Abstract
Background and Objectives: This prospective cohort study aimed to evaluate the onset and severity of pain and other complications following lower impacted third molar extraction and to identify potential risk predictors. Materials and Methods: Twenty-five patients were treated with at least one lower impacted third molar extraction. The primary outcome was the onset of post-operative pain, evaluated at 6 h, 12 h, 24 h, 48 h, 72 h, and 7 days. The secondary outcomes (trismus, edema, alveolitis, dehiscence, neuralgic injury, and suppuration) were recorded at 3, 7 and 21 days after oral surgery. A correlation analysis was performed to identify potential associations between patient- and tooth-related factors and VAS (Visual Analogue Scale) scale. When a statistically significant correlation was identified, a regression analysis was performed. Results: Most of the patients were female (84%) with a mean age of 25 ± 3 years; the reason for oral surgery was dysodontiasis in 60% of cases, while the most frequent Pell and Gregory class was BII (36%). The VAS scale showed the onset of mild pain at 6 h (44%), 12 h (48%), 24 h (68%) and 48 (68%) after surgery. Trismus, edema, and alveolitis were observed at 3-day (20%, 64% and 12%, respectively) and at 7-day (16%, 12% and 4%, respectively) follow-up. Neuralgic injury was reported in one case (4%). The linear regression analysis showed a statistically significant association (p < 0.05) between the duration of oral surgery and VAS scores at 6 and 12 h. Finally, the binary logistic regression identified systemic disease, Pell and Gregory classification, duration of oral surgery, VAS at 6 and 12 h, trismus, and edema at 3 and 7 days as predictive factors of post-operative complications. Conclusions: Within their limits, the results of this study suggest that the onset of post-operative complications increases in proportion to the duration of the surgical procedure.
Keywords: impacted tooth, third molar, tooth extraction, oral surgery complications, inferior alveolar nerve injury
1. Introduction
Third molars are the most commonly impacted teeth, with a frequency of up to 75% in the young adult population [1]. The reason is related to human evolution, as there has been a development of the neurocranium to the detriment of the splanchnocranium, whose dimensions have remained almost unchanged and consequently made the third molars redundant [2]. Therefore, the lack of space has meant that the eruption of third molars occurs very frequently in anomalous positions, often determining clinical symptoms such as pain, otalgia, odynophagia, and dysphagia [3]. Consequently, surgical avulsion represents one of the most common procedures in oral and maxillofacial surgery [1,4].
Several indications for impacted third molar (IMTM) extraction are described in the literature, including orthodontic and periodontal reasons, caries, pain, infections, association with cysts or tumors, damage to the neighboring teeth, and proximity to mandibular fracture lines or orthognathic surgical sites [5,6].
Pre-surgical planning is essential in order to perform the extraction safely, reducing discomfort and complications. Winter and Pell and Gregory classifications [6,7] are the most-used methods to evaluate the extractive pattern of IMTMs, giving information on the angulation and the position in the mandibular ramus, respectively. According to Bui et al. [8], the mesio-angular position is associated with a higher risk of post-operative complications. However, these classifications are based on the orthopantomographic aspect only, which is not enough to define the difficulty level of the surgery [9,10]. For this reason, many authors [6,11,12] reported classification systems based on cone-beam computed tomography (CBCT) analysis, offering more detailed information on radicular morphology and on the relationship with the inferior alveolar nerve (IAN) [13].
Bone density, age, sex, previous gnathological pathologies, and surgeon experience are other pre-operatory variables that can influence the difficulty of the intervention, although there is not a single classification that includes all these necessary parameters [9,10].
The main objective of IMTM surgery is avulsion with minimal intra- and post-operative complications. After careful pre-operative planning, access to the third molar should be performed from the buccal side by incision of a mucoperiosteal flap, and it is preferable to have an accurate odontomy rather than osteotomy, removing undercuts and safeguarding the alveolar bone [14]. In the case of multi-rooted teeth, it could be convenient to separate them using either a diamond bur mounted on a straight handpiece or piezo-surgical instruments [15].
Subsequently, after having achieved adequate mobility, the tooth can be extracted, removing any periapical lesion and smoothing the sharp bone edges. Finally, the suture must be performed without tension in order to give stability to the flap and the clot [16].
Moreover, Barone et al. [17] reported other techniques, including cryotherapy, piezo-surgery, and the application of platelet concentrates in the surgical site, in order to minimize early and post-operative complications [17].
Post-intervention pharmacological therapy is also described in the literature to reduce the risk of infection and to inhibit the release of the inflammatory mediators responsible for the acute response. The most common antibiotic therapy involves the use of amoxicillin: 500 mg every 8 h (or three times a day) after surgery for a total of 5 days [18].
While some authors concluded that antibiotic prophylaxis does not seem to contribute to better wound healing, less pain, and greater mouth opening after surgery [19,20,21], a recent systematic review reports that the most common protocol (i.e., amoxicillin and clavulanate per os) seems to guarantee high predictability and safety [22]. Therefore, while the routine use of antibiotics for IMTM remains debated, it is very common in dental practices.
Non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are instead indicated to prevent and/or reduce post-surgery discomfort such as pain and swelling [23]. Among these, the most used are ketoprofen, ibuprofen, paracetamol, and prednisone, used with different dosages depending on the patient [24].
Approximately about 65% of IMTM interventions are performed with minimal intra- and post-operative discomforts (pain, edema), and less commonly, they incur major complications (post-operative bleeding, trismus, bone fractures, and paresthesia) [13]. Mantovani et al. [15] reported a high incidence of alveolar osteitis, dehiscence, and suppuration in the immediate post-operative period [15]. The onset of these complications could have a substantial impact on the patient’s quality of life, thus making a correct surgical and preventive approach necessary [25].
Currently, there are many studies in the literature that evaluate the onset of complications following IMTM avulsion, although few of these focus on the analysis of possible predictive risk factors [26].
Others, while reporting the association with pre-operative risk factors, did not analyze patients’ perceptions of quality of life after intervention [24]. On the other hand, many studies identified only individual variables, reporting descriptive assessments and making the interpretation difficult because of a lack of data [25].
Therefore, the aim of this study was to evaluate the onset and severity of pain and other complications following IMTM surgery and to identify potential risk predictors.
2. Materials and Methods
The study was designed as a prospective cohort study. Patients who needed the avulsion of at least one impacted lower third molar from October 2019 to October 2021 were selected at the Department of Oral Surgery—University of Naples Federico II.
2.1. Eligibility Criteria
The patients were selected based on the following inclusion criteria:
Male and female;
Age ≥ 18 years;
Non-smoking patients;
Complete root formation of lower IMTMs based on computed tomography scan (CT scan);
Followed up to 3-, 7- and 21-day post-intervention.
The exclusion criteria were:
Pregnant or breastfeeding;
Alcohol or drug abuse;
Upper third molars;
Presence of suspected neoplastic lesion close to the impacted tooth (based on CT scan);
Presence of relevant medical history contraindicating surgical therapy;
Patients who have not undergone periodical follow-up.
