Abstract
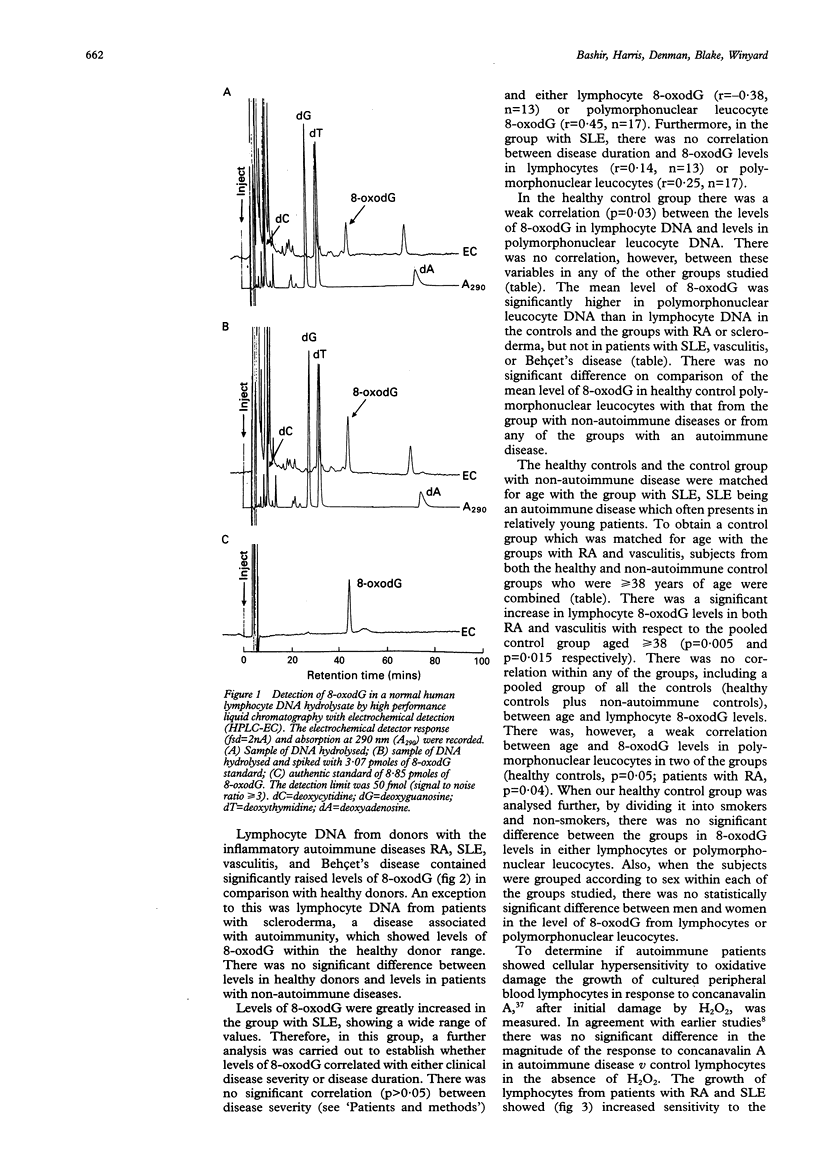
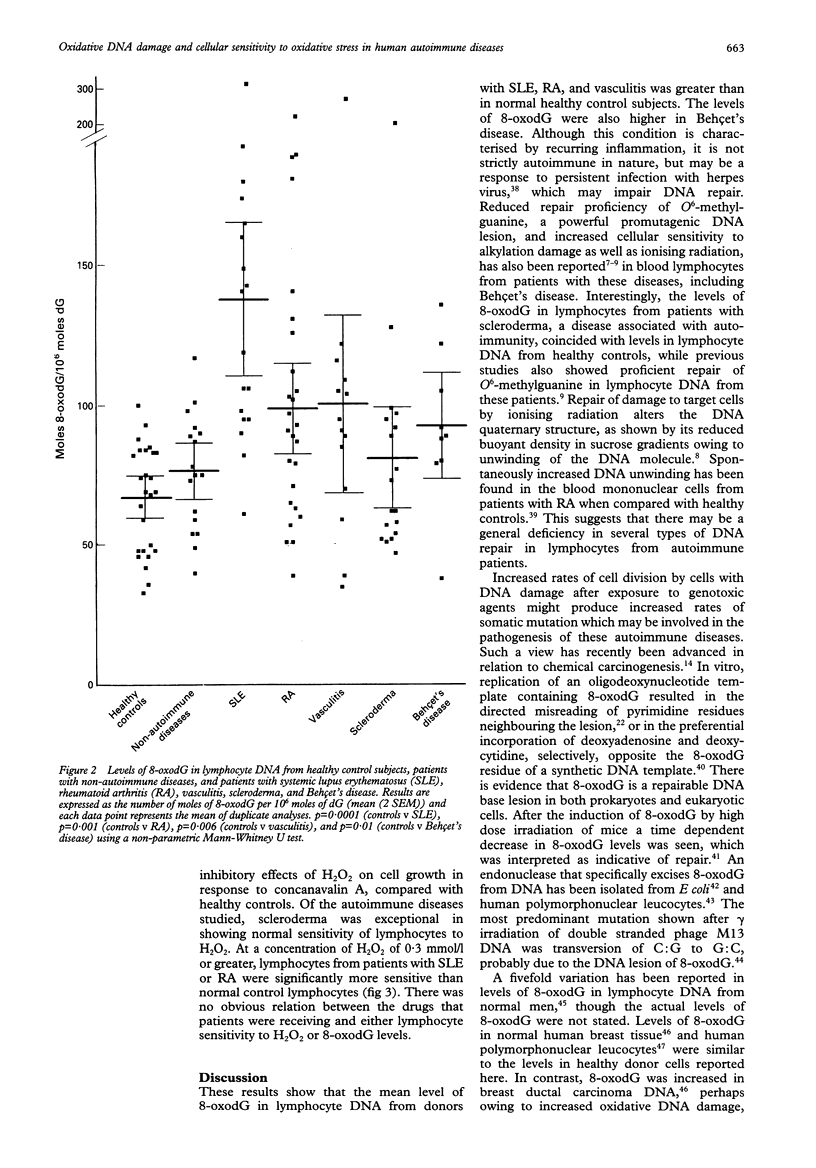
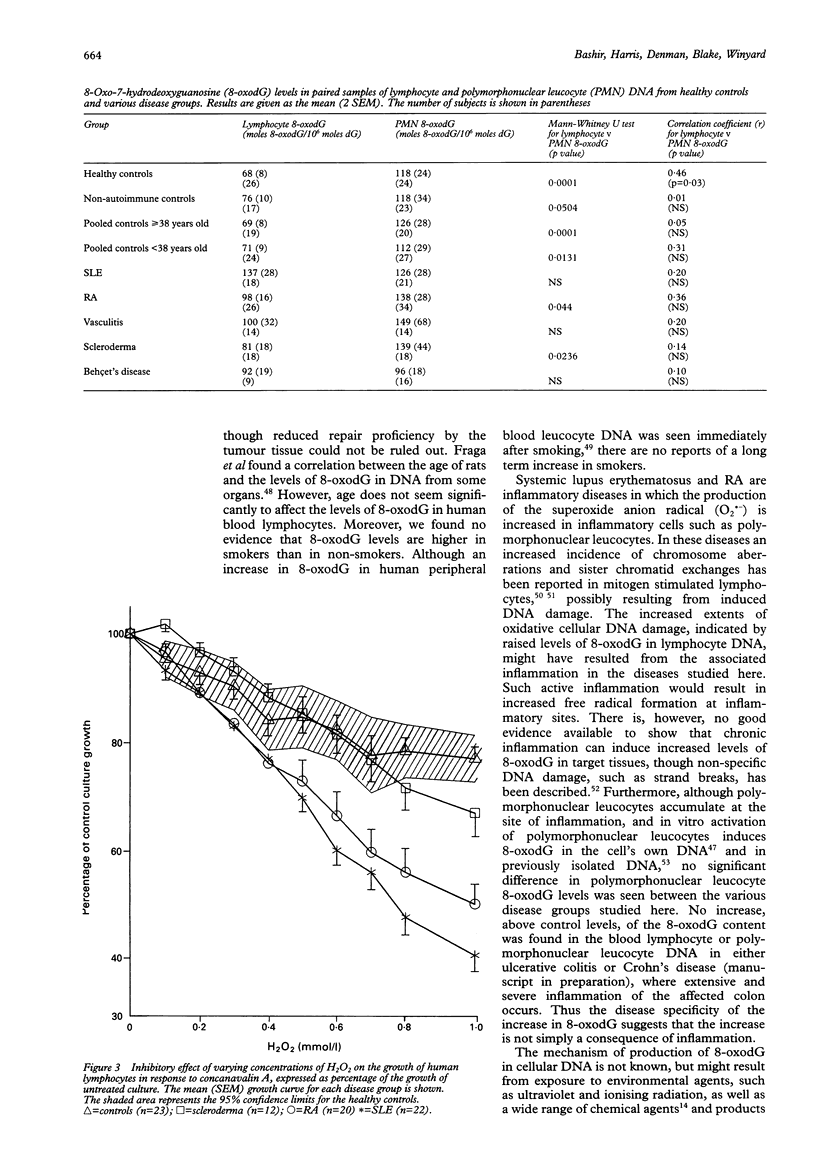
OBJECTIVES--To estimate the extent of genomic DNA damage and killing of lymphocytes by reactive oxygen intermediates in autoimmune diseases. METHODS--8-Oxo-7-hydrodeoxyguanosine (8-oxodG), a promutagenic DNA lesion induced by reactive oxygen intermediates, was measured by high performance liquid chromatography, coupled with electrochemical detection, in hydrolysates of DNA which had been extracted from lymphocyte and polymorphonuclear leucocyte fractions of human blood. In addition, human primary blood lymphocytes stimulated by concanavalin A were assayed for cytotoxicity induced by hydrogen peroxide on day 0, by assessing cell proliferation during seven days of culture. RESULTS--Constitutive 8-oxodG was detectable (mean (2 SEM) moles 8-oxodG/10(6) moles deoxyguanosine) in DNA isolated from normal human blood lymphocytes (68 (8), n = 26) and polymorphonuclear leucocytes (118 (24), n = 24). Lymphocyte DNA from donors with the following inflammatory autoimmune diseases contained significantly higher levels of 8-oxodG than that from healthy donors: rheumatoid arthritis (98 (16)), systemic lupus erythematosus (137 (28)), vasculitis (100 (32)), and Behçet's disease (92 (19)). Lymphocyte 8-oxodG levels in non-autoimmune controls and patients with scleroderma were not significantly different from those of healthy controls. The levels of 8-oxodG were significantly higher in the DNA from normal polymorphonuclear leucocytes than in paired DNA samples from normal lymphocytes, but there were no differences between levels of 8-oxodG in polymorphonuclear leucocytes from normal subjects and the patients studied. Levels of 8-oxodG did not correlate with disease duration, disease severity, or age. Lymphocytes from patients with systemic lupus erythematosus and rheumatoid arthritis, but not those with scleroderma, also showed cellular hypersensitivity to the toxic effects of hydrogen peroxide. CONCLUSION--There was increased genomic DNA damage, and increased susceptibility to cytotoxic killing by hydrogen peroxide, in lymphocytes from