Abstract
Objectives
Long-term results of hepaticojejunostomy (HJ) for complex bile duct injury (BDI) remain under-reported. The objective of this study was to assess short-term and long-term outcomes of HJ for post-cholecystectomy BDI.
Methods
This was a retrospective cohort study and included patients who underwent Roux-en-Y HJ for BDI (n = 87). Short-term (90-day) and long-term morbidity and mortality were assessed.
Results
At presentation, 42 (48.2%) patients had E3 or E4 BDI, 27 (31%) patients had vascular injury, and liver resection was performed in 12 (13.7%) patients. The 90-day morbidity was 51.7% (n = 45), and the 90-day mortality was 2.3% (n = 2). The long-term mortality was 3.4% (n = 3). The 10-year estimated stricture-free survival was 95%. The 10-year estimated overall survival rate was 100% in patients who underwent major hepatectomy and 91% in patients who did not. The 10-year estimated overall survival rate was 100% in patients with vasculobiliary injury and was not reached in patients without vascular injury.
Conclusions
Vascular injury with proximal BDI is not uncommon. Excellent long-term outcomes might be achieved with Roux-en-Y HJ for BDI with vascular injury and in patients requiring liver resection.
Keywords: Cholecystectomy, hepaticojejunostomy, morbidity, hepatectomy, mortality, bile duct injury, liver resection
Introduction
Laparoscopic cholecystectomy (LC) is the gold standard for treating symptomatic gallstones.1–3 Bile duct injury (BDI) occurs more frequently with LC than with the traditional open approach.4–7 However, because of improved pain control and cosmesis, a reduced length of hospital stay, and an early return to baseline activity, LC is preferred over the open approach.4–9 While the rate of post-cholecystectomy BDI ranges from 0.1% to 2.3%,4,5,10 it is associated with a mortality of 3% to 12%.5,10,11 In patients who develop BDI, a poor quality of life and financial burden are major long-term problems.12,13 The subsequent litigation can become a liability for the hospital or the surgeon directly involved in the patient’s care.14 The complex nature of this problem requires surgeons, gastroenterologists, and radiologists to work together in a multi-disciplinary setting.15 When managing this complex surgical entity, experience of the operating surgeon is a strong predictor of the outcome.1,15 The reported outcomes after the management of BDI remain conflicting. This controversy is due to a low overall BDI rate, diversity of options available for management, and incomplete follow-ups.16 There are limited data on the eventual outcome of vasculobiliary injury, and the utility of liver resection in these patients remains under-reported.17–18 Therefore, the current study aimed to determine short-term and long-term results of hepaticojejunostomy (HJ) for BDI.
Methods
Patients
This was a retrospective cohort study and included all consecutive patients who underwent Roux-en-Y HJ as definitive management of post-cholecystectomy BDI at our center between January 2011 and August 2021. The patients underwent Roux-en-Y HJ during this period at our center, and all patients had cholecystectomy performed elsewhere.
Surgery and follow-up
Patients with post-cholecystectomy BDI were evaluated in a weekly multi-disciplinary meeting. When feasible, we routinely performed liver dynamic computed tomography (CT) and magnetic resonance cholangiopancreatography in these patients. Preoperative CT combined with intraoperative findings improved the assessment of the extent of vascular injury. When surgery was considered appropriate, an end-to-side Roux-en-Y HJ was performed. The bile ducts were exposed proximal to the stricture or to the site of bile leakage and divided to ensure an adequate blood supply. In most patients with dilated ducts, a continuous polydioxanon-suture (PDS) 4/0 or 5/0 suture was used for the bilioenteric anastomoses under loupe magnification. When small ducts were encountered, we preferred a posterior continuous layer with Prolene 6/0 and an anterior interrupted layer with PDS 6/0 (Ethicon, Johnson & Johnson Medical N.V., Brussels, Belgium). This technique was adopted from our living donor liver transplantation experience where duct to duct anastomoses were performed in this fashion. We did not observe any difference in the stricture rate in living donor liver transplants between Prolene and PDS in small ducts. We usually used a posterior layer with continuous Prolene because PDS tends to curl around itself when used in a continuous fashion. The anterior layer was achieved with PDS in an interrupted fashion. We used the same technique in patients with a biliary injury and small ducts. We did not place transanastomotic stents. The abdominal cavity was drained with a 28 French drain placed under the HJ near the inferior surface of the liver. The drain was removed on the 5th postoperative day. Occasionally, patients underwent HJ elsewhere. In these patients, we preferred to use the same loop, and the HJ was performed at a slightly different location. Liver resection was considered when there was symptomatic right lobe atrophy, usually associated with vascular injury. The other indication for liver resection (segment 4 resection) was to gain access to healthy proximal right or left hepatic ducts. A 27 French plastic drain was placed under the HJ. On postoperative day 5, if the drain output was serous and had <500 mL/24 hours, it was removed. After discharge, patients visited our center at weeks 1, 4, and 12, and then annually. Baseline tests including liver function tests were performed at all visits. In addition, ultrasonography was performed in the first 3 months and later if required. We performed magnetic resonance cholangiopancreatography or CT if it was required and the patient was symptomatic.
Patients’ characteristics
The patients’ demographics, the open versus laparoscopic approach for cholecystectomy, prior surgical management, the Strasberg grade of BDI,19 concomitant vascular injury, the timing of definitive surgery, and postoperative outcomes were assessed. Short-term and long-term outcomes were reported. We recorded 90-day morbidity and mortality for short-term outcomes. All grade ≥3 complications as determined by Clavien–Dindo grading were recorded.20 The long-term morbidity and mortality were documented. More specifically, we investigated the development of bilioenteric anastomotic stricture after HJ at our center.
Statistical analysis
Data are presented as the frequency and percentage for categorical variables, and as the median with interquartile range for continuous variables. The stricture-free survival (SFS) rate was calculated by subtracting the date of documentation of HJ stricture from the date of surgery at our center. The estimated 10-year SFS and overall survival (OS) were determined using Kaplan–Meier curves, and the log rank test was used to determine significance. A two tailed P value <0.05 was considered statistically significant. With regard to SFS, only patients with a minimum follow-up of 3 years were considered. All analysis was performed using SPSS version 20 (IBM Corp., Armonk, NY, USA). Verbal informed consent was provided by the participants, and the ethics committee of Shifa International Hospital approved the study (IRB # 0224-22). The reporting of this study conforms to the STROBE guidelines.21
Results
Patients’ characteristics
We included 87 patients in this study. The median age of the patients was 43 years (33–53 years). LC was attempted in 55 (63.2%) patients, acute cholecystitis was the indication in 20 (23%) patients, and surgery was performed at a tertiary care center in 23 (26.4%) patients (Table 1). Among the 55 patients who underwent LC, conversion to the open method was required in 21 (38.1%) patients. Among 19 patients who presented with bile leakage, 10 patients had biliary peritonitis, 5 had bile in the drain, and localized abdominal collections were present in 4 patients. At the time of presentation, the median number of surgical attempts was 2 (1–5), and in 36 (41.3%) patients, bile duct repair was attempted. The median duration from the initial event to presentation at our center was 8 months (1–28 months), with a maximum of 241 months. At presentation, 40 (45.9%) patients had persistent symptoms after BDI, and the remaining 47 (54.1%) patients had a symptom-free interval of 8 months (3–23 months) before becoming symptomatic again.
Table 1.
Characteristics of patients referred after post-cholecystectomy bile duct injury.
| Patient characteristics | n (%)(n = 87) |
|---|---|
| Sex | |
| Male | 19 (21.8) |
| Female | 68 (78.2) |
| Comorbidities | |
| Diabetes mellitus | 14 (16.1)) |
| Hypertension | 12 (13.8) |
| Ischemic heart disease | 3 (3.4) |
| Asthma | 1 (1.1) |
| Chronic liver disease | 1 (1.1) |
| ECOG | |
| 1 | 63 (72.4) |
| 2 | 11 (12.6) |
| 3 | 13 (14.9) |
| Sepsis at presentation | |
| No | 53 (60.9) |
| Yes | 34 (39.1) |
| Clinical presentation | |
| Bile leakage | 19 (21.8) |
| Jaundice | 65 (74.7) |
| Initial surgery | |
| Bile leakage and jaundice | 3 (3.4) |
| Open cholecystectomy | 32 (36.8) |
| Laparoscopic cholecystectomy | 55 (63.2) |
| Conversion to open | 21 (38.1) |
| Reason for conversion (n = 21) | |
| Operative difficulty | 5 (23.8) |
| Intraoperative bleeding | 3 (14.3) |
| Bile duct injury | 13 (61.9) |
| Surgery at a tertiary facility | |
| Yes | 23 (26.4) |
| No | 64 (73.6) |
| Prior ERCP | |
| Performed | 36 (41.4) |
| Prior PTBD | |
| Performed | 10 (11.5) |
| Prior surgical management | |
| None | 35 (40.2) |
| Laparotomy and drainage | 16 (18.4) |
| Primary repair of the bile duct | 2 (2.3) |
| T-tube repair | 10 (11.5) |
| Choledochoduodenostomy | 4 (4.6) |
| Hepaticojejunostomy | 20 (23) |
ECOG, Eastern Cooperative Oncology Group; ERCP, endoscopic retrograde cholangiopancreatography; PTBD, percutaneous transhepatic biliary drainage.
Details of bile duct injury
Forty-two (48.2%) patients had E3 or E4 BDI (Table 2). In addition, vascular injuries were identified in 27 (31.0%) patients. These included injuries to the right hepatic artery (RHA) in 25 (28.7%) and injury to the portal vein in 6 (6.9%) patients.
Table 2.
Pattern of vasculobiliary injury at the time of presentation.
| Grade of bile duct injury (Strasberg) | n (%) (n = 87) |
|---|---|
| C | 2 (2.3) |
| D | 5 (5.7) |
| E1 | 14 (16.1) |
| E2 | 24 (27.6) |
| E3 | 29 (33.4) |
| E4 | 13 (14.9) |
| Concomitant vascular injury | 27 (31.0) |
| Hepatic arterial injury | 21 (24.1) |
| Complete thrombosis of the RHA | 15 (17.2) |
| Attenuated RHA | 6 (6.9) |
| Portal venous injury | 2 (2.3) |
| Occluded RPV with marked narrowing of the LPV | 1 (1.1) |
| Chronic occlusion of the RPV with recanalization | 1 (1.1) |
| Combined | 4 (4.6) |
| Thrombosed RHA/RAPV narrowing | 1 (1.1) |
| Thrombosed RHA/MPV narrowing | 1 (1.1) |
| Thrombosed RHA/LPV narrowing | 1 (1.1) |
| Thrombosed RHA/occluded RPPV | 1 (1.1) |
RHA, right hepatic artery; RPV, right portal vein; LPV, left portal vein RAPV, right anterior portal vein; RPPV, right posterior portal vein.
Surgical management
A single bilioenteric anastomosis was performed in 79 (90.8%) patients (Table 3). Hepaticojejunostomy was performed on the aberrant right hepatic duct in both patients with type C injury. In five (5.7%) patients, ductoplasty was performed to achieve a single bilioenteric anastomosis. Liver resection was performed in 12 (13.7%) patients. Among seven (8%) patients who underwent right hepatectomy, five (5.7%) patients had vasculobiliary injury (hepatic artery = 3, portal vein = 1, combined artery and portal vein = 1), and the remaining patients had right lobe atrophy with persistent upper abdominal pain. Segment 4 resection was performed in five (5.7%) patients to gain exposure to healthy right and left hepatic ducts in E3–E4 BDI. Iatrogenic injury to the RHA occurred in two patients. End-to-end arterial reconstruction between the RHA-RHA and the RHA-gastroduodenal artery was performed in these patients. Both patients remained well postoperatively.
Table 3.
Details of surgical management.
| n (%) (n = 87) | |
|---|---|
| Surgical procedure | |
| Standard Roux-en-Y loop HJ | 79 (90.8) |
| Right and left duct HJ | 3 (3.4) |
| RHD to LHD ductoplasty | 3 (3.4) |
| RAHD to CHD ductoplasty | 1 (1.1) |
| RAHD-RPHD-LHD ductoplasty | 1 (1.1) |
| Right hepatectomy | 7 (8.0) |
| Segment 4 resection | 5 (5.7) |
| Vascular reconstruction | 2(2.3) |
| Perioperative details | |
| Duration of surgery, minutes, median (range) | 270 (85–720) |
| Blood loss, mL, median (range) | 300 (100–4000) |
| Days in the ICU, median (range) | 2 (0–22) |
| Days in hospital, median (range) | 6 (2–32) |
HJ, hepaticojejunostomy; RHD, right hepatic duct; LHD, left hepatic duct; CHD, common hepatic duct; RAHD, right anterior hepatic duct; RPHD, right posterior hepatic duct; ICU, intensive care unit.