2.2. Outcome Measures
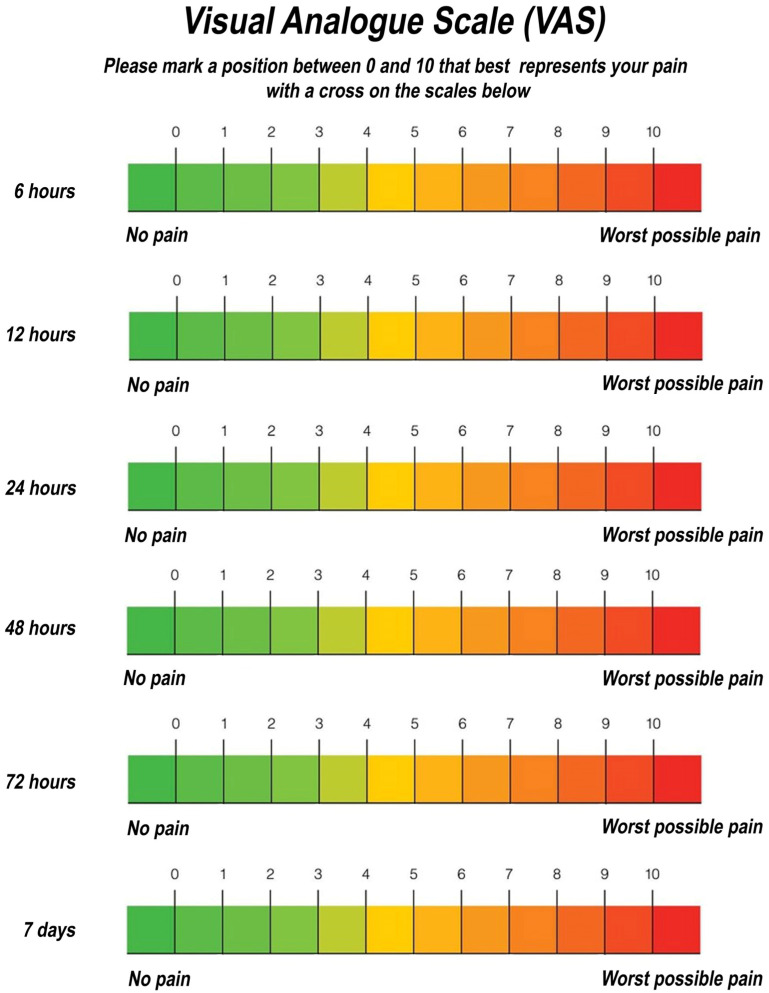
The primary outcome was the onset post-operative pain, evaluated with a Visual Analogue Scale (VAS) at 6 h, 12 h, 24 h, 48 h, 72 h and 7 days (Figure 1). The VAS scores were recorded as follows:
0–1: absence of pain;
2–4: mild pain;
5–7: moderate pain;
8–9: severe pain;
10: extremely severe pain.
Figure 1.
VAS scale.
The secondary outcomes were the presence of trismus, edema, and other post-operative complications (alveolitis, dehiscence, neuralgic injury, and suppuration), all evaluated during periodical follow-up.
Trismus was assessed by measuring the difference between the interincisal distance pre-operatively and post-operatively at 3, 7, and 21 days (Figure 2). The measurements were performed using a millimeter ruler from the incisal edge of the maxillary central incisors to the incisal edge of the mandibular central incisors at the midline when the mouth was open as wide as possible (roughly 40–55 mm) [27].
Figure 2.
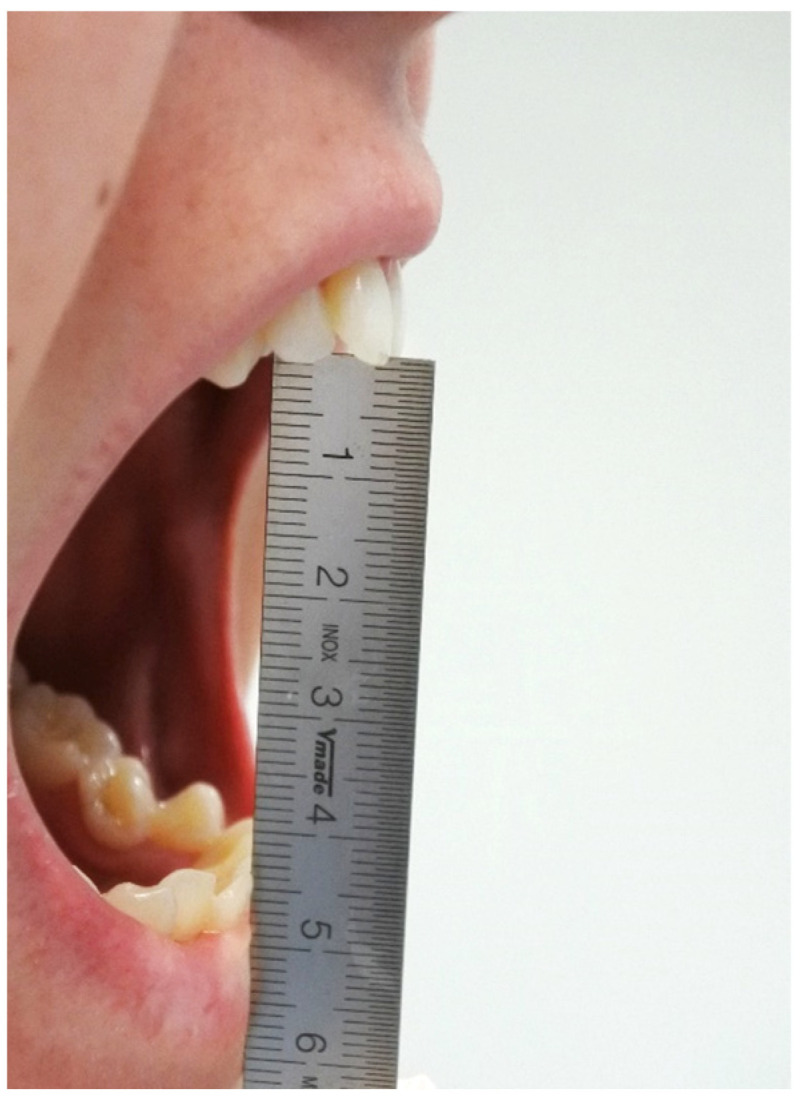
Measurement of the interincisal distance for trismus evaluation.
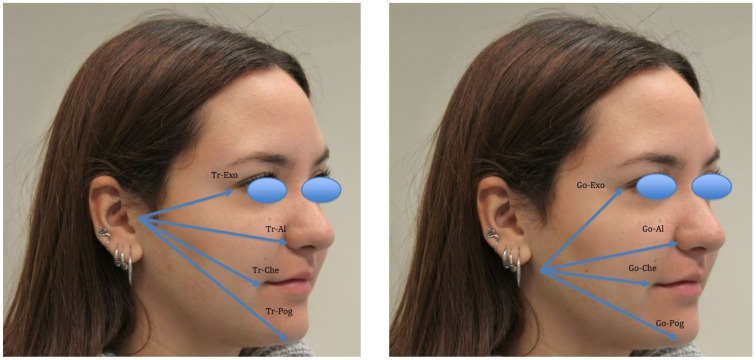
Post-operative edema was evaluated by analysing the differences between pre- and post-operative values (at 3, 7 and 21 days) of specific gnathological and facial measurements [28,29], performed with facial arches and millimeter rulers (Figure 3). The measurements were as follows:
Trago to nasal border (Tr-Al);
Trago to anatomic pogonion (TR-Pog);
Trago to eye’s external corner (Tr-Exo);
Trago to labial commissure (Tr-Che);
Anatomic gonion to anatomic pogonion (Go-Pog);
Anatomic gonion to eye’s external corner (Go-Exo);
Anatomic gonion to nasal border (Go-Al);
Anatomic gonion to labial commissure (Go-Che).
