Abstract
Nitrogen (N) and potassium (K) are essential macronutrients for plants. Sufficient N and K uptake from the environment is required for successful growth and development. However, how N and K influence each other at the molecular level in plants is largely unknown. In this study, we found loss-of-function mutation in SLAH3 (SLAC1 HOMOLOGUE 3), encoding a NO3− efflux channel in Arabidopsis thaliana, enhanced tolerance to high KNO3 concentrations. Surprisingly, slah3 mutants were less sensitive to high K+ but not NO3−. Addition of NO3− led to reduced phenotypic difference between wild-type and slah3 plants, suggesting SLAH3 orchestrates NO3−-K+ balance. Non-invasive Micro-test Technology analysis revealed reduced NO3− efflux and enhanced K+ efflux in slah3 mutants, demonstrating that SLAH3-mediated NO3− transport and SLAH3-affected K+ flux are critical in response to high K +. Further investigation showed that two K+ efflux channels, GORK (GATED OUTWARDLY-RECTIFYING K+ CHANNEL) and SKOR (STELAR K+ OUTWARD RECTIFIER), interacted with SLAH3 and played key roles in high K+ response. The gork and skor mutants were slightly more sensitive to high K+ conditions. Less depolarization occurred in slah3 mutants and enhanced depolarization was observed in gork and skor mutants upon K+ treatment, suggesting NO3−/K+ efflux-mediated membrane potential regulation is involved in high K+ response. Electrophysiological results showed that SLAH3 partially inhibited the activities of GORK and SKOR in Xenopus laevis oocytes. This study revealed that the anion channel SLAH3 interacts with the potassium channels GORK and SKOR to modulate membrane potential by coordinating N–K balance.
The anion channel SLAC1 HOMOLOGUE 3 (SLAH3) interacts with the potassium channels GORK and SKOR to modulate the membrane potential by coordinating the balance between nitrogen and potassium.
IN A NUTSHELL.
Background: The nitrogen (N)–potassium (K) balance not only profoundly affects plant growth but also serves as an essential signal to regulate plant–environment interactions. However, the detailed molecular mechanisms that maintain the N–K balance are largely unknown.
Question: How does the nitrate efflux channel SLAH3 regulate the N–K balance in plants?
Findings: The anion channel SLAH3 mediates NO3− efflux under high K+ conditions. The accelerated depolarization next causes the opening of the K+ channels GORK and SKOR to mediate K+ efflux. The channel–channel interaction may also be important for manipulating K+ channel activities. We found that the N–K balance is regulated by N and K channels, with both the membrane potential and the interactions between these membrane proteins modulating this regulation.
Next steps: As anion and cation channels function coordinately to regulate the N–K balance, the corresponding genes could be used to construct novel crop variants that can withstand diverse N–K environments through molecular breeding.
Introduction
Nitrogen (N) is an essential element for plants. N is the component of numerous organic compounds such as proteins, chlorophyll, plant hormones, and alkaloids (O'Brien et al., 2016). Plants absorb both inorganic and organic forms of N. Inorganic N mainly includes ammonium (NH4+) and nitrate (NO3−), whereas the organic forms of N include amino acids, amides, and urea. Inorganic N is the major N form absorbed by plants and nitrate is usually the most abundant source of inorganic N (Miller et al., 2007). The potassium ion (K+) is the most abundant cation in plants. K+ is involved in a variety of enzymatic reactions, protein synthesis, and photosynthesis. K+ also acts as an essential factor affecting cell turgor, xylem and phloem transport, pH homeostasis, and membrane potential. In addition, maintaining appropriate K+ concentrations in the plant cell cytoplasm is crucial for balancing the fluxes of cations and anions (Schachtman and Shin, 2007; Wang and Wu, 2013).
Most plants are sensitive to salt stress caused by high concentrations of sodium chloride (NaCl) (van Zelm et al., 2020). In contrast, the plant cells absorb K+ from the environment and maintain relatively high concentrations of K+ in cytosol. As a result, the concentrations of K+ in plant cell are usually higher than those in the soil (Maathuis, 2009). Therefore, most studies have focused on how plants respond to low K+ in the medium or soil, while little is known whether excess K+ causes salt stress in plants. Despite that plant growth and development are greatly affected by low K+, high concentrations of K+ can also cause toxicity in plants (Matoh et al., 1986; Ramos et al., 2004; Pantha et al., 2022). The molecular mechanisms underlying high K +-induced stress are not determined yet.
Earlier studies have shown that N and K, two indispensable macronutrients for plants, are closely related in terms of absorption, transplantation, and signaling (Armengaud et al., 2004; Zhang et al., 2010; Tsay et al., 2011; Vidal et al., 2020). The N–K balance not only dramatically affects plant growth and development by regulating cation–anion homeostasis but also functions as an essential signal for stress responses. However, most of the studies on N–K balance in plants are limited to physiological levels. The molecular mechanisms of N–K homeostasis regulation still await in-depth investigations. A few key components regulating N–K balance were previously identified. For instance, the nitrate transporter NRT1.5 has been shown to function in N–K balance in plants (Drechsler et al., 2015; Meng et al., 2016; Li et al., 2017; Chen et al., 2021). NRT1.5 acts as a K+/NO3− symporter to directly mediate K+ efflux and NO3− loading into xylem (Lin et al., 2008; Li et al., 2017). Moreover, a recent report indicated nitrate transporter NRT1.1 plays an important role in K+ absorption and relocation under low K+ conditions (Fang et al., 2020).
Plasma membrane (PM) separates the intracellular contents from apoplastic environment and also functions as an electrical barrier. Due to the activity of H +-ATPase localized to PM, the movement of positively charged protons (H+) across PM and H+ accumulation in apoplast create membrane potential that serves as a driving force for ion transportations through channels and transporters (Palmgren, 2001; Hedrich et al., 2016). The membrane potential is closely related to the external K+ concentrations as well (Wang and Wu, 2013). It is worth noting that the activation of anion channels mediated PM depolarization is crucial for dealing with the environmental stresses (Wayne, 1994; Felle and Zimmermann, 2007; Roelfsema et al., 2012; Lehmann et al., 2021). For example, in guard cell, upon the presence of signaling molecules such as abscisic acid (ABA), action potential is quickly provoked, which is initiated by depolarization caused by anion efflux. Next, the outward transportations of anions such as NO3− and Cl− further depolarize the membrane potential, which subsequently activates voltage-gated K+ channels that mediate K+ flux from cytosol to apoplast (Roelfsema et al., 2012). The neutralization of K+ and anions eventually leads to repolarizing to resting status. Thus, positively charged K+ and negatively charged anions play central roles in modulating membrane potential during responding to various extracellular and intracellular signals in plant cell (Felle and Zimmermann, 2007; Hedrich et al., 2016). However, the detailed molecular mechanisms underlying how K+ and anion channels contribute to membrane potential regulation are still largely unknown.
The SLOW ANION CHANNEL-ASSOCIATED/SLAC1 HOMOLOGUE (SLAC/SLAH) gene family was identified to encode a group of slow anion channels in Arabidopsis thaliana (Negi et al., 2008; Vahisalu et al., 2008; Hedrich and Geiger, 2017). There are five members in this family including SLAC1 and SLAH1 to 4 (Dreyer et al., 2012). The protein products of SLAC/SLAH genes contain 10 transmembrane helices with both N and C termini in cytosol. SLAC1 was the first functionally characterized gene member that is specifically expressed in guard cell (Negi et al., 2008; Vahisalu et al., 2008). SLAC1 plays a central role in regulating stomatal movement by mediating the efflux of anions such as Cl− and NO3− in Ca2+-dependent and Ca2+-independent manners (Geiger et al., 2009; Geiger et al., 2010; Guzel Deger et al., 2015; Zheng et al., 2018). Similarly, SLAH3, the closest homolog of SLAC1, was found to be expressed in guard cell and exerts important functions in stomatal movement upon biotic and abiotic stimuli (Geiger et al., 2011; Guzel Deger et al., 2015; Zheng et al., 2018). Different from SLAC1, SLAH3 exhibits a much higher affinity for NO3− over Cl− and is therefore considered a NO3− efflux channel (Geiger et al., 2011; Wang et al., 2012). In pollen tube, SLAH3 acts as a critical component regulating anion homeostasis (Gutermuth et al., 2013).
Our earlier studies revealed that SLAH3 also showed strong expression in root, suggesting its additional functions besides its roles in guard cell and pollen tube (Zheng et al., 2015; Sun et al., 2021). We previously showed that the null mutants of SLAH3 exhibited enhanced sensitivity to high NH4+ and low NO3− culture conditions. These results indicated SLAH3 acts as a key regulator in the process of nitrate-dependent alleviation of ammonium toxicity (Zheng et al., 2015; Sun et al., 2021; Xiao et al., 2022). Of note, a recent report indicated SLAH3 likely functions as a pH-sensor that can be activated by cytoplasmic H+ (Lehmann et al., 2021), which is consistent with our results that the enhanced ammonium toxic phenotype of slah3 mutants is caused by acidic conditions (Zheng et al., 2015; Sun et al., 2021; Xiao et al., 2022). The discoveries of the versatility of SLAH3 implicated its additional roles in regulating physiological processes other than controlling stomatal movement.
In this study, we found slah3 mutants were less sensitive to high concentrations of KNO3 compared to wild-type (WT) plants. Unexpectedly, slah3 mutants showed enhanced tolerance to high concentrations of K+, instead of NO3−. Moreover, the phenotypic difference between slah3 and WT upon high K+ treatment was reduced by exogenously applied NO3−, indicating SLAH3 plays a key role in N–K balance. The K +-induced membrane potential depolarization was reduced in slah3 mutants, suggesting NO3−-mediated membrane potential regulation is involved in this process. Furthermore, K+ channels GORK (GATED OUTWARDLY-RECTIFYING K+ CHANNEL) and SKOR (STELAR K+ OUTWARD RECTIFIER) were found to interact with SLAH3 and the gork and skor loss-of-function mutants showed slightly enhanced sensitivity to high K+ conditions. The K +-induced membrane potential depolarization was enhanced in gork and skor mutants. Consistently, the electrophysiological results indicated SLAH3 partially inhibited the channel activities of GORK and SKOR in Xenopus laevis oocytes. Overall, our findings reveal that NO3− channel SLAH3 functions together with K+ channels GORK and SKOR to influence N–K homeostasis and modulate membrane potential in plants.
Results
Loss-of-function slah3 mutants show enhanced tolerance to high concentrations of K+ but not NO3−
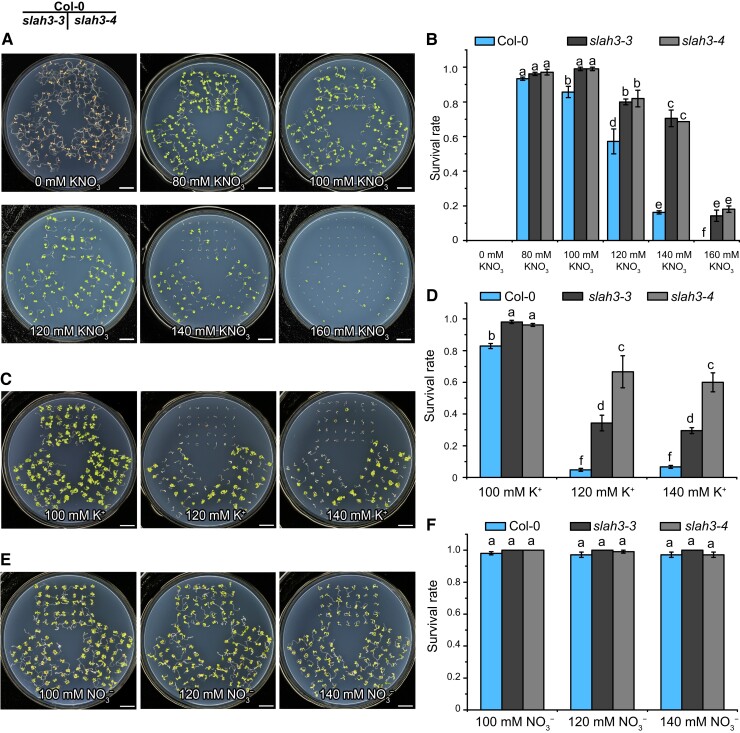
Our previous studies revealed that SLAH3 mediates NO3− efflux to alleviate ammonium toxicity (Zheng et al., 2015; Sun et al., 2021; Xiao et al., 2022). Considering that SLAH3 is a NO3− efflux channel, we set to investigate whether slah3 mutants exhibit different phenotypes from WT plants under high concentrations of NO3−. We cultured two null slah3 mutant alleles, slah3-3 and slah3-4, and Col-0 WT plants on N-free 1/2 MS medium supplemented with different concentrations of KNO3 as sole N source. High concentrations of KNO3 (120 and 140 mM) resulted in growth inhibition and reduced survival rates in Col-0 plants (Figure 1A). Compared to Col-0, slah3 mutants showed significantly enhanced resistance to high concentrations of KNO3 (Figure 1B). We postulated that the phenotypic difference between the Col-0 and slah3 was caused by excessive NO3− in the medium.
Figure 1.