Short-term morbidity and mortality
Overall, 90-day ≥ grade 2 morbidity was 51.7% (n = 45) (Table 4). Five (5.7%) patients had post-HJ bile leak. Conservative management was performed in four (4.5%) patients, while laparotomy for bile leak from a duodenal injury was required in one patient. The 90-day mortality was 2.3% (n = 2), and both mortalities were attributed to septic shock. Both of these patients had biliary peritonitis at the time of the operation with proximal BDI, progressive deterioration despite an attempt at preoperative optimization, and a history of attempted repair at BDI before being referred.
Table 4.
Short-term morbidity and mortality.
| Short-term morbidity and mortality | n (%) |
|---|---|
| 90-day morbidity | 45 (51.7) |
| Grade 2 | 22 (25.3) |
| Blood transfusion | 2 (2.3) |
| Additional antibiotics | 20 (23) |
| Grade 3 A | 7 (8) |
| Bile leakage | 4 (4.5) |
| Pleural effusion | 3 (3.5) |
| Grade 3 B | 3 (3.5) |
| Burst abdomen | 1 (1.1) |
| Duodenal injury | 1 (1.1) |
| Bleeding from the IVC | 1 (1.1) |
| Grade 4 A | 9 (10.3) |
| Septic shock | 2 (2.2) |
| Acute renal failure | 3 (3.5) |
| Type 1 respiratory failure | 2 (2.2) |
| Vasovagal shock | 1 (1.1) |
| NSTEMI | 1 (1.1) |
| Grade 4 B | 4 (4.5) |
| Septic shock, ARDS, acute renal failure | 4 (4.5) |
| 90-day mortality | 2 (2.3) |
IVC, inferior vena cava; NSTEMI, non-ST elevation myocardial infarction; ARDS, adult respiratory distress syndrome.
Long-term morbidity and mortality
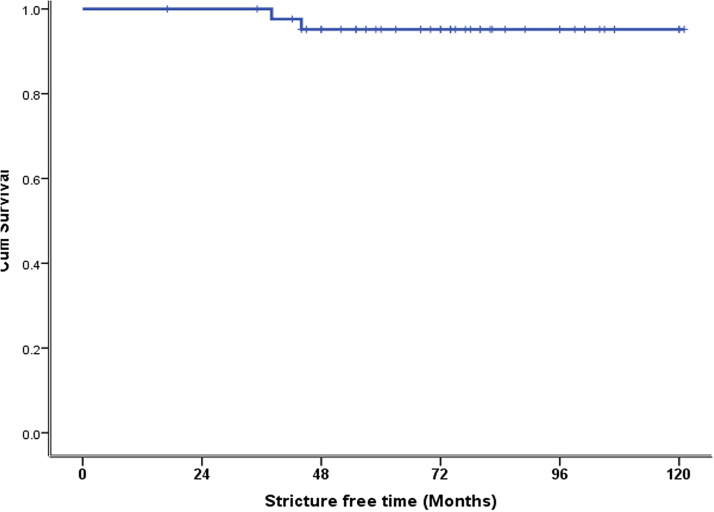
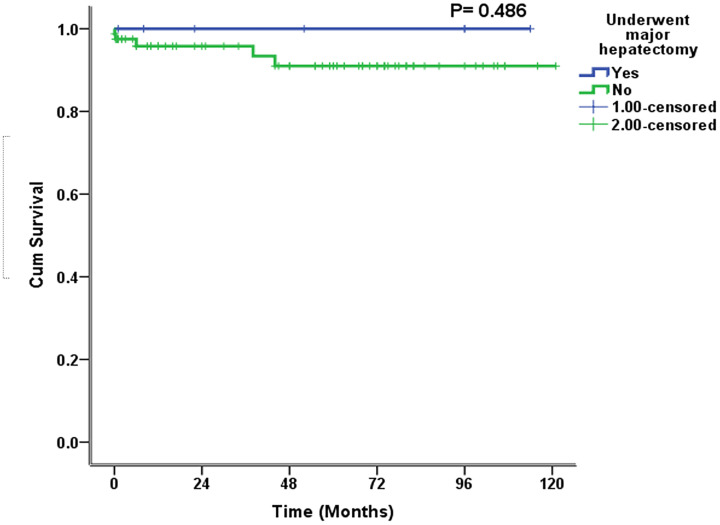
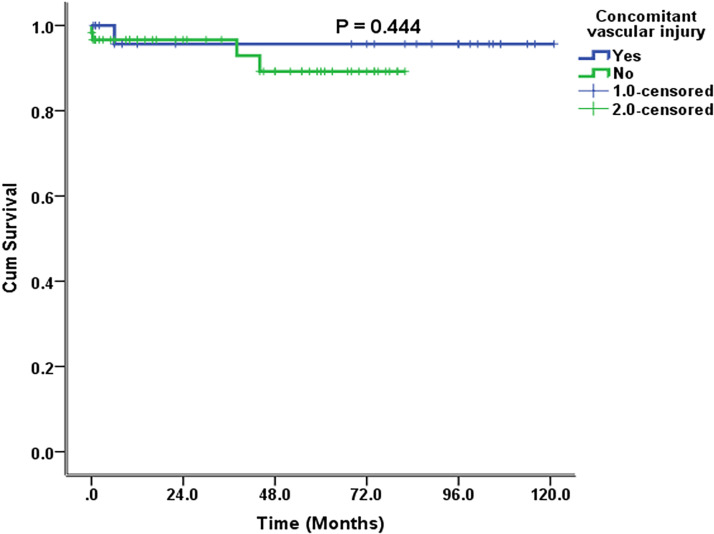
The median follow-up was 38 (2–74) months, and the longest follow-up was 121 months. The long-term morbidity was 13.7% (n = 12) (Table 5). This morbidity included HJ stricture and decompensated liver disease. Among the patients with HJ stricture, one developed HJ stricture in <12 months, two in <24 months, one in >24 months, and one after 5 years. Three (3.5%) of these patients underwent revisional surgery and two (2.3%) of these required a right hepatectomy (Table 5). The other two patients with HJ stricture refused surgery and were managed with percutaneous transhepatic biliary drainage. One patient with decompensated liver disease and diffuse porto-mesenteric thrombosis was managed with anticoagulation. The other patient refused liver transplantation and died 38 months after surgery. There was one mortality due to inoperable cholangiocarcinoma 41 months after HJ, and in one patient, who was 6 months post-HJ, no obvious reason for death could be determined. The 10-year estimated SFS was 95% (Figure 1). The 10-year estimated OS rate was 100% in patients who underwent major hepatectomy and 91% in patients who did not (Figure 2). The 10-year estimated OS rate was 100% in patients with concomitant vascular injury and this rate was not reached in patients without vascular injury) (Figure 3).
