Abstract
Endoscopic ultrasound-guided biliary drainage (EUS-BD) has become comparable to endoscopic retrograde cholangiopancreatography and is now considered a first-line intervention for certain biliary obstructions. Although analysis of experience-related factors may help achieve better outcomes and contribute to its wider adoption, no concrete evidence exists regarding the required operator or institutional experience levels. This study aimed to analyze experience-related factors at beginner multicenters. Patients who underwent EUS-BD using self-expandable metal stents and/or dedicated plastic stents during the study period (up to the first 25 cases since introducing the technique) were retrospectively enrolled from seven beginner institutions and operators. Overall, 90 successful (technical success without early adverse events) and 22 failed (technical failure and/or early adverse events) cases were compared. EUS-BD-related procedures conducted at the time of applicable EUS-BD by each institution/operator were evaluated. The number of institution-conducted EUS-BD procedures (≥7) and operator-conducted EUS screenings (≥436), EUS-guided fine-needle aspirations (FNA) (≥93), and EUS-guided drainages (≥13) significantly influenced improved EUS-BD outcomes (p = 0.022, odds ratio [OR], 3.0; p = 0.022, OR, 3.0; p = 0.022, OR, 3.0; and p = 0.028, OR, 2.9, respectively). Our threshold values, which significantly divided successful and failed cases, were assessed using receiver operating characteristic curve analysis and may provide useful approximate indications for successful EUS-BD.
Keywords: adverse events, choledochoduodenostomy, endoscopic ultrasound-guided biliary drainage, experience, hepaticogastrostomy
1. Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) with transpapillary stent placement is a first-line treatment for both benign and malignant biliary obstructions [1,2]. Recently, the use of endoscopic ultrasound-guided biliary drainage (EUS-BD) has increased as an alternative treatment for patients undergoing difficult ERCP owing to malignant luminal obstruction, surgically altered anatomy, and cannulation failure [3,4]. Moreover, EUS-BD may be superior to percutaneous drainage thanks to its high success rate, resulting in high quality of life and a low risk of adverse events and reinterventions [5,6].
EUS-BD with transluminal stenting, including EUS-guided hepaticogastrostomy (EUS-HGS), choledochoduodenostomy (EUS-CDS), and antegrade biliary stenting (EUS-ABS), is recommended, especially in patients with malignant biliary obstructions; its effectiveness may be comparable with that of ERCP as the first-line management of certain biliary obstructions [7,8,9,10,11]. Since its first description in 2001 [12], the procedure has improved and shown high technical and clinical success rates of approximately 95% and 92–94%, respectively [3,10]. Considering the prevention of adverse events, metal stents [3,4] and newly developed dedicated plastic stents for EUS-BD are being used more widely than conventional plastic stents [13]. In addition, organized technical tips have been designed and widely recognized [4,14].
A recent review reported a relatively lower adverse event rate of 9.3–16% for EUS-BD than that reported in previous studies [3,6,10,15]. As the technique becomes standardized and safer, EUS-BD should be more widely and routinely employed, and its introduction may become possible in beginner centers. As EUS-BD is generally required in only 0.6% of patients with a native papilla undergoing ERCP [16], a more efficient introduction of EUS-BD is required in beginner centers. A thorough analysis of the factors involved in achieving better EUS-BD outcomes and technical success without adverse events may contribute to wider employment of the procedure [17]. Particularly, factors related to experience with the EUS-BD procedure may affect its success. Previous studies reported that technique-related factors may influence the rate of adverse events; however, there is no concrete evidence regarding experience-related factors required by beginner institutions and operators to achieve better EUS-BD outcomes [3,18,19]. Therefore, this multicenter study aimed to clarify potential experience-related factors for achieving better EUS-BD outcomes in beginner multicenters.
