Abstract
Many ant species are equipped with chemical defenses, although how these compounds impact nervous system function is unclear. Here, we examined the utility of Caenorhabditis elegans chemotaxis assays for investigating how ant chemical defense compounds are detected by heterospecific nervous systems. We found that C. elegans respond to extracts from the invasive Argentine Ant ( Linepithema humile ) and the osm-9 ion channel is required for this response. Divergent strains varied in their response to L. humile extracts, suggesting genetic variation underlying chemotactic responses. These experiments were conducted by an undergraduate laboratory course, highlighting how C. elegans chemotaxis assays in a classroom setting can provide genuine research experiences and reveal new insights into interspecies interactions.
Figure 1. Argentine Ant ( Linepithema humile ) extracts induce an osm-9 dependent chemotaxis response in C. elegans .
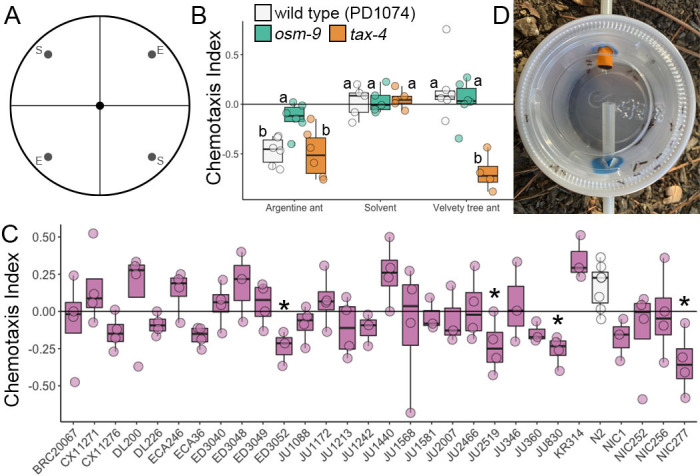
(A) Chemotaxis assays were performed on circular plates divided into quadrants, where worms placed in the center were exposed to ant extracts (E) and solvent (S). (B) The chemotaxis response of wild-type worms ( PD1074 , white), osm-9 ( ky10 ) worms (orange), and tax-4 ( p678 ) worms (blue) were tested in response to Argentine ant ( Linepithema humile) and Velvety tree ant ( Liometopum occidentale ) extracts. Wild-type PD1074 and tax-4 knockout worms, but not osm-9 knockout worms, were repelled by Argentine ant extracts. Wild-type PD1074 and osm-9 knockout worms did not respond to Velvety tree ant extracts, whereas tax-4 knockout worms were repelled. Groups not connected by the same letter are significantly different. (C) The response to Argentine ant extract was measured across a divergent strain set. Stars indicate significant differences between genetically diverse strains (purple) from wild type ( N2 , white). (D) Students constructed simple aspirators to collect ants.
Description
Ants use a diverse array of chemicals for communication, such as identifying nestmates or as chemical defense against competitors (Fox and Adams, 2022; Schmidt, 1986). However, the receptors that bind these compounds and the classes of neurons that respond to these chemical messengers to influence behavior are not well understood, as probing the neurogenetic basis of ant chemical communication remains difficult (but see (Trible et al., 2017)). Here, we explore (1) using C. elegans chemotaxis assays as a simple screen for identifying genes and neurons responsive to ant chemical defenses and (2) using this approach in an undergraduate classroom setting to engage students in authentic scientific research. Chemotaxis assays using C. elegans are a convenient and practical approach, as this species has a short generation time, are easy to culture in large quantities, have many readily available genetic tools, and rely on chemosensation to navigate their environment (Bargmann, 2006; Corsi et al., 2015). Specifically, C. elegans chemosensory neurons can be silenced by removing essential ion channels such as OSM-9 (Colbert et al., 1997) or TAX-4 (Komatsu et al., 1996), allowing for relatively simple chemotaxis mutant screens that quickly identify a subset of neurons important for chemosensation of various compounds. These features render C. elegans a potentially promising organism for screening ant-derived compounds for behavior-altering molecules, determining the neural and molecular mechanisms underlying the behavioral response, and conducting these experiments in an undergraduate laboratory classroom.
