota-gut-brain axis in the pathogenesis and microbiota-targeting therapies for depression. FMT, Fecal microbiota transplantation; HPA, Hypothalamic–pituitary–adrenal; SCFAs, Short-chain fatty acids; LPS, Lipopolysaccharide; TMAO, Trimethylamine-N-oxide; IL-6, Interleukin-6; IL-1β, Interleukin-1β; TNF-α, Tumor necrosis factor-alpha.
The interactions between gut microbiota and antidepressants
The effects of antidepressants on gut microbiota composition
Currently, drugs interventions are still the therapeutic mainstays in depression. Drugs, especially those taken orally, have a significant effect on gut microbial composition and function. Interactions between drugs and microbiota can alter bacterial metabolism and drug activity and efficacy. There is evidence that clinically utilized antidepressants have antimicrobial effects, especially on gram-positive bacteria.60 Selective serotonin reuptake inhibitor antidepressants (SSRIs), tricyclic antidepressants, and others alter the microbial diversity and composition (Table 1).60, 61, 62, 63, 64, 65, 66, 67, 68 As reported, SSRIs treatment increase the abundance of intestinal Eubacterium ramulus while tricyclic antidepressants increase the abundance of Clostridium leptum.69 Duloxetine also markedly increases the abundance of Eubacterium rectale by more than 100-fold when compared to a no-drug group.70 Since these bacteria are known to produce anti-inflammatory butyrate during bacterial metabolism, their increased abundance may impose an adjuvant effect on antidepressant therapy, thereby increasing the beneficial drug effects.71 These data suggest that antidepressants alter gut microbial composition and function, and that this may explain part of their antidepressant effects. In addition, a prospective cohort study demonstrated that gut microbiota may be used as a predictor of antidepressant treatment outcome in geriatric depression; enrichment of Faecalibacterium, Roseburia and Agathobacter relative to Lachnoclostridium (a reference frame) at baseline was associated with depression remission.72
Table 1.
Effects of antidepressants on the composition of gut microbiota and its metabolites.
| Antidepressant drugs | Effect on α-diversity | Effect on β-diversity | Decreased taxa | Increased taxa | Involved metabolites |
|---|---|---|---|---|---|
| SSRIs antidepressants | |||||
| Paroxetine | Faith's PD↓62 | Significant65 | p_Firmicutes,62 c_Vampirivibrionia,65 o_Clostridia UCG-014,65 o_Clostridiales,65 o_Gastranaerophilales,65 f_Clostridia UCG-014,65 f_Clostridiaceae,65 f_Gastranaerophilales,65 g_Clostridia UCG-014,65 g_Clostridium sensu stricto 1,65 g_Acetatifactor,65 g_Papillibacter65 | p_Bacteroidetes,62 f_Enterococcaceae,65 g_Erysipelatoclostridium,65 g_Faecalibaculum,65 g_Enterococcus,65 g_Frisingicoccus,65 g_Lachnospiraceae UCG-001,65 g_Anaerotroncus,65 g_Paludicola,65 s_Eubacterium ramulus,69 s_Streptococcus salivarius69 | Bile acids,62 neuroactive steroids65 |
| Sertraline | NA | NA | s_Staphylococcus aureus,67 s_Bacillus subtilus,67 s_Escherichia coli,67 s_Shigella dysenteriae,67 s_Salmonella spp.,67 s_Vibrio cholerae,67 s_Candida albicans,67 s_Candida tropicalis,67 s_Candida parapsilosis,67 s_Aspergillus spp.,67 s_Corynebacterium urealyticum67 | NA | NA |
| Citalopram | NA | NA | g_Akkermansia,66 s_Escherichia coli,60 s_Eubacterium rectale,60 s_Faecalibacterium prausnitzii,60 s_Pseudomonas aeruginosa60 | NA | 5-HT and 5-HIAA66 |
| Escitalopram | Ace↑,64 Faith's PD↓73 | Significant64,73 | p_Firmicutes,68 s_Escherichia coli,60 s_Lactobacillus rhamnosus,60 s_Pseudomonas aeruginosa,67 s_Klebsiella pseumoniae,67 s_Proteus mirabilis,67 s_Enterobacter cloacae,67 s_Ruminococcus flavefaciens,73 s_Adlercreutzia equolifaciens73 | p_Bacteroidetes,68 g_Bilophila,73 g_Desulfovibrio,73 g_Christensenellaceae_R-7_group,68 g_[Eubacterium] _ruminantium_group,68 g_Fusobacterium68 | NA |
| Fluoxetine | Shannon↑,61 Faith's PD↓73 | Significant61,73 | p_Firmicutes,61 g_Prevotella,63 g_Ruminococcus,63 s_ Escherichia coli,60 s_Lactobacillus rhamnosus,60 s_Ruminococcus flavefaciens,73 s_Adlercreutzia equolifaciens,73 s_Candida albicans,60 s_Haemophilus influenzae,67 s_Moraxella catarrhales,67 s_Campylobacter jejuni,67 s_Aspergillus spp.,67 s_Candida parapsilosis67 | p_Bacteroidetes,61 f_Porphyromonadaceae,61 f_Mogibacteriaceae,73 g_Bacteroides,63 g_Parabacteroides,61 g_Butyricimonas,61 g_Alistipes,61 g_Acetatifactor,61 g_Candidatus_Arthromitus,73 | Amino acids, bile acids, and short-chain fatty acids63 |
| SNRIs antidepressants | |||||
| Venlafaxine | Faith's PD↓73 | Significant73 | s_ Escherichia coli,60 s_Lactobacillus rhamnosus,60 s_Pseudomonas aeruginosa,60 s_Ruminococcus flavefaciens,73 s_Adlercreutzia equolifaciens73 | NA | NA |
| Duloxetine | Faith's PD↓73 | Significant73 | s_Ruminococcus flavefaciens,73 s_Adlercreutzia equolifaciens73 | s_ Eubacterium rectale70 | NA |
| Tricyclic antidepressants | |||||
| Amitriptyline | Observed diversity↑61 | Significant61 | g_Ruminococcaceae_UCG-014,61 s_Staphylococcus spp.,60 s_Bacillus spp.,60 s_Vibrio cholerae,60,67 s_Klebsiella pneumoniae,67 s_Shigella spp.,67 s_Bacillus spp.,67 s_Salmonella spp.,67 s_Escherichia coli,67 s_Vibrio parahaemolyticus,67 s_Pseudomonas aeruginosa,67 s_Lactobacillus sporogenes,67 s_Citrobacter spp.,67 s_Cryptococcus spp.67 | f_Porphyromonadaceae,61 f_Bacteroidaceae,61 g_Bacteroides,61 g_Parabacteroides,61 g_Butyricimonas,61 g_Alistipes,61 g_Tyzzerella,61 g_Ruminiclostridium_161 | NA |
| Desipramine | NA | Significant73 | g_ Ruminococcus,60 g_ Adlercreutzia,60 s_Akkermansia muciniphila,60 s_Escherichia coli,60 s_Ruminococcus flavefaciens73 | F_Lactobacillaceae,73 g_Lactobacillus,73 g_Dorea73 | NA |
| Imipramine | NA | NA | s_Escherichia coli,60 s_Yersinia enterocolitica60 | NA | NA |
| Maprotiline | NA | NA | g_Francisella60 | NA | NA |
| Clomipramine | NA | NA | g_Francisella,60 s_ Francisella novicida60 | NA | NA |
| MAOIs antidepressants | |||||
| Phenelzine | NA | NA | s_Eubacterium rectale,60 s_Faecalibacterium prausnitzii,60 | NA | NA |
Notes: SSRIs, Selective serotonin reuptake inhibitors; SNRIs, Serotonin and noradrenalin reuptake inhibitors; MAOIs, Monoamine oxidase inhibitors; PD, Phylogenetic diversity; 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-Hydroxyindole-3-acetic acid.
