Abstract
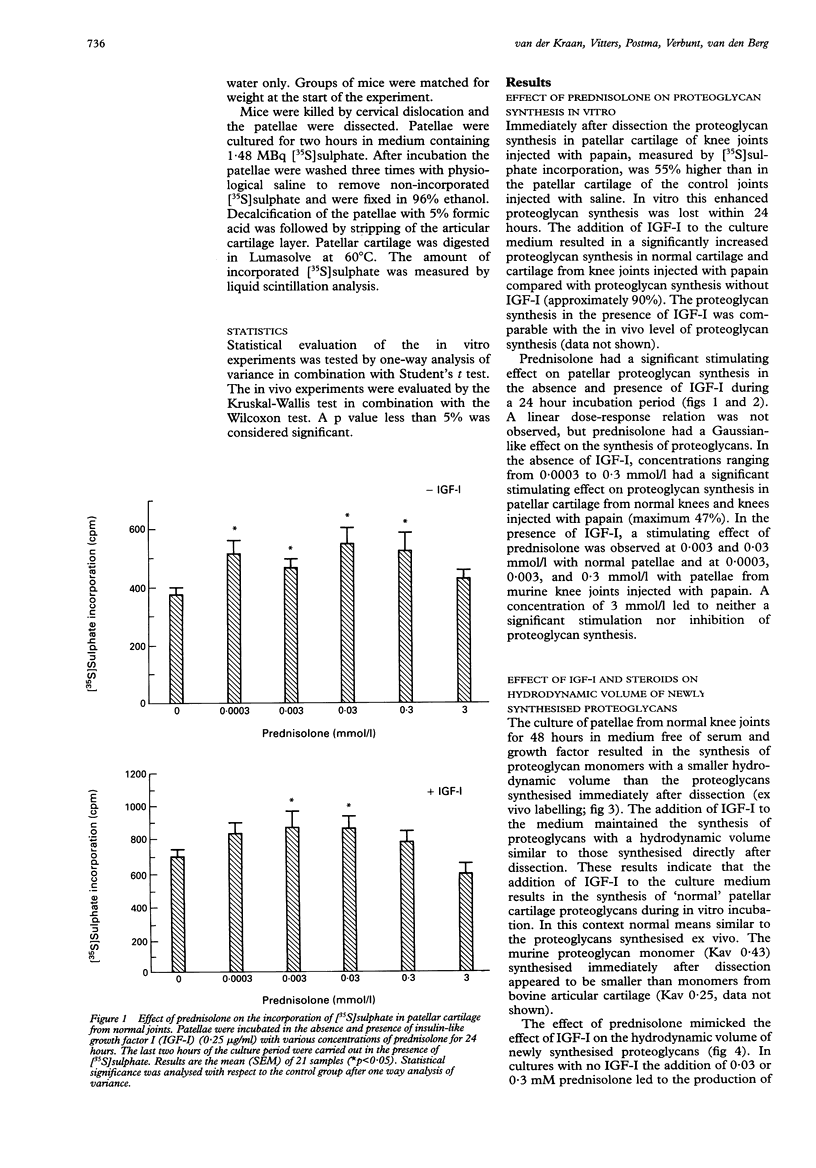
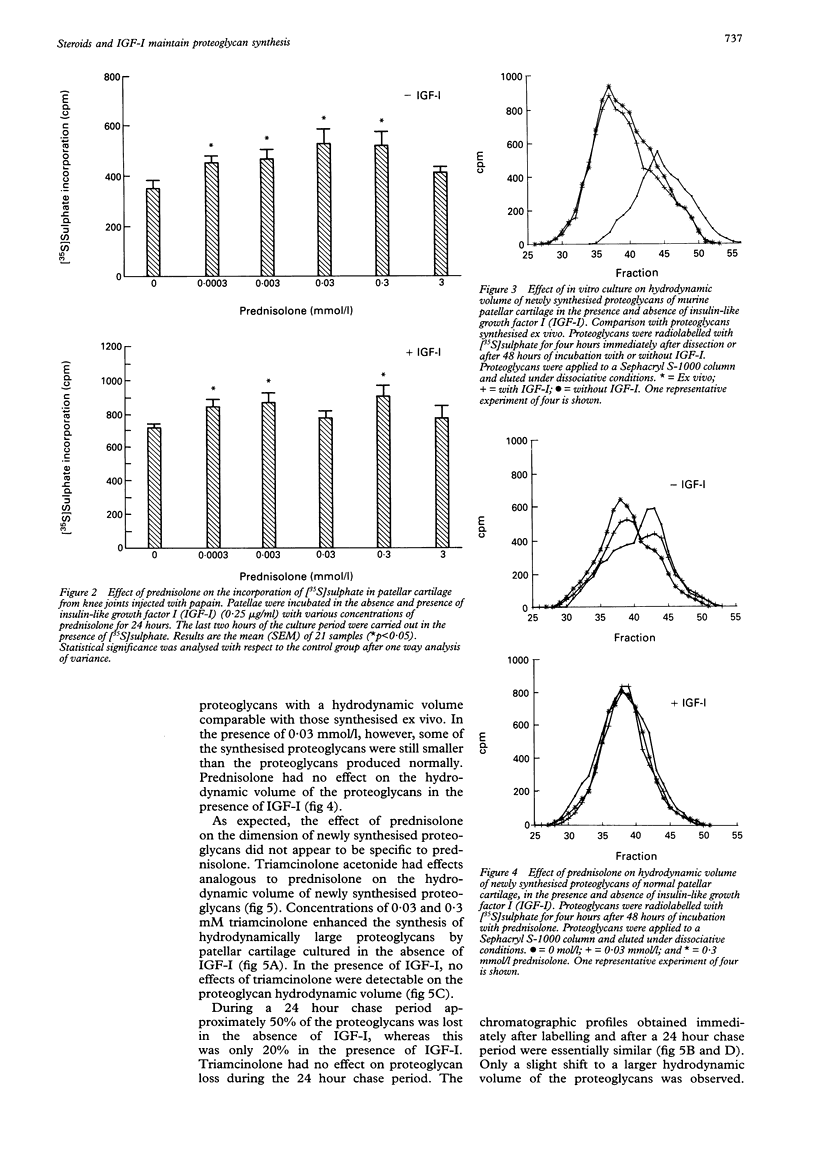
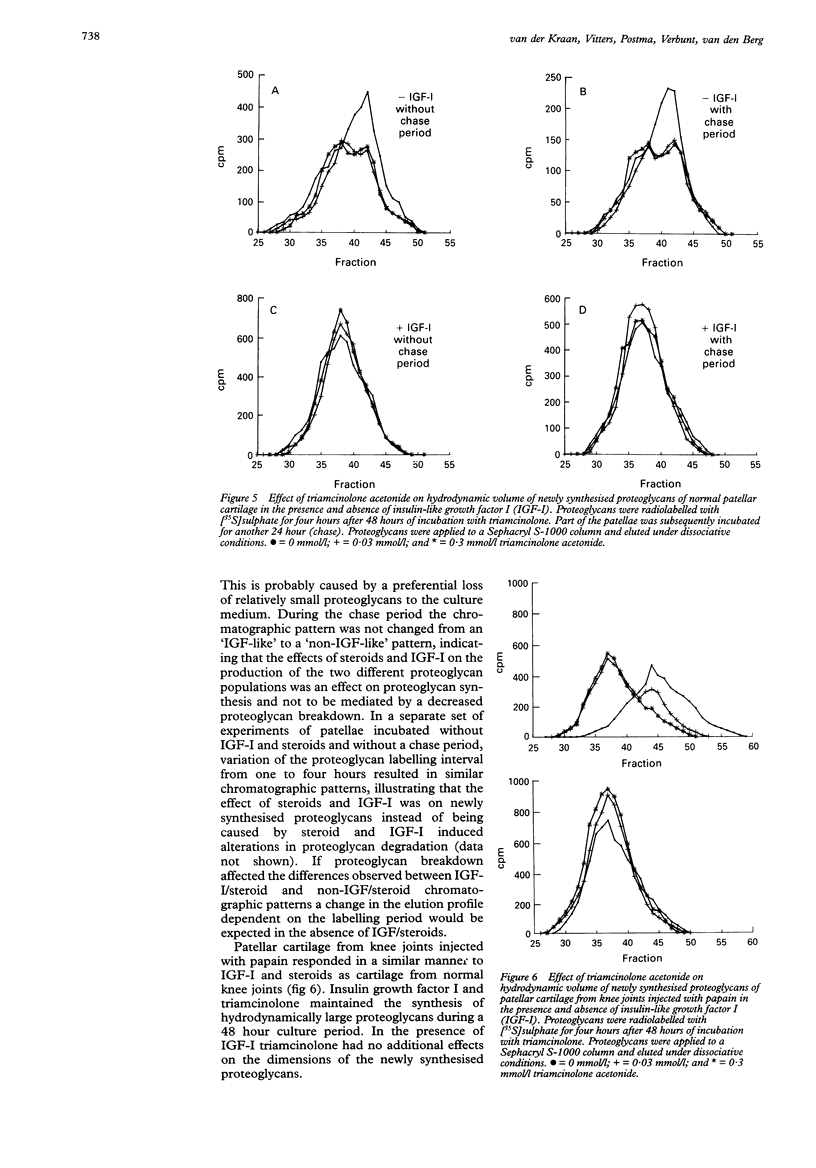
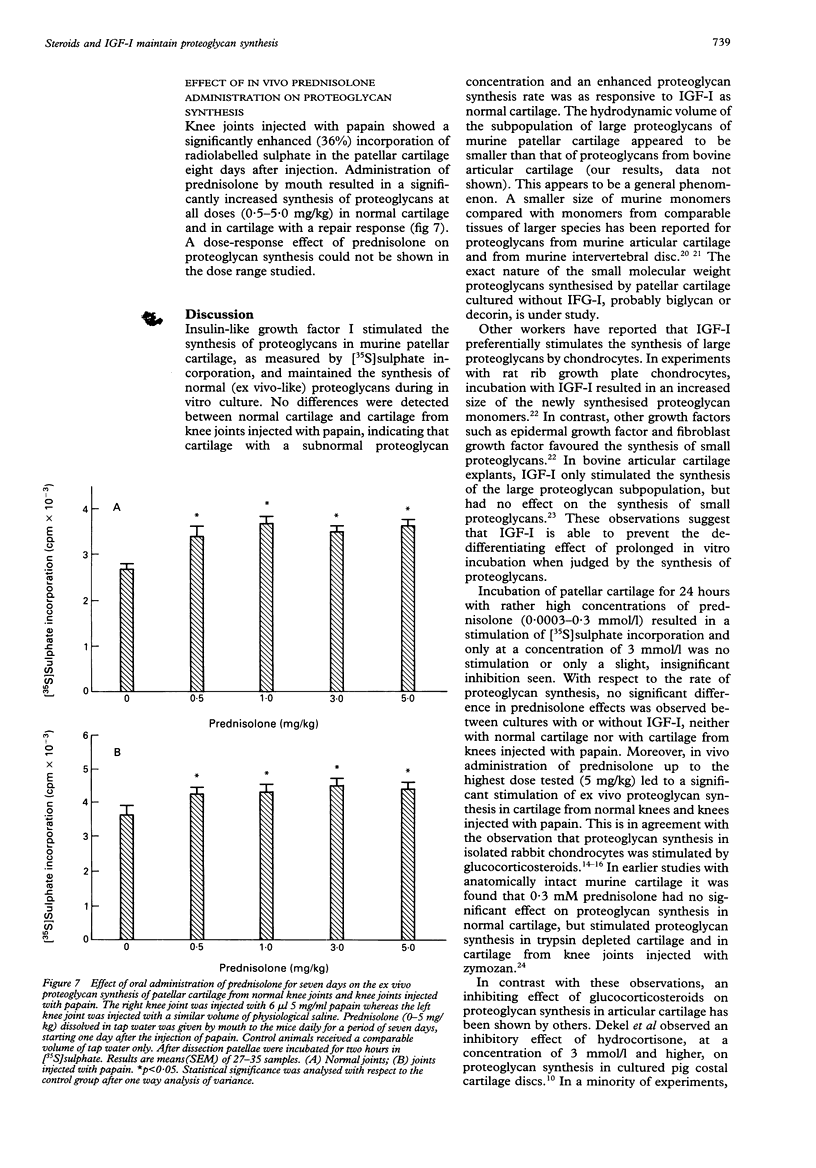
OBJECTIVES--The exact regulation of the synthesis of cartilage specific molecules, such as collagen type II and aggrecan, by articular chondrocytes is unknown, but growth factors and hormones probably play an important part. The effects of glucocorticosteroids (prednisolone and triamcinolone), in combination with insulin-like growth factor I (IGF-I), on the synthesis and hydrodynamic volume of proteoglycans from murine patellar cartilage were investigated. METHODS--The in vitro effect of IGF-I and steroids on proteoglycan synthesis in murine patellar cartilage was evaluated by [35S]sulphate incorporation in combination with dissociative gel chromatography using a Sephacryl S-1000 column. The impact of in vivo prednisolone (0-5 mg/kg) on proteoglycan synthesis in murine patellar cartilage was analysed by [35S]sulphate incorporation immediately after dissection from the knee joint. RESULTS--Prednisolone stimulated proteoglycan synthesis in murine patellar cartilage from normal knees and in cartilage from knees injected with papain in vitro in the absence and presence of IGF-I. Moreover, oral administration of prednisolone for seven days to C57Bl10 mice resulted in enhanced proteoglycan synthesis in patellar cartilage. The incubation of patellar cartilage for 48 hours without serum or growth factors led to the synthesis of proteoglycans with a smaller hydrodynamic volume than those synthesised immediately after dissection of the patellae. This could either be circumvented by the