Abstract
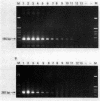
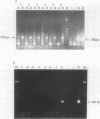
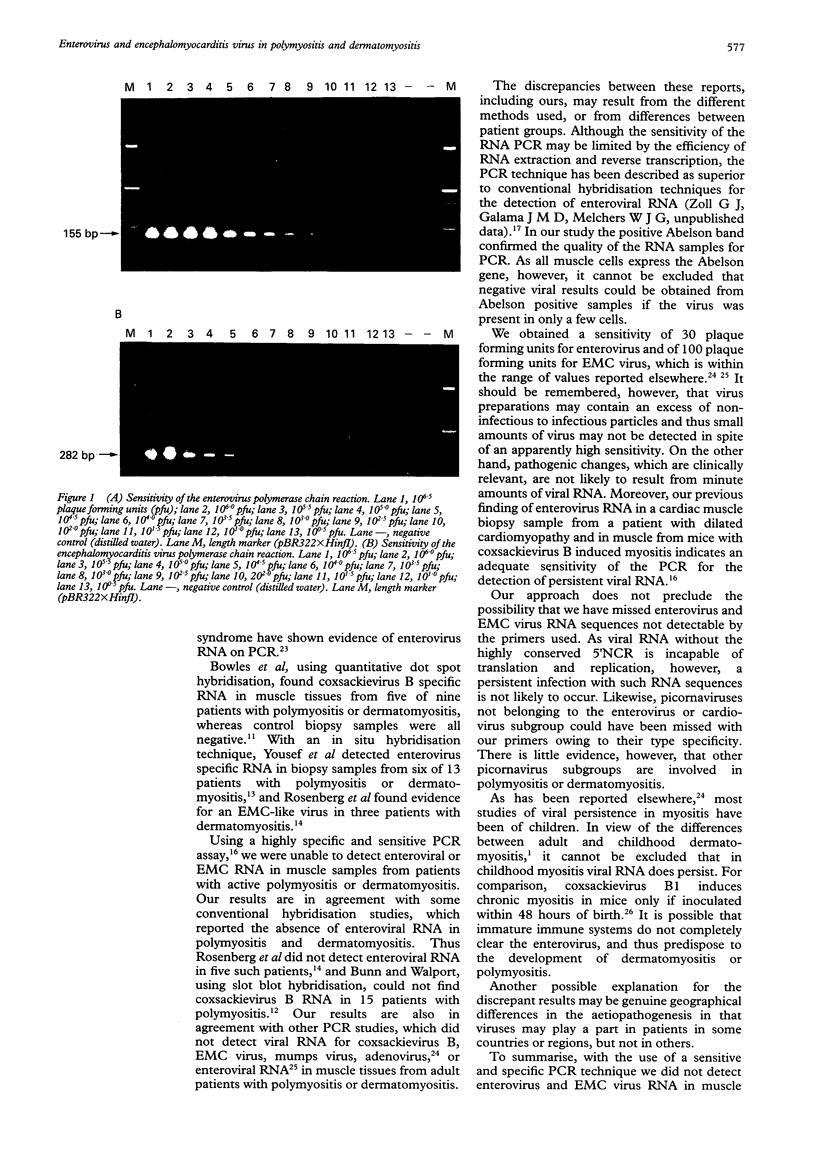
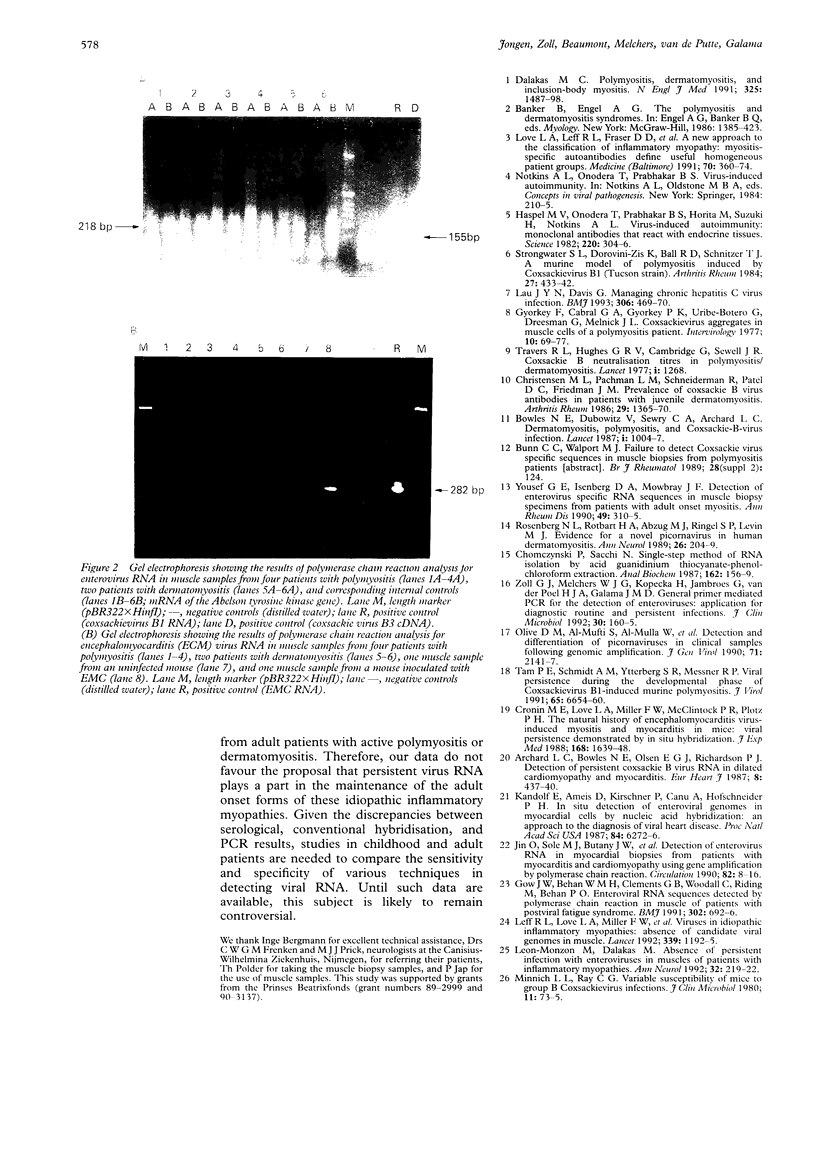
OBJECTIVES--A persistent infection of enteroviruses and cardioviruses has been implicated in polymyositis and dermatomyositis, but conventional hybridisation studies of the presence of enterovirus RNA and encephalomyocarditis (EMC) virus RNA in affected muscle have yielded conflicting results. To investigate further the possibility of viral persistence, the presence of viral RNA in muscle from patients with adult onset polymyositis and dermatomyositis was investigated using a polymerase chain reaction (PCR) technique. METHODS--Muscle tissue was obtained from 10 patients with polymyositis and five patients with dermatomyositis, all with adult onset active disease. A PCR was performed using primers with high specificity for enterovirus and EMC virus RNA, followed by Southern blot hybridisation with an oligonucleotide probe directed against the internal portion of the amplified product. A PCR directed against the Abelson tyrosine kinase mRNA served as an internal control for the presence and quality of RNA. RESULTS--A specific amplification for enterovirus or for EMC virus could not be seen in any of the muscle biopsy samples, despite a sensitivity of about 30 plaque forming units for enterovirus and of 100 plaque forming units for EMC virus. Southern blot hybridisation confirmed these results in that positive controls hybridised with the oligonucleotide probe, but no signal was obtained with the muscle specimens. CONCLUSION--A sensitive and specific PCR technique showed no evidence of the presence of enterovirus or EMC virus RNA in muscle samples from patients with polymyositis or dermatomyositis. These data do not support the proposal that viral RNA persistence plays a part in these idiopathic inflammatory myopathies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles N. E., Dubowitz V., Sewry C. A., Archard L. C. Dermatomyositis, polymyositis, and Coxsackie-B-virus infection. Lancet. 1987 May 2;1(8540):1004–1007. doi: 10.1016/s0140-6736(87)92271-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christensen M. L., Pachman L. M., Schneiderman R., Patel D. C., Friedman J. M. Prevalence of Coxsackie B virus antibodies in patients with juvenile dermatomyositis. Arthritis Rheum. 1986 Nov;29(11):1365–1370. doi: 10.1002/art.1780291109. [DOI] [PubMed] [Google Scholar]
