Abstract
Photosensitizer-based photodynamic therapy (PDT) has been considered as a promising modality for fighting diverse types of cancers. PDT directly inhibits local tumors by a minimally invasive strategy, but it seems to be incapable of achieving complete eradication and fails to prevent metastasis and recurrence. Recently, increasing events proved that PDT was associated with immunotherapy by triggering immunogenic cell death (ICD). Upon a specific wavelength of light irradiation, the photosensitizers will turn the surrounding oxygen molecules into cytotoxic reactive oxygen species (ROS) for killing the cancer cells. Simultaneously, the dying tumor cells release tumor-associated antigens, which could improve immunogenicity to activate immune cells. However, the progressively enhanced immunity is typically limited by the intrinsic immunosuppressive tumor microenvironment (TME). To overcome this obstacle, immuno-photodynamic therapy (IPDT) has come to be one of the most beneficial strategies, which takes advantage of PDT to stimulate the immune response and unite immunotherapy for inducing immune-OFF tumors to immune-ON ones, to achieve systemic immune response and prevent cancer recurrence. In this Perspective, we provide a review of recent advances in organic photosensitizer-based IPDT. The general process of immune responses triggered by photosensitizers (PSs) and how to enhance the antitumor immune pathway by modifying the chemical structure or conjugating with a targeting component was discussed. In addition, future perspectives and challenges associated with IPDT strategies are also discussed. We hope this Perspective could inspire more innovative ideas and provide executable strategies for future developments in the war against cancer.
Keywords: photosensitizer, immunogenic cell death, immuno-photodynamic therapy, apoptosis, necroptosis, pyroptosis
1. Introduction
Cancer is one of the major threats to human life and health.1,2 The research to efficiently cure cancer has always been the focus of attention. In recent years, with a deeper understanding of cancer immunology, cancer immunotherapy has emerged as a promising modality for cancer treatment.3−5 It is accomplished by stimulating or regulating inherent autoimmune systems to identify, attack, eliminate tumor cells, and eventually contribute to the long-term control of the disease.6,7 Current immunotherapeutic strategies mainly include immune checkpoint blockade (ICB) therapy,8 chimeric antigen receptor T cells (CAR-T) therapy,9 and cancer vaccines,10,11 all intended to imitate the body’s natural antitumor immune defenses to fight against tumors and the prolong life of patients.12,13 However, immunotherapy cannot work on all patients due to the inadequate immunogenicity of solid tumors and low clinical objective response rates.14 Hence, developing a strategy to promote the immunogenicity and improve antitumor efficacy could be significant for efficient cancer immunotherapy.
To overcome these defects of mono-immunotherapy, combinational therapy such as immuno-photodynamic therapy (IPDT) has become a revolutionary approach,15 due to the increasing number of studies which have confirmed that PDT could initiate an antitumor immune response by the immunogenic cell death (ICD) mechanism.16 This approach is expected to offset the shortfalls of each mode of therapy and elicit an intact immune system for cancer treatment, which can not only bring strong immunogenicity for immunotherapy but also inhibit the growth of remaining tumor cells in the body left behind after PDT.17 During IPDT, the photosensitizers were excited by light in the presence of oxygen to generate reactive oxygen species (ROS) which could lead to apoptosis, necroptosis, pyroptosis, and microvascular damage, and then the dying cells induced ICD by releasing the damaged-associated molecular patterns (DAMPs), tumor-specific antigens, and proinflammatory cytokines, which could lead to more immunogen exposure and activation of the immune system.18−20 These biological events, similar to in situ “tumor vaccine”, enhance the tumor infiltration of immune cells and amplify the antitumor immune responses against solid tumors.21,22
Given the fact that photosensitizers (PSs) play such vital role in the success of IPDT, it is hardly surprising that considerable effort has gone into the development of better PSs.23 In order to meet the actual demand, excellent PSs should have the following characteristics: (1) Minimal dark toxicity, little allergic reactions, ideal biocompatibility, high tumor selectivity. (2) Rapid clearance from the normal tissue, avoiding photosensitivity damage and metabolic burden. (3) Strong absorption in the therapeutic-window region (650–1350 nm) but weak absorption in the range of strong emission from the sun (400–600 nm), avoiding phototoxic side effects. (4) High yield of ROS under physiological conditions ensuring effective IPDT. (5) Pure substances, easily prepared and stable enough under the storage condition. To date, some PSs have showed superior advantages in clinical applications or research experiments for IPDT, including porphyrin, chlorin, or phthalocyanine derivatives, with macrocycle tetrapyrrole structures, all of which absorb light efficiently and generate ROS.24,25 Additionally, methylene blue, cyanine dyes, rose bengal, and hypericin with photodynamic properties conducive to oncological applications have been utilized in clinical applications or trials.26,27
In recent years, researchers have focused on exploring new IPDT pathways with effective PSs.28,29 Due to the fast development and widespread coverage of the topic, we summarize the mechanism of organic PSs for more sensitive IPDT. We believe that this discussion of the new IPDT concepts and design strategies underscores the rational basis for further development of PS-mediated fluorescence imaging, cancer therapy, and other related biological applications.30,31 Moreover, current challenges and future outlooks are also discussed at the end of this Perspective.
2. General Process and Mechanisms of Action for IPDT
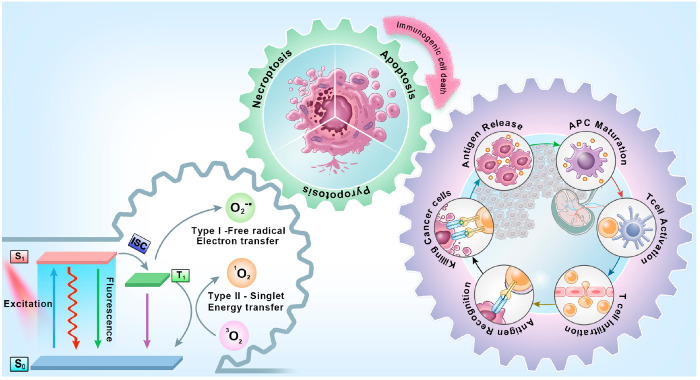
IPDT is a synergistic therapy process that has been found to achieve satisfactory results in the treatment of in situ and metastatic cancer by a systemic immunostimulatory response.32 As shown in Figure 1, PDT provides the first line of defense against the tumor.33 Upon light irradiation, the PSs is activated from the ground singlet state (S0) to the excited singlet state (S1) and followed by intersystem crossing (ISC) to reach triplet state (T1). According to the photochemical and photophysical mechanisms, the PDT mainly goes through two pathways. The first is the Type-I mechanism, where the PSs in T1 experience hydrogen abstraction or an electron transfer process with the biological substrate to generate free radicals, such as superoxide anions (O2–•). And then one electron reduction or dismutation of O2–• generates more ROS including hydrogen peroxide (H2O2) and hydroxyl radicals (·OH). For Type-II mechanism, the photoinduced energy transfers from PSs in T1 to ground-state oxygen (3O2) directly, for generating cytotoxic singlet oxygen (1O2).34 By interacting with endogenous biomolecules, ROS leads to an avalanche of damage to cancerous lesions.35 In addition, if the PSs are localized in several cellular structures (the cell nucleus, mitochondria, lysosomes, endoplasmic reticulum, cytomembrane, etc.), the photochemical reaction will drive more effective oxidative stress, causing irreversible damage in tumor cells.36
Figure 1.
Schematic illustration of general procedure and mechanisms of action for IPDT and summary of three major strategies to induce ICD for IPDT.
Meanwhile, the ideal IPDT could not only kill tumor cells directly but also deliver endogenous danger signals during cancer cell death by the process of ICD,37 could activate the immune system by a series of mechanisms including recruiting immune cells, promoting the antigen presentation by antigen-presenting cells, increasing the release of antitumor cytokines, and further stimulating antigen-specific immune responses against a broad spectrum of solid tumors.38−40 Specifically, IPDT-dominated ICD release of the danger-associated molecular patterns (DAMPs) is considered to be a golden rule in the immunogenicity of tumor cells, the well-known DAMPs related to include the translocation of calreticulin (CRT) onto the surface,41 the release of high mobility group box 1 (HMGB1) from the nucleus,42 and the secretion of adenosine triphosphate (ATP).43 This upregulation of proinflammatory cytokines results in the promotion of antigen presentation for dendritic cells (DCs) maturation, and then DCs migrate to the lymph nodes, where cross-presentation of the antigens triggers native T cells differentiation into cytotoxic CD8+ T cells for recognizing and attacking tumor cells.44 Meanwhile, these effects usually synergize with the immunotherapies that either consistently provide the immunogenicity of the tumor or reduce immunosuppression in the tumor microenvironment (TME).45 In addition, certain cytokines like interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and various interleukins including IL-2, IL-12, and IL-6 were also reported to increase, contributing to immune response.46−49 Therefore, IPDT-induced antitumor immune responses have the potential to effectively eradicate the target tumor as well as any metastatic or heterogeneous tumor cells, which could also trigger systemic immunity and provide the possibility of preventing tumor recurrence permanently.50−53
2.1. Immunogenic Cell Death Induced by IPDT
From the point of view of immunity, the dying cancer cells are the source of tumor immunogenicity, which could provide the antigens for stimulating tumor-specific immunological responses.54,55 As a type of regulated cell death, ICD is considered as an alternative approach for activating an adaptive immune response in homologous hosts with normal immune function.56 Legrand et al. defined ICD as a successful dialogue between the dying tumor cells and an appropriately disposed immune system.57 Thus, it bridges the gap between PDT and immunotherapy, providing a rejuvenated immune system for the patients via IPDT. Increasing evidence has shown that upon PDT the dying cells actively upregulate immune responses.58−62 However, the induction of cell death usually determines the level of immunogenicity and its downstream molecular pathways. Thus, investigation of the mechanisms of IPDT-induced ICD in cell levels is of great importance to understand how the immune system is activated and, ultimately, to make IPDT more realistic.63−66 In this section, we discuss several kinds of cell death modes (apoptosis, necroptosis, and pyroptosis); they are commonly described to induce ICD.
2.1.1. Apoptosis
Apoptosis is a caspase-dependent process of programmed cell death, which controls the development, homeostasis, and immunity in multicellular organisms.67,68 Facilitating apoptosis is one of the most active regulatory pathways to induce ICD. Due to the cancer cells being more sensitive to ROS than normal cells, apoptosis induced by IPDT would be more effective and avoid overtreatment.69 The main characteristics of IPDT evoked apoptosis are chromatin condensation, cellular fragmentation, and protease activation. Fortunately, effective anticancer therapy relies on just that. In tumor cells, the apoptosis-initiated ICD is commonly related to the localization of PSs, such as the mitochondria-mediated pathway, oxidative stress promoted DNA damage, and endoplasmic reticulum (ER) stress.70 Among them, mitochondrial-localized PSs are the most widely studied in inducing apoptosis. When exposed to irritant signals such as ROS, the mitochondrial outer membrane becomes more permeable to release hemeprotein cytochrome C for activating caspase proteases that carry out the apoptosis process.71 Another classic signal transduction pathway is endoplasmic reticulum (ER) stress causing apoptosis. The ER is the largest membranous intracellular organelle, and it borders the nucleus and connects with the cell membrane.72 Functionally, the ER participates in the process of protein synthesis and maturation, and it also maintains intracellular Ca2+ balance. During the ROS generation process, the ER suffers from toxic attack and environmental changes. If the cells cannot regulate the ER stress to a tolerable level in time, it may lead to ER stress directly or indirectly, ultimately resulting in cell apoptosis. Meanwhile, apoptosis-related proteins such as caspase-3, caspase-7, GRP78, and CHOP have been identified as the key mediators of cell apoptosis; they are important for various of reactions, from cell migration, differentiation, and eventually provoking ICD.73
2.1.2. Necroptosis
Necroptosis is a type of lytic regulated cell death, featuring the cytoplasmic swelling, disintegration of the plasma membrane, and leakage of intracellular contents induced by specific stress.74 As it accompanies the disintegration of intact cytosolic components, which triggers a range of inflammatory responses, necroptosis is considered an inherently immunogenic form of cell death.75 Usually, necroptosis-induced ICD occurs in the cell membrane targeting PSs, with the photoreaction mediating the kinases RIPK1 and RIPK3 assembly into the necrosome, which causes the rapid loss of membrane integrity and then release of immunogenic DAMPs that robustly activate the innate and adaptive immune systems.76 In addition, death receptors such as tumor necrosis factor alpha (TNF-α), interferon-gamma (IFN-γ), and TLR4 detecting bacterial liposolysaccharide (LPS) are activated constantly, which is positively correlated with the immunogenicity of tumor cells. In contrast to apoptosis, the remarkable thing is that necroptosis is found more efficiently in the process of IPDT-induced ICD, which can boost antitumor immunity because the tumor-specific antigens are directly released without exposure to further oxidation and proteolysis by organelles. This contributes to antigen uptake by tumor-associated macrophages and induces DCs maturation leading to efficient CD8+ T cell cross-priming, and then antigen-specific native CD8+ T cells are activated, differentiated into cytotoxic T lymphocytes, and involved in antitumor immunity. Thus, targeted stimulation of dying tumor cells into necroptosis represents a feasible treatment approach in cancer therapy, especially for apoptosis-resistant tumors.77
2.1.3. Pyroptosis
Since it was first reported in 2001, pyroptosis has become the center of attention in a variety of physiological and pathological studies. Recently, an increasing number of reports introduced a new prospect of pyroptosis inducing ICD for IPDT tumor ablation.78 Comparing with cell apoptosis and necroptosis, pyroptosis is more likely to trigger extremely inflammatory consequences through intracellular proinflammatory mediators including interleukin-1β (IL-1β), interleukin-18 (IL-18), and lactate dehydrogenase (LDH). During the photochemical reaction, the PSs induce the generation of a cytosolic complex called “inflammasomes” in tumor cells, which activates caspase-1 to cleave gasdermin-D (GSDMD) and release gasdermin-N domains (N-GSDMD), following the N-GSDMD move into the cell membrane where it forms membrane pores, driving cell swelling, membrane rupture, and cell death.79 In addition, a capase-1-independent pyroptosis pathway with GSDME-triggered pyroptosis via caspase-3 also followed. Significantly, GSDME expression in cancer cells is not the only direction for ICD, but it prevents tumor growth by enhancing the phagocytosis of tumor cells and the activation of tumor-infiltrating natural killer (NK) and CD8+ T cells, facilitating the application of PS-induced pyroptosis in cancer therapy.80 Overall, pyroptosis-caused ICD is able to cure cancer through participation in two key processes: providing immunogenicity and restoring the activity of T cells, largely promoting the therapeutic effect. Therefore, PSs inducing pyroptosis is considered as the most potential IPDT pathway.