NA represents no data.
The effects of gut microbiota on individual's response to antidepressants
Gut microbiota can also enzymatically alter the bioactivity, bioavailability, or toxicity of antidepressant drugs, subsequently altering their efficacy and adverse effect profiles.74 Studies have reported that various pathobionts such as Streptococcus salivarius, B. uniformis, B. thetaiotaomicron, and E. coli IAI1 increase the bioaccumulation of duloxetine, which might reduce its efficacy by decreasing its bioavailability.70 In addition, the Ruminococcus flavefaciens-duloxetine interaction also has been shown to attenuate the antidepressant effects of this drug.73 A multicenter antidepressant effectiveness study (the STAR∗D cohort) in patients with MDD reported on a subset of patients with a high risk for treatment resistance,75 which is a key issue in depression research and clinical management. As expected, antidepressant treatment-resistance is associated with a specific microbial composition; for instance, an increased abundance of bacteria assigning to Proteobacteria phylum, such as Citrobacter and Yersinia.76 Ketamine shows long-lasting antidepressant effects and can be effective for treatment-resistant patients. Gut microbiota is an important modulator of the antidepressant effects of Ketamine and its enantiomer R-ketamine.77 Phylum Actinobacteria, class Coriobacteriia, and SCFAs producing genera, such as Butyricimonas and Turicibacter, might contribute to the robust antidepressant effects of ketamine and its enantiomer in depression.78,79 Furthermore, due to the effects of microbiota on drug toxicity, the chronic use of most drugs, from all categories, potentially causes various adverse effects in some patients,80 including an increased risk of diabetes, obesity, gastrointestinal symptoms and cardiovascular diseases. Correcting gut dysbiosis by using probiotics or other interventional strategies might help reverse the antidepressant-associated adverse effects.80
It is worth mentioning that most previous studies only assessed the drug–microbiota interactions for individual drugs. However, patients with MDD are often prescribed multiple antidepressants and medications for comorbidities; this polypharmacy approach may cause bias in the interpretation of drug–microbiota interactions. Importantly, the actual drug–microbiota interactions are far more complex than suggested by current reductionist findings from preclinical studies. With the adoption of pharmacomicrobiomics in depression, a deeper understanding of the bidirectional interactions between drugs and the gut microbiome will emerge that explains how antidepressants affect gut microbial composition and function and how the microbiome, in turn, metabolizes drugs to improve or impede their efficacy. Slowly, this knowledge will build a theoretical foundation of how we can exploit the gut microbiome to improve the therapeutic efficacy of antidepressants.
The clinical application prospects of gut microbiota
Unlike the host's genome, the gut microbiome is dynamic, diverse, and capable of external modulation. Dietary control, supplementation with live microorganisms (single or mixed species), and taking molecular substances that are beneficial for microbiota growth can manipulate the microbial composition and function to maintain a healthy balance in the gut. This highlights the possibility of the gut microbiota as a novel therapeutic target for depression. Accumulating evidence supports the efficacy of various microbiota-target therapeutics in alleviating depression, including dietary interventions, FMT, probiotics, prebiotics, synbiotics, and postbiotics.
Dietary interventions
Diet is a key determinant of gut microbial composition and function. The associations between diet, gut microbiota, and depression have been increasingly explored. The Mediterranean diet (MD), which is rich in dietary polyphenols and fibers and has anti-inflammatory properties, is thought to be broadly beneficial to human health. After an average 20.4-year follow-up of 49,261 Swedish women, a lower risk of depression was found in individuals who adhered more closely to an MD dietary pattern,81 suggesting that MD may possess antidepressant properties. A 12-week randomized controlled trial (RCT) found that MD significantly relieved depressive symptoms and increased the quality of life in young males with clinical depression.82 The MD diet is associated with a higher gut microbial diversity, lower levels of gastrointestinal inflammation, and the integrity of the intestinal barrier.83 Microbiota-derived SCFAs, especially propionate and butyrate, act as a key mechanistic link between MD and gut pathophysiology.83 Yang et al.84 evaluated the relationship between relative carbohydrate intake and depression based on genetic information from over 400,000 people and found that a 16% increase in carbohydrate-based calories was associated with a 58% decrease in the risk of depression; this protective effect has some causality. In general, a healthy dietary pattern that includes high levels of plant compounds, vitamins, minerals, polyunsaturated fatty acids, and dietary fibers was recommend for patients who have or are predisposed to depression. Due to individual differences in the genome and gut microbiome, the use of a personalized diet was suggested. However, dietary interventions alone should be used cautiously in the treatment of clinical depression; these need to be combined with other interventions, such as antidepressant drugs, psychological interventions, and lifestyle regulation.
FMT
FMT is a rapid approach to reshaping the patient's gut microbiota by the administration of fecal flora from healthy donors; this makes it a promising intervention strategy for chronic diseases associated with dysbiosis. In preclinical studies, FMT has been widely documented as being able to alleviate depressive-like behavior. Transplanting microbiota from healthy donors relieved alcohol-induced depressive-like behavior in mice,85 and also improved a stress-induced depression phenotype in rats by suppressing neuroinflammation, correcting the imbalance in gut microbiota, and repairing intestinal barrier damage.86 Bacteriophages may act as a mediator in the efficacy of FMT.87 In addition to the global microbiota transplantation during FMT procedure, mono-colonization with Lactobacillus plantarum protects against depression-like states in Drosophila.88 The vagus nerve might be an important signaling route in the gut-brain axis regulating the protective effects of FMT on depression.89 In patients with diarrhea-predominant irritable bowel syndrome (IBS-D), FMT treatment gradually improved depression symptoms, regardless of gastrointestinal symptom remission.90 These positive therapeutic effects in patients with concomitant IBS-D, anxiety, and depression were further verified in an RCT.91 Another RCT assessed the efficacy of oral frozen FMT-capsules as an add-on therapy in MDD patients and found that depressive symptoms improved significantly 4 weeks after transplantation.92 Similar to autism, FMT therapy for depression also improves the gastrointestinal symptoms and rebalances the gut ecosystem.93 Although some articles have reported the adverse effects and complications of FMT therapy,94 this treatment approach is gaining traction in scientific research and clinical fields, especially an alternative pill derived from human feces that achieves the same effects with less invasive and more standardized.95
Probiotics
Probiotics are live microorganisms that benefit host health when administered in adequate amounts. Probiotics are the most studied microbiota-targeting therapies for depression. A previous comprehensive study identified 178 species and subspecies probiotics that can attenuate depression; most probiotic regimens for depression treatment are based on Lactobacillus spp. and Bifidobacterium spp.3 RCTs have demonstrated the efficacy of various probiotic species in the treatment of clinical depression (Table 2)96, 97, 98, 99, 100, 101, 102, 103; these include L. helveticus, L. rhamnosus, B. longum, and B. breve CCFM1025. However, it is important to note that some studies have reported negative results.104,105 Although unknown, heterogeneity in the cohort characteristics and/or the interventions may underlie these mixed findings. An umbrella meta-analysis reported a robust effect of probiotics on relieving depression symptoms in adults only when administered for >8 weeks at a dosage of >10 × 109 colony forming units.106 However, to accurately screen probiotics for use in MDD treatment, we must understand the structure and function of gut microbiota and the effects of various confounding factors (e.g., geographical region, age, comorbidity) in patients with depression. Moreover, the interaction of multiple species can produce synergistic effects, enhancing the antidepressant efficacy of probiotics, suggesting that the judicious use of multi-species probiotics may be more effective than single species.107 Furthermore, in recent years, a group of microorganisms that perform special functions in the host and are very difficult to cultivate have shown great probiotic potential; these are called the “next generation probiotics” and include Akkermansia spp. and Clostridium spp. A. muciniphila and C. butyricum miyairi 588 were shown to ameliorate stress-induced depressive-like behavior in mice.99,108,109 However, there is still a gap between laboratory research and clinical application. To achieve clinical translation, researchers must first overcome this obstacle.
Table 2.
Antidepressant efficacy of probiotics assessed by randomized controlled trials.
| Study | Population | Intervention | Control | Treatment detail | Outcome measure | Depressive symptom score changes | Microbiota changes | Gut-brain axis changes |
|---|---|---|---|---|---|---|---|---|
| Akkasheh G et al. 201696 | MDD (N = 40) | Probiotics (N = 20): Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum | Placebo (N = 20): starch | 8 weeks, 2 × 109 CFU/g/day for each species | BDI | Significant decrease in BDI score in probiotic group compared to placebo over 8 weeks. | NA | Serum insulin↓, hs-CRP↓, plasma glutathione↑ |
| Kazemi A et al. 201997 | MDD (N = 110) | Probiotics (N = 38): Lactobacillus helveticus R0052, Bifidobacterium longum R0175 Prebiotics (N = 36): galactooligosaccharide | Placebo (N = 36): excipients | 8 weeks, 10 × 109 CFU/5 g/day | BDI | Significant decrease in BDI score in probiotic group compared to prebiotic and placebo over 8 weeks. | NA | Serum kynurenine/tryptophan ratio↓, tryptophan/isoleucine ratio↑ |
| Messaoudi M et al. 201198 | Health volunteers (N = 55) | Probiotics (N = 26): Lactobacillus helveticus R0052, Bifidobacterium longum R0175 | Placebo (N = 29): excipients | 30 days, 3 × 109 CFU/1.5 g/day | HSCL-90 | Significant decrease in HSCL-90 depression subscore in probiotic group compared to placebo over 30 days. | NA | NA |
| Miyaoka T et al. 201899 | TR-MDD (N = 40) | Probiotics (N = 20): Clostridium butyricum MIY AIRI 588 (CBM588) + antidepressants | Placebo (N = 20): antidepressants | 8 weeks, 60 mg/day | HAMD-17 | Significant decrease in HAMD-17 score in probiotic group compared to placebo over 8 weeks. | NA | NA |
| Schaub AC et al. 2022100 | MDD (N = 60) | Probiotics (N = 30):Streptococcus thermophilus NCIMB 30438, Bifidobacterium breve NCIMB30441, Bifidobacterium longum NCIMB 30435, Bifidobacterium infantis NCIMB 30436, Lactobacillus acidophilus NCIMB 30442, Lactobacillus plantarum NCIMB 30437, Lactobacillus paracasei NCIMB 30439, Lactobacillus delbrueckii subsp.Bulgaricus NCIMB 30440 | Placebo (N = 30): maltose | 4 weeks (31 days), 9 × 1011 CFU/day | HAMD | A significant stronger decrease of HAMD scores in the probiotics compared to the placebo group over 4 weeks | Lactobacillus↑ | Probiotics increase gray matter volume in calcarine sulcus and alter putamen's activation during emotion processing |
| Slykerman RF et al. 2017101 | Pregnancy woman (N = 423) | Probiotics (N = 212): Lactobacillus rhamnosus HN001 | Placebo (N = 211): corn-derived maltodextrin | From enrolment until 6 months postpartum if breastfeeding, 6 × 109 CFU/day | EPDS | Significant lower depression scores in probiotic group compared to placebo from enrolment until 6 months postpartum if breastfeeding | NA | NA |
| Tian P et al. 2022102 | MDD (N = 51) | Probiotics (N = 25): Bifidobacterium breve CCFM1025 | Placebo (N = 26): maltodextrin | 4 weeks, 1 × 1010 CFU/day | HDRS-24 | Significant decrease in HDRS-24 score in probiotic group compared to placebo over 4 weeks | Desulfovibrio↑, Faecalibaculum↑ | Serum 5-HIAA↓ |
| Zhang X et al. 2021103 | MDD with constipation (N = 82) | Probiotics (N = 41): Lacticaseibacillus paracaseiYIT 9029 | Placebo (N = 41) | 9 weeks, 1.0 × 1010 CFU/day | BDI | Significant decrease in BDI score in probiotic group over 9 weeks. | Veillonella↑, Neisseria↑, Ralstonia↑, Eggerthella↑, Rikenellaceae_RC9_gut_group↓, Sutterella↓, Oscillibacter↓, Rothia ↓ | Serum IL-1β↓, IL-6↓, and TNF-α↓ |
Notes: MDD, Major depressive disorder; TR-MDD, Treatment-resistant major depressive disorder; CFU, Colony forming units; BDI, Beck Depression Inventory; HSCL-90, Hopkins Symptom Checklist-90; HAMD, Hamilton Depression Rating Scale; EPDS, Edinburgh Postnatal Depression Scale; HDRS-24, Hamilton Depression Rating scale-24; hs-CRP, Hypersensitive c-reactive protein; 5-HIAA, 5-Hydroxyindole-3-acetic acid; IL-1β, Interleukin-1β; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor-α.