Figure 3.
Facial and gnathological measurements for edema evaluation.
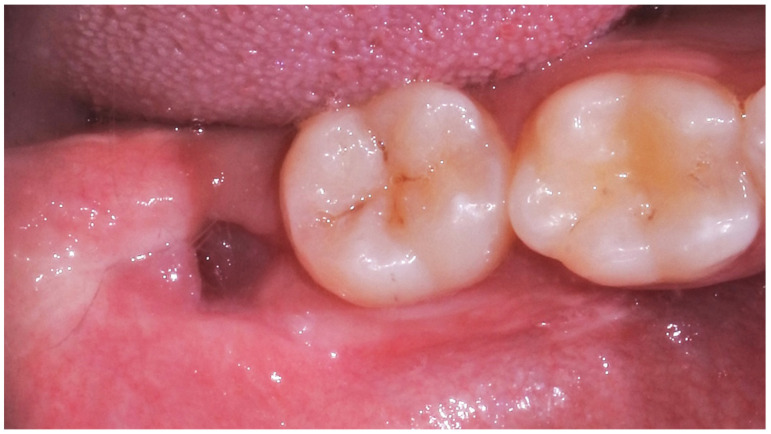
Alveolitis, dehiscence and suppuration were also assessed through a detailed inspection of the post-surgical site, evaluating the presence of osteitis and/or purulent exudate (Figure 4).
Figure 4.
Post-surgical site (4.8) evaluation at 21-day follow-up.
Furthermore, the presence of neuralgic injury was evaluated using the light touch test, which consists of tracking the course of the inferior alveolar nerve by exercising a light pressure (“light touch”) at several points with a cotton swab. The patient reports the feeling of pressure or not, and consequentially, the area of paresthesia can be mapped. If this complication occurred, the patient was monitored regularly in order to assess the pattern of recovery after 1 month, 3 months, and 6 months, according to the standardized assessments [30,31,32].
2.3. Clinical Procedure
Before oral surgery intervention, all patients received non-surgical periodontal therapy coupled with oral hygiene instructions and motivation. At 7 days, tooth extraction was performed following a standardized surgical approach.
After local anesthesia with mepivacaine 2%, access to the third molar was achieved from the buccal aspect, and bone was eventually removed with a tungsten carbide round bur on a straight handpiece under continuous irrigation with sterile saline solution. If necessary, sectioning of the crown and roots was performed with a diamond fissure bur. After reaching adequate dental mobility through the dislocation maneuver, the tooth avulsion was performed. Any periapical lesion was removed with the use of an alveolar spoon during the alveolar revision, waiting for the clot to form.
All sharp bony edges were smoothened. At last, a tension-free closure of the alveolar socket was realized with horizontal mattress sutures and single-interrupted sutures 3/0 silk. Then, an ice pack was applied to the patient’s face for 20 min.
Based on the results of a recent systematic review [22], all the patients underwent systemic antibiotic prophylaxis (amoxicillin 875 mg + clavulanic acid 125 mg every 12 h per os) starting from 2 days before up to 4 days after surgery. Furthermore, an anti-inflammatory therapy (ibuprofen 600 mg per os; one tablet after 2 h and another one 6 h after surgery) was prescribed.
Patients were instructed to rinse with 0.20% chlorhexidine-based mouthwash three times a day for 10 days (starting 24 h after surgery). Finally, a module was delivered on how to report the intensity of the pain accused (VAS scale), post-operative instructions, and reminders of the follow-ups. The sutures were removed during the 7-day follow-up, and the pain module (VAS scale) was withdrawn.
2.4. Data Collection
For each selected patient, the following data were recorded at baseline: gender, age, systemic diseases, the reason for dental extraction, inclusion type (partial or total), and surgical site (dx or sin). The characteristics of lower IMTMs (angulation, root anatomy, IAN relationship, and presence of pericoronaritis) were evaluated on CT scan and classified according to Pell and Gregory classification (Table 1). Furthermore, the duration of oral interventions was also recorded.
Table 1.
Pell and Gregory classification of lower impacted third molars (1933).
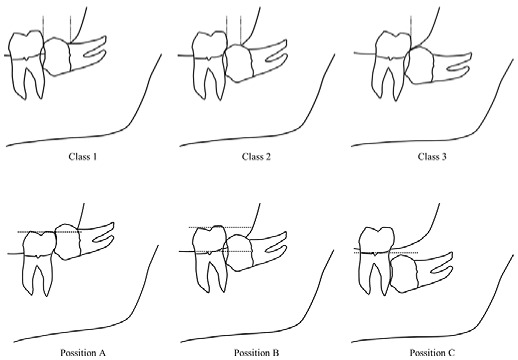
|
Class | |
| Class I | There is enough space between the ramus and the distal surface of the second molar for the accommodation of the mesiodistal size of the crown of the third molar | |
| Class II | The space between the ramus and the distal surface of the second molar is less than the mesiodistal size of the crown of the third molar | |
| Class III | All or most of the third molar is located within the ramus | |
| Position | ||
| Position A | The highest point of the tooth is on level or above the occlusal plane of the second molar | |
| Position B | The highest point of the tooth is below the occlusal plane but above the cervical line of the second molar | |
| Position C | The highest point of the tooth is below the cervical line of the second molar | |
Interincisal distance and all measurements for the evaluation of edema (Tr-Al, TR-Pog, Tr-Exo, Tr-Che, Go-Pog, Go-Exo, Go-Al, and Go-Che) were recorded at baseline and during follow-up (3, 7, and 21 days).
Finally, other complications (alveolitis, dehiscence, suppuration, and neuralgic injury) were assessed during clinical controls through an oral inspection of the post-surgical site.
2.5. Statistical Analysis
The data analysis was performed using a commercially available statistical software (IBM SPSS Statistics v.25, IBM Inc., Armonk, NY, USA). Gender, systemic diseases, reason for surgery, and characteristics of IMTMs (inclusion type, surgical site, Pell and Gregory classification, angulation, root anatomy, IAN relationship, and the presence of pericoronaritis) were recorded as frequencies and percentages, while mean and standard deviations (SDs) were calculated for age (years), duration of oral surgery (minutes), trismus (millimeters), and edema (millimeters).
Pain was registered with a VAS scale, and all other post-operative complications (trismus, edema, alveolitis, dehiscence, neuralgic injury, and suppuration) were reported as frequencies and percentages. Furthermore, the presence of edema was assessed by changing at least one of the facial or gnathological measurements.
A correlation analysis was performed to identify potential associations between pre- (systemic diseases, Pell and Gregory classification, angulation, IAN relationship), peri- (surgical time), and post- (trismus, edema) operative factors and VAS scores during follow-up. When a statistically significant correlation was identified, a regression analysis was performed. A p-value < 0.05 was accepted to identify a statistically significant difference.