The pathway of IPDT inducing ICD on a cellular level is complex, but there are still some regular events that can be observed to allow us to carry out the treatment strategy better. (1) The primary position of ROS generation directly affects the efficacy of IPDT. Thus, the organelle-targeted PSs generally show better ICD inducibility than that randomly distributed in tumor tissue. (2) Influenced by cellular signaling factors, multiple mechanisms of cell damage may coexist to induce ICD. (3) When operating with high doses of IPDT, necroptosis is the dominant form of cell death. More importantly, all of the above could facilitate antigen presentation and recruit immune cells, increasing the release of cytokines and further stimulating adaptive immune responses.
2.2. Research Progress and Clinical Application
As a treatment mode of stimulus response, IPDT can provide very precise nonphysical and noninvasive external stimulation to induce systematic therapeutic effects. In particular, nearly all strategies of IPDT aim to establish an immunogenic tumor microenvironment, which significantly improves the efficiency of cancer immunotherapy. To date, there have been two main strategies used in IPDT: (1) Increasing tumor immunogenicity, after PDT induced ICD, immunotherapy provided sustained immunogenicity to evoke strong and enduring immune responses combating metastatic tumors. (2) Relieving immunoregulatory suppression, when antitumor immunity triggered by PDT-induced ICD, abundant immunosuppressive factors and cells were also aroused, so the efficacy of IPDT was not strong enough to eradicate tumor residuals.
As a prevalent modality appearing over the past few decades, PDT has already been approved for clinical use in tumors. Currently, IPDT has been used to treat cancer in the per-clinic or clinical research. In 2015, Kleinovink et al. reported the combination of Bremachlorin-based PDT with immunity therapeutic peptide vaccination in a per-clinic study.81 The results showed that PDT ablated established tumors and led to a significant CD8+ T cell response against the tumors. Importantly, the combination treatment led to subsequent eradication of the remaining or unrecognizable tumors to the naked eye and offered protection from repeated tumor occurrence. Therefore, this successful application of IPDT, resulting in a strong antitumor response and immunologic memory, suggests a novel treatment strategy for advanced cancer. Subsequently, the case of IPDT in human head and neck cancer was illustrated by Santos et al. in 2018. Redaporfin, a kind of PS approved by the U. S. Food and Drug Administration (FDA), was used for multiple treatments in order to achieve maximal destruction to tumors. Combined with immunotherapy, it successfully treated head and neck cancer, improving outcomes and prolonging survival.82 Besides, the first IPDT drug (a complex of PSs “IR700” and artificial antibodies “Akalux”) developed for the treatment of malignant tumors of the head and neck was approved in 2020. It could play a role in treatment accompanied by local irradiation and show satisfactory results.83 We believe in technological advances to broaden indications of IPDT and increase its the safety and efficacy.
Meanwhile, the development of various photodynamic systems in clinical treatments enhance the accuracy for IPDT. For example, the ordinary diffuser fiber is designed in the shape of a balloon or diffused to deliver light to the lesion area, including tracheal or esophageal tissues.84 Injectable optical devices can be implanted into deep tissue which activates the photosensitizers and induces large and deep-situated tumor damage.85 Moreover, the burgeoning pulsed laser technology offers greater specificity for the diagnosis and treatment of diverse diseases, which inspires extensive photodynamic materials as well as novel strategies.86
3. Organic Photosensitizers Used for IPDT
Cancer immunotherapy has emerged as a novel anticancer strategy. However, a tumor is generally immunosuppressive and it is usually challenging to stimulate a sufficient immune response to evoke immunotherapy.87,88 Fortunately, PDT can offset the “cool” immunosuppressive environment of tumors into a “hot” immune-active spot. Based on the excellent IPDT performance of organic PSs, which can be used as inducers for ICD or the macrophage polarization process for stimulating the immune system, they can be considered as a feasible way to achieve satisfactory anticancer efficacy.89 Until now, some available organic dyes include phthalocyanine (Pc) derivatives, boron dipyrromethene (BODIPY) derivatives, cyanine dyes, and so on. They are introduced in this section and play a pivotal role in this field.90
3.1. Transition Metal Complex
Transition metal complexes (Ru, Ir, and Re) are of increasing interest as PSs by virtue of their tunable photophysicochemical characteristics and structure flexibility to the realm of biosensing, bioimaging, and cancer therapy.91 To date, a large number of these ICD inducers are transition metal-based complexes, which give rise to an immune response against tumor cells.92 Meanwhile, the advances have also been made in terms of exploiting combinatorial IPDT strategies.
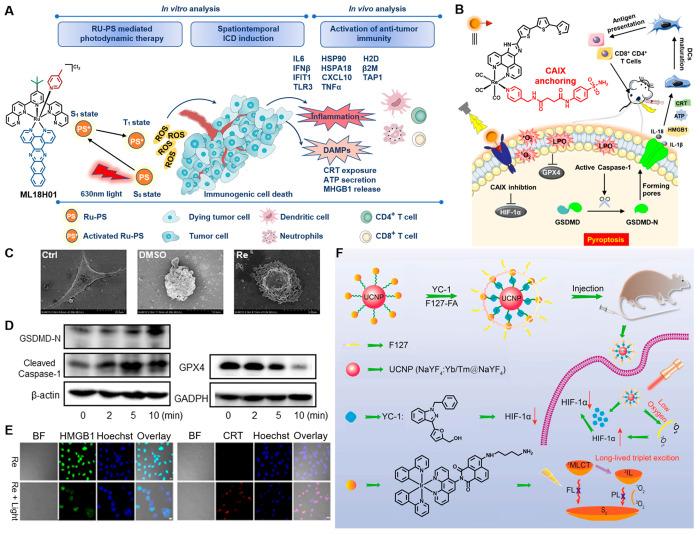
With great potential in anticancer therapeutics, Ru-based PSs are undergoing clinical trials. Meanwhile, the potential of Ru compounds as ICD inducer was also verified by researchers. The first report on the Ru-based PS for IPDT was published by Konda et al. in 2020, following activation by NIR light the PSs witnessed potent cytotoxicity and induced ICD in melanoma cells, which could establish protective antitumor immunity for cancer therapeutic (Figure 2A).93 In order to improve the efficiency of combination therapy, the optimal combination of ligands on Ru was identified in this work, achieving NIR-absorbing singlet states and lowest energy triplet states for generating ROS as much as feasible.
Figure 2.
(A) Chemical structures of the Ru complexes ML18H01 and its IPDT mechanism in vitro and in vivo. (B) Schematic diagram of the CA-Re inducing pyroptosis for IPDT. (C) Morphological features of normal and dying MDA-MB-231 cells revealed by SEM (D) GSDMD-N fragment, cleaved caspase-1 and GPX4 expression were observed by Western blot assay. (E) CLSM examination of CRT and HMGB1 in MDA-MB-231 cells. Reproduced from ref (94). Copyright 2022 Wiley-VCH. (F) Schematic illustration of the preparation procedure of UCNP@IrYCF127 and its working principle in anticancer pattern. Reproduced from ref (95). Copyright 2020 American Chemical Society.
The optimal anticancer strategy should take note of both destroying primary tumors and improving the immunogenicity of the TME to achieve a gratifying antitumor immune effect. Based on such an objective, a carbonic anhydrase IX (CAIX)-anchored Re(I) photosensitizer CA-Re was designed by Mao et al. (Figure 2B), which not only performs both type-I and type-II PDT with high efficiency phototoxicity under hypoxia, but also evokes gasdermin D (GSDMD)-mediated pyroptosis to effectively stimulate tumor immunogenicity (Figures 2C and D).94 As the first metal complex-based pyroptosis inducer, CA-Re enhanced the maturation and antigen-presenting ability of DCs by proinflammatory cytokines-induced ICD (Figure 2E), and fully activated T cells dependent anittumor immune response in vivo, eventually eliminating destroyed primary and distant tumors simultaneously.
Ir-based PSs have been recently considered as potential PSs for application in PDT, since Ir has a strong spin–orbit coupling constant, which leads Ir-based PSs easily to reach triplet excited states and more suitable for tumor ablation. Thus, Gou’s group reported a smart therapy method for effectively IPDT in the hypoxic environment (Figure 2F).95 First, a long-lived Ir PSs was developed by attaching 4[(4′-aminobutyl) amino]-1,8-naphthalimide to Ir complex, then combination with HIF-1α inhibitor (YC-1) via oleic acid-capping UCNPs. It not only enhanced the ability of light capture converts of Ir-based PSs, but converted PDT-induced tumor hypoxia into an advantage for therapy, opening up ideas to overcome the hypoxia in IPDT.
3.2. D−π–A Structure PSs
Organic PSs with the D−π–A structure were introduced by Tang and co-workers in 2001. During the two decades of development, the rapid progress of D−π–A structure PS research in tumor treatment was witnessed.96,97 Owing to intrinsic photophysical signatures, they show the advantages of high emission efficiency and photosensitivity in the aggregation state, making them excellent candidates for biological studies. Especially, therapeutic strategies that combine D−π–A structure PSs with immunotherapy are intended to suppress the side effects of traditional treatments and improve the cancer therapy effect.98
The cell membrane is composed of phospholipids, glycoproteins, glycolipids, and proteins. It protects the cells just like a “city wall” and maintains the intracellular metabolism of the cells. Once the cell membrane is damaged, the abundant cellular content and inflammatory cytokines are activated in the immunogenic reaction. Thus, Liu et al. designed a series of cell membranes with the labeling ability of AIE PSs of TBD-1C, TBD-2C, and TBD-3C, which could generate locally high concentrations of ROS and ablate various cancer tumors by the pyroptosis mechanism under light irradiation (Figure 3A).99 The authors proposed that, with the increasing numbers of cationic chains, these PSs exhibit better membrane-anchoring capability (Figure 3B). Pyroptosis promoted the immunostimulatory release of cell contents and inflammatory cytokines, which had great potential to activate antitumor immune responses. It was confirmed for the first time that the pyroptosis pathway could induce D−π–A structure PSs and play an important role in cancer cell ablation. Another cell membrane anchoring D−π–A structure PS, TBMPEI, was reported by Wang and co-workers (Figure 3C). With the capability of ablating cancer cells by the necroptosis pathway, TBMPEI enabled cell membrane rupture and DNA degradation, which endowed it with impressive performance both in vitro and in vivo.100
Figure 3.
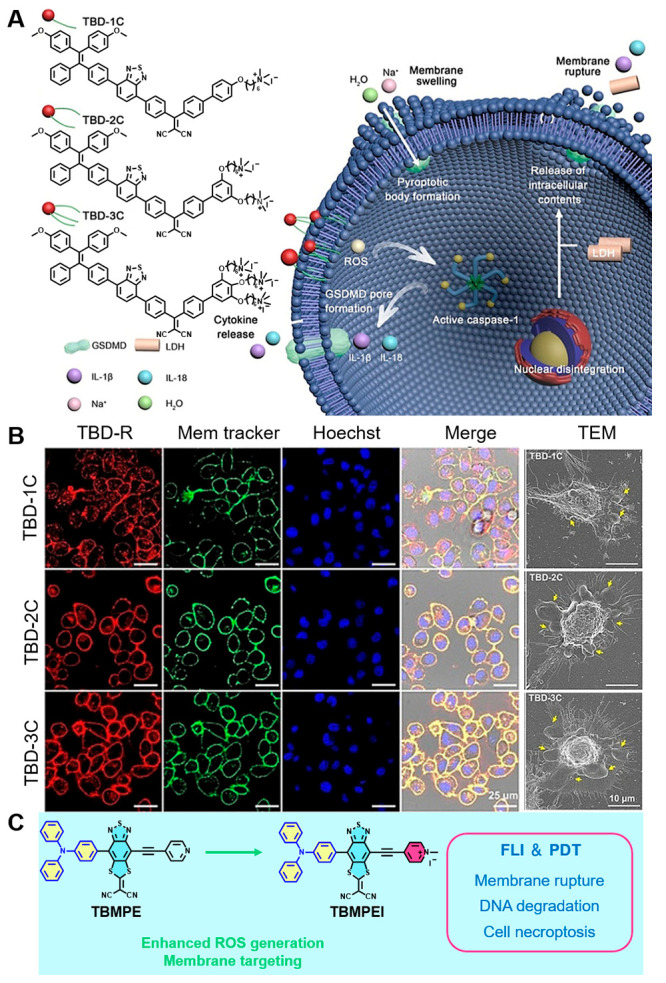
(A) Chemical structures of the PS TBD-R and illustration of its pyroptosis pathway. (B) CLSM and TEM imagines for evaluation of cell membrane localization and arousing pyroptosis. Reproduced from ref (99). Copyright 2021 Wiley-VCH. (C) Molecular design on a cell membrane targeting D−π–A structure photosensitizer for IPDT. Reproduced with permission from ref (100). Copyright 2022 [Benzhong Tang et al.], some rights reserved; exclusive licensee Royal Society of Chemistry. Distributed under a Creative Commons Attribution License 3.0 (CC BY) https://creativecommons.org/licenses/by/3.0/.
