Abstract
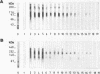
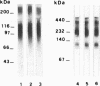
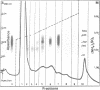
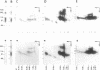
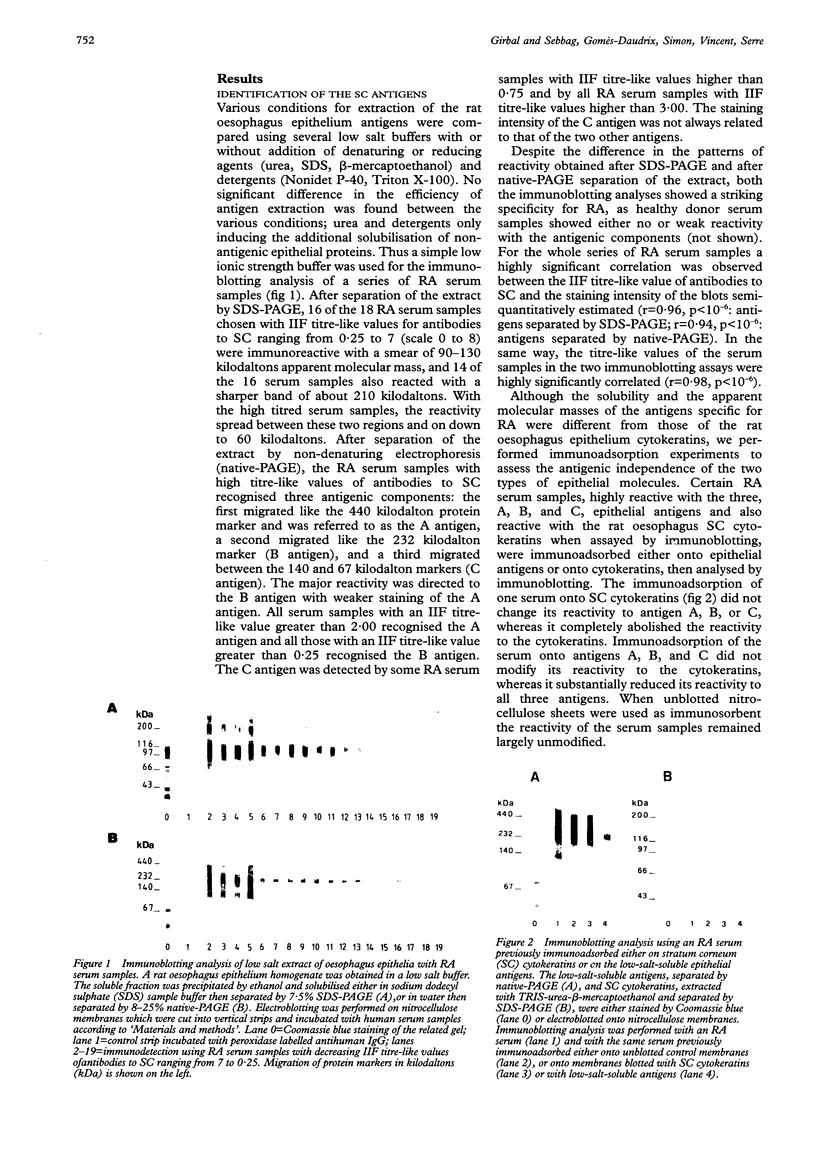
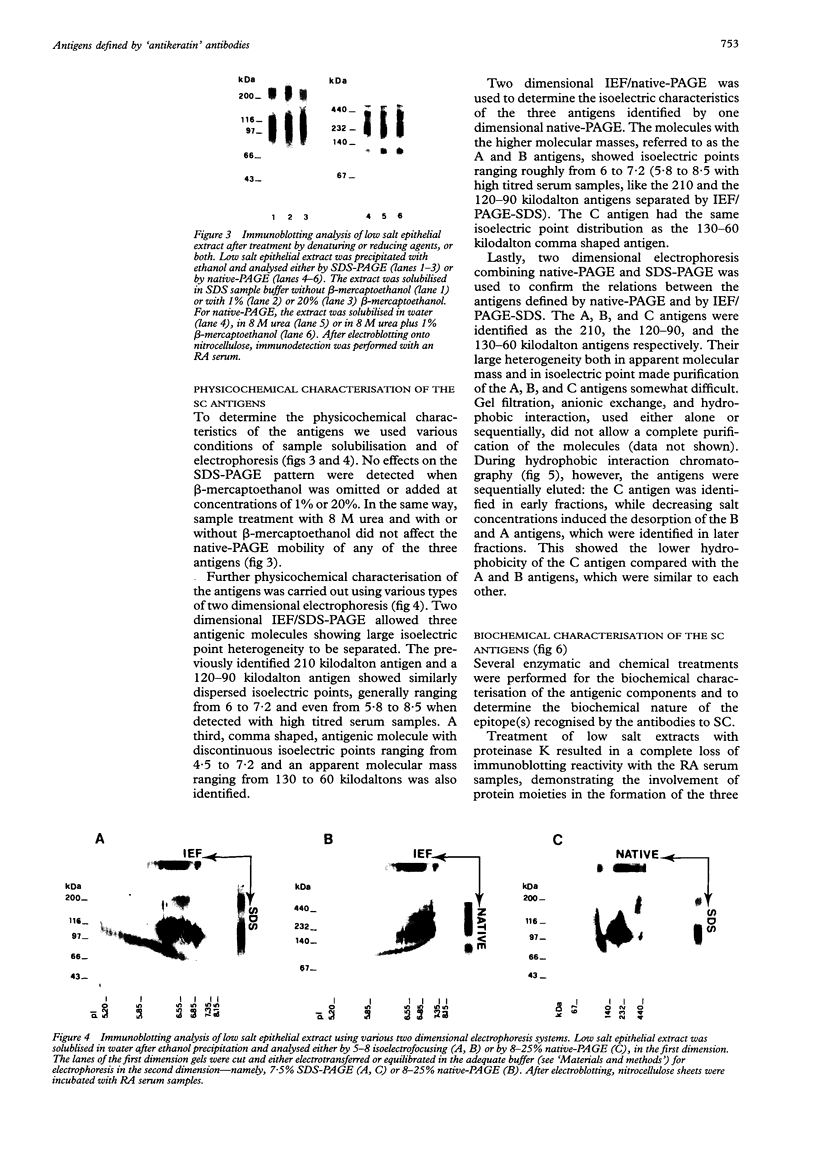
OBJECTIVES--An attempt was made to characterise the antigens recognised by serum IgG antibodies directed to the stratum corneum of rat oesophagus epithelium, the so-called 'antikeratin antibodies', which were shown to be highly specific for rheumatoid arthritis (RA) and thus to have an actual diagnostic value. METHODS--Immunoblotting was performed with RA serum samples on different extracts of rat oesophagus epithelium separated by various monodimensional and two dimensional electrophoreses. RESULTS--Three low-salt-soluble antigens sensitive to proteinase K and, therefore, of protein nature were identified. Two proteins, with apparent molecular masses of 210 and 120-90 kilodaltons, shared isoelectric points ranging from 5.8 to 8.5; the third protein exhibited isoelectric points from 4.5 to 7.2 while its molecular mass ranged from 130 to 60 kilodaltons. Immunoadsorption of RA serum samples onto cytokeratins extracted from the stratum corneum of rat oesophagus epithelium did not change their immunoreactivity towards the three antigenic proteins. Widely used deglycosylation and dephosphorylation methods failed to modify either the electrophoretic migration of the proteins or their immunoreactivity with RA serum samples. CONCLUSION--The so-called 'antikeratin antibodies' do not react with cytokeratins. They specifically recognise three late epithelial differentiation proteins which had not been previously described. These proteins may be related to (pro)filaggrin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Ball R. D., Walker G. K., Bernstein I. A. Histidine-rich proteins as molecular markers of epidermal differentiation. J Biol Chem. 1978 Aug 25;253(16):5861–5868. [PubMed] [Google Scholar]
- Berger E. G., Buddecke E., Kamerling J. P., Kobata A., Paulson J. C., Vliegenthart J. F. Structure, biosynthesis and functions of glycoprotein glycans. Experientia. 1982 Oct 15;38(10):1129–1162. doi: 10.1007/BF01959725. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Roth T. F. Staining of phospho-proteins on acrylamide gel electropherograms. Anal Biochem. 1973 Aug;54(2):386–394. doi: 10.1016/0003-2697(73)90367-9. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Lonsdale-Eccles J. D., Lynley A. M. Two-dimensional analysis of proteins of rat oral epithelia and epidermis. Arch Oral Biol. 1982;27(7):529–533. doi: 10.1016/0003-9969(82)90066-8. [DOI] [PubMed] [Google Scholar]
