Abstract
Purpose: The present retrospective study aimed to explore the relationship between pancreatitis and pancreatic cancer in the population cohort of the UK Biobank (UKB) (https://www.ukbiobank.ac.uk). Methods: From the 500 thousand population cohort of UKB, according to the age and gender of patients with pancreatic cancer 1:10, matching the control without pancreatic cancer, the binary Logistic regression model was used to analyze the relationship between pancreatitis and pancreatic cancer, and subgroup analyses were used to identify potential effect modifiers. Results: A total of 1538 patients with pancreatic cancer were compared with 15 380 controls. In the fully adjusted model, patients with pancreatitis had a significantly increased risk of pancreatic cancer compared with no pancreatitis. The risk of pancreatitis and pancreatic cancer increased with the age of pancreatitis, and the risk of pancreatic cancer was highest in the 61 to 70 age group. In addition, in the first 3 years of acute pancreatitis, the risk of pancreatic cancer increased significantly with the increase in the duration of the disease (odds ratio [OR] 29.13, 95% confidence interval [CI]: 16.34-51.93), after 3 years, the trend of increase decreased. After more than 10 years, there was no significant correlation between the risk of acute pancreatitis and pancreatic cancer. However, patients with chronic pancreatitis were significantly associated with an increased risk of pancreatic cancer only in the first 3 years (OR 28.14, 95% CI: 14.86-53.31). Conclusion: Pancreatitis may associate with an increased risk of pancreatic cancer. The older the age of pancreatitis, the higher the risk of pancreatic cancer. The risk of pancreatic cancer increases significantly in the first 3 years of the course of pancreatitis. This may provide an alternative strategy for the early identification of individuals at high risk of pancreatic cancer.
Keywords: pancreatic cancer, pancreatitis, acute pancreatitis, chronic pancreatitis, UKB
Introduction
Pancreatic cancer (PC) is a highly invasive malignant tumor with a fatal risk.1 Over the past few decades, the overall 5-year survival rate for PC has changed little and is expected to be the second leading cause of cancer-related death in Western countries over the next decade.2 It was reported that in 2012, 8% of the world's population was over 65 years old, and it was the highest-risk age group for PC. In the next 30 years, this proportion is expected to double to 16.7%, especially in Asia, Australia, Europe, and Latin America.3 As global health improves and the human lifespan increases, the overall incidence of PC continues to rise. There is no previously recommended screening tool for PC,4 and the difficulty remains in how to effectively reduce PC incidence and mortality.
Pancreatitis is an inflammatory disease of the pancreas. Acute pancreatitis (AP) is an unpredictable and potentially fatal disease of variable severity and course, with gallstone disease and alcohol consumption as its main causes.5 The incidence of AP has been increasing worldwide in recent years and it is one of the most common causes of gastrointestinal hospitalization in the United States.6 The relationship between AP and PC is still unclear, and although AP may be the first manifestation of PC, the long-term risk of PC in patients with AP is still uncertain. Chronic pancreatitis (CP) is a syndrome involving inflammation, fibrosis, and loss of acinar and islet cells and can present with persistent abdominal pain, malnutrition, and exocrine and endocrine insufficiency, the most common of which is due to excessive alcohol consumption, smoking, or genetic mutations.7,8 CP is known to be a risk factor for PC, and long-term repeated chronic inflammation would promote pancreatic cell renewal and stellate cell proliferation, which would promote the formation of a cancerous pancreatic tissue microenvironment.9 Autoimmune pancreatitis (AIP) is a chronic fibroinflammatory disease of the pancreas that may increase the risk of malignancy compared to other inflammatory phases, and although rare cases of pancreaticobiliary malignancy have been reported in patients with AIP, overall, the lifetime risk of developing PC does not appear to be elevated and long-term follow-up data are limited.10,11 Therefore, in order to more clearly understand the relationship between AP, CP, and PC, we investigated the relationship between pancreatitis and PC risk through a case-control study in the UK Biobank (UKB) 500 000 population cohort and determined the role of acute and chronic pancreatitis in the development of PC.