addition of IGF-I or by the addition of glucocorticosteroids (prednisolone or triamcinolone) to the culture medium. CONCLUSIONS--These results show that in a dose range of 0.0003-0.3 mmol/l, glucocorticosteroids, like IGF-I, stimulate proteoglycan synthesis and maintain the synthesis of hydrodynamically large proteoglycans by chondrocytes from murine articular cartilage. This indicates that glucocorticosteroids might play a part in the preservation of matrix integrity in articular cartilage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOSTROEM H., BERNTSEN K., WHITEHOUSE M. W. BIOCHEMICAL PROPERTIES OF ANTI-INFLAMMATORY DRUGS-II. SOME EFFECTS ON SULPHATE-35S METABOLISM IN VIVO. Biochem Pharmacol. 1964 Mar;13:413–420. doi: 10.1016/0006-2952(64)90161-3. [DOI] [PubMed] [Google Scholar]
- Blondelon D., Adolphe M., Zizine L., Lechat P. Evidence for glucocorticoid receptors in cultured rabbit articular chondrocytes. FEBS Lett. 1980 Aug 11;117(1):195–199. doi: 10.1016/0014-5793(80)80943-4. [DOI] [PubMed] [Google Scholar]
- Dekel S., Falconer J., Francis M. J. The effect of anti-inflammatory drugs on glycosaminoglycan sulphation in pig cartilage. Prostaglandins Med. 1980 Mar;4(3):133–140. doi: 10.1016/0161-4630(80)90074-9. [DOI] [PubMed] [Google Scholar]
- DiBattista J. A., Martel-Pelletier J., Wosu L. O., Sandor T., Antakly T., Pelletier J. P. Glucocorticoid receptor mediated inhibition of interleukin-1 stimulated neutral metalloprotease synthesis in normal human chondrocytes. J Clin Endocrinol Metab. 1991 Feb;72(2):316–326. doi: 10.1210/jcem-72-2-316. [DOI] [PubMed] [Google Scholar]