NA represents no data.
Prebiotics
Prebiotics are substrates that are beneficial for host health by being selectively utilized by gut microbiota. This can promote the growth of some beneficial bacteria. A previous study summarized the preclinical and clinical evidence for the antidepressant effects of various prebiotics.3 Fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) are the most documented prebiotics in the treatment of depression, along with polyphenols, inulin, and compounds from vegetables, herbs, and plants. For example, chronic prebiotic FOS and GOS administration improves stress-induced depressive-like behaviors in C57BL/6J male mice; this is accompanied by the normalization of gut microbiota, reduced pro-inflammatory cytokines, and an increase of cecal acetate and propionate, these changes are related to behavior improvement.110 However, the efficacy of prebiotics in the treatment of depression has not been consistent across studies. A previous RCT compared the effects of probiotic (L. helveticus and B. longum) and prebiotic (GOS) supplementation on depression remission in MDD patients and found that 8 weeks of supplementation with probiotics, but not prebiotics, improved the depression symptoms.97 Consistently, administration of 4G-β-d-galactosylsucrose for 24 weeks improved self-efficacy in patients with depression in an RCT, but not their depressive symptoms.111 Prebiotics do not exert a direct effect on the body but rather indirectly benefit host health by promoting the growth of probiotics. Thus, prebiotics have often been used together with probiotics in depression treatment.
Synbiotics
The development of probiotics and prebiotics has led to the development of synbiotics which combine the advantages of both. A synbiotic supplementation (containing L. acidophilus, L. casei and B. bifidum plus inulin) improved depressive symptoms in overweight or obese adults,112 and another symbiotic supplement (L. acidophilus strain T16, B. bifidum strain BIA-6, B. lactis strain BIA-7 and B. longum strain BIA-8 plus FOS, GOS, and inulin) was found to decrease depression scores in hemodialysis patients and increase serum levels of brain-derived neurotrophic factor in a subgroup of patients with depression.113,114 Given the symbiotic relationships between probiotics and prebiotics, synbiotics are likely to drive the next breakthroughs in depression treatment. The simultaneous administration of probiotics and prebiotics is believed to improve the activity of beneficial gut bacteria. However, the formulation of effective synbiotics is contingent on the careful selection of the correct probiotic strains and prebiotics.
Postbiotics
The inanimate microbial cells and/or their components and metabolites that are beneficial to host health are defined as postbiotics. This class offers a novel microbial therapeutic approach for depression. Studies have reported that pretreatment with heat-killed probiotic L. plantarum-derived postbiotics, particularly their metabolites, protected mice from Salmonella-induced depressive-like behavior by modulating the gut-brain axis.115 Some bacterial metabolites such as SCFAs and BAs act as postbiotics. Oral treatment with sodium butyrate restored paclitaxel-induced alterations in microbiota composition and gut barrier integrity and relieved depressive-like behaviors in mice.116 Despite the apparent antidepressant effects of probiotics, the potential adverse effects associated with administrating live bacteria to seriously ill patients or other vulnerable populations remain unknown. At present, there is still a lack of sufficient scientific evidence to properly eliminate this concern. Therefore, postbiotics that exert similar health benefits to probiotics might offer a novel and safer alternative to probiotics for the treatment of depression. Although there are few studies on postbiotic-based depression treatments, postbiotics offer a promising approach to utilizing microbial therapeutics for this condition.
Limitations
Some limitations of this review need to be mentioned. Firstly, because of the limited space, discussion on some content was not expanded, while this does not affect the academic reading comprehension. Furthermore, under the current trend of research, this review focused more on the potential of gut microbiota in the treatment of depression. Therefore, we did not discussed the gut-brain pathological mechanisms involved in gut microbiota regulation of depression with a large space. Finally, narrative review has its own limitations, in order to better evaluate the effects of gut microbiota on antidepressant drugs and the efficacy of gut microbiota-targeted intervention strategies for depression, relevant systematic reviews were required in the future.
Summary and prospects
Gut microbiota plays a vital role in regulating human health. In recent years, abundant research has uncovered the effects of gut microbiota and its metabolites on the pathogenesis of depression. Microbiota-based diagnostics and therapeutics for depression are the future directions of research. In moving the field forward, many key challenges need to be addressed and some recommendations provided might be useful: (1) Exploring the causal relationship between gut microbiome and depression. More prospective studies are required to analyze the interplay between the development of dysbiosis and depression, and drug–microbiota interactions, to uncover the causal relationships between dysbiosis, depression, and treatment. In particular, we need to better understand if the antidepressant effects of drugs can be enhanced by modulating gut microbiota; (2) Elaborating the pathological mechanism in MGB axis and identifying the key strains. Growing preclinical and clinical evidence suggests that microbiota-based therapies can result in the remission of depression, but little is known about their mechanisms of action, the key microbiota, and how these bacteria interact; (3) Isolating the bacteria to construct the disease-based gut microbial biobank. More studies are required that utilize high-throughput sequencing, multi-omics approaches, and microbial culture technology to isolate the pathogenic and beneficial strains involved in depression, identify the exact functions of these strains, and uncover the underlying mechanisms involved in the gut-brain axis, to build a disease-based strain resource database; (4) Analyzing the confounding effects to promote the development of microbiota-based diagnostics and therapeutics. The effects of various confounding factors should not be ignored when analyzing the changes of microbial structure and function in depression. We must better understand how the composition, proportion, and abundance changes of gut microbiota contribute to various cases of MDD. This will build a foundation for targeted therapeutic manipulation and finally impel the uptake of microbiota-based personalized precision medicine for depression; (5) Developing new tools. New methodological tools such as isotope tracing, engineered and CRISPR-edited strains are instrumental to moving the development of microbiota-based treatment strategies forward in depression, and this is a next frontier in microbiome research.