Materials and Methods
Research Design and Overall
This is a retrospective study, all clinical data for the PC group and the no PC group in this case-control study were obtained from the UKB, application number: 69476. UKB’s clinically relevant data was obtained after registration on the website (https://www.ukbiobank.ac.uk).
| Pancreas disease | AP: (ICD10:K85,K85.0,K85.1,K85.2,K85.3,K85.8,K85.9;ICD9: 5770) CP: (ICD10: K86, K86.1,K86.2,K86.3,K86.9,K87.1;ICD9: 5711) PC (ICD10: C25,C25.0,C25.1,C25.2,C25.3,C25.4,C25.7,C25.8,C25.9;ICD9: 1570) |
| Other disorders | Hypertension(ICD10:I10,I11,I12,I13,I15;ICD9:401,402,403,404,405) Diabetes (ICD10:E10,E11,E12,E13,E14;ICD9:250) |
| Other basic information | Age21003, Sex31 (code9)、Race21000(code1001)、Townsend index189, age of completion of full-time education845(code100306), BMI21001 drinking history20117(code90),smoking history20116 (code90) Date of first hospitalization (ICD10: 41262; ICD9: 41263) |
Between 2006 and 2010, UKB recruited 500 000 people aged 40 to 69 across the United Kingdom who underwent extensive body measurements, provided information about their lifestyle and medical history, donated blood, urine, and saliva samples for future analysis, and agreed to follow up through health-related records. Related disease codes, other baseline information acquisition serial numbers and codes are shown in Table 1.
Table 1.
General Distribution and Single Factor Analysis of Research Objects.
| Baseline data | PC group (n = 1538) | Control group (n = 15 380) | P value | OR 95%CI |
|---|---|---|---|---|
| Age (years) | 62 (58-66) | 62 (58-66) | .93 | 1.0 |
| Gender (%) | 1.0 | 1.0 | ||
| Male | 809 (52.6) | 8090 (52.6) | ||
| Female | 729 (47.4) | 7290 (47.4) | ||
| Race | .74 | |||
| Caucasian | 1410 (91.7) | 14 193 (92.3) | 1.0 | |
| Mixed | 54 (3.5) | 501 (3.3) | 1.09 (0.82, 1.44) | |
| Asian | 48 (3.1) | 465 (3.0) | 1.04 (0.77, 1.41) | |
| Black | 8 (0.5) | 50 (0.3) | 1.61 (0.76, 3.40) | |
| Other ethnic | 18 (1.2) | 171 (1.1) | 1.06 (0.65, 1.73) | |
| Townsend index (%) | −2 (−4, 1) | −2 (−4, 0) | .003 | 1.03 (1.02, 1.05) |
| Education level (%) | .95 | |||
| ≤14 years | 22 (1.4) | 214 (1.4) | 1.0 | |
| 15-18 years | 910 (59.2) | 9143 (59.4) | 1.04 (0.66, 1.63) | |
| >18 years | 137 (8.9) | 1305 (8.5) | 1.08 (0.67, 1.75) | |
| Unknown | 469 (30.5) | 4718 (30.7) | 0.98 (0.62, 1.56) | |
| BMI (%) | .000 | |||
| <25 kg/m2 | 342 (22.2) | 3877 (25.2) | 1.0 | |
| 25-29.9 kg/m2 | 685 (44.5) | 7130 (46.4) | 1.06 (0.95, 1.25) | |
| ≥30 kg/m2 | 511 (33.2) | 4373 (28.4) | 1.33 (1.15, 1.53) | |
| Drinking history (%) | .47 | |||
| Never | 70 (4.6) | 668 (4.3) | 1.0 | |
| Before | 69 (4.5) | 598 (3.9) | 1.10 (0.78, 1.56) | |
| Current | 1399 (91.0) | 14 114 (91.8) | 0.95 (0.74, 1.22) | |
| Smoking history (%) | .000 | |||
| Never | 701 (46.5) | 7834 (50.9) | 1.0 | |
| Before | 594 (38.6) | 6148 (40.0) | 1.08 (0.96, 1.21) | |
| Current | 243 (15.8) | 1398 (9.1) | 1.94 (1.66, 2.27) | |
| Hypertension (%) | .000 | |||
| Yes | 643 (32.7) | 5732 (37.3) | 1.21 (1.09, 1.35) | |
| No | 895 (67.3) | 9648 (62.7) | 1.0 | |
| Diabetes (%) | .000 | |||
| Yes | 324 (21.1) | 1069 (10.5) | 2.28 (2.00-2.61) | |
| No | 1214 (78.9) | 13 771 (89.5) | ||
| Pancreatitis history (%) | .000 | |||
| Yes | 139 (9.0) | 148 (1.0) | 10.23 (8.06-12.97) | |
| No | 1399 (91.0) | 15 232 (99.0) | 1.0 |
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio;PC, pancreatic cancer.