The slah3 mutants show enhanced tolerance to high concentrations of K +. A, The phenotypes of Col-0 and slah3 mutants grown on the medium supplemented with different concentrations of KNO3. Seeds were germinated on ½ N-free MS medium supplemented with 0–160 mM KNO3. Seven-day-old seedlings were photographed. Bars = 1 cm. The phenotypes of Col-0 and slah3 mutants grown on the medium supplemented with different concentrations of K +. Seeds were germinated on 1/2 N-free MS medium supplemented with 100, 120, or 140 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1). Seven-day-old seedlings were photographed. Bars = 1 cm. E, The phenotypes of Col-0 and slah3 mutants grown on the medium supplemented with different concentrations of NO3−. Seeds were germinated on 1/2 N-free MS medium supplemented with 100, 120, or 140 mM NO3− ([KNO3]: [NaNO3]: [Mg(NO3)2] = 2:2:1). Seven-day-old seedlings were photographed. Bars = 1 cm. B, D, and F, The survival rates of plants grown on the KNO3 (B), K+ (D), and NO3− medium (F). Data are mean ± SD, n = 3 plates (105 seedlings). Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05).
To test the influence of K+, Col-0 and slah3 plants were cultured on N-free 1/2 MS medium supplemented with different concentrations of K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1) and NO3− ([KNO3]: [NaNO3]: [Mg(NO3)2] = 2:2:1), respectively. Unexpectedly, there was no phenotypic difference between slah3 and Col-0 on the NO3− medium, while slah3 mutants exhibited significantly higher survival rates than Col-0 on the K+ medium (Figure 1, C, D, E, and F). These results surprisingly suggested that slah3 is more tolerant to high concentrations of K+ and that NO3− alone causes no phenotypic difference between slah3 and Col-0.
Next, five-day-old Col-0 and slah3 seedlings germinated on 1/2 MS medium were transferred to high K+ (150–170 mM) or 1/2 MS medium for additional seven days. After the plants were transferred to 1/2 MS medium, the shoots and the roots of Col-0 and slah3 mutants exhibited no obvious phenotypic difference. When the plants were transferred to the high K+ medium, the shoots of Col-0 but not slah3 showed chlorosis (Supplemental Figure 1A). The lateral root length, lateral root density, and primary root length of slah3 were greater than those in Col-0 (Supplemental Figure 1, B, C, and D).
Since slah3 exhibited no noticeable phenotypic difference from Col-0 on the medium with 100 mM KNO3 but showed significant resistance on the medium with 120 mM KNO3 (Figure 1A), it suggested extra 20 mM K+ or NO3− distinguished the phenotypes of slah3 from WT plants. Therefore, in N-free 1/2 MS medium supplemented with 100 mM KNO3, 20 mM KCl, or 20 mM NaNO3 were supplied. In total, 20 mM NaCl was added as a control (Supplemental Figure 2A). The difference in survival rates between Col-0 and slah3 could only be recapitulated on the 100 mM KNO3 medium supplemented with 20 mM KCl (Supplemental Figure 2B).
To determine whether SLAH3 plays its role in K+ response in root or in shoot, we cultured the WT and slah3 mutant plants on 1/2 MS medium for five days. The seedlings were transferred to 180 mM K+ medium for an additional week. Only the roots, but not the shoots, of the plants were in contact with the culture medium. The slah3 mutant plants showed enhanced resistance to high K+ condition compared to WT (Supplemental Figure 3A). This phenotype was verified by the statistical analyses on primary root length, lateral root length, and lateral root density of the plants (Supplemental Figure 3, B, C, and D). These results indicated SLAH3 regulates high K+ responses mainly in root.
SLAH3 belongs to SLAC/SLAH family and has four homologous genes including SLAC1, SLAH1, SLAH2, and SLAH4. We explored the phenotypes of the null mutants of other SLAC/SLAH genes under high K+ conditions. Except for slah3-4, no additional slac/slah mutants showed altered phenotype to high K+ compared to Col-0 (Supplemental Figure 4, A and B). SLAH3 is likely the only SLAC/SLAH gene regulating high K+ response or plays dominant role in this process. The above results show that slah3 mutants specifically resist high potassium stress.
The tolerance of slah3 to high concentrations of K+ is not related to NA+, CL− or osmotic stress
In some cases, K+ and Na+, two major monovalent cations, show some similar effects in plants. For instance, high concentrations of either K+ or Na+ can cause osmotic stress in plants (Ramos et al., 2004). Besides NO3−, SLAC/SLAH also transport Cl−, which is critical for osmotic regulation as well. We tested whether Na+, Cl−, and/or osmotic stress can cause similar phenotype as high K+ did in slah3. The WT and slah3 mutant plants were grown on N-free 1/2 MS medium supplemented with 20 mM NaNO3 as N source plus different concentrations of NaCl (Supplemental Figure 5A). As a control, the plants were grown on 1/2 N-free MS medium supplemented with 20 mM KNO3 as N source plus different concentrations of KCl (Supplemental Figure 5B). The survival rates of slah3 on K +-containing medium were significantly higher than those of Col-0, while no phenotypic difference between slah3 and Col-0 was detected on Na +-containing medium (Supplemental Figure 5, C and D). The slah3 mutant plants seem to be specifically tolerant to high K+ but not high Na+ stress.
SLAH3 was previously reported to function in chloride loading in the root by interacting with SLAH1 (Cubero-Font et al., 2016). We thus examined whether Cl− affects slah3 phenotype. As a control, slah3 plants were obviously resistant to high K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1) conditions compared to Col-0 (Supplemental Figure 6A). The WT and slah3 mutant plants were grown on the medium supplemented with three chlorine salts. No phenotypic difference between Col-0 and slah3 was found under high Cl− ([KCl]: [NaCl]: [MgCl2] = 2:2:1) conditions (Supplemental Figure 6B). The statistical results also supported high K+, but not high Cl−, was able to distinguish Col-0 and slah3 (Supplemental Figure 6, C and D).
Under high K+ conditions, osmotic stress can be induced and affect plant growth. We examined whether the K +-triggered phenotypic difference in Col-0 and slah3 was related to osmotic stress. When WT and slah3 mutant plants were grown on the medium supplemented with 40 mM KNO3 as N source and 140 mM sorbitol in which the ionic strength equals 120 mM K+ (40 mM KNO3 + 40 mM KCl + 20 mM K2SO4), no phenotypic difference was found, suggesting the tolerance of slah3 to high K+ is unrelated to osmotic stress (Supplemental Figure 7, A and B).
SLAH3 is involved in nitrogen-potassium homeostasis
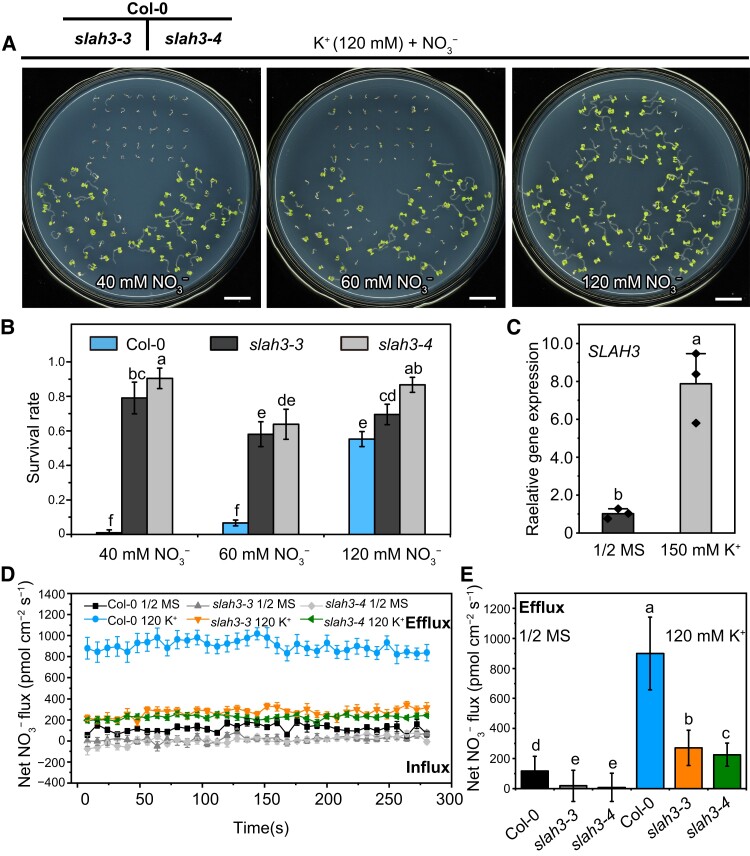
Next, the WT and slah3 plants were grown on the medium supplemented with fixed concentration of 120 mM K+ plus different concentrations of NO3− (Figure 2A). Interestingly, the application of NO3− greatly rescued the sensitive phenotype of WT plants under high K+ condition. When the concentration ratio of K+ to NO3− was 1:1 (120 mM K+ + 120 mM NO3−), the phenotypic difference between WT and slah3 mutant plants nearly disappeared (Figure 2, A and B). In addition, we found SLAH3 transcripts can be markedly induced upon high K+ treatment (Figure 2C).
Figure 2.
NO3− is involved in SLAH3-mediated high K+ tolerance. A, The phenotypes of Col-0 and slah3 mutants grown on the medium supplemented with different K+/NO3− ratios. Seeds were germinated on 1/2 N-free MS medium containing 120 mM K+ supplemented with 40 mM, 60 mM, or 120 mM NO3−. The ratios of K+/NO3− are 1:0.3, 1:0.5, and 1:1. Seven-day-old seedlings were photographed. Bars = 1 cm. B, The survival rates of the plants presented in (A). Data are mean ± Sd, n = 3 plates (105 seedlings). Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05). C, The SLAH3 transcripts are induced by high K +. qPCR was used to detect the expression levels of SLAH3 in Col-0 roots. The relative gene expression was normalized to the expression of ACT2 and was calculated according to the ΔΔCT method. The seven-day-old Col-0 seedlings were transferred to the 1/2 MS or 1/2 MS containing 150 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1) medium for 12 h. Data are mean ± Sd, n = 3 biological replicates. Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05). D, The net NO3− fluxes in the root mature zone. The time course of net NO3− fluxes of Col-0 and slah3 was detected using NMT. Col-0 and slah3 mutants were pretreated in liquid 1/2 MS 1/2 MS added with 120 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1) for 5 h. The experiment was then carried out in the test solution (1 mM KNO3, 0.1 mM KCl, 0.1 mM CaCl2, 0.3 mM MES and pH 6.0). Data are mean ± Se, n = 5 seedlings. E, The mean value of net NO3− fluxes in the root mature zone. Data are mean ± Sd, n = 5 seedlings. Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05).
To further verify the involvement of NO3− in the high K+ response mediated by SLAH3, the NO3− flow rates in the root mature zone of Col-0 and slah3 were tested by using Non-invasive Micro-test Technology (NMT). The plants were pretreated with or without 120 mM K+ in liquid 1/2 MS for 5 h. Without K+ treatment, the flow rates of NO3− efflux of slah3-3 and slah3-4 were obviously lower than those in Col-0, which is consistent with the function of SLAH3 as a NO3− efflux channel. The flow rates of NO3− efflux of Col-0 and slah3 dramatically elevated upon 120 mM K+ pre-treatment, suggesting high K+ induces NO3− efflux. The flow rates of NO3− efflux of Col-0 were significantly higher than slah3-3 and slah3-4, indicating the NO3− efflux in the plants pretreated with high K+ requires SLAH3 (Figure 2, D and E). These results indicated appropriate NO3− can alleviate high K+ toxicity and implicated SLAH3 mediates NO3− efflux under high K+ stress.
The channel activity of SLAH3 is important for its function in high potassium response
The complementation lines in slah3 background were next generated. Transformation of SLAH3 gene driven by its native promoter (ProSLAH3:SLAH3) in slah3-4 can restore its phenotype to WT-like under high K+ condition (Figure 3A). Quantitative real-time PCR (qPCR) was used to analyze the expression of SLAH3 in slah3-4 background (Figure 3B). The survival rates were greatly decreased (Figure 3C) and the NO3− efflux was recovered in the complementation lines (Figure 3D).
Figure 3.
The channel activity of SLAH3 is required for its role in high K+ response. A, E, and I, Col-0, slah3-4, and two independent complementation lines (ProSLAH3:SLAH3 (A), ProSLAH3:SLAH3F517L (E), and ProSLAH3:SLAH3G264D (I) in slah3-4) were germinated on 1/2 N-free MS medium supplemented with 120 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1). Seven-day-old seedlings were photographed. Bars = 1 cm. B, F, and J, qPCR was used to detect the expression levels of SLAH3 in Col-0, slah3-4, and the complementation lines (SLAH3 (B), SLAH3F517L (F), and SLAH3G264D (J) in slah3-4) under 120 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1) conditions. The relative gene expression was normalized to the expression of ACT2 and was calculated according to the ΔΔCT method. Data are mean ± Sd, n = 3 biological replicates. Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05). C, G, and K, The survival rates of Col-0, slah3-4, and the complementation lines (SLAH3 (C), SLAH3F517L (G), and SLAH3G264D (K) in slah3-4) under high K+ conditions. Data are mean ± Sd, n = 3 plates (90 seedlings). Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05). D, H, and L, The net NO3− fluxes in the root mature zone. The NO3− flow rates of Col-0, slah3-4, and complementation lines (SLAH3 (D), SLAH3F517L (H), and SLAH3G264D (L) in slah3-4) were detected using NMT. The plants were pretreated in liquid 1/2 MS added with 120 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1) for 5 h. The experiment was then carried out in the test solution (1 mM KNO3, 0.1 mM KCl, 0.1 mM CaCl2, 0.3 mM MES and pH 6.0). Data are mean ± Sd, n = 3 seedlings. Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05).