2. Materials and Methods
2.1. Eligibility Criteria
We searched the electronic medical records of seven participating institutions to enroll patients undergoing EUS-BD with transmural stenting as an alternative treatment for ERCP due to malignant luminal obstruction, surgically altered anatomy, ERCP failure, and insufficient first drainage by ERCP between January 2014 and June 2021. The subtypes of the EUS-BD procedures included in this multicenter study were EUS-HGS, HGS combined with ABS, CDS using a self-expandable metal stent (SEMS), and EUS-BD with dedicated plastic stents. EUS-guided rendezvous procedures were excluded. The participating institutions were relatively low-volume centers with ≤500 ERCP cases per year, no previous experience performing EUS-BD, and only one beginner EUS-BD operator in each. The first 25 patients from each institution admitted since the introduction of EUS-BD were enrolled. All operators had previously performed >500 ERCP procedures at the time of their first EUS-BD; additionally, they had studied the EUS-BD procedures by observation in high-volume centers before their first EUS-BD. Most assistants had little to no experience with EUS-BD or assisting with it. All institutions had surgeons or radiologists standing by to handle a potential emergency.
This study was designed and conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was registered by the University Hospital Medical Network Clinical Trials Registry (UMIN000047698) as a retrospective study after receiving approval from our Institutional Review Board (IRB protocol number, 2021001K). The need for informed consent was waived owing to the retrospective nature of the study.
2.2. Definition and Study Outcome
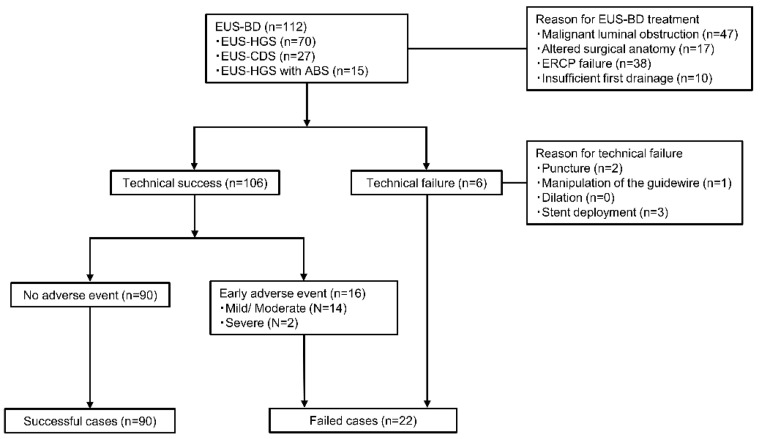
This study compared two groups of patients undergoing EUS-BD: successful (technical success without early adverse events) and failed (technical failure and/or early adverse events) cases. Technical success was defined as successful stent placement between the biliary tree and the gastrointestinal lumen. Early adverse events were defined as procedure-related adverse events occurring within 2 weeks of stent placement that required endoscopic, surgical, or radiological intervention, or additional conservative treatment. The adverse events included bleeding, cholangitis, bile peritonitis, and pneumoperitoneum. The severity of adverse events was evaluated according to the American Society of Gastrointestinal Endoscopy classification [20]. Figure 1 depicts the decision-making process for the distinction between successful and failed cases.
Figure 1.
Flow diagram of the study design. ABS, antegrade biliary stenting; BD, biliary drainage; CDS, choledochoduodenostomy; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; HGS, hepaticogastrostomy.
Overall, 112 patients who underwent EUS-BD were enrolled, and 90 patients who underwent technically successful EUS-BD and completed treatment without early adverse events were classified as successful. The technical success rate was 96.4%, and the early adverse event rate was 14.3%. A total of 22 patients, including 6 patients who experienced technical failure and 16 patients who experienced technical success with early adverse events, were classified as failed cases.
Experience with EUS-BD was defined as the number of procedures conducted by each institution and operator by the time of the applicable EUS-BD procedure. The operators’ EUS-related procedural experiences were evaluated by analyzing the number of pancreatobiliary screening EUS, EUS-guided fine-needle aspiration (FNA), and other EUS-guided drainage procedures (cyst, abscess, and gallbladder) that had been conducted by the time of applicable EUS-BD procedure introduction.
The primary aim of the study was to analyze the experience-related factors of EUS-BD/EUS-BD-related procedures for beginner institutions and operators that may affect the occurrence of successful cases. The secondary aim was to clarify the significant procedure-related factors involved in the occurrence of successful cases.