Moral Statement
The ethical approval and patient consent were not required for our study, and all patient information was de-identified, and the reporting of this study conforms to RECORD guidelines.
Covariate Selection
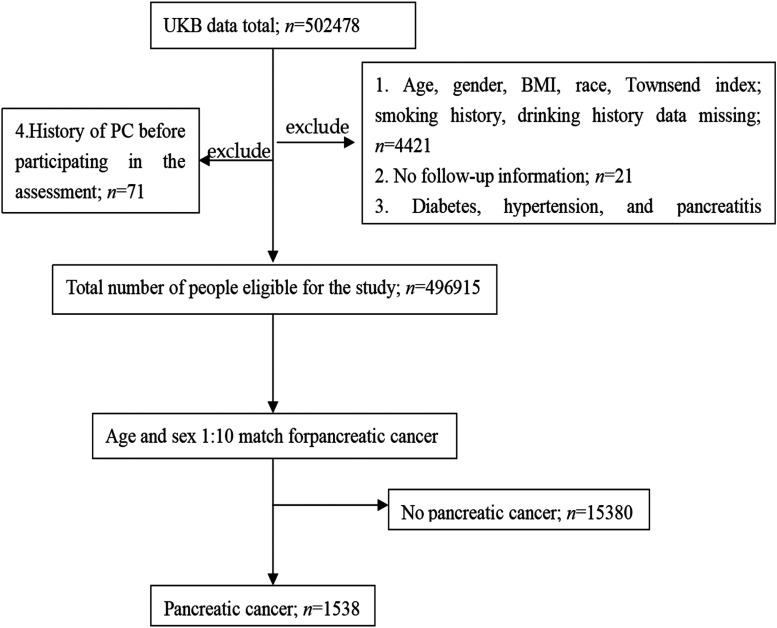
To obtain more reliable results, we included as much as possible baseline information. The baseline information of the subjects included: age (age of first participation in the assessment), gender, race, Townsend index (Townsend index represents the economic and social status of the individual, the greater the value represents the lower the economic and social status of the individua12), education level (age of completion of full-time education: ≤14, 15-18, >18 years), body mass index (BMI) (<25, 25-29.9, ≥30 kg/m2), drinking history (never, before, current), smoking history (never, before, current), hypertension (yes, no), diabetes (yes, no), and pancreatitis (yes, no). Study exclusion criteria: (1) age, gender, BMI, race, and other information missing; (2) no follow-up information; (3) diabetes, hypertension, and pancreatitis occurred after PC; and (4) a history of PC before baseline assessment. The screening process and results are shown in Figure 1.
Figure 1.
Screening process and results of research objects. Abbreviations: PC, pancreatic cancer; UKB, UK Biobanks.
Statistical Methods
SPSS25.0 statistical software was used for statistical analysis. The categorical data were expressed as a percentage N (%) and compared by chi-square test and fisher test. Continuous data were expressed as the median and interquartile range, and the U test was selected. A binary logistic regression model was used to analyze the relationship between pancreatitis and PC, and the odds ratio (OR) and 95% confidence interval (CI) were calculated. When P < .05, the difference was statistically significant. Subgroup analysis was used to identify potential effect modifiers.
Result
General Distribution and Single-Factor Analysis of Research Objects
We retrospectively and randomly collected the data of 500 000 participants in UKB from 2006 to 2010. A total of 1358 patients with PC and 13 580 controls without PC were included in this study (Figure 1). The general distribution of patients in the PC group and the control group is shown in Table 1. There was no significant difference in age and gender between the PC group and the control group (P > .05), and the data were comparable. The PC group had statistically significant differences in Townsend index, BMI, smoking history, and the prevalence of hypertension, diabetes, and pancreatitis compared with the control group (P < .05), whereas there were no significant differences between the 2 groups in terms of race, education, and drinking history (P > .05). The median age of the 2 groups was 62 years old, mainly male patients, accounting for about 52.6% (Table 1). The majority of patients are white, accounting for about 90%. Approximately 77.7% and 74.8% of patients in the PC and control groups, respectively, had a BMI ≥ 25 kg/m2. More than 90% of patients in both groups had a history of drinking. Patients with a history of smoking in the PC group and the control group accounted for about 53.5% and 49.1%, respectively. In addition, more than half of the patients in the 2 groups had no history of hypertension, diabetes, and pancreatitis (Table 1). Finally, univariate logistic regression analysis showed that there were significant differences in Townsend index, BMI ≥ 30 kg/m2, current smoking, hypertension, diabetes, and pancreatitis between the PC group and the control group (P < .05), and the above may be related to the incidence of PC (Table 1).