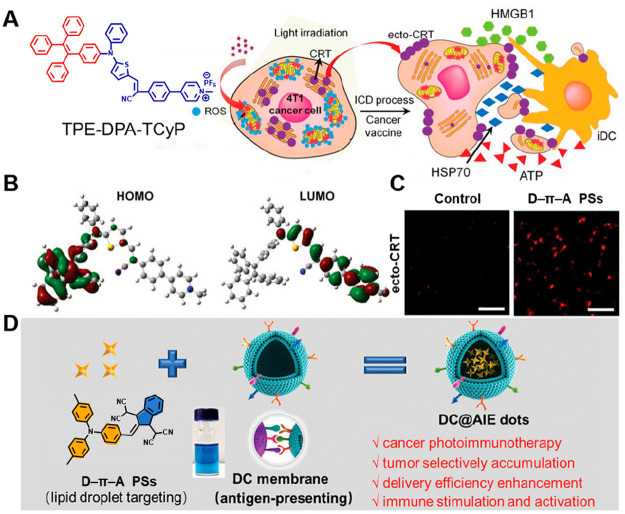
ICD provides significant theoretical principles for IPDT. Aiming for the development of a PS-based ICD inducer, Ding et al. designed a D−π–A structure PS (TPE-DPA-TCyP) (Figure 4A), with a twisted molecular structure. The HOMO of TPE-DPA-TCyP is almost entirely localized in the TPE amino group, while the LUMO is mainly distributed in its cyanostyrylthiophene core and pyridinium unit (Figure 4B).101 The effective separation of HOMO–LUMO distribution can reduce ΔEST and thus improve the ISC, which gives the specific ability for effectively causing focused mitochondrial oxidative stress and generating ecto-CRT (Figure 4C). This PS as a super ICD inducer as demonstrated using a prophylactic tumor vaccination model; meanwhile, the underlying antitumor mechanism of the TPE-DPA-TCyP was verified by immune cells analyses.
Figure 4.
(A) Chemical structures of the PSs TPE-DPA-TCyP and illustration of its ICD progress. (B) HOMO–LUMO distributions by DFT calculations of TPE-DPA-TCyP. (C) Ecto-CRT expression as shown by CLSM images. Reproduced from ref (101). Copyright 2019 Wiley-VCH. (D) Preparation and assembly of D−π–A structure photosensitizers with dendritic cell membrane coating. Reproduced from ref (102). Copyright 2021 Wiley-VCH.
During immuno-photodynamic treatment, it is one of the most critical steps that stimulates and proliferates T cells for anticancer responses, which depends on the efficient presentation of tumor antigens and the delivery of costimulatory signals by DCs. Thus, Tang et al. proposed lipid droplet-targeted AIE PSs with a D−π–A structure, coating dendritic cell membranes (DC@AIEdots) to improve antigen-presenting and hitchhiking abilities for killing the tumor cells and stimulating the immune system (Figure 4D). Notably, with the action of DC membranes, the tumor delivery efficiency of the PSs was increased from 25.12% to 39.77%. The combined effect of PDT and artificially presented antigens on T cells can effectively reduce the in situ tumor size, which also activates the immune system to inhibit the growth of both primary and distant tumors while having long-lasting effects.102
3.3. Porphyrins and Their Derivatives
Porphyrins and their derivatives are the most ubiquitously PSs used in cancer treatment, due to the long-lived triplet excited state, visible range of absorption spectrum, and efficient phototoxicity toward cancer cell porphyrins, and are popular in clinical practice. As early as the 1950s, they were used in disease diagnosis and were shown to accumulate in tumor tissues.103 Modern science introduces them into immunotherapy, giving new hope for IPDT.104,105
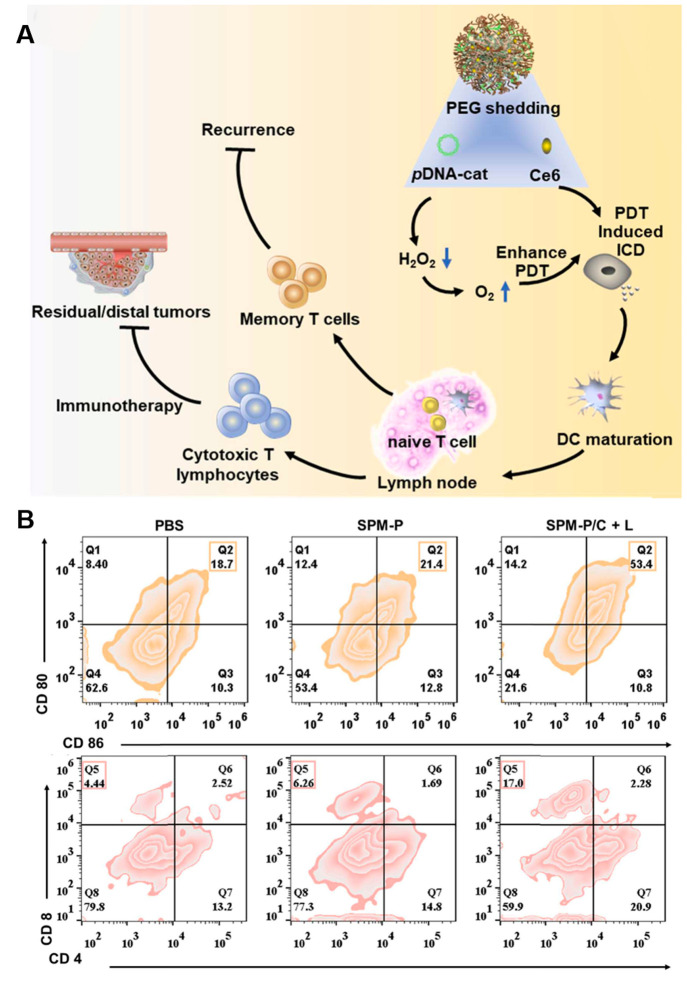
Chlorin e6 (Ce6) is a common porphyrin PS. It can rapidly generate cytotoxic ROS under red-light irradiation, thus enhancing immunogenicity, which promotes the maturation (DCs) and tumor infiltration of T cells, which helps improve the effect of tumor immunotherapy. Encouraged by this advantage, Peng et al. reported a multifunctional nanomedicine (SPM-P/C) by incorporating a plasmid DNA encoding catalase gene (pDNA-cat) and Ce6 (Figure 5A). SPM-P/C could elicit robust immunity such as DC maturation and antitumor T cell infiltration via hypoxia-relieving PDT (Figure 5B).106
Figure 5.
(A) Therapeutic mechanism of the nanomedicine SPM-P/C PSs. (B) Quantitative analysis of DC maturation and CD8+ T in mice model. Reproduced from ref (106). Copyright 2022 Elsevier Ltd.
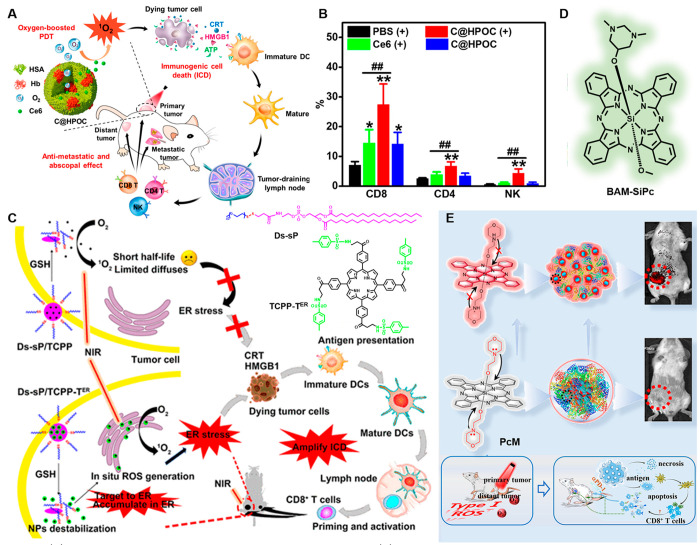
Also based on Ce6, Zheng and co-workers provided an ideal cancer therapeutic strategy to ablate primary tumors and prevent distant metastases or relapse. This research structured an oxygen self-sufficient nanocarrier by hybridizing human serum albumin with hemoglobin and then encapsulating Ce6 (C@HPOC) for IPDT (Figure 6A).107 Specifically, in the hypoxia TME, C@HPOC realized the tumor-targeted codelivery of PSs and oxygen, raising the blood oxygen level and improving the tumor hypoxic conditions. Additionally, the increased oxygen in the tumor not only improves the efficacy of PDT but also increases the infiltration of cytotoxic T lymphocytes and natural killer (NK) cells (Figure 6B); meanwhile, C@HPOC-mediated IPDT could elicit effective antimetastatic and abscopal effects in a 4T1 mTNBC murine model achieving enhanced antitumor immunity.
Figure 6.
(A) Schematic depiction of oxygen-augmented IPDT via C@HPOC. (B) Flow cytometric analysis of CD8, CD4, and NK cells in tumors. Reproduced from ref (107). Copyright 2018 American Chemical Society. (C) Schematic diagram of ER-targeting PS TCPP-TER amplifying ICD and its chemical structure. Reproduced from ref (108). Copyright 2020 American Chemical Society. (D) Chemical structure of BAM-SiPc. (E) Schematic illustration of the structure of PS PcM, the mechanism of its tumor-targeted fluorescence imaging, and the synergistic antitumor effect. Reproduced from ref (110). Copyright 2022 American Chemical Society.
As described earlier, the ER plays an essential role in maintaining the signaling function of cells. Therefore, exploring the ER targeting porphyrin agent is an optimal scheme to effectively induce IPDT. For example, Deng et al. developed an intelligent ER-targeted porphyrin and loaded it in a reduction-sensitive polymer. Under NIR light irradiation, Ds-sP NPs could induce ER stress, evoke ICD, and promote the release of DMAPs (Figure 6C).108 Additionally, the secretion of cytokines and the infiltration of CD8+ T cells in the tumor site also increased, suggesting that the combination PDT strategy can activate immune cells and improve the efficiency of immunotherapy.
In addition, as another kind of porphyrin-based compound, phthalocyanines exhibit superior photoproperties that assist them to become a very attractive class of PSs for PDT. BAM-SiPc, a silicon(IV) phthalocyanine was verified could induce immunogenic necroptosis in the tumor cells from Fong and co-workers (Figure 6D).109 Later, Yoon’s group published a report on the self-assembly of morpholine-substituted silicon phthalocyanine with albumin for fluorescence imaging and IPDT (Figure 6E). Depending on the acid-induced abolition of the photoinduced electron transfer effect and breakup of the nanostructure, the NanoPcM showed a fluorescent turn-on which realized a high signal-to-noise ratio and tumor-targeted imaging. With a superior immunogenic PDT NanoPcM that works effectively against solid tumors, the combination of NanoPcM-based PDT with αPD-1-induced immunotherapy can efficiently inhibit tumor growth, reduce spontaneous lung metastasis, and trigger abscopal effects. This study provides a perspective for the choice of PSs in the design of nanomaterials as promising phototheranostics for cancer imaging and IPDT.110
3.4. Phenothiazinium Dyes
Phenothiazine dyes are a class of cationic compounds composed of a tricyclic skeleton including Nile blue analogues and methylene blue derivatives.111,112 All of them possess favorable water solubility and display intensive absorption between 600 and 800 nm, which are increasingly utilized in cancer treatment.113
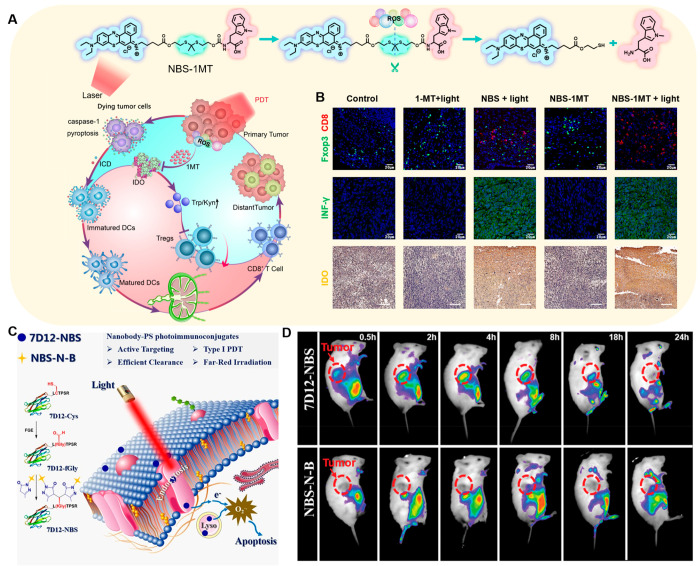
The combination of Nile blue analogues and immunotherapy using IPDT activator NBS-1MT was demonstrated by our group. NBS-1MT synthesized through a ROS sensitive linker united PSs and IDO pathway inhibitor 1MT. Upon 660 nm red laser irradiation, NBS-IMT activated caspase-1 and then removed gasdermin D (GSDMD), leading to pyroptosis to trigger ICD and further improve immunogenicity and stimulate DC maturation (Figure 7A).114 The PDT-produced ROS cut the linker releasing the 1-MT into the tumor microenvironment, which participates in the IDO pathway, reducing the accumulation of kynurenine (Kyn) to limit the activity of regulatory T cells (Tregs) and recover the antitumor function of cytotoxic T lymphocytes (CTLs). NBS-1MT achieved a pyroptosis-mediated immune reaction, which led to a significant reduction in the level of Kyn and population of Tregs in 4T1 tumor-bearing BALB/c mice (Figure 7B). Using primary and distant tumors to explore systemic antitumor immune responses, NBS-1MT could effectively inhibit tumor growth by increasing the CTLs, playing an important role in immunomodulation. Another work based on Nile blue was reported,115 in order to improve the selectivity of PSs for tumors, avoiding the side effects to nearby normal tissues. Antibodies as the active targeting ligand were developed for more precise IPDT. Conjugating Nile blue analogues to anti-EGFR nanobody 7D12 led to 7D12-NBS (Figure 7C), which displayed satisfactory active targeting ability and possessed potent phototoxicity both in normoxia and hypoxia, offering significant advantages in IPDT (Figure 7D).