patients with certain autoimmune diseases. These results might be explained by defective repair of DNA damage or by increased production of reactive oxygen intermediates in inflammation. Although more direct studies are needed, the evidence available favours the former explanation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gold L. S. Chemical carcinogenesis: too many rodent carcinogens. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7772–7776. doi: 10.1073/pnas.87.19.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Gajewski E., Dizdaroglu M. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem J. 1991 Feb 1;273(Pt 3):601–604. doi: 10.1042/bj2730601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Gajewski E., Dizdaroglu M. Damage to the bases in DNA induced by hydrogen peroxide and ferric ion chelates. J Biol Chem. 1989 Dec 5;264(34):20509–20512. [PubMed] [Google Scholar]
- Bhusate L. L., Herbert K. E., Scott D. L., Perrett D. Increased DNA strand breaks in mononuclear cells from patients with rheumatoid arthritis. Ann Rheum Dis. 1992 Jan;51(1):8–12. doi: 10.1136/ard.51.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Seto S., Wasson D. B., Carrera C. J. DNA strand breaks, NAD metabolism, and programmed cell death. Exp Cell Res. 1986 Jun;164(2):273–281. doi: 10.1016/0014-4827(86)90028-5. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Seto S., Wasson D. B. Lymphocyte dysfunction after DNA damage by toxic oxygen species. A model of immunodeficiency. J Exp Med. 1986 Mar 1;163(3):746–751. doi: 10.1084/jem.163.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y. C., Heppner G. H., Paul L. A., Fulton A. M. Macrophage-mediated induction of DNA strand breaks in target tumor cells. Cancer Res. 1989 Dec 1;49(23):6652–6657. [PubMed] [Google Scholar]
- Chung M. H., Kasai H., Jones D. S., Inoue H., Ishikawa H., Ohtsuka E., Nishimura S. An endonuclease activity of Escherichia coli that specifically removes 8-hydroxyguanine residues from DNA. Mutat Res. 1991 Jan;254(1):1–12. doi: 10.1016/0921-8777(91)90035-n. [DOI] [PubMed] [Google Scholar]