3. Results
A total of 25 participants were selected from October 2019 to October 2021 at the Department of Oral Surgery—University of Naples Federico II. The characteristics of patients at baseline are shown in Table 2. Most participants were female (21; 84%) with a mean age of 25 ± 3 years. Systemic diseases were represented by multiple sclerosis (8%) and fibromyalgia (4%), while 88% of patients were in healthy condition (ASA index ≤ 2). The reasons for oral surgery were dysodontiasis (15; 60%), severe tooth decay (3; 12%), orthodontics (4; 16%) and periodontal (3; 12%). The inclusion type was partial in 15 patients (60%) and total in 10 (40%). Moreover, the lower IMTM was the tooth 4.8 in 56% of the cases and 3.8 in 44%.
Table 2.
Characteristics of selected patients at baseline.
| Patients (N = 25) | % | ||
|---|---|---|---|
| Gender | Female = 21 Male = 4 |
84% 16% |
|
| Mean age | 25 ± 3 years | ||
| Systemic disease | Healthy (ASA Index ≤ 2) = 22 Multiple sclerosis (MS) = 2 Fibromyalgia (FMS) = 1 |
88% 8% 4% |
|
| Reason of surgery | Dysodontiasis Severe tooth decay Orthodontics Periodontal |
=15 =3 =4 =3 |
60% 12% 16% 12% |
| Inclusion type | Partially impacted Totally impacted |
=15 =10 |
60% 40% |
| Surgical site | Third molar dx (4.8) Third molar sin (3.8) |
=14 = 11 |
56% 44% |
Table 3 reports the characteristics of lower IMTMs analysed on CT scans. The most frequent classes, according to Pell and Gregory classification, were: BII (9; 36%), AII (7; 28%), BIII (5; 20%) and CII (4; 16%). The tooth angulations were vertical in 40% of the cases, mesio-inclined in 28%, and horizontal in 32%.
Table 3.
Characteristics of lower impacted third molars (IMTMs).
| Patients (N = 25) | % | ||
|---|---|---|---|
| Classification (Pell and Gregory) |
BII AII BIII CII |
=9 =7 =5 =4 |
36% 28% 20% 16% |
| Angulation | Vertical Mesio-inclined Horizontal |
=10 =7 =8 |
40% 28% 32% |
| Root anatomy | Fused roots Separated roots Buttoned roots |
=13 =11 =1 |
52% 44% 4% |
| IAN relationship | Proximity > 2 mm Proximity ≤ 2 mm Continuity |
=14 =8 =3 |
56% 32% 12% |
| Pericoronaritis | Yes No |
=15 =10 |
60% 40% |
| Average duration of oral surgery | 35 ± 5 min | ||
Radicular anatomy was represented in 52% of patients as fused, 44% as separated, and 4% as buttoned. The relationship with the inferior alveolar nerve (IAN) was, respectively, 56% of proximity >2 mm, 32% of proximity ≤2 mm, and 12% of continuity.
Pericoronaritis was recorded in 15 patients (60%) before oral surgery. At last, the average duration of intervention was 35 ± 5 min.
Post-operative pain was recorded using the Visual Analogic Scale, as reported in Table 4. The VAS values showed that most patients had mild pain (VAS score: 2–4) at 6 h (44%), 12 h (48%), 24 h (68%), and 48 (68%) after surgery. Pain and discomfort were reported in only 6 cases (24%; VAS score 2–4) at 72 h, while a VAS score of 0–1 was recorded in all patients after 7 days.
Table 4.
VAS Scores.
| Patients (N = 25) | ||||||
|---|---|---|---|---|---|---|
| VAS Score | 6 h | 12 h | 24 h | 48 h | 72 h | 7 Days |
| 0–1 | 4 (16%) | 4 (16%) | 4 (16%) | 4 (16%) | 19 (76%) | 25 (100%) |
| 2–4 | 11 (44%) | 12 (48%) | 17 (68%) | 17 (68%) | 6 (24%) | 0 (0%) |
| 5–7 | 8 (32%) | 8 (32%) | 4 (16%) | 4 (16%) | 0 (0%) | 0 (0%) |
| 8–9 | 2 (8%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 10 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Table 5 shows the average interincisal distance differences from the baseline. Trismus was observed in 5 patients (20%) at 3-day follow-up, with an average reduction of mouth opening (interincisal distance) of 7 mm ± 3.
Table 5.
Average interincisal distance difference from baseline.
| Patients (N = 25) | |||
|---|---|---|---|
| Follow Up | Trismus | Average Interincisal Distance Difference (from Baseline) | |
| 3-day | Yes No |
=5 (20%) =20 (80%) |
7 mm ± 3 |
| 7-day | Yes No |
=4 (16%) =21 (84%) |
3 mm ± 3 |
| 21-day | Yes No |
=0 (0%) =25 (100%) |
0 mm ± 3 |
This reduction was 3 mm ± 3 at 7-day follow-up but only in 4 patients (16%), while no reduction of the physiological range of mouth opening (40–55 mm) was registered at 21 days.
Facial and gnathological measurements for the evaluation of edema are described in Table 6. The majority of patients (64%) showed edema at 3-day follow-up, with an increase in all parameters. However, these values decreased at 7-day follow-up as well as the number of patients with swelling (12%). All facial and gnathological measurements returned at baseline values at 21 days.
Table 6.
Average facial and gnathological measurements differences from baseline.
| Patients (N = 25) | |||
|---|---|---|---|
| Follow Up | Edema | Facial Measurements | Average Facial and Gnathological Measurements Differences (from Baseline) |
| 3-day | Yes = 16 (64%) No = 9 (36%) |
|
|
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
| 7-day | Yes = 3 (12%) No = 22 (88%) |
|
|
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
| 21-day | Yes = 0 (0%) No = 25 (100%) |
/ | / |
Table 7 reports other post-operative complications observed during the clinical inspection of the surgical site. While dehiscence and suppuration were not recorded, alveolitis was instead present in 12% of patients at 3-day follow-up, in 4% at 7 days, and in no case at 21 days.
Table 7.
Other post-operative complications.
| Follow up | Complication | Patients (N = 25) | % |
|---|---|---|---|
| 3-day | Alveolitis | Yes = 3 No = 22 |
12% 88% |
| Dehiscence | Yes = 0 No = 25 |
0% 100% |
|
| Nevralgic injury | Yes = 1 No = 24 |
4% 96% |
|
| Suppuration | Yes = 0 No = 25 |
0% 100% |
|
| 7-day | Alveolitis | Yes = 1 No = 24 |
4% 96% |
| Dehiscence | Yes = 0 No = 25 |
0% 100% |
|
| Nevralgic injury | Yes = 1 No = 24 |
4% 96% |
|
| Suppuration | Yes = 0 No = 25 |
0% 100% |
|
| 21-day | Alveolitis | Yes = 0 No = 25 |
0% 100% |
| Dehiscence | Yes = 0 No = 25 |
0% 100% |
|
| Nevralgic injury | Yes = 1 No = 24 |
4% 96% |
|
| Suppuration | Yes = 0 No = 25 |
0% 100% |
|
Neuralgic injury represented by temporary paraesthesia was reported in one case (4%) at 7-day follow-up, where a buttoned root was in a continuity relationship with IAN. The area of paraesthesia was mapped by a light touch test using a cotton swab. The patient was monitored regularly at the Department of Oral Surgery—University of Naples Federico II, and spontaneous healing was observed after 3 months.