Outstanding questions
Dysbiosis is associated with depression, although specific gut microbial changes are not consistently identified across studies. Gut microbiota is easily influenced by external factors such as diet; the compositions of gut microbiota in two healthy individuals are likely to be markedly different. Therefore, the effects of various confounding factors on gut microbiota should be clarified in future studies. In addition, large-scale prospective studies are required to (1) identify the characteristic microbial changes of depression that change in parallel with depression symptoms, (2) determine the pathogenic and beneficial strains involved in depression and their cause-effect relationships, and (3) elaborate the underlying gut-brain mechanisms that are involved in the pathogenesis of depression. Ultimately, this work should generate a disease-related strain resource database. Furthermore, in the complex intestinal environment, there are myriad bacteria–bacteria and bacteria–metabolite interactions that need to be considered when exploring the function of specific bacterial strains and developing gut microbiota-target therapeutics. Much remains unknown about the microbiota-host relationships and future work is needed to further describe these interactions and the possible influence of interventions targeting microbiota on the host. Indeed, there are still many challenges to developing gut microbiota-target therapeutics for depression, and we hope that more new technologies and approaches will emerge in this field to address these challenges.
Search strategy and selection criteria.
Data for this review were identified by searches of PubMed and references from relevant articles using the search terms: “microbiome” or “microbiota” or “metabolites” or “fecal microbiota transplantation” or “FMT” or “probiotics” or “prebiotics” or “synbiotics” or “postbiotics” and “depression” or “depressive” or “antidepressant∗”. Only articles published in English up to Oct 14, 2022 were included.
Contributors
Lanxiang Liu and Peng Xie contributed to the conception and design of the review. Lanxiang Liu and Haiyang Wang written the first draft of the manuscript. Xueyi Chen, Yangdong Zhang and Hanping Zhang created all the figures and tables. Peng Xie critically revised the manuscript. Lanxiang Liu and Haiyang Wang contributed equally to this article, and all authors approved the submitted version.
Declaration of interests
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Natural Science Foundation Project of China (No. 82201683 and No. 81820108015), the China Postdoctoral Science Foundation (No. 2020TQ0393, No. 2020M683634XB, and No. 2021M693926), the Chongqing Science and Technology Commission (No. cstc2021jcyj-bshX0201, No. cstc2021jcyj-bshX0150), and Special Funding for Chongqing Postdoctoral Research Projects (No. 2021XMT001). The funders had no involvement in paper design, data collection, data analysis, interpretation, writing of the paper.
References
- 1.Santomauro D.F., Mantilla Herrera A.M., Shadid J., et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng P., Zeng B., Zhou C., et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- 3.Liu L., Wang H., Zhang H., et al. Towards a deeper understanding of gut microbiome in depression: the promise of clinical applicability. Adv Sci (Weinh) 2022;9 doi: 10.1002/advs.202203707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohler O., Petersen L., Mors O., et al. Infections and exposure to anti-infective agents and the risk of severe mental disorders: a nationwide study. Acta Psychiatr Scand. 2017;135:97–105. doi: 10.1111/acps.12671. [DOI] [PubMed] [Google Scholar]
- 5.Lavebratt C., Yang L.L., Giacobini M., et al. Early exposure to antibiotic drugs and risk for psychiatric disorders: a population-based study. Transl Psychiatry. 2019;9:317. doi: 10.1038/s41398-019-0653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lurie I., Yang Y.-X., Haynes K., Mamtani R., Boursi B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry. 2015;76:1522–1528. doi: 10.4088/JCP.15m09961. [DOI] [PubMed] [Google Scholar]
- 7.Pouranayatihosseinabad M., Bezabih Y., Hawrelak J., Peterson G.M., Veal F., Mirkazemi C. Antibiotic use and the development of depression: a systematic review. J Psychosom Res. 2023;164 doi: 10.1016/j.jpsychores.2022.111113. [DOI] [PubMed] [Google Scholar]
- 8.Guida F., Turco F., Iannotta M., et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav Immun. 2018;67:230–245. doi: 10.1016/j.bbi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Marx W., Lane M., Hockey M., et al. Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry. 2021;26:134–150. doi: 10.1038/s41380-020-00925-x. [DOI] [PubMed] [Google Scholar]
- 10.Herselman M.F., Bailey S., Bobrovskaya L. The effects of stress and diet on the "Brain-Gut" and "Gut-Brain" pathways in animal models of stress and depression. Int J Mol Sci. 2022;23:2013. doi: 10.3390/ijms23042013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raes J. Nifty new tools for microbiome treatment design. Nat Rev Gastroenterol Hepatol. 2023;20:77–78. doi: 10.1038/s41575-022-00735-2. [DOI] [PubMed] [Google Scholar]
- 12.Nikolova V.L., Hall M.R.B., Hall L.J., Cleare A.J., Stone J.M., Young A.H. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry. 2021;78:1343–1354. doi: 10.1001/jamapsychiatry.2021.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson C.A., Diaz-Arteche C., Eliby D., Schwartz O.S., Simmons J.G., Cowan C.S.M. The gut microbiota in anxiety and depression–a systematic review. Clin Psychol Rev. 2021;83 doi: 10.1016/j.cpr.2020.101943. [DOI] [PubMed] [Google Scholar]
- 14.Zheng P., Yang J., Li Y., et al. Gut microbial signatures can discriminate unipolar from bipolar depression. Adv Sci (Weinh) 2020;7 doi: 10.1002/advs.201902862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J., Zheng P., Li Y., et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuinness A.J., Davis J.A., Dawson S.L., et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psychiatry. 2022;27:1920–1935. doi: 10.1038/s41380-022-01456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martino C., Dilmore A.H., Burcham Z.M., Metcalf J.L., Jeste D., Knight R. Microbiota succession throughout life from the cradle to the grave. Nat Rev Microbiol. 2022;20(12):707–720. doi: 10.1038/s41579-022-00768-z. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y., Nguyen M., Khetrapal V., et al. Within-host evolution of a gut pathobiont facilitates liver translocation. Nature. 2022;607:563–570. doi: 10.1038/s41586-022-04949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J.-J., He S., Fang L., et al. Age-specific differential changes on gut microbiota composition in patients with major depressive disorder. Aging (Albany NY) 2020;12:2764–2776. doi: 10.18632/aging.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly J.R., Borre Y., O' Brien C., et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Chidambaram S.B., Essa M.M., Rathipriya A.G., et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: tales of a vicious cycle. Pharmacol Ther. 2022;231 doi: 10.1016/j.pharmthera.2021.107988. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Fan Q., Hou Y., et al. Bacteroides species differentially modulate depression-like behavior via gut-brain metabolic signaling. Brain Behav Immun. 