To examine the influence of ant-derived compounds on C. elegans chemotaxis behavior, we tested the response of osm-9 ( ky10 ) (Colbert et al., 1997), tax-4 ( p678 ) ( Komatsu et al., 1996), and wild-type worms ( PD1074 , (Yoshimura et al., 2019)) to extracts derived from two different ant species: the invasive Argentine ant ( Linepithema humile) and the native Velvety tree ant ( Liometopum occidentale ) (Figure 1A-B). We found a significant interaction between strains and compounds (2-way ANOVA, Strain*Compound: F(4) = 9.3459, p < 0.001). The strains did not differ in their response to solvent, but there were significant differences in responses to the ant extracts. PD1074 animals were indifferent to solvent and extracts from the Velvety tree ants but avoided Argentine ant extracts ( PD1074 : solvent vs Argentine ant extract, t(40) = −4.223, p = 0.003). Argentine ant extracts also repelled tax-4 mutants ( tax-4 : solvent vs Argentine ant extract, t(40) = −4.615, p = 0.002), but not the osm-9 mutants ( osm-9 : solvent vs Argentine ant extract, t(40) = −1.434, p = 0.239). Within groups exposed to Argentine ant compounds, the response of osm-9 mutants was significantly different from both PD1074 (t(40) = −2.990, p = 0.010) and tax-4 mutants (t(40) = 3.189, p = 0.006). Together, these data suggest that C. elegans sense Argentine ant extracts and osm-9 -expressing chemosensory neurons likely mediate this response. As Argentine ants are an invasive species, their chemicals have been extensively studied and include fatty acids, hydrocarbons, and defensive chemicals such as the terpenoid iridomyrmecin (Cavill and Houghton, 1974; Cavill et al., 1980, 1976), that are used in intra- and inter-species interactions (Welzel et al., 2018). Future experiments could include the presentation of extract fractions or commercially available extract compounds to examine which components influence C. elegans behavior.
Extracts of the Velvety tree ant showed a different pattern of behavioral response, in which tax-4 mutants were repelled by the extracts ( tax-4 : solvent vs Velvety tree ant extract, t(40) = −5.645, p < 0.001), but osm-9 mutants ( osm-9 : solvent vs Velvety tree ant extract, t(40) = 0.018, p = 0.985) and the wild type ( PD1074 : solvent vs Velvety tree ant extract, t(40) = −1.096, p = 0.373) were not. Within groups exposed to Velvety tree ant compounds, the response of tax-4 mutants was significantly different from both PD1074 (t(40) = 6.705, p < 0.001) and osm-9 mutants (t(40) = 5.718, p < 0.001). As tax-4 and osm-9 are co-expressed in only a subset of chemosensory neurons, it is possible that co-expression masks neural responses to exogenous compounds and that a behavioral phenotype is only observed when tax-4 is removed. Inclusion of a double knockout of both tax-4 and osm-9 in future experiments would help clarify if there are compensatory relationships between osm-9 and tax-4 chemosensory neurons that would lead to avoidance in tax-4 mutants, but not in the PD1074 strain. In addition, Velvety tree ants have a variety of hydrocarbons and fatty acids present in their extracts but lack the terpenoid iridomyrmecin present in Argentine ants (Moskowitz et al., 2022). Fractionation of Velvety tree ant extracts for future chemotaxis experiments would be the next step towards identifying compounds that elicit avoidance.