A linear regression analysis between VAS scores and patient- and tooth-related factors was performed, showing a statistically significant association (p < 0.05) between the duration of oral surgery and VAS scores at 6 (Table 8) and 12 h (Table 9).
Table 8.
Correlation between VAS scores at 6 h and patient- and tooth-related factors.
| Model Summary | ||||||
| Model | R | R-square | Adapted R-square | Std. error of the estimate | ||
| 1 | 0.828 a | 0.685 | 0.496 | 0.605 | ||
| ANOVA | ||||||
| 1 | Sum of squares | df | Mean Square | F | Sign. | |
| Regression | 11.946 | 9 | 1.327 | 3.624 | 0.14 a | |
| Residual | 5.494 | 15 | 0.366 | |||
| Total | 17.440 | 24 | ||||
| Coefficients | ||||||
| 1 | Non-standardized coefficients | Standardized coefficients | ||||
| B | Standard Error | Beta | t | Sign. | ||
| (Constant) | 3.384 | 2.127 | 0.133 | |||
| Pell and Gregory classification | 0.255 | 0.299 | 0.243 | 0.854 | 0.406 | |
| IAN relationship | 0.392 | 0.280 | 0.327 | 1.401 | 0.182 | |
| Duration of oral surgery | 1.279 | 0.384 | 0.808 | 3.329 | 0.005 | |
| Systemic disease | 0.405 | 0.428 | 0.284 | 0.946 | 0.359 | |
| Edema at 3-day | 0.037 | 0.309 | ||||
| Edema at 7-day | 0.089 | 0.489 | ||||
| Trismus at 3-day | 0.550 | 0.385 | 0.319 | 1.428 | 0.174 | |
| Trismus at 7-day | 0.689 | 0.855 | ||||
| Angulation | 0.107 | 0.204 | 0.108 | 0.525 | 0.607 | |
a Predictors: (costant) Pell and Gregory classification, IAN relationship, duration of oral surgery, systemic diseases, edema at 3- and 7- day, trismus at 3- and 7- day, angulation.
Table 9.
Correlation between VAS scores at 12 h and patient- and tooth-related factors.
| Model Summary | ||||||
| Model | R | R-square | Adapted R-square | Std. error of the estimate | ||
| 1 | 0.839a | 0.703 | 0.526 | 0.537 | ||
| ANOVA | ||||||
| 1 | Sum of squares | df | Mean Square | F | Sign. | |
| Regression | 10.242 | 9 | 1.138 | 3.954 | 0.009 a | |
| Residual | 4.138 | 15 | 0.288 | |||
| Total | 14.560 | 24 | ||||
| Coefficients | ||||||
| 1 | Non-standardized coefficients | Standardized coefficients | ||||
| B | Standard Error | Beta | t | Sign. | ||
| (Constant) | 3.971 | 1.886 | 0.052 | |||
| Pell and Gregory classification | 0.119 | 0.265 | 0.124 | 0.448 | 0.660 | |
| IAN relationship | 0.234 | 0.248 | 0.214 | 0.944 | 0.360 | |
| Duration of oral surgery | 1.209 | 0.341 | 0.836 | 3.351 | 0.003 | |
| Systemic disease | 0.380 | 1.858 | 0.083 | |||
| Edema at 3-day | 0.032 | 0.600 | ||||
| Edema at 7-day | 0.034 | 0.079 | 0.115 | 0.429 | 0.674 | |
| Trismus at 3-day | 0.458 | 0.342 | 0.290 | 1.340 | 0.200 | |
| Trismus at 7-day | 0.582 | 0.611 | 0.305 | 0.952 | 0.356 | |
| Angulation | 0.117 | 0.181 | 0.129 | 0.646 | 0.528 | |
a Predictors: (costant) Pell and Gregory classification, IAN relationship, duration of oral surgery, systemic diseases, edema at 3- and 7- day, trismus at 3- and 7- day, angulation.
Moreover, the logistic binary regression analysis identified systemic disease, Pell and Gregory classification, duration of oral surgery, VAS at 6 and 12 h, and trismus and edema at 3 and 7 days as predictive factors of post-operative complications (Table 10).
Table 10.
Logistic binary regression analysis.
| Classification Table a,b | |||||||||
| Observed | Predicted | ||||||||
| Complications | Percentage correct | ||||||||
| No (0) | Yes (1) | ||||||||
| Step 0 | Complications | No (0) | 21 | 0 | 100.0 | ||||
| Yes (1) | 4 | 0 | 0.0 | ||||||
| Overall percentage | 84.0 | ||||||||
| Variables in the Equation | |||||||||
| Step 0 | B | S.E. | Wald | df | Sign. | Exp (B) | |||
| Constant | 0.546 | 9.239 | 1 | 0.002 | 0.190 | ||||
| Variables not in the Equation a | |||||||||
| Step 0 | Score | df | Sign. | ||||||
| Variables | Angulation | 0.043 | 1 | 0.836 | |||||
| Pell and Gregory classification | 6.361 | 1 | 0.12 | ||||||
| IAN relationship | 1.895 | 1 | 1.169 | ||||||
| Duration of oral surgery | 8.622 | 1 | 0.003 | ||||||
| Systemic disease | 14.187 | 1 | 0.000 | ||||||
| VAS at 6-h | 9.505 | 1 | 0.002 | ||||||
| VAS at 12-h | 4.723 | 1 | 0.030 | ||||||
| VAS at 24-h | 3.720 | 1 | 0.054 | ||||||
| VAS at 48-h | 3.720 | 1 | 0.054 | ||||||
| VAS at 72-h | 1.765 | 1 | 0.184 | ||||||
| Trismus at 3-day | 6.857 | 1 | 0.009 | ||||||
| Trismus at 7-day | 9.003 | 1 | 0.003 | ||||||
| Edema at 3-day | 11.049 | 1 | 0.001 | ||||||
| Edema at 7-day | 6.512 | 1 | 0.011 | ||||||
a Constant is included in the model. b The cut value is 0.500.
4. Discussion
The aim of this study was to evaluate the onset of complications following lower impacted third molar (IMTM) extraction. More specifically, it was sought to assess the patient’s perception related to post-operative pain and to identify potential risk predictors.
In this study, the majority of patients were female and needed IMTM extraction due to dysodontiasis. These data are confirmed by Kruger et al. [33], who reported that lower third molar inclusion more frequently affects the female gender.
Furthermore, Juodzbalys and Daugela [6] stated in their review that dysodontiasis is one of the most frequent causes of extraction, followed by carious lesions, resorption of adjacent teeth, pericoronaritis, periapical abscess, and the presence of a cyst or neoplasm.
Regarding the variables related to inclusion type, this study adopted Pell and Gregory classification, showing a prevalence of type BII (9; 36%), followed by AII (7; 28%), BIII (5; 20%), and CII (4; 16%). These patterns are in line with Santos et al. [34], who reported BII as the most frequent class of inclusion (26.4%) [34]. Moreover, class BII is the second most common position associated with secondary caries, preceded by class AII.
Position CIII was not reported in our study, although it is the most difficult position in terms of oral surgery procedure, as well as the most common class related to early and post-operative complications [34].
In this study, the majority of the third molars were located in vertical angulation (10; 40%). In contrast, Santos et al. [34] highlighted a majority of mesio-angular positions (41.8%) on a total of 1055 third lower molars analysed [34].
The IAN relationship was 56% of proximity > 2 mm, 32% of proximity ≤ 2 mm, and 12% of continuity.