- Dale B. A. Purification and characterization of a basic protein from the stratum corneum of mammalian epidermis. Biochim Biophys Acta. 1977 Mar 28;491(1):193–204. doi: 10.1016/0005-2795(77)90055-1. [DOI] [PubMed] [Google Scholar]
- Ferris J., Cooper S., Roessner K., Hochberg M. Antibodies to denatured type II collagen in rheumatoid arthritis: negative association with IgM rheumatoid factor. J Rheumatol. 1990 Jul;17(7):880–884. [PubMed] [Google Scholar]
- Florman H. M., Wassarman P. M. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985 May;41(1):313–324. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M., Hiraoka B. Y., Fukasawa K., Fukasawa K. M. Phosphoprotein phosphatase activity of bovine intestinal alkaline phosphatase. Experientia. 1981 Jun;37(6):547–548. doi: 10.1007/BF01990040. [DOI] [PubMed] [Google Scholar]
- Hassfeld W., Steiner G., Hartmuth K., Kolarz G., Scherak O., Graninger W., Thumb N., Smolen J. S. Demonstration of a new antinuclear antibody (anti-RA33) that is highly specific for rheumatoid arthritis. Arthritis Rheum. 1989 Dec;32(12):1515–1520. doi: 10.1002/anr.1780321204. [DOI] [PubMed] [Google Scholar]
- Haydock P. V., Dale B. A. Filaggrin, an intermediate filament-associated protein: structural and functional implications from the sequence of a cDNA from rat. DNA Cell Biol. 1990 May;9(4):251–261. doi: 10.1089/dna.1990.9.251. [DOI] [PubMed] [Google Scholar]
- Haydock P. V., Dale B. A. The repetitive structure of the profilaggrin gene as demonstrated using epidermal profilaggrin cDNA. J Biol Chem. 1986 Sep 25;261(27):12520–12525. [PubMed] [Google Scholar]
- Kirstein H., Hjarvard K., Hansen T. M. Antikeratin antibodies in synovial fluid in rheumatoid arthritis. APMIS. 1989 Feb;97(2):185–189. doi: 10.1111/j.1699-0463.1989.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Kirstein H., Mathiesen F. K. Antikeratin antibodies in rheumatoid arthritis. Methods and clinical significance. Scand J Rheumatol. 1987;16(5):331–338. doi: 10.3109/03009748709102504. [DOI] [PubMed] [Google Scholar]
- Kurki P., Aho K., Palosuo T., Heliövaara M. Immunopathology of rheumatoid arthritis. Antikeratin antibodies precede the clinical disease. Arthritis Rheum. 1992 Aug;35(8):914–917. doi: 10.1002/art.1780350810. [DOI] [PubMed] [Google Scholar]