Table 5.
Long-term morbidity and mortality after HJ.
| Long-term morbidity | n (%) |
|---|---|
| HJ stricture | 5 (5.7) |
| Decompensated liver disease | 1 (1.1) |
| Decompensated liver disease with portal vein thrombosis | 1 (1.1) |
| Incisional hernia | 4 (4.6) |
| Sub-acute intestinal obstruction | 1 (1.1) |
| Mortality | 3 (3.4) |
HJ, hepaticojejunostomy.
Figure 1.
Estimated 10-year stricture-free survival in 44 patients with a minimum follow-up of 36 months.
Cum, cumulative.
Figure 2.
Estimated 10-year overall survival in patients undergoing repair of bile duct injury with or without hepatectomy.
Cum, cumulative.
Figure 3.
Estimated 10-year overall survival in patients undergoing repair of bile duct injury with or without vasculobiliary injury.
Cum, cumulative.
Discussion
Post-cholecystectomy BDI is an uncommon but complex clinical problem.15,17,22–24 The extent of BDI, associated vascular injuries, delayed presentation, previous attempts at surgical repair, and diversity in management options makes this a challenging clinical situation requiring multidisciplinary management. In approximately 40% of patients, bile duct repair was attempted before referral to our center, and HJ was the most common procedure performed. Vasculobiliary injuries were not uncommon and were observed in 27 (31%) patients, and a liver resection was required in more than 10% of patients. Despite a high percentage of patients with complex BDI, the short-term outcomes and long-term SFS and OS were acceptable.12
While vascular injury might occur during initial cholecystectomy, exploration with attempted bile duct repair is an important risk factor.25 Vascular injuries might occur after conversion and not during the index operation.26 A lack of appropriate surgical equipment compromises outcomes.27 Every failed attempt leads to the loss of bile duct length and might convert an E2 injury to E3 or E4 BDI. This situation makes subsequent attempts at repair a challenging task, and occasionally liver resection might be required. Consequently, “drain now and fix later” appears to be a safe strategy, and early referral to specialist centers cannot be overemphasized.23 When BDI is identified during LC, drains can be placed laparoscopically and patients can be referred without conversion.28,29 Scarring from previous surgeries leads to the loss of planes, and an inadvertent vascular injury can occur. There were two patients who had iatrogenic RHA injury at our center. Both of these patients were managed successfully with arterial reconstruction.
With regard to the long-term results, development of biliary-enteric anastomotic (BEA) strictures and SFS represents one of the most important measures of the outcome. Between 4% and 69% patients develop BEA strictures when followed in the long term.1,14,29 Bansal and colleagues showed that concomitant vascular injury, the time period between injury and repair, and a high BDI at or above the confluence were associated with poor outcomes after definitive repair.18 In our cohort, with a median follow-up of 38 months, the long-term BEA stricture rate was <6%. The median time to development of BEA strictures is between 11 and 30 months.1,12,14,29 Approximately 66% of these develop within 3 years, 80% within 5 years, and 90% within 7 years.22,30 Long-term mortality following treatment for BDI varies from 1.8% to 4.6%, with unrecognized or untreated strictures leading to secondary biliary cirrhosis, its sequelae, and death.12,15,22,31 Guidelines for uniform reporting of outcomes after BDI repair have been recently proposed. The patency rate or SFS has been used to determine the efficacy of treatment and long-term results.32 In our study, patients with a minimum follow-up of 3 years were considered for the assessment of 10-year SFS. With a median follow-up of 74 months, the 10-year SFS rate was 95%. The 10-year OS rate in patients who had concomitant vascular injuries and those who underwent major hepatectomy was 100%. Despite the complex nature of BDI in most patients, we believe that our extensive living donor liver transplant experience might have contributed to these acceptable outcomes. Familiarity with the porta hepatis during donor and recipient operations and small duct-to-duct biliary anastomoses using 7/0 sutures may have been responsible for these outcomes.33–35
Liver resection is required in only 0.8% to 1.4% patients with complex vasculobiliary injury or proximal BDI.12,17 A recent systematic review showed that only 67 patients underwent right hepatectomy for BDI.17 Liver resection is a useful therapeutic option in patients with symptomatic liver atrophy or hepatic abscesses secondary to BDI. Anatomical resection, such as right hepatectomy, is safe in these patients because of hypertrophy of the contralateral side.17,30 Additionally, partial segment 4 to 5 resection allows access to healthy proximal bile ducts in cases of E3 or E4 BDI, and improves the surgical space, which enables secure, tension-free anastomoses.32 With regard to proximal BDI, surgical experience is an important predictor of SFS.1,2,12 Identification of healthy ducts, familiarity with ductal anatomy, and tension-free anastomoses are critical steps in achieving acceptable long-term results.6,30 Along with surgical experience, factors such as adequate retraction, proper instrumentation, loupe magnification, and prior exposure to hepatic and vascular resection and reconstruction might play an important role in long-term success. We found excellent survival in patients who underwent major hepatectomy, and no short-term or long term-mortality was observed.
The limitations of this study include its retrospective design and the potential to have missed pertinent data. Although the surgical technique was uniform, and all patients had Roux-en-Y HJ, the spectrum of presentation was diverse. Many patients had previous attempts at bile duct repair, and vascular injuries were frequent. Outcomes after single definitive repair are better than multiple attempts.1 Because there is no national policy for referring patients with BDI to specialist centers, some patients had undergone repair of BDI before presenting to our center. Because of the same reason for patients undergoing repair of BDI before presenting to our center, accurate grading of BDI and determination of the time interval between injury and repair were not possible in many patients. Nevertheless, the best time for repair of BDI is yet to be determined.36 Therefore, in patients with previous BDI repair, we classified BDI on the basis of the location of the stricture at the time of presentation to our center. The actual injury might have been less complex and distal than what we actually had to deal with. We could not determine independent predictors of the long-term outcome owing to low rates of stricture and mortality. However, our study suggested that outcomes in patients with major hepatectomy and vascular injuries are excellent.
