Abstract
For nearly 20 years, dental stem cells (DSCs) have been successfully isolated from mature/immature teeth and surrounding tissue, including dental pulp of permanent teeth and exfoliated deciduous teeth, periodontal ligaments, dental follicles, and gingival and apical papilla. They have several properties (such as self-renewal, multidirectional differentiation, and immunomodulation) and exhibit enormous potential for clinical applications. To date, many clinical articles and clinical trials using DSCs have reported the treatment of pulpitis, periapical lesions, periodontitis, cleft lip and palate, acute ischemic stroke, and so on, and DSC-based therapies obtained satisfactory effects in most clinical trials. In these studies, no adverse events were reported, which suggested the safety of DSC-based therapy. In this review, we outline the characteristics of DSCs and summarize clinical trials and their safety as DSC-based therapies. Meanwhile, we also present the current limitations and perspectives of DSC-based therapy (such as harvesting DSCs from inflamed tissue, applying DSC-conditioned medium/DSC-derived extracellular vesicles, and expanding-free strategies) to provide a theoretical basis for their clinical applications.
Keywords: Dental stem cells, Adult stem cells, Dental pulp, Tissue regeneration
Core Tip: Since dental pulp stem cells were first isolated and identified in 2000, a variety of dental stem cells (DSCs) have been reported. DSCs have shown satisfactory clinical effects in the treatment of a variety of diseases and have great potential for clinical application. This paper will summarize DSC-based clinical trials and put forward the current limitations and perspectives to accelerate and extend the clinical application of DSCs.
INTRODUCTION
Mesenchymal stem cells (MSCs) are a population of unspecialized cells characterized by the properties of self-renewal and multidirectional differentiation[1,2]. Currently, MSCs are currently being explored for the treatment of many diseases, such as cardiovascular disease, neurodegenerative diseases, dental diseases, and metabolic diseases[1].
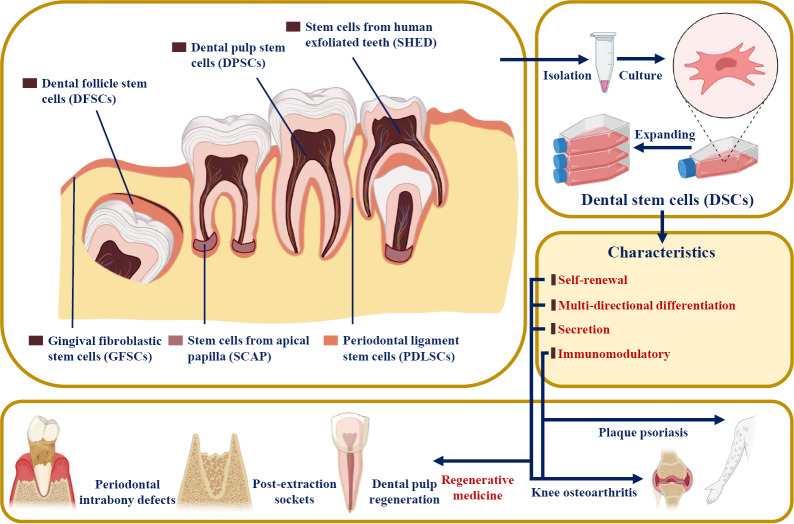
Dental SCs (DSCs) were reported to have similar features to MSCs[3]. Since dental pulp SCs (DPSCs) were first successfully isolated from the extracted third molar in 2000[4], multiple DSC types have been harvested from mature and immature teeth and their surrounding tissues, including periodontal ligament stem cells (PDLSCs), stem cells from apical papilla (SCAP), stem cells from exfoliated deciduous teeth (SHED), gingiva-derived mesenchymal SCs (GMSCs), and dental follicle progenitor cells (DFPCs)[5-7] (Figure 1). DSCs develop from the neural crest and express both stem cell markers and neural markers[8,9]. It was reported that DSCs have the potential for multipotent differentiation into osteogenic, chondrogenic, adipogenic, neurogenic, odontogenic, dentinogenic cells, and so on[10]. In addition to their self-renewal and differentiation properties, DSCs have also been reported to be involved in secretion, immunomodulation, and tumor processes[3,11]. Based on the characteristics of DSCs, many clinical articles and clinical trials have used DSCs in tissue regeneration and the treatment of various diseases, such as pulpitis, periapical lesions, and periodontitis[12].
Figure 1.
Tissue origin, harvest, characteristics, and clinical application potential of the various populations of dental stem cells. Dental pulp stem cells and stem cells from exfoliated deciduous teeth can be isolated from the inner dental pulp of permanent teeth and deciduous exfoliated teeth, respectively. Stem cells from apical papilla can be extracted from the apical papilla; periodontal ligament stem cells can be harvested from the periodontal ligament; and dietary fiber supplementation combinations can be derived from the dental follicle. Gingiva-derived mesenchymal stem cells can be extracted from gingiva. Citation: Sharpe PT. Dental mesenchymal stem cells. Development 2016; 143: 2273-2280[139]. Copyright ©The Authors 2016. Published by The Company of Biologists Ltd. The authors have obtained the permission for figure using from the Company of Biologists Ltd (Supplementary material).
In this study, the current status of clinical articles and clinical trials using DSCs in the treatment of various diseases and conditions are reviewed. In addition, current limitations and perspectives, including harvesting DSCs from inflamed tissue, applying DSC-conditioned medium (CM) and DSC-derived extracellular vesicles (EVs), and expanding-free strategies, are also discussed.
CHARACTERISTICS OF DSCS
Based on their various sources, DSCs are divided into DPSCs, SHED, PDLSCs, SCAP, GMSCs, and DFPSCs (Figure 1). DSCs are known to express not only mesenchymal and embryonic stem cell markers (such as CD44, STRO-1, and Nanog) but also neuronal markers because they originate from embryonic neural crests[8,9] (Table 1). However, they do not express CD34, CD45, or CD11b, which are defined as hematopoietic markers[7].
Table 1.
Characteristics of different types of dental stem cells
|
Cell types
|
Markers
|
Multidirectional differentiations
|
||
|
Cell surface markers
|
Embryonic stem cell markers
|
Nerual markers
|
||
| DPSCs | CD13, CD29, CD44, CD59, CD73, CD90, CD105, CD146, STRO-1[7], CD81, CD49f[140], CD40, CD120a, CD261, CD262, CD264, CD266, CD121a, CD130, CD213a1, CD217, CDw210b[141] | OCT-4, Nanog[142], SSEA-1, SEEA-4[140], SOX-2[143] | βIII-tubulin, NFM, Nestin, CNPase[144], S100, CD271[17] | Osteogenic, Odontogenic[145], Dentinogeni, Chondrogenic, Neurogenic, Myogenic, Adipogenic[13], Hepatogenic[146] |
| PDLSCs | CD13, CD29, CD44, CD49, CD73, CD90, CD105, CD146, CD166, CD271[147], CD10[7], STRO-1[148] | SSEA-1, SSEA-3, SSEA-4, TRA-1-60, TRA-1-81, OCT-4, Nanog, SOX-2, REX1, and ALP[149] | Nestin, OCT-4, SSEA-4[9] CD271, SOX-10[147], SOX-2[149] | Osteogenic, Cementogenic, Adipogenic, Chondrogenic, Neurogenic[13], Hepatogenic[149], Cardiac myogenic, Endothelial-like, Islet-like, Retinal ganglion-like[147] |
| SCAP | CD13, CD24, CD29, CD44, CD49, CD51, CD56, CD61, CD73, CD90, CD105, CD106, CD146, CD166, STRO-1, NOTCH-3[150], CD81, CD49f[151] | OCT-4, Nanog, SOX2[143], CD49f[151] | βIII-tubulin, NFM, Nestin, CNPase[144], SOX-2[143], Vimentin, Survivin[150] | Osteogenic, Dentinogenic, Adipogenic[15], Neurogenic[13], Chondrogenic, Hepatogenic[150] |
| SHED | CD29, CD73, CD90, CD166[13], STRO-1, CD44[145], CD105[152], NOTCH-1, CD10, CD13, CD34, CD106, CD146, CD166, CD271[102] | OCT-4, Nanog, SSEA-3[153], SSEA-4[152], NOTCH-1, OCT-4, SOX-2[102] | βIII-tubulin, NFM, Nestin, CNPase, GAD, NeuN, GFAP[154], CD271, Vimentin, OCT-4, PAX-6, NSE, MAP-2, PSA-NCAM, TH[102] | Osteogenic, Odontogenic[145], Dentinogenic, Chondrogenic, Neurogenic, Myogenic, Adipogenic[13], Hepatogenic[155] |
| DFPCs | CD13, CD29, CD59, CD90[7], CD105, CD146[142], CD44, CD73, NOTCH-1, STRO-1[156] | OCT-4, Nanog[142], NOTCH-1, SOX-2[156] | OCT-4, SOX2[9], Nestin, SOX-2[156] | Osteogenic, Cementogenic, Odontogenic, Adipogenic, Chondrogenic[13], Hepatogenic[146] |
| GMSCs | CD13, CD29, CD44, CD73, CD90, CD105, CD146, STRO-1[16] | SSEA-4[16], OCT-4, Nanog[157] | Nestin, SOX10[16], βIII-tubulin, NFM, CNPase[144] | Osteogenic, Adipogenic, Chondrogenic, Neurogenic, Endothelial-like, Odontogenic[16], Myogenic[158] |
ALP: Alkaline phosphatase; CD: Cluster of differentiation; CNPase: 2’,3’-cyclic nucleotide 3’-phosphodiesterase; GAD: Glutamic acid decarboxylase; GFAP: Glial fibrillary acidic protein; MAP-2: Microtubule associated protein 2; NeuN: Neuronal nuclei; NFM: Neurofilament medium chain; NGFR: Nerve growth factor receptor; NSE: Neuron-specific enolase; OCT: Octamer-binding transcription factor; PAX-6: Paired Box 6; PSA-NCAM: Polysialylated neural cell adhesion molecule; REX-1: RNA exonuclease 1 homolog; SOX: Sex determining region Y-box; SSEA: Stage-specific embryonic antigen; TH: Tyrosine hydroxylase; SHED: Stem cells from exfoliated deciduous teeth.
Similar to mesenchymal stem cells, DSCs showed the ability of self-renewal and multidirectional differentiation, such as osteogenic, chondrogenic, adipogenic, neurogenic, odontogenic, dentinogenic, cementogenic, and myogenic differentiation[13-16] (Table 1). In addition, even in the undifferentiated state, DSCs were able to secrete several angiogenic and neurotrophic factors, including vascular endothelial growth factor (VEGF), ciliary neurotrophic factor (CNTF), brain-derived neurotrophic factor (BDNF), glia-derived neurotrophic factor (GDNF), and β-nerve growth factor (β-NGF), to promote angiogenesis and tissue regeneration[17,18].
In addition, the immunomodulatory features of DSCs have also been the focus of a number of studies. First, it was reported that DSCs, like mesenchymal stem cells, faintly express the MHC class II antigen HLA-DR and maintain low immunogenicity[19-21]. Second, local tissue regeneration and inflammation could be influenced by the secretome of DSCs (including the production of inflammatory and anti-inflammatory cytokines and the regulation of immune cells), which is also regulated by the local inflammatory microenvironment[21-24]. Finally, the inflammatory microenvironment could impact the behaviors of DSCs, such as proliferation potential, migration, homing, and differentiation[22].
Based on the characteristics of DSCs, they have been widely studied in regenerative medicine and tissue engineering and have shown an amazing therapeutic effect on oral-facial, neurologic, corneal, cardiovascular, hepatic, diabetic, renal, muscular, tenogenic, dystrophic and autoimmune conditions in both animal and human models[21,25-27]. For example, the proliferation, paracrine effect, and multidirectional differentiation potential of DSCs support the application of DSCs in regenerative medicine (e.g., dental pulp and bone tissue regeneration)[28,29]. The anti-inflammatory, immunomodulatory, and immunoevasive properties of DSCs also help in the treatment of plaque psoriasis[30] (Figure 1). DSC-based therapies have broad prospects for clinical application.