However, there are other radiological classifications in order to evaluate the distance from the mandibular canal, as reported by the study of W.P. Smith [35] et al., in which on a total of 1589 teeth, 466 (29%) showed a distant relationship, 869 (55%) were close to the canal, and only 254 (16%) were deemed to be intimate to the canal [35]. Despite different evaluations, these results are partially in line with our study.
Pericoronaritis is a pathological condition due to the inflammation of the pericoronary follicle, reported in this study in 15 patients (60%). This inflammatory process is widely reported in the literature, and it is considered the most common reason for pain and discomfort. Smith et al. [35] stated the presence of pericoronaritis in 772 third molars (49%) that required dental avulsion [35].
A linear regression analysis was performed to identify potential associations between patient- and tooth-related factors and VAS scale. The results showed a positive correlation only for the duration of intervention with VAS at 6 and 12 h post-intervention. No correlations were found in subsequent follow up (24 h, 48 h, 72 h, and 7 days).
These data are in line with several other studies, which have demonstrated how the length of the surgical intervention is strictly related to the onset of early pain, as well as being a possible predictive factor of a longer recovery when the time of surgery is over 30 min [36,37]. Furthermore, Alvira-González et al. [38] reported that surgical time could also be considered a predictive variable for determining extraction difficulty [38,39].
A logistic binary regression analysis was also performed in order to evaluate potential predictive factors related to IMTM complications. The analysis showed a positive correlation for the following variables: systemic disease, Pell and Gregory classification, duration of oral surgery, VAS at 6 and 12 h, and trismus and edema at 3 and 7 days.
Many authors showed a correlation between post-operative complications and systemic diseases, not only with fibromyalgia but also with multiple sclerosis [40]. These pathological conditions indeed determine a systemic inflammatory state, with a massive release of IL-6 and a leukocyte activity deficit mainly detected from 2 to 6 h post-intervention. All these phenomena lead to a delay in the healing process and an increased risk of complications [27].
The position of IMTM, evaluated with Pell and Gregory classification, was shown to be, in this study, a predictive factor of post-operative discomfort. According to Bui et al. [8], mesio-angular impacted teeth were associated with a higher risk for post-operative complications. Moreover, Yuasa et al. [41] found that depth and ramus relationship/space available were associated with a more difficult extraction and, consequently, pain and discomfort.
Duration of oral surgery was also a predictive factor of pain, trismus, and edema. According to Alkadi et al. [16], the greater the operative time, the greater the probability of post-operative complications.
The assessment of trismus, measured by comparing the interincisal distance difference from baseline, showed a percentage of 20% at 3 days and 16% at 7 days, while there was a complete recovery by the 21st day of follow-up. These results are in accordance with De Menezes et al. [42], although it is very probable that these values have been achieved due to the use of non-steroidal anti-inflammatory drugs (NSAIDs). In our study, NSAIDs were administrated to all patients (ibuprofen 600 mg per os; one tablet after 2 h and another one 6 h after surgery), so due to the lack of a control group, we were not able to assess any statistical correlations.
With regard to swelling values, the results of this study showed the presence of edema in 64% of patients at 3 days after surgery, values that are significantly reduced at 7-day follow-up (12%). The swelling can be explained by the inflammatory and edema responses that occur as a result of surgical trauma.
This mechanism occurs mainly through the production of prostaglandins and cyclooxygenases, which are synthesized following arachidonic acid release from the cell membrane of cells at the surgical site [43]. In contrast, Yuasa et al. [41] reported high rates of edema from the third day after surgery to the seventh day, with a gradual onset and a peak at 48-h post-intervention.
Nevertheless, in this study, alveolar osteitis (AO) did not prove to be statistically significant, although it is one of the most common complications related to impacted third molars extraction. Therefore, Bartuli et al. [44] reported an incidence of AO between 4.1% to 32.6%, relating this to mild and severe pain.
Suppuration and dehiscence were also not detected, although these complications are often reported in the literature. Generally, in these cases, the treatment involves antibiotic therapy (clindamycin or amoxicillin) or a re-opening of the surgical site and curettage in case of refractoriness to therapy [45]. Rahpeyma et al. [46] highlighted how wound dehiscence could be avoided with a more conservative flap; nevertheless, this depends on the clinical case.
In this study, inferior alveolar nerve (IAN) continuity did not show to have any correlation with post-operative complications, although Albuquerque et al. [23] reported a positive association with a greater probability of nerve injury.
In our study, only one patient showed temporary paresthesia, which healed spontaneously after 3 months. Many studies in the literature report that paresthesia, mainly due to post-operative edema and swelling, generally tends to heal after 2–6 months [47]. Possible therapies to facilitate recovery after nerve injury include the administration of neurotrophic factors such as B12 vitamin, mecobalamin, or a stellate ganglion block, as suggested by Nogami et al. [47], despite being an invasive procedure.
The main objective of IMTM surgery is avulsion with minimal intra- and post-operative complications, performing the most conservative technique possible and supporting the patient in the post-intervention period.
There is much evidence of a relationship between surgical trauma and post-intervention complications [37]. Additionally, the size of the mucoperiosteum flap and the quantity of the osteotomy influenced the severity of pain and swelling [47]. Grossi et al. [48] demonstrated that raising a small flap without bone removal and/or tooth/root sectioning could cause patients to suffer more severe pain, presumably because of the excessive soft tissue damage and delayed wound healing [49]. Furthermore, Lo Giudice et al. [50] highlighted how an ostectomy performed with an ultrasonic tip showed the best results, preserving the bone morphology in both quantitative and qualitative analyses [50].
Therefore, a meticulous surgical planification with a CT exam is essential in order to minimize complications, especially intra-operatively, and to perform a correct ostectomy and odontotomy [49].
5. Conclusions
Although the small number of patients, the use of a subjective pain assessment method (VAS scale), and the prescription of an anti-inflammatory therapy represent limitations, the outcomes of this prospective cohort study suggest that the onset of pain and other post-operative complications increase in proportion to the duration of the surgical procedure, in accordance with the literature. Hence, an accurate evaluation of risk predictors and a conservative surgical technique will minimize complications or at least reduce recovery times and patient symptoms. Further investigations with a large sample and a more objective pain assessment method are needed to elucidate the best preventive and surgical protocols for the management of the complications related to third molar avulsion.