Although long-term outcomes after the repair of BDI at a specialist center might be satisfactory, BDI is associated with considerable morbidity. Many patients undergo repair of BDI at non-specialist centers and are referred only when these attempts do not succeed. Consequentially, proximal migration of BDI and concomitant vascular injury might be observed more frequently, mandating liver resection. There is a need to better identify factors that have a prognostic effect on long-term outcomes of BDI repair, and early referral to specialist centers needs to be promoted.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605231162444 for Outcomes of hepaticojejunostomy for post-cholecystectomy bile duct injury by Humaid Ahmad, Haseeb Haider Zia, Mohammad Salih, Muhammad Naseer, Nusrat Yar Khan and Abu Bakar Hafeez Bhatti in Journal of International Medical Research
Author contributions: HA and AHB contributed to the concept and design, data collection, analysis, drafting, and finalizing the manuscript. HHZ, MS, MN, and NYK contributed to the design, drafting, and finalizing the manuscript. All authors have read and approved the final manuscript.
The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Abu Bakar Hafeez Bhatti https://orcid.org/0000-0002-4875-603X
Data availability statement
The data supporting the findings of this study are available on request from the corresponding author.
References
- 1.De’Angelis N, Catena F, Memeo R, et al. 2020 WSES guidelines for the detection and management of bile duct injury during cholecystectomy. W J Emerg Surg 2021; 16: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diaz-Martinez J, Chapa-Azuela O, Roldan-Garcia JA, et al. Bile duct injuries after cholecystectomy, analysis of constant risk. Ann Hepatobiliary Pancreat Surg 2020; 24: 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun K. Recent classifications of common bile duct injury. Korean J Hepatobiliary Pancreat Surg 2014; 18: 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boerma D, Rauws EAJ, Keulemans YCA, et al. Impaired Quality of Life 5 Years after Bile Duct Injury During Laparoscopic Cholecystectomy. Ann Surg 2001; 6: 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sicklick JK, Camp MS, Lillemore KD, et al. Surgical Management of Bile Duct Injuries Sustained During Laparoscopic Cholecystectomy. Ann Surg 2005; 241: 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felekouras E, Petrou A, Neofytou K, et al. Early or delayed intervention for bile duct injuries following laparoscopic cholecystectomy? A dilemma looking for an answer. Gastroenterol Res Pract 2015; 2015: 104235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haung ZQ, Haung XQ. Changing patterns of traumatic bile duct injuries: a review of forty years experience. World J Gastroenterol 2002; 8: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillemore KD, Martin SA, Cameron JL, et al. Major Bile Duct Injuries During Laparoscopic Cholecystectomy: Follow up After Combined Surgical and Radiologic Management. Ann Surg 1997; 225: 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergman JJG, Van den Brink GR, Rauws EAJ, et al. Treatment of bile duct lesions after laparoscopic cholecystectomy. Gut 1996; 38: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JT, Charpentier KP, Beard RE. An update on iatrogenic bile duct injuries: identification, classification, and management. Surg Clin North Am 2019; 99: 283–299. [DOI] [PubMed] [Google Scholar]
- 11.Hussain M, Khan AW, Khan MA, et al. Managing iatrogenic bile duct injuries through a multidisciplinary team approach: a SIUT case series. J Pak Med Assoc 2019; 69: 1559–1564. [PubMed] [Google Scholar]
- 12.Schreuder AM, Busch OR, Besselink MG, et al. Long-term impact of iatrogenic bile duct injury. Dig Surg 2020; 37: 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pucher PH, Brunt ML, Davies N, et al. Outcome trends and safety measures after 30 years laparoscopic cholecystectomy: a systemic review and pooled data analysis. Surg Endosc 2018; 18: 2175–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perera MT, Silva MA, Shah AJ, et al. Risk factors for litigation following major transactional bile duct injury sustained at laparoscopic cholecystectomy. World J Surg 2010; 34: 2635–2641. [DOI] [PubMed] [Google Scholar]
- 15.McPartland KJ, Pomposelli JJ. Iatrogenic bile duct injuries: classification, identification, and management. Surg Clin North Am 2008; 88: 1329–1343. [DOI] [PubMed] [Google Scholar]
- 16.Lindemann J, Jones E, Kotze U, et al. Evolution of bile duct repair in a low and middle-income country (LMIC): a comparison of diagnosis, referral, management and outcomes in repair of bile duct injury after laparoscopic cholecystectomy from 1991 to 2004 and 2005 to 2017. HPB (Oxford) 2020; 22: 391–397. [DOI] [PubMed] [Google Scholar]
- 17.Furtado R, Yoshino O, Muralidharan V, et al. Hepatectomy after bile duct injury: a systematic review. HPB (Oxford) 2022; 2: 161–168. [DOI] [PubMed] [Google Scholar]
- 18.Bansal VK, Krishna A, Misra MC, et al. Factors Affecting Short-Term and Long-Term Outcomes After Bilioenteric Reconstruction for Post-cholecystectomy Bile Duct Injury: Experience at a Tertiary Care Centre. Indian J Surg 2015; 77: 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 1995; 180: 101–125. [PubMed] [Google Scholar]
- 20.Clavien PA, Barkun J, De Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250: 187–196. [DOI] [PubMed] [Google Scholar]
- 21.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 22.De Reuver PR, Grossman I, Busch OR, et al. Referral pattern and timing of repair are risk factors for complications after reconstructive surgery for bile duct injury. Ann Surg 2007; 245: 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabłońska B, Lampe P. Reconstructive Biliary Surgery in the Treatment of Iatrogenic Bile Duct Injuries, New Advances in the Basic and Clinical Gastroenterology, Thomas Brzozowski, IntechOpen, doi: 10.5772/30733. Available from: https://www.intechopen.com/chapters/35463.