Relationship between AP, CP, and PC Risk
Through binary logistic regression model analysis, before adjusting for confounding factors, AP and CP were associated with PC risk, and the age of onset of AP and CP (<50, 50-60, 61-70, >70 years), disease duration within 10 years of AP and CP within 3 years were associated with PC (Table 2). After adjustment for Townsend index, BMI, smoking history, hypertension, and diabetes mellitus, patients with AP and CP all had an increased risk of PC compared with patients without pancreatitis (ORAP7.75, 95% CI: [5.77-10.41]; ORCP14.70, 95% CI: [9.48-22.79]), particularly patients with CP, the risk of PC was as high as 14.70 times (Table 2). The risk of AP and CP with PC also increases continuously with the age of disease onset in AP and CP, with the highest risk for PC at ages 61 to 70 years (ORAP 9.44, 95% CI:[5.92-15.07]; ORCP 30.47, 95% CI: [12.11-76.67]) (Table 2). In addition, the association between AP, CP, and PC risk correlated with both disease duration. In the first 3 years of AP, the risk of PC increased significantly with time (OR 29.13, 95% CI : 16.34-51.93), and a decreasing trend after 3 years, with no significant association between AP and PC risk beyond 10 years (OR 0.88, 95% CI: 0.35-2.22, P > .05). The risk of PC in patients with CP increased significantly only in the first 3 years of CP (OR 28.14, 95% CI: 14.86-53.31), and after more than 3 years, no correlation between CP and PC risk was observed (P > .05) (Table 2).
Table 2.
OR and 95% CI of AP, CP, and PC Risk.
| Variable | OR1 95% CI | OR2 95%CI | OR3 95%CI |
|---|---|---|---|
| Pancreatitis | |||
| No | 1.0 | 1.0 | 1.0 |
| AP | 7.78 (5.83-10.38) | 7.73 (5.77-10.35) | 7.75 (5.77-10.41) |
| CP | 17.06 (11.10-26.20) | 16.76 (10.87-25.85) | 14.70 (9.48-22.79) |
| Age with AP | |||
| <50 | 3.49 (0.71-17.35) | 3.61 (0.72-18.08) | 3.46 (0.68-17.47) |
| 50-60 | 5.50 (2.65-11.42) | 5.66 (2.71-11.81) | 5.29 (2.51-11.15) |
| 61-70 | 9.94 (6.28-15.73) | 9.31 (5.86-14.78) | 9.44 (5.92-15.07) |
| >70 | 7.59 (4.87-11.84) | 7.11 (4.54-11.14) | 7.35 (4.66-11.59) |
| Age with CP | |||
| <50 | 10.36 (3.34-32.15) | 7.69 (2.44-24.29) | 6.06 (1.89-19.47) |
| 50-60 | 10.36 (2.59-41.45) | 7.39 (1.81-30.17) | 8.08 (1.98-32.93) |
| 61-70 | 36.24 (14.61-89.93) | 35.14 (14.09-87.64) | 30.47 (12.11-76.67) |
| >70 | 14.38 (7.38-26.42) | 14.47 (7.85-26.68) | 12.67 (6.82-23.53) |
| Duration of AP (years) | |||
| No | 1.0 | 1.0 | 1.0 |
| <3 | 29.79(16.83-52.76) | 28.01 (15.76-49.75) | 29.13 (16.34-51.93) |
| 3-10 | 3.29(1.80-6.03) | 3.03 (1.65-5.56) | 2.94 (1.59-5.47) |
| >10 | 0.96 (0.38-2.40) | 0.96 (0.38-2.41) | 0.88 (0.35-2.22) |
| Duration of CP (years) | |||
| No | 1.0 | 1.0 | 1.0 |
| <3 | 30.83 (16.42-57.88) | 31.81 (16.89-59.91) | 28.14 (14.86-53.31) |
| 3-10 | 1.52 (0.53-4.35) | 1.36 (0.47-3.92) | 1.21 (0.42-3.49) |
| >10 | 2.28 (0.77-6.76) | 1.87 (0.62-5.59) | 1.60 (0.53-4.84) |
Notes: 1. Unadjusted; 2. Adjusted for: Townsend index, BMI, smoking history, hypertension; 3. Adjusted for: Townsend index, BMI, smoking history, hypertension, diabetes.