Figure 7.
(A) Schematic depiction of NBS-1MT inducing pyroptosis and its chemical structure. (B) Immune cell fluorescence imaging in tumors. Reproduced from ref (114). Copyright 2021 Elsevier Ltd. (C) Proposed mechanism of 7D12-NBS for IPDT. (D) PS biodistribution in vivo. Reproduced from ref (115). Copyright 2021 Wiley-VCH.
In 2022, an engineered stem cell-based IPDT platform (MB/IL12-MSCs) incorporating immune gene plasmid IL12 (pIL12) and PS methylene blue (MB) was developed by Yang’s group to realize cancer treatment (Figure 8A). First, pIL12 and MB were loaded in magnetic mesoporous silica nanoparticles and then internalized into mesenchymal stem cells (MSCs) via magnetic targeting eventually to acquire MB/IL12-MSCs.116 The IPDT platform allowed a more sensible expression and distribution of IL12 at the tumor site, eliciting sufficient anticancer immunity. In the in vitro experiment, treatment exhibited moderate cell death when given laser irradiation, while negligible cytotoxicity was found when laser irradiation was absent. Subsequently, the in vivo therapeutic effect was further investigated and PDT efficiently induced apoptosis in the tumor tissues. Besides, tumor-localized elevation of IL12 secretion stimulated the production of IFN-γ and TNF-α, promoting the maturation of DCs and facilitating the proliferation of CD4+ and CD8+ T cells, which contribute to reversing the tumor immunosuppression and restoring the immunological function. This work offers an alternative and complementary strategy to overcome the tumor-induced immunosuppression and provide a reinforced synergistic therapy. In another example, Sun et al. developed MB-based nanomaterials which could realize self-destruction by introducing ROS-cleavable diselenide bonds for synergistic IPDT.117 Upon red-light irradiation, the ROS produced by MB-triggered matrix degradation and boosted ICD (Figure 8B). It not only cleaned the carrier materials but also elicited long-term systemic antitumor immunity. In addition, remarkable inhibition effects of abscopal tumor and lung metastasis with the help of the programmed cell death-1 (PD-1) checkpoint blockade proved that this strategy promoted photodynamic effects and reinforced immune responses.
Figure 8.
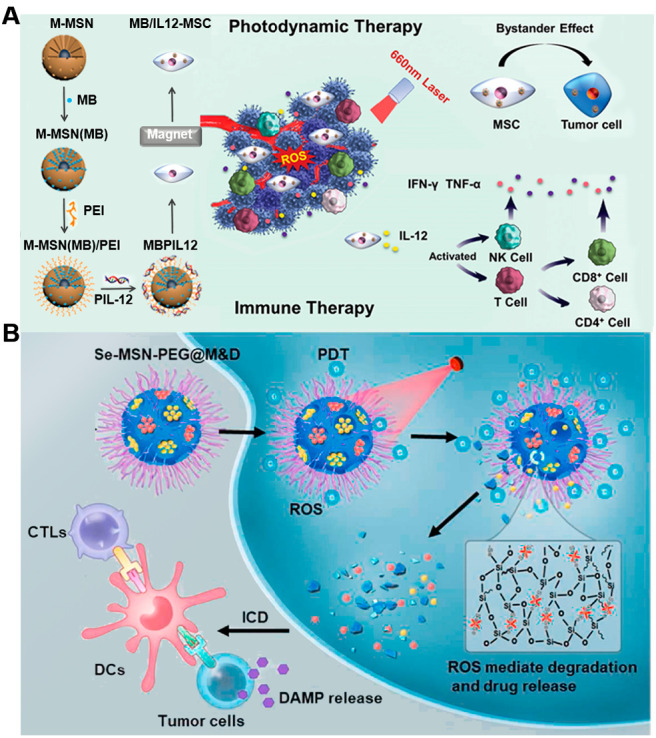
(A) Schematic diagram of the MB/IL-12-MSC for enhanced IPDT. Reproduced from ref (116). Copyright 2022 Wiley-VCH. (B) Schematic of the red-light-triggered self-destructive nanoparticles for immune cells activation and IPDT. Reproduced from ref (117). Copyright 2022 Elsevier Ltd.
3.5. BODIPY
BODIPY-based PSs proved useful in tumor treatment due to their excellent photostability and outstanding PDT efficacy. Meanwhile, some photochemical properties such as excitation wavelength, molar extinction coefficient, and 1O2 quantum yield can be adjusted in a very straightforward manner for the development of more ideal PSs to meet practical needs.118−120
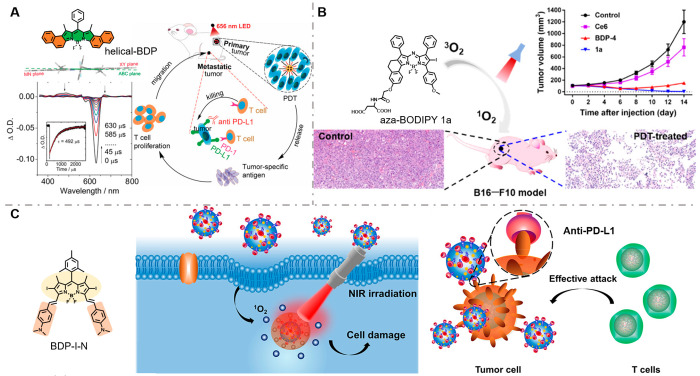
Recently, researchers have confirmed that BODIPY can also be used in IPDT. Zhao et al. developed metal/heavy atom-free BODIPY helicene PSs (helical-BDP) which handle both efficient triplet state quantum yield and long triplet excited state lifetime, thereby prolonging the reaction duration of oxygen and PSs, benefiting concomitant 1O2 generation (Figure 9A).121 This modification strategy overcomes the challenge of hypoxic TME to PDT and amplifies antitumor immunity in a record low dose (0.25 μg kg–1) with an ultralow light dose (6 J cm–2). By the joint actions of anti PD-L1, the helical-BDP mediated IPDT was verified on CT26 tumors in BALB/c mice. Except for the obvious inhibition in tumor volume, the IPDT group effectively prevented tumor metastasis with the increase of tumor-infiltrating T-cells (CD4+ CD8+) and IFN-γ. We believe that this study brings new thinking to PS molecular structural modification, promoting the progress of cancer treatment.
Figure 9.
(A) Chemical structure and side view of the RHF-optimized ground structure of helical-BDP and schematic diagram of the proposed mechanism with anti-PD-L1 to combat a tumor. Reproduced from ref (121). Copyright 2020 Wiley-VCH. (B) Chemical structure of BDP-I-N and its tumor suppressor effect in the B16-F-10 mouse model via IPDT. Reproduced from ref (124). Copyright 2022 American Chemical Society. (C) Chemical structure of the BDP-I-N and its immune efficacy for tumor cells. Reproduced from ref (125). Copyright 2021 American Chemical Society.
Another well-known BODIPY-based PS, aza-BODIPY-type PS (ADPM06), was disclosed by O’Shea and co-workers and achieved a complete cure without recurrence during 8 weeks of observations.122,123 However, poor aqueous solubility required the use of an emulsifier for effective antitumor activity, which limited its further applications in the clinic. Thus, a series of amino-acid-modified aza-BODIPY PSs to enhance the aqueous solubility were synthesized and evaluated by Zhu et al. for the treatment of melanoma (Figure 9B).124 The 1O2 generation efficiency and photodark toxicity ratio were also improved after conjugation of the amino acid in PS aza-BODIPT 1a, which were favorable for inducing ICD. This approach received extensive attention owing to the outstanding antitumor efficacies by enhancement aqueous solubility.
At present, another limitation for PSs-based IPDT is light penetration into deep tissues. The long wavelength PSs (NIR-II, 1000–1700 nm) offer high spatial resolution at tissue depths, solving technical barriers. Wang and co-workers developed a NIR-II PS based on the BODIPY parent structure; it exhibits high intensity fluorescence in the NIR-II therapy window and high 1O2 quantum yield, through self-assembly with the PD-L1 monoclonal antibody. It eliminated MC38 tumors in mice, with no tumor recurrence within a period of 40 days (Figure 9C).125
3.6. Cyanine Dyes
Cyanines dyes are the most active studied PSs for developing phototherapy because of their favorable optical properties including large absorption coefficients at 600–800 nm, while there is almost absorption in the visible light range, avoiding phototoxic side effects.126
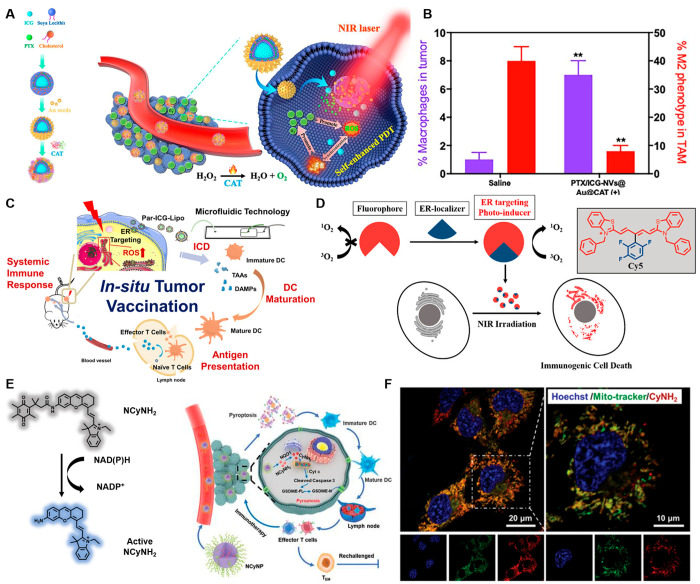
Among the currently reported cyanine-based IPDT agents, indocyanine green (ICG) is of particular interest due to its favorable biocompatibility and its approval by the FDA for clinical applications. In 2019, Gao and co-workers constructed promising oxygen generation to overcome tumor hypoxia and break the immune inhibition of tumor cells (Figure 10A). This work utilizes the photophysical process of ICG, driving the catalysis of hydrogen peroxide in the TME by gold nanoshells, which promote the polarization of tumor-associated macrophages from M2 to M1 type, removing the immune inhibition (Figure 10B).127 The multifunctional oxygen nanogenerator solved a vital issue for the limitation of M2 resulting from hypoxia. It heightened the efficacy of cancer treatment and provided a whole new perspective on IPDT.
Figure 10.
(A) Schematic diagram of the ICG nanoparticles. Reproduced from ref (127). Copyright 2019 Elsevier Ltd. (B) Macrophage infiltration and polarization within tumors. (C) Hematic illustration of antitumor immune responses induced by Par-ICG-Lipo. Reproduced from ref (128). Copyright 2022 American Chemical Society. (D) Chemical structure of the ER-targeting Cy5 and the ER localizer mediating ICD for IPDT. Reproduced from ref (129). Copyright 2022 American Chemical Society. (E) Chemical structure of the NCyNH2 and its IPDT pathway. (F) Intracellular localization of CyNH2 in 4T1 cells. Reproduced from ref (133). Copyright 2021 Wiley-VCH.
Based on the superior properties of ICG, Luo et al. reported ER-targeting Par-ICG-Lipo, loading ICG into a virus-like nonvirus nanocarrier (Par-Lipo) by a microfluidic technique with a high encapsulation efficiency for IPDT. Par-ICG-Lipo could target ER via moving along the cytoskeleton and following caveolin-mediated endocytosis, which provided a powerful treatment for triggering ER oxidative stress and inducing ICD under laser irradiation (Figure 10C).128 And then, in order to increase intratumoral DCs to maintain durable immunogenicity, immature DCs were injected into the proximal tumor as the “DC vaccines” after irradiation. As a result, the number of mature DCs was significantly increased due to the injection of DC vaccines in the Par-PDT+DC group, indicating that this approach could improve the immune cell infiltration. Meanwhile, the antigen-presenting capacity of DCs in lymph nodes also improved obviously, which was beneficial for the activation of the immune system, inhibited the growth of distant tumors significantly, and supported the validation of a tumor rechallenge experiment. It suggested that ER-targeting IPDT could provide a memorable systemic immune response.