Author Contributions
Conceptualization, L.R.; methodology, A.B.; validation, A.C.; formal analysis, G.I.; investigation, R.M. and V.I.-S.; data curation, A.B.; writing—original draft preparation, A.C.; writing—review and editing, G.I.; supervision, L.R.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The protocol was approved by the Commission on Research Ethics of the University of Naples Federico II (approval number 131/18, approval date 18 July 2018). The study was conducted according to the Principles of the Declaration of Helsinki on experimentation involving human subjects.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Eklund S.A., Pittman J.L. Third-molar removal patterns in an insured population. J. Am. Dent. Assoc. 2001;132:469–475. doi: 10.14219/jada.archive.2001.0209. [DOI] [PubMed] [Google Scholar]
- 2.Ramiro-Verdugo J., De Vicente-Corominas E., Montiel-Company J.M., Gandía-Franco J.L., Bellot-Arcís C. Association between third molar agenesis and craniofacial structure development. Am. J. Orthod. Dentofac. Orthop. 2015;148:799–804. doi: 10.1016/j.ajodo.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Brickley M., Kay E., Shepherd J.P., Armstrong R.A. Decision Analysis for lower-third-molar Surgery. Med. Decis. Mak. 1995;15:143–151. doi: 10.1177/0272989X9501500207. [DOI] [PubMed] [Google Scholar]
- 4.Costa M.G., Pazzini C.A., Pantuzo M.C. Is there justification for prophylactic extraction of third molars? A systemic review. Braz. Oral Res. 2013;27:183–188. doi: 10.1590/S1806-83242013000100024. [DOI] [PubMed] [Google Scholar]
- 5.Guerrouani A., Zeinoun T., Vervaet C., Legrand W. A Four-Year Monocentric Study of the Complications of Third Molars Extractions under General Anesthesia: About 2112 Patients. Int. J. Dent. 2013;2013:763837. doi: 10.1155/2013/763837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juodzbalys G., Daugela P. Mandibular Third Molar Impaction: Review of Literature and a Proposal of a Classification. J. Oral Maxillofac. Res. 2013;4:e1. doi: 10.5037/jomr.2013.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susarla S., Dodson T.B. Estimating third molar extraction difficulty: A comparison of subjective and objective factors. J. Oral Maxillofac. Surg. 2005;63:427–434. doi: 10.1016/j.joms.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Bui C.H., Seldin E.B., Dodson T.B. Types, frequencies, and risk factors for complications after third molar extraction. J. Oral Maxillofac. Surg. 2003;61:1379–1389. doi: 10.1016/j.joms.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Blondeau F., Daniel N.G. Extraction of impacted mandibular third molars: Postoperative complications and their risk factors. J. Can. Dent. Assoc. 2007;73:325–327. [PubMed] [Google Scholar]
- 10.Xu G.-Z., Yang C., Fan X.-D., Yu C.-Q., Cai X.-Y., Wang Y., He D. Anatomic relationship between impacted third mandibular molar and the mandibular canal as the risk factor of inferior alveolar nerve injury. Br. J. Oral Maxillofac. Surg. 2013;51:e215–e219. doi: 10.1016/j.bjoms.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Garcia A.G., Sampedro F.G., Rey J.G. Pell Gregory classification is unreliable as a predictor of difficulty in extracting impacted lower third molars. Br. J. Oral Maxillofac. Surg. 2000;38:585–587. doi: 10.1054/bjom.2000.0535. [DOI] [PubMed] [Google Scholar]
- 12.Diniz-Freitas M., Lago-Méndez L., Gude-Sampedro F., Martín J.M.S., Gándara-Rey J.M., Garcia-Garcia A. Pederson scale fails to predict how difficult it will be to extract lower third molars. Br. J. Oral Maxillofac. Surg. 2007;45:23–26. doi: 10.1016/j.bjoms.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z.-L., Jiang E.-S., Cui L.-Y., Li J.-X. Cone-Beam Computed Tomography Analysis on the Relationship between the Mandibular Third Molar and the Position of the Mandibular Canal in Koreans from the Yanbian Area and the Han People. Int. J. Clin. Pract. 2023;2023:9563476. doi: 10.1155/2023/9563476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sifuentes-Cervantes J.S., Carrillo-Morales F., Castro-Núñez J., Cunningham L.L., Van Sickels J.E. Third molar surgery: Past, present, and the future. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021;132:523–531. doi: 10.1016/j.oooo.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani E., Arduino P.G., Schierano G., Ferrero L., Gallesio G., Mozzati M., Russo A., Scully C., Carossa S. A Split-Mouth Randomized Clinical Trial to Evaluate the Performance of Piezosurgery Compared with Traditional Technique in Lower Wisdom Tooth Removal. J. Oral Maxillofac. Surg. 2014;72:1890–1897. doi: 10.1016/j.joms.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Alkadi S., Stassen L. Effect of One-Suture and Sutureless Techniques on Postoperative Healing After Third Molar Surgery. J. Oral Maxillofac. Surg. 2019;77:703–716. doi: 10.1016/j.joms.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Barone A., Marconcini S., Giacomelli L., Rispoli L., Calvo J.L., Covani U. A Randomized Clinical Evaluation of Ultrasound Bone Surgery Versus Traditional Rotary Instruments in Lower Third Molar Extraction. J. Oral Maxillofac. Surg. 2010;68:330–336. doi: 10.1016/j.joms.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 18.López-Cedrún J.L., Pijoan J.I., Fernández S., Santamaria J., Hernández G. Efficacy of Amoxicillin Treatment in Preventing Postoperative Complications in Patients Undergoing Third Molar Surgery: A Prospective, Randomized, Double-Blind Controlled Study. J. Oral Maxillofac. Surg. 2011;69:e5–e14. doi: 10.1016/j.joms.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Monaco G., Staffolani C., Gatto M.R., Checchi L. Antibiotic therapy in impacted third molar surgery. Eur. J. Oral Sci. 1999;107:437–441. doi: 10.1046/j.0909-8836.1999.eos107604.x. [DOI] [PubMed] [Google Scholar]
- 20.Poeschl P.W., Eckel D., Poeschl E. Postoperative prophylactic antibiotic treatment in third molar surgery—A necessity? J. Oral Maxillofac. Surg. 2004;62:3–8. doi: 10.1016/j.joms.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 21.D’Ambrosio F., Di Spirito F., Amato A., Caggiano M., Giudice R.L., Martina S. Attitudes towards Antibiotic Prescription and Antimicrobial Resistance Awareness among Italian Dentists: What Are the Milestones? Healthcare. 2022;10:1585. doi: 10.3390/healthcare10081585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cervino G., Cicciù M., Biondi A., Bocchieri S., Herford A.S., Laino L., Fiorillo L. Antibiotic Prophylaxis on Third Molar Extraction: Systematic Review of Recent Data. Antibiotics. 2019;8:53. doi: 10.3390/antibiotics8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albuquerque A., Fonteles C., Val D.D., Chaves H., Bezerra M., Pereira K., Silva P.D.B., de Lima B., Soares E., Ribeiro T., et al. Effect of pre-emptive analgesia on clinical parameters and tissue levels of TNF-α and IL-1β in third molar surgery: A triple-blind, randomized, placebo-controlled study. Int. J. Oral Maxillofac. Surg. 2017;46:1615–1625. doi: 10.1016/j.ijom.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Gazal G., Al-Samadani K.H. Comparison of paracetamol, ibuprofen, and diclofenac potassium for pain relief following dental extractions and deep cavity preparations. Saudi Med. J. 2017;38:284–291. doi: 10.15537/smj.2017.3.16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath C., Comfort M., Lo E., Luo Y. Changes in life quality following third molar surgery, the immediate postoperative period. Br. Dent. J. 2003;195:265–268. doi: 10.1038/sj.bdj.4809930. [DOI] [PubMed] [Google Scholar]