Abbreviations: AP, acute pancreatitis; BMI, body mass index; CI, confidence interval; CP, chronic pancreatitis; OR, odds ratio; PC, pancreatic cancer.
Subgroup Analysis
After grouping by gender (Table 3), in patients with AP, men have a higher risk of PC than women. In patients with CP, women had a higher risk of PC than men, but this trend was not significant (AP: Pinteraction = .33, CP: Pinteraction = .48) (Table 3). After stratified by smoking (Table 4), patients with AP with previous smoking and current smoking had a higher risk of PC than nonsmoking patients, and patients with AP with current smoking had a higher risk of PC (OR 11.35, 95% CI: 4.72-27.26) (Table 4). Among patients with CP, previous smokers had a higher risk of PC (OR 17.08, 95% CI: 8.73-33.45), while current smokers had a lower risk of PC than nonsmokers, and this trend was not significant (AP: Pinteraction = .67, CP: Pinteraction = .38) (Table 4).
Table 3.
OR and 95% CI of Pancreatitis and PC Stratified by Sex.
| Event | OR1 95%CI | OR2 95%CI | P interaction | |
|---|---|---|---|---|
| Female | ||||
| No | 678 | 1.0 | 1.0 | |
| AP | 35 | 6.89 (4.46-10.63) | 6.48 (4.14-10.12) | .33 |
| CP | 16 | 20.43 (8.71-47.89) | 19.05 (7.99-45.41) | .48 |
| Male | ||||
| No | 721 | 1.0 | 1.0 | |
| AP | 48 | 8.59 (5.82-12.66) | 9.04 (6.09-13.42) | |
| CP | 40 | 16.13 (9.79-26.58) | 14.04 (8.44-23.36) | |
Notes: 1. unadjusted; 2. adjusted for: Townsend index, BMI, smoking history, hypertension, diabetes.
Abbreviations: AP, acute pancreatitis; BMI, body mass index; CI, confidence interval; CP, chronic pancreatitis; OR, odds ratio; P, P value; PC, pancreatic cancer.
Table 4.
OR and 95% CI of Pancreatitis and PC Risk Stratified by Smoking.
| Event | OR1 95%CI | OR2 95%CI | P interaction | |
|---|---|---|---|---|
| Never smoke | ||||
| No | 642 | 1.0 | 1.0 | |
| AP | 38 | 7.07 (4.69-10.66) | 7.09 (4.68-10.75) | .67 |
| CP | 21 | 18.58 (9.26-37.26) | 16.48 (8.11-33.49) | .38 |
| Before smoked | ||||
| No | 539 | 1.0 | 1.0 | |
| AP | 30 | 7.92 (4.91-12.79) | 7.99 (4.90-13.03) | |
| CP | 25 | 19.25 (9.95-37.24) | 17.08 (8.73-33.45) | |
| Current smoking | ||||
| No | 218 | 1.0 | 1.0 | |
| AP | 15 | 11.43 (4.79-27.27) | 11.35 (4.72-27.26) | |
| CP | 10 | 8.53 (3.21-22.63) | 7.80 (2.01-20.94) | |
Notes: 1. unadjusted; 2. adjusted for: Townsend index, BMI, hypertension, diabetes.
Abbreviations: AP, acute pancreatitis; BMI, body mass index; CI, confidence interval; CP, chronic pancreatitis; OR, odds ratio; P, P value; PC, pancreatic cancer.
Discussion
Based on the UKB 500 000 prospective population cohort, this study found that both AP and CP were associated with an increased risk of PC through a case-control study. The older the age of AP and CP, the higher the risk of PC, especially the patients aged 61 to 70 years. The risk of PC was highest in the first 3 years of the course of AP and CP, and then the correlation gradually weakened or disappeared over time.