2.3. Statistical Analyses
Patient characteristics, experience-related factors, and procedure-related factors were compared between successful and failed cases. As appropriate, categorical parameters were compared using the chi-square test or Fisher’s exact test. Continuous variables are expressed as mean ± standard deviation, depending on the normality of the distribution. The thresholds for EUS-BD and EUS-BD-related procedures that significantly divided successful and failed cases were determined using the receiver operating characteristic curve (ROC) analysis. The threshold was defined as the cross point of the true and false positive rates of the ROC. The odds ratios (OR) between the thresholds were further evaluated with 95% confidence intervals (CI). All statistical analyses were conducted using SPSS version 28.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at p < 0.05.
3. Results
Table 1 summarizes the patients’ characteristics for the successful and failed cases. Other than sex, there are no significant differences between the groups, as shown in Table 1.
Table 1.
Patient characteristics in successful and failed cases.
| Successful Cases (n = 90) |
Failed Cases (n = 22) |
p-Value | |
|---|---|---|---|
| Age, mean (±SD), y | 76.0 ± 12.8 | 75.5 ± 13.9 | 0.822 |
| Male sex, n (%) | 42 (46.7) | 17 (77.2) | 0.010 |
| Etiology of biliary obstruction, n (%) | |||
| Malignant | 84 (93.3) | 18 (81.8) | 0.090 |
| Benign | 6 (6.7) | 4 (18.2) | |
| Presence of jaundice, n (%) | 72 (80.0) | 15 (68.2) | 0.233 |
| Serum bilirubin (mean ± SD), mg/dL | 7.5 ± 6.7 | 6.5 ± 7.3 | 0.520 |
| Presence of cholangitis, n (%) | 35 (38.9) | 9 (40.9) | 0.862 |
| C-reactive protein (mean ± SD), mg/dL | 5.4 ± 5.4 | 3.5 ± 4.7 | 0.249 |
| Presence of ascites, n (%) | 15 (16.7) | 3 (13.6) | 0.729 |
| Reasons for EUS-BD, n (%) | 0.301 | ||
| Malignant luminal obstruction | 38 (42.2) | 9 (40.9) | |
| Altered surgical anatomy | 11 (12.2) | 6 (27.3) | |
| Failed ERCP | 33 (36.7) | 5 (22.7) | |
| Insufficient previous drainage | 8 (8.9) | 2 (9.1) |
EUS-BD, endoscopic ultrasound-guided biliary drainage; ERCP, endoscopic retrograde cholangiopancreatography; SD; standard deviation.
Table 2 summarizes the number of EUS-BD and EUS-BD-related procedures conducted by each institution and operator at the time of the applicable EUS-BD procedure.
Table 2.
Number of EUS procedures conducted and institution and operator experience-related factors.
| Successful Cases (n = 90) |
Failed Cases (n = 22) |
p-Value | Odds Ratio (95% CI) |
|
|---|---|---|---|---|
| Conducted EUS-BD (mean ± SD) | NA | NA | ||
| Institution | 10.1 ± 6.7 | 6.1 ± 4.7 | ||
| Operator | 9.0 ± 7.1 | 5.6 ± 5.0 | ||
| Conducted EUS-BD, n (%) | ||||
| Institution ≥ 7 cases | 57 (63.3) | 8 (36.4) | 0.022 | 3.0 (1.1–8.0) |
| Operator ≥ 6 cases | 54 (60.0) | 10 (45.5) | 0.217 | 1.8 (0.7–4.6) |
| Operator-conducted EUS-BD related procedures (mean ± SD) | NA | NA | ||
| EUS screening | 712.1 ± 422.1 | 586.4 ± 487.4 | ||
| EUS-FNA | 133.7 ± 96.9 | 82.6 ± 54.9 | ||
| EUS-guided drainage | 17.1 ± 10.3 | 10.9 ± 8.5 | ||
| Operator-conducted procedures, n (%) | ||||
| Screening EUS ≥ 436 cases | 57 (63.3) | 8 (36.4) | 0.022 | 3.0 (1.1–8.0) |
| EUS-FNA ≥ 93 cases | 57 (63.3) | 8 (36.4) | 0.022 | 3.0 (1.1–8.0) |
| EUS-guided drainage ≥ 13 cases | 56 (60.0) | 8 (36.4) | 0.028 | 2.9 (1.1–7.6) |
CI, confidence interval; EUS, endoscopic ultrasound; EUS-BD, endoscopic ultrasound-guided biliary drainage; EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration; NA, not assessed; SD, standard deviation.