- Menzel J., Steffen C., Kolarz G., Eberal G., Frank O., Thumb N. Demonstration of antibodies to collagen and of collagen-anticollagen immune complexes in rheumatoid arthritis synovial fluids. Ann Rheum Dis. 1975 Oct;35(5):446–450. doi: 10.1136/ard.35.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Fabregas D., Cyna L., Ryckewaert A. Les anticorps anti-kératine. Un marqueur des polyarthrites rhumatoïdes évolutives. Rev Rhum Mal Osteoartic. 1986 Nov;53(11):601–605. [PubMed] [Google Scholar]
- Murozuka T., Fukuyama K., Epstein W. L. Immunochemical comparison of histidine-rich protein in keratohyalin granules and cornified cells. Biochim Biophys Acta. 1979 Aug 28;579(2):334–345. doi: 10.1016/0005-2795(79)90061-8. [DOI] [PubMed] [Google Scholar]
- NIENHUIS R. L., MANDEMA E. A NEW SERUM FACTOR IN PATIENTS WITH RHEUMATOID ARTHRITIS; THE ANTIPERINUCLEAR FACTOR. Ann Rheum Dis. 1964 Jul;23:302–305. doi: 10.1136/ard.23.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordeig J., Guardia J. Diagnostic value of antikeratin antibodies in rheumatoid arthritis. J Rheumatol. 1984 Oct;11(5):602–604. [PubMed] [Google Scholar]
- Paimela L., Gripenberg M., Kurki P., Leirisalo-Repo M. Antikeratin antibodies: diagnostic and prognostic markers for early rheumatoid arthritis. Ann Rheum Dis. 1992 Jun;51(6):743–746. doi: 10.1136/ard.51.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre G., Vincent C., Fournié B., Lapeyre F., Soleilhavoup J. P., Fournié A. Anticorps anti-stratum corneum d'oesophage de rat, auto-anticorps anti-kératines épidermiques et anti-épiderme dans la polyarthrite rhumatoïde et différentes affections rhumatologiques. Intérêt diagnostique, aspects fondamentaux. Rev Rhum Mal Osteoartic. 1986 Nov;53(11):607–614. [PubMed] [Google Scholar]
- Serre G., Vincent C., Viraben R., Soleilhavoup J. P. Natural IgM and IgG autoantibodies to epidermal keratins in normal human sera. I: ELISA-titration, immunofluorescence study. J Invest Dermatol. 1987 Jan;88(1):21–27. doi: 10.1111/1523-1747.ep12464810. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Plummer T. H., Jr Enzymatic approaches for studying the structure, synthesis, and processing of glycoproteins. Methods Cell Biol. 1989;32:111–139. doi: 10.1016/s0091-679x(08)61169-3. [DOI] [PubMed] [Google Scholar]
- Terato K., Shimozuru Y., Katayama K., Takemitsu Y., Yamashita I., Miyatsu M., Fujii K., Sagara M., Kobayashi S., Goto M. Specificity of antibodies to type II collagen in rheumatoid arthritis. Arthritis Rheum. 1990 Oct;33(10):1493–1500. doi: 10.1002/art.1780331006. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuaillon N., Muller S., Pasquali J. L., Bordigoni P., Youinou P., Van Regenmortel M. H. Antibodies from patients with rheumatoid arthritis and juvenile chronic arthritis analyzed with core histone synthetic peptides. Int Arch Allergy Appl Immunol. 1990;91(3):297–305. doi: 10.1159/000235131. [DOI] [PubMed] [Google Scholar]