- Hunneyball I. M. Some further effects of prednisolone and triamcinolone hexacetonide on experimental arthritis in rabbits. Agents Actions. 1981 Nov;11(5):490–498. doi: 10.1007/BF02004711. [DOI] [PubMed] [Google Scholar]
- Itagane Y., Inada H., Fujita K., Isshiki G. Interactions between steroid hormones and insulin-like growth factor-I in rabbit chondrocytes. Endocrinology. 1991 Mar;128(3):1419–1424. doi: 10.1210/endo-128-3-1419. [DOI] [PubMed] [Google Scholar]
- Joosten L. A., Helsen M. M., van den Berg W. B. Protective effect of rimexolone on cartilage damage in arthritic mice: a comparative study with triamcinolone hexacetonide. Agents Actions. 1990 Aug;31(1-2):135–142. doi: 10.1007/BF02003233. [DOI] [PubMed] [Google Scholar]
- Kato Y., Gospodarowicz D. Stimulation by glucocorticoid of the synthesis of cartilage-matrix proteoglycans produced by rabbit costal chondrocytes in vitro. J Biol Chem. 1985 Feb 25;260(4):2364–2373. [PubMed] [Google Scholar]
- Kolibas L. M., Goldberg R. L. Effect of cytokines and anti-arthritic drugs on glycosaminoglycan synthesis by bovine articular chondrocytes. Agents Actions. 1989 Jun;27(3-4):245–249. doi: 10.1007/BF01972787. [DOI] [PubMed] [Google Scholar]
- Makower A. M., Wroblewski J., Pawlowski A. Effects of IGF-I, EGF, and FGF on proteoglycans synthesized by fractionated chondrocytes of rat rib growth plate. Exp Cell Res. 1988 Dec;179(2):498–506. doi: 10.1016/0014-4827(88)90287-x. [DOI] [PubMed] [Google Scholar]
- Nagi M. N., Cook L., Laguna J. C., Cinti D. L. Dual action of 2-decynoyl coenzyme A: inhibitor of hepatic mitochondrial trans-2-enoyl coenzyme A reductase and peroxisomal bifunctional protein and substrate for the mitochondrial beta-oxidation system. Arch Biochem Biophys. 