- 24.Hadi A, Aman Z, Khan SA, et al. Surgical management of bile duct injuries following open or laparoscopic surgery. J Pak Med Assoc 2013; 63: 1008–1012. [Google Scholar]
- 25.Mesleh MG, Asbun HJ. Management of common bile duct injury. In: Díaz-Martínez J, Chapa-Azuela O, Roldan-García JA, et al (eds). The SAGES manual of biliary surgery. Switzerland: Springer, 2020, pp. 213–231.
- 26.Strasberg SM, Gouma DJ. ‘Extreme’ vasculobiliary injuries: association with fundus-down cholecystectomy in severely inflamed gallbladders. HPB (Oxford) 2012; 14: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heise M, Schmidt SC, Adler A, et al. Management of bile duct injuries following laparoscopic cholecystectomy. Zentralbl Chir 2003; 128: 944–951. [DOI] [PubMed] [Google Scholar]
- 28.Krige JE, Bornman PC, Kahn D. Bile leaks and sepsis: drain now, fix later. Arch Surg 2010; 145: 763. [DOI] [PubMed] [Google Scholar]
- 29.Karanikas M, Bozali F, Vamvakerou V, et al. Biliary tract injuries after lap cholecystectomy – types, surgical intervention and timing. Ann Transl Med 2016; 4: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perini MV, Herman P, Montagnini AL, et al. Liver resection for the treatment of post-cholecystectomy biliary stricture with vascular injury. World J Gastroenterol 2015; 21: 2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt SC, Langrehr JM, Hintze RE, et al. Long-term results and risk factors influencing outcome of major bile duct injuries following cholecystectomy. Br J Surg 2005; 92: 76–82. [DOI] [PubMed] [Google Scholar]
- 32.Cho JY, Baron TH, Carr-Locke DL, et al. Proposed standards for reporting outcomes of treating biliary injuries. HPB (Oxford) 2018; 20: 370–378. [DOI] [PubMed] [Google Scholar]
- 33.Bhatti ABH, Naqvi W, Ali N, et al. Textbook outcome among voluntary donors undergoing major living donor hepatectomy. Langenbecks Arch Surg 2022; 407: 2905–2913. doi: 10.1007/s00423-022-02578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dar FS, Bhatti ABH, Qureshi AI, et al. Living Donor Liver Transplantation in South Asia: Single Center Experience on Intermediate-Term Outcomes. World J Surg 2018; 42: 1111–1119. doi: 10.1007/s00268-017-4259-1. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhao J, Chai S, et al. Reliable reconstruction of the complex high-location bile duct injury: a novel hepaticojejunostomy. BMC Surg 2019; 19: 176. doi.org/10.1186/s12893-019-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kambakamba P, Cremen S, Möckli B, et al. Timing of surgical repair of bile duct injuries after laparoscopic cholecystectomy: A systematic review. World J Hepatol 2022; 14: 442–455. doi: 10.4254/wjh.v14.i2.442. PMID: 35317176; PMCID: PMC8891678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231162444 for Outcomes of hepaticojejunostomy for post-cholecystectomy bile duct injury by Humaid Ahmad, Haseeb Haider Zia, Mohammad Salih, Muhammad Naseer, Nusrat Yar Khan and Abu Bakar Hafeez Bhatti in Journal of International Medical Research
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author.