2022;102:11–22. doi: 10.1016/j.bbi.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Medina-Rodriguez E.M., Madorma D., O'Connor G., et al. Identification of a signaling mechanism by which the microbiome regulates Th17 cell-mediated depressive-like behaviors in mice. Am J Psychiatry. 2020;177:974–990. doi: 10.1176/appi.ajp.2020.19090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin Y., Havulinna A.S., Liu Y., et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54:134–142. doi: 10.1038/s41588-021-00991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li D., Liu R., Wang M., et al. 3beta-Hydroxysteroid dehydrogenase expressed by gut microbes degrades testosterone and is linked to depression in males. Cell Host Microbe. 2022;30:329–339.e5. doi: 10.1016/j.chom.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Ludington W.B. Higher-order microbiome interactions and how to find them. Trends Microbiol. 2022;30:618–621. doi: 10.1016/j.tim.2022.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Shkoporov A.N., Turkington C.J., Hill C. Mutualistic interplay between bacteriophages and bacteria in the human gut. Nat Rev Microbiol. 2022;20(12):737–749. doi: 10.1038/s41579-022-00755-4. [DOI] [PubMed] [Google Scholar]
- 28.Wu J., Chai T., Zhang H., et al. Changes in gut viral and bacterial species correlate with altered 1,2-diacylglyceride levels and structure in the prefrontal cortex in a depression-like non-human primate model. Transl Psychiatry. 2022;12:74. doi: 10.1038/s41398-022-01836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H.-Y., Pan L.-Y., Zhang X., Zhang Z., Zhou Y.-Y., Ruan B. Altered gut bacterial-fungal interkingdom networks in patients with current depressive episode. Brain Behav. 2020;10 doi: 10.1002/brb3.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musumeci S., Coen M., Leidi A., Schrenzel J. The human gut mycobiome and the specific role of Candida albicans: where do we stand, as clinicians? Clin Microbiol Infect. 2022;28:58–63. doi: 10.1016/j.cmi.2021.07.034. [DOI] [PubMed] [Google Scholar]
- 31.Nicolas G.R., Chang P.V. Deciphering the chemical lexicon of host-gut microbiota interactions. Trends Pharmacol Sci. 2019;40:430–445. doi: 10.1016/j.tips.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skonieczna-Żydecka K., Grochans E., Maciejewska D., et al. Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients. 2018;10:1939. doi: 10.3390/nu10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van de Wouw M., Boehme M., Lyte J.M., et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596:4923–4944. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caspani G., Kennedy S., Foster J.A., Swann J. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microb Cell. 2019;6:454–481. doi: 10.15698/mic2019.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun N., Zhang J., Wang J., et al. Abnormal gut microbiota and bile acids in patients with first-episode major depressive disorder and correlation analysis. Psychiatry Clin Neurosci. 2022;76:321–328. doi: 10.1111/pcn.13368. [DOI] [PubMed] [Google Scholar]
- 36.Mudimela S., Vishwanath N.K., Pillai A., et al. Clinical significance and potential role of trimethylamine N-oxide in neurological and neuropsychiatric disorders. Drug Discov Today. 2022;27 doi: 10.1016/j.drudis.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meinitzer S., Baranyi A., Holasek S., et al. Sex-specific associations of trimethylamine-N-oxide and Zonulin with signs of depression in carbohydrate malabsorbers and nonmalabsorbers. Dis Markers. 2020;2020 doi: 10.1155/2020/7897240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li T., Zheng L.-N., Han X.-H. Fenretinide attenuates lipopolysaccharide (LPS)-induced Blood-Brain Barrier (BBB) and depressive-like behavior in mice by targeting Nrf-2 signaling. Biomed Pharmacother. 2020;125 doi: 10.1016/j.biopha.2019.109680. [DOI] [PubMed] [Google Scholar]
- 39.Rudzki L., Stone T.W., Maes M., Misiak B., Samochowiec J., Szulc A. Gut microbiota-derived vitamins–underrated powers of a multipotent ally in psychiatric health and disease. Prog Neuropsychopharmacol Biol Psychiatry. 2021;107 doi: 10.1016/j.pnpbp.2020.110240. [DOI] [PubMed] [Google Scholar]
- 40.Ortega M.A., Alvarez-Mon M.A., García-Montero C., et al. Gut microbiota metabolites in major depressive disorder-deep insights into their pathophysiological role and potential translational applications. Metabolites. 2022;12:50. doi: 10.3390/metabo12010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 42.Cryan J.F., O'Riordan K.J., Cowan C.S.M., et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh S., Whitley C.S., Haribabu B., Jala V.R. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol Hepatol. 2021;11:1463–1482. doi: 10.1016/j.jcmgh.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kronsten V.T., Tranah T.H., Pariante C., Shawcross D.L. Gut-derived systemic inflammation as a driver of depression in chronic liver disease. J Hepatol. 2022;76:665–680. doi: 10.1016/j.jhep.2021.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Kiecolt-Glaser J.K., Wilson S.J., Bailey M.L., et al. Marital distress, depression, and a leaky gut: translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology. 2018;98:52–60. doi: 10.1016/j.psyneuen.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slyepchenko A., Maes M., Jacka F.N., et al. Gut microbiota, bacterial translocation, and interactions with diet: pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother Psychosom. 2017;86:31–46. doi: 10.1159/000448957. [DOI] [PubMed] [Google Scholar]
- 47.Fried S., Wemelle E., Cani P.D., Knauf C. Interactions between the microbiota and enteric nervous system during gut-brain disorders. Neuropharmacology. 2021;197 doi: 10.1016/j.neuropharm.2021.108721. [DOI] [PubMed] [Google Scholar]
- 48.Pu Y., Tan Y., Qu Y., et al. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav Immun. 2021;94:318–326. doi: 10.1016/j.bbi.2020.12.032. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J., Ma L., Chang L., Pu Y., Qu Y., Hashimoto K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry. 2020;10:186. doi: 10.1038/s41398-020-00878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aaronson S.T., Sears P., Ruvuna F., et al. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am J Psychiatry. 2017;174:640–648. doi: 10.1176/appi.ajp.2017.16010034. [DOI] [PubMed] [Google Scholar]
- 51.Jiang Y., Liu Y., Gao M., Xue M., Wang Z., Liang H. Nicotinamide riboside alleviates alcohol-induced depression-like behaviours in C57BL/6J mice by altering the intestinal microbiota associated with microglial activation and BDNF expression. Food Funct. 2020;11:378–391. doi: 10.1039/c9fo01780a. [DOI] [PubMed] [Google Scholar]
- 52.Li W., Ali T., He K., et al. Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain Behav Immun. 2021;92:10–24. doi: 10.1016/j.bbi.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Lynch C.M.K., Nagpal J., Clarke G., Cryan J.F. Wrapping things up: recent developments in understanding the role of the microbiome in regulating myelination. Curr Opin Physiol. 2021;23 [Google Scholar]