- Cronin M. E., Love L. A., Miller F. W., McClintock P. R., Plotz P. H. The natural history of encephalomyocarditis virus-induced myositis and myocarditis in mice. Viral persistence demonstrated by in situ hybridization. J Exp Med. 1988 Nov 1;168(5):1639–1648. doi: 10.1084/jem.168.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas M. C. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991 Nov 21;325(21):1487–1498. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- Gow J. W., Behan W. M., Clements G. B., Woodall C., Riding M., Behan P. O. Enteroviral RNA sequences detected by polymerase chain reaction in muscle of patients with postviral fatigue syndrome. BMJ. 1991 Mar 23;302(6778):692–696. doi: 10.1136/bmj.302.6778.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorkey F., Cabral G. A., Gyorkey P. K., Uribe-Botero G., Dreesman G. R., Melnick J. L. Coxsackievirus aggregates in muscle cells of a polymyositis patient. Intervirology. 1978;10(2):69–77. doi: 10.1159/000148970. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Onodera T., Prabhakar B. S., Horita M., Suzuki H., Notkins A. L. Virus-induced autoimmunity: monoclonal antibodies that react with endocrine tissues. Science. 1983 Apr 15;220(4594):304–306. doi: 10.1126/science.6301002. [DOI] [PubMed] [Google Scholar]
- Jin O., Sole M. J., Butany J. W., Chia W. K., McLaughlin P. R., Liu P., Liew C. C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990 Jul;82(1):8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J. Y., Davis G. L. Managing chronic hepatitis C virus infection. BMJ. 1993 Feb 20;306(6876):469–470. doi: 10.1136/bmj.306.6876.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff R. L., Love L. A., Miller F. W., Greenberg S. J., Klein E. A., Dalakas M. C., Plotz P. H. Viruses in idiopathic inflammatory myopathies: absence of candidate viral genomes in muscle. Lancet. 1992 May 16;339(8803):1192–1195. doi: 10.1016/0140-6736(92)91134-t. [DOI] [PubMed] [Google Scholar]