Previous study showed that the F450 amino acid residue was the key gating site for SLAC1, and SLAC1F450L was inactive in Xenopus oocytes (Chen et al., 2010). SLAC1G194D was also shown to have no channel activity by genetic and electrophysiological methods (Negi et al., 2008; Chen et al., 2010). The corresponding mutations in SLAH3 (F517L and G264D) also led to reduced and completely abolished activity in oocytes, respectively (Cubero-Font et al., 2016). To explore whether the channel activity of SLAH3 is required for its function in high K+ response, we generated slah3 mutant plants complemented with two SLAH3 mutant variants, F517L and G264D, respectively. Col-0, slah3-4, and slah3-4 complemented with SLAH3F517L or SLAH3G264D were grown on 120 mM K+ medium for seven days. SLAH3F517L can partially restore slah3 phenotype, while SLAH3G264D failed to alter slah3 phenotype (Figure 3, E G, I, and K). qPCR was used to verify the SLAH3 transcripts in the slah3 complementation plants (Figure 3, F and J). Next, we used NMT to analyze the NO3− flow rates of Col-0, slah3-4, and the complementation lines after pretreated in liquid 1/2 MS with 120 mM K+ for 5 h. In line with the phenotypes observed, the NMT results indicated SLAH3F517L possesses partial channel activity and SLAH3G264D is almost inactive (Figure 3, H and L). Taken together, these data demonstrated that the channel activity of SLAH3 is required for its role in responding to high K+ stress.
There are no significant differences in K+ and NO3− contents between Col-0 and slah3 under high K+ conditions
In order to understand whether the accumulation of K+ and NO3− affects the phenotype of slah3, we analyzed the K+ and NO3− contents in Col-0 and slah3 mutants. Col-0, slah3-3, and slah3-4 were cultured under 1/2 MS hydroponic condition for three weeks, and then the seedlings were transferred to 1/2 MS hydroponic solution with or without 150 mM K+ followed by measuring the K+ contents. After 150 mM K+ treatment for two days, the K+ concentrations in Col-0 and slah3 mutants were significantly higher than those grown under 1/2 MS condition. The K+ contents in slah3 mutants were almost the same as those in Col-0 (Figure 4A). Thus, the chlorosis phenotype of Col-0 grown on the high K+ medium is unlikely caused by the accumulation of excessive K +.
Figure 4.
There are no significant differences in K+ and NO3− contents between Col-0 and slah3 under high K+ conditions. A, The measurements of K+ contents in Col-0 and slah3 mutants. Seeds were cultured under 1/2 MS hydroponic conditions for three weeks, and then the seedlings were transferred to 1/2 MS or 1/2 MS hydroponic solution containing 150 mM K+ (50 mM KNO3 + 50 mM KCl + 25 mM K2SO4) for two days to detect the K+ contents in shoots and roots. B, The measurements of NO3− contents in Col-0 and slah3 mutants. Seeds were cultured under 1/2 N-free MS hydroponic medium with 10 mM KNO3 as nitrogen source for two weeks, and then the seedlings were transferred to 1/2 N-free MS hydroponic solution containing 50 mM K+ (50 mM KNO3) or 150 mM K+ (50 mM KNO3 + 50 mM KCl + 25 mM K2SO4) for five days to detect the NO3− contents in shoots and roots. Data in (A) and (B) are mean ± Sd, n = 3 biological replicates. Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05).
To analyze the NO3− contents, the plants were cultured in 1/2 N-free MS hydroponic medium supplemented with 10 mM KNO3 as N source for two weeks. The seedlings were transferred to 1/2 N-free MS hydroponic solution containing 50 mM K+ (50 mM KNO3) or 150 mM K+ (50 mM KNO3 + 50 mM KCl + 25 mM K2SO4) for five days to examine the NO3− contents in shoots and roots. High K+ treatment caused decreased NO3− abundance in the shoots of both WT plants and slah3 mutants. The NO3− concentration was slightly higher in slah3 mutants compared to that in WT upon 150 mM K+ treatment in the shoots. In the roots, high K+ led to increased NO3− in Col-0. The NO3− contents were higher in slah3 mutants than those in Col-0 before 150 mM K+ treatment. The NO3− contents in the roots were less affected by high K+ condition in slah3 mutants compared to Col-0 (Figure 4B). These results were consistent with previous report showing SLAH3 contributes to nitrate loading in the roots (Cubero-Font et al., 2016).
Since no significant differences of K+ and NO3− contents were detected in slah3 and WT plants under high K+ conditions, the phenotypic difference between Col-0 and slah3 is unlikely caused by excessive/deficient K+ and NO3− in plants.
K +-induced membrane potential depolarization is reduced in slah3 mutants
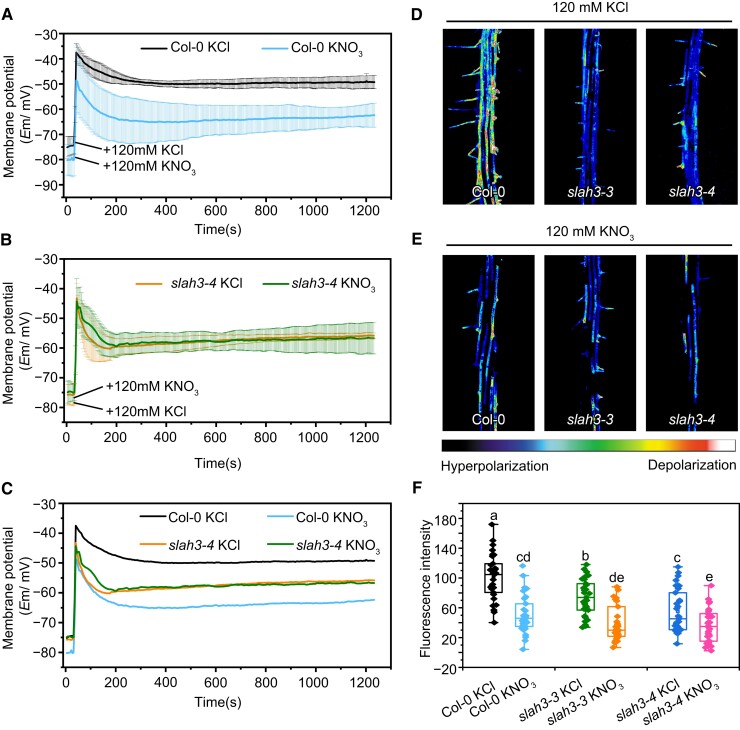
Cations and anions play crucial roles in the establishment and maintenance of membrane potential in plant cell. The changes of K+ concentrations in cytoplasm serve as a critical factor affecting membrane potential in plants (Wang and Wu, 2013). Similarly, the activation of anion efflux channels is necessary in the process of cell membrane potential depolarization (Felle and Zimmermann, 2007; Roelfsema et al., 2012). We thus investigated whether SLAH3 contributes to membrane potential by regulating NO3− and K+ balance. We analyzed the membrane potentials upon the treatment of high concentrations of KCl and KNO3 in the root cells of Col-0 and slah3 utilizing impaled microelectrodes. The plants were placed on 1/2 MS medium followed by the treatment of 120 mM KCl or 120 mM KNO3. In Col-0, prompt and severe membrane potential depolarization was induced when treated with high concentration of KCl, while the application of high concentration of KNO3 led to a lower level of depolarization (Figure 5, A and C). It indicated K+ leads to membrane potential depolarization and NO3− helps to repolarize membrane potential. By contrast, KCl caused less membrane potential depolarization in slah3. Intriguingly, KNO3 resulted in a membrane potential depolarization in slah3 almost identical to KCl, suggesting K +-induced depolarization is impaired in slah3 (Figure 5, B and C).
Figure 5.
Depolarization in slah3 is reduced upon K+ treatment. A, and B, Time courses of root membrane potential changes of Col-0 (A) and slah3-4 (B) in 1/2 MS medium followed by the treatment of 120 mM KCl or KNO3. An impaled microprobe was used to detect the membrane potential changes in the roots of seven-day-old seedlings. Data are mean ± Se, n = 8 seedling (C) Merged images of membrane potential diagrams of Col-0 (A) and slah3-4 (B). D, and E, Fluorescent dye DiBAC4(3) was used to detect the membrane potential. Five-day-old seedlings were placed in a treatment solution (pH 5.8) containing 15 μM DiBAC4(3) with 120 mM KCl (D) or 120 mM KNO3 (E), for 15 min. Laser scanning confocal microscopy was used for observation. Fluorescent pseudocolors represent membrane potential states. The warm colors indicate depolarization, and the cool colors indicate hyperpolarization. Pseudocolor range is shown at the bottom. F, The statistical analyses of the fluorescence intensities in Col-0 and slah3, n = 31 seedlings. Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05).
The involvement of SLAH3 in regulating membrane potential was further verified using the fluorescent dye DiBAC4(3). Depolarization of membrane potential causes transfer of additional DiBAC4(3) into the plasma membrane, leading to an increase of the fluorescence signal. Fluorescent pseudocolors represent membrane potential states. The warm colors indicate depolarization, and the cool colors indicate hyperpolarization. Treated with 120 mM KCl or 120 mM KNO3, the WT and slah3 mutant plants were stained with DiBAC4(3). The DiBAC4(3) staining results revealed that Col-0 root significantly depolarized compared to slah3 root under 120 mM KCl condition, but no obvious difference was observed when the plants were treated with 120 mM KNO3 (Figure 5, D E, and F). Instead, compared to WT plants, the slah3 mutants appeared to be insensitive to both KCl and KNO3 treatment.
The membrane potential results supported the observed phenotype that slah3 showed less sensitivity to high K+ alone. Exogenously applied NO3− contributed to membrane repolarization. We speculated that NO3− serves as an important anion for plant membrane potential regulation and that, more importantly, SLAH3 plays an essential role in modulating membrane potential by mediating NO3− efflux.
GORK and SKOR are involved in plant response to high potassium stress
The discovery of the involvement of SLAH3 in membrane potential regulation upon high K+ conditions inspired us to seek potential potassium channels functioning together with SLAH3 in this process. It is well established that potassium efflux channels are activated to release K+ to apoplast during repolarization in guard cell (Wang and Wu, 2013). We thus tested whether two outward-rectifying shaker-like potassium channels, GORK and SKOR, are involved in SLAH3-mediated membrane potential regulation, since GORK and SKOR are highly expressed in root and activated during depolarization (Wang and Wu, 2013).
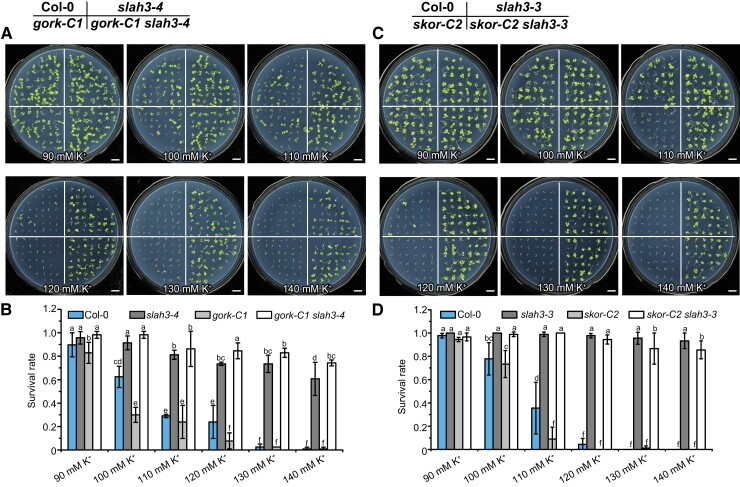
The gork-C1 and skor-C2 null mutants were generated by CRISPR/Cas9-mediated genome editing. The double mutants gork-C1 slah3-4 and skor-C2 slah3-3 were next generated (Supplemental Data Set 2). The WT and mutant plants were cultured on high K+ medium for seven days before the phenotypes were observed. gork-C1 exhibited a slightly more sensitive phenotype under 100 mM K+ compared to Col-0. The phenotype of gork-C1 slah3-4 was identical to slah3-4 (Figure 6, A and B). Similarly, skor-C2 showed reduced resistance phenotype under 110 mM K+ compared to Col-0. The double-mutant skor-C2 slah3-3 displayed a phenotype similar to slah3-3 (Figure 6, C and D). The gork-C1 skor-C2 double-mutant plants exhibited an additive phenotype compared to gork and skor single mutants. The triple-mutant gork-C1 skor-C2 slah3-4 displayed a phenotype similar to slah3-4 (Supplemental Figure 8, A and B). The genetic evidence suggested K+ efflux channels GORK and SKOR are involved in the response to high K+ stress. SLAH3 likely functions together with GORK and SKOR in the same physiological process and is epistatic to GORK and SKOR.
Figure 6.