AP may be a risk factor for PC. A Danish cohort study13 showed that age >50 years was a predictor of PC in patients with AP, and patients aged 56 to 70 years had the highest risk, which was similar to our findings. Our results also showed that the risk of CP in patients with AP increased significantly (OR 7.75, 95% CI: 5.77-10.41), and the risk of PC increased with time in the first 10 years of AP, and there was no correlation between the 2 after more than 10 years. Similarly, a cohort study in Sweden14 showed that the risk of PC increased significantly in the first few years after AP, but gradually decreased over time, reaching levels comparable to those in people without pancreatitis more than 10 years later, and a meta-analysis by Zhang et al15 showed similar results. Contrary to this view in our results, a case study by Fernandez et al16 showed that 5 years or more after the diagnosis of pancreatitis (RR = 6.9) was significantly higher than the previous 4 years (RR = 2.1). However, in this case-control study of Fernandez et al,16 the main disease duration node is 5 years, which is different from the time node of our study, cannot be directly compared, and does not provide sufficient evidence to determine the effect of course of disease >10 years on the risk estimation of PC in their population.
CP is a recognized risk factor for PC. At present, several studies17–22 have shown that CP is significantly associated with an increased risk of PC, and our study concludes the same, However, due to the differences in research design and measurement endpoints, the effect of reporting is also quite different. Our study also showed that the older the age of CP, the higher the risk of PC. Consistently, a retrospective study19 showed that the higher the age of onset of CP, the greater the risk of PC. When the age of onset of CP was greater than 50 years, the risk of PC increased by 1.55 times for every 5 years of increase. In addition, several studies23–25 have shown that the risk of PC increases with the increase of the duration of CP, and the risk of PC still increases at 2, 5, and 10 years after CP diagnosis. This is contrary to our research results that the risk relationship between CP and PC is only significantly correlated within the first 3 years of CP. Considering the different times of disease duration demarcation and the limited number of study participants with CP in our data, our research results may be accidental.
AIP is a rare form of CP, the pathogenesis of AIP is still unclear, its clinical manifestations are nonspecific, and it overlaps with PC in pathology.26–28 So it is difficult to differentiate AIP from PC, and the relationship between them is still controversial. Type I AIP is a form of IgG4-related disease, and although elevated IgG4 has high sensitivity and specificity in the case of AIP, in some patients with PC, even at twice the normal limit, there is no AIP.29 A retrospective analysis of patients undergoing pancreatectomy found that 6 of the 15 patients diagnosed with AIP were diagnosed with PC, indicating that PC and AIP may occur simultaneously, and preoperative diagnosis of AIP does not exclude the presence of PC at the same time.30 Whereas another study found that, in 31 patients with type 1 AIP, PC and AIP occurred simultaneously in 11 cases (33%) and metachronous in 22 cases (67%).31 In the metachronous group most cancers (86%) occurred more than 2 years after AIP diagnosis, and in most patients (70%) cancer originated from the pancreatic part affected by AIP.31 In our study, due to the limitations of the data itself, it was not possible to further identify patients with AIP and explore the relationship between AIP and PC, resulting in certain limitations in the research results.
Furthermore, in our subgroup analysis, male patients with AP had a higher risk of PC than females, and female patients with CP had a higher risk of PC than males. The patients with AP with previous smoking and current smoking have a higher risk of PC than nonsmokers, while patients with CP with current smoking have a lower risk of PC than not only never-smokers but also current smokers in AP, which may be related to the low incidence of CP in this study and is accidental. Due to the trend P > .05 in the subgroup analysis, this trend is not significant and has no substantial impact on the research results. Similarly, a cohort study by Kirkegård et al32 showed that smoking history did not have a substantial effect on the results, but that there was a significant difference in the correlation between AP and PC between men and women in the gender subgroup. This inconsistency may be due to differences in sample size and covariate adjustments. Therefore, the results of subgroup analysis need to be further replicated by larger sample studies.
The strengths of this study include (1) effective case selection based on ICD9 and ICD10 confirmation of PC; (2) the study population was from the UKB ‘s 500 000 prospective cohort, and matched for PC by gender and age 1: 10, reducing the differences caused by other confounding factors between the groups; (3) our study adds supporting evidence to other studies related to pancreatitis and PC. However, our research also has limitations: (1) the research subjects are mainly Caucasian, which cannot represent other races or even ordinary people; (2) in subgroup analysis, the distribution of PC cases was sparse, which may affect the reliability of the relevant results; (3) due to data limitations, the etiology of AP and CP was not further stratified analysis; and (4) due to data limitations, the relevant data of AIP were not collected for further analysis.