- 26.Phillips C., White R.P., Jr., Shugars D.A., Zhou X. Risk factors associated with prolonged recovery and delayed healing after third molar surgery. J. Oral Maxillofac. Surg. 2003;61:1436–1448. doi: 10.1016/j.joms.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham S., Hunt N., Feinmann C. Perceptions of outcome following orthognathic surgery. Br. J. Oral Maxillofac. Surg. 1996;34:210–213. doi: 10.1016/S0266-4356(96)90271-5. [DOI] [PubMed] [Google Scholar]
- 28.Costa F., Soares E., Esses D., Silva P.D., Bezerra T., Scarparo H., Ribeiro T., Fonteles C. A split-mouth, randomized, triple-blind, placebo-controlled study to analyze the pre-emptive effect of etoricoxib 120mg on inflammatory events following removal of unerupted mandibular third molars. Int. J. Oral Maxillofac. Surg. 2015;44:1166–1174. doi: 10.1016/j.ijom.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Isola G., Matarese M., Ramaglia L., Cicciù M. Evaluation of the efficacy of celecoxib and ibuprofen on postoperative pain, swelling, and mouth opening after surgical removal of impacted third molars: A randomized, controlled clinical trial. Int. J. Oral Maxillofac. Surg. 2019;48:1348–1354. doi: 10.1016/j.ijom.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Stone H.H., Hooper C.A., Kolb L.D., Geheber C.E., Dawkins E.J. Antibiotic Prophylaxis in Gastric, Biliary and Colonic Surgery. Ann. Surg. 1976;184:443–452. doi: 10.1097/00000658-197610000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Classen D.C., Evans R.S., Pestotnik S.L., Horn S.D., Menlove R.L., Burke J.P. The Timing of Prophylactic Administration of Antibiotics and the Risk of Surgical-Wound Infection. N. Engl. J. Med. 1992;326:281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 32.Isola G., Matarese M., Ramaglia L., Iorio-Siciliano V., Cordasco G., Matarese G. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: A randomized, triple-blind, controlled clinical trial. Clin. Oral Investig. 2019;23:2443–2453. doi: 10.1007/s00784-018-2690-9. [DOI] [PubMed] [Google Scholar]
- 33.Kruger E., Thomson W.M., Konthasinghe P. Third molar outcomes from age 18 to 26: Findings from a population-based New Zealand longitudinal study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001;92:150–155. doi: 10.1067/moe.2001.115461. [DOI] [PubMed] [Google Scholar]
- 34.Santos K.K., Lages F.S., Maciel C.A.B., Glória J.C.R., Douglas-De-Oliveira D.W. Prevalence of Mandibular Third Molars According to the Pell & Gregory and Winter Classifications. J. Maxillofac. Oral Surg. 2022;21:627–633. doi: 10.1007/s12663-020-01473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith W. The relative risk of neurosensory deficit following removal of mandibular third molar teeth: The influence of radiography and surgical technique. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013;115:18–24. doi: 10.1016/j.oooo.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Gidron Y., McGrath P.J., Goodday R. The physical and psychosocial predictors of Adolescents’ recovery from oral surgery. J. Behav. Med. 1995;18:385–399. doi: 10.1007/BF01857662. [DOI] [PubMed] [Google Scholar]
- 37.Lago-Méndez L., Diniz-Freitas M., Senra-Rivera C., Gude-Sampedro F., Rey J.M.G., García-García A. Relationships Between Surgical Difficulty and Postoperative Pain in Lower Third Molar Extractions. J. Oral Maxillofac. Surg. 2007;65:979–983. doi: 10.1016/j.joms.2006.06.281. [DOI] [PubMed] [Google Scholar]
- 38.Alvira-Gonzalez J., Figueiredo R., Valmaseda-Castellon E., Quesada-Gomez C., Escoda C.G. Predictive factors of difficulty in lower third molar extraction: A prospective cohort study. Med. Oral Patol. Oral Y Cir. Bucal. 2017;22:e108–e114. doi: 10.4317/medoral.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharif K., Watad A., Bragazzi N.L., Lichtbroun M., Amital H., Shoenfeld Y. Physical activity and autoimmune diseases: Get moving and manage the disease. Autoimmun. Rev. 2018;17:53–72. doi: 10.1016/j.autrev.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Peterson L.J., Miyawaki T., Maeda S., Shimada M. Elevation of plasma interleukin-6 level in patients undergoing oral and maxillofacial surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology. 1996;81:15–20. doi: 10.1016/S1079-2104(96)80140-5. [DOI] [PubMed] [Google Scholar]
- 41.Yuasa H., Sugiura M. Clinical postoperative findings after removal of impacted mandibular third molars: Prediction of postoperative facial swelling and pain based on preoperative variables. Br. J. Oral Maxillofac. Surg. 2004;42:209–214. doi: 10.1016/j.bjoms.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 42.De Menezes S., Cury P. Efficacy of nimesulide versus meloxicam in the control of pain, swelling and trismus following extraction of impacted lower third molar. Int. J. Oral Maxillofac. Surg. 2010;39:580–584. doi: 10.1016/j.ijom.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Barbalho J., Vasconcellos R., de Morais H., Santos L., Almeida R.D.A., Rêbelo H., Lucena E., de Araújo S. Effects of co-administered dexamethasone and nimesulide on pain, swelling, and trismus following third molar surgery: A randomized, triple-blind, controlled clinical trial. Int. J. Oral Maxillofac. Surg. 2017;46:236–242. doi: 10.1016/j.ijom.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Bartuli F.N., Luciani F., Caddeo F. Piezosurgery vs High Speed Rotary Handpiece: A comparison between the two techniques in the impacted third molar surgery. Oral Implantol. 2013;6:5–10. doi: 10.11138/orl/2013.6.1.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaposvári I., Körmöczi K., Csurgay K., Horváth F., Ashourioun A.H., Buglyó A., Turai A.R., Joób-Fancsaly Á. Delayed-onset infections after lower third molar surgery: A Hungarian case-control study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021;132:641–647. doi: 10.1016/j.oooo.2021.04.052. [DOI] [PubMed] [Google Scholar]
- 46.Rahpeyma A., Khajehahmadi S., Ilkhani S. Wound Dehiscence after Wisdom Tooth Removal in Mandibular Mesioangular Class IB Impactions: Triangular Transposition Flap versus Envelope Flap. J. Dent. Res. Dent. Clin. Dent. Prospect. 2015;9:175–180. doi: 10.15171/joddd.2015.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nogami S., Yamauchi K., Shiiba S., Kataoka Y., Hirayama B., Takahashi T. Evaluation of the Treatment Modalities for Neurosensory Disturbances of the Inferior Alveolar Nerve Following Retromolar Bone Harvesting for Bone Augmentation. Pain Med. 2015;16:501–512. doi: 10.1111/pme.12618. [DOI] [PubMed] [Google Scholar]
- 48.Grossi G.B., Maiorana C., Garramone R.A., Borgonovo A., Creminelli L., Santoro F. Assessing Postoperative Discomfort After Third Molar Surgery: A Prospective Study. J. Oral Maxillofac. Surg. 2007;65:901–917. doi: 10.1016/j.joms.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 49.Sarikov R., Juodzbalys G. Inferior Alveolar Nerve Injury after Mandibular Third Molar Extraction: A Literature Review. J. Oral Maxillofac. Res. 2014;5:e1. doi: 10.5037/jomr.2014.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo Giudice R., Puleio F., Rizzo D., Alibrandi A., Lo Giudice G., Centofanti A., Fiorillo L., Di Mauro D., Nicita F. Comparative Investigation of Cutting Devices on Bone Blocks: An SEM Morphological Analysis. Appl. Sci. 2019;9:351. doi: 10.3390/app9020351. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy restrictions.