Gork and skor show slight hypersensitivity to high K+ conditions. A, The phenotypes of Col-0, slah3-4, gork-C1, and gork-C1 slah3-4 grown on the medium supplemented with different concentrations of K +. Seeds were germinated on 1/2 N-free MS medium supplemented with 90–140 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1). Seven-day-old seedlings were photographed. Bars = 1 cm. B, The survival rates of the plants presented in (A). Data are mean ± Sd, n = 3 plates (90 seedlings). Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05). C, The phenotypes of Col-0, slah3-3, skor-C2, and skor-C2 slah3-3 grown on the medium supplemented with different concentrations of K +. Seeds were germinated on 1/2 N-free MS medium supplemented with 90–140 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1). Seven-day-old seedlings were photographed. Bars = 1 cm. D, The survival rates of the plants present in (C). Data are mean ± Sd, n = 3 plates (90 seedlings). Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05).
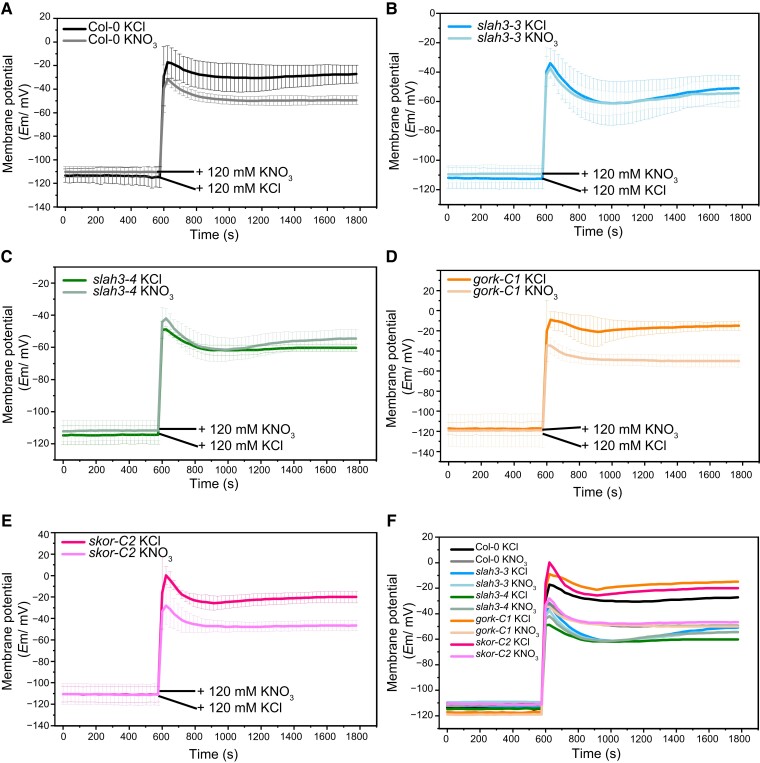
K +-induced membrane potential depolarization is enhanced in gork and skor mutants
Since depolarization in slah3 mutants is reduced upon K+ treatment, we next analyzed whether GORK and SKOR contribute to membrane potential modulation under high K+ conditions. Instead of measuring the plants in the 1/2 MS medium (Figure 5, A, B, and C), we verified the results by examining the membrane potential in a K +-, NO3−-, and Cl−-free solution. The membrane potential was detected by impaled microelectrodes in 0.5 g L−1 MES (pH 5.8) solution and then 120 mM KNO3 or 120 mM KCl was added into the solutions. Consistent with our earlier results, KCl caused stronger depolarization in Col-0 compared to KNO3 (Figure 7A). Upon KCl treatment, slah3 showed reduced depolarization which was similar to the treatment with KNO3 (Figure 7, B, C, and F). Compared to WT, enhanced depolarization was found in both gork and skor when K+ was supplied (Figure 7, D, E, and F). In addition, the membrane potential changes were detected by using DiBAC4(3). Similarly, slah3 showed reduced depolarization but gork and skor exhibited significantly stronger depolarization when treated with KCl (Supplemental Figure 9, A and B). The skor slah3 and gork slah3 double mutants displayed responses similar to slah3 single mutant, indicating SLAH3 functions upstream to GORK and SKOR in membrane potential regulation. The statistical analysis on fluorescence intensities supported that slah3 was less sensitive but gork and skor were more sensitive to high KCl conditions (Supplemental Figure 9C).
Figure 7.
K +-induced membrane potential depolarization is enhanced in gork and skor mutants. Time courses of root membrane potential changes in Col-0 (A), slah3-3 (B), slah3-4 (C), gork-C1 (D), and skor-C2 (E). The membrane potentials were measured in the roots of seven-day-old seedlings using an impaled microprobe in 0.5 g L−1 MES (pH 5.8) solution upon the treatment of 120 mM KCl or 120 mM KNO3. Data are mean ± Se, n = 4 seedlings. F, Merged images of membrane potential diagrams.
SLAH3 interacts with GORK and SKOR
GORK is expressed in root hair and epidermis to mediate K+ efflux and SKOR is expressed in root stellar tissues responsible for loading K+ into shoots (Gaymard et al., 1998; Ivashikina et al., 2001). We constructed transgenic plants harboring ProSLAH3: NLS-YFP to detect the detailed expression patterns of SLAH3 in root. We found SLAH3 was expressed in root hair, epidermis, and stele, indicating SLAH3 shows expression patterns similar to GORK or SKOR (Supplemental Figure 10, A and B).
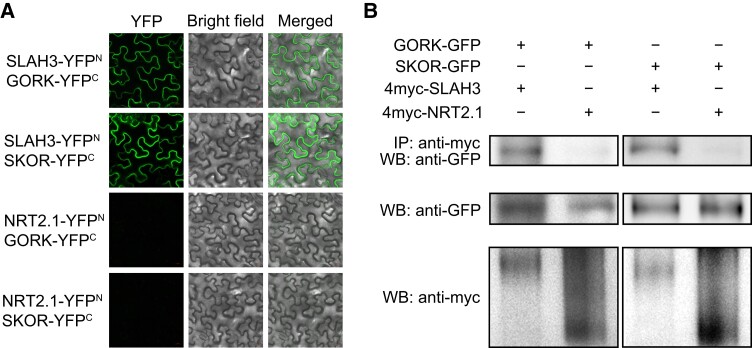
Since SLAH3 expression overlaps with that of GORK and SKOR, we analyzed whether SLAH3 directly interacts with GORK and SKOR. First, bimolecular fluorescence complementation (BiFC) assay was performed in the epidermal cells of Nicotiana benthamiana. The BiFC results showed that SLAH3 associated with both GORK and SKOR in vivo (Figure 8A). Next, co-immunoprecipitation (Co-IP) assay was utilized to confirm the interactions of SLAH3 with GORK and SKOR in planta (Figure 8B). SLAH4, a homolog of SLAH3, was also examined. Despite that the interactions of SLAH4 with GORK or SKOR were not detectable in BiFC, we found it associated with both GORK and SKOR in Co-IP assay (Supplemental Figure 11, A and B). In addition, NRT2.1, a PM-localized nitrate transporter, showed no interaction with GORK and SKOR in BiFC or Co-IP assay (Figure 8). These results demonstrated SLAH3 likely forms a complex with GORK or SKOR to modulate membrane potential.
Figure 8.
SLAH3 interacts with GORK and SKOR. A, In BiFC assay, SLAH3 interacts with GORK and SKOR in N. benthamiana epidermal cells. NRT2.1 was used as a negative control. YFP signal was detected by laser scanning confocal microscopy. The leaves of 20-day-old N. benthamiana plants were used for the experiment. These experiments were repeated at least three times with similar results. B, In Co-IP assay, SLAH3 interacts with GORK and SKOR in planta. NRT2.1 was used as a negative control. Membrane proteins were extracted from 20-day-old N. benthamiana leaves. Proteins immunoprecipitated with an α-myc antibody were analyzed with an α-GFP or α-myc antibodies. These experiments were repeated at least three times with similar results.
SLAH3 inhibits GORK and SKOR in Xenopus laevis oocytes
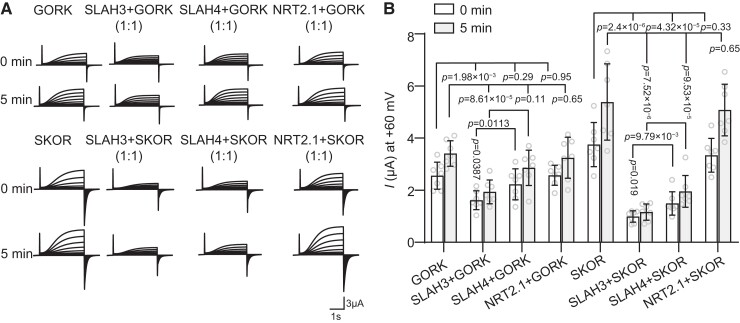
An earlier report showed that SLAH3 interacted with K+ channel KAT1 in guard cell and partly inhibited KAT1 channel activity in Xenopus laevis oocytes (Zhang et al., 2016). Therefore, we investigated whether SLAH3 also affects the channel activities of GORK and SKOR. Equal amounts of SLAH3 and GORK or SKOR cRNA were injected in X. laevis oocytes followed by voltage-clamp assays. The electrophysiological results showed that SLAH3 suppressed the channel activities of both GORK and SKOR (Figure 9, A and B). We also analyzed this suppression by using different [cRNA] ratios. When the [cRNA] ratio of SLAH3: GORK or SKOR was 3:1 or 1:0.3, SLAH3 still significantly repressed the channel activities of GORK and SKOR (Supplemental Figures 12 and 13). By contrast, NRT2.1 failed to affect the activities of GORK or SKOR (Figure 9, A and B). Even though we showed SLAH4 can interact with both GORK and SKOR, it only partially suppressed the activity of SKOR but not GORK (Figure 9, A and B). It indicated not all SLAH-GORK/SKOR associations can cause K+ channel repression.
Figure 9.
SLAH3 inhibits the activities of GORK and SKOR. A, TEVC recording from Xenopus laevis oocytes expressing GORK (16.1 ng cRNA), SKOR (16.1 ng cRNA), and co-expressing SLAH3 (16.1 ng cRNA)/SLAH4 (8.05 ng cRNA)/NRT2.1 (16.1 ng cRNA) and GORK (16.1 ng cRNA)/SKOR (16.1 ng cRNA), in the bath solution containing 90 mM KCl. The test voltages ranged from −90 to + 60 mV in 10 mV increments. The holding potential was −100 mV. B, Summary of the currents at + 60 mV from multiple recordings as in (A). Bars show mean ± Sd, n = 7 oocytes, Two-tails with Student's t test.
We next investigated whether this inhibition of GORK and SKOR depends on SLAH3 structure or SLAH3 channel activity. In oocytes, SLAH3G246D and SLAH3F517L were found to still partially inhibit the channel activities of GORK and SKOR (Figure 10A). The repression was also confirmed by quantification and statistical analysis (Figure 10B).
Figure 10.
The channel activity of SLAH3 is not required for inhibiting GORK and SKOR. A, TEVC recording from Xenopus laevis oocytes expressing GORK (16.1 ng cRNA), SKOR (16.1 ng cRNA), and co-expressing SLAH3 mutant variants (48.3 ng cRNA) and GORK (16.1 ng cRNA) or SKOR (16.1 ng cRNA) under the bath solution containing 90 mM KCl. The test voltages ranged from −90 to + 60 mV in 10 mV increments. The holding potential was −100 mV. B, Summary of the currents at + 60 mV from multiple recordings as in (A) (mean ± Sd, n = 8 oocytes) (Two-tails with Student's t test).
To rule out the possibility that SLAH3 inhibits GORK and SKOR by affecting their protein abundance, we analyzed the expression of GORK and SKOR upon SLAH3 co-expression. Three different [cRNA] ratios of SLAH3 and GORK/SKOR (1:0.3/1:1/1:1.5) were analyzed. Our results indicated SLAH3 had no effect on protein abundance of GORK or SKOR in oocytes at all GORK/SKOR dilutions (Supplemental Figure 14A). The fluorescence intensities of GORK and SKOR were statistically analyzed (Supplemental Figure 14B).
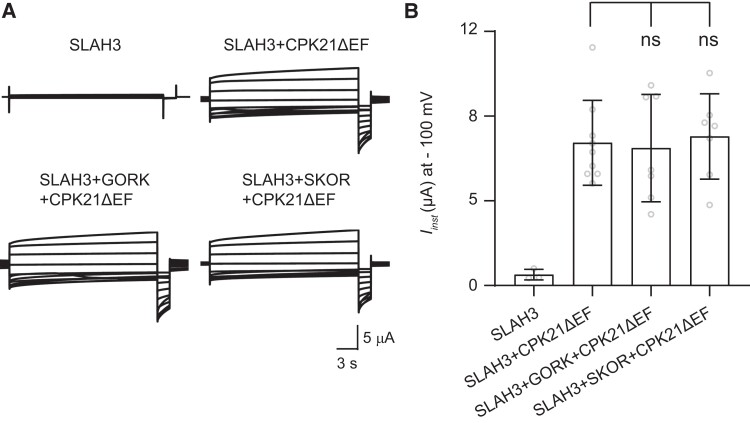
Moreover, we tested whether SLAH3-GORK/SKOR interaction affects SLAH3 channel activity in oocytes. Since SLAH3 is a silent channel, we used a constitutively active variant of CPK21 (CALCIUM-DEPENDENT PROTEIN KINASE 21), CPK21ΔEF, to open SLAH3 in oocytes. Neither GORK nor SKOR was able to affect the channel activity of SLAH3 (Figure 11, A and B).
Figure 11.