Conclusion
We observed that both AP and CP were may associated with an increased risk of PC. When the age group was 61 to 70 years old with AP and CP, the risk of PC was the highest, and the risk increased significantly within the first 3 years of the course of the disease. This study may adds evidence to the prevention of PC and helps to further identify high-risk groups of PC. In addition, the data distribution of subgroup analysis by gender and smoking history in this study was sparse, so these findings need to be further replicated and verified in larger sample studies in the future.
Acknowledgment
All authors are very grateful for to help in the statistical analysis of this study.
Abbreviations
- AIP
autoimmune pancreatitis
- AP
acute pancreatitis
- CI
confidence interval
- CP
chronic pancreatitis
- MREC
North West Multi-Centre Research Ethics Committee
- OR
odds ratio
- P
P value
- PC
pancreatic cancer
- UKB
UK Biobanks.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed the following financial support for the research, authorship, and/or publication of this article: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the National Nature Science Foundation of China (grant number 82070668).
Ethics Approval: The retrospective single-center study was conducted in accordance with the principles of the Declaration of Helsinki. All clinical data for the PC group and the no PC group in this case-control study were obtained from the UKB, application number: 69476. UKB’s clinically relevant data was obtained after registration on the website (https://www.ukbiobank.ac.uk), therefore ethical approval and patient consent were not required for our study, and all patient information was de-identified, and our study is in line with TARGET guidelines.
ORCID iD: Dong-Mei Ma https://orcid.org/0000-0002-5584-5671
References
- 1.Moore A, Donahue T. Pancreatic cancer. JAMA. 2019;322(14):1426. doi: 10.1001/jama.2019.14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):333‐348. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 3.Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18(7):493‐502. doi: 10.1038/s41575-021-00457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens DK, Davidson KW, Krist AH, et al. Screening for pancreatic cancer: US preventive services task force reaffirmation recommendation statement. JAMA. 2019;322(5):438‐444. doi: 10.1001/jama.2019.10232. [DOI] [PubMed] [Google Scholar]
- 5.Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325(4):382‐390. doi: 10.1001/jama.2020.20317. [DOI] [PubMed] [Google Scholar]
- 6.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(8):479‐496. doi: 10.1038/s41575-019-0158-2. [DOI] [PubMed] [Google Scholar]
- 7.Umans DS, Hoogenboom SA, Sissingh NJ, Lekkerkerker SJ, Verdonk RC, van Hooft JE. Pancreatitis and pancreatic cancer: a case of the chicken or the egg. World J Gastroenterol. 2021;27(23):3148‐3157. doi: 10.3748/wjg.v27.i23.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pham A, Forsmark C. Chronic pancreatitis: review and update of etiology, risk factors, and management. F1000Res. 2018;7:F1000. 10.12688/f1000research.12852.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Algül H, Treiber M, Lesina M, Schmid RM. Mechanisms of disease: chronic inflammation and cancer in the pancreas–a potential role for pancreatic stellate cells? Nat Clin Pract Gastroenterol Hepatol. 2007;4(8):454‐462. doi: 10.1038/ncpgasthep0881. [DOI] [PubMed] [Google Scholar]
- 10.Majumder S, Takahashi N, Chari ST. Autoimmune pancreatitis. Dig Dis Sci. 2017;62(7):1762‐1769. doi: 10.1007/s10620-017-4541-y. [DOI] [PubMed] [Google Scholar]