- 54.Młynarska E., Gadzinowska J., Tokarek J., et al. The role of the microbiome-brain-gut axis in the pathogenesis of depressive disorder. Nutrients. 2022;14:37. doi: 10.3390/nu14091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chevalier G., Siopi E., Guenin-Macé L., et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat Commun. 2020;11:6363. doi: 10.1038/s41467-020-19931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu L., Wang H., Rao X., et al. Comprehensive analysis of the lysine acetylome and succinylome in the hippocampus of gut microbiota-dysbiosis mice. J Adv Res. 2021;30:27–38. doi: 10.1016/j.jare.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H., Liu L., Rao X., et al. Integrated phosphoproteomic and metabolomic profiling reveals perturbed pathways in the hippocampus of gut microbiota dysbiosis mice. Transl Psychiatry. 2020;10:346. doi: 10.1038/s41398-020-01024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng P., Wu J., Zhang H., et al. The gut microbiome modulates gut-brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression. Mol Psychiatry. 2021;26:2380–2392. doi: 10.1038/s41380-020-0744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J., Vitetta L. Mitochondria could be a potential key mediator linking the intestinal microbiota to depression. J Cell Biochem. 2020;121:17–24. doi: 10.1002/jcb.29311. [DOI] [PubMed] [Google Scholar]
- 60.Ait Chait Y., Mottawea W., Tompkins T.A., Hammami R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci Rep. 2020;10 doi: 10.1038/s41598-020-74934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang W., Qu W., Wang H., Yan H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl Psychiatry. 2021;11:131. doi: 10.1038/s41398-021-01254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dethloff F., Vargas F., Elijah E., et al. Paroxetine administration affects microbiota and bile acid levels in mice. Front Psychiatry. 2020;11:518. doi: 10.3389/fpsyt.2020.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramsteijn A.S., Jašarević E., Houwing D.J., Bale T.L., Olivier J.D.A. Antidepressant treatment with fluoxetine during pregnancy and lactation modulates the gut microbiome and metabolome in a rat model relevant to depression. Gut Microbes. 2020;11:735–753. doi: 10.1080/19490976.2019.1705728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duan J., Huang Y., Tan X., et al. Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment. Transl Psychiatry. 2021;11:303. doi: 10.1038/s41398-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diviccaro S., Giatti S., Cioffi L., et al. Paroxetine effects in adult male rat colon: focus on gut steroidogenesis and microbiota. Psychoneuroendocrinology. 2022;143 doi: 10.1016/j.psyneuen.2022.105828. [DOI] [PubMed] [Google Scholar]
- 66.Deng Y., Zhou M., Wang J., et al. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes. 2021;13:1–16. doi: 10.1080/19490976.2020.1869501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macedo D., Filho A.J.M.C., Soares de Sousa C.N., et al. Antidepressants, antimicrobials or both? gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J Affect Disord. 2017;208:22–32. doi: 10.1016/j.jad.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Shen Y., Yang X., Li G., Gao J., Liang Y. The change of gut microbiota in MDD patients under SSRIs treatment. Sci Rep. 2021;11 doi: 10.1038/s41598-021-94481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vich Vila A., Collij V., Sanna S., et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020;11:362. doi: 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klunemann M., Andrejev S., Blasche S., et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature. 2021;597:533–538. doi: 10.1038/s41586-021-03891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang R., Wang Y., Liu J., et al. Gut microbiota is involved in the antidepressant effects of adipose-derived mesenchymal stem cells in chronic social defeat stress mouse model. Psychopharmacology (Berl) 2022;239:533–549. doi: 10.1007/s00213-021-06037-w. [DOI] [PubMed] [Google Scholar]
- 72.Lee S.M., Dong T.S., Krause-Sorio B., et al. The intestinal microbiota as a predictor for antidepressant treatment outcome in geriatric depression: a prospective pilot study. Int Psychogeriatr. 2022;34:33–45. doi: 10.1017/S1041610221000120. [DOI] [PubMed] [Google Scholar]
- 73.Lukić I., Getselter D., Ziv O., et al. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl Psychiatry. 2019;9:133. doi: 10.1038/s41398-019-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weersma R.K., Zhernakova A., Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69:1510–1519. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perlis R.H. A clinical risk stratification tool for predicting treatment resistance in major depressive disorder. Biol Psychiatry. 2013;74:7–14. doi: 10.1016/j.biopsych.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fontana A., Manchia M., Panebianco C., et al. Exploring the role of gut microbiota in major depressive disorder and in treatment resistance to antidepressants. Biomedicines. 2020;8:311. doi: 10.3390/biomedicines8090311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y., Xu X., Luo A., Yang C. Ketamine. Springer; Singapore: 2020. The role of gut microbiota in the antidepressant effects of ketamine; pp. 127–141. [Google Scholar]
- 78.Huang N., Hua D., Zhan G., et al. Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol Biochem Behav. 2019;176:93–100. doi: 10.1016/j.pbb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 79.Yang C., Qu Y., Fujita Y., et al. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry. 2017;7:1294. doi: 10.1038/s41398-017-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson D., Thurairajasingam S., Letchumanan V., Chan K.-G., Lee L.-H. Exploring the role and potential of probiotics in the field of mental health: major depressive disorder. Nutrients. 2021;13:1728. doi: 10.3390/nu13051728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yin W., Löf M., Chen R., Hultman C.M., Fang F., Sandin S. Mediterranean diet and depression: a population-based cohort study. Int J Behav Nutr Phys Act. 2021;18:153. doi: 10.1186/s12966-021-01227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bayes J., Schloss J., Sibbritt D. The effect of a Mediterranean diet on the symptoms of depression in young males (the "AMMEND: A Mediterranean Diet in MEN with Depression" study): a randomized controlled trial. Am J Clin Nutr. 2022;116:572–580. doi: 10.1093/ajcn/nqac106. [DOI] [PubMed] [Google Scholar]
- 83.Seethaler B., Nguyen N.K., Basrai M., et al. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: data from the randomized controlled LIBRE trial. Am J Clin Nutr. 2022;116:928–942. doi: 10.1093/ajcn/nqac175. [DOI] [PubMed] [Google Scholar]
- 84.Yao S., Zhang M., Dong S.-S., et al. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between relative carbohydrate intake and depression. Nat Hum Behav. 2022;6(11):1569–1576. doi: 10.1038/s41562-022-01412-9. [DOI] [PubMed] [Google Scholar]
- 85.Xu Z., Liu Z., Dong X., et al. Fecal microbiota transplantation from healthy donors reduced alcohol-induced anxiety and depression in an animal model of chronic alcohol exposure. Chin J Physiol. 2018;61:360–371. doi: 10.4077/CJP.2018.BAH633. [DOI] [PubMed] [Google Scholar]
