Abstract
This open-label randomized clinical trial assessed the 12-month risk of disease activity flares after discontinuation of conventional synthetic DMARDs (csDMARDs) compared with continuing half-dose csDMARDs in adult Norwegian patients with rheumatoid arthritis and excellent disease control.
With advances in rheumatoid arthritis (RA) therapy, more patients achieve sustained remission. Treatment recommendations suggest considering dosage tapering of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) in these patients.1 Although flares appear more frequently when tapering csDMARDs vs full-dose therapy,2,3 tapering could be successful in some patients.4 However, it is uncertain whether discontinuation of csDMARDs is realistic and sustainable.
We assessed the 12-month risk of disease activity flares when discontinuing csDMARDs compared with continuing half-dose csDMARDs in patients with RA and excellent disease control.
Methods
This randomized, open-label trial is part of the ARCTIC REWIND project, which included adults (aged 18-80 years) with RA (American College of Rheumatology/European Alliance of Associations for Rheumatology 2010 criteria) in sustained remission according to Disease Activity Score (DAS; range, 0-10, with higher scores indicating greater disease activity) based on assessment of 28 (DAS28) or 44 (DAS) joints for 12 months or longer with stable csDMARD therapy, and with no swollen joints (of 44 assessed) and DAS remission at inclusion. Patients enrolled at 10 Norwegian hospitals were randomized 1:1 by computer-based center-stratified block randomization. In study period 1 (inclusion from June 17, 2013, to June 18, 2018), patients were allocated to half-dose or stable csDMARDs.2 Those who tapered medication to half-dose and remained flare-free for 1 year were eligible for the second (current) study period (last visit June 26, 2020), with randomization to csDMARD discontinuation or continued half-dose. Visits occurred every 4 months and additionally if disease activity increased. Full-dose csDMARD was reinstated in the event of a flare. The South-Eastern Norway Regional Ethics Committee approved the study; patients provided written informed consent.
The primary analysis tested superiority of discontinuing csDMARDs or maintaining a stable half-dose regarding disease flare during 12 months of follow-up, using a center-stratified risk difference estimator. Flare was defined as a combination of DAS greater than 1.6, change in DAS of 0.6 units or greater, and 2 or more swollen joints. Additionally, flare could be registered if the physician and patient agreed that a clinically significant flare occurred. Secondary end points, including reachievement of remission after flare, radiographic progression (change in the van der Heijde modified Sharp score ≥1 unit per year), and adverse events, were analyzed using logistic or linear mixed-effects models as appropriate. Sample size was determined with respect to the initial comparison in study period 1.2 Primary and secondary efficacy analyses were performed in randomized patients attending 1 or more efficacy evaluation, with robustness analyses for the primary end point in patients without major protocol deviations and in those using methotrexate monotherapy. Stata version 16.0 (StataCorp) and R version 4.0.3 (R Foundation) were used for analyses; 95% CIs around the risk difference that did not include 0 defined statistical significance.
Results
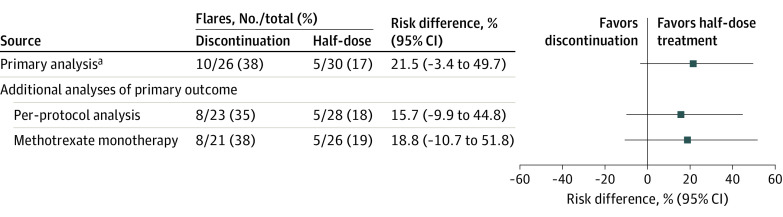
Of 78 patients randomized to half-dose therapy in study period 1, 56 were eligible for the current study. Baseline characteristics were mostly well balanced (Table). A flare within 12 months was experienced by 10 of 26 patients (38.5%) discontinuing csDMARD, compared with 5 of 30 (16.7%) continuing a half-dose (risk difference, 21.5% [95% CI, −3.4% to 49.7%]) (Figure). Comparable results were found in the per-protocol population and methotrexate monotherapy users. Median time to flare among those with flare was 179 (IQR, 99-245) days in the discontinuation group and 133 (IQR, 126-217) days in the half-dose group.