It is worth noting that the naming of mesenchymal stem cells and mesenchymal stromal cells remains controversial. Based on the position paper issued by The International Society for Cell & Gene Therapy (ISCT) Mesenchymal Stromal Cell (ISCT MSC) in 2005, mesenchymal stem cells are not equivalent or interchangeable with mesenchymal stromal cells[31]. Mesenchymal stem cells refer to progenitor cell populations with obvious self-renewal and differentiation functions, while mesenchymal stromal cells refer to large populations with significant secretion, immune regulation, and homing properties[32-34]. As we have just summarized, dental stem cells share some of the characteristics of both mesenchymal stem cells and mesenchymal stromal cells, and more consensus articles may be needed to further define the naming of dental stem cells.
DSC-BASED CLINICAL TRIALS FROM PUBLISHED ARTICLES
Pulpitis and pulp necrosis
Four studies were reported to treat pulp necrosis or irreversible pulpitis using autologous DPSCs or SHED, including a randomized controlled trial (RCT), two case series, and a case report[28,35-37] (Table 2). Xuan et al[28] applied SHED in the treatment of pulp necrosis caused by trauma and observed dental pulp tissue regeneration at 12 mo and 24 mo after transplantation. Meanwhile, the results also showed increased dental root length and decreased apical foramen width compared with traditional apexification treatment. Two case series reported by Nakashima et al[35,37] indicated that DPSCs transplanted with granulocyte colony-stimulating factor and gelatin sponges could increase pulp sensitivity and mineralization and recover the signal intensity (SI) of regenerated pulp tissue on MRI examination. Meza et al[36] transplanted DPSCs and leukocyte platelet-rich fibrin (L-PRF) harvested from autologous inflamed dental pulp and blood, respectively, to the root canal of irreversible pulpitis teeth and observed dentin bridge formation and a response to the cold test and electric pulp test.
Table 2.
Dental stem cell-based clinical trials from published articles
|
Ref.
|
Registration ID
|
Conditions/diseases
|
Study design
|
Cell source
|
Administration route
|
Interventions
|
Follow-up period
|
Outcomes
|
|
|
Test group
|
Control group
|
||||||||
| Xuan et al[28], 2018 | NCT01814436 | Pulp necrosis | RCT | Autologous deciduous pulp | Implanted into injured teeth | SHED (n = 26) | Traditional apexification treatment (n = 10) | 12 mo; 24 mo | Dental pulp tissue regeneration; no adverse events observed; the length of the root (↑); the width of the apical foramen (↓) |
| Nakashima et al[35], 2022 | None | Irreversible pulpitis | Case series | Autologous dental pulp | Transplanted into the root canal | DPSCs + Gelatin sponge + G-CSF (n = 5) | None | 1, 2, 4, 12, 24, 28, 32 wk | Pulp sensibility (↑); MRI examination showed similar SI between test teeth and untreated controls |
| Nakashima et al[37], 2017 | None | Irreversible pulpitis | Case series | Autologous dental pulp | Transplanted into the root canal | DPSCs + Gelatin sponge + G-CSF (n = 2) | None | 1, 4, 12, 24, and 48 wk | MRI examination showed similar SI between test teeth and untreated controls; mineralized tissue deposition (↑) |
| Meza et al[36], 2019 | None | Irreversible pulpitis | A case report | Autologous inflamed dental pulp | Transplanted into the root canal | DPSCs + L-PRF (n = 1) | None | 6 mo; 3 year | Delayed response to the cold test; positive response to electric pulp testing; dentin bridge formation |
| Shiehzadeh et al[38], 2014 | None | Periapical lesions | Case series | Case 1 and case 3: Autologous apical papilla; case 2: Deciduous pulp | Case 1 and Case 3: Injected from root apex to cavity; case 2: Injected into the defect via a surgical approach | Case 1 and Case 3: SCAP + PEG-PLGA scaffold (n = 2); case 2: SHED + PEG-PLGA scaffold (n = 1) | None | Case 1: 30 d, 3 mo, 1 year; 2 year; case 2: 3, 6, 18 mo; case 3: 3, 6, 12, 24 mo | Developed mature apices; periapical tissue healing (↑) |
| Prasad et al[39], 2017 | None | Periapical lesions | Case series | Allogeneic deciduous pulp | Transplanted into the root canal | SHED + Bioglass (n = 2) | None | 7, 30, 90, 180, 365 d | Closure of open apex; periapical tissue healing; positive response to electric pulp testing and cold testing |
| Prasad et al[40], 2019 | None | Periapical lesions | A case report | Allogeneic deciduous pulp | Transplanted into the root canal and periapical area | SHED + Bioglass (n = 1) | None | 2 wk; 4, 12, 24 mo | Periapical tissue healing; positive response to electric pulp testing |
| Ferrarotti et al[41], 2018 | NCT03386877 | Periodontal intrabony defects | RCT | Autologous dental pulp | Implanted into bone defect sites consisted of MIST | Pulp micrografts + Collagen sponge (n = 15) | Collagen sponge (n = 14) | 6 and 12 mo | PD (↓); CAL (↓); bone defect fill (↑); residual PD < 5 mm and CAL gain ≥ 4 mm (↑) |
| Sánchez et al[42], 2020 | ISRCTN13093912 | Periodontal intrabony defects | CCT | Autologous periodontal ligament | Implanted into bone defect sites via surgical approach | PDLSCs + β-TCP (n = 9) | β-TCP (n = 10) | 1, 3, 6, 9, 12 mo | CAL (-); PPD (-) |
| Feng et al[43], 2010 | None | Periodontal intrabony defects | Case series | Autologous periodontal ligament | Implanted into bone defect sites via surgical approach | PDLPs + HA/TCP (n = 3) | None | 3, 6, 12, 32, 42, and 72 mo | CAL (↓); PD (↓); GR (↑) |
| Chen et al[29], 2016 | NCT01357785 | Periodontal intrabony defects | RCT | Autologous periodontal ligament | Implanted into bone defect sites via surgical approach | PDLSCs sheets + DBBM (n = 20) | DBBM (n = 21) | 2 wk; 3, 6, 12 mo | CAL (-); PD (-); GR (-) |
| Iwata et al[44], 2018 | UMIN000005027 | Periodontal intrabony defects | Case series | Autologous periodontal ligament | Implanted into bone defect sites via surgical approach | PDL-derived cell sheets + β-TCP (n = 10) | None | 3, 6, 55 ± 19 mo | CAL (↓); PD (↓); bone height (↑) |
| Vandana et al[125], 2015 | None | Periodontal intrabony defects | A case report | Autologous periodontal ligament | Implanted into bone defect sites via surgical approach | Periodontal ligament soft tissue + Gelatin sponge + Cementum scrapings (n = 1) | None | 1 wk; 3, 6, 12 mo | CAL (↓); PD (↓); BMD (↑) |
| Aimetti et al[47], 2014 | None | Periodontal intrabony defects | A case report | Autologous dental pulp | Implanted into bone defect sites via surgical approach | Pulp micrografts + Collagen sponge (n = 1) | None | 6 mo; 1 year | PPD (↓); bone fill (↑) |
| Aimetti et al[46], 2018 | None | Periodontal intrabony defects | Case series | Autologous dental pulp | Implanted into bone defect sites via surgical approach | Pulp micrografts + Collagen sponge (n = 11) | None | 1 year | CAL (↓); PD (↓); bone fill (↑) |
| Aimetti et al[49], 2015 | None | Periodontal intrabony defects | Case series | Autologous dental pulp | Implanted into bone defect sites via surgical approach | Pulp micrografts + Collagen sponge (n = 4) | None | 6, 12 mo | PD (↓); CAL (↓); bone fill (↑) |
| Hernández-Monjaraz et al[48], 2018 | ISRCTN12831118 | Periodontal intrabony defects | A case report | Allogeneic dental pulp | Implanted into bone defect sites via surgical approach | DPSCs + Lyophilized collagen-polyvinylpyrrolidone sponge scaffold (n = 1) | None | 3, 6 mo | PD (↓); TM (↓); bone fill (↑) |
| Barbier et al[57], 2018 | EudraCT database 2014-001913-18 | Post-extraction sockets | Split-mouth RCT | Autologous dental pulp | Implanted into postextraction sockets | Pulp micrografts + collagen matrix (n = 30) | Collagen matrix (n = 30) | 6 mo | BMD (-); interdental septum height (-) |
| Cubuk et al[62], 2023 | NCT04641533 | Post-extraction sockets | Split-mouth RCT | Autologous dental pulp | Implanted into postextraction sockets | Pulp micrografts + L-PRF (n = 13) | L-PRF (n = 13) | 7 d; 6 mo | PPD (-); CAL (-); vertical bone loss (-); relative bone density (-) |
| d’Aquino et al[58], 2009 | None | Post-extraction sockets | Split-mouth CCT | Autologous dental pulp | Implanted into postextraction sockets | Dental pulp stem/progenitor cells + collagen sponge (n = 7) | Collagen sponge (n = 7) | 7 d; 1, 2, 3, 12 mo | Rate of mineralization (↑); levels of cortical bone (↑); CAL (↓); BMP-2, VEGF (↑) |
| Tanikawa et al[63], 2020 | NCT03766217 | Cleft lip and palate | Historical control study | Autologous deciduous pulp | Placed into the alveolar defect via surgical approach | SHED + Hydroxyapatite-collagen sponge (n = 6) | rhBMP-2 + Hydroxyapatite-collagen sponge (Group I n = 8); Iliac crest bone graft (Group II n = 8) | 6, 12 mo | Bone filling percentage (↑, compared with Group I at the 6-mo follow-up) |
| Manimaran et al[59], 2014 | None | Mandibular osteoradionecrosis | A case report | Allogeneic dental pulp | Inserted into the defect after surgical curettage | DPSCs + PRP + TCP (n = 1) | None | 2, 6 mo | Bone formation (↑) |
| Manimaran et al[60], 2016 | None | Bone defect left by the resection of mandibular ameloblastoma | A case report | Autologous dental pulp | Packed inside the mesh and placed over the mandible after tumor resection | DPSCs + β-TCP + PRF + SVF (n = 1) | None | 1, 10 mo; 1.5 years | Bone regeneration (↑); no recurrence of tumor |
| Brunelli et al[61], 2013 | None | Sinus lifting | A case report | Autologous dental pulp | Implanted into sinus cavity | Pulp micrografts + Collagen sponge (n = 1) | None | 4 mo | BMD (↑) |
| Koga et al[64], 2022 | None | Erectile dysfunction | Case series | Allogeneic deciduous pulp | Injected into the penis | SHED-CM (n = 38) | None | After every injection | IIEF-5 score (↑) |
| Silva et al[65], 2022 | NCT02728115 | Huntington’s disease with preexisting pulmonary nodule | A case report | Allogeneic deciduous pulp | Intravenous administrations | SHED (n = 1) | None | 15, 30 d; 7, 24, 32 mo | Unified Huntington’s disease rating scale (↓); not show long-term tropism or homing for the lung adenocarcinoma |
| Wang et al[93], 2010 | None | Plaque psoriasis | A case report | Allogeneic gingival | Bolus injection | GMSCs (n = 1) | None | 3 years | Psoriatic lesions fully cleared; no recurrence |
| Suda et al[67], 2022 | NCT04608838; JapicCTI194570 | Acute ischemic stroke | Study protocol | Allogeneic dental pulp | Intravenous administration | DPSCs | Placebo | Per 15 min (1-4 h); per 30 min (4-6 h); 12, 24 h; 2, 3, 8, 31, 91, 181, 366 d | No results |
| Nagpal et al[68], 2016 | None | Chronic disability after stroke | Study protocol | Autologous dental pulp | Implanted into peri-infarct region via neurosurgical procedure | DPSCs | None | 1, 6, 9, 12 mo | No results |
| Ye et al[69], 2020 | ChiCTR2000031319: NCT04336254 | COVID-19 | Study protocol | Allogeneic dental pulp | Intravenous administration | DPSCs | Saline | 2 h ± 30 min; 24 h ± 30 min; 90 d ± 3 d | No results |
BMD: Bone mineral density; CAL: Clinical attachment level; CCT: Controlled clinical trials; COVID-19: Coronavirus disease 2019; DBBM: Deproteinized bovine bone mineral; GR: Gingival recession; G-CSF: Granulocyte colony stimulating factor; HA/TCP: Hydroxyapatite/tricalcium phosphate; IIEF: International index of erectile function; L-PRF: Leukocyte-platelet rich fibrin; MIST: Minimally invasive surgical technique; MRI: Magnetic resonance imaging; PD: Probing depth; PDL: Periodontal ligament; PDLPs: Periodontal ligament progenitor cells; PPD: Periodontal probing depth; PRF: Platelet rich fibrin; PRP: Platelet-rich plasma; PEG-PLGA: Poly (lactide-co glycolide)-polyethylene glycol; RCT: Random clinical trial; rh-BMP: Recombinant human bone morphogenetic protein; SI: Signal intensity; SVF: Stromal vascular fraction: TCP: Tricalcium phosphate; TM: Tooth mobility; VEGF: Vascular endothelial growth factor; SHED-CM: Stem cells from exfoliated deciduous teeth conditioned medium.