Encouraged by the idea that ER stress was responsible for efficient ICD, Peng et al. were devoted to seek ER-localizable PSs for IPDT. The thio-pentamethine cyanine dye (TCy5) as a classic imaging chromophore was favorable among PSs because of its good water solubility and versatile chemical structure. When the polyfluorophenyl was modified on the meso-position of TCy5, it not only targets PSs to ER, but takes an electron-withdrawing group to enhance the ROS generation ability (Figure 10D). To induce cell death mode, the ICD could be divided into two categories: Type I, which involves non-ER target cell damage; Type II, which acts directly on the ER and triggers associated immunogenicity via ER stress. Under mild NIR irradiation, the PS TCy5-Ph-3 evoked ER stress, which could give rise to mature DCs and further recruit CTLs to accomplish IPDT. These results confer practical applicability in IPDT and provide a guideline for inducing ICD, which also enables the expansion of organic molecule applications for cancer immunotherapy.129
As a subclass of cyanine dyes, hemicyanine dyes preserve the excellent photophysical properties of cyanines while enhancing the light stability via the rigid conjugate structure.130−132 Yuan and co-workers synthesized a NIR hemicyanine PS (CyNH2) that selectively induced cancer cells pyrolysis for boosting antitumor immunity by recognition of the quinone oxidoreductase isozyme 1 (NQO1) (Figure 10E). The PS was also loaded in PEG-b-PLGA, forming NCyNP for systemic and real-time administration (Figure 10F). Finally, the NCyNPs were synergized with αPD1 to further enhance the antitumor effect.133
3.7. Organic Semiconducting Photosensitizers
Efficient PSs are highly desirable in photoactivated disease diagnosis and treatment, which is beneficial not only to generate ROS effectively killing cancer cells but also to induce more sufficient ICD to accelerate the development of antitumor immunotherapy.134 As we know, the 1O2 yield is considered as one of the most crucial indicators in the process of photosensitization, which can be controlled by adjusting the ISC for better performance in cancer treatment.135 Organic semiconducting polymers have been found to have an advantage in promoting the ISC process, which exhibits a higher 1O2 generation efficiency and stronger light-harvesting ability than small-molecular analogues do. In this section, several cases of organic semiconducting polymers were exemplified owing to their splendid cancer treatment ability.
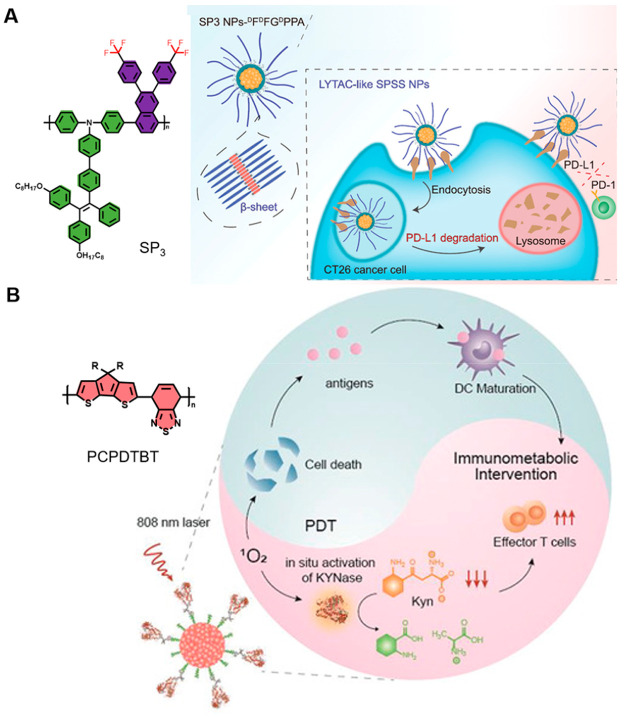
In 2018, Tang’s group put forward two efficient strategies to enhance the photosensitization efficiency: (1) polymerization-facilitated photosensitization; (2) the D–A even–odd effect.136 Based on this, Ding et al. constructed a new kind of semiconducting polymer (SPSS NPs) which worked along double lines, enhanced the photosensitization by organic semiconducting polymer SP3, and activated cytotoxic T lymphocytes to amplify immunotherapy by β-sheet protein degrading PD-L1 (Figure 11A).137 Unlike traditional ICB therapy, this brand-new approach avoiding some potential problems such as high cost, difficulty for storage, high risk of immunogenicity, and short half-life in biological systems further guarantees its application in IPDT. The experimental results indicated that SPSS NPs can not only produce high-level ROS but also elicit strong ICD to release DAMPs, which could evoke long-term immunological memory against tumor rechallenge. This work offers a new idea by combining well-designed photosensitizer-based ICD induction and proteins, rendering great promise for cancer immunotherapy.
Figure 11.
(A) Schematic illustration of SPSS NPs for self-synergistic cancer immunotherapy and its chemical structure. Reproduced from ref (137). Copyright 2022 Wiley-VCH. (B) Chemical structure of PCPDTBT and the potential IPDT process of SPNK. Reproduced from ref (138). Copyright 2021 Wiley-VCH.
In addition, a different IPDT modality “immunometabolic photodynamic therapy” triggered by organic semiconducting polymers was proposed by Pu and co-workers. This approach aims to modulate the cancer metabolic pathway by releasing immunotherapeutic drugs that bond with specific targets to promote the proliferation of effector T cells and suppress immune escape, which could minimize side effects like immune-related adverse events (Figure 11B).138 Thus, a KYNase-conjugated semiconducting polymer nanozyme (SPNK) was designed for IPDT. SPNK consisted of a NIR absorbing semiconducting polymer and hydrophilic PEG conjugated with KYNase via a singlet oxygen cleavable linkage. Upon 808 nm laser irradiation, SPNK could generate 1O2 to cleave the linkage between KYNase and PEG, releasing KYNase which could further degrade Kyn and improving the proliferation of effector T cells.
4. Conclusion and Perspective
Cancer immunotherapy has great potential for the development of tumor therapy. However, problems such as low response rates and immune-related adverse effects limit its clinical application. One popular method is PDT, which has emerged as an excellent tool to complement immunotherapies for a more efficacious cancer treatment. In this Perspective, we have provided insights on the close links between PDT and immunotherapy while focusing on the character of organic PSs and their cascade reaction in TME. We always believe that the structure and function of PSs occupied the main position in IPDT, which not only affects the degree of tumor ablation but also stimulates the body’s immune response. In recent years, several ideal IPDT means have been developed by targeting the immunogenic organelles or reprogramming the TME, such as PDT cooperation with an immunologic adjuvant, immune checkpoint blockade, and development of DC vaccine. Despite many systematic investigations and promising outcomes, the development of IPDT is still in its nascent and proof of concept stages. When it advances into the clinic, there will still be some challenges need to be resolved. First, the penetration depth of light is so limited that IPDT cannot play a full role for tumors located in situi. Thus, potential operating procedures and optical delivery systems include but are not limited to laser optical devices, flexible and deformable optical devices, injectable optical devices, pulsed lasers, and so on. They are expected to be developed for clinical application scopes of IPDT from skin carcinoma to organ cancer. Second, insufficient resistance to photobleaching discounts the PDT effects while the structure and cytotoxicity of the photolysis products may decide its clinical effect. Therefore, improving the photoconversion performance of proven PSs is still an inevitable requirement for perfecting IPDT. Meanwhile, mass production and safe application of diverse PSs are still great challenges. Third, the in vivo biocompatibility, biodistribution, and long-term toxicity of these IPDT drugs also need to be systemically assessed before their clinical translation. The detail mechanism of combination therapy is also not completely clear, and the interaction of multiple components is difficult to fully explain in a temporal and spatial sequence. Finally, preclinical tumor models are urgently needed to evaluate the therapeutic index of deep PDT. Current research is mostly based on mouse models which is not enough to provide clear guidelines for clinical treatment. Further patient derived xenograft experimental models should be established, and this will gradually drive IPDT from the laboratory to the clinic. In the future, more work is needed to understand the tracer dynamic immune response pathway contribution to the specific impact of each component in the TME, to achieve the goal of providing an accurate approach for patients.
The IPDT stimulateed immune response against tumors is usually an extremely complicated process, which needs the full cooperation of multiple pathways. The absence of any step will weaken the antitumor effect. Therefore, we propose several possible directions for its future developments. (1) Organic NIR PSs with advanced functions will become an active area for thorough eradication of tumors, such as specific targeting, improved tissue penetration, or less oxygen-dependent properties. Future research should look more forward to shift research modes from molecular screening to PS intelligent management. It requires extending the pool of PSs to control and use the high-quality PSs, which could open up innovative and exciting possibilities. (2) The induction of ICD and timing of immunotherapies should be investigated in more detail which will have a profound impact on the therapeutic efficacy. There is still a need for suitable ICD induction strategies for general and practical clinical use in the future. (3) For combined treatment, immune-related adverse events caused by immune drug abuse should be prevented. A new idea is breaking the growth balance of tumors by adjusting the homeostasis of TME (oxygen, pH, capillaries, and interstitial pressure) for suppressing tumors. The systematic study of the collaborative effects between PDT and immunological therapy is crucial for further optimizing therapeutic efficiency. This may be instructive for the reasonable use of the tumor immune pathway, rather than randomly combining some treatment strategies. (4) One should highlight the single agent efficacy and explore multifunctional PSs to realize tumor ablation and immunotherapy integration. Comparing with nanoparticles, the single molecule agent is more beneficial to realize the mass production. (5) Cell and gene therapy modulates other types of immune cells, such as NK cells and B cells, which provides additional channels with more targets and longer-lasting efficacy for cancer immunotherapy.
In general, the PSs as the core of IPDT show great promise for precise tumor treatment for future clinic practices. This Perspective provides the substantial progress made in this field in recent years as well as the opportunities and challenges faced. Additionally, some efforts may be made to explore their feasibility for treatments of other diseases such as Alzheimer’s disease, thromboembolism, and atherosclerotic plaque. We believe that there will be great progress in organic PS-based precise IPDT in curing tumors through the joint efforts of scientific researchers and clinicians.
Acknowledgments
National Natural Science Foundation of China (Project No. 22090011 and U1908202) and the Fundamental Research Funds for the Central Universities of China (DUT22LAB608).
Author Contributions
Y.L., W.S., J.J.D., J.L.F., and X.J.P. wrote and revised the paper and the reference format. CRediT: Yang Lu conceptualization, software, writing-original draft; Wen Sun supervision, writing-original draft; Jianjun Du supervision, writing-original draft; Jiangli Fan resources, supervision, writing-original draft; Xiaojun Peng conceptualization, resources, supervision, writing-original draft.
The authors declare no competing financial interest.
References
- Alexandrov L. B.; Kim J.; Haradhvala N. J.; Huang M. N.; Tian Ng A. W.; Wu Y.; Boot A.; Covington K. R.; Gordenin D. A.; Bergstrom E. N.; Islam S. M. A.; Lopez-Bigas N.; Klimczak L. J.; McPherson J. R.; Morganella S.; Sabarinathan R.; Wheeler D. A.; Mustonen V.; Getz G.; Rozen S. G.; Stratton M. R. The repertoire of mutational signatures in human cancer. Nature 2020, 578, 94–101. 10.1038/s41586-020-1943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani S.; Varma R. S. MXenes for Cancer Therapy and Diagnosis: Recent Advances and Current Challenges. ACS Biomater. Sci. Eng. 2021, 7, 1900–1913. 10.1021/acsbiomaterials.0c01763. [DOI] [PubMed] [Google Scholar]