Table. Baseline Characteristics of the Study Populationa.
| Characteristics | Discontinued csDMARDs (n = 26) | Maintained half-dose csDMARDs (n = 30) |
|---|---|---|
| Age, mean (SD), y | 55.6 (11.2) | 56.3 (12.8) |
| Sex, No. (%) | ||
| Female | 15 (58) | 22 (73) |
| Male | 11 (42) | 8 (27) |
| Time since first swollen joint, median (IQR), y | 4.2 (3.4-4.9) | 3.8 (3.3-5.0) |
| Positivity, No. (%)b | ||
| For anticitrullinated peptide antibodies | 22 (85) | 23 (77) |
| For rheumatoid factor | 18 (69) | 19 (63) |
| Body mass index, median (IQR)b | 24.2 (22.5-26.4) | 28.4 (22.8-35.8) |
| Current smoking, No. (%) | 7 (27) | 3 (10) |
| Measures of disease activity | ||
| Disease Activity Score, mean (SD)c | 0.8 (0.4) | 0.9 (0.4) |
| Simplified Disease Activity Index, median (IQR)d | 1.0 (0.5-2.5) | 0.8 (0.4-2.0) |
| ACR/EULAR remission, No. (%)e | 19 (76) | 22 (73) |
| SJC, mean (SD)f | 0.1 (0.3) | 0.1 (0.3) |
| TJC, median (IQR)g | 0.0 (0.0-0.0) | 0.0 (0.0-1.0) |
| Erythrocyte sedimentation rate, median (IQR), mm/hh | 7.0 (3.0-15.0) | 7.0 (5.0-13.0) |
| CRP, median (IQR), mg/dLi | 0.2 (0.1-0.3) | 0.2 (0.1-0.4) |
| Global assessment (scale, 0-10), median (IQR)j | ||
| Patient-reported | 0.4 (0.1-1.3) | 0.4 (0.1-1.1) |
| Physician-reported | 0.3 (0.0-0.7) | 0.0 (0.0-0.5) |
| Functional outcomes, median (IQR) | ||
| PROMIS physical function, mean (SD)k | 55.7 (7.7) | 53.8 (7.3) |
| Fatiguel | 13.0 (2.0-29.0) | 3.5 (1.0-25.0) |
| Joint painl | 5.5 (1.0-14.0) | 2.0 (1.0-9.0) |
| Radiographic joint damage, median (IQR) | ||
| Total van der Heijde modified Sharp scorem | 1.0 (0.0-2.5) | 1.5 (0.5-6.0) |
| van der Heijde Sharp erosion score | 1.0 (0.0-1.5) | 1.0 (0.5-2.0) |
| van der Heijde Sharp joint space narrowing score | 0.0 (0.0-0.0) | 0.0 (0.0-3.0) |
| Ultrasound outcomesn | ||
| Total power Doppler signal score, median (IQR) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) |
| Total gray scale score, median (IQR) | 0.0 (0.0-2.0) | 2.0 (0.0-3.0) |
| No power Doppler signal in any joint, No. (%) | 23 (92) | 30 (100) |
| Medication | ||
| Methotrexate monotherapy, No. (%) | 21 (81) | 26 (87) |
| Oral | 18 (69) | 19 (63) |
| Subcutaneous | 3 (12) | 7 (23) |
| Methotrexate/sulfasalazine/hydroxychloroquine, mean dose (SD) | 3 (12) | 2 (7) |
| Methotrexate, mg/wk | 10.2 (1.8) | 10.2 (2.1) |
| Sulfasalazine, mg/d | 750 (289) | 1000 (0) |
| Hydroxychloroquine, mg/d | 200 (0) | |
| Other monotherapies or duotherapies, No. (%) | 5 (19) | 4 (13) |
Abbreviations: ACR, American College of Rheumatology; CRP, C-reactive protein; csDMARD, conventional synthetic disease-modifying antirheumatic drug; EULAR, European Alliance of Associations for Rheumatology; PROMIS, Patient-Reported Outcomes Measurement Information Score; RAI, Ritchie Articular Index; SJC, swollen joint count; TJC, tender joint count; VAS, visual analog scale.
All randomized patients who attended at least 1 efficacy evaluation.
Collected at the first visit in study period 1. Body mass index was calculated as weight in kilograms divided by height in meters squared.
Disease Activity Score (range, 0-10) includes an SJC of 44, assessment of tender joints by the RAI, erythrocyte sedimentation rate (ESR), and patient global assessment of disease activity (PGA) on a VAS (0-100 mm). It is calculated as 0.54 × (RAI)1/2 + 0.065 × (SJC44) + 0.33 × ln(ESR) + 0.0072 × PGA. Remission is defined as any value less than 1.6, low disease activity as 1.6 to 2.4, moderate disease activity as greater than 2.4 to 3.7, and high disease activity as greater than 3.7; higher scores thus indicate more disease activity.
Simplified Disease Activity Index (range, 0-86) includes a swollen joint and tender joint count of 28, CRP level, and patient and physician global assessment of disease activity (PGA) on a VAS (0-100 mm). It is calculated as TJC28 + SJC28 + PGA/10 + PGA/10 + CRP. Remission is defined as a score of 3.3 or lower, with higher scores indicating more disease activity.