1988 Nov 15;267(1):1–12. doi: 10.1016/0003-9861(88)90001-x. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Cloutier J. M., Martel-Pelletier J. In vitro effects of tiaprofenic acid, sodium salicylate and hydrocortisone on the proteoglycan metabolism of human osteoarthritic cartilage. J Rheumatol. 1989 May;16(5):646–655. [PubMed] [Google Scholar]
- Rayan V., Hardingham T. E. Proteoglycan metabolism in chondrocytes of young pig articular cartilage and its modulation by therapeutic agents. Biochem Soc Trans. 1990 Oct;18(5):972–972. doi: 10.1042/bst0180972. [DOI] [PubMed] [Google Scholar]
- Rostand K. S., Baker J. R., Caterson B., Christner J. E. Isolation and characterization of mouse articular cartilage proteoglycans using preformed CsCl density gradients in the Beckman Airfuge. A rapid semi-micro procedure for proteoglycan isolation. J Biol Chem. 1982 Jan 25;257(2):703–707. [PubMed] [Google Scholar]
- Schalkwijk J., Joosten L. A., van den Berg W. B., van Wyk J. J., van de Putte L. B. Insulin-like growth factor stimulation of chondrocyte proteoglycan synthesis by human synovial fluid. Arthritis Rheum. 1989 Jan;32(1):66–71. doi: 10.1002/anr.1780320111. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., Joosten L. A., van den Berg W. B., van de Putte L. B. Chondrocyte nonresponsiveness to insulin-like growth factor 1 in experimental arthritis. Arthritis Rheum. 1989 Jul;32(7):894–900. [PubMed] [Google Scholar]