- Chung M. H., Kim H. S., Ohtsuka E., Kasai H., Yamamoto F., Nishimura S. An endonuclease activity in human polymorphonuclear neutrophils that removes 8-hydroxyguanine residues from DNA+. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1472–1478. doi: 10.1016/0006-291x(91)91059-l. [DOI] [PubMed] [Google Scholar]
- Diamond B., Scharff M. D. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M., Nackerdien Z., Chao B. C., Gajewski E., Rao G. Chemical nature of in vivo DNA base damage in hydrogen peroxide-treated mammalian cells. Arch Biochem Biophys. 1991 Mar;285(2):388–390. doi: 10.1016/0003-9861(91)90378-v. [DOI] [PubMed] [Google Scholar]
- Eglin R. P., Lehner T., Subak-Sharpe J. H. Detection of RNA complementary to herpes-simplex virus in mononuclear cells from patients with Behçet's syndrome and recurrent oral ulcers. Lancet. 1982 Dec 18;2(8312):1356–1361. doi: 10.1016/s0140-6736(82)91268-5. [DOI] [PubMed] [Google Scholar]
- Emerit I., Michelson A. M. Chromosome instability in human and murine autoimmune disease: anticlastogenic effect of superoxide dismutase. Acta Physiol Scand Suppl. 1980;492:59–65. [PubMed] [Google Scholar]
- Fiala E. S., Conaway C. C., Mathis J. E. Oxidative DNA and RNA damage in the livers of Sprague-Dawley rats treated with the hepatocarcinogen 2-nitropropane. Cancer Res. 1989 Oct 15;49(20):5518–5522. [PubMed] [Google Scholar]
- Floyd R. A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990 Sep;11(9):1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French D. L., Laskov R., Scharff M. D. The role of somatic hypermutation in the generation of antibody diversity. Science. 1989 Jun 9;244(4909):1152–1157. doi: 10.1126/science.2658060. [DOI] [PubMed] [Google Scholar]
- Harris G., Asbery L., Lawley P. D., Denman A. M., Hylton W. Defective repair of 0(6)-methylguanine in autoimmune diseases. Lancet. 1982 Oct 30;2(8305):952–956. doi: 10.1016/s0140-6736(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Harris G., Cramp W. A., Edwards J. C., George A. M., Sabovljev S. A., Hart L., Hughes G. R., Denman A. M., Yatvin M. B. Radiosensitivity of peripheral blood lymphocytes in autoimmune disease. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Jun;47(6):689–699. doi: 10.1080/09553008514550931. [DOI] [PubMed] [Google Scholar]