The threshold of EUS-BD conducted by an institution that significantly divided the successful and failed cases was seven cases. Similarly, the threshold of EUS-BD conducted by an operator was six cases, and that of EUS screening, EUS-FNA, and EUS-guided drainage conducted by the operator were 436, 93, and 13 cases, respectively.
The number of EUS-BD cases conducted by the institution (≥7) is a significant factor in the occurrence of successful cases (63.6% vs. 36.7%; p = 0.022; OR, 0.33 [95% CI, 0.13–0.87]). Conversely, the number of EUS-BD cases conducted by the operator (≥6) is not a significant factor for the occurrence of successful cases (54.5% vs. 40.0%; p = 0.217; OR, 0.56 [0.22–1.42]). In addition, the number of EUS screenings cases (≥436), EUS-FNA cases (≥93), and EUS-guided drainage cases (≥13) conducted by the operator are all significant factors for successful cases (63.6% vs. 36.7%, p = 0.022, OR, 3.0 [1.1–8.0]; 63.6% vs. 36.7%, p = 0.022, OR, 3.0 [1.1–8.0]; and 63.6% vs. 37.8%, p = 0.028, OR, 2.9 [1.1–7.6], respectively). Only 13 EUS-BD procedures were conducted by assistants who had already performed or assisted with ≥6 EUS-BD cases; however, the experience is not significant for the occurrence of successful cases (13.3% vs. 4.5%, p = 0.249).
Table 3 summarizes the procedure-related factors. Naturally, the location of the biliary obstruction/puncture site for EUS-CDS was the extrahepatic bile duct/common bile duct. Among the subtypes of EUS-BD, location of biliary obstruction, assistant (non-doctor or doctor), puncture site, puncture bile duct diameter, type of guidewire, type of fistula dilation, stent diameter/length, and location of biliary obstruction (extrahepatic bile duct obstruction) were the only significant factors involved in successful cases (73.3% vs. 40.9%; p = 0.004; OR, 4.0 [1.5–10.5]).
Table 3.
Procedure-related factors.
| Successful Cases (n = 90) |
Failed Cases (n = 22) |
p-Value | |
|---|---|---|---|
| EUS-BD, n (%) | 0.539 | ||
| EUS-HGS | 54 (60.0) | 16 (72.8) | |
| EUS-CDS | 23 (25.6) | 4 (18.2) | |
| EUS-HGS with ABS | 13 (14.4) | 2 (9.0) | |
| Location of biliary obstruction, n (%) | 0.004 * | ||
| Extrahepatic bile duct | 66 (73.3) | 9 (40.9) | |
| Intrahepatic bile duct | 24 (26.7) | 13 (59.1) | |
| Assistant, n (%) | 0.109 | ||
| Non-doctor | 15 (16.7) | 7 (31.8) | |
| Doctor | 75 (83.3) | 15 (68.2) | |
| Puncture site, n (%) | 0.561 | ||
| B2 | 17 (18.9) | 3 (13.6) | |
| B3 | 50 (55.6) | 15 (68.2) | |
| Common bile duct | 23 (25.5) | 4 (18.2) | |
| Puncture bile duct diameter, mean (± SD), mm | 8.0 ± 4.5 | 7.1 ± 3.4 | 0.239 |
| Guidewire, n (%) | 0.198 | ||
| 0.025 inch | 76 (84.4) | 16 (72.7) | |
| 0.035 inch | 14 (15.6) | 6 (27.3) | |
| Fistula dilation, n (%) | 0.555 | ||
| Without dilation | 9 (10.0) | 4 (18.2) | |
| Blunt dilation (bougie or balloon) | 60 (66.7) | 13 (59.1) | |
| Electrical dilation | 21 (23.3) | 5 (22.7) | |
| Type of stent, n (%) | 0.756 | ||
| Dedicated plastic stent | 20 (22.2) | 3/16 (18.8) | |
| SEMS | 70 (77.8) | 13/16 (81.2) | |
| Stent diameter, n (%) | 0.803 | ||
| 7 Fr | 20 (22.2) | 3/16 (18.8) | |
| 6/8 mm | 33 (36.7) | 5/16 (31.2) | |
| 10 mm | 37 (41.1) | 8/16 (50.0) | |
| Stent length, n (%) | 0.158 | ||
| 60/80 mm | 30 (33.3) | 2/16 (12.5) | |
| 100/120 mm | 40 (44.4) | 11/16 (68.8) | |
| 140 mm | 20 (22.3) | 3/16 (18.7) |
* p < 0.05, compared to the success and failure cases. EUS-BD, endoscopic ultrasound-guided biliary drainage; EUS-CDS, endoscopic ultrasound-guided choledochoduodenostomy; EUS-HGS, endoscopic ultrasound-guided hepaticogastrostomy; ABS, antegrade biliary stenting; CDS, choledochoduodenostomy; SD, standard deviation; SEMS, self-expandable metal stent.
Table 4 summarizes the details of technical failures and early adverse events of failed cases.
Table 4.
Details of technical failure and early adverse events.
| Technical failure step | 6 (cases) |
| Puncture | 2 |
| Manipulation of the guidewire | 1 |
| Dilation | 0 |
| Stent deployment | 3 |
| Early adverse events | 16 (cases) |
| Peritonitis | 9 |
| Bleeding | 2 |
| Pneumoperitoneum | 2 |
| Cholangitis | 2 |
| Pancreatitis | 1 |
| Severity of adverse events | |
| Mild | 10 |
| Moderate | 4 |
| Severe | 2 |
4. Discussion
The results of this study performed at EUS-BD beginner institutions with relatively similar conditions suggest that various experience-related factors (number of EUS-BD conducted by the institution and EUS screening, EUS-FNA, and EUS-guided drainage conducted by the operator) may affect the occurrence of successful EUS-BD outcomes, whereas the operator’s experience with EUS-BD itself may not.
Some studies analyzed the learning curve of EUS-BD of a single operator in a high-volume center [21,22]. They recommend that 7–32 cases are required to achieve stable, successful EUS-BD or a reduction in procedural time [21,22,23]. Another study of 101 patients reported that the first half of enrolled patients had a lower mortality rate than the second half [24]. In another study, the success rate in the first 3 years was lower than that in the last 2 years [25]. The analysis of the learning curve or the period of gaining EUS-BD experience was reported for some institutions; however, most previous studies were conducted in high-volume centers and performed by a single expert operator. In addition, a learning curve was defined to achieve quicker EUS-BD [21].
Conversely, other studies reported that the accumulation of operator experience over time did not yield significant reductions in EUS-BD-related adverse events [17,26]. Moreover, experience with EUS-guided interventions may be associated with fewer EUS-BD-related adverse events due to the technical similarities between EUS-guided pseudocyst drainage and EUS-BD [27]. The findings of these reports support our results.
It has been recommended that EUS-BD should be attempted only by experts in high-volume centers with appropriate training [3] owing to its lower technical success rate [17], higher adverse event rate compared with ERCP [3], and seriousness of technical failures/adverse events [24,28]. In contrast, a review showed a relatively lower adverse event rate of 9.3–16% for EUS-BD [10,15] compared with that of 23% reported previously [3]. In addition, increased success rates and decreased adverse events were observed in studies published after 2013, probably because of the newly designed safer stents [29,30], organized technical tips/algorithms for EUS-BD [4,14], and greater experience of endoscopists [10]. Considering these facts, the widespread introduction of EUS-BD may be feasible in appropriate conditions. Certainly, EUS-BD should be performed with surgical and radiological backup [3].
Worldwide societies of gastrointestinal endoscopy recommend that several cases of EUS screening and EUS-FNA should be performed for learning EUS procedures [19,31]. EUS-BD trainees should be trained in convex EUS handling and be allowed to simulate the procedures using simulators and models for EUS-BD before EUS-BD implementation [32]. They should begin the EUS-BD procedures under expert supervision and, if possible, complete training programs in experienced institutions [18]. One expert center recommends that at least 20 EUS-BD procedures should be performed by trainees under expert supervision [32]. However, it is difficult for all institutions to perform EUS-BD under similar conditions. While step-by-step training for EUS-BD and actual procedures under supervision are essential, a more efficient EUS-BD introduction model is required.
Our results suggest that the institution-conducted experience with EUS-BD and operator-conducted EUS-BD-related procedures have a greater influence on better outcomes than the operator-conducted EUS-BD experience, indicating the importance of the institution-conducted experience, likely because EUS-BD experience may reflect the experience of the assistant doctors and engineers in addition to that of the operators. Further, operator experience in EUS-BD-related procedures may compensate for the operator’s lack of EUS-BD experience itself. The threshold values calculated in this study may be used as a rough indicator for the achievement of successful EUS-BD outcomes at a reasonable number of procedures.
Regarding procedure-related factors, one study reported fistula dilation with a needle knife as a significant factor responsible for adverse events [26]. However, no specific procedure-related factors were confirmed in another systematic review [3]. This study showed that the predictor of the bile duct obstruction location (extrahepatic bile duct obstruction compared to intrahepatic obstruction) was only relevant in successful cases. A study of the initial experience with EUS-BD reported that manipulating the guidewire into the duct was the most difficult aspect of the procedure [17]. Manipulating the guidewire across the intrahepatic bile duct may be more difficult than crossing the extrahepatic duct, which may have affected the results of this study. A beginner operator may need to be careful in EUS-BD procedures for intrahepatic bile duct obstruction cases.
Comprehensively, from our results, a certain level of institutional experience with EUS-BD and operator experience with EUS-BD related procedures may be required for the occurrence of a successful case for the very first EUS-BD procedure performed by a beginner. In addition, EUS-BD beginners may need to avoid intrahepatic obstruction cases.
This study has the following strengths. To the best of our knowledge, this is the first multicenter study to report on the various experience-related factors that lead to better EUS-BD outcomes with the corresponding threshold values. In addition, participating beginner institutions had relatively similar conditions. However, this study also has some limitations. The retrospective design, the variation in the number of cases, and the dataset validation between institutions may have introduced selection bias. In addition, the indications for the procedure and technical methods for EUS-BD were not completely unified among the institutions. Ideally, experience- and procedure-related factors should be evaluated for each EUS-BD procedure because of the slight differences between them. In the future, further studies with a greater number of institutions under similar procedural conditions and of enrolled cases should be carried out.
5. Conclusions
The number of institution-conducted EUS-BD procedures and operator-conducted EUS screening, FNA, and EUS-guided drainage led to significantly better EUS-BD outcomes at beginner centers. Our threshold values may provide reasonable and approximate indications for successful EUS-BD.
Author Contributions
Conceptualization, R.S., K.M. (Kazuhiro Mizukami), K.O. and K.M. (Kazunari Murakami); methodology, R.S. and K.O.; software, R.S.; validation, R.S., K.O. and K.M. (Kazuhiro Mizukami); formal analysis, R.S.; investigation, R.S., K.O., C.N., T.S., H.N., Y.K., M.W., Y.O., S.F., H.T. and N.K.; data curation, R.S. and K.S.; writing—original draft preparation, R.S., K.O., C.N., T.S., H.N., Y.K., M.W., Y.O., S.F., H.T. and N.K.; writing—review and editing, K.M. (Kazuhiro Mizukami); visualization, R.S.; supervision, K.M. (Kazunari Murakami); project administration, R.S., K.O., K.M. (Kazuhiro Mizukami) and K.M. (Kazunari Murakami). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was designed and conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was registered by the University Hospital Medical Network Clinical Trials Registry (UMIN000047698) as a retrospective study after receiving approval from our Institutional Review Board (IRB protocol number, 2021001K).
Informed Consent Statement
Patient consent was waived owing to the retrospective nature of this study.
Data Availability Statement
The dataset used during the current study is available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fogel E.L., Sherman S., Devereaux B.M., Lehman G.A. Therapeutic biliary endoscopy. Endoscopy. 2001;33:31–38. doi: 10.1055/s-2001-11186. [DOI] [PubMed] [Google Scholar]
- 2.Freeman M.L., Guda N.M. ERCP cannulation: A review of reported techniques. Gastrointest. Endosc. 2005;61:112–125. doi: 10.1016/S0016-5107(04)02463-0. [DOI] [PubMed] [Google Scholar]
- 3.Wang K., Zhu J., Xing L., Wang Y., Jin Z., Li Z. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest. Endosc. 2016;83:1218–1227. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Minaga K., Kitano M. Recent advances in endoscopic ultrasound-guided biliary drainage. Dig. Endosc. 2018;30:38–47. doi: 10.1111/den.12910. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen-Tang T., Binmoeller K.F., Sanchez-Yague A., Shah J.N. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232–236. doi: 10.1055/s-0029-1243858. [DOI] [PubMed] [Google Scholar]
- 6.Sharaiha R.Z., Khan M.A., Kamal F., Tyberg A., Tombazzi C.R., Ali B., Tombazzi C., Kahaleh M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest. Endosc. 2017;85:904–914. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Bang J.Y., Navaneethan U., Hasan M., Hawes R., Varadarajulu S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos) Gastrointest. Endosc. 2018;88:9–17. doi: 10.1016/j.gie.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Paik W.H., Lee T.H., Park D.H., Choi J.H., Kim S.O., Jang S., Kim D.U., Shim J.H., Song T.J., Lee S.S., et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018;113:987–997. doi: 10.1038/s41395-018-0122-8. [DOI] [PubMed] [Google Scholar]
- 9.Kakked G., Salameh H., Cheesman A.R., Kumta N.A., Nagula S., DiMaio C.J. Primary EUS-guided biliary drainage versus ERCP drainage for the management of malignant biliary obstruction: A systematic review and meta-analysis. Endosc. Ultrasound. 2020;9:298–307. doi: 10.4103/eus.eus_10_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han S.Y., Kim S.O., So H., Shin E., Kim D.U., Park D.H. EUS-guided biliary drainage versus ERCP for first-line palliation of malignant distal biliary obstruction: A systematic review and meta-analysis. Sci. Rep. 2019;9:16551. doi: 10.1038/s41598-019-52993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J.K., Woo Y.S., Noh D.H., Yang J.I., Bae S.Y., Yun H.S., Lee J.K., Lee K.T., Lee K.H. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest. Endosc. 2018;88:277–282. doi: 10.1016/j.gie.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Giovannini M., Moutardier V., Pesenti C., Bories E., Lelong B., Delpero J.R. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 13.Mukai S., Itoi T., Sofuni A., Tsuchiya T., Tanaka R., Tonozuka R., Honjo M., Fujita M., Yamamoto K., Nagakawa Y. EUS-guided antegrade intervention for benign biliary diseases in patients with surgically altered anatomy (with videos) Gastrointest. Endosc. 2019;89:399–407. doi: 10.1016/j.gie.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Ogura T., Higuchi K. Endoscopic Ultrasound-Guided Hepaticogastrostomy: Technical Review and Tips to Prevent Adverse Events. Gut Liver. 2021;15:196–205. doi: 10.5009/gnl20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artifon E.L.A., Visconti T.A.C., Brunaldi V.O. Choledochoduodenostomy: Outcomes and limitations. Endosc. Ultrasound. 2019;8:S72–S78. doi: 10.4103/eus.eus_62_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt B.A., Hawes R., Hasan M., Canipe A., Tharian B., Navaneethan U., Varadarajulu S. Biliary drainage: Role of EUS guidance. Gastrointest. Endosc. 2016;83:160–165. doi: 10.1016/j.gie.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Vila J.J., Pérez-Miranda M., Vazquez-Sequeiros E., Abadia M.A., Pérez-Millán A., González-Huix F., Gornals J., Iglesias-Garcia J., De la Serna C., Aparicio J.R., et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest. Endosc. 2012;76:1133–1141. doi: 10.1016/j.gie.