- Veale R. B., Thornley A. L., Scott E., Antoni A., Segal I. Quantitation of autoantibodies to cytokeratins in sera from patients with squamous cell carcinoma of the oesophagus. Br J Cancer. 1988 Dec;58(6):767–772. doi: 10.1038/bjc.1988.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C., Serre G., Basile J. P., Lestra H. C., Girbal E., Sebbag M., Soleilhavoup J. P. Subclass distribution of IgG antibodies to the rat oesophagus stratum corneum (so-called anti-keratin antibodies) in rheumatoid arthritis. Clin Exp Immunol. 1990 Jul;81(1):83–89. doi: 10.1111/j.1365-2249.1990.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent C., Serre G., Fournié B., Fournié A., Soleilhavoup J. P. Natural IgG to epidermal cytokeratins vs IgG to the stratum corneum of the rat oesophagus epithelium, so-called 'antikeratin antibodies', in rheumatoid arthritis and other rheumatic diseases. J Autoimmun. 1991 Jun;4(3):493–505. doi: 10.1016/0896-8411(91)90161-5. [DOI] [PubMed] [Google Scholar]
- Vincent C., Serre G., Lapeyre F., Fournié B., Ayrolles C., Fournié A., Soleilhavoup J. P. High diagnostic value in rheumatoid arthritis of antibodies to the stratum corneum of rat oesophagus epithelium, so-called 'antikeratin antibodies'. Ann Rheum Dis. 1989 Sep;48(9):712–722. doi: 10.1136/ard.48.9.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F., Cathey M. A., Roberts F. K. The latex test revisited. Rheumatoid factor testing in 8,287 rheumatic disease patients. Arthritis Rheum. 1991 Aug;34(8):951–960. doi: 10.1002/art.1780340804. [DOI] [PubMed] [Google Scholar]
- Yonekura H., Oguri K., Itoh K., Tanabe K., Takahashi N., Nakanishi Y., Okayama M. Sulfated glycoproteins synthesized by the corneal epithelium of chick embryo having the same molecular weights as cytokeratin polypeptides. J Biochem. 1988 Oct;104(4):648–657. doi: 10.1093/oxfordjournals.jbchem.a122527. [DOI] [PubMed] [Google Scholar]
- Youinou P., Le Goff P., Colaco C. B., Thivolet J., Tater D., Viac J., Shipley M. Antikeratin antibodies in serum and synovial fluid show specificity for rheumatoid arthritis in a study of connective tissue diseases. Ann Rheum Dis. 1985 Jul;44(7):450–454. doi: 10.1136/ard.44.7.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. J., Mallya R. K., Leslie R. D., Clark C. J., Hamblin T. J. Anti-keratin antibodies in rheumatoid arthritis. Br Med J. 1979 Jul 14;2(6182):97–99. doi: 10.1136/bmj.2.6182.97. [DOI] [PMC free article] [PubMed] [Google Scholar]