The NO3− channel activity of SLAH3 is not affected by GORK or SKOR. A, TEVC recording from Xenopus laevis oocytes expressing SLAH3 (16.1 ng cRNA), co-expressing SLAH3 (16.1 ng cRNA) and CPK21ΔEF (16.1 ng cRNA), co-expressing SLAH3 (16.1 ng cRNA), CPK21ΔEF (16.1 ng cRNA) and GORK (16.1 ng cRNA) or SKOR (16.1 ng cRNA) under nitrate-based buffer. B, Instantaneous currents (Iinst) recorded at −100 mV of oocytes expressing SLAH3 and co-expressing SLAH3, CPK21ΔEF, GORK or SKOR (mean ± Sd., SLAH3 n = 3 oocytes, SLAH3 + CPK21ΔEF n = 9 oocytes, SLAH3 + CPK21ΔEF + GORK, SLAH3 + CPK21ΔEF + SKOR n = 7 oocytes) (Two-tails with Student's t test).
To summarize, similar to SLAH3-KAT1 regulation, the formation of SLAH3-GORK/SKOR complexes causes reduced activities of these K+ channels, which ultimately leads to elevated activation thresholds of K+ efflux channels during membrane potential repolarization.
SLAH3 contributes to K +-induced NO3− and K+ effluxes
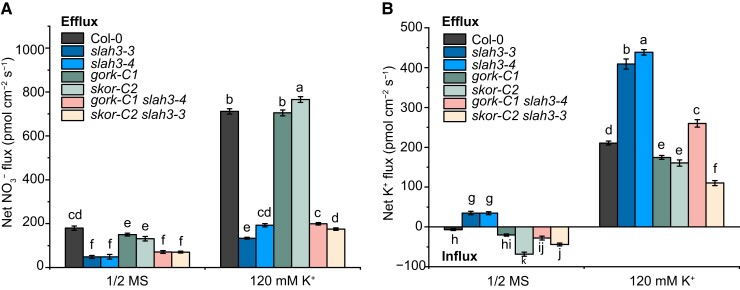
To verify the results from oocytes, we analyzed NO3− and K+ effluxes in mutants. The WT, slah3, gork, skor, gork slah3, and skor slah3 plants were pretreated with or without 120 mM K+ in liquid 1/2 MS for 5 h and the net fluxes of NO3− and K+ were measured by using NMT. The Col-0 plants pretreated with high K+ showed remarkable increase of net NO3− efflux. The K +-triggered NO3− efflux was significantly decreased in slah3. The gork and skor mutants showed same NO3− efflux as WT. This result is consistent with the electrophysiological data that neither GORK nor SKOR affects the activity of SLAH3 channel (Figure 11). Whereas, the gork slah3 and skor slah3 double mutants showed NO3− efflux similar to slah3 (Figure 12A).
Figure 12.
The net flow rates of NO3− and K+ in WT and mutant plants. A, The net NO3− fluxes in the root mature zone. The net NO3− fluxes of Col-0, slah3, gork-C1, skor-C2, gork-C1 slah3-4, and skor-C2 slah3-3 were detected using NMT. The plants were pretreated in liquid 1/2 MS or 1/2 MS supplemented with 120 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1) for 5 h. The experiment was then carried out in the test solution (1 mM KNO3, 0.1 mM KCl, 0.1 mM CaCl2, 0.3 mM MES and pH 6.0). Data are mean ± Sd, n = 4 seedlings. Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05). B, The net K+ fluxes in root mature zone. The net K+ fluxes of Col-0, slah3, gork-C1, skor-C2, gork-C1 slah3-4, and skor-C2 slah3-3 were detected using NMT. The plants were pretreated in liquid 1/2 MS or 1/2 MS supplemented with 120 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1) for 5 h. The experiment was then carried out in the test solution (1 mM KCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM NaCl, 0.3 mM MES, 0.2 mM Na2SO4, pH 6.0). Data are mean ± Sd, n = 4 seedlings. Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05).
High K+ pre-treatment also induced elevated K+ efflux in WT. The K+ efflux was slightly weaker in gork and skor mutants than that in Col-0 upon high K+ pre-treatment. Of note, the K+ efflux triggered by high K+ pre-treatment was enhanced in slah3, suggesting SLAH3 represses the function of GORK and SKOR (Figure 12B). Next, we examined the K+ flux in Col-0, two slah3 lines, and two independent slah3 lines expressing SLAH3G246D. We found upon high K+ pre-treatment, SLAH3G264D restored the K+ efflux in slah3 to WT-like levels (Supplemental Figure 15). Therefore, the ion flux analysis verified the results of electrophysiological assays, demonstrating SLAH3 inhibits GORK and SKOR activation through physical associations to regulate K +-induced anion and cation fluxes.
Discussion
SLAH3 and GORK/SKOR modulate each other through membrane potential regulation and physical interaction
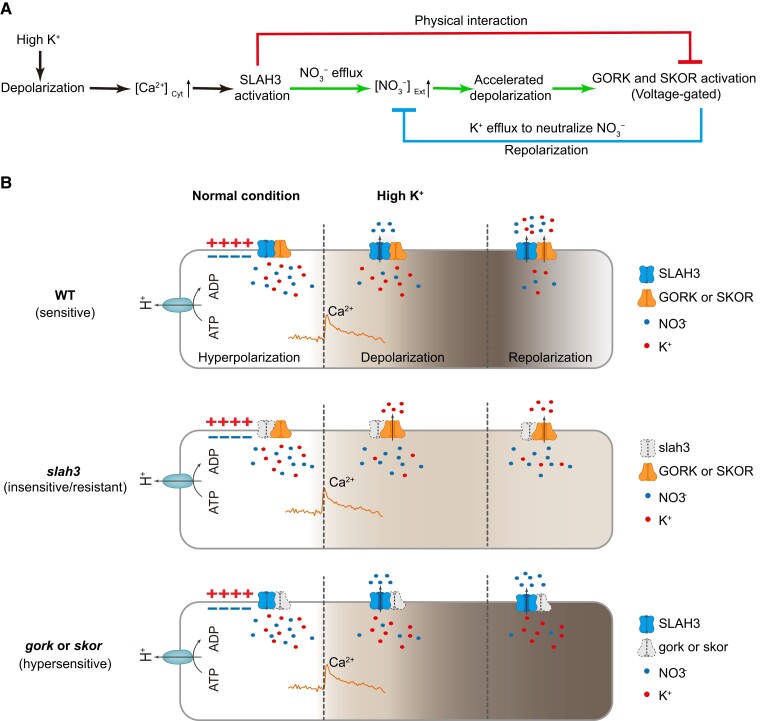
In this study, we investigated the interplays between NO3− channel SLAH3 and K+ channels GORK and SKOR and revealed a mechanism underlying how N and K are balanced via membrane potential modulation in plants. In the hypothetical model, high K+ condition causes depolarization and subsequent Ca2+ signal activation. Ca2+ signatures activate SLAH3, likely through CPKs and/or CIPKs (CBL-INTERACTING PROTEIN KINASE), to mediate NO3− efflux, leading to deeper depolarization. As a result, GORK and SKOR are activated to mediate K+ efflux, which ultimately neutralizes extracellular anions and causes repolarization. SLAH3-GORK/SKOR regulation is mediated through three routes. First, once silent channel SLAH3 is opened, it mediates NO3− efflux and causes accelerated depolarization. The voltage-gated channels GORK and SKOR are sequentially activated. SLAH3 activates GORK and SKOR through membrane potential modulation. Second, the activated GORK and SKOR mediate the efflux of K+ that neutralizes apoplastic NO3− and eventually causes repolarization. Thus, the SLAH3-induced depolarization is counterbalanced by GORK and SKOR-mediated repolarization. GORK and SKOR antagonize SLAH3 through membrane potential regulation. Third, SLAH3 associates with GORK and SKOR to repress their channel activities through direct structural inhibition (Figure 13A).
Figure 13.
Schematic model for SLAH3- and GORK/SKOR-mediated membrane potential regulation upon high K+ stimuli. A, Flow chart of membrane potential changes under high K+ conditions in root. High K+ condition causes depolarization that leads to activation of Ca2+ singling in cytosol. Silent channel SLAH3 is opened by Ca2+-activated components such as CPKs and CIPKs. In the green route, activated SLAH3 mediates NO3− efflux, resulting in accelerated depolarization. Subsequently, the voltage-gated K+ channels GORK and SKOR are activated. SLAH3 functions upstream to activate GORK and SKOR through membrane potential modulation. In the blue route, activated GORK and SKOR mediate K+ efflux to neutralize apoplastic anions, causing repolarization. GORK and SKOR thus antagonize SLAH3-mediated depolarization through membrane potential regulation. In the red route, SLAH3 directly interacts with GORK and SKOR to inhibit their channel activities. This repression helps to increase the gating thresholds for GORK and SKOR activation. B, Hypothetical model of membrane potential changes in WT, slah3, and gork or skor plant cells upon high K +. The deeper depolarization is indicated by the darker color in the cytosol. In the WT plants, high K+ causes SLAH3-mediated depolarization, GORK and SKOR cause repolarization. In slah3, the NO3− efflux-induced depolarization is impaired under high K+ conditions. Meanwhile, the inhibition on GORK and SKOR by SLAH3 is released. The more active GORK and SKOR induce prompt repolarization. The slah3 plants thus show insensitivity/resistance to high K +. In gork and skor mutants, high K+ promotes SLAH3-mediated NO3− efflux and leads to depolarization. Repolarization fails to occur due to the lack of GORK and SKOR-mediated K+ efflux, causing enhanced and prolonged depolarization. The gork and skor plants thus show hypersensitivity to high K +.
Upon high K+ conditions, WT plants show sensitive phenotype. In the slah3 mutant plants, the anion efflux-induced depolarization is partially repressed under high K+ conditions. In addition, GORK and SKOR are more active due to the lack of their inhibitor, SLAH3. The GORK and SKOR-mediated K+ efflux further causes prompt repolarization. As a result, the membrane potential change in slah3 is dramatically reduced compared to WT. Accordingly, the slah3 plants exhibit insensitivity/resistance to high K +. In gork and skor mutants, SLAH3 can be activated to mediate NO3− efflux and induce enhanced depolarization upon high K+ conditions. Due to the absence of GORK or SKOR, the repolarization largely fails to occur, causing enhanced and elongated depolarization in plant cell. The gork and skor mutants are thus hypersensitive to high K+ (Figure 13B).
In guard cell, it has been long proposed that SLAC/SLAH mediate anion efflux to induce depolarization in response to distinct stimuli during stomatal closure. Such depolarization activates GORK to cause repolarization (Schroeder et al., 2001; Roelfsema and Hedrich, 2005; Ward et al., 2009; Waadt et al., 2022). However, the evidence showing direct regulations between slow anion channels and outward-rectifying K+ channels is still insufficient. In this study, we demonstrated SLAH3 not only regulates GORK and SKOR through membrane potential alterations, but also physically represses GORK and SKOR channel activities in root. It will be interesting to explore whether similar mechanisms apply to the modulation between SLAC1/SLAH3 and GORK in guard cell.
The NMT results indicated that the plants pretreated with high K+ showed obvious K+ efflux. Given that the concentration of K+ in cytosol of plant cell is around 100 mM while the K+ at the root surface ranges from 0.1 to 1 mM under natural condition (Wang and Wu, 2013), plant cell can accumulate 100 to 1000-fold higher K+ in cytoplasm compared to apoplast. GORK and SKOR are silent channels that are closed under normal condition, which helps to avoid K +. The opening of GORK and SKOR, therefore, is not likely triggered by K+ gradients. Instead, depolarization serves as the determining factor to activate the voltage-gated channels GORK and SKOR to mediate K+ outward transport.
Upon 120 mM K+ treatment, the membrane potential of WT plants can reach around −17 mV and is maintained at an average membrane potential about −28 mV (Figure 7B). Based on the calculation of Nernst equilibrium potential for K+ (Ek = (RT/ZF) ln([C]out/[C]in), the cytoplasmic K+ needs to be about 230 and 350 mM or higher to trigger K+ efflux when the membrane potentials are −17 mV and −28 mV, respectively. Considering plant cell is able to accumulate as high as 100 mM K+ in cytoplasm when extracellular K+ is very low (0.1–1 mM) (Wang and Wu, 2013), we speculate the cytosolic K+ can reach 200–300 mM, at least for a short period of time, leading to K+ efflux during high K +-induced depolarization.
SLAH3 forms complexes with GORK and SKOR
An earlier report showed SLAH3 was capable of associating with KAT1 and partially inhibiting KAT1 activity in guard cell (Zhang et al., 2016). In addition, the channel heteromerization of SLAH3 and its homolog, SLAH1, activates SLAH3 to mediate chloride loading in the xylem of root (Cubero-Font et al., 2016). Moreover, our recent study revealed that SLAH3 associates with nitrate transporter NRT1.1 to form a channel-transporter complex in response to high ammonium and acidic conditions (Xiao et al., 2022).
Similarly, our current findings revealed SLAH3 forms complexes with GORK or SKOR in root. First, SLAH3-GORK/SKOR complexes allow sensitive perceptions and prompt responses to local environmental changes, instead of sensing whole-cell ionic and electric alterations. This strategy may render plant cell efficient and rapid responses to stress conditions when the ion transports occur locally. Second, the voltage-clamp results showed that SLAH3 inhibited the channel activity of GORK and SKOR. The suppression of SLAH3 on GORK and SKOR likely leads to elevated gating thresholds to activate GORK and SKOR channel in root, avoiding readily activated GORK and SKOR and K+ loss under resting and mild depolarization conditions.
Although the SLAH4-GORK/SKOR interactions were not detected in BiFC, we found SLAH4 indeed associated with GORK and SKOR in Co-IP assays. Of note, SLAH4 can suppress the channel activity of SKOR, but not GORK, indicating not all SLAH-GORK/SKOR interactions can cause K+ channel repression. SLAC/SLAH members show similar structures and are functionally redundant in terms of biochemical features. For instance, slac1 single mutant was shown to be insensitive to CO2 or ABA treatments while overexpression of SLAH1 or SLAH3 fully complemented the stomatal phenotype of slac1 upon CO2 or ABA treatments (Negi et al., 2008). These findings, together with our result that only slah3 showed obvious high-K+ phenotype (Supplemental Figure 4), indicate the specificity of SLAC/SLAH genes may mainly depend on the spatiotemporal expression and/or specific regulators.
A recent study showed the activity of GORK is regulated by its clustering status determined by its N-terminal association. The polymerization of GORK leads to the alteration of channel activity (Horaruang et al., 2022). Similarly, our results demonstrated that channel–channel interaction serves as a regulatory mechanism in GORK and SKOR activity modulation. Future studies should focus on the detailed mechanisms how GORK and SKOR are suppressed by SLAH3. For instance, whether the binding of SLAH3 to GORK and SKOR affects clustering status of GORK or SKOR to regulate their activities.
SLAH3 functions upstream of GORK and SKOR
In our model, SLAH3 plays a central role in depolarization while GORK and SKOR are critical for repolarization. The gork and skor single mutants exhibited phenotype opposite to slah3 under high K+ conditions, but the gork-C1 slah3-4 and skor-C2 slah3-3 double-mutant plants displayed phenotype identical to slah3 (Figure 6). Likewise, even though K+-induced depolarization was enhanced in gork and skor mutants, the gork slah3 and skor slah3 double mutants showed reduced depolarization, similar to slah3 (Figure 7 and Supplemental Figure 9). Whereas, K +-efflux was remarkably increased in slah3, and gork slah3 and skor slah3 showed K+ efflux similar to gork or skor, suggesting SLAH3 also negatively regulates GORK and SKOR (Figure 12B). These results verified SLAH3 is epistatic to GORK and SKOR and confirmed SLAH3 functions in the same signaling pathway with GORK and SKOR. SLAH3-mediated signaling ultimately counterbalanced itself and is regulated through a negative feedback loop.
The gork and skor mutant plants show slightly enhanced sensitivity to high K+ conditions
The slah3 mutant plants showed obvious phenotypic difference compared to the WT under high K+ conditions. However, gork and skor mutants merely exhibited slightly enhanced sensitivity to high K+ treatment. According to our model, the mutation in SLAH3 causes less membrane potential alterations upon K+ treatment, which is generally unharmful to plants. By contrast, the absence of GORK or SKOR leads to significantly enhanced and endured depolarization under high K+, potentially resulting in severe dysfunctions in plant cell. We speculate that the plant cell will counterbalance the deepened depolarization in gork and skor by utilizing alternative strategies such as promoting temporary H+, Na+, Ca2+ efflux, and/or anion influx to eventually cause repolarization for a longer period.
The response of SLAH3 to high K+ is independent of known signaling pathways
SLAH3 exerts its functions in guard cell and pollen tube (Geiger et al., 2011; Gutermuth et al., 2013; Guzel Deger et al., 2015; Zheng et al., 2018). Moreover, SLAH3 was shown to respond to acidic stresses in root (Zheng et al., 2015; Lehmann et al., 2021; Sun et al., 2021; Xiao et al., 2022). Under high K+ conditions, the medium pH alterations failed to affect the sensitive phenotype of slah3 compared to WT (Supplemental Figure 16, A, B, C, and D). We also measured the pH values of the medium after the WT and slah3 were grown on a high K+ medium for one week. There was no obvious difference in the pH values of WT and slah3 medium (Supplemental Figure 16E). Thus, the high K+ response mediated by SLAH3 is independent of the known signaling pathways regulated by SLAH3.
Nitrogen and potassium are closely coordinated
The molecular mechanisms regulating N–K homeostasis have been previously described. First, K+ was found to be able to alleviate NH4+ toxicity (Cao et al., 1993; Szczerba et al., 2008). K+ transporters and channels are capable of transporting NH4+ (ten Hoopen et al., 2010; Tsay et al., 2011). Second, a common regulator was identified to manipulate N and K transports. A Ca2+-activated protein kinase CIPK23 phosphorylates and regulates NRT1.1, AKT1 (K+ TRANSPORTER 1), and AMT1; 1 (AMMONIUM TRANSPORTER 1; 1), demonstrating the uptakes of NO3−, K+ and NH4+ are closely linked (Xu et al., 2006; Ho et al., 2009; Straub et al., 2017). Third, the functional analyses on NRT1.5 provide additional insights into N–K balance (Lin et al., 2008; Drechsler et al., 2015; Meng et al., 2016; Li et al., 2017; Chen et al., 2021). Mutations in NRT1.5 cause impaired root-to-shoot NO3− transport (Lin et al., 2008). Moreover, NRT1.5 affects root-to-shoot NO3− transport by coordinating SKOR in the xylem loading (Drechsler et al., 2015). NRT1.5 also acts as a K+/NO3− symporter to directly modulate NO3−-K+ homeostasis and distribution (Li et al., 2017). However, our results indicated SLAH3 is unlikely involved in the stellar K+ transportation and translocation since no obvious K+ content difference was found in root and shoot in slah3 mutants compared to WT plants under high K conditions (Figure 4A). In this study, we revealed additional mechanisms of N–K balance by showing that membrane potential modulation is crucial in this process.
Elucidation of SLAH3-GORK/SKOR regulation potentially benefits agricultural improvements
High K+ often causes significant N-starvation in Arabidopsis, compared to halophytes (Pantha et al., 2022). The high K-induced N depletion can be largely alleviated by internal regulations such as promoting the functions of NRT1.7/NRT1.8 to remobilize NO3−, and/or exogenously applied N (Zhang et al., 2010; O'Brien et al., 2016; Pantha et al., 2022). Our results showed that the high K +-induced growth inhibition was alleviated by addition of NO3− in Col-0. By contrast, the high K +-resistant phenotype of slah3 was not affected by NO3− application (Figure 2). Balanced application of K and N fertilizers are important in agricultural practice. Our results showing membrane potential regulation modulated by nitrate and potassium channels provides additional insights into the molecular mechanisms underlying how nitrogen detoxifies high K+ and, more importantly, how nutrient elements are efficiently utilized by plants.
The slah3 loss-of-function mutation confers enhanced resistance to stress conditions in Arabidopsis. It potentially provides useful solutions for stress resistance and environmental adaptions in crops. This study provides a theoretical basis for improving nitrogen and potassium utilization efficiency (NUE and KUE) in plants to increase crop yields and reduce environmental pollution.
Materials and methods
Plant materials and growth conditions
In this study, all Arabidopsis thaliana plants are in Col-0 background. T-DNA insertion plant lines were obtained from The Arabidopsis Biological Resource Center (ABRC), including slah3-3 (SALK_10605), slah3-4 (SALK_111623), and slac1-3 (SALK_099139). The gork-C1, skor-C2, slah1-C1, slah2-C1, and slah4-C1 mutants were generated by CRISPR/Cas9-mediated genome editing. The CRISPR/Cas9 method has been described by a previous study (Wang et al., 2015). The primers used for genotype identification and CRISPR/Cas9 gene editing are listed in Supplemental Data Set 1. The gene editing results are listed in Supplemental Data Set 2.
After two-day vernalization, the seeds of Col-0 and mutants were planted in soil, 1/2 MS agar medium, or 1/2 N-free MS agar medium (Caisson) containing different concentrations of anions and cations and grown in an incubator at 22°C with 16 h-light (120 μmol s−1 m−2 light intensity provided by white LED lamps)/8 h-dark.
Molecular cloning and generation of transgenic plants
The coding sequences (CDS) and promoter sequences of SLAH3, GORK, SKOR, NRT2.1, and SLAH4 were PCR-amplified, and the PCR products were recombined into the donor vector pDONR/Zeo using the Gateway BP reaction followed by LR reaction into destination vectors (Invitrogen). SLAH3F517L and SLAH3G264D were generated using the site-directed mutagenesis method described previously (Reikofski and Tao, 1992). The destination vectors were transformed into plants via Agrobacterium tumefaciens strain GV3101 using the floral-dip method (Clough and Bent, 1998). T3 generation homozygous transgenic plants were obtained for experiment. For BiFC assay, the SLAH3, SLAH4, NRT2.1, GORK, and SKOR CDS entry vectors were recombined into the destination vector pEarley Gate201-nYFP or pEarley Gate202-cYFP (Ou et al., 2022). For Co-IP experiments, the SLAH3, SLAH4, and NRT2.1 CDS entry vectors were recombined into the destination vector pGWB418, and the GORK and SKOR CDS entry vectors were recombined into the destination vector pBIB-BASTA-35S-GWR-GFP. For SLAH3 tissue-specific expression analysis, the SLAH3 promoter (1511 bp) entry vector was recombined into the destination vector pFYTAG (Zhang et al., 2005).
RNA isolation and qPCR
The total RNA of Arabidopsis plants was obtained using an RNA simple Total RNA Kit (TIANGEN) according to the manufacturer's instructions. First-strand cDNA was obtained by using the reverse-transcriptase M-MLV (Takara). The gene expression levels were analyzed by qPCR. qPCR assays were performed using SYBR Premix ExTaq II (Takara) on a StepOnePlus Real-Time PCR System (Applied Biosystems). The relative gene expression was normalized to the expression of ACT2. Relative expression was calculated according to the ΔΔCT method (Livak and Schmittgen, 2001). All experiments were performed in three independent biological replicates. The primers used for qPCR are listed in Supplemental Data Set 1.
Phenotypic and statistical analyses
Seeds were germinated on N-free 1/2 MS medium supplemented with different reagents (KNO3, K+, different K+/NO3− ratio, NaCl, KCl, Cl−, and sorbitol) for seven days. The survival rates of the plants were analyzed.
In the transplanting experiment, the five-day-old Col-0 and slah3 seedlings grown on 1/2 MS medium were transferred to 1/2 MS or high K+ (150–180 mM) medium for seven days. The lateral root length, lateral root density, and primary root length of the plants were measured using ImageJ software.
In the pH-related test experiment, the phenotypes of Col-0 and slah3 mutants grown on the ½ MS or 120 mM K+ medium with different pH were observed. Four-week-old plants were photographed.
All values are presented as mean and standard deviation (Sd). Different letters indicate a significant difference following one-way ANOVA with Fisher's LSD multiple comparison test (P < 0.05) by using the Software OriginPro2019b. The significance of differences for pairwise comparisons was estimated by two-tailed Student's t test in Microsoft Excel 2019. The statistical analysis results are listed in Supplemental Data Set 3.
Measurements of the net K+ and NO3− fluxes using NMT
The net K+ and NO3− fluxes were measured by NMT, (Xuyue Beijing Science and Technology Company). The NMT system is NMT150S and the software is imFluxesV2.0 (YoungerUSA LLC, Amherst, MA 01002, USA). The protocol was described in previous report (Sun et al., 2009). The selective cocktails of NO3− is NO3−LIX (NMT selective liquid ion-exchange cocktails, XY-SJ-NO3-25). The NO3− backfilling buffer contains 10 mM KNO3. And the selective cocktails of K+ is K+ LIX (XY-SJ-K-10). The K+ backfilling buffer contains 100 mM KCl. The solution of reference electrode of NMT equipment is 3 M KCl. 3 M KCl solution is poured into the plastic casing and silver wire is inserted to form a salt bridge to make the device form a path.
After two-day vernalization, the seeds of Col-0 and mutants were planted in ½ MS agar medium and grown in an incubator at 22°C with 16 h-light/8 h-dark for 5 days. The WT and mutants were pretreated with liquid ½ MS medium or ½ MS supplemented with 120 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1) for 5 h, respectively. After immobilization in the test solution for 10 min, the plants were measured. The mature zone of plant root where the root hair emerges was measured.
The measuring solution for K+ contains 1 mM KCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM NaCl, 0.3 mM MES, 0.2 mM Na2SO4, pH 6.0. The calibration solution for K+ contains 0.1 mM KCl/1 mM KCl/10 mM KCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM NaCl, 0.3 mM MES, 0.2 mM Na2SO4, pH 6.0. The measuring solution for NO3− contains 1 mM KNO3, 0.1 mM KCl, 0.1 mM CaCl2, 0.3 mM MES, pH 6.0. The calibration solution for NO3− contains 0.1 mM KNO3/1 mM KNO3/10 mM KNO3, 0.1 mM KCl, 0.1 mM CaCl2, 0.3 mM MES, pH 6.0.
Measurements of K+ and NO3− content in plant tissues
Cleaned the treated plants with double-distilled water, and dried the plant surface. Collected the roots and shoots of plants, respectively. Put the samples in the oven at 105°C for 10 min, then baked them at 80°C for 48 h until they were completely dried. Finally, put the samples into the dryer to cool to room temperature, and ground and crushed the dry samples of plant tissues with mortar and pestle.
For the measurement of K+ contents, seeds were cultured under ½ MS hydroponic conditions for three weeks, and then the seedlings were transferred to ½ MS or ½ MS hydroponic solution containing 150 mM K+ (50 mM KNO3; 50 mM KCl; 25 mM K2SO4) for two days to detect the K+ contents in shoots and roots. Weighed an appropriate amount of crushed plant tissues, transferred them into a test tube filled with 10 mL of pre-cooled 100 mM glacial acetic acid. Shaked the test tube to ensure that the samples were completely immersed in glacial acetic acid. Placed the test tube in a 90°C water bath for 2 h, cooled and filtered to obtain the total ion solution in the tissue. After appropriate dilution, K+ contents were determined by an inductively coupled plasma emission spectrometer (ICP-OES, PE Avio 500) (Wang et al., 2007).
For the measurement of NO3− contents, seeds were cultured under N-free ½ MS hydroponic medium add 10 mM KNO3 as nitrogen source for two weeks, and then the seedlings were transferred to N-free ½ MS hydroponic solution containing 50 mM K+ (50 mM KNO3) or 150 mM K+ (50 mM KNO3 + 50 mM KCl + 25 mM K2SO4) (the same concentration of NO3− was added) for 5 days to detect the NO3− contents in shoots and roots. Weighed 2.5 mg of the dry weight of the samples, put it into a 1.5 mL EP tube, added 1 mL ddH2O, and heated it at 80°C for 20 min. The heated sample was cooled on ice and centrifuged for 10,000 g/15 min. Added 50 μl of supernatant (including sample solution and standard solution) into 200 μl of 5% salicylic acid (w/v) sulfuric acid EP tube, fully mixed, and stood in dark for 20 min. Added 250 μl of the mixture in the previous step to 4.75 mL of 2 mM NaOH solution, mixed well, and cooled to room temperature. In total, 200 μl of the mixed solution was added and measured at OD 410 nm (Thermo) (Zhao and Wang, 2017).
Membrane potential recording
NMT was used to detect root membrane potential. The membrane potential of the mature zone was measured by 0.2 μm glass Ag/AgCl microelectrode perfused with 1 M KCl (Shabala et al., 2005; Serre et al., 2021). Using NMT equipment (NMT Physiolyzer, Younger, USA; Xuyue Company), the membrane potential changes of the plant root in real-time before and after treatment with 120 mM KCl or 120 mM KNO3 were measured. 3 M KCl solution was used as the reference and was poured into the plastic casing and silver wire was inserted to form a salt bridge to make the device form a path. Directly read and output membrane potential data through imFluxes V2.0 software (Xuyue Company). The seven-day-old Arabidopsis seedlings were immobilized in the test solution pre-cultured for 20 min. Placed the microelectrode on the youngest root mature zone and recorded the extra-membrane potential Eout. Then pierced the electrode into the root epidermal cell and recorded the membrane potential, that is, the intracellular potential Ein. The membrane potential of Arabidopsis root epidermal cells before treatment E = Ein−Eout. Replaced the test solution with the treatment solution and immediately recorded the change in the intracellular membrane potential Eint. The membrane potential after treatment Et = Eint−Eout.
For the measurements in Figure 5, seedlings were immobilized in the liquid ½ MS solution (pH 5.8, buffered with MES) for 20 min. The treatment solution was the ½ MS solution supplemented with 120 mM KCl or 120 mM KNO3 (pH 5.8, buffered with MES). For the measurements in Figure 7, seedlings were immobilized in 0.5 g L−1 MES solution (pH 5.8) for 20 min. The treatment solution was 0.5 g L−1 MES solution supplemented with 120 mM KCl or 120 mM KNO3 (pH 5.8).
For detecting membrane potential with fluorescent dye DiBAC4(3) (US EVERBRIGHT INC), five-day-old Arabidopsis seedlings were placed in a 120 mM KCl or 120 mM KNO3, pH 5.8 (MES) treatment solution containing 15 μM DiBAC4(3) for 15 min. Laser confocal microscopy was used for observation (ZEISS/LSM880). The fluorescent dye DiBAC4(3) was excited at 488 nm and the signals at 500–600 nm were collected. The fluorescence intensities were analyzed by using ZEN 3.2 (blue edition) software. When DiBAC4(3) enters the cell, the intracellular fluorescence intensity increases, that is, the membrane potential increases, indicating cell depolarization. On the contrary, if the intracellular fluorescence intensity decreases, that is, the membrane potential decreases, indicating cell hyperpolarization.
Protein–protein interaction assays
For BiFC assay, Agrobacterium tumefaciens strain GV3101 containing vector SLAH3-YN, SLAH4-YN, or NRT2.1-YN was mixed with the strain containing GORK-YC or SKOR-YC and infiltrated into the leaves of 20-day-old Nicotiana benthamiana plants. SLAH4 and NRT2.1 were used as controls. The YFP signal was detected by a confocal microscope (ZEISS/LSM880) with a Plan-Apochromat 20×/0.8 M27 objective lens. Fluorescence images were captured using the ZEN software with an excitation wavelength at 514 nm and the emission was collected at 519–620 nm.
For Co-IP assay, Agrobacterium tumefaciens strain GV3101 containing vector 4myc-SLAH3, 4myc-SLAH4 or 4myc-NRT2.1 was mixed with strain containing GORK-GFP or SKOR-GFP and infiltrated into the young leaves of Nicotiana benthamiana. After two days, the total protein of tobacco leaves (about 10 g) was extracted by membrane protein extraction buffer (MPEB: 0.05 M HEPES, 0.15 M KCl, 1 mM EDTA, 10% [v/v] glycerol, 0.2%[v/v] Triton X-100, 1:100 complete protease inhibitor cocktail [Roche; 04693132001]), followed by high-speed centrifugation (100,000 g/1 h) to obtain membrane protein, and dissolved the membrane protein in 2 mL membrane protein solution buffer (MPSB: 0.05 M HEPES, 0.15 M KCl, 1 mM EDTA, 10% [v/v] glycerol, 1% [v/v] Triton X-100, 1:100 complete protease inhibitor cocktail [Roche; 04693132001]). Added 8 mL MPEB (without Triton X-100) to dilute 1% Triton X-100% to 0.2%. Took 1 mL lysate, added 15 μL myc beads (KT HEALTH, KTSM1306) or 15 μL GFP beads (KT HEALTH, KTSM1301), and incubated at 4°C for 3 h with gentle shaking as the input to ensure that the protein was expressed. Added 30 μL myc beads to the remaining 8 mL lysate and incubated at 4°C for 3 h as Co-IP samples. Used MPEB (without Triton X-100) to wash the beads five times and added 30 μl 2×SDS loading buffer, and then in a water bath at 95°C for 5 min to obtain the destination protein. The samples were separated on 10% SDS–PAGE gels and detected by immunoblotting using horseradish peroxidase (PerkinElmer; Western Lightning Plus ECL, lot NEL105001EA). For immunoblotting, the antibody α-myc (Abmart; M20002L, lot 324086, 1:3000) and α-GFP (Roche; 11814460001, lot 19958500, 1:3000) were used.
Two-Electrode Voltage-Clamp (TEVC) assays
The full-length cDNAs of SLAH3 (BamHI/EcoRI), GORK (XmaI/XbaI), SKOR (BamHI/XbaI), CPK21ΔEF (BamHI/EcoRI), SLAH4 (XmaI/EcoRI), and NRT2.1 (BamHI/XbaI) were cloned into the pGEMHE vectors. And construction pGEMHE vectors of SLAH3 F517L and SLAH3 G264D by site-directed mutagenesis. The capped RNAs (cRNAs) were prepared from the linearized DNA templates using the mMACHINE high-yield capped RNA T7 kit (Thermo, Invitrogen).
For the K+ channel activity recording, SLAH3, GORK, SKOR, SLAH3 + GORK (1:1, 3:1, or 1:0.3), SLAH3 + SKOR (1:1, 3:1, or 1:0.3), SLAH3 F517L + GORK (3:1), SLAH3 G264D +SKOR (3:1), SLAH4 + GORK (1:1), NRT2.1 + GORK (1:1), SLAH4 + SKOR (1:1), and NRT2.1 + SKOR (1:1) were injected into one Xenopus laevis oocyte. Whole oocyte currents were recorded two days after cRNA injection using two-electrode voltage-clamp. Electrodes were filled with 3 M KCl. The oocytes were continuously perfused during the voltage-clamp experiment. The standard bath solution contained 10 mM KCl, 90 mM NaCl, 1 mM CaCl2, 4 mM MgCl2, and 10 mM HEPES pH 7.4. Voltage steps were applied from −90 to +60 mV in 10 mV increments during 3 s. The holding potential was −100 mV. Oocytes were voltage-clamped using a TEV 200A amplifier (Dagan) and monitored by computer through Digidata 1440A converter (Axon) and pCLAMP 10.7 software (Axon).
For the NO3− channel activity recording, SLAH3, SLAH3 + CPK21ΔEF (1:1), SLAH3 + CPK21ΔEF + GORK (1:1:1), or SLAH3 + CPK21ΔEF + SKOR (1:1:1) were injected into one oocyte that were perfused with Tris/Mes-based buffers. The nitrate-based buffer contained 10 mM Tris/Mes (pH 5.6), 1 mM Ca(gluconate)2, 1 mM Mg(gluconate)2, 1 mM LaCl3, 30 mM NaNO3, and adjusted osmotic to 220 mmol/kg by Na(gluconate). The test voltages ranged from −140 to +60 mV in 20 mV increments. The holding potential was 0 mV. Instantaneous currents (Iinst) were extracted immediately after the voltage jumped from the holding potential of 0 to 50 mV test pulses ranging from −140 to + 60 mV.
Fluorescence intensity analysis in Xenopus oocytes
The yellow fluorescent protein (YFP) was fused to the C-terminus of GORK and SKOR. The cRNAs were prepared using the same plasmid and kit in the TEVC method. GORK-YFP, SKOR-YFP, SLAH3 + GORK-YFP, and SLAH3 + SKOR-YFP was injected into one oocyte at different [cRNA] ratios. The oocytes were incubated two days at 18°C. And then the oocytes were imaged with Leica 10x/0.45 dry objective on a Leica TCS SP8 STED (Leica) confocal microscope with Leica Application Suite X software. YFP was excited with a white laser at 514 nm, the emissions were collected at 523–546 nm on a HyD detector.
Determination of pH in the agar rooting medium
The experiment was performed followed by a previously described method for measuring pH in agar medium (Zhu et al., 2019). Col-0 and slah3 mutant seeds were grown in 1/2 N-free MS agar medium containing 120, 140, or 160 mM K+ ([KNO3]: [KCl]: [K2SO4] = 2:2:1) for seven days, and the rhizosphere agar medium was collected into a 50 mL centrifuge tube. Froze the obtained medium at −20°C overnight, and thawed it at room temperature to release water. The released liquid was filtered, and the pH values were measured with a pH meter.
Accession numbers
The gene sequences in this study can be obtained in the TAIR database (https://www.arabidopsis.org). The accession numbers are: SLAH3, AT5G24030; GORK, AT5G37500; SKOR, AT3G02850; SLAC1, AT1G12480; SLAH1, AT1G62280; SLAH2, AT4G27970; SLAH4, AT1G62262; CPK21, AT4G04720; ACT2, AT3G18780 and NRT2.1, AT1G08090.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. The slah3 mutants show enhanced tolerance phenotype than WT after transferring to high concentrations of K+ medium.
Supplemental Figure S2. The slah3 mutants show enhanced tolerance to high concentrations K+ but not NO3−.
Supplemental Figure S3. SLAH3 mainly functions in root to cope with high K+ stress.
Supplemental Figure S4. SLAH3 is the only SLAC/SLAH gene regulating high K+ response.
Supplemental Figure S5. The slah3 mutants show no phenotypic difference to Col-0 under high Na+ conditions.
Supplemental Figure S6. The slah3 mutants show no phenotypic difference to Col-0 under high Cl− conditions.
Supplemental Figure S7. The tolerance of slah3 to high K+ is not related to osmotic stress.
Supplemental Figure S8. The gork skor mutants show subtle hypersensitivity to high K+ conditions.
Supplemental Figure S9. The enhanced depolarization in gork and skor upon high K+ depends on SLAH3.
Supplemental Figure S10. The expression patterns of SLAH3 in the root.
Supplemental Figure S11. SLAH4 interacts with GORK and SKOR.
Supplemental Figure S12. SLAH3 inhibits the activity of GORK and SKOR when the ratio of [cRNA] of SLAH3-GORK/SKOR is 3:1.
Supplemental Figure S13. SLAH3 inhibits the activity of GORK and SKOR when the ratio of [cRNA] of SLAH3-GORK/SKOR is 1:0.3.
Supplemental Figure S14. The expression of GORK and SKOR is not affected by SLAH3 in Xenopus laevis oocytes.
Supplemental Figure S15. The net flow rates of K+ in WT and mutant plants.
Supplemental Figure S16. The phenotypic difference between Col-0 and slah3 on high K+ medium is not affected by medium pH.
Supplemental Data Set S1. Primers used in this study.
Supplemental Data Set S2. The CRISPR/Cas9 gene editing results.
Supplemental Data Set S3. Summary of statistical analyses.
Supplementary Material
Acknowledgments
We thank Liang Peng, Liping Guan, Yahu Gao, Xueyao Zhang, Lei Wang, Yang Zhao, and Li Xie (Core Facility for Life Science Research, Lanzhou University) for technical support.