Defined as a TJC of 1 or less, SJC of 1 or less, and CRP level of 1 mg/dL or lower, and patient global assessment score of 1 or less (on a 0-10 scale).
Number of swollen joints of 44 joints assessed.
Calculated by the RAI assessing tenderness of 26 joint regions. The RAI ranges from 0 to 3 for individual measures and the sum of 0 to 78 overall, with higher scores indicating more tenderness.
Reference, less than 17 mm/h in women and less than 12 mm/h in men. Reference values may vary among laboratories.
Reference, less than 0.4 mg/dL. Reference values may vary among laboratories.
Overall assessments of disease with use of a VAS (0-10 mm), with higher scores indicating more severe disease.
Scores range from 0 to 100, with scores lower than 50 indicating disability worse than average.
Self-reported with use of a VAS (0 to 100 mm), with higher scores indicating more severe fatigue or joint pain.
Rheumatoid arthritis inflammatory disease activity is associated with radiographic joint damage progression, which in turn might lead to functional decline. The van der Heijde modified Sharp scoring method assesses erosions in 16 joints of each hand (range, 0-5 for each joint) and 6 joints of each foot (range, 0-10 per joint), and joint space narrowing in 15 joints for each hand, as well as 6 joints for each foot (range, 0-4 per joint). This gives scores for erosions on a scale from 0 to 280 and joint-space narrowing on a scale from 0 to 168; thus, the total van der Heijde Sharp scores range from 0 to 448, with higher scores indicating greater joint damage.
Two aspects of synovitis can be assessed by ultrasound; morphology and quantity using a gray scale and synovial vascularity using power Doppler. The ultrasound examination was performed using 0 to 3 semiquantitative scoring systems for both gray scale and power Doppler in 32 joints.
Figure. Flare Rate Within 12 Months in Patients Discontinuing vs Continuing Half-Dose Antirheumatic Treatment.
aFlare was defined as a combination of Disease Activity Score (DAS) above the cutoff for remission (1.6), an increase in the DAS of at least 0.6, and at least 2 swollen joints, or agreement by both the treating physician and the patient that a clinically significant flare had occurred. Six of 15 flares were by consensus (2 in the stable half-dose group, 4 in the discontinuation group). After all statistical testing had been completed, a thorough review of the flares defined by consensus revealed definite signs of increased inflammatory disease activity in these patients (eg, ultrasound findings, corresponding increase in C-reactive protein level).
At the first visit after flare, 8 of 10 patients (80.0% [95% CI, 44.4%-97.5%]) in the discontinuation group and 2 of 3 (66.7% [95% CI, 9.5%-99.2%]) in the half-dose group regained DAS remission. Radiographic joint damage progression was absent in 84.0% of patients who discontinued therapy and 69.0% of those who continued half-dose therapy (risk difference, 13.9% [95% CI, −10.6% to 38.3%]). Total adverse events numbered 22 in the discontinuation group and 26 in the half-dose group, including 1 serious adverse event (infection).
Discussion
For patients with RA in sustained remission while receiving half-dose csDMARD treatment, continuation of a csDMARD half-dose was not found to be superior to withdrawal regarding disease flare risk. Although a numerically higher number of flares was observed in the discontinuation group, most patients achieved drug-free remission for 1 year or longer. The data suggest that csDMARD withdrawal might be viable in some patients and that structured follow-up of DMARD treatment–free patients is advisable, because most patients with flare regained remission by restarting full-dose csDMARDs.5 Study limitations include the open-label design and limited sample size. The results provide a basis for shared decision-making in treatment of patients with RA achieving remission.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Senior Editor.
Data Sharing Statement
References
- 1.Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3-18. doi: 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 2.Lillegraven S, Paulshus Sundlisæter N, Aga AB, et al. Effect of half-dose vs stable-dose conventional synthetic disease-modifying antirheumatic drugs on disease flares in patients with rheumatoid arthritis in remission: the ARCTIC REWIND randomized clinical trial. JAMA. 2021;325(17):1755-1764. doi: 10.1001/jama.2021.4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haschka J, Englbrecht M, Hueber AJ, et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy. Ann Rheum Dis. 2016;75(1):45-51. doi: 10.1136/annrheumdis-2014-206439 [DOI] [PubMed] [Google Scholar]
- 4.Curtis JR, Ogdie A, George MD. Treatment strategies for patients with immune-mediated inflammatory diseases. JAMA. 2021;325(17):1726-1728. doi: 10.1001/jama.2021.2740 [DOI] [PubMed] [Google Scholar]
- 5.Verstappen M, van Mulligen E, de Jong PHP, van der Helm-Van Mil AHM. DMARD-free remission as novel treatment target in rheumatoid arthritis. RMD Open. 2020;6(1):e001220. doi: 10.1136/rmdopen-2020-001220 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement