Abstract
Natriuretic peptides (NPs) encompass a family of structurally related hormone/paracrine factors acting through the natriuretic peptide system regulating cell proliferation, vessel tone, inflammatory processes, neurohumoral pathways, fluids, and electrolyte balance. The three most studied peptides are atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-Type natriuretic peptide (CNP). ANP and BNP are the most relevant NPs as biomarkers for the diagnosis and prognosis of heart failure and underlying cardiovascular diseases, such as cardiac valvular dysfunction, hypertension, coronary artery disease, myocardial infarction, persistent arrhythmias, and cardiomyopathies. Cardiac dysfunctions related to cardiomyocytes stretching in the atria and ventricles are primary elicitors of ANP and BNP release, respectively. ANP and BNP would serve as biomarkers for differentiating cardiac versus noncardiac causes of dyspnea and as a tool for measuring the prognosis of patients with heart failure; nevertheless, BNP has been shown with the highest predictive value, particularly related to pulmonary disorders. Plasma BNP has been reported to help differentiate cardiac from pulmonary etiologies of dyspnea in adults and neonates. Studies have shown that COVID-19 infection also increases serum levels of N-terminal pro b-type natriuretic peptide (NT-proBNP) and BNP. This narrative review assesses aspects of ANP and BNP on their physiology, and predictive values as biomarkers. We present an overview of the NPs’ synthesis, structure, storage, and release, as well as receptors and physiological roles. Following, considerations focus on ANP versus BNP, comparing their relevance in settings and diseases associated with respiratory dysfunctions. Finally, we compiled data from guidelines for using BNP as a biomarker in dyspneic patients with cardiac dysfunction, including its considerations in COVID-19.
Keywords: Natriuretic peptides, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), Biomarker, Heart Failure, Respiratory Disease, COVID-19, Pharmacology/name of drugs
Introduction
Natriuretic peptides (NPs) encompass a family of structurally related hormone/paracrine factors acting through the natriuretic peptide system (NPS) that regulate cell proliferation, vessel tone, inflammatory processes, neurohumoral pathways, fluid, and electrolyte balance [1,2]. The three most studied peptides currently are atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-Type natriuretic peptide (CNP) [3].
ANP, the first identified member, was originally discovered in the cardiac atrium when de Bold and colleagues showed rats’ atrial extracts contained a component that decreased blood pressure by increasing renal sodium and water secretion [4]. BNP, called B-type, was discovered next and was isolated from porcine brain extracts by Sudoh et al. in the 1980s [5]. Lastly, in the 1990s, CNP, which causes smooth muscle relaxation, was first identified from porcine brain extracts [2,6,7].
Besides ANP, BNP, and CNP, two other NPs: urodilatin and dendroaspis natriuretic peptide (DNP), were identified. Urodilatin is a product of proANP, expressed in the kidney with identical ring structures and similar properties to ANP [8]. It binds to the same receptors as ANP in the kidneys, acting as a potent natriuretic and diuretic peptide. DNP was isolated from the venom of green mamba snakes (Dendroaspis angusticeps) [9]. It has been demonstrated that DNP acts like ANP and BNP by stimulating ANP receptors. Its intrinsic physiological actions are under investigation [7].
ANP and BNP are the most relevant NP biomarkers for diagnosing and prognosis of heart failure and underlying cardiovascular diseases usually associated with increased ventricular stress and congestive disorders [10]. Although ANP and BNP diagnose other diseases and comorbidities related to heart failure, their relevance is sometimes questionable [10,11].
This review covers aspects of ANP and BNP’s physiology, their predictive values as biomarkers, and their suitability regarding diagnosis and prognosis. We first present an overview of the synthesis, structure, storage, and release of NPs, followed by ANP and BNP receptors and physiological roles, which leads to the following topics: ANP and BNP in various settings and conditions and consideration guidelines for the use of BNP as a biomarker in dyspneic patients with cardiac dysfunction, including consideration about COVID-19.
Search Methods
For this narrative review, pertinent studies were examined from seven electronic databases (PubMed, Google Scholar, Cochrane Library, Web of Science, Science Citation Index, and EMBASE) using the common keyword Natriuretic peptides, Atrial natriuretic peptide (ANP), Brain Natriuretic Peptide (BNP), Biomarker, Heart Failure, Respiratory Disease/COVID-19, Prognosis, Diagnosis.
ANP, BNP: Synthesis, Structure, Storage, Release
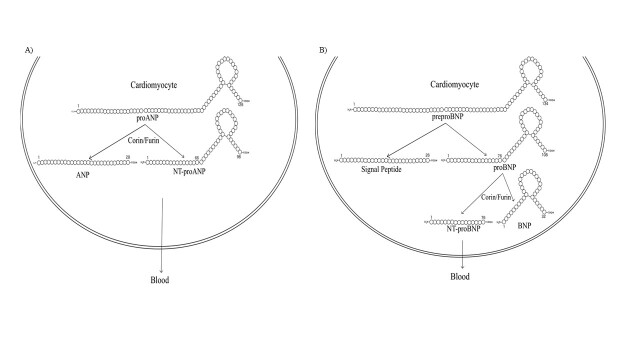
All natriuretic peptides (NPs) are synthesized as preprohormones (Figure 1) [12]. The NPs are found intracellularly as inactive precursor polypeptides (pro-peptide), which are converted to biologically active forms [13]. Each NP’s active form shares a typical 17-amino acid disulfide ring structure with variable C-terminal and N-terminal tails; however, their precursors are encoded by separate genes [2]. The genes that encode ANP and BNP are NPPA and NPPB [14].
ANP is mainly synthesized in the cardiac atria and is primarily expressed and stored in the granules of cardiomyocytes [14]. However, at lower concentrations, the NPPA gene is also expressed in other tissues, such as the ventricles, lungs, brain, and kidneys.
The primary stimulus for ANP release is atrial wall stretch resulting from increased intravascular volume [15,16]. Secretion of ANP is also stimulated by hormones and neurotransmitters, such as endothelin, arginine vasopressin, and catecholamines, which facilitate its distribution to its various target organs in an endocrine manner [17-19].
BNP is secreted as a prohormone in a pulsatile pattern under volume and pressure stress [20]. The half-life of BNP active hormone is about 22 minutes, whereas N-terminal pro b-type natriuretic peptide (NT-proBNP) has a half-life of about 60 to 120 minutes [20]. The tissue concentrations of BNP are much higher in the atria than in the ventricles, which is a potential cause of atrial fibrillation and atrial overload in some cases [21].
BNP can be stored in low concentrations along with ANP in cardiac atria but is found in greater concentrations in the cardiac ventricles [2]. Additionally, BNP is usually transcribed as needed in response to cardiac stress rather than stored in cardiac granules, like ANP [2].
Figure 1.
ANP and BNP synthesis in human cardiomyocytes. A) ProANP (126 aa) is cleaved into the 28 amino-acid active ANP and the 98 amino-acid NT-proANP by the enzymes corin and furin. Human preproANP is 151 amino acids in length. Cleavage of the amino-terminal signal sequence results in the 126-amino-acid proANP, the predominant form stored in atrial granules [22]. ANP is primarily expressed and stored in granules in the atria, although it is present at lower concentrations in tissues such as the ventricles and kidneys. B) BNP is synthesized in various organs but primarily in the left ventricular myocardium following mechanical stretching caused by volume overload [23]. PreproBNP (134 aa) is first cleaved in the sarcoplasmic reticulum to pre-pro (26-amino-acid signaling peptide) and the proBNP (108 aa). ProBNP is then cleaved by convertase enzymes (corin and furin) into the biologically active BNP NT-proBNP (an inactive signal peptide), being both released into the blood [10,23].
New studies are attempting to understand the role of NPs outside the cardiovascular and renal systems. Although cardiomyocyte stress is the chief stimulus to release ANP from the atria and BNP from the ventricles, other stimuli are also relevant [22,23]. Tissues other than cardiomyocytes, such as the uterus, spleen, thyroid, and prostate, also produce ANP and BNP [24]. Hormones such as endothelin, angiotensin, and arginine-vasopressin (AVP) stimulate ANP release, as do water immersion and head-down tilt [25-30]. It has been described that, as well as ANP, hormones, such as catecholamines, angiotensin II, and endothelin, and disease-associated conditions, such as hypoxia, enhance the secretion of NT-proBNP [31,32].
ANP and BNP Receptors and Intracellular Signaling
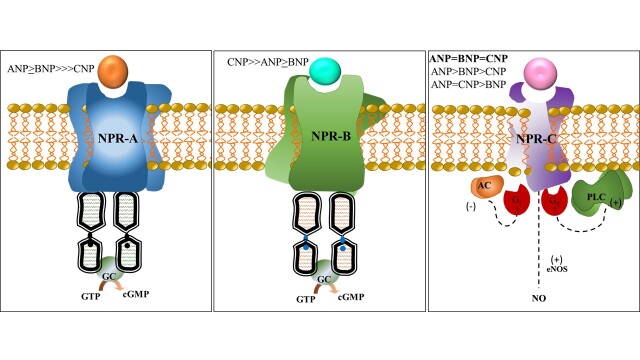
NPs’ effects, discussed in the next session, are mainly mediated by stimulation of transmembrane receptors, members of G (guanylyl) proteins [12,27,33,34]. NP receptors (NPRs) stimulation activates guanylyl cyclase (GC), leading to the production of the second messenger cyclic guanosine monophosphate (cGMP) (Figure 2). The NPRs consist of three subtypes: natriuretic peptide receptor-A (NPR-A or guanylyl cyclase-A), natriuretic receptor-B (NPR-B or guanylyl cyclase-B), and natriuretic peptide receptor-C (NPR-C or clearance receptor) [12].
Figure 2.
Natriuretic Peptide Receptors A, B, and C. NPR-A and NPR-B have a similar structure and are linked to the cGMP-dependent signaling cascade. NPR-C does not possess a GC domain and has no direct effect on cGMP levels [35-37]. Although the extracellular domain of NPR-C is 30% identical to NPR-A and NPR-B, it is not coupled to GC. Affinity to NPR-A: ANP ≥ BNP >>> CNP [12]. Affinity to NPR-B: CNP >> ANP ≥ BNP. Affinity to NPR-C is controversial: ANP≥CNP≥BNP; ANP=CNP=BNP; ANP=CNP>BNP [12,36,38-41]. Although there are controversies regarding the peptides’ affinity to NPR-C, it is assumed that ANP has the highest and BNP has the lowest affinity for NPR-C [36,38-41]. Hypothesis for NPR-C and investigations/development of selective agonists are under course. It might involve inhibitory (Gi) and/or stimulatory protein (Gq) activation, as well as activation of e-NOS leading to NO production. GC: Guanylyl Cyclase. cGMP: cyclic Guanosine Monophosphate. AC: Adenylyl Cyclase. e-NOS: endothelial Nitric Oxide Synthase. NO: Nitric Oxide.
Human NPR-A mRNA is highly expressed in kidneys, adrenal glands, terminal ileum, adipose, aortic, and lung tissues [12]. NPR-B is the primary NP receptor in the brain; however, its mRNA is found in the lungs, brain, adrenal glands, kidneys, uterus, and ovaries. NPR-C is primarily expressed in kidneys, and adipocytes are its second-largest expression site and the main target for endogenous agonists [42].
A dual intracellular mechanism has been proposed for NPR-C signaling [38,40,41]. The NPR-C lacks guanylyl cyclase (GC) activity; instead, receptor activation is coupled to the inhibition of adenylyl cyclase (AC) and/or activation of PLC. The inhibition of one pathway may thus be compensated for by the upregulation of the other [43,44]. Using selective NPR-C agonists, molecular studies on signaling transduction have shown that NPs activate Gi proteins and the consequent AC activity inhibition (via the α subunit) results in a stimulation of PLCβ signaling (via the βγ subunits) [35].
In addition, some studies suggest that NPR-C activation is indirectly involved with endothelial nitric oxide synthase (e-NOS)/nitric oxide production (NO). This would lead to cardioprotective roles through its activation and signaling mediated by NO, particularly in the coronaries [44]. This may be clinically relevant in heart failure patients with expressive endothelial dysfunction since NPR-C stimulation could lead to coronary vessel relaxation.
The physiological functions mediated by NPs receptors’ activation are presented in the following section and compiled in Table 1.
ANP and BNP: Physiological Roles
NPs influence many physiological functions: vasodilation, antiproliferative effects, vascular remodeling, and modulation of the renin-angiotensin-aldosterone system (RAAS) [2,12,38,45].
ANP and BNP act on target organs to promote fluid regulation and cardiovascular homeostasis. These NPs increase the glomerular filtration rate (GFR), thus increasing diuresis by afferent renal arteriole relaxation and efferent renal arteriole constriction [17].
NPR-A stimulation in the kidneys by ANP increases natriuresis by decreasing the open probability of epithelial sodium channels (ENaCs) [46]. In addition, ANP is a key player in the aldosterone escape phenomenon [26], a natriuretic compensatory mechanism triggered by extracellular fluid volume expansion in response to the sodium-retaining effects of aldosterone [47].
ANP and BNP participate in the homeostasis of cardiovascular functions by regulating cellular growth, cellular proliferation, and vascular permeability [48]. They reduce cardiac remodeling and hypertrophy, which are crucial to avoiding the long-term effects of increased blood volumes and counteracting the progression of heart diseases such as congestive heart failure [48].
ANP and BNP levels increase in response to myocardial infarction, promoting protective actions against neutrophil-induced endothelial damage. In this sense, ANP and BNP suppress neutrophil adhesiveness to human endothelial cells, CD18 expression on neutrophils, and the release of elastase from neutrophils [49]. Following myocardial ischemia and coronary reperfusion, ANP and BNP decrease cardiovascular tissue necrosis by stimulating cGMP synthesis, preventing excessive cardiac myocytes’ contracture and death [49-51].
Besides being critical factors in maintaining the homeostasis of cardiovascular and renal functions, ANP and BNP are synthesized and act in the organs where their receptors are expressed (as described above). ANP and BNP activate hormone-sensitive lipase in adipose cells, which express NPR-A and NPR-C, resulting in the breakdown of triglycerides [52,53]. In humans and rats, ANP released from enterochromaffin cells of the stomach stimulates somatostatin secretion [54]. NPR-C, stimulated by ANP, BNP, and CNP, functions as clearance receptors for those respective natriuretic peptides [28].
Table 1. Effects Mediated by Natriuretic Peptide Receptor A, B, and C Stimulation.
| Natriuretic Peptide Receptors | |||
| Target Organs/System | NPR-A | NPR-B | NPR-C (clearance receptor) |
| CNS | ↓ Thirst ↓ Water intake ↓ Sympathetic activity |
||
| Renal | ↓ Na+ reabsorption ↓ Water reabsorption ↓ Renin secretion ↓ Adrenal aldosterone secretion ↑ Afferent arteriolar vasodilation ↑ Efferent arteriolar vasoconstriction ↑ Renal blood flow ↑ GFR |
↓ Ability to concentrate* | |
| Adipose | ↑ Lipolysis | ↓ Lipolysis* | |
| Vascular | ↑ Arterial dilatation ↑ Capillary endothelial permeability ↑ Vascular smooth muscle relaxation ↑ Venous dilation ↑ Vascular regeneration |
↑ Vascular smooth muscle relaxation ↑ Venous dilation ↑ Vascular regeneration |
|
| Pulmonary | ↓ Pulmonary hypertension & fibrosis | ||
| Musculoskeletal | ↑ Endochondral bone growth | ↓ Endochondral bone growth* | |
| Cardiac | ↓ Myocyte hypertrophy ↓ Cardiac fibrosis |
↓ Myocyte hypertrophy ↓ Cardiac fibrosis |
|
*Studies have shown that knock-out mutations of the NPR-C receptor have been found to have the opposite effect. This is supposed to be due to the observed increase in the half-life of natriuretic peptides following the knock-out mutation of the NPR-C. These effects mediated by NPR-C activation are based on the literature data collection [28,52,55,56]. CNS: Central Nervous System. GFR: glomerular filtration rate. (Adapted from Zakeri and Burnett and Matsukawa et al [3,28]).
Generally, hormones or growth factors that stimulate vasoconstriction or promote cellular growth or proliferation oppose the actions of natriuretic peptides. Angiotensin II, Vasopressin, lysophosphatidic acid, sphingosine-1-phosphate, platelet-derived growth factor, fibroblast growth factor, and endothelin have all been found to inhibit NPR-A and/or NPR-B receptors [12,34,57-59]. Lysophosphatidic acid and Sphingosine-1-phosphate have been found to cause NPR-B receptor desensitization by inducing dephosphorylation of NPR-B guanylyl cyclase [34,57]. The inhibition of NPR-B by growth factors has been correlated with the dephosphorylation of the phosphoserine and phosphothreonine residues of the receptor [59]. Vasopressin, Angiotensin II, and endothelin bind to their respective receptors, which are coupled to Gq, activating phospholipase C-β (PLC-β). This leads to protein kinase C’s activity and intracellular calcium’s elevation. The elevated intracellular calcium results in vasoconstriction, which opposes the actions of natriuretic peptides [58]. Angiotensin II has also decreased the expression of gene coding for NPR-A receptor-dependent guanylyl cyclase activity [33].
NPR-C signaling in heart diseases is under investigation. There are suggestive results on the hypothesis that, in heart failure, an increase in NPR-C intracellular pathway activation indicates (1) an increase in NPR-C expression, (2) increased recruitment, or (3) activation of NPR-C already expressed [38,51,57].
ANP and BNPs as Biomarkers
Plasma levels of ANP in healthy subjects are reported to be 5.2 ± 2.8 pmol/L, whereas plasma levels of BNP in healthy subjects were measured to be 2.5 ± 2.4 pmol/L [60]. In addition, Cowie et al. reported that BNP concentration is a more suitable indicator than ANP in patients with imminent heart failure [61]. ANP plasma levels in patients with heart failure with underlying cardiovascular diseases such as coronary artery disease and dilated cardiomyopathy were 41.4 ± 53.1 pmol/L, whereas BNP plasma levels were 210 ± 286.3 pmol/L [60]. In patients with heart failure and those underlying cardiovascular diseases, the level of BNP is ~84 times lower than in healthy subjects, whereas ANP was only ~8 times lower in healthy subjects [60].
Plasma BNP levels are shown to increase due to the progression of heart failure and the slow and steady increase of BNP release [62]. However, although BNP levels are increased in patients with CHF, no significant change is noted in the exercise duration [62]. This finding is essential since it demonstrates that the kinetics of BNP is not as effective in regulating plasma BNP levels in patients [62]. Plasma levels of ANP in healthy patients are approximately 10 fmol/ml and are elevated 10- to 30-fold in patients with congestive heart failure [63,64].
A report by the American College of Cardiology Solution Set Oversight Committee released in 2021 highlights updated guidelines for managing heart failure and indications for utilizing natriuretic peptides in heart failure.
Current clinical guidelines give a Class I recommendation to measure BNP or NT-proBNP when attempting to diagnose heart failure, assess disease severity, and establish prognosis [65,66]. Guidelines released by the Heart Failure Association of the European Society of Cardiology in 2019 and by an Italian consensus document in 2005 advise performing a BNP/NT-proBNP assay to confirm the diagnosis of heart failure in patients with vague symptoms that could be confused with pathologies such as chronic obstructive pulmonary disease [67,68]. However, they highlight the necessity to account for the prevalence of pulmonary hypertension and right ventricular dysfunction in those patients and that serum levels of BNP may be in the “gray zone.” However, the accuracy of natriuretic peptides in diagnosing heart failure in patients with pre-existing pulmonary diseases remains unchanged [68,69].
ANP and BNP in Various Settings and Conditions
Cardiac dysfunctions related to cardiomyocytes stretching in the atria and ventricles are primary elicitors of ANP and BNP release, respectively. Cardiac valvular dysfunction, hypertension, coronary artery disease, myocardial infarction, persistent arrhythmias, and cardiomyopathies are pathologies mainly associated with heart failure, either as causes or consequences, and are known to activate NPs [70]. Acute dysrhythmias such as atrial fibrillation have increased ANP and BNP due to increased blood pooling in the heart chambers [71,72].
Cardiac amyloidosis also increases serum ANP and BNP levels through a similar mechanism of increased intracardiac blood volume. It is proposed to be directly involved with myocardial damage due to regional mechanical stress caused by extracellular amyloid deposition [29,73,74].
Myocardial depression secondary to septic shock is believed to be caused by the release of various cytokines that results in systolic dysfunction. This causes an increase in ventricular blood volume and cardiomyocyte stretch resulting in the release of ANP and BNP [30,75,76]. Renal pathologies such as chronic kidney disease (CKD) decrease GFR, resulting in increased blood volume and thus increased BNP plasma levels, regardless of whether that individual has heart failure [77]. GFR is inversely related to ANP and BNP concentrations [78].
Little atrial ANP is produced by ventricular tissue in normal adults, but it is present in the ventricular tissue of fetuses and neonates and the hypertrophied ventricle. Aging is also a critical non-modifiable factor associated with the progressive increase in levels of ANP and BNP [29,79]. Age-related reduction in GFR, systolic dysfunction, cardiac hypertrophy, and decreased diastolic function all contribute to the rise of ANP and BNP with age [29,79].
Studies have shown that the prevalence of chronic obstructive pulmonary disease (COPD) is two to three times higher in individuals over 60 than in younger individuals. Evidence of aging, such as cellular senescence and telomere attrition, is present in the lungs of individuals with COPD. These findings have shown a correlation between aging and the development of COPD [80,81].
Cor pulmonale-associated disorders, such as pulmonary embolisms and COPD, also result in increased blood volume in the heart chambers due to impaired forward blood flow out of the right heart chambers, eliciting ANP and BNP release [71,82,83].
Plasma BNP has been reported to help differentiate cardiac from pulmonary etiologies of dyspnea in adults [84] and neonates [85].
BNP as a Prognostic Parameter in Dyspneic Patients with Cardiovascular Dysfunctions
Pre-existing co-morbidities such as chronic kidney disease, hypertension, and pulmonary disease impact the baseline levels of BNP, and variabilities amongst individuals such as sex, age, and weight have illustrated the need for standardized guidelines in the use of ANP and BNP in diagnosing heart failure. BNP levels tend to increase with age, and females tend to have slightly higher levels of BNP than males. Obesity, however, has been correlated with lower levels of BNP [29,73].
Dyspnea is one of the major symptoms of heart failure [64]. Impaired contractility of the left ventricle not only results in the release of ANP and BNP due to stretching of the cardiomyocytes but also increases pressure in the pulmonary vasculature due to impaired forward flow of blood in the heart resulting in dyspnea, cough, and wheezing [63].
Although both ANP and BNP would serve as biomarkers for differentiating cardiac versus noncardiac causes of dyspnea and as a tool for measuring the prognosis of patients with heart failure, BNP has been shown with the highest predictive value, particularly in pulmonary disorders [84-86].
In fact, NT-proBNP is recognized as a critical player in maintaining cardiopulmonary homeostasis. Its levels are directly associated with hemodynamic variables regulating the heart (left and right ventricles) and pulmonary functional capacities [86]. Literature has displayed a fair amount of data evaluating BNP levels in the management of pulmonary hypertension (PH) and COPD management [86-90].
Serum concentrations of NT-proBNP are recommended as prognostic parameters for PH [85,86]. A recent review indicated NT-proBNP has strong diagnostic efficacy in predicting morbidity in patients with acute exacerbations with COPD and PH. The authors concluded that 175.14 pg/ mL is a predictive NTproBNP threshold, with a weak correlation with the severity of PH with COPD [86].
The multiparametric risk assessment approach has been included in systematic reviews to evaluate NTproBNP levels in the PH patient management scope [88]. Persistent NT-proBNP levels are highly prognostic of PH progression and indicate patients at the highest risk [86,89,90]. NT-proBNP is a powerful independent predictor of death and adverse events in heart failure, a broad range of cardiovascular conditions, even in asymptomatic individuals. Many studies have attempted to determine the predictability of adverse outcomes in patients with heart failure. These studies have found that patients with higher levels of BNP have a positive association with mortality [25,91,92].
The prognosis and diagnosis of cardiovascular disease in patients with COPD are critical to reducing morbidity and mortality [86]. Time-intensive management of patients with elevated NT-proBNP helps identify cardiovascular disease, stratify risk, and guide the treatment of COPD [87].
Considering admission and all-cause mortality [93], including respiratory dysfunction, the time frame for prognostic values of BNP and NT-proBNP mean levels (pg/mL) are described in Table 2.
Table 2. BNP and NT-proBNP Mean Levels (pg/mL) at Admission and All-cause Mortality in Patients with Acute Decompensated Heart Failure.
| Time Frame | BNP | NT-proBNP |
| 15 - 31 Days | 549 - 1,189 | 5,165 |
| 2 - 3 Months | 764 - 766 | 4,077 - 9,488 |
| 6 - 11 Months | 237 - 311 | 7,685 - 10,283 |
| 12 - 23 Months | Not Reported | 3,512 - 7,863 |
| 24 Months – 6.8 years | 441 - 658 | 1,668 - 5,734 |
(Adapted from Santaguida et al [93]).
Although optimal discriminatory values have not been determined, studies have attempted to establish broad inferences between certain levels of BNP and its strength in diagnosing heart failure interwoven with respiratory dysfunctions. Limitations to achieving the optimal levels of BNP as a predictive biomarker are often related to the lack of a variety of stratified correlations, ie, there are considerations in patients based on comorbidities but not based on age. A correlation between dyspneic patients with heart failure and associated BNP values above 400 pg/mL was observed. BNP levels below 100 pg/mL have a very high negative predictive value for heart failure as the cause of dyspnea compared to good standard clinical diagnostic criteria, such as ejection fraction. Levels between 100 and 400 pg/mL have low sensitivity and specificity for diagnosing heart failure as the cause of dyspnea [16]. Measurement of plasma BNP/NT-proBNP levels is recommended in all patients with acute dyspnea and suspected acute heart failure. While there is no absolute “diagnostic level” of these biomarkers for acute heart failure (AHF), most assays have three ranges. For the typical BNP assay, a BNP less than or equal to 100 pg/mL is strongly suggestive of non-heart failure etiology for the dyspnea, BNP 100 to 400 pg/mL is indeterminate, and BNP more significant than 400 pg/mL is strongly supportive of AHF.
Although dyspnea is the most relevant clinical presentation in which NPs are frequently used to support the diagnosis of HF, several confounders must be considered when interpreting the predictive values since they might elevate or reduce BNP/NT-proBNP levels beyond heart failure [73,94-97] (Table 3).
Table 3. Selected Cardiac and Noncardiac Comorbidities Influencing Factors in the Predictive Values of BNP and NT-ProBNP Levels as Diagnostic Parameters in Heart Failure.
| Causes of Elevated peptide levels | Causes of reduced peptide levels | |||||||||||||
| Cardiac | •Heart failure (including RV syndromes) •Acute coronary syndrome •Heart muscle disease, including LVH •Valvular heart disease •Pericardial disease •Atrial fibrillation •Myocarditis •Cardiac surgery •Cardioversion | •Cardiac tamponade •Mitral stenosis •End-Stage cardiomyopathy | ||||||||||||
| Noncardiac | Advancing age* •Anemia •Renal failure • Pulmonary: obstructive sleep apnea, severe pneumonia, pulmonary hypertension •Critical illness •Bacterial sepsis •Severe burns •Toxic-metabolic factors (ie: cancer chemotherapy) | •Flash pulmonary edema; •Genetic polymorphisms •Obesity**
|
*Grey zone when analyzing levels of NT-proBNP according to age: <50 years old 300 450 pg/mL; 50–75 years 300–900 pg/mL; >75 years 300–1800 pg/mL [95]. **Obesity and BMI as predictive parameters for heart failure: independent of BMI, the diagnostic value is negative (‘rule out’), if BNP levels are lower than 100 ng/L and NT-proBNP is lower than 300 ng/L. In addition, independent of BMI, if BNP levels are higher than 400 pg/mL, the diagnostic value is positive (‘rule in’). (Adapted from Reinmann et al. [73], Chow et al. [96], and Maisel et al. [97]).
With BNP levels below 100 pg/ml having a high negative predictive value and levels above 400 pg/ml having a high positive predictive value, a gray area remains in between that is left for interpretation by the diagnostician caring for a patient. Individuals found to be in this gray area cannot be assigned to a particular group, dyspnea due to heart failure or dyspnea not due to heart failure. Currently, the literature does not present clear evidence to rule out pulmonary edema, pneumonia, embolism, and core pulmonale in patients with high BNP. The ambiguous levels of BNP limit their predictability in diagnosing heart failure, as the cause of dyspnea may result from pre-existing conditions and individual variability.
BNP as a Biomarker for COVID-19
Recent studies have examined the impact of COVID-19 on levels of BNP and NT-proBNP and their correlation with the prognosis of infected patients. A thorough search of PubMed did not find any research looking at the impact of COVID-19 on levels of serum ANP. It is accepted that since the circulating concentration of BNP is less than 20% of that of ANP in normal subjects but can equal or exceed that of ANP in patients with heart failure, BNP is more valuable than ANP in the evaluation of cardiac dysfunction and volume overload [16,98].
Pneumonia has long been known to cause an increase in BNP levels [99]. Serum levels of BNP have also been shown to correlate with the severity of pneumonia [100]. Several hypotheses exist for the correlation between pneumonia and increased BNP levels. Some studies have suggested that right ventricular overload due to tissue hypoxia, pulmonary vasoconstriction, and pulmonary hypertension seen in pneumonia cause the secretion of BNP [32,101,102]. Other studies have found that specific proinflammatory cytokines such as IL-1b, IL-6, and TNF-α induce the release of BNP from cardiomyocytes cultured in vitro [103,104].
Studies have shown that COVID-19 infection also increases serum levels of NT-proBNP and BNP [22,105]. Three hypotheses about the increased BNP and NT-proBNP levels in COVID-19-infected patients have been made. Pneumonia and mechanical ventilators are known to increase pulmonary vascular tone, which may increase right ventricular afterload and wall tension, stimulating the release of BNP and NT-proBNP [22]. Direct involvement of the myocardium in COVID-19 infection has also been shown to increase levels of NT-proBNP [105].
Lastly, COVID-19 infection can result in acute renal failure, impairs clearance, resulting in elevated levels of NT-proBNP [106]. Elevated levels of NT-proBNP correlate with more severe cases of infection and higher mortality rates [106]. A study that looked at the prognostic significance of NT-proBNP in patients with COVID-19 without any previous history of heart failure concluded that increased NT-proBNP levels are independently linked to higher mortality rates in patients with COVID-19 pneumonia and no heart failure [15]. These findings illustrate the need for further investigation in patients with dyspnea, elevated levels of serum BNP, and signs of systemic infection. Such results may be more indicative of pneumonia than primary cardiac dysfunction. These new findings may also indicate the need for assessing cardiac biomarkers in COVID-19-infected patients, as they may enable the timely diagnosis of cardiac injury, the monitoring of disease progression, and aid in implementing appropriate treatment plans.
Conclusions
Plasma levels of BNP/NT-proBNP are a potential biomarker in diagnosing and making prognostic assessments of cardiopulmonary-related diseases. Nevertheless, further studies are needed to narrow its predictive value. The most common aspects that limit the achievement of the optimal values (or range of values) are related to study designs, such as retrospective, cross-sectional (transversal – short-term studies), single-center, and the lack of healthy control groups. Therefore, due to these limitations, many studies miss the populational stratification regarding conditional and non-conditional variability of respiratory diseases associated with cardiovascular and metabolic co-morbidities. The lack of stratification of subjects in studies based on criteria such as age, sex, and medical history may be a limiting factor in increasing the specificity of the biomarker. Also, when it comes to respiratory dysfunctions, a combination of patients with chronic distress and those with acute dyspnea may bias the observations. As with all laboratory tests, BNP concentrations need to be interpreted in the context of the individual patient. Some assays provide different diagnostic ranges based on a patient’s sex/gender, age, and renal function. In addition, there are known factors other than CHF that can result in high BNP levels (eg, age, renal dysfunction, myocardial infarction, acute pulmonary embolism, and high output states such as cirrhosis) and lower-than-expected BNP levels (eg, obesity, within 1 hour of flash pulmonary edema, acute mitral regurgitation, and mitral stenosis).
Future research may need to focus on the stratification of patients based on age, sex, weight, and medical history, and the impact of those confounding variables on serum levels of BNP in patients presenting with dyspnea. Differences between laboratory-conducted and point-of-care assays may also play a role in this variability. Therefore, it is vital to ensure that laboratories and assay kits are subjected to strict quality control and assessment criteria.
Acknowledgments
To Michigan State University (MSU): College of Osteopathic Medicine and the Department of Pharmacology and Toxicology. The authors declare that they have no conflict of interest.
Glossary
- NPs
natriuretic peptides
- NPS
natriuretic peptide system
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- CNP
C-Type natriuretic peptide
Author Contributions
CBAR conceived the project and coordinated manuscript production, edited the final version. CBAR, MS, and SM participated in the literature search. CBAR, MS, and SM contributed equally to writing the manuscript. CBAR, MS, and SM critically revised and approved the manuscript’s final version. CBAR, MS, and SM are responsible for publishing the final version’s intellectual content.
References
- Silver MA. The natriuretic peptide system: kidney and cardiovascular effects. Curr Opin Nephrol Hypertens. 2006. Jan;15(1):14–21. 10.1097/01.mnh.0000199008.49176.37 [DOI] [PubMed] [Google Scholar]
- Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic Peptides: Their Structures. Handbook of Experimental Pharmacology. Receptors, Physiologic Functions and Therapeutic Applications; 2009. pp. 341–66. 10.1007/978-3-540-68964-5_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeri R, Burnett JC Jr. Designer natriuretic peptides: a vision for the future of heart failure therapeutics. Can J Physiol Pharmacol. 2011. Aug;89(8):593–601. 10.1139/y11-048 [DOI] [PubMed] [Google Scholar]
- de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981. Jan;28(1):89–94. 10.1016/0024-3205(81)90370-2 [DOI] [PubMed] [Google Scholar]
- Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988. Mar;332(6159):78–81. 10.1038/332078a0 [DOI] [PubMed] [Google Scholar]
- Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990. Apr;168(2):863–70. 10.1016/0006-291X(90)92401-K [DOI] [PubMed] [Google Scholar]
- Dickey DM, Burnett JC Jr, Potter LR. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem. 2008. Dec;283(50):35003–9. 10.1074/jbc.M804538200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly RT, Weissmann N, Enke B, Ghofrani HA, Forssmann WG, Grimminger F, et al. Urodilatin, a natriuretic peptide stimulating guanylate cyclase, and the phosphodiesterase five inhibitor dipyridamole attenuate experimental pulmonary hypertension. Am J Respir Cell Mol Biol. 2001;25:219–25. 10.1165/ajrcmb.25.2.4256 [DOI] [PubMed] [Google Scholar]
- Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps). J Biol Chem. 1992. Jul;267(20):13928–32. 10.1016/S0021-9258(19)49658-0 [DOI] [PubMed] [Google Scholar]
- Nishikimi T, Kuwahara K, Nakao K. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J Cardiol. 2011. Mar;57(2):131–40. 10.1016/j.jjcc.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Baba M, Yoshida K, Ieda M. Clinical Applications of Natriuretic Peptides in Heart Failure and Atrial Fibrillation. Int J Mol Sci. 2019. Jun;20(11):2824. 10.3390/ijms20112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006. Feb;27(1):47–72. 10.1210/er.2005-0014 [DOI] [PubMed] [Google Scholar]
- Cea LB. Natriuretic peptide family: new aspects. Curr Med Chem Cardiovasc Hematol Agents. 2005. Apr;3(2):87–98. 10.2174/1568016053544309 [DOI] [PubMed] [Google Scholar]
- Song W, Wang H, Wu Q. Atrial natriuretic peptide in cardiovascular biology and disease (NPPA). Gene. 2015. Sep;569(1):1–6. 10.1016/j.gene.2015.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selçuk M, Keskin M, Çınar T, Günay N, Doğan S, Çiçek V, et al. Prognostic significance of N-Terminal Pro-BNP in patients with COVID-19 pneumonia without previous history of heart failure. J Cardiovasc Thorac Res. 2021;13(2):141–5. 10.34172/jcvtr.2021.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel A. B-type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what’s next? Circulation. 2002. May;105(20):2328–31. 10.1161/01.cir.0000019121.91548.c2 [DOI] [PubMed] [Google Scholar]
- Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998. Jul;339(5):321–8. 10.1056/NEJM199807303390507 [DOI] [PubMed] [Google Scholar]
- Vesely DL, Douglass MA, Dietz JR, Gower WR Jr, McCormick MT, Rodriguez-Paz G, et al. Three peptides from the atrial natriuretic factor prohormone amino terminus lower blood pressure and produce diuresis, natriuresis, and/or kaliuresis in humans. Circulation. 1994. Sep;90(3):1129–40. 10.1161/01.cir.90.3.1129 [DOI] [PubMed] [Google Scholar]
- Gu J, D’Andrea M, Seethapathy M. Atrial natriuretic peptide and its messenger ribonucleic acid in overloaded and overload-