We next tested whether there is genetic variation in chemotaxis responses elicited by ant extracts by comparing genetically diverse C. elegans strains from the CeNDR collection (Cook et al., 2017) (Figure 1C). We focused on extracts of Argentine ants since it elicited a strong response in wild-type worms in the previous assay, and because Argentine ants are highly abundant and easily collected by undergraduate students (see Methods). Across thirty strains, including an N2 strain, there was a significant effect of Argentine ant extract on chemotaxis behavior (ANOVA, F(29) = 2.3364, p = 0.001). Four strains showed a significantly different response when compared to N2 , including ED3052 (t(84) = −2.862, p = 0.039), JU2519 (t(84) = −3.091, p = 0.026), JU830 (t(84) = −3.309, p = 0.020), and NIC277 (t(84) = −3.956, p = 0.004). Notably, the N2 strain responded differently than the PD1074 strain from the previous experiment. It is possible that genetic differences between PD1074 and other N2 strains underlie the different responses to Argentine ant extracts, as N2 strains propagated by various labs can be genetically variable whereas PD1074 is a derived clonal strain that is genetically homogenous (Yoshimura et al., 2019). Alternatively, this difference may be due to the Argentine ant extract being different across experiments, as the extracts were prepared at different times. The concentration of compounds likely varied across different preparations, which could have induced opposing chemotaxis results (Yoshida et al., 2012). Moreover, differences in extract composition could influence worm chemotaxis responses, as ant diet, which can be different across colonies or seasons, can influence ant chemistry (Liang and Silverman, 2000). Regardless, the diversity in chemotaxis responses across strains suggests that future genome-wide association studies could identify additional molecular factors important in this behavioral response (Cook et al., 2017).
In summary, we have shown that extracts of the Argentine ant influence C. elegans chemotaxis, osm-9 chemosensory neurons likely mediate this response, and there are variable responses to Argentine ant extracts across divergent C. elegans strains. Moreover, we have uncovered a complex interaction in chemotaxis responses to extracts from Velvety tree ants. Future studies will include more mutant and divergent worm strains and more ant species with consistent collection and extraction methods that reduce response variability. Importantly, this work establishes the feasibility of using C. elegans chemotaxis assays as a tool for learning more about the biochemistry mediating interspecies interactions within classroom undergraduate research experience courses.
Methods
Worm Strains
Strains were obtained from the Caenorhabditis Genetics Center (CGC) at the University of Minnesota and the Caenorhabditis elegans Natural Diversity Resource CeNDR at Northwestern University (see Reagents). Animals were maintained in 20°C incubators and nematodes were synchronized by bleaching adults to obtain eggs. Roughly 300-500 eggs were pipetted onto 55 mm Nematode Growth Media plates spread with OP50 E. coli . NGM plates were made as described (Stiernagle, 2006) with the addition of uracil at 2μg/mL. Hatched eggs were kept at 20°C for roughly 3 days when the population reached a young adult stage and were used for chemotaxis assays.
Ant extracts
The invasive Argentine ant ( Linepithema humile) and the Velvety tree ant ( Liometopum occidentale ) were collected on the Stanford University campus. In the first screening set, ant extracts that had been previously collected and analyzed with gas chromatography / mass spectrometry were used, as detailed in Moskowitz et al. (2022). To acquire more Argentine ant extract for the divergent screen, students built aspirators to collect ants using plastic ketchup cups, plastic straws, putty, and strips of pantyhose fabric (Figure 1D). After collection, ants were incubated at -80C for 15 minutes and then placed in methanol for 24 hours at 20C. Ants were removed from methanol samples before evaporation under a constant flow of nitrogen gas to dryness. Evaporated ant samples were then resuspended in dimethyl sulfoxide (DMSO).
Chemotaxis Assays
Undergraduate students in a laboratory course performed chemotaxis assays while unaware of compound or strain being tested until data was submitted to the instructor. Chemotaxis plates [5mM KPO 4 (pH 6), 1mM CaCl 2 , 1mM MgSO 4 , 2% agar] were divided into four quadrants (Figure 1A). Compounds (5 µL, ant extract concentration unknown) were placed on dots located in two non-adjacent quadrants (E, experimental) while 5 µL of DMSO was placed on dots in the other two non-adjacent quadrants (S, solvent). Plates were then incubated for 30 minutes to allow for the establishment of a chemical gradient. During this incubation period, worms were removed from their plate and washed three times with Chemotaxis Assay Buffer [5mM KPO 4 (pH 6), 1mM CaCl 2 , 1mM MgSO 4 ]. Following compound incubation, 2 µL 0.5 M sodium azide solution was applied to each of the quadrant dots to serve as a worm paralytic. Then, roughly 100 worms were placed in the center of each plate and the excess buffer was removed using a KimWipe. Worms were allowed to roam the plate for one hour and then were counted manually in each quadrant under a dissecting microscope using a tally counter. Worms in the center dot of overlapping quadrants were not counted for any quadrant but were included in the total number of worms. Eighteen students conducted all the experiments and each assay was replicated 3-6 times.
Data Analysis
The Chemotaxis Index (CI) was calculated for each plate: CI = (Number of worms in the two experimental quadrants – Number of worms in the two solvent quadrants) / Total number of worms on the entire plate. Thus, a positive CI indicates attraction and a negative CI indicates repulsion to the experimental compounds. Plates were removed from the data set prior to analysis if there were less than 20 worms on the plate or if a student noted a technical error in the plate setup, such as mistakes in pipetting compounds or worms onto the appropriate locations.
Data analysis and visualization were performed in R (version 4.1.2). For the screen with two ant species and three worm strains, a 2-way ANOVA was used to detect significant differences between groups, with chemotaxis index as dependent variable and compound, worm strain, and their interaction as independent variables. For the divergent screen, a one-way ANOVA was used to detect significant differences across groups, with chemotaxis index as dependent variable and worm strain as the independent variables. Parametric assumptions were met by both datasets, including homogeneity of variance confirmed with Levene’s test (leveneTest) and normality of residuals (visualized with qqp functions). Posthoc analyses were performed using emmeans (version 1.7.2) and grafify (version 3.0.0) packages with false discovery rate (fdr) adjustment of p-values to account for multiple testing. Pairwise posthoc tests were run for the initial screen while divergent strains were only compared to the N2 wild-type group. Boxplots were generated using the ggplot2 (version 3.3.5) package.
Classroom pedagogy
We conducted the experiments described here across two laboratory sessions. These sessions were preceded with two training sessions where students learned how to conduct chemotaxis assays using known attractants (isoamyl alcohol) and repellants (carvone) (Ellington et al., 2020). An additional laboratory session involved conducting “field work” by having students construct aspirators and collect the ants needed for the divergent strain experiments. Weekly homework included reading relevant literature, data analysis and visualization, and writing the results and interpretations. The final project was to write this journal-style article.
Reagents
|
Strain Name |
Genotype |
Source |
|
Wild type |
Caenorhabditis Genetics Center (CGC) at the University of Minnesota |
|
|
Caenorhabditis Genetics Center (CGC) at the University of Minnesota |
||
|
Caenorhabditis Genetics Center (CGC) at the University of Minnesota |
||
|
N2 , BRC20067 , CX11271 , CX11276 , DL200 , DL226 , ECA246 , ECA36 , ED3040 , ED3048 , ED3049 , ED3052 , JU1088 , JU1172 , JU1213 , JU1242 , JU1440 , JU1568 , JU1581 , JU2007 , JU2466 , JU2519 , JU346 , JU360 , JU830 , KR314 , NIC1 , NIC252 , NIC256 , NIC277 |
Wild isolates; N2 (Bristol) |
CeNDR at Northwestern University |
Acknowledgments
Acknowledgments
The authors would like to thank Emily Fryer, Tessa Logan, Miriam Goodman and the Stanford NeuroPlant team for advice and assistance with reagents as well as Griselda Morales for additional help in preparing plates.
Funding
This work was supported by an NSF CAREER grant (IOS-1845651) and a New York Stem Cell Foundation grant to LAO. LEC is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-2428-21). MDS is supported by a K99 Pathway to Independence award from the NIH (NIGMS K99GM135489). KF is supported by the National Science Foundation Postdoctoral Research Fellowship in Biology (NSF BIO-2109400). LAO is a New York Stem Cell Foundation – Robertson Investigator.