- Takano T., Takigawa M., Suzuki F. Stimulation by glucocorticoids of the differentiated phenotype of chondrocytes and the proliferation of rabbit costal chondrocytes in culture. J Biochem. 1985 Apr;97(4):1093–1100. doi: 10.1093/oxfordjournals.jbchem.a135153. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Takano T., Nakagawa K., Sakuda M., Suzuki F. Hydrocortisone stimulation of proliferation and glycosaminoglycan synthesis in rabbit craniofacial chondrocytes in vitro. Arch Oral Biol. 1988;33(12):893–899. doi: 10.1016/0003-9969(88)90019-2. [DOI] [PubMed] [Google Scholar]
- Tesch G. H., Handley C. J., Cornell H. J., Herington A. C. Effects of free and bound insulin-like growth factors on proteoglycan metabolism in articular cartilage explants. J Orthop Res. 1992 Jan;10(1):14–22. doi: 10.1002/jor.1100100103. [DOI] [PubMed] [Google Scholar]
- de Vries B. J., van den Berg W. B., Vitters E., van de Putte L. B. Effects of NSAIDs on the metabolism of sulphated glycosaminoglycans in healthy and (post) arthritic murine articular cartilage. Drugs. 1988;35 (Suppl 1):24–32. doi: 10.2165/00003495-198800351-00007. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B. Impact of NSAID and steroids on cartilage destruction in murine antigen induced arthritis. J Rheumatol Suppl. 1991 Feb;27:122–123. [PubMed] [Google Scholar]
- van der Kraan P. M., Vitters E. L., van de Putte L. B., van den Berg W. B. Development of osteoarthritic lesions in mice by "metabolic" and "mechanical" alterations in the knee joints. Am J Pathol. 1989 Dec;135(6):1001–1014. [PMC free article] [PubMed] [Google Scholar]
- van der Kraan P. M., Vitters E. L., van den Berg W. B. Inhibition of proteoglycan synthesis by transforming growth factor beta in anatomically intact articular cartilage of murine patellae. Ann Rheum Dis. 1992 May;51(5):643–647. doi: 10.1136/ard.51.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]