- 86.Rao J., Xie R., Lin L., et al. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur J Neurosci. 2021;53:3598–3611. doi: 10.1111/ejn.15192. [DOI] [PubMed] [Google Scholar]
- 87.Lam S., Bai X., Shkoporov A.N., et al. Roles of the gut virome and mycobiome in faecal microbiota transplantation. Lancet Gastroenterol Hepatol. 2022;7:472–484. doi: 10.1016/S2468-1253(21)00303-4. [DOI] [PubMed] [Google Scholar]
- 88.Mayneris-Perxachs J., Castells-Nobau A., Arnoriaga-Rodríguez M., et al. Microbiota alterations in proline metabolism impact depression. Cell Metab. 2022;34:681–701.e610. doi: 10.1016/j.cmet.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 89.Marcondes Ávila P.R., Fiorot M., Michels M., et al. Effects of microbiota transplantation and the role of the vagus nerve in gut-brain axis in animals subjected to chronic mild stress. J Affect Disord. 2020;277:410–416. doi: 10.1016/j.jad.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 90.Kurokawa S., Kishimoto T., Mizuno S., et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord. 2018;235:506–512. doi: 10.1016/j.jad.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 91.Lin H., Guo Q., Wen Z., et al. The multiple effects of fecal microbiota transplantation on diarrhea-predominant Irritable Bowel Syndrome (IBS-D) patients with anxiety and depression behaviors. Microb Cell Fact. 2021;20:233. doi: 10.1186/s12934-021-01720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doll J.P.K., Vázquez-Castellanos J.F., Schaub A.-C., et al. Fecal Microbiota Transplantation (FMT) as an adjunctive therapy for depression-case report. Front Psychiatry. 2022;13 doi: 10.3389/fpsyt.2022.815422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kang D.W., Adams J.B., Gregory A.C., et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dailey F.E., Turse E.P., Daglilar E., Tahan V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol. 2019;49:29–33. doi: 10.1016/j.coph.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 95.Servick K. Alternatives to fecal transplants near approval. Science. 2022;375:368–369. doi: 10.1126/science.ada0539. [DOI] [PubMed] [Google Scholar]
- 96.Akkasheh G., Kashani-Poor Z., Tajabadi-Ebrahimi M., et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32:315–320. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Kazemi A., Noorbala A.A., Azam K., Eskandari M.H., Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr. 2019;38:522–528. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 98.Messaoudi M., Lalonde R., Violle N., et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 99.Miyaoka T., Kanayama M., Wake R., et al. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: a prospective open-label trial. Clin Neuropharmacol. 2018;41:151–155. doi: 10.1097/WNF.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 100.Schaub A.C., Schneider E., Vazquez-Castellanos J.F., et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: a randomized controlled trial. Transl Psychiatry. 2022;12:227. doi: 10.1038/s41398-022-01977-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Slykerman R.F., Hood F., Wickens K., et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. eBioMedicine. 2017;24:159–165. doi: 10.1016/j.ebiom.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tian P., Chen Y., Zhu H., et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: a randomized clinical trial. Brain Behav Immun. 2022;100:233–241. doi: 10.1016/j.bbi.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 103.Zhang X., Chen S., Zhang M., et al. Effects of fermented milk containing lacticaseibacillus paracasei strain shirota on constipation in patients with depression: a randomized, double-blind, placebo-controlled trial. Nutrients. 2021;13:2238. doi: 10.3390/nu13072238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Browne P.D., Bolte A.C., Besseling-van der Vaart I., Claassen E., de Weerth C. Probiotics as a treatment for prenatal maternal anxiety and depression: a double-blind randomized pilot trial. Sci Rep. 2021;11:3051. doi: 10.1038/s41598-021-81204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reininghaus E.Z., Platzer M., Kohlhammer-Dohr A., et al. PROVIT: supplementary probiotic treatment and vitamin B7 in depression-a randomized controlled trial. Nutrients. 2020;12:3422. doi: 10.3390/nu12113422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Musazadeh V., Zarezadeh M., Faghfouri A.H., et al. Probiotics as an effective therapeutic approach in alleviating depression symptoms: an umbrella meta-analysis. Crit Rev Food Sci Nutr. 2022:1–9. doi: 10.1080/10408398.2022.2051164. [DOI] [PubMed] [Google Scholar]
- 107.Chapman C.M.C., Gibson G.R., Rowland I. In vitro evaluation of single- and multi-strain probiotics: inter-species inhibition between probiotic strains, and inhibition of pathogens. Anaerobe. 2012;18:405–413. doi: 10.1016/j.anaerobe.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Ding Y., Bu F., Chen T., et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol. 2021;105:8411–8426. doi: 10.1007/s00253-021-11622-2. [DOI] [PubMed] [Google Scholar]
- 109.Tian T., Xu B., Qin Y., et al. Clostridium butyricum miyairi 588 has preventive effects on chronic social defeat stress-induced depressive-like behaviour and modulates microglial activation in mice. Biochem Biophys Res Commun. 2019;516:430–436. doi: 10.1016/j.bbrc.2019.06.053. [DOI] [PubMed] [Google Scholar]
- 110.Burokas A., Arboleya S., Moloney R.D., et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82:472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 111.Tarutani S., Omori M., Ido Y., Yano M., Komatsu T., Okamura T. Effects of 4G-beta-D-Galactosylsucrose in patients with depression: a randomized, double-blinded, placebo-controlled, parallel-group comparative study. J Psychiatr Res. 2022;148:110–120. doi: 10.1016/j.jpsychires.2022.01.059. [DOI] [PubMed] [Google Scholar]
- 112.Hadi A., Sepandi M., Marx W., Moradi S., Parastouei K. Clinical and psychological responses to synbiotic supplementation in obese or overweight adults: a randomized clinical trial. Complement Ther Med. 2019;47 doi: 10.1016/j.ctim.2019.102216. [DOI] [PubMed] [Google Scholar]
- 113.Haghighat N., Rajabi S., Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr Neurosci. 2021;24:490–499. doi: 10.1080/1028415X.2019.1646975. [DOI] [PubMed] [Google Scholar]
- 114.Haghighat N., Mohammadshahi M., Shayanpour S., Haghighizadeh M.H., Rahmdel S., Rajaei M. The effect of synbiotic and probiotic supplementation on mental health parameters in patients undergoing hemodialysis: a double-blind, randomized, placebo-controlled trial. Indian J Nephrol. 2021;31:149–156. doi: 10.4103/ijn.IJN_341_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu Y., Wang Y., Hu A., et al. Lactobacillus plantarum-derived postbiotics prevent Salmonella-induced neurological dysfunctions by modulating gut-brain axis in mice. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.946096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cristiano C., Cuozzo M., Coretti L., et al. Oral sodium butyrate supplementation ameliorates paclitaxel-induced behavioral and intestinal dysfunction. Biomed Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113528. [DOI] [PubMed] [Google Scholar]