Periapical lesions
In a case report and two case series, SCAP/SHED combined with a polyethylene glycol polylactic-polyglycolic acid (PEG-PLGA) scaffold and SHED combined with bioglass were used for the treatment of periapical lesions[38-40] (Table 2). Periapical tissue healing was found in the follow-up examinations of all three studies. It was reported a positive response in the test of dental pulp activity after SHED transplantation, suggesting the regeneration of pulp or pulp-like tissue, which does not occur in traditional root canal therapy[39,40].
Periodontal intrabony defects
There are two RCTs, a controlled clinical trial (CCT), three case series, and two case reports of DSC-based treatment for periodontal intrabony defects[29,41-46] (Table 2). The RCT of Ferrarotti et al[41] indicated that pulp micrografts applied with collagen sponges could significantly reduce PD and CAL and promote the regeneration of bone defects when compared with collagen sponges alone. Three case series and a case report using pulp micrografts/DPSCs and collagen sponges also reported similar results of periodontal benefits[46-49]. It was reported a novel approach using periodontal ligament soft tissue, gelatin sponges, and cementum scrapings, which reduced the CAL and PD of periodontitis teeth in their case report[45].
Although two case series demonstrated the periodontal benefits of PDLPs and PDL-derived cell sheets[43,44], significant differences in periodontal indices (including PD and CAL) were not observed between the test groups and control groups in the other two CCTs that applied PDLSC and PDLSC sheets[29,42]. Several factors might have contributed to the lack of significant differences in the outcomes, such as satisfactory scaffold material properties and small sample sizes. In these four studies, β-TCP, HA/TCP, and deproteinized bovine bone mineral (Bio-oss®) were applied as scaffold materials. Although some studies reported abilities to provide support for PDLSCs on osteogenic differentiation of these scaffoldsin vitro and in vivo[50-53], only using these scaffolds also achieved great clinical benefits in the treatment of periodontitis[54-56]. The excellent performance of the scaffold may have overshadowed the contribution by PDLSCs. More clinical studies at multiple centers with different amounts and types of DSCs, more follow-up time points, and larger sample sizes are necessary, and the results of such studies would be meaningful.
Bone defects caused by other conditions
In addition to periodontal intrabony defects, DSCs were also used for the treatment of post-extraction sockets, mandibular osteoradionecrosis, bone defects after ameloblastoma resection, and sinus lifting[57-61] (Table 2). Two split-mouth RCTs reported by Barbier et al[57] and Cubuk et al[62] did not find significant differences in BD or interdental septum height between the pulp micrograft + scaffold (collagen matrix/L-PRF) group and the scaffold (collagen matrix/L-PRF) group after implantation into post-extraction sockets. However, in another split-mouth CCT designed for regenerating post-extraction sockets, DPSCs combined with collagen sponges promoted the rate of mineralization, the levels of cortical bone, and the expression of bone morphogenetic protein-2 (BMP-2) and VEGF when compared with collagen sponge treatment alone[58]. Tanikawa et al[63] reported a historical control study comparing the effects of SHED, rhBMP, and iliac crest bone grafts in treating cleft lip and palate. The SHED group showed similar satisfactory performance in bone healing compared with iliac crest bone grafts and a higher bone filling percentage compared with the rhBMP group at the 6-mo follow-up[63].
Two case reports indicated that DPSCs combined with TCP could increase the bone regeneration of bone defects caused by osteoradionecrosis and ameloblastoma[59,60]. A case report by Brunelli et al[61] demonstrated that pulp micrografts + collagen sponges increased the BD in newly formed bone when applied for sinus lifting.
Other conditions
Koga et al[64] reported a case series that applied SHED conditioned medium (SHED-CM) to treat erectile dysfunction. In this study, the international index of erectile function (IIEF-5), which is clinically used to screen for erectile function and to assess treatment efficacy, was increased after SHED-CM injection into the corpus cavernosum of erectile dysfunction patients[64]. A case report indicated that SHED intravenous administrations could decrease the scale of unified Huntington’s disease rating, which is designed to assess clinical performance and capacity in patients with Huntington’s disease[65,66]. Meanwhile, the patient with Huntington’s disease also suffered from preexisting pulmonary nodules, and SHED injection did not result in long-term tropism or homing for the patient’s lung adenocarcinoma[65]. In a case report by Wang et al[30], GMSCs were used to treat plaque psoriasis via bolus injection, and they observed fully cleared psoriatic lesions without recurrence.
Three clinical study protocols using DSCs have been published in recent years, including the treatment of acute ischemic stroke, chronic disability after stroke, and COVID-19[67-69].
DSC-BASED CLINICAL TRIALS FROM CLINICAL DATABASES
ClinicalTrials.gov (https://clinicaltrials.gov/) and the International Clinical Trials Registry Platform (ICTRP, https://trialsearch.who.int/) were screened for DSC-based clinical trials.
To date, there have been 21 clinical trials registered on ClinicalTrials.gov evaluating the use of DSCs in treating periodontitis (33.3%, 7/21), post-extraction sockets (4.8%, 1/21), edentulous alveolar ridge (4.8%, 1/21), cleft lip and palate (9.5%, 2/21), knee osteoarthritis (4.8%, 1/21), dental pulp necrosis (4.8%, 1/21), liver cirrhosis (4.8%, 1/21), type 1 diabetes (4.8%, 1/21), acute ischemic stroke (4.8%, 1/21), Huntington’s disease (14.3%, 3/21), and COVID-19 (9.5%, 2/21) (Table 3). In addition to the 6 studies reported in ClinicalTrials.gov, 7 clinical trials were registered on the ICTRP using DSCs in the treatment of periodontitis (57.1%, 4/7), wrinkles (28.6%, 2/7), and hair loss (14.3%, 1/7) (Table 4). In all, 28 clinical trials were registered on these two platforms.
Table 3.
Dental stem cell-based clinical trials registered at clinicaltrials.gov
| Ref. |
Registration ID
|
Status
|
Diseases
|
Study design
|
Cell source
|
Administration route
|
Number of patients
|
Interventions
|
Follow-upperiod
|
Phase
|
Outcomes
|
|
|
Test group
|
Control group
|
|||||||||||
| - | NCT04983225 | Recruiting | Periodontitis | Randomized; parallel assignment; double-blind (participant, investigator) | Dental pulp | Injecting into the periodontal defect site | 36 | DPSCs (1 × 106)/site; DPSCs (5 × 106)/site; DPSCs (3-4 × 107)/three or four sites; DPSCs (1 × 107)/site; DPSCs (2 × 107)/two sites | Saline solution | 90, 180, 360, 720 d | Phase 1 | |
| - | NCT02523651 | Unknown | Periodontitis | Randomized; parallel assignment; triple-blind (participant, investigator, outcomes Assessor) | Allogeneic dental pulp | Injecting into the periodontal defect site | 40 | DPSCs (1 × 106) | Saline solution | 1 year | Phase 1/2 | |
| - | NCT03386877 | Completed | Periodontitis | Randomized; parallel assignment; triple-blind (participant, investigator, outcomes assessor) | Autologous dental pulp | Delivering into intrabony defect via minimally invasive surgical technique | 29 | Micrografts of DPSCs + Collagen sponge | Collagen sponge | 6, 12 mo | Not applicable | |
| - | NCT01082822 | Unknown | Periodontitis | Nonrandomized; parallel assignment; open label | Periodontal ligament | Implanted into bone defect sites via surgical approach | 80 | PDLSCs sheet fragment + DBBM (Bio-oss); PDLSCs sheet pellets + DBBM (Bio-oss); DBBM (Bio-oss) | Sham comparator | 4, 12, 24 wk; 1 year | Phase 1/2 | |
| - | NCT03638154 | Completed | Periodontitis | Randomized; parallel assignment; double-blind (care provider, outcomes assessor) | Gingival | Implanted into bone defect sites via surgical approach | 20 | GFs + GMSCs + β-TCP | β-TCP | 1, 3, 7, 14 d; 6 mo | Not applicable | |
| - | NCT03137979 | Unknown | Periodontitis | Randomized; parallel assignment | Gingival | Implanted into bone defect sites via surgical approach | 30 | GMSCs + Collagen scaffolds; collagen scaffolds | Open flap debridement | 1, 3, 6 mo | Phase1/2 | |
| Chen et al[29], 2016 | NCT01357785 | Unknown | Periodontitis | Randomized; parallel assignment; open label | Autologous periodontal ligament | 35 | None | 3-12 mo | Phase1 | |||
| Cubuk et al[62], 2023 | NCT04641533 | Completed | Post-extraction sockets | Split-mouth; randomized; crossover assignment; double-blind (investigator, outcomes assessor) | Dental pulp | Placing into the extraction socket | 13 | DPSCs + L-PRF | L-PRF | 7 d; 6 mo | Not applicable | |
| - | NCT02731586 | Unknown | Edentulous alveolar ridge | Single group assignment; open label | Allogeneic dental pulp | Introducing dental pulp-derived mesenchymal stem cells during placement of dental implants | 10 | Dental pulp-derived MSCs | None | 3 mo | Early Phase 1 | |
| Tanikawa et al[63], 2020; Pinheiro et al[70], 2019 | NCT03766217 | Completed | Cleft lip and palate | Randomized; parallel assignment; single-blind (outcomes assessor) | Autologous deciduous pulp | Placed into the alveolar defect via surgical approach | 62 | SHED + Hydroxyapatite-collagen sponge | Iliac crest autogenous bone graft | 15 d; 3, 6, 12 mo | Phase3 | |
| Tanikawa et al[63], 2020 | NCT01932164 | Completed; Has results | Cleft lip and palate | Single group assignment; open label | Autologous deciduous pulp | Maxillary alveolar graft by tissue engineering | 5 | SHED + Hydroxyapatite-collagen sponge | None | 3, 6 mo | Not applicable | Percentage of bone filling at 6 mo postoperatively: 89.5% |
| - | NCT04130100 | Unknown | Knee osteoarthritis | Randomized; parallel assignment; open label | Dental pulp | Intraarticular injection | 60 | Low dose of DPSCs; high dose of DPSCs | Sodium hyaluronate | 12 mo | Early phase 1 | |
| - | NCT01814436 | Unknown | Dental pulp necrosis | Single group assignment;open label | Autologous deciduous pulp | 80 | Scaffold-free SHED-derived pellet | None | 3-12 mo | Not applicable | ||
| - | NCT03957655 | Unknown | Liver cirrhosis | Randomized; parallel assignment; single-blind (outcomes assessor) | Autologous deciduous pulp | Peripheral vein infusion | 40 | SHED (1 × 106 cells/kg body weight) | Standard medication for viral hepatitis and cirrhosis | 4, 8, 12, 16, 24 wk | Early phase 1 | |
| - | NCT03912480 | Unknown | Type 1 diabetes | Single group assignment; open label | Deciduous pulp | Intravenous drip | 24 | SHED (0.11 IU/kg body weight) + Insulin + oral hypoglycemic drugs | None | 1, 2, 6 wk; 2, 3, 6, 9, 12 mo | Early phase 1 | |
| Suda et al[67], 2022 | NCT04608838 | Completed | Acute ischemic stroke | Randomized;Parallel assignment;Quadruple-blind (Participant, Care Provider, Investigator, Outcomes Assessor); | Allogeneic dental pulp | Intravenously infusion | 79 | DPSCs (JTR-161, 1 × 108 cells); DPSCs (JTR-161, 3 × 108 cells) | Placebo | 91, 366 d | Phase 1/2 | |
| - | NCT02728115 | Active, not recruiting | Nonrandomized; parallel assignment; open label | Allogeneic deciduous pulp | Intravenous administration | 6 | SHED (Cellavita HD, 1 × 106 cells); SHED (Cellavita HD, 2 × 106 cells) | None | 1, 4 years | Phase 1 | ||
| - | NCT04219241 | Active, not recruiting | Huntington’s disease | Single group assignment; open label | Allogeneic deciduous pulp | Intravenous administration | 35 | SHED (Cellavita HD, 2 × 106 cells) | None | 1, 2 years | Phase 2/3 | |
| Wenceslau et al[71], 2022 | NCT03252535 | Completed | Huntington’s disease | Randomized; parallel assignment; triple-blind (participant, investigator, outcomes assessor) | Allogeneic deciduous pulp | Intravenous administration | 35 | SHED (Cellavita HD, 1 × 106 cells); SHED (Cellavita HD, 2 × 106 cells) | Physiological solution without cells | Monthly for 14 mo | Phase 2 | |
| Ye et al[69], 2020 | NCT04336254 | Recruiting | COVID-19 | Randomized; parallel assignment; triple-blind (participant, investigator, outcomes assessor) | Allogeneic dental pulp | Intravenous injection | 20 | DPSCs (3 × 107 cells) | Saline | 28 d | Phase 1/2 | |
| - | NCT04302519 | Unknown | COVID-19 | Single group assignment; open label | Dental pulp | Intravenous injection | 24 | DPSCs (1 × 107 cells/kg body weight) | None | 3, 7, 14, 28, 360 d | Early phase 1 | |
DBBM: Deproteinized bovine bone mineral; GFs: Gingival fibroblast; PRF: Platelet-rich fibrin; TCP: Tricalcium phosphate; DPSCs: Dental pulp stem cells; SHED: Stem cells from exfoliated deciduous teeth.
Table 4.
Dental stem cell-based clinical trials registered on the International Clinical Trials Registry Platform
| Ref. |
Registration ID
|
Status
|
Diseases
|
Study design
|
Cell source
|
Administration route
|
Number of patients
|
Interventions
|
Follow-up period
|
Phase
|
Outcomes
|
|
|
Test group
|
Control group
|
|||||||||||
| - | JPRN-UMIN000042791 | Complete: Follow-up complete | Periodontitis | Randomized; parallel assignment; single-blind (participants) | Deciduous pulp | Gargle | 30 | Mouthwash containing SHED culture supernatant | Mouthwash without SHED culture supernatant | 1 mo | Not applicable | |
| - | ChiCTR2100051466 | Recruiting | Periodontitis | Randomized; parallel assignment; open label | Dental pulp | Bilateral multipoint injection on a single tooth | 96 | DPSCs (1 × 107 cells) for once; DPSCs (1 × 107 cells) for twice | Saline | 90, 180, 360 d | Phage 0 | |
| - | ChiCTR2100049178 | Pending | Periodontitis | Randomized; parallel assignment; double-blind | Dental pulp | Local injection | 36 | DPSCs (1 × 106 cells) for single injection; DPSCs (5 × 106 cells) for single injection; DPSCs (1 × 107 cells) for single injection; DPSCs (1 × 107 cells) for single injection in 2 locations; DPSCs (1 × 107 cells) for single injection in 3-4 locations | None | Phage 1 | ||
| Sánchez et al[42], 2020 | ISRCTN13093912 | Completed | Periodontitis | Randomized; parallel assignment; single-blind (patients and examiners) | Dental pulp | Implanted into bone defect sites via surgical approach | 20 | DPSCs (1 × 107 cells) + hydroxyapatite-collagen scaffold | Hydroxyapatite-collagen scaffold | 1, 2, 4, 12, 24, 36 wk; 12, 24, 36, 48, 60 mo | Not applicable | |
| - | JPRN-UMIN000045926 | Complete: Follow-up complete | Wrinkles | Randomized; parallel assignment; single-blind (outcomes assessor) | Dental pulp | 12 | All-in-one gel containing immortalized DPSCs-CM solution and various beauty ingredients | No treatment | 4 wk | Not applicable | ||
| - | JPRN-UMIN000043528 | Complete: Follow-up complete | Wrinkles | Randomized; parallel assignment; single-blind (outcomes assessor) | Dental pulp | 12 | All-in-one gel containing immortalized DPSC-CM solution and the latest peptide raw materials | No treatment | 4 wk | Not applicable | ||
| - | JPRN-UMIN000045897 | Complete: Follow-up continuing | Hair loss | Nonrandomized; parallel assignment; open label | Deciduous pulp | Injection | 22 | SHED-CM; after SHED-CM injection, one dose of micrografts (Rigenera) followed by another SHED-CM injection; SHED-CM injection after one dose of micrografts (Rigenera) | None | 6 mo | Not applicable | |
DPSCs: Dental pulp stem cells; SHED-CM: Stem cells from exfoliated deciduous teeth conditioned medium.
Several registered clinical trials applied two stages in one work. The most frequently appearing trial phases were phase 1 (42.9%, 12/28), followed by phase 2 (25%, 7/28), Phase 3 (7.1%, 2/28), and Phase 0 (3.6%, 1/28). There were 10 trials (35.7%) in which the phase design was not applied or not selected. One clinical trial reported the outcomes both on the registry platform and in a published article[63] (NCT01932164), and the published articles of seven trials stated the registered ID[29,42,62,63,67,69-71], while other trials did not publish any data.
Consistent with the literature, the proportion of clinical trials using DSCs to treat periodontitis was the highest. Eleven registered clinical trials researched the effect of DSCs on periodontitis (39.3%, 11/28). In these trials, various amounts, types, and injection times of DSCs and different application modes (such as DSCs, micrografts, cell sheet pellets, and cell sheet fragments) were applied. In addition, several scaffolds were used in combination with DSCs, including collagen sponges, deproteinized bovine bone minerals, β-TCP scaffolds, and hydroxyapatite-collagen scaffolds.
SAFETY ISSUES REGARDING DSC-BASED THERAPY
Although encouraging treatment effects on diseases have been achieved, the safety issues of stem cell-based therapy remain controversial, especially in long-term follow-up[72]. At present, the limitations of stem cell-based therapy are mainly focused on non-directional differentiation, accelerating tumor progression.
In addition, uncontrolled non-directional differentiation may have a great impact on the safety of stem cell transplantation. Breitbach et al[73] found that the encapsulated structures in the infarcted areas contained calcifications and/or ossifications in myocardial infarction mice after MSC injection. In another study, unselected bone marrow cells injected directly induced significant intramyocardial calcification in acutely infarcted myocardium[74].
Similar to the regeneration of damaged tissue, tumors exert chemotactic effects on MSCs, affecting their recruitment to tumor sites[75-77]. Current studies have shown that MSCs have bidirectional, anti-cancer and pro-cancer, regulatory effects, which raises safety concerns for clinical application. On the one hand, MSCs are the major component of the tumor microenvironment and can be reprogrammed to the pro-tumorigenic phenotype by the tumor[78]. MSCs have been revealed to participate in the initiation, development, progression, and metastasis of multiple cancers[79]. The pro-cancer effect of stem cells may be achieved by secreting molecules that affect the phenotype of tumor cells, promoting tumor angiogenesis, cancer-associated fibroblast differentiation, cell-to-cell contact, or cell engulfment[76]. In recent studies, DPSCs and their conditioned medium were reported to promote the proliferation and carcinogenic properties of prostate cancer, oral cancer, breast cancer, and melanoma cellsin vitro[80-82].
On the other hand, there is also evidence that MSCs can inhibit the growth of a variety of tumors, including breast cancer, Kaposi’s sarcoma, hepatoma, glioma, and melanoma[76,83-85]. DPSCs and their conditioned medium also showed a suppressive effect on the development and migration of colorectal cancer cells through mitogen-activated protein kinase pathways[86]. In fact, there are few reports of primary pulp malignancies[87]. In a genome-wide RNA-seq study, phosphatase and tensin homolog (PTEN) expression in DPSCs was higher than that in BMSCs[88]. PTEN, a phosphatase, can metabolize phosphatidylinositol 3,4,5-triphosphate and directly oppose the activation of the oncogenic PI3K/AKT/mTOR signaling network[89]. At present, the regulatory effects of stem cells on cancer are still controversial, and the difference in results may be related to cell lines, cell doses, animal models, cancer types, treatment duration time, and other factors.
In conclusion, no adverse events were reported in the published clinical articles or clinical trials using DSCs, which suggested the safety of DSC-based therapy. However, based on current concerns about the safety of stem cell therapy, more in vivo studies on the safety of DSC-based therapies are of great significance.
CURRENT LIMITATIONS AND PERSPECTIVES
Harvesting DSCs from inflamed tissue
Most studies applied stem cells extracted from healthy dental tissue for treatment, but additional surgery (such as third molar extraction) might increase patient suffering. Harvesting stem cells from inflamed dental tissue could be an alternative method, although stem cell abilities might be affected[36,90,91].
Several studies have researched the different biological properties of DPSCs derived from normal and inflamed pulps (iDPSCs), and the results are still in dispute[92-98]. In some studies, DPSCs showed better self-renewal ability[92,93] and multidirectional differentiation capacities than iDPSCs[92], while in other studies, no significant difference was observed[94,95,98]. A study by Nie et al[97] indicated that DPSCs showed higher colony-forming, proliferative, and osteo/dentinogenesis abilities, while iDPSCs demonstrated enhanced chondrogenesis, neurogenesis, angiogenesis, and adipogenesis capacities. Park et al[96] reported that iDPSCs appear to have higher osteogenic differentiation potential and lower neurogenic differentiation potential than DPSCs.
Differences in inflammation levels may explain the discrepancy in the biological properties of DPSCs and iDPSCs in various studies. Intense and rapid inflammatory stimulation irreversibly initiates pulp necrosis, while low insult levels of inflammation are able to cause reversible pulpitis and promote dentine regeneration[99]. DPSCs are a suitable source of stem cells for pulp nerve regeneration because of their neuronal differentiation potential. It was reported that acute inflammation with a high level of proinflammatory cytokines could reduce neural precursor cell (NPC) survival and inhibit the neuronal differentiation of NPCs, while chronic inflammation expressed a potentially neuroprotective phenotype and supported neuronal differentiation[100]. Meanwhile, age, sex, tooth position, and sample size are also confounding factors affecting the function of DPSCs, which should be considered in subsequent studies and clinical practice.
DSC-CM and DSC-EVs
The culture medium collected from cells in culture is known as CM. CM is applied as an alternative therapy for tissue regeneration, which is a less ethical issue because it uses cells indirectly. Koga et al[64] applied SHED-CM in the treatment of erectile dysfunction, which is the only record of its clinical use to the best of our knowledge.
DSC-CM contains a variety of cytokines associated with vascular and nerve tissue regeneration, such as VEGF, BDNF, β-NGF, GDNF and neurotrophin-3 (NT-3)[101,102]. To date, DSC-CM has been reported to have the potential to promote bone regeneration[103], periodontal regeneration[104], angiogenesis[105], pulp regeneration[106], and nerve protection/regeneration[105,107-109] with great possibilities for clinical application.
In addition, DSC-CM showed satisfactory anti-inflammatory and immunoregulatory effects. Several in vivo studies based on various animal models reported that intravenous injection or intranasal administration of SHED-CM improved liver fibrosis[110], acute liver failure[111], acute lung injury[112], Alzheimer’s disease, temporomandibular joint osteoarthritis[113], Sjögren’s syndrome[114], and rheumatoid arthritis[115] by exerting anti-inflammatory effects. Meanwhile, studies have also reported the effect of SHED-CM on promoting Treg cell differentiation[114] and M2-like macrophage induction[111,112], as well as inhibiting Th17 cell differentiation[114] and inflammatory macrophage activation[116].
In addition to DSC-CM, DSC-EVs harvested from cell-culture medium have also been deeply studied in recent years. Multiple studies have indicated the promotion effect of DSC-EVs on jawbone and calvarial bone regeneration[117,118], angiogenesis and cutaneous wound healing in vivo[119,120]. Li et al[121] also reported that DSC-EVs could alleviate cerebral ischemia-reperfusion by suppressing the inflammatory response, which is related to the inhibition of the HMGB1/TLR4/MyD88/NF-κB pathway.
The poor survival rate of implanted DSCs and host immunogenic reactions are the main drawbacks of applying DSCs directly. In some comparative studies, stem cell-derived CM showed similar and even better treatment effects on acute lung injury, Parkinsonism, and type 1 diabetes than the direct use of stem cells[112,122,123]. DSC-CM and its components (such as EVs) provide several key advantages over cell-based applications, including avoiding the risk of host immunogenic reactions, cost-effectiveness, long-term storage capacity, and simpler evaluation of safety and efficacy[104,124]. Accumulating evidence indicates the great potential of DSC-CM/DSC-EV-based treatment in clinical applications.
Expanding-free strategy
Despite encouraging results of differentiation and tissue regeneration, DSCs still require rigorous cell-expanding procedures to obtain a sufficient number of cells for treatment, which is costly with great technique sensitivity, often taking tens of days. The ex vivo expansion of stem cells often reduces their self-renewal and proliferation abilities[125]. Direct mechanical digestion or tissue transplantation are promising solutions to these limitations.
In recent years, using mechanical disaggregation of dental tissues instead of cell-expending procedures was successful for harvesting autologous pulp micrografts rich in progenitor cells[41,126]. In 2016, Monti et al[126] indicated that DSCs harvested by mechanical digestion (Rigenera® system, HBW, Turin, Italy) were fully comparable to stem cells obtained after enzymatic digestion. In this study, mechanical digestion-obtained DPSCs showed osteogenic, adipogenic, and chondrogenic differentiation abilitiesin vitro and were able to increase the regeneration of post-extraction sockets in vivo when applied with the collagen sponge[126].
Pulp micrografts harvested by mechanical digestion were also applied in the treatment of sinus lifting, post-extraction sockets, and periodontal intrabony defects[46,47,49,57,61,62]. One clinical trial using pulp micrografts was also designed for periodontitis management (NCT03386877), but the outcome was not reported. Different systems of mechanical disaggregation were applied in these studies, including BD Medimachine (BD Biosciences San Jose, CA, United States)[62], the Rigenera® system (HBW, Turin, Italy)[46,57,61], and the Medimachine System (Consul TS, Orbassano, Italy)[47,49]. In brief, dental pulp is first collected from extracted teeth and then sent to the mechanical disaggregation system to obtain pulp micrografts. After filtration or without filtration, pulp micrografts are combined with the scaffold for transplantation.
In addition, Vandana et al[125] described a novel approach using stem cell assistance in the periodontal regeneration technique (SAI-PRT), which contained periodontal ligament soft tissue gelatin sponge scaffolds and cementum scrapings. In their research, SAI-PRT successfully bypassedin vitro culture and expanded PDLSCs, resulting in satisfactory defect filling of periodontal intrabony defects[125].
Embryonic stem cells, PSCs, and DSCs
Embryonic stem cells (ESCs) are pluripotent cells of great significance to developmental biology. They give rise to all types of germ layer cells in the embryo. The self-renewal ability and plasticity of ESCs make it possible to generate unlimited numbers of different types of cells in vitro[127]. Similar to embryonic cells, PSCs derived from different somatic cells also have the ability to immortalize and differentiate into the three germ layers[128]. The properties of these two cell types make them promising sources for stem cell-based therapy for various diseases and injuries. However, due to the limitations of ESCs and PSCs, adult stem cells (such as DSCs) still possess high application value.
First, ethical issues regarding the use of ESCs make their clinical application challenging[128]. Second, the preparation of autologous PSCs takes a long time (more than 3 mo) and has high medical cost, and the immune rejection issue of allotransplantation should be considered[129]. In addition, teratomas are germ cell tumors containing cells of two or three germ lines that always occur via uncontrollable stem cell proliferation and differentiation[130,131]. In experimental studies, stem cell transplants (especially ESC and PSC transplants) have been found to increase the risk of teratomas, raising safety concerns[131-133]. Previously, viral vector integration and contamination of animal-derived components also posed obstacles to the use of PSCs, but these problems have been addressed by innovative techniques, such as integration-free methods and xeno-free culture[134-136].
DSCs did not show unlimited proliferation potential and demonstrated poorer differentiation ability than PSCs and ESCs[137]. However, the advantages of DSCs over ESCs and PSCs, such as fewer ethical issues and lower teratoma risk[87,88,138], lower cost and shorter preparation period, harvesting from medical waste, and implementing therapeutic effects without gene editing, grant them greater potential for clinical applications in the future.
CONCLUSION
Many clinical articles and clinical trials of autologous and allogeneic DSCs have aimed to evaluate their therapeutic effects on various diseases, such as pulpitis, periapical lesions, periodontitis, cleft lip and palate and Huntington’s disease. In most studies, satisfactory clinical treatment results were obtained, while clinical benefits of using DSCs were not found in some research. Although safety risks exist for stem cell-based therapies, safety issues have not been reported in the clinical applications of DSCs. In the future, in addition to continuing to study the efficacy and safety of DSC-based treatment, harvesting DSCs from inflammatory tissues, expanding-free strategies, and applying DSC-CM or DSC-EVs should be studied, as they have strong research value and application potential. Taken together, DSC-based therapy is a promising tool for the treatment of various diseases and can be further promoted.
ACKNOWLEDGEMENTS
We thank our friend Han-Yi Dong for designing and drawing Figure 1.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 5, 2022
First decision: January 11, 2023
Article in press: March 8, 2023
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Collart-Dutilleul PY, France; Ventura C, Italy S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
Contributor Information
Wen-Peng Song, Department of Stomatology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Lu-Yuan Jin, Department of General Dentistry and Integrated Emergency Dental Care, Beijing Stomatological Hospital, Capital Medical University, Beijing 100050, China.
Meng-Di Zhu, Department of General Dentistry and Integrated Emergency Dental Care, Beijing Stomatological Hospital, Capital Medical University, Beijing 100050, China.
Hao Wang, Department of Stomatology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Deng-Sheng Xia, Department of General Dentistry and Integrated Emergency Dental Care, Beijing Stomatological Hospital, Capital Medical University, Beijing 100050, China. dsxia@mail.ccmu.edu.cn.
References
- 1.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85:3–10. doi: 10.1159/000345615. [DOI] [PubMed] [Google Scholar]
- 3.Gan L, Liu Y, Cui D, Pan Y, Zheng L, Wan M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020;2020:8864572. doi: 10.1155/2020/8864572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizisin AP, Wiley CA, Hughes RA, Powell HC. Peripheral nerve demyelination in rabbits after inoculation with Freund's complete adjuvant alone or in combination with lipid haptens. J Neuroimmunol. 1987;16:381–395. doi: 10.1016/0165-5728(87)90112-3. [DOI] [PubMed] [Google Scholar]
- 6.Mai Z, Chen H, Ye Y, Hu Z, Sun W, Cui L, Zhao X. Translational and Clinical Applications of Dental Stem Cell-Derived Exosomes. Front Genet. 2021;12:750990. doi: 10.3389/fgene.2021.750990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aydin S, Şahin F. Stem Cells Derived from Dental Tissues. Adv Exp Med Biol. 2019;1144:123–132. doi: 10.1007/5584_2018_333. [DOI] [PubMed] [Google Scholar]
- 8.Heng BC, Jiang S, Yi B, Gong T, Lim LW, Zhang C. Small molecules enhance neurogenic differentiation of dental-derived adult stem cells. Arch Oral Biol. 2019;102:26–38. doi: 10.1016/j.archoralbio.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Bonaventura G, Incontro S, Iemmolo R, La Cognata V, Barbagallo I, Costanzo E, Barcellona ML, Pellitteri R, Cavallaro S. Dental mesenchymal stem cells and neuro-regeneration: a focus on spinal cord injury. Cell Tissue Res. 2020;379:421–428. doi: 10.1007/s00441-019-03109-4. [DOI] [PubMed] [Google Scholar]
- 10.Nuti N, Corallo C, Chan BM, Ferrari M, Gerami-Naini B. Multipotent Differentiation of Human Dental Pulp Stem Cells: a Literature Review. Stem Cell Rev Rep. 2016;12:511–523. doi: 10.1007/s12015-016-9661-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhai Q, Dong Z, Wang W, Li B, Jin Y. Dental stem cell and dental tissue regeneration. Front Med. 2019;13:152–159. doi: 10.1007/s11684-018-0628-x. [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y, Nakamura-Yamada S, Konoki R, Baba S. Promising advances in clinical trials of dental tissue-derived cell-based regenerative medicine. Stem Cell Res Ther. 2020;11:175. doi: 10.1186/s13287-020-01683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 15.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Lee AE, Xu Q, Zhang Q, Le AD. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine - A Comprehensive Review. Front Immunol. 2021;12:667221. doi: 10.3389/fimmu.2021.667221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014;28:1634–1643. doi: 10.1096/fj.13-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattei V, Martellucci S, Pulcini F, Santilli F, Sorice M, Delle Monache S. Regenerative Potential of DPSCs and Revascularization: Direct, Paracrine or Autocrine Effect? Stem Cell Rev Rep. 2021;17:1635–1646. doi: 10.1007/s12015-021-10162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junior AL, Pinheiro CCG, Tanikawa DYS, Ferreira JRM, Amano MT, Bueno DF. Mesenchymal Stem Cells from Human Exfoliated Deciduous Teeth and the Orbicularis Oris Muscle: How Do They Behave When Exposed to a Proinflammatory Stimulus? Stem Cells Int. 2020;2020:3670412. doi: 10.1155/2020/3670412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fageeh HN. Preliminary Evaluation of Proliferation, Wound Healing Properties, Osteogenic and Chondrogenic Potential of Dental Pulp Stem Cells Obtained from Healthy and Periodontitis Affected Teeth. Cells. 2021;10 doi: 10.3390/cells10082118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin L, Du L. The role of secreted factors in stem cells-mediated immune regulation. Cell Immunol. 2018;326:24–32. doi: 10.1016/j.cellimm.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhou LL, Liu W, Wu YM, Sun WL, Dörfer CE, Fawzy El-Sayed KM. Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters. Stem Cells Int. 2020;2020:1327405. doi: 10.1155/2020/1327405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan ZR, Roberts AI, Shi S, Le AD, Shi Y. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan F, Liu O, Zhang H, Zhou Y, Zhou D, Zhou Z, He Y, Tang Z, Wang S. Human dental pulp stem cells regulate allogeneic NK cells' function via induction of anti-inflammatory purinergic signalling in activated NK cells. Cell Prolif. 2019;52:e12595. doi: 10.1111/cpr.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botelho J, Cavacas MA, Machado V, Mendes JJ. Dental stem cells: recent progresses in tissue engineering and regenerative medicine. Ann Med. 2017;49:644–651. doi: 10.1080/07853890.2017.1347705. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Cao Y, Xie Y, Wang H, Fan Z, Wang J, Zhang C, Wu CT, Wang S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res Ther. 2016;7:130. doi: 10.1186/s13287-016-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, An Y, Zhang C, Wang S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010;28:1829–1838. doi: 10.1002/stem.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, Liu S, Liu W, Hu C, Shi S, Jin Y. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aaf3227. [DOI] [PubMed] [Google Scholar]
- 29.Chen FM, Gao LN, Tian BM, Zhang XY, Zhang YJ, Dong GY, Lu H, Chu Q, Xu J, Yu Y, Wu RX, Yin Y, Shi S, Jin Y. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. doi: 10.1186/s13287-016-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang SG, Hsu NC, Wang SM, Wang FN. Successful Treatment of Plaque Psoriasis with Allogeneic Gingival Mesenchymal Stem Cells: A Case Study. Case Rep Dermatol Med. 2020;2020:4617520. doi: 10.1155/2020/4617520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 32.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Chan CKF, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, Ransom RC, Reinisch A, Wearda T, Murphy M, Brewer RE, Koepke LS, Marecic O, Manjunath A, Seo EY, Leavitt T, Lu WJ, Nguyen A, Conley SD, Salhotra A, Ambrosi TH, Borrelli MR, Siebel T, Chan K, Schallmoser K, Seita J, Sahoo D, Goodnough H, Bishop J, Gardner M, Majeti R, Wan DC, Goodman S, Weissman IL, Chang HY, Longaker MT. Identification of the Human Skeletal Stem Cell. Cell. 2018;175:43–56.e21. doi: 10.1016/j.cell.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, Nolta J, Phinney DG, Sensebe L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima M, Fukuyama F, Iohara K. Pulp Regenerative Cell Therapy for Mature Molars: A Report of 2 Cases. J Endod. 2022;48:1334–1340.e1. doi: 10.1016/j.joen.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Meza G, Urrejola D, Saint Jean N, Inostroza C, López V, Khoury M, Brizuela C. Personalized Cell Therapy for Pulpitis Using Autologous Dental Pulp Stem Cells and Leukocyte Platelet-rich Fibrin: A Case Report. J Endod. 2019;45:144–149. doi: 10.1016/j.joen.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima M, Iohara K, Murakami M, Nakamura H, Sato Y, Ariji Y, Matsushita K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther. 2017;8:61. doi: 10.1186/s13287-017-0506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiehzadeh V, Aghmasheh F, Shiehzadeh F, Joulae M, Kosarieh E. Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: new method and three case reports. Indian J Dent Res. 2014;25:248–253. doi: 10.4103/0970-9290.135937. [DOI] [PubMed] [Google Scholar]
- 39.Prasad MGS, Ramakrishna J, Babu DN. Allogeneic stem cells derived from human exfoliated deciduous teeth (SHED) for the management of periapical lesions in permanent teeth: Two case reports of a novel biologic alternative treatment. J Dent Res Dent Clin Dent Prospects. 2017;11:117–122. doi: 10.15171/joddd.2017.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghana Shyam Prasad M, Juvva R, Babu Duvvi N. Towards a New Era in the Management of Large Periapical Lesion in Permanent Tooth Using Stemcells: A 2-Year Clinical Application Report. J Dent (Shiraz) 2019;20:137–140. doi: 10.30476/DENTJODS.2019.44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrarotti F, Romano F, Gamba MN, Quirico A, Giraudi M, Audagna M, Aimetti M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J Clin Periodontol. 2018;45:841–850. doi: 10.1111/jcpe.12931. [DOI] [PubMed] [Google Scholar]
- 42.Sánchez N, Fierravanti L, Núñez J, Vignoletti F, González-Zamora M, Santamaría S, Suárez-Sancho S, Fernández-Santos ME, Figuero E, Herrera D, García-Sanz JA, Sanz M. Periodontal regeneration using a xenogeneic bone substitute seeded with autologous periodontal ligament-derived mesenchymal stem cells: A 12-month quasi-randomized controlled pilot clinical trial. J Clin Periodontol. 2020;47:1391–1402. doi: 10.1111/jcpe.13368. [DOI] [PubMed] [Google Scholar]
- 43.Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, Wang BB, Huang GT, Wang S, Shi S. Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 2010;16:20–28. doi: 10.1111/j.1601-0825.2009.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwata T, Yamato M, Washio K, Yoshida T, Tsumanuma Y, Yamada A, Onizuka S, Izumi Y, Ando T, Okano T, Ishikawa I. Periodontal regeneration with autologous periodontal ligament-derived cell sheets - A safety and efficacy study in ten patients. Regen Ther. 2018;9:38–44. doi: 10.1016/j.reth.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kl V, Ryana H, Dalvi PJ. Autologous periodontal stem cell assistance in periodontal regeneration technique (SAI-PRT) in the treatment of periodontal intrabony defects: A case report with one-year follow-up. J Dent Res Dent Clin Dent Prospects. 2017;11:123–126. doi: 10.15171/joddd.2017.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aimetti M, Ferrarotti F, Gamba MN, Giraudi M, Romano F. Regenerative Treatment of Periodontal Intrabony Defects Using Autologous Dental Pulp Stem Cells: A 1-Year Follow-Up Case Series. Int J Periodontics Restorative Dent. 2018;38:51–58. doi: 10.11607/prd.3425. [DOI] [PubMed] [Google Scholar]
- 47.Aimetti M, Ferrarotti F, Cricenti L, Mariani GM, Romano F. Autologous dental pulp stem cells in periodontal regeneration: a case report. Int J Periodontics Restorative Dent. 2014;34 Suppl 3:s27–s33. doi: 10.11607/prd.1635. [DOI] [PubMed] [Google Scholar]
- 48.Hernández-Monjaraz B, Santiago-Osorio E, Ledesma-Martínez E, Alcauter-Zavala A, Mendoza-Núñez VM. Retrieval of a periodontally compromised tooth by allogeneic grafting of mesenchymal stem cells from dental pulp: A case report. J Int Med Res. 2018;46:2983–2993. doi: 10.1177/0300060518773244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aimetti M, Ferrarotti F, Mariani GM, Cricenti L, Romano F. Use of Dental Pulp Stem Cells/Collagen Sponge Biocomplex in the Treatment of Non-Contained Intrabony Defects: A Case Series. Clin Adv Periodontics. 2015;5:104–109. doi: 10.1902/cap.2013.130047. [DOI] [PubMed] [Google Scholar]
- 50.He H, Yu J, Cao J, E L, Wang D, Zhang H, Liu H. Biocompatibility and Osteogenic Capacity of Periodontal Ligament Stem Cells on nHAC/PLA and HA/TCP Scaffolds. J Biomater Sci Polym Ed. 2011;22:179–194. doi: 10.1163/092050609X12587018007767. [DOI] [PubMed] [Google Scholar]
- 51.Xia L, Zhang Z, Chen L, Zhang W, Zeng D, Zhang X, Chang J, Jiang X. Proliferation and osteogenic differentiation of human periodontal ligament cells on akermanite and β-TCP bioceramics. Eur Cell Mater. 2011;22:68–82; discussion 83. doi: 10.22203/ecm.v022a06. [DOI] [PubMed] [Google Scholar]
- 52.Yu BH, Zhou Q, Wang ZL. Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio-Oss scaffolds for ectopic and in situ bone formation: A comparative study in the rat. J Biomater Appl. 2014;29:243–253. doi: 10.1177/0885328214521846. [DOI] [PubMed] [Google Scholar]
- 53.Fu X, Jin L, Ma P, Fan Z, Wang S. Allogeneic stem cells from deciduous teeth in treatment for periodontitis in miniature swine. J Periodontol. 2014;85:845–851. doi: 10.1902/jop.2013.130254. [DOI] [PubMed] [Google Scholar]
- 54.Maroo S, Murthy KR. Treatment of periodontal intrabony defects using β-TCP alone or in combination with rhPDGF-BB: a randomized controlled clinical and radiographic study. Int J Periodontics Restorative Dent. 2014;34:841–847. doi: 10.11607/prd.2030. [DOI] [PubMed] [Google Scholar]
- 55.Zafiropoulos GK, Hoffmann O, Kasaj A, Willershausen B, Weiss O, Van Dyke TE. Treatment of Intrabony Defects Using Guided Tissue Regeneration and Autogenous Spongiosa Alone or Combined With Hydroxyapatite/β-Tricalcium Phosphate Bone Substitute or Bovine-Derived Xenograft. J Periodontol. 2007;78:2216–2225. doi: 10.1902/jop.2007.070146. [DOI] [PubMed] [Google Scholar]
- 56.Camelo M, Nevins ML, Schenk RK, Simion M, Rasperini G, Lynch SE, Nevins M. Clinical, radiographic, and histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Gide. Int J Periodontics Restorative Dent. 1998;18:321–331. [PubMed] [Google Scholar]
- 57.Barbier L, Ramos E, Mendiola J, Rodriguez O, Santamaria G, Santamaria J, Arteagoitia I. Autologous dental pulp mesenchymal stem cells for inferior third molar post-extraction socket healing: A split-mouth randomised clinical trial. Med Oral Patol Oral Cir Bucal. 2018;23:e469–e477. doi: 10.4317/medoral.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.d'Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 59.Manimaran K, Sankaranarayanan S, Ravi VR, Elangovan S, Chandramohan M, Perumal SM. Treatment of osteoradionecrosis of mandible with bone marrow concentrate and with dental pulp stem cells. Ann Maxillofac Surg. 2014;4:189–192. doi: 10.4103/2231-0746.147130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manimaran K, Sharma R, Sankaranarayanan S, Perumal SM. Regeneration of mandibular ameloblastoma defect with the help of autologous dental pulp stem cells and buccal pad of fat stromal vascular fraction. Ann Maxillofac Surg. 2016;6:97–100. doi: 10.4103/2231-0746.186128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brunelli G, Motroni A, Graziano A, D'Aquino R, Zollino I, Carinci F. Sinus lift tissue engineering using autologous pulp micro-grafts: A case report of bone density evaluation. J Indian Soc Periodontol. 2013;17:644–647. doi: 10.4103/0972-124X.119284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cubuk S, Oduncuoglu BF, Alaaddinoglu EE. The effect of dental pulp stem cells and L-PRF when placed into the extraction sockets of impacted mandibular third molars on the periodontal status of adjacent second molars: a split-mouth, randomized, controlled clinical trial. Oral Maxillofac Surg. 2023;27:59–68. doi: 10.1007/s10006-022-01045-2. [DOI] [PubMed] [Google Scholar]
- 63.Tanikawa DYS, Pinheiro CCG, Almeida MCA, Oliveira CRGCM, Coudry RA, Rocha DL, Bueno DF. Deciduous Dental Pulp Stem Cells for Maxillary Alveolar Reconstruction in Cleft Lip and Palate Patients. Stem Cells Int. 2020;2020:6234167. doi: 10.1155/2020/6234167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koga S, Horiguchi Y. Efficacy of a cultured conditioned medium of exfoliated deciduous dental pulp stem cells in erectile dysfunction patients. J Cell Mol Med. 2022;26:195–201. doi: 10.1111/jcmm.17072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.da Silva JM, Araldi RP, Colozza-Gama GA, Pagani E, Sid A, Valverde CW, Kerkis I. Human Immature Dental Pulp Stem Cells Did Not Graft into a Preexisting Human Lung Adenocarcinoma. Case Rep Oncol. 2022;15:413–422. doi: 10.1159/000523896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Unified Huntington's Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 67.Suda S, Nito C, Ihara M, Iguchi Y, Urabe T, Matsumaru Y, Sakai N, Kimura K J- REPAIR trial group. Randomised placebo-controlled multicentre trial to evaluate the efficacy and safety of JTR-161, allogeneic human dental pulp stem cells, in patients with Acute Ischaemic stRoke (J-REPAIR) BMJ Open. 2022;12:e054269. doi: 10.1136/bmjopen-2021-054269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagpal A, Kremer KL, Hamilton-Bruce MA, Kaidonis X, Milton AG, Levi C, Shi S, Carey L, Hillier S, Rose M, Zacest A, Takhar P, Koblar SA. TOOTH (The Open study Of dental pulp stem cell Therapy in Humans): Study protocol for evaluating safety and feasibility of autologous human adult dental pulp stem cell therapy in patients with chronic disability after stroke. Int J Stroke. 2016;11:575–585. doi: 10.1177/1747493016641111. [DOI] [PubMed] [Google Scholar]
- 69.Ye Q, Wang H, Xia X, Zhou C, Liu Z, Xia ZE, Zhang Z, Zhao Y, Yehenala J, Wang S, Zhou G, Hu K, Wu B, Wu CT, He Y. Safety and efficacy assessment of allogeneic human dental pulp stem cells to treat patients with severe COVID-19: structured summary of a study protocol for a randomized controlled trial (Phase I / II) Trials. 2020;21:520. doi: 10.1186/s13063-020-04380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinheiro CCG, Leyendecker Junior A, Tanikawa DYS, Ferreira JRM, Jarrahy R, Bueno DF. Is There a Noninvasive Source of MSCs Isolated with GMP Methods with Better Osteogenic Potential? Stem Cells Int. 2019;2019:7951696. doi: 10.1155/2019/7951696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wenceslau CV, de Souza DM, Mambelli-Lisboa NC, Ynoue LH, Araldi RP, da Silva JM, Pagani E, Haddad MS, Kerkis I. Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington's Disease 3-NP Rat Model. Cells. 2022;11 doi: 10.3390/cells11101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36–45. doi: 10.7150/ijms.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 74.Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–3157. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 75.Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front Bioeng Biotechnol. 2020;8:43. doi: 10.3389/fbioe.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14:195. doi: 10.1186/s13045-021-01208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Merckx G, Lo Monaco M, Lambrichts I, Himmelreich U, Bronckaers A, Wolfs E. Safety and Homing of Human Dental Pulp Stromal Cells in Head and Neck Cancer. Stem Cell Rev Rep. 2021;17:1619–1634. doi: 10.1007/s12015-021-10159-1. [DOI] [PubMed] [Google Scholar]
- 78.Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol. 2018;35:69–79. doi: 10.1016/j.smim.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16:31. doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doğan A, Demirci S, Apdik H, Apdik EA, Şahin F. Dental pulp stem cells (DPSCs) increase prostate cancer cell proliferation and migration under in vitro conditions. Tissue Cell. 2017;49:711–718. doi: 10.1016/j.tice.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Raj AT, Kheur S, Khurshid Z, Sayed ME, Mugri MH, Almasri MA, Al-Ahmari MM, Patil VR, Bhandi S, Testarelli L, Patil S. The Growth Factors and Cytokines of Dental Pulp Mesenchymal Stem Cell Secretome May Potentially Aid in Oral Cancer Proliferation. Molecules. 2021;26 doi: 10.3390/molecules26185683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raj AT, Kheur S, Bhonde R, Mani VR, Baeshen HA, Patil S. Assessing the effect of human dental pulp mesenchymal stem cell secretome on human oral, breast, and melanoma cancer cell lines. Saudi J Biol Sci. 2021;28:6556–6567. doi: 10.1016/j.sjbs.2021.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, Kang SK, Lee YS, Kang KS. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289–298, 1 p following 298. doi: 10.1080/14653240902807026. [DOI] [PubMed] [Google Scholar]
- 84.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L, Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 86.Nikkhah E, Kalalinia F, Asgharian Rezaee M, Tayarani-Najaran Z. Suppressive effects of dental pulp stem cells and its conditioned medium on development and migration of colorectal cancer cells through MAPKinase pathways. Iran J Basic Med Sci. 2021;24:1292–1300. doi: 10.22038/ijbms.2021.58273.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Neuhaus KW. Teeth: malignant neoplasms in the dental pulp? Lancet Oncol. 2007;8:75–78. doi: 10.1016/S1470-2045(06)71013-0. [DOI] [PubMed] [Google Scholar]
- 88.Shen WC, Lai YC, Li LH, Liao K, Lai HC, Kao SY, Wang J, Chuong CM, Hung SC. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat Commun. 2019;10:2226. doi: 10.1038/s41467-019-10197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Álvarez-Garcia V, Tawil Y, Wise HM, Leslie NR. Mechanisms of PTEN loss in cancer: It's all about diversity. Semin Cancer Biol. 2019;59:66–79. doi: 10.1016/j.semcancer.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Li X, Makarov SS. An essential role of NF-kappaB in the "tumor-like" phenotype of arthritic synoviocytes. Proc Natl Acad Sci U S A. 2006;103:17432–17437. doi: 10.1073/pnas.0607939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 92.Alongi DJ, Yamaza T, Song Y, Fouad AF, Romberg EE, Shi S, Tuan RS, Huang GT. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med. 2010;5:617–631. doi: 10.2217/rme.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Z, Pan J, Wright JT, Bencharit S, Zhang S, Everett ET, Teixeira FB, Preisser JS. Putative stem cells in human dental pulp with irreversible pulpitis: an exploratory study. J Endod. 2010;36:820–825. doi: 10.1016/j.joen.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pereira LO, Rubini MR, Silva JR, Oliveira DM, Silva IC, Poças-Fonseca MJ, Azevedo RB. Comparison of stem cell properties of cells isolated from normal and inflamed dental pulps. Int Endod J. 2012;45:1080–1090. doi: 10.1111/j.1365-2591.2012.02068.x. [DOI] [PubMed] [Google Scholar]
- 95.Attar A, Eslaminejad MB, Tavangar MS, Karamzadeh R, Dehghani-Nazhvani A, Ghahramani Y, Malekmohammadi F, Hosseini SM. Dental pulp polyps contain stem cells comparable to the normal dental pulps. J Clin Exp Dent. 2014;6:e53–e59. doi: 10.4317/jced.51305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park YT, Lee SM, Kou X, Karabucak B. The Role of Interleukin 6 in Osteogenic and Neurogenic Differentiation Potentials of Dental Pulp Stem Cells. J Endod. 2019;45:1342–1348. doi: 10.1016/j.joen.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 97.Nie SC, Yang K, Luan NN, Lian XL, Dai XH, Liang SX, Yan YB. Unveiling the Differences in Biological Properties of Dental Pulp Stem Cells from Normal and Inflamed Pulp: A Comprehensive Comparative Study. Med Sci Monit. 2022;28:e934511. doi: 10.12659/MSM.934511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tomasello L, Mauceri R, Coppola A, Pitrone M, Pizzo G, Campisi G, Pizzolanti G, Giordano C. Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: a potential application for bone formation. Stem Cell Res Ther. 2017;8:179. doi: 10.1186/s13287-017-0633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zaky SH, Shehabeldin M, Ray H, Sfeir C. The role of inflammation modulation in dental pulp regeneration. Eur Cell Mater. 2021;41:184–193. doi: 10.22203/eCM.v041a13. [DOI] [PubMed] [Google Scholar]
- 100.Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56:412–425. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- 101.Kato M, Tsunekawa S, Nakamura N, Miura-Yura E, Yamada Y, Hayashi Y, Nakai-Shimoda H, Asano S, Hayami T, Motegi M, Asano-Hayami E, Sasajima S, Morishita Y, Himeno T, Kondo M, Kato Y, Izumoto-Akita T, Yamamoto A, Naruse K, Nakamura J, Kamiya H. Secreted Factors from Stem Cells of Human Exfoliated Deciduous Teeth Directly Activate Endothelial Cells to Promote All Processes of Angiogenesis. Cells. 2020;9 doi: 10.3390/cells9112385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hochuli AHD, Senegaglia AC, Selenko AH, Fracaro L, Brofman PRS. Dental Pulp from Human Exfoliated Deciduous Teeth-derived Stromal Cells Demonstrated Neuronal Potential: In vivo and In Vitro Studies. Curr Stem Cell Res Ther. 2021;16:495–506. doi: 10.2174/1574888X16666210215160402. [DOI] [PubMed] [Google Scholar]
- 103.Hiraki T, Kunimatsu R, Nakajima K, Abe T, Yamada S, Rikitake K, Tanimoto K. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. 2020;26:381–390. doi: 10.1111/odi.13244. [DOI] [PubMed] [Google Scholar]
- 104.Lin H, Chen H, Zhao X, Chen Z, Zhang P, Tian Y, Wang Y, Ding T, Wang L, Shen Y. Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration. J Transl Med. 2021;19:456. doi: 10.1186/s12967-021-03125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Makino E, Nakamura N, Miyabe M, Ito M, Kanada S, Hata M, Saiki T, Sango K, Kamiya H, Nakamura J, Miyazawa K, Goto S, Matsubara T, Naruse K. Conditioned media from dental pulp stem cells improved diabetic polyneuropathy through anti-inflammatory, neuroprotective and angiogenic actions: Cell-free regenerative medicine for diabetic polyneuropathy. J Diabetes Investig. 2019;10:1199–1208. doi: 10.1111/jdi.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Cara SPHM, Origassa CST, de Sá Silva F, Moreira MSNA, de Almeida DC, Pedroni ACF, Carvalho GL, Cury DP, Câmara NOS, Marques MM. Angiogenic properties of dental pulp stem cells conditioned medium on endothelial cells in vitro and in rodent orthotopic dental pulp regeneration. Heliyon. 2019;5:e01560. doi: 10.1016/j.heliyon.2019.e01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ueda T, Ito T, Inden M, Kurita H, Yamamoto A, Hozumi I. Stem Cells From Human Exfoliated Deciduous Teeth-Conditioned Medium (SHED-CM) is a Promising Treatment for Amyotrophic Lateral Sclerosis. Front Pharmacol. 2022;13:805379. doi: 10.3389/fphar.2022.805379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sugimura-Wakayama Y, Katagiri W, Osugi M, Kawai T, Ogata K, Sakaguchi K, Hibi H. Peripheral Nerve Regeneration by Secretomes of Stem Cells from Human Exfoliated Deciduous Teeth. Stem Cells Dev. 2015;24:2687–2699. doi: 10.1089/scd.2015.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsuruta T, Sakai K, Watanabe J, Katagiri W, Hibi H. Dental pulp-derived stem cell conditioned medium to regenerate peripheral nerves in a novel animal model of dysphagia. PLoS One. 2018;13:e0208938. doi: 10.1371/journal.pone.0208938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hirata M, Ishigami M, Matsushita Y, Ito T, Hattori H, Hibi H, Goto H, Ueda M, Yamamoto A. Multifaceted Therapeutic Benefits of Factors Derived From Dental Pulp Stem Cells for Mouse Liver Fibrosis. Stem Cells Transl Med. 2016;5:1416–1424. doi: 10.5966/sctm.2015-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matsushita Y, Ishigami M, Matsubara K, Kondo M, Wakayama H, Goto H, Ueda M, Yamamoto A. Multifaceted therapeutic benefits of factors derived from stem cells from human exfoliated deciduous teeth for acute liver failure in rats. J Tissue Eng Regen Med. 2017;11:1888–1896. doi: 10.1002/term.2086. [DOI] [PubMed] [Google Scholar]
- 112.Wakayama H, Hashimoto N, Matsushita Y, Matsubara K, Yamamoto N, Hasegawa Y, Ueda M, Yamamoto A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy. 2015;17:1119–1129. doi: 10.1016/j.jcyt.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 113.Ogasawara N, Kano F, Hashimoto N, Mori H, Liu Y, Xia L, Sakamaki T, Hibi H, Iwamoto T, Tanaka E, Yamamoto A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental temporomandibular joint osteoarthritis. Osteoarthritis Cartilage. 2020;28:831–841. doi: 10.1016/j.joca.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 114.Matsumura-Kawashima M, Ogata K, Moriyama M, Murakami Y, Kawado T, Nakamura S. Secreted factors from dental pulp stem cells improve Sjögren's syndrome via regulatory T cell-mediated immunosuppression. Stem Cell Res Ther. 2021;12:182. doi: 10.1186/s13287-021-02236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ishikawa J, Takahashi N, Matsumoto T, Yoshioka Y, Yamamoto N, Nishikawa M, Hibi H, Ishigro N, Ueda M, Furukawa K, Yamamoto A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental rheumatoid arthritis. Bone. 2016;83:210–219. doi: 10.1016/j.bone.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 116.Muto H, Ito T, Tanaka T, Yokoyama S, Yamamoto K, Imai N, Ishizu Y, Maeda K, Honda T, Ishikawa T, Kato A, Ohshiro T, Kano F, Yamamoto A, Sakai K, Hibi H, Ishigami M, Fujishiro M. Conditioned medium from stem cells derived from human exfoliated deciduous teeth ameliorates NASH via the Gut-Liver axis. Sci Rep. 2021;11:18778. doi: 10.1038/s41598-021-98254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Imanishi Y, Hata M, Matsukawa R, Aoyagi A, Omi M, Mizutani M, Naruse K, Ozawa S, Honda M, Matsubara T, Takebe J. Efficacy of extracellular vesicles from dental pulp stem cells for bone regeneration in rat calvarial bone defects. Inflamm Regen. 2021;41:12. doi: 10.1186/s41232-021-00163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee AE, Choi JG, Shi SH, He P, Zhang QZ, Le AD. DPSC-Derived Extracellular Vesicles Promote Rat Jawbone Regeneration. J Dent Res. 2023;102:313–321. doi: 10.1177/00220345221133716. [DOI] [PubMed] [Google Scholar]
- 119.Zhou Z, Zheng J, Lin D, Xu R, Chen Y, Hu X. Exosomes derived from dental pulp stem cells accelerate cutaneous wound healing by enhancing angiogenesis via the Cdc42/p38 MAPK pathway. Int J Mol Med. 2022;50 doi: 10.3892/ijmm.2022.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Merckx G, Hosseinkhani B, Kuypers S, Deville S, Irobi J, Nelissen I, Michiels L, Lambrichts I, Bronckaers A. Angiogenic Effects of Human Dental Pulp and Bone Marrow-Derived Mesenchymal Stromal Cells and their Extracellular Vesicles. Cells. 2020;9 doi: 10.3390/cells9020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li S, Luo L, He Y, Li R, Xiang Y, Xing Z, Li Y, Albashari AA, Liao X, Zhang K, Gao L, Ye Q. Dental pulp stem cell-derived exosomes alleviate cerebral ischaemia-reperfusion injury through suppressing inflammatory response. Cell Prolif. 2021;54:e13093. doi: 10.1111/cpr.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abdelwahab S, Elsebay SAG, Fouli Gaber M, Abdel-Hafez SMN. Comparative study between bone marrow mesenchymal stem cell and their conditioned medium in the treatment of rat model of Parkinsonism. J Cell Physiol. 2021;236:440–457. doi: 10.1002/jcp.29872. [DOI] [PubMed] [Google Scholar]
- 123.Hashemi SM, Hassan ZM, Hossein-Khannazer N, Pourfathollah AA, Soudi S. Investigating the route of administration and efficacy of adipose tissue-derived mesenchymal stem cells and conditioned medium in type 1 diabetic mice. Inflammopharmacology. 2020;28:585–601. doi: 10.1007/s10787-019-00661-x. [DOI] [PubMed] [Google Scholar]
- 124.Kovach TK, Dighe AS, Lobo PI, Cui Q. Interactions between MSCs and immune cells: implications for bone healing. J Immunol Res. 2015;2015:752510. doi: 10.1155/2015/752510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vandana KL, Desai R, Dalvi PJ. Autologous Stem Cell Application in Periodontal Regeneration Technique (SAI-PRT) Using PDLSCs Directly From an Extracted Tooth···An Insight. Int J Stem Cells. 2015;8:235–237. doi: 10.15283/ijsc.2015.8.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Monti M, Graziano A, Rizzo S, Perotti C, Del Fante C, d'Aquino R, Redi CA, Rodriguez Y Baena R. In Vitro and In vivo Differentiation of Progenitor Stem Cells Obtained After Mechanical Digestion of Human Dental Pulp. J Cell Physiol. 2017;232:548–555. doi: 10.1002/jcp.25452. [DOI] [PubMed] [Google Scholar]
- 127.Vazin T, Freed WJ. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor Neurol Neurosci. 2010;28:589–603. doi: 10.3233/RNN-2010-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020;27:523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 129.Ohnuki M, Takahashi K. Present and future challenges of induced pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140367. doi: 10.1098/rstb.2014.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Anisimov SV, Morizane A, Correia AS. Risks and mechanisms of oncological disease following stem cell transplantation. Stem Cell Rev Rep. 2010;6:411–424. doi: 10.1007/s12015-010-9134-5. [DOI] [PubMed] [Google Scholar]
- 131.Wang H, Gong P, Li J, Fu Y, Zhou Z, Liu L. Role of CD133 in human embryonic stem cell proliferation and teratoma formation. Stem Cell Res Ther. 2020;11:208. doi: 10.1186/s13287-020-01729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Han L, He H, Yang Y, Meng Q, Ye F, Chen G, Zhang J. Distinctive Clinical and Pathologic Features of Immature Teratomas Arising from Induced Pluripotent Stem Cell-Derived Beta Cell Injection in a Diabetes Patient. Stem Cells Dev. 2022;31:97–101. doi: 10.1089/scd.2021.0255. [DOI] [PubMed] [Google Scholar]
- 133.Lee MO, Moon SH, Jeong HC, Yi JY, Lee TH, Shim SH, Rhee YH, Lee SH, Oh SJ, Lee MY, Han MJ, Cho YS, Chung HM, Kim KS, Cha HJ. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A. 2013;110:E3281–E3290. doi: 10.1073/pnas.1303669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 135.Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K, Morizane A, Doi D, Takahashi J, Nishizawa M, Yoshida Y, Toyoda T, Osafune K, Sekiguchi K, Yamanaka S. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep. 2014;4:3594. doi: 10.1038/srep03594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miyazaki T, Futaki S, Suemori H, Taniguchi Y, Yamada M, Kawasaki M, Hayashi M, Kumagai H, Nakatsuji N, Sekiguchi K, Kawase E. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat Commun. 2012;3:1236. doi: 10.1038/ncomms2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suman S, Domingues A, Ratajczak J, Ratajczak MZ. Potential Clinical Applications of Stem Cells in Regenerative Medicine. Adv Exp Med Biol. 2019;1201:1–22. doi: 10.1007/978-3-030-31206-0_1. [DOI] [PubMed] [Google Scholar]
- 138.Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396:489–497. doi: 10.1007/s00423-011-0762-2. [DOI] [PubMed] [Google Scholar]
- 139.Sharpe PT. Dental mesenchymal stem cells. Development. 2016;143:2273–2280. doi: 10.1242/dev.134189. [DOI] [PubMed] [Google Scholar]
- 140.Bakopoulou A, Apatzidou D, Aggelidou E, Gousopoulou E, Leyhausen G, Volk J, Kritis A, Koidis P, Geurtsen W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects "stemness" properties. Stem Cell Res Ther. 2017;8:247. doi: 10.1186/s13287-017-0705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Niehage C, Karbanová J, Steenblock C, Corbeil D, Hoflack B. Cell Surface Proteome of Dental Pulp Stem Cells Identified by Label-Free Mass Spectrometry. PLoS One. 2016;11:e0159824. doi: 10.1371/journal.pone.0159824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shoi K, Aoki K, Ohya K, Takagi Y, Shimokawa H. Characterization of pulp and follicle stem cells from impacted supernumerary maxillary incisors. Pediatr Dent. 2014;36:79–84. [PubMed] [Google Scholar]
- 143.Hu L, Zhao B, Gao Z, Xu J, Fan Z, Zhang C, Wang J, Wang S. Regeneration characteristics of different dental derived stem cell sheets. J Oral Rehabil. 2020;47 Suppl 1:66–72. doi: 10.1111/joor.12839. [DOI] [PubMed] [Google Scholar]
- 144.Li D, Zou XY, El-Ayachi I, Romero LO, Yu Z, Iglesias-Linares A, Cordero-Morales JF, Huang GT. Human Dental Pulp Stem Cells and Gingival Mesenchymal Stem Cells Display Action Potential Capacity In Vitro after Neuronogenic Differentiation. Stem Cell Rev Rep. 2019;15:67–81. doi: 10.1007/s12015-018-9854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sabbagh J, Ghassibe-Sabbagh M, Fayyad-Kazan M, Al-Nemer F, Fahed JC, Berberi A, Badran B. Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. J Dent. 2020;101:103413. doi: 10.1016/j.jdent.2020.103413. [DOI] [PubMed] [Google Scholar]
- 146.Kumar A, Kumar V, Rattan V, Jha V, Pal A, Bhattacharyya S. Molecular spectrum of secretome regulates the relative hepatogenic potential of mesenchymal stem cells from bone marrow and dental tissue. Sci Rep. 2017;7:15015. doi: 10.1038/s41598-017-14358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Trubiani O, Pizzicannella J, Caputi S, Marchisio M, Mazzon E, Paganelli R, Paganelli A, Diomede F. Periodontal Ligament Stem Cells: Current Knowledge and Future Perspectives. Stem Cells Dev. 2019;28:995–1003. doi: 10.1089/scd.2019.0025. [DOI] [PubMed] [Google Scholar]
- 148.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 149.Kawanabe N, Murata S, Murakami K, Ishihara Y, Hayano S, Kurosaka H, Kamioka H, Takano-Yamamoto T, Yamashiro T. Isolation of multipotent stem cells in human periodontal ligament using stage-specific embryonic antigen-4. Differentiation. 2010;79:74–83. doi: 10.1016/j.diff.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 150.Kang J, Fan W, Deng Q, He H, Huang F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. Biomed Res Int. 2019;2019:6104738. doi: 10.1155/2019/6104738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bakopoulou A, Leyhausen G, Volk J, Koidis P, Geurtsen W. Comparative characterization of STRO-1(neg)/CD146(pos) and STRO-1(pos)/CD146(pos) apical papilla stem cells enriched with flow cytometry. Arch Oral Biol. 2013;58:1556–1568. doi: 10.1016/j.archoralbio.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 152.Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, Wang S, Shi S. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1:5. doi: 10.1186/scrt5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–116. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 154.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamaza T, Alatas FS, Yuniartha R, Yamaza H, Fujiyoshi JK, Yanagi Y, Yoshimaru K, Hayashida M, Matsuura T, Aijima R, Ihara K, Ohga S, Shi S, Nonaka K, Taguchi T. In vivo hepatogenic capacity and therapeutic potential of stem cells from human exfoliated deciduous teeth in liver fibrosis in mice. Stem Cell Res Ther. 2015;6:171. doi: 10.1186/s13287-015-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bi R, Lyu P, Song Y, Li P, Song D, Cui C, Fan Y. Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules. 2021;11 doi: 10.3390/biom11070997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang Q, Nguyen AL, Shi S, Hill C, Wilder-Smith P, Krasieva TB, Le AD. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev. 2012;21:937–947. doi: 10.1089/scd.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ansari S, Chen C, Xu X, Annabi N, Zadeh HH, Wu BM, Khademhosseini A, Shi S, Moshaverinia A. Muscle Tissue Engineering Using Gingival Mesenchymal Stem Cells Encapsulated in Alginate Hydrogels Containing Multiple Growth Factors. Ann Biomed Eng. 2016;44:1908–1920. doi: 10.1007/s10439-016-1594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]