- Gu J.; Huang Y.; Yan Z.; He D.; Zhang Y.; Xu J.; Li Y.; Xie X.; Xie J.; Shi D.; Abagyan R.; Zhang J.; Tan Q. Biomimetic Membrane-Structured Nanovesicles Carrying a Supramolecular Enzyme to Cure Lung Cancer. ACS Appl. Mater. Interfaces 2020, 12, 31112–31123. 10.1021/acsami.0c06207. [DOI] [PubMed] [Google Scholar]
- Hwang J.; An E.; Kim S.; Zhang W.; Jin J. Escherichia coli Mimetic Gold Nanorod-Mediated Photo- and Immunotherapy for Treating Cancer and Its Metastasis. ACS Nano 2022, 16, 8472–8483. 10.1021/acsnano.2c03379. [DOI] [PubMed] [Google Scholar]
- Anand S.; Chan T.; Hasan T.; Maytin E. Current Prospects for Treatment of Solid Tumors via Photodynamic, Photothermal, or Ionizing Radiation Therapies Combined with Immune Checkpoint Inhibition (A Review). Pharmaceuticals 2021, 14, 447. 10.3390/ph14050447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B. V.; Connors T. J.; Farber D. L. Human T Cell Development, Localization, and Function throughout Life. Immunity (Cambridge, Mass.) 2018, 48, 202–213. 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiak M.; Lysenko L.; Gerber H.; Nowak R. Cell reactions and immune responses to photodynamic therapy in oncology. Postepy Hig Med. Dosw (Online) 2016, 70, 735–742. 10.5604/17322693.1208196. [DOI] [PubMed] [Google Scholar]
- Han X.; Li H.; Zhou D.; Chen Z.; Gu Z. Local and Targeted Delivery of Immune Checkpoint Blockade Therapeutics. Acc. Chem. Res. 2020, 53, 2521–2533. 10.1021/acs.accounts.0c00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P.; Wang Y.; Yao Z.; Gao X.; Liu C.; Wang X.; Wu H.; Ding X.; Hu J.; Lin B.; Li Q.; Li M.; Li X.; Chen X.; Qi W.; Li W.; Xue J.; Xu H. Enhanced Safety and Antitumor Efficacy of Switchable Dual Chimeric Antigen Receptor-Engineered T Cells against Solid Tumors through a Synthetic Bifunctional PD-L1-Blocking Peptide. J. Am. Chem. Soc. 2020, 142, 18874–18885. 10.1021/jacs.0c08538. [DOI] [PubMed] [Google Scholar]
- Lin M. J.; Svensson-Arvelund J.; Lubitz G. S.; Marabelle A.; Melero I.; Brown B. D.; Brody J. D. Cancer vaccines: the next immunotherapy frontier. Nature Cancer 2022, 3, 911–926. 10.1038/s43018-022-00418-6. [DOI] [PubMed] [Google Scholar]
- Das R.; Hardie J.; Joshi B. P.; Zhang X.; Gupta A.; Luther D. C.; Fedeli S.; Farkas M. E.; Rotello V. M. Macrophage-Encapsulated Bioorthogonal Nanozymes for Targeting Cancer Cells. JACS Au 2022, 2, 1679–1685. 10.1021/jacsau.2c00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.; Zhang Z.; Liu Q.; Zhao Y.; Zheng C.; Hao J.; Yi K.; Wang Y.; Wang C.; Zhao X.; Shi L.; Kang C.; Liu Y. Multifunctional Nanomodulators Regulate Multiple Pathways To Enhance Antitumor Immunity. ACS Appl. Mater. Interfaces 2020, 3, 4635–4642. 10.1021/acsabm.0c00513. [DOI] [PubMed] [Google Scholar]
- Han X.; Cheng K.; Xu Y.; Wang Y.; Min H.; Zhang Y.; Zhao X.; Zhao R.; Anderson G. J.; Ren L.; Nie G.; Li Y. Modularly Designed Peptide Nanoprodrug Augments Antitumor Immunity of PD-L1 Checkpoint Blockade by Targeting Indoleamine 2,3-Dioxygenase. J. Am. Chem. Soc. 2020, 142, 2490–2496. 10.1021/jacs.9b12232. [DOI] [PubMed] [Google Scholar]
- Chin A. L.; Jiang S.; Jang E.; Niu L.; Li L.; Jia X.; Tong R. Implantable optical fibers for immunotherapeutics delivery and tumor impedance measurement. Nat. Commun. 2021, 12, 5138. 10.1038/s41467-021-25391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X.; Chan C.; Guo N.; Han W.; Weichselbaum R. R.; Lin W. Photodynamic Therapy Mediated by Nontoxic Core–Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. 10.1021/jacs.6b09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z.; Tang J.; Gu Z.; Wang Y.; Yang Y.; Yang Y.; Yu C. Eliciting Immunogenic Cell Death via a Unitized Nanoinducer. Nano Lett. 2020, 20, 6246–6254. 10.1021/acs.nanolett.0c00713. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Zhao Y.; Ma H.; Sun Y.; Cao J. How to improve photodynamic therapy-induced antitumor immunity for cancer treatment?. Theranostics 2022, 12, 4629–4655. 10.7150/thno.72465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger A.; Brandhonneur N.; Molard Y.; Cordier S.; Kowouvi K.; Amela-Cortes M.; Dollo G. From molecules to nanovectors: Current state of the art and applications of photosensitizers in photodynamic therapy. Int. J. Pharmaceut. 2021, 604, 120763. 10.1016/j.ijpharm.2021.120763. [DOI] [PubMed] [Google Scholar]
- Mardi A.; Shirokova A. V.; Mohammed R. N.; Keshavarz A.; Zekiy A. O.; Thangavelu L.; Mohamad T. A. M.; Marofi F.; Shomali N.; Zamani A.; Akbari M. Biological causes of immunogenic cancer cell death (ICD) and anti-tumor therapy; Combination of Oncolytic virus-based immunotherapy and CAR T-cell therapy for ICD induction. Cancer Cell Int. 2022, 22, 168. 10.1186/s12935-022-02585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Hua P.; He C.; Chen M. Non-apoptotic cell death-based cancer therapy: Molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharmaceutica Sinica B 2022, 12, 3567–3593. 10.1016/j.apsb.2022.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X.; Chan C.; Lin W. Durch Nanopartikel vermittelter immunogener Zelltod ermöglicht und verstärkt die Immuntherapie gegen Krebs. Angew. Chem., Int. Ed. 2019, 131, 680–691. 10.1002/ange.201804882. [DOI] [Google Scholar]
- Gong H.; Chao Y.; Xiang J.; Han X.; Song G.; Feng L.; Liu J.; Yang G.; Chen Q.; Liu Z. Hyaluronidase To Enhance Nanoparticle-Based Photodynamic Tumor Therapy. Nano Lett. 2016, 16, 2512–2521. 10.1021/acs.nanolett.6b00068. [DOI] [PubMed] [Google Scholar]
- Li X.; Lee S.; Yoon J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. 10.1039/C7CS00594F. [DOI] [PubMed] [Google Scholar]
- Li X.; Lovell J. F.; Yoon J.; Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nature reviews. Clinical oncology 2020, 17, 657–674. 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- Li X.; Kwon N.; Guo T.; Liu Z.; Yoon J. Innovative Strategien für die photodynamische Therapie hypoxischer Tumore. Angew. Chem., Int. Ed. 2018, 130, 11694–11704. 10.1002/ange.201805138. [DOI] [Google Scholar]
- Wang M.; Rao J.; Wang M.; Li X.; Liu K.; Naylor M. F.; Nordquist R. E.; Chen W. R.; Zhou F. Cancer photo-immunotherapy: from bench to bedside. Theranostics 2021, 11, 2218–2231. 10.7150/thno.53056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán Hernández I.; Yu Y.; Ossendorp F.; Korbelik M.; Oliveira S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. Journal of Clinical Medicine 2020, 9, 333. 10.3390/jcm9020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.; Lei S.; Pan K.; Chen T.; Lin J.; Ni G.; Liu J.; Zeng X.; Chen Q.; Dan H. Application of photodynamic therapy in immune-related diseases. Photodiagn. Photodyn. 2021, 34, 102318. 10.1016/j.pdpdt.2021.102318. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Chen M.; Liu Z. Local biomaterials-assisted cancer immunotherapy to trigger systemic antitumor responses. Chem. Soc. Rev. 2019, 48, 5506–5526. 10.1039/C9CS00271E. [DOI] [PubMed] [Google Scholar]
- Li H.; Kim D.; Yao Q.; Ge H.; Chung J.; Fan J.; Wang J.; Peng X.; Yoon J. Activity-Based NIR Enzyme Fluorescent Probes for the Diagnosis of Tumors and Image-Guided Surgery. Angew. Chem., Int. Ed. 2021, 60, 17268–17289. 10.1002/anie.202009796. [DOI] [PubMed] [Google Scholar]
- Wang R.; Li X.; Yoon J. Organelle-Targeted Photosensitizers for Precision Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 19543–19571. 10.1021/acsami.1c02019. [DOI] [PubMed] [Google Scholar]
- Nguyen V.; Yan Y.; Zhao J.; Yoon J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. 10.1021/acs.accounts.0c00606. [DOI] [PubMed] [Google Scholar]
- Ng C. W.; Li J.; Pu K. Recent Progresses in Phototherapy-Synergized Cancer Immunotherapy. Adv. Funct. Mater. 2018, 28, 1804688. 10.1002/adfm.201804688. [DOI] [Google Scholar]
- Zhao X.; Liu J.; Fan J.; Chao H.; Peng X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. 10.1039/D0CS00173B. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Song J.; Nie L.; Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. 10.1039/C6CS00271D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Lei Q.; Zhang X. Recent advances in photonanomedicines for enhanced cancer photodynamic therapy. Prog. Mater. Sci. 2020, 114, 100685. 10.1016/j.pmatsci.2020.100685. [DOI] [Google Scholar]
- Kroemer G.; Galassi C.; Zitvogel L.; Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022, 23, 487–500. 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
- Ahmed A.; Tait S. W. G. Targeting immunogenic cell death in cancer. Molecular oncology 2020, 14, 2994–3006. 10.1002/1878-0261.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Lai X.; Fu S.; Ren L.; Cai H.; Zhang H.; Gu Z.; Ma X.; Luo K. Immunogenic Cell Death Activates the Tumor Immune Microenvironment to Boost the Immunotherapy Efficiency. Adv. Sci. 2022, 9, 2201734. 10.1002/advs.202201734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S.; Hufnagel S.; Maier E. Y.; Aguilar I.; Selvakumar J.; DeVore J. E.; Lynch V. M.; Arumugam K.; Cui Z.; Sessler J. L.; Arambula J. F. Rationally Designed Redox-Active Au(I) N-Heterocyclic Carbene: An Immunogenic Cell Death Inducer. J. Am. Chem. Soc. 2020, 142, 20536–20541. 10.1021/jacs.0c09753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W.; Dong W.; Li M.; Guo Z.; Wang Q.; Liu Y.; Bi Y.; Zhou H.; Wang Y. Nitric Oxide Induces Immunogenic Cell Death and Potentiates Cancer Immunotherapy. ACS Nano 2022, 16, 3881–3894. 10.1021/acsnano.1c09048. [DOI] [PubMed] [Google Scholar]
- Tan J.; Ding B.; Zheng P.; Chen H.; Ma P.; Lin J. Hollow Aluminum Hydroxide Modified Silica Nanoadjuvants with Amplified Immunotherapy Effects through Immunogenic Cell Death Induction and Antigen Release. Small 2022, 18, 2202462. 10.1002/smll.202202462. [DOI] [PubMed] [Google Scholar]
- Wang F.; Liu J.; Hong X.; Tan C.; Zhang L.; Chen W.; Sadler P. J.; Mao Z. Anion-Responsive Manganese Porphyrin Facilitates Chloride Transport and Induces Immunogenic Cell Death. CCS Chemistry 2022, 4, 2409–2419. 10.31635/ccschem.021.202101212. [DOI] [Google Scholar]
- Fan Y.; Kuai R.; Xu Y.; Ochyl L. J.; Irvine D. J.; Moon J. J. Immunogenic Cell Death Amplified by Co-localized Adjuvant Delivery for Cancer Immunotherapy. Nano Lett. 2017, 17, 7387–7393. 10.1021/acs.nanolett.7b03218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musetti S.; Huang L. Nanoparticle-Mediated Remodeling of the Tumor Microenvironment to Enhance Immunotherapy. ACS Nano 2018, 12, 11740–11755. 10.1021/acsnano.8b05893. [DOI] [PubMed] [Google Scholar]
- Xiong W.; Qi L.; Jiang N.; Zhao Q.; Chen L.; Jiang X.; Li Y.; Zhou Z.; Shen J. Metformin Liposome-Mediated PD-L1 Downregulation for Amplifying the Photodynamic Immunotherapy Efficacy. ACS Appl. Mater. Interfaces 2021, 13, 8026–8041. 10.1021/acsami.0c21743. [DOI] [PubMed] [Google Scholar]
- Wang T.; Zhang H.; Han Y.; Liu H.; Ren F.; Zeng J.; Sun Q.; Li Z.; Gao M. Light-Enhanced O2-Evolving Nanoparticles Boost Photodynamic Therapy To Elicit Antitumor Immunity. ACS Appl. Mater. Interfaces 2019, 11, 16367–16379. 10.1021/acsami.9b03541. [DOI] [PubMed] [Google Scholar]
- Lin B.; Liu J.; Wang Y.; Yang F.; Huang L.; Lv R. Enhanced Upconversion Luminescence-Guided Synergistic Antitumor Therapy Based on Photodynamic Therapy and Immune Checkpoint Blockade. Chem. Mater. 2020, 32, 4627–4640. 10.1021/acs.chemmater.0c01031. [DOI] [Google Scholar]
- Li X.; Zhen Y.; Li S. Review on combination strategy of immune checkpoint blockade, photodynamic therapy and nanomedicine against solid tumor. Materials & Design 2021, 209, 109958. 10.1016/j.matdes.2021.109958. [DOI] [Google Scholar]