- Harris G., Lawley P. D., Olsen I. Mode of action of methylating carcinogens: comparative studies of murine and human cells. Carcinogenesis. 1981;2(5):403–411. doi: 10.1093/carcin/2.5.403. [DOI] [PubMed] [Google Scholar]
- Hoebee B., Brouwer J., van de Putte P., Loman H., Retèl J. 60Co gamma-rays induce predominantly C/G to G/C transversions in double-stranded M13 DNA. Nucleic Acids Res. 1988 Aug 25;16(16):8147–8156. doi: 10.1093/nar/16.16.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P., de Vere Tyndall A., Ansell B. M., Platts-Mills T., Gumpel J. M., Mertin J., Smith D. S., Denman A. M. Intensive immunosuppression versus prednisolone in the treatment of connective tissue diseases. Ann Rheum Dis. 1982 Dec;41(6):557–562. doi: 10.1136/ard.41.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. H., Gajewski E., Schraufstatter I. U., Hyslop P. A., Fuciarelli A. F., Cochrane C. G., Dizdaroglu M. Damage to the bases in DNA induced by stimulated human neutrophils. J Clin Invest. 1989 Nov;84(5):1644–1649. doi: 10.1172/JCI114342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. Isolation and characterization of Escherichia coli K-12 mutants unable to induce the adaptive response to simple alkylating agents. J Bacteriol. 1979 Sep;139(3):783–791. doi: 10.1128/jb.139.3.783-791.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of deoxyribonucleic acids; evidence on the nature of bonds between deoxyribonucleic acid and protein. Biochem J. 1957 Jul;66(3):495–504. doi: 10.1042/bj0660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Crain P. F., Kuchino Y., Nishimura S., Ootsuyama A., Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986 Nov;7(11):1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- Kasai H., Nishimura S. DNA damage induced by asbestos in the presence of hydrogen peroxide. Gan. 1984 Oct;75(10):841–844. [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosawa H., Suko M., Okudaira H., Murata K., Miyamoto T., Chung M. H., Kasai H., Nishimura S. Cigarette smoking induces formation of 8-hydroxydeoxyguanosine, one of the oxidative DNA damages in human peripheral leukocytes. Free Radic Res Commun. 1990;11(1-3):23–27. doi: 10.3109/10715769009109664. [DOI] [PubMed] [Google Scholar]