References
- Bargmann CI. Chemosensation in C. elegans. WormBook. 2006 Oct 25;:1–29. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavill GW, Houghton E. Volatile constituents of the Argentine ant, Iridomyrmex humilis. J Insect Physiol. 1974 Oct 1;20(10):2049–2059. doi: 10.1016/0022-1910(74)90112-7. [DOI] [PubMed] [Google Scholar]
- Cavill G. W. K., Davies N. W., McDonald F. J. Characterization of aggregation factors and associated compounds from the argentine ant,Iridomyrmex humilis. Journal of Chemical Ecology. 1980 Mar 1;6(2):371–384. doi: 10.1007/bf01402915. [DOI] [Google Scholar]
- Cavill G.W.K., Houghton E., McDonald F.J., Williams P.J. Isolation and characterization of dolichodial and related compounds from the argentine ant, Iridomyrmex humilis. Insect Biochemistry. 1976 Jan 1;6(5):483–490. doi: 10.1016/0020-1790(76)90072-x. [DOI] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997 Nov 1;17(21):8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DE, Zdraljevic S, Roberts JP, Andersen EC. CeNDR, the Caenorhabditis elegans natural diversity resource. Nucleic Acids Res. 2016 Oct 3;45(D1):D650–D657. doi: 10.1093/nar/gkw893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi AK, Wightman B, Chalfie M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics. 2015 Jun 1;200(2):387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington C, Hayden A, LaGrange Z, Luccioni M, Osman M, Ramlan L, Vogt M, Guha S, Goodman M, O'Connell L. The plant terpenoid carvone is a chemotaxis repellent for C. elegans . . MicroPubl Biol. 2020 Mar 13;2020 doi: 10.17912/micropub.biology.000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EGP, Adams RMM. On the Biological Diversity of Ant Alkaloids. Annu Rev Entomol. 2021 Oct 22;67:367–385. doi: 10.1146/annurev-ento-072821-063525. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996 Oct 1;17(4):707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- Liang D, Silverman J. "You are what you eat": diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften. 2000 Sep 1;87(9):412–416. doi: 10.1007/s001140050752. [DOI] [PubMed] [Google Scholar]
- Moskowitz NA, D'Agui R, Alvarez-Buylla A, Fiocca K, O'Connell LA. Poison frog dietary preference depends on prey type and alkaloid load. PLoS One. 2022 Dec 1;17(12):e0276331–e0276331. doi: 10.1371/journal.pone.0276331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JO. 1986. Chemistry, Pharmacology, and Chemical Ecology of Ant Venoms. Venoms of the Hymenoptera.
- Stiernagle T. Maintenance of C. elegans. WormBook. 2006 Feb 11;:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trible W, Olivos-Cisneros L, McKenzie SK, Saragosti J, Chang NC, Matthews BJ, Oxley PR, Kronauer DJC. orco Mutagenesis Causes Loss of Antennal Lobe Glomeruli and Impaired Social Behavior in Ants. Cell. 2017 Aug 10;170(4):727–735.e10. doi: 10.1016/j.cell.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzel KF, Lee SH, Dossey AT, Chauhan KR, Choe DH. Verification of Argentine ant defensive compounds and their behavioral effects on heterospecific competitors and conspecific nestmates. Sci Rep. 2018 Jan 24;8(1):1477–1477. doi: 10.1038/s41598-018-19435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Hirotsu T, Tagawa T, Oda S, Wakabayashi T, Iino Y, Ishihara T. Odour concentration-dependent olfactory preference change in C. elegans. Nat Commun. 2012 Mar 13;3:739–739. doi: 10.1038/ncomms1750. [DOI] [PubMed] [Google Scholar]
- Yoshimura J, Ichikawa K, Shoura MJ, Artiles KL, Gabdank I, Wahba L, Smith CL, Edgley ML, Rougvie AE, Fire AZ, Morishita S, Schwarz EM. Recompleting the Caenorhabditis elegans genome. . Genome Res. 2019 May 23;29(6):1009–1022. doi: 10.1101/gr.244830.118. [DOI] [PMC free article] [PubMed] [Google Scholar]