- Murciano-Goroff Y. R.; Warner A. B.; Wolchok J. D. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovedi S. J.; Adlard A. L.; Lipowska-Bhalla G.; McKenna C.; Jones S.; Cheadle E. J.; Stratford I. J.; Poon E.; Morrow M.; Stewart R.; Jones H.; Wilkinson R. W.; Honeychurch J.; Illidge T. M. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014, 74, 5458–5468. 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- Ngwa W.; Irabor O. C.; Schoenfeld J. D.; Hesser J.; Demaria S.; Formenti S. C. Using Immunotherapy to Boost the Abscopal Effect. Nat. Rev. Cancer 2018, 18 (5), 313–322. 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. P.; Zheng R. R.; Kong R. J.; Huang C. Y.; Rao X. N.; Yang N.; Chen A. L.; Yu X. Y.; Cheng H.; Li S. Y. Self-Delivery Ternary Bioregulators for Photodynamic Amplified Immunotherapy by Tumor Microenvironment Reprogramming. ACS Nano 2022, 16, 1182–1197. 10.1021/acsnano.1c08978. [DOI] [PubMed] [Google Scholar]
- Liu J.; Liew S. S.; Wang J.; Pu K. Bioinspired and Biomimetic Delivery Platforms for Cancer Vaccines. Adv. Mater. 2022, 34, 2103790. 10.1002/adma.202103790. [DOI] [PubMed] [Google Scholar]
- Banchereau J.; Palucka K. Cancer Vaccines on the Move. Nat. Rev. Clin. Oncol. 2018, 15, 9–10. 10.1038/nrclinonc.2017.149. [DOI] [PubMed] [Google Scholar]
- Krysko D. V.; Garg A. D.; Kaczmarek A.; Krysko O.; Agostinis P.; Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- Legrand A. J.; Konstantinou M.; Goode E. F.; Meier P. The Diversification of Cell Death and Immunity: Memento Mori. Mol. Cell 2019, 76, 232–242. 10.1016/j.molcel.2019.09.006. [DOI] [PubMed] [Google Scholar]
- Ma D.; Bian H.; Long S.; Zhou P.; Tian R.; Wu Y.; Ge H.; Li M.; Du J.; Fan J.; Zhang Y.; Peng X. Se-sensitized NIR hot band absorption photosensitizer for anti-Stokes excitation deep photodynamic therapy. Sci. China Chem. 2022, 65, 563–573. 10.1007/s11426-021-1179-7. [DOI] [Google Scholar]
- Wu Q.; Chen Y.; Li Q.; Chen J.; Mo J.; Jin M.; Yang Q.; Rizzello L.; Tian X.; Luo L. Time Rules the Efficacy of Immune Checkpoint Inhibitors in Photodynamic Therapy. Adv. Sci. 2022, 9, 2200999. 10.1002/advs.202200999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Zhang Y.; Gao Y.; Wang P.; He G.; Blum N. T.; Lin J.; Liu Q.; Wang X.; Huang P. Six Birds with One Stone: Versatile Nanoporphyrin for Single-Laser-Triggered Synergistic Phototheranostics and Robust Immune Activation. Adv. Mater. 2020, 32, 2004481. 10.1002/adma.202004481. [DOI] [PubMed] [Google Scholar]
- Meng Z.; Zhou X.; Xu J.; Han X.; Dong Z.; Wang H.; Zhang Y.; She J.; Xu L.; Wang C.; Liu Z. Light-Triggered In Situ Gelation to Enable Robust Photodynamic-Immunotherapy by Repeated Stimulations. Adv. Mater. 2019, 1900927. 10.1002/adma.201900927. [DOI] [PubMed] [Google Scholar]
- Li J.; Gao H.; Liu R.; Chen C.; Zeng S.; Liu Q.; Ding D. Endoplasmic reticulum targeted AIE bioprobe as a highly efficient inducer of immunogenic cell death. Sci. China Chem. 2020, 63, 1428–1434. 10.1007/s11426-020-9846-4. [DOI] [Google Scholar]
- Donohoe C.; Senge M. O.; Arnaut L. G.; Gomes-da-Silva L. C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2019, 1872, 188308. 10.1016/j.bbcan.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Yao C.; Li Y.; Wang Z.; Song C.; Hu X.; Liu S. Cytosolic NQO1 Enzyme-Activated Near-Infrared Fluorescence Imaging and Photodynamic Therapy with Polymeric Vesicles. ACS Nano 2020, 14, 1919–1935. 10.1021/acsnano.9b08285. [DOI] [PubMed] [Google Scholar]
- Xu J.; Xu L.; Wang C.; Yang R.; Zhuang Q.; Han X.; Dong Z.; Zhu W.; Peng R.; Liu Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 11, 4463–4474. 10.1021/acsnano.7b00715. [DOI] [PubMed] [Google Scholar]
- Matsuoka K.; Yamada M.; Sato M.; Sato K. Near-Infrared Photoimmunotherapy for Thoracic Cancers: A Translational Perspective. Biomedicines 2022, 10, 1662. 10.3390/biomedicines10071662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Chen H.; Wang B.; Luo Y.; Yang G.; Zhang S.; Li S. Triarylboron-Based High Photosensitive Probes for Apoptosis Detection, Tumor-Targeted Imaging, and Selectively Inducing Apoptosis of Tumor Cells by Photodynamics. Anal. Chem. 2022, 94, 8483–8488. 10.1021/acs.analchem.2c01364. [DOI] [PubMed] [Google Scholar]
- Nath S.; Obaid G.; Hasan T. The course of immune stimulation by photodynamic therapy: bridging fundamentals of photochemically induced immunogenic cell death to the enrichment of T-cell repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. 10.1111/php.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; Jin X.; Gao M.; Zhang Y.; Tang B. Z. A Self-Reporting Fluorescent Salicylaldehyde–Chlorambucil Conjugate as a Type-II ICD Inducer for Cancer Vaccines. Adv. Mater. 2022, 34, 2205701. 10.1002/adma.202205701. [DOI] [PubMed] [Google Scholar]
- Garg A. D.; Galluzzi L.; Apetoh L.; Baert T.; Birge R. B.; Bravo-San Pedro J. M.; Breckpot K.; Brough D.; Chaurio R.; Cirone M.; Coosemans A.; Coulie P. G.; De Ruysscher D.; Dini L.; de Witte P.; Dudek-Peric A. M.; Faggioni A.; Fucikova J.; Gaipl U. S.; Golab J.; Gougeon M.; Hamblin M. R.; Hemminki A.; Herrmann M.; Hodge J. W.; Kepp O.; Kroemer G.; Krysko D. V.; Land W. G.; Madeo F.; Manfredi A. A.; Mattarollo S. R.; Maueroder C.; Merendino N.; Multhoff G.; Pabst T.; Ricci J.; Riganti C.; Romano E.; Rufo N.; Smyth M. J.; Sonnemann J.; Spisek R.; Stagg J.; Vacchelli E.; Vandenabeele P.; Vandenberk L.; Van den Eynde B. J.; Van Gool S.; Velotti F.; Zitvogel L.; Agostinis P. Molecular and Translational Classifications of DAMPs in Immunogenic Cell Death. Frontiers in Immunology 2015, 6, 588. 10.3389/fimmu.2015.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.; Gao M.; Xing H.; Du Z.; Wu Z.; Liu J.; Li T.; Cao J.; Yang X.; Li R.; Wang W.; Wang J.; Luo S. Rationally Designed Heptamethine Cyanine Photosensitizers that Amplify Tumor-Specific Endoplasmic Reticulum Stress and Boost Antitumor Immunity. Small 2022, 18, 2202728. 10.1002/smll.202202728. [DOI] [PubMed] [Google Scholar]
- Shi H.; Kwok R. T. K.; Liu J.; Xing B.; Tang B. Z.; Liu B. Real-Time Monitoring of Cell Apoptosis and Drug Screening Using Fluorescent Light-Up Probe with Aggregation-Induced Emission Characteristics. J. Am. Chem. Soc. 2012, 134, 17972–17981. 10.1021/ja3064588. [DOI] [PubMed] [Google Scholar]
- Grootjans S.; Vanden Berghe T.; Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 2017, 24, 1184–1195. 10.1038/cdd.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinlich R.; Oberst A.; Beere H. M.; Green D. R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 2017, 18 (2), 127–136. 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]
- Kamiya M.; Mizoguchi F.; Kawahata K.; Wang D.; Nishibori M.; Day J.; Louis C.; Wicks I. P.; Kohsaka H.; Yasuda S. Targeting necroptosis in muscle fibers ameliorates inflammatory myopathies. Nat. Commun. 2022, 13, 166. 10.1038/s41467-021-27875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annibaldi A.; Meier P. Checkpoints in TNF-Induced Cell Death: Implications in Inflammation and Cancer. Trends Mol. Med. 2018, 24, 49–65. 10.1016/j.molmed.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Xiao Y.; Zhang T.; Ma X.; Yang Q. C.; Yang L. L.; Yang S. C.; Liang M.; Xu Z.; Sun Z. J. Microenvironment-Responsive Prodrug-Induced Pyroptosis Boosts Cancer Immunotherapy. Adv. Sci. 2021, 8, 2101840. 10.1002/advs.202101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P.; Pelegrín P.; Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Zhang Y.; Xia S.; Kong Q.; Li S.; Liu X.; Junqueira C.; Meza-Sosa K. F.; Mok T. M. Y.; Ansara J.; Sengupta S.; Yao Y.; Wu H.; Lieberman J. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420. 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X.; Chen L.; Li Y.; Hu Z.; He F. Ferroptosis, necroptosis, and pyroptosis in the tumor microenvironment: Perspectives for immunotherapy of SCLC. Semin. Cancer Biol. 2022, 86, 273–285. 10.1016/j.semcancer.2022.03.009. [DOI] [PubMed] [Google Scholar]
- Kleinovink J. W.; van Driel P. B.; Snoeks T. J.; Prokopi N.; Fransen M. F.; Cruz L. J.; Mezzanotte L.; Chan A.; Löwik C. W.; Ossendorp F. Combination of Photodynamic Therapy and Specific Immunotherapy Efficiently Eradicates Established Tumors. Clin. Cancer Res. 2016, 22, 1459–1468. 10.1158/1078-0432.CCR-15-0515. [DOI] [PubMed] [Google Scholar]
- Santos L. L.; Oliveira J.; Monteiro E.; Santos J.; Sarmento C. Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: A Clinical Case Report. Case Reports in Oncology 2018, 11, 769–776. 10.1159/000493423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-da-Silva L. C.; Kepp O.; Kroemer G. Regulatory approval of photoimmunotherapy: photodynamic therapy that induces immunogenic cell death. Oncoimmunology 2020, 9, 1841393. 10.1080/2162402X.2020.1841393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. M.; Darafsheh A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. 10.1111/php.13219. [DOI] [PubMed] [Google Scholar]
- Seung Lee J.; Kim J.; Ye Y.; Kim T. Materials and device design for advanced phototherapy systems. Adv. Drug Delivery Rev. 2022, 186, 114339. 10.1016/j.addr.2022.114339. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Zhan M.; Hu X. Pulsed Laser Excited Photoacoustic Effect for Disease Diagnosis and Therapy. Chem.—Eur. J. 2022, 28, e202200042. 10.1002/chem.202200042. [DOI] [PubMed] [Google Scholar]
- Castano A. P.; Mroz P.; Hamblin M. R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo P.-C.; Rodríguez-Morgade M. S.; Pandey R. K.; Ng D. K. P.; Torres T.; Dumoulin F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. 10.1039/C9CS00129H. [DOI] [PubMed] [Google Scholar]
- Liang C.; Zhang X.; Yang M.; Wang W.; Chen P.; Dong X. Remodeling Tumor Microenvironment by Multifunctional Nanoassemblies for Enhanced Photodynamic Cancer Therapy. ACS Mater. Lett. 2020, 2, 1268–1286. 10.1021/acsmaterialslett.0c00259. [DOI] [Google Scholar]
- Xu F.; Ge H.; Xu N.; Yang C.; Yao Q.; Long S.; Sun W.; Fan J.; Xu X.; Peng X. Radical induced quartet photosensitizers with high 1O2 production for in vivo cancer photodynamic therapy. Sci. China Chem. 2021, 64, 488–498. 10.1007/s11426-020-9922-3. [DOI] [Google Scholar]
- Wu Y.; Li S.; Chen Y.; He W.; Guo Z. Recent advances in noble metal complex based photodynamic therapy. Chem. Sci. 2022, 13, 5085–5106. 10.1039/D1SC05478C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Guan R.; Xie L.; Liao X.; Xiong K.; Rees T. W.; Chen Y.; Ji L.; Chao H. An ER-Targeting Iridium(III) Complex That Induces Immunogenic Cell Death in Non-Small-Cell Lung Cancer. Angew. Chem., Int. Ed. 2021, 60, 4657–4665. 10.1002/anie.202013987. [DOI] [PubMed] [Google Scholar]
- Konda P.; Lifshits L. M.; Roque J. R.; Cole H. D.; Cameron C. G.; McFarland S. A.; Gujar S. Discovery of immunogenic cell death-inducing ruthenium-based photosensitizers for anticancer photodynamic therapy. Oncoimmunology 2021, 10, 1863626. 10.1080/2162402X.2020.1863626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X.; Wang W. J.; Cao Q.; Zhang H.; Liu B.; Ling Y.; Zhou X.; Mao Z. W. A Carbonic Anhydrase IX (CAIX)-Anchored Rhenium(I) Photosensitizer Evokes Pyroptosis for Enhanced Anti-Tumor Immunity. Angew. Chem., Int. Ed. 2022, 61, e202115800. 10.1002/anie.202115800. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Sun S.; Li X.; Zhang W.; Gou S. Enhancing Photodynamic Therapy Efficacy of Upconversion-Based Nanoparticles Conjugated with a Long-Lived Triplet Excited State Iridium(III)-Naphthalimide Complex: Toward Highly Enhanced Hypoxia-Inducible Factor-1. ACS Applied Bio Materials 2020, 3, 252–262. 10.1021/acsabm.9b00774. [DOI] [PubMed] [Google Scholar]
- Ding D.; Tang B. Z. Advances in Improving Healthcare with Aggregation-Induced Emission. Adv. Healthc. Mater. 2021, 10, 2102499. 10.1002/adhm.202102499. [DOI] [PubMed] [Google Scholar]
- Wei D.; Chen Y.; Huang Y.; Li P.; Zhao Y.; Zhang X.; Wan J.; Yin X.; Liu T.; Yin J.; Liu Z.; Zhang Q.; Wang J.; Xiao H. NIR-light triggered dual-cascade targeting core-shell nanoparticles enhanced photodynamic therapy and immunotherapy. Nano Today 2021, 41, 101288. 10.1016/j.nantod.2021.101288. [DOI] [Google Scholar]
- Chen Y.; Ai W.; Guo X.; Li Y.; Ma Y.; Chen L.; Zhang H.; Wang T.; Zhang X.; Wang Z. Mitochondria-Targeted Polydopamine Nanocomposite with AIE Photosensitizer for Image-Guided Photodynamic and Photothermal Tumor Ablation. Small 2019, 15, 1902352. 10.1002/smll.201902352. [DOI] [PubMed] [Google Scholar]