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Isayama H., Nakai Y., Itoi T., Yasuda I., Kawakami H., Ryozawa S., Kitano M., Irisawa A., Katanuma A., Hara K., et al. Clinical practice guidelines for safe performance of endoscopic ultrasound/ultrasonography-guided biliary drainage: 2018. J. Hepatobiliary Pancreat. Sci. 2019;26:249–269. doi: 10.1002/jhbp.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polkowski M., Larghi A., Weynand B., Boustière C., Giovannini M., Pujol B., Dumonceau J.M. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 20.Cotton P.B., Eisen G.M., Aabakken L., Baron T.H., Hutter M.M., Jacobson B.C., Mergener K., Nemcek A., Jr., Petersen B.T., Petrini J.L., et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Tyberg A., Mishra A., Cheung M., Kedia P., Gaidhane M., Craig C., Tarnasky P.R., Ardengh J.C., Kahaleh M. Learning curve for EUS-guided biliary drainage: What have we learned? Endosc. Ultrasound. 2020;9:392–396. doi: 10.4103/eus.eus_42_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh D., Park D.H., Song T.J., Lee S.S., Seo D.W., Lee S.K., Kim M.H. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap. Adv. Gastroenterol. 2017;10:42–53. doi: 10.1177/1756283X16671671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyberg A., Kats D., Choi A., Gaidhane M., Nieto J., Kahaleh M. Endoscopic Ultrasound Guided Gastroenterostomy: What Is the Learning Curve? J. Clin. Gastroenterol. 2021;55:691–693. doi: 10.1097/MCG.0000000000001400. [DOI] [PubMed] [Google Scholar]
- 24.Poincloux L., Rouquette O., Buc E., Privat J., Pezet D., Dapoigny M., Bommelaer G., Abergel A. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy. 2015;47:794–801. doi: 10.1055/s-0034-1391988. [DOI] [PubMed] [Google Scholar]
- 25.Attasaranya S., Netinasunton N., Jongboonyanuparp T., Sottisuporn J., Witeerungrot T., Pirathvisuth T., Ovartlarnporn B. The Spectrum of Endoscopic Ultrasound Intervention in Biliary Diseases: A Single Center’s Experience in 31 Cases. Gastroenterol. Res. Pract. 2012;2012:680753. doi: 10.1155/2012/680753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park D.H., Jang J.W., Lee S.S., Seo D.W., Lee S.K., Kim M.H. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest. Endosc. 2011;74:1276–1284. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 27.Will U., Thieme A., Fueldner F., Gerlach R., Wanzar I., Meyer F. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007;39:292–295. doi: 10.1055/s-2007-966215. [DOI] [PubMed] [Google Scholar]
- 28.Dhir V., Itoi T., Khashab M.A., Park D.H., Yuen Bun Teoh A., Attam R., Messallam A., Varadarajulu S., Maydeo A. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest. Endosc. 2015;81:913–923. doi: 10.1016/j.gie.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 29.Itonaga M., Kitano M., Hatamaru K., Tamura T., Nuta J., Kawaji Y., Takenaka M., Minaga K., Kudo M., Ogura T., et al. Endoscopic ultrasound-guided choledochoduodenostomy using a thin stent delivery system in patients with unresectable malignant distal biliary obstruction: A prospective multicenter study. Dig. Endosc. 2019;31:291–298. doi: 10.1111/den.13300. [DOI] [PubMed] [Google Scholar]
- 30.Amato A., Sinagra E., Celsa C., Enea M., Buda A., Vieceli F., Scaramella L., Belletrutti P., Fugazza A., Cammà C., et al. Efficacy of lumen-apposing metal stents or self-expandable metal stents for endoscopic ultrasound-guided choledochoduodenostomy: A systematic review and meta-analysis. Endoscopy. 2021;53:1037–1047. doi: 10.1055/a-1324-7919. [DOI] [PubMed] [Google Scholar]
- 31.Dhir V., Itoi T., Fockens P., Perez-Miranda M., Khashab M.A., Seo D.W., Yang A.M., Lawrence K.Y., Maydeo A. Novel ex vivo model for hands-on teaching of and training in EUS-guided biliary drainage: Creation of “Mumbai EUS” stereolithography/3D printing bile duct prototype (with videos) Gastrointest. Endosc. 2015;81:440–446. doi: 10.1016/j.gie.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Hara K., Yamao K., Mizuno N., Hijioka S., Imaoka H., Tajika M., Tanaka T., Ishihara M., Okuno N., Hieda N., et al. Endoscopic ultrasonography-guided biliary drainage: Who, when, which, and how? World J. Gastroenterol. 2016;22:1297–1303. doi: 10.3748/wjg.v22.i3.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used during the current study is available from the corresponding author upon reasonable request.