- Kohda K., Tada M., Kasai H., Nishimura S., Kawazoe Y. Formation of 8-hydroxyguanine residues in cellular DNA exposed to the carcinogen 4-nitroquinoline 1-oxide. Biochem Biophys Res Commun. 1986 Sep 14;139(2):626–632. doi: 10.1016/s0006-291x(86)80036-5. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Topper R., Denman A. M., Hylton W., Hill I. D., Harris G. Increased sensitivity of lymphocytes from patients with systemic autoimmune diseases to DNA alkylation by the methylating carcinogen N-methyl-N-nitrosourea. Ann Rheum Dis. 1988 Jun;47(6):445–451. doi: 10.1136/ard.47.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malins D. C., Haimanot R. Major alterations in the nucleotide structure of DNA in cancer of the female breast. Cancer Res. 1991 Oct 1;51(19):5430–5432. [PubMed] [Google Scholar]
- Mason R. M., Barnes C. G. Behçet's syndrome with arthritis. Ann Rheum Dis. 1969 Mar;28(2):95–103. doi: 10.1136/ard.28.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980 May;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I., Hyslop P. A., Jackson J. H., Cochrane C. G. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988 Sep;82(3):1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Umemura T., Sai K., Takagi A., Hasegawa R., Kurokawa Y. Formation of 8-hydroxydeoxyguanosine (8-OH-dG) in rat kidney DNA after intraperitoneal administration of ferric nitrilotriacetate (Fe-NTA). Carcinogenesis. 1990 Feb;11(2):345–347. doi: 10.1093/carcin/11.2.345. [DOI] [PubMed] [Google Scholar]
- Weitberg A. B., Weitzman S. A., Destrempes M., Latt S. A., Stossel T. P. Stimulated human phagocytes produce cytogenetic changes in cultured mammalian cells. N Engl J Med. 1983 Jan 6;308(1):26–30. doi: 10.1056/NEJM198301063080107. [DOI] [PubMed] [Google Scholar]
- Winyard P. G., Faux S. P., Smith A. J., Davies J. E., Chipman J. K. Bleomycin-induced unscheduled DNA synthesis in non-permeabilized human and rat hepatocytes is not paralleled by 8-oxo-7,8-dihydrodeoxyguanosine formation. Biochem Pharmacol. 1992 Oct 6;44(7):1255–1260. doi: 10.1016/0006-2952(92)90523-l. [DOI] [PubMed] [Google Scholar]