- Leon-Monzon M., Dalakas M. C. Absence of persistent infection with enteroviruses in muscles of patients with inflammatory myopathies. Ann Neurol. 1992 Aug;32(2):219–222. doi: 10.1002/ana.410320215. [DOI] [PubMed] [Google Scholar]
- Love L. A., Leff R. L., Fraser D. D., Targoff I. N., Dalakas M., Plotz P. H., Miller F. W. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991 Nov;70(6):360–374. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- Rosenberg N. L., Rotbart H. A., Abzug M. J., Ringel S. P., Levin M. J. Evidence for a novel picornavirus in human dermatomyositis. Ann Neurol. 1989 Aug;26(2):204–209. doi: 10.1002/ana.410260204. [DOI] [PubMed] [Google Scholar]
- Strongwater S. L., Dorovini-Zis K., Ball R. D., Schnitzer T. J. A murine model of polymyositis induced by coxsackievirus B1 (Tucson strain). Arthritis Rheum. 1984 Apr;27(4):433–442. doi: 10.1002/art.1780270411. [DOI] [PubMed] [Google Scholar]
- Tam P. E., Schmidt A. M., Ytterberg S. R., Messner R. P. Viral persistence during the developmental phase of Coxsackievirus B1-induced murine polymyositis. J Virol. 1991 Dec;65(12):6654–6660. doi: 10.1128/jvi.65.12.6654-6660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers R. L., Hughes G. R., Cambridge G., Sewell J. R. Coxsackie B neutralisation titres in polymyositis/dermatomyositis. Lancet. 1977 Jun 11;1(8024):1268–1268. doi: 10.1016/s0140-6736(77)92487-4. [DOI] [PubMed] [Google Scholar]
- Yousef G. E., Isenberg D. A., Mowbray J. F. Detection of enterovirus specific RNA sequences in muscle biopsy specimens from patients with adult onset myositis. Ann Rheum Dis. 1990 May;49(5):310–315. doi: 10.1136/ard.49.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoll G. J., Melchers W. J., Kopecka H., Jambroes G., van der Poel H. J., Galama J. M. General primer-mediated polymerase chain reaction for detection of enteroviruses: application for diagnostic routine and persistent infections. J Clin Microbiol. 1992 Jan;30(1):160–165. doi: 10.1128/jcm.30.1.160-165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]