- 11.Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology. 2015;149(1):39‐51. doi: 10.1053/j.gastro.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Forrest LF, Adams J, Wareham H, Rubin G, White M. Socioeconomic inequalities in lung cancer treatment: systematic review and meta-analysis. PLoS Med. 2013;10(2):e1001376. doi: 10.1371/journal.pmed.1001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkegård J, Mortensen FV, Heide-Jørgensen U, Cronin-Fenton D. Predictors of underlying pancreatic cancer in patients with acute pancreatitis: a Danish nationwide cohort study. HPB. 2020;22(4):553‐562. doi: 10.1016/j.hpb.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(11):1711‐1719. doi: 10.1038/s41395-018-0255-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, An R, Tian H, Zhao J. Increased risk of pancreatic cancer after acute pancreatitis: a meta-analysis of prospective cohort studies. Clin Res Hepatol Gastroenterol. 2019;43(3):e39‐e41. doi: 10.1016/j.clinre.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez E, La Vecchia C, Porta M, Negri E, d'Avanzo B, Boyle P. Pancreatitis and the risk of pancreatic cancer. Pancreas. 1995;11(2):185‐189. doi: 10.1097/00006676-199508000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Yang MW, Zhang LD. Analysis of prevalence and risk factors of pancreatic cancer. Chin J Med Front. 2017;9(04):122‐124. [Google Scholar]
- 18.Pang TS, Cao LP, Ling TY, et al. Multivariate analysis of risk factors for pancreatic cancer. Chin J Pract Surg. 2014;34(10):962‐966. [Google Scholar]
- 19.Korpela T, Udd M, Mustonen H, et al. Association between chronic pancreatitis and pancreatic cancer: a 10-year retrospective study of endoscopically treated and surgical patients. Int J Cancer. 2020;147(5):1450‐1460. doi: 10.1002/ijc.32971. [DOI] [PubMed] [Google Scholar]
- 20.Bo X, Shi J, Liu R, et al. Using the risk factors of pancreatic cancer and their interactions in cancer screening: a case-control study in Shanghai, China. Ann Glob Health. 2019;85(1). doi: 10.5334/aogh.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon CY, Chen Q, Yu W, et al. Identification of individuals at increased risk for pancreatic cancer in a community-based cohort of patients with suspected chronic pancreatitis. Clin Transl Gastroenterol. 2020;11(4):e00147. doi: 10.14309/ctg.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bracci PM, Wang F, Hassan MM, Gupta S, Li D, Holly EA. Pancreatitis and pancreatic cancer in two large pooled case-control studies. Cancer Causes Control. 2009;20(9):1723‐1731. doi: 10.1007/s10552-009-9424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakorafas GH, Sarr MG. Pancreatic cancer after surgery for chronic pancreatitis. Dig Liver Dis. 2003;35(7):482‐485. doi: 10.1016/s1590-8658(03)00221-4. [DOI] [PubMed] [Google Scholar]
- 24.Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112(9):1366‐1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 25.Tong GX, Geng QQ, Chai J, et al. Association between pancreatitis and subsequent risk of pancreatic cancer: a systematic review of epidemiological studies. Asian Pac J Cancer Prev. 2014;15(12):5029‐5034. doi: 10.7314/apjcp.2014.15.12.5029. [DOI] [PubMed] [Google Scholar]
- 26.Poddighe D. Autoimmune pancreatitis and pancreatic cancer: epidemiological aspects and immunological considerations. World J Gastroenterol. 2021;27(25):3825‐3836. doi: 10.3748/wjg.v27.i25.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaho M, Dítě P, Kunovský L, Kianička B. Autoimmune pancreatitis—an ongoing challenge. Adv Med Sci. 2020;65(2):403‐408. doi: 10.1016/j.advms.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto A, Watanabe T, Kamata K, Minaga K, Kudo M. Recent updates on the relationship between cancer and autoimmune pancreatitis. Intern Med. 2019;58(11):1533‐1539. doi: 10.2169/internalmedicine.2210-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bojková M, Dítě P, Dvořáčková J, et al. Immunoglobulin G4, autoimmune pancreatitis and pancreatic cancer. Dig Dis. 2015;33(1):86‐90. doi: 10.1159/000368337. [DOI] [PubMed] [Google Scholar]
- 30.Macinga P, Pulkertova A, Bajer L, et al. Simultaneous occurrence of autoimmune pancreatitis and pancreatic cancer in patients resected for focal pancreatic mass. World J Gastroenterol. 2017;23(12):2185‐2193. doi: 10.3748/wjg.v23.i12.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macinga P, Bajer L, Del Chiaro M, et al. Pancreatic cancer in patients with autoimmune pancreatitis: a scoping review. Pancreatology. 2021;21(5):928‐937. doi: 10.1016/j.pan.2021.03.007 [DOI] [PubMed] [Google Scholar]
- 32.Kirkegård J, Cronin-Fenton D, Heide-Jørgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(6):1729‐1736. doi: 10.1053/j.gastro.2018.02.011. [DOI] [PubMed] [Google Scholar]