Contributor Information
Beibei Liu, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China.
Changxin Feng, College of Life Sciences, Capital Normal University, Beijing 100048, China.
Xianming Fang, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China.
Zhen Ma, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China.
Chengbin Xiao, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China.
Shuaishuai Zhang, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China.
Zhenzhen Liu, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China.
Doudou Sun, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China.
Hongyong Shi, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China; School of Life Sciences, Guangzhou University, Guangzhou 510006, China.
Xiaoqin Ding, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China.
Chenyang Qiu, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China.
Jia Li, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China; School of Life Sciences, Guangzhou University, Guangzhou 510006, China.
Sheng Luan, Department of Plant and Microbial Biology, University of California, Berkeley, California 94720, USA.
Legong Li, College of Life Sciences, Capital Normal University, Beijing 100048, China.
Kai He, Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou 730000, China.
Funding
This work was supported by National Natural Science Foundation of China (grant nos. 32170280, 31870235), Science and Technology Program of Gansu Province (grant no. 22JR5RA432), the 111 Project (grant no. B16022), and the Fundamental Research Funds for the Central Universities (grant nos. lzujbky-2022-it29, lzujbky-2020-kb05, lzujbky-2021-it22).
References
- Armengaud P, Breitling R, Amtmann A (2004) The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol 136(1): 2556–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Glass AD, Crawford NM (1993) Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutations aux1, axr1, and axr2. Plant Physiol 102(3): 983–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Hu L, Punta M, Bruni R, Hillerich B, Kloss B, Rost B, Love J, Siegelbaum SA, Hendrickson WA (2010) Homologue structure of the SLAC1 anion channel for closing stomata in leaves. Nature 467(7319): 1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang Q, Wang X, Zhang J, Ismail AM, Zhang Z (2021) Nitrogen form-mediated ethylene signal regulates root-to-shoot K+ translocation via NRT1.5. Plant Cell Environ 44(12): 3576–3588 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6): 735–743 [DOI] [PubMed] [Google Scholar]
- Cubero-Font P, Maierhofer T, Jaslan J, Rosales MA, Espartero J, Diaz-Rueda P, Muller HM, Hurter AL, Al-Rasheid KA, Marten I, et al. (2016) Silent S-type anion channel subunit SLAH1 gates SLAH3 open for chloride root-to-shoot translocation. Curr Biol 26(16): 2213–2220 [DOI] [PubMed] [Google Scholar]
- Drechsler N, Zheng Y, Bohner A, Nobmann B, von Wirén N, Kunze R, Rausch C (2015) Nitrate-Dependent control of shoot K homeostasis by the nitrate Transporter1/peptide transporter family member NPF7.3/NRT1.5 and the stelar K+ outward rectifier SKOR in Arabidopsis. Plant Physiol 169(4): 2832–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer I, Gomez-Porras JL, Riaño-Pachón DM, Hedrich R, Geiger D (2012) Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front Plant Sci 3: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang XZ, Liu XX, Zhu YX, Ye JY, Jin CW (2020) The K+ and NO3− interaction mediated by NITRATE TRANSPORTER1.1 ensures better plant growth under K+-limiting conditions. Plant Physiol 184(4): 1900–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH, Zimmermann MR (2007) Systemic signalling in barley through action potentials. Planta 226(1): 203–214 [DOI] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferrière N, Thibaud JB, Sentenac H (1998) Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94(5): 647–655 [DOI] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KA, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, et al. (2011) Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal 4(173): ra32. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KAS, Grill E, et al. (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci U S A 107(17): 8023–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KA, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci U S A 106(50): 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutermuth T, Lassig R, Portes MT, Maierhofer T, Romeis T, Borst JW, Hedrich R, Feijó JA, Konrad KR (2013) Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20. Plant Cell 25(11): 4525–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzel Deger A, Scherzer S, Nuhkat M, Kedzierska J, Kollist H, Brosché M, Unyayar S, Boudsocq M, Hedrich R, Roelfsema MR (2015) Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol 208(1): 162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Geiger D (2017) Biology of SLAC1-type anion channels-from nutrient uptake to stomatal closure. New Phytol 216(1): 46–61 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Salvador-Recatalà V, Dreyer I (2016) Electrical wiring and long-distance plant communication. Trends Plant Sci 21(5): 376–387 [DOI] [PubMed] [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF (2009) CHL1 Functions as a nitrate sensor in plants. Cell 138(6): 1184–1194 [DOI] [PubMed] [Google Scholar]
- Horaruang W, Klejchova M, Carroll W, Silva-Alvim FAL, Waghmare S, Papanatsiou M, Amtmann A, Hills A, Alvim JC, Blatt MR, et al. (2022) Engineering a K+ channel ‘sensory antenna’ enhances stomatal kinetics, water use efficiency and photosynthesis. Nat Plants 8(11): 1262–1274 [DOI] [PubMed] [Google Scholar]
- Ivashikina N, Becker D, Ache P, Meyerhoff O, Felle HH, Hedrich R (2001) K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett 508(3): 463–469 [DOI] [PubMed] [Google Scholar]
- Lehmann J, Jørgensen ME, Fratz S, Müller HM, Kusch J, Scherzer S, Navarro-Retamal C, Mayer D, Böhm J, Konrad KR, et al. (2021) Acidosis-induced activation of anion channel SLAH3 in the flooding-related stress response of Arabidopsis. Curr Biol 31(16): 3575–3585 [DOI] [PubMed] [Google Scholar]
- Li H, Yu M, Du XQ, Wang ZF, Wu WH, Quintero FJ, Jin XH, Li HD, Wang Y (2017) NRT1.5/NPF7.3 Functions as a proton-coupled H+/K+ antiporter for K+ loading into the Xylem in Arabidopsis. Plant Cell 29(8): 2016–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Kuo HF, Canivenc G, Lin CS, Lepetit M, Hsu PK, Tillard P, Lin HL, Wang YY, Tsai CB, et al. (2008) Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20(9): 2514–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Method 25(4): 402–408 [DOI] [PubMed] [Google Scholar]
- Maathuis FJ (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12(3): 250–258 [DOI] [PubMed] [Google Scholar]
- Matoh T, Watanabe J, Takahashi E (1986) Effects of sodium and potassium salts on the growth of a halophyte Atriplex Gmelini. Soil Sci Plant Nutr 32(3): 451–459 [Google Scholar]
- Meng S, Peng JS, He YN, Zhang GB, Yi HY, Fu YL, Gong JM (2016) Arabidopsis NRT1.5 mediates the suppression of nitrate starvation-induced leaf senescence by modulating foliar potassium level. Mol Plant 9(3): 461–470 [DOI] [PubMed] [Google Scholar]
- Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM (2007) Nitrate transport and signalling. J Exp Bot 58(9): 2297–2306 [DOI] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 Regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452(7186): 483–486 [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Vega A, Bouguyon E, Krouk G, Gojon A, Coruzzi G, Gutierrez RA (2016) Nitrate transport, sensing, and responses in plants. Mol Plant 9(6): 837–856 [DOI] [PubMed] [Google Scholar]
- Ou Y, Tao B, Wu Y, Cai Z, Li H, Li M, He K, Gou X, Li J (2022) Essential roles of SERKs in the ROOT MERISTEM GROWTH FACTOR-mediated signaling pathway. Plant Physiol 189(1): 165–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG (2001) PLANT PLASMA MEMBRANE H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52(1): 817–845 [DOI] [PubMed] [Google Scholar]
- Pantha P, Oh DH, Longstreth D, Dassanayake M (2022) Living with high potassium: balance between nutrient acquisition and K-induced salt stress signaling. Plant Physiol kiac564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J, López MJ, Benlloch M (2004) Effect of NaCl and KCl salts on the growth and solute accumulation of the halophyte Atriplex nummularia. Plant Soil 259(1/2): 163–168 [Google Scholar]
- Reikofski J, Tao BY (1992) Polymerase chain reaction (PCR) techniques for site-directed mutagenesis. Biotechnol Adv 10(4): 535–547 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R (2005) In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol 167(3): 665–691 [DOI] [PubMed] [Google Scholar]
- Roelfsema MR, Hedrich R, Geiger D (2012) Anion channels: master switches of stress responses. Trends Plant Sci 17(4): 221–229 [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Shin R (2007) Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol 58(1): 47–69 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52(1): 627–658 [DOI] [PubMed] [Google Scholar]
- Serre NBC, Kralik D, Yun P, Slouka Z, Shabala S, Fendrych M (2021) AFB1 Controls rapid auxin signalling through membrane depolarization in Arabidopsis thaliana root. Nat Plants 7(9): 1229–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala L, Cuin TA, Newman IA, Shabala S (2005) Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 222(6): 1041–1050 [DOI] [PubMed] [Google Scholar]
- Straub T, Ludewig U, Neuhauser B (2017) The kinase CIPK23 inhibits ammonium transport in Arabidopsis thaliana. Plant Cell 29(2): 409–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Chen SL, Dai SX, Wang RG, Li NY, Shen X, Zhou XY, Lu CF, Zheng XJ, Hu ZM, et al. (2009) NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol 149(2): 1141–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Fang X, Xiao C, Ma Z, Huang X, Su J, Li J, Wang J, Wang S, Luan S, et al. (2021) Kinase SnRK1.1 regulates nitrate channel SLAH3 engaged in nitrate-dependent alleviation of ammonium toxicity. Plant Physiol 186(1): 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Balkos KD, Kronzucker HJ (2008) Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and -insensitive components of NH4+ transport. J Exp Bot 59(2): 303–313 [DOI] [PubMed] [Google Scholar]
- ten Hoopen F, Cuin TA, Pedas P, Hegelund JN, Shabala S, Schjoerring JK, Jahn TP (2010) Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. J Exp Bot 61(9): 2303–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay YF, Ho CH, Chen HY, Lin SH (2011) Integration of nitrogen and potassium signaling. Annu Rev Plant Biol 62(1): 207–226 [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al. (2008) SLAC1 Is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452(7186): 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annu Rev Plant Biol 71(1): 403–433 [DOI] [PubMed] [Google Scholar]
- Vidal EA, Alvarez JM, Araus V, Riveras E, Brooks MD, Krouk G, Ruffel S, Lejay L, Crawford NM, Coruzzi GM, et al. (2020) Nitrate in 2020: thirty years from transport to signaling networks. Plant Cell 32(7): 2094–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Seller CA, Hsu PK, Takahashi Y, Munemasa S, Schroeder JI (2022) Plant hormone regulation of abiotic stress responses. Nat Rev Mol Cell Biol 23(10): 680–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Hsu PK, Tsay YF (2012) Uptake, allocation and signaling of nitrate. Trends Plant Sci 17(8): 458–467 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu WH (2013) Potassium transport and signaling in higher plants. Annu Rev Plant Biol 64(1): 451–476 [DOI] [PubMed] [Google Scholar]
- Wang ZP, Xing HL, Dong L, Zhang HY, Han CY, Wang XC, Chen QJ (2015) Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol 16(1): 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SM, Zhang JL, Flowers TJ (2007) Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiol 145(2): 559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Maser P, Schroeder JI (2009) Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol 71(1): 59–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R (1994) The excitability of plant cells: with a special emphasis on characean internodal cells. Bot Rev 60(3): 265–367 [DOI] [PubMed] [Google Scholar]
- Xiao C, Sun D, Liu B, Fang X, Li P, Jiang Y, He M, Li J, Luan S, He K (2022) Nitrate transporter NRT1.1 and anion channel SLAH3 form a functional unit to regulate nitrate-dependent alleviation of ammonium toxicity. J Integr Plant Biol 64(4): 942–957 [DOI] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125(7): 1347–1360 [DOI] [PubMed] [Google Scholar]
- Zhang CQ, Gong FC, Lambert GM, Galbraith DW (2005) Cell type-specific characterization of nuclear DNA contents within complex tissues and organs. Plant Methods 1(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Niu JF, Zhang WF, Chen XP, Li CJ, Yuan LX, Xie JC (2010) Potassium nutrition of crops under varied regimes of nitrogen supply. Plant Soil 335(1-2): 21–34 [Google Scholar]
- Zhang A, Ren HM, Tan YQ, Qi GN, Yao FY, Wu GL, Yang LW, Hussain J, Sun SJ, Wang YF (2016) S-type anion channels SLAC1 and SLAH3 function as essential negative regulators of inward K+ channels and stomatal opening in Arabidopsis. Plant Cell 28(4): 949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wang Y (2017) Nitrate assay for plant tissues. Bio Protoc 7(2): e2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, He K, Kleist T, Chen F, Luan S (2015) Anion channel SLAH3 functions in nitrate-dependent alleviation of ammonium toxicity in Arabidopsis. Plant Cell Environ 38(3): 474–486 [DOI] [PubMed] [Google Scholar]
- Zheng X, Kang S, Jing Y, Ren Z, Li L, Zhou JM, Berkowitz G, Shi J, Fu A, Lan W, et al. (2018) Danger-associated peptides close stomata by OST1-independent activation of anion channels in guard cells. Plant Cell 30(5): 1132–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Fang XZ, Dai YJ, Zhu YX, Chen HS, Lin XY, Jin CW (2019) Nitrate transporter 1.1 alleviates lead toxicity in Arabidopsis by preventing rhizosphere acidification. J Exp Bot 70(21): 6363–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.