- Wu M.; Liu X.; Chen H.; Duan Y.; Liu J.; Pan Y.; Liu B. Activation of Pyroptosis by Membrane-Anchoring AIE Photosensitizer Design: New Prospect for Photodynamic Cancer Cell Ablation. Angew. Chem., Int. Ed. 2021, 60, 9093–9098. 10.1002/anie.202016399. [DOI] [PubMed] [Google Scholar]
- Niu N.; Yu Y.; Zhang Z.; Kang M.; Wang L.; Zhao Z.; Wang D.; Tang B. Z. A cell membrane-targeting AIE photosensitizer as a necroptosis inducer for boosting cancer theranostics. Chem. Sci. 2022, 13, 5929–5937. 10.1039/D2SC01260J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Ni X.; Jia S.; Liang Y.; Wu X.; Kong D.; Ding D. Massively Evoking Immunogenic Cell Death by Focused Mitochondrial Oxidative Stress using an AIE Luminogen with a Twisted Molecular Structure. Adv. Mater. 2019, 31, 1904914. 10.1002/adma.201904914. [DOI] [PubMed] [Google Scholar]
- Xu X.; Deng G.; Sun Z.; Luo Y.; Liu J.; Yu X.; Zhao Y.; Gong P.; Liu G.; Zhang P.; Pan F.; Cai L.; Tang B. Z. A Biomimetic Aggregation-Induced Emission Photosensitizer with Antigen-Presenting and Hitchhiking Function for Lipid Droplet Targeted Photodynamic Immunotherapy. Adv. Mater. 2021, 33, 2102322. 10.1002/adma.202102322. [DOI] [PubMed] [Google Scholar]
- Kwon N.; Kim H.; Li X.; Yoon J. Supramolecular agents for combination of photodynamic therapy and other treatments. Chem. Sci. 2021, 12, 7248–7268. 10.1039/D1SC01125A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.; Zheng R.; Huang J.; Chen X.; Deng F.; Liu Y.; Huang C.; Yu X.; Cheng H.; Li S. Self-Delivery Photo-Immune Stimulators for Photodynamic Sensitized Tumor Immunotherapy. ACS Nano 2020, 14, 17100–17113. 10.1021/acsnano.0c06765. [DOI] [PubMed] [Google Scholar]
- Um W.; Ko H.; You D. G.; Lim S.; Kwak G.; Shim M. K.; Yang S.; Lee J.; Song Y.; Kim K.; Park J. H. Necroptosis-Inducible Polymeric Nanobubbles for Enhanced Cancer Sonoimmunotherapy. Adv. Mater. 2020, 32, 1907953. 10.1002/adma.201907953. [DOI] [PubMed] [Google Scholar]
- Huang J.; Xiao Z.; Chen G.; Li T.; Peng Y.; Shuai X. A pH-sensitive nanomedicine incorporating catalase gene and photosensitizer augments photodynamic therapy and activates antitumor immunity. Nano Today 2022, 43, 101390. 10.1016/j.nantod.2022.101390. [DOI] [Google Scholar]
- Chen Z.; Liu L.; Liang R.; Luo Z.; He H.; Wu Z.; Tian H.; Zheng M.; Ma Y.; Cai L. Bioinspired Hybrid Protein Oxygen Nanocarrier Amplified Photodynamic Therapy for Eliciting Anti-tumor Immunity and Abscopal Effect. ACS Nano 2018, 12, 8633–8645. 10.1021/acsnano.8b04371. [DOI] [PubMed] [Google Scholar]
- Deng H.; Zhou Z.; Yang W.; Lin L.; Wang S.; Niu G.; Song J.; Chen X. Endoplasmic Reticulum Targeting to Amplify Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 2020, 20, 1928–1933. 10.1021/acs.nanolett.9b05210. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Cheung Y.; Ng D. K. P.; Fong W. Immunogenic necroptosis in the anti-tumor photodynamic action of BAM-SiPc, a silicon (IV) phthalocyanine-based photosensitizer. Cancer Immunology, Immunotherapy 2021, 70, 485–495. 10.1007/s00262-020-02700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.; Kim K.; Yoo J.; Li X.; Kwon N.; Jeon Y.; Shin S.; Han S. S.; Lee D.; Yoon J. A Nanostructured Phthalocyanine/Albumin Supramolecular Assembly for Fluorescence Turn-On Imaging and Photodynamic Immunotherapy. ACS Nano 2022, 16, 3045–3058. 10.1021/acsnano.1c10565. [DOI] [PubMed] [Google Scholar]
- Li M.; Xiong T.; Du J.; Tian R.; Xiao M.; Guo L.; Long S.; Fan J.; Sun W.; Shao K.; Song X.; Foley J. W.; Peng X. Superoxide Radical Photogenerator with Amplification Effect: Surmounting the Achilles’ Heels of Photodynamic Oncotherapy. J. Am. Chem. Soc. 2019, 141, 2695–2702. 10.1021/jacs.8b13141. [DOI] [PubMed] [Google Scholar]
- Li M.; Xia J.; Tian R.; Wang J.; Fan J.; Du J.; Long S.; Song X.; Foley J. W.; Peng X. Near-Infrared Light-Initiated Molecular Superoxide Radical Generator: Rejuvenating Photodynamic Therapy against Hypoxic Tumors. J. Am. Chem. Soc. 2018, 140, 14851–14859. 10.1021/jacs.8b08658. [DOI] [PubMed] [Google Scholar]
- Xiong T.; Li M.; Chen Y.; Du J.; Fan J.; Peng X. A singlet oxygen self-reporting photosensitizer for cancer phototherapy. Chem. Sci. 2021, 12, 2515–2520. 10.1039/D0SC05495J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.; Xu F.; Wang Y.; Shi C.; Sha Y.; He G.; Yao Q.; Shao K.; Sun W.; Du J.; Fan J.; Peng X. Cancer immunogenic cell death via photo-pyroptosis with light-sensitive Indoleamine 2,3-dioxygenase inhibitor conjugate. Biomaterials 2021, 278, 121167. 10.1016/j.biomaterials.2021.121167. [DOI] [PubMed] [Google Scholar]
- Xiong T.; Peng Q.; Chen Y.; Li M.; Du J.; Fan J.; Jia L.; Peng X. A Novel Nanobody–Photosensitizer Conjugate for Hypoxia Resistant Photoimmunotherapy. Adv. Funct. Mater. 2021, 31, 2103629. 10.1002/adfm.202103629. [DOI] [Google Scholar]
- Zhang H.; Feng Y.; Xie X.; Song T.; Yang G.; Su Q.; Li T.; Li S.; Wu C.; You F.; Liu Y.; Yang H. Engineered Mesenchymal Stem Cells as a Biotherapy Platform for Targeted Photodynamic Immunotherapy of Breast Cancer. Adv. Healthc. Mater. 2022, 11, 2101375. 10.1002/adhm.202101375. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Chen F.; Xu N.; Yao Q.; Wang R.; Xie X.; Zhang F.; He Y.; Shao D.; Dong W.; Fan J.; Sun W.; Peng X. Red-light-triggered self-destructive mesoporous silica nanoparticles for cascade-amplifying chemo-photodynamic therapy favoring antitumor immune responses. Biomaterials 2022, 281, 121368. 10.1016/j.biomaterials.2022.121368. [DOI] [PubMed] [Google Scholar]
- Li M.; Long S.; Kang Y.; Guo L.; Wang J.; Fan J.; Du J.; Peng X. De Novo Design of Phototheranostic Sensitizers Based on Structure-Inherent Targeting for Enhanced Cancer Ablation. J. Am. Chem. Soc. 2018, 140, 15820–15826. 10.1021/jacs.8b09117. [DOI] [PubMed] [Google Scholar]
- Zou Y.; Long S.; Xiong T.; Zhao X.; Sun W.; Du J.; Fan J.; Peng X. Single-Molecule Förster Resonance Energy Transfer-Based Photosensitizer for Synergistic Photodynamic/Photothermal Therapy. ACS Cent. Sci. 2021, 7, 327–334. 10.1021/acscentsci.0c01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.; Li M.; Zhang Z.; Yao Q.; Shao K.; Xu F.; Xu N.; Li H.; Fan J.; Sun W.; Du J.; Long S.; Wang J.; Peng X. Catalase-based liposomal for reversing immunosuppressive tumor microenvironment and enhanced cancer chemo-photodynamic therapy. Biomaterials 2020, 233, 119755. 10.1016/j.biomaterials.2020.119755. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Huang L.; Yan Y.; El Zohry A. M.; Toffoletti A.; Zhao J.; Barbon A.; Dick B.; Mohammed O. F.; Han G. Elucidation of the Intersystem Crossing Mechanism in a Helical BODIPY for Low-Dose Photodynamic Therapy. Angew. Chem., Int. Ed. 2020, 59, 16114–16121. 10.1002/anie.202005269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A.; Killoran J.; O’Shea C.; Kenna T.; Gallagher W. M.; O’Shea D. F. In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy. J. Am. Chem. Soc. 2004, 126, 10619–10631. 10.1021/ja047649e. [DOI] [PubMed] [Google Scholar]
- Byrne A. T.; O’Connor A. E.; Hall M.; Murtagh J.; O’Neill K.; Curran K. M.; Mongrain K.; Rousseau J. A.; Lecomte R.; McGee S.; Callanan J. J.; O’Shea D. F.; Gallagher W. M. Vascular-targeted photodynamic therapy with BF2-chelated Tetraaryl-Azadipyrromethene agents: a multi-modality molecular imaging approach to therapeutic assessment. Br. J. Cancer 2009, 101, 1565–1573. 10.1038/sj.bjc.6605247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Wang H.; Chen Z.; Dong X.; Zhao W.; Shi Y.; Zhu Q. Discovery of an Amino Acid-Modified Near-Infrared Aza-BODIPY Photosensitizer as an Immune Initiator for Potent Photodynamic Therapy in Melanoma. J. Med. Chem. 2022, 65, 3616–3631. 10.1021/acs.jmedchem.1c02154. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Tian J.; Tian Y.; Sun Q.; Sun D.; Wang F.; Xu H.; Ying G.; Wang J.; Yetisen A. K.; Jiang N. Near-Infrared-II Nanoparticles for Cancer Imaging of Immune Checkpoint Programmed Death-Ligand 1 and Photodynamic/Immune Therapy. ACS Nano 2021, 15, 515–525. 10.1021/acsnano.0c05317. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Yao Q.; Long S.; Chi W.; Yang Y.; Tan D.; Liu X.; Huang H.; Sun W.; Du J.; Fan J.; Peng X. An Approach to Developing Cyanines with Simultaneous Intersystem Crossing Enhancement and Excited-State Lifetime Elongation for Photodynamic Antitumor Metastasis. J. Am. Chem. Soc. 2021, 143, 12345–12354. 10.1021/jacs.1c06275. [DOI] [PubMed] [Google Scholar]
- He Y.; Cong C.; He Y.; Hao Z.; Li C.; Wang S.; Zhao Q.; He H.; Zhu R.; Li X.; Gao D. Tumor hypoxia relief overcomes multidrug resistance and immune inhibition for self-enhanced photodynamic therapy. Chem. Eng. J. 2019, 375, 122079. 10.1016/j.cej.2019.122079. [DOI] [Google Scholar]
- Liu X.; Liu Y.; Li X.; Huang J.; Guo X.; Zhang J.; Luo Z.; Shi Y.; Jiang M.; Qin B.; Du Y.; Luo L.; You J. ER-Targeting PDT Converts Tumors into In Situ Therapeutic Tumor Vaccines. ACS Nano 2022, 16, 9240–9253. 10.1021/acsnano.2c01669. [DOI] [PubMed] [Google Scholar]
- Ma H.; Lu Y.; Huang Z.; Long S.; Cao J.; Zhang Z.; Zhou X.; Shi C.; Sun W.; Du J.; Fan J.; Peng X. ER-Targeting Cyanine Dye as an NIR Photoinducer to Efficiently Trigger Photoimmunogenic Cancer Cell Death. J. Am. Chem. Soc. 2022, 144, 3477–3486. 10.1021/jacs.1c11886. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Li H.; Shi C.; Xu F.; Zhang Z.; Yao Q.; Ma H.; Sun W.; Shao K.; Du J.; Long S.; Fan J.; Wang J.; Peng X. An APN-activated NIR photosensitizer for cancer photodynamic therapy and fluorescence imaging. Biomaterials 2020, 253, 120089. 10.1016/j.biomaterials.2020.120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Sun W.; Tian R.; Cao J.; Tian Y.; Gurram B.; Fan J.; Peng X. Smart J-aggregate of cyanine photosensitizer with the ability to target tumor and enhance photodynamic therapy efficacy. Biomaterials 2021, 269, 120532. 10.1016/j.biomaterials.2020.120532. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zhao X.; Xiong T.; Du J.; Sun W.; Fan J.; Peng X. NIR photosensitizers activated by γ-glutamyl transpeptidase for precise tumor fluorescence imaging and photodynamic therapy. Sci. China Chem. 2021, 64, 808–816. 10.1007/s11426-020-9947-4. [DOI] [Google Scholar]
- Wang K.; Xiao X.; Jiang M.; Li J.; Zhou J.; Yuan Y. An NIR-Fluorophore-Based Theranostic for Selective Initiation of Tumor Pyroptosis-Induced Immunotherapy. Small 2021, 17, 2102610. 10.1002/smll.202102610. [DOI] [PubMed] [Google Scholar]
- Zhou W.; He X.; Wang J.; He S.; Xie C.; Fan Q.; Pu K. Semiconducting Polymer Nanoparticles for Photoactivatable Cancer Immunotherapy and Imaging of Immunoactivation. Biomacromolecules 2022, 23, 1490–1504. 10.1021/acs.biomac.2c00065. [DOI] [PubMed] [Google Scholar]
- Li J.; Pu K. Semiconducting Polymer Nanomaterials as Near-Infrared Photoactivatable Protherapeutics for Cancer. Acc. Chem. Res. 2020, 53, 752–762. 10.1021/acs.accounts.9b00569. [DOI] [PubMed] [Google Scholar]
- Liu S.; Zhang H.; Li Y.; Liu J.; Du L.; Chen M.; Kwok R. T. K.; Lam J. W. Y.; Phillips D. L.; Tang B. Z. Strategies to Enhance the Photosensitization: Polymerization and the Donor–Acceptor Even–Odd Effect. Angew. Chem., Int. Ed. 2018, 57, 15189–15193. 10.1002/anie.201810326. [DOI] [PubMed] [Google Scholar]
- Qi J.; Jia S.; Kang X.; Wu X.; Hong Y.; Shan K.; Kong X.; Wang Z.; Ding D. Semiconducting Polymer Nanoparticles with Surface-Mimicking Protein Secondary Structure as Lysosome-Targeting Chimaeras for Self-Synergistic Cancer Immunotherapy. Adv. Mater. 2022, 34, 2203309. 10.1002/adma.202203309. [DOI] [PubMed] [Google Scholar]
- Zeng Z.; Zhang C.; Li J.; Cui D.; Jiang Y.; Pu K. Activatable Polymer Nanoenzymes for Photodynamic Immunometabolic Cancer Therapy. Adv. Mater. 2021, 33, 2007247. 10.1002/adma.202007247. [DOI] [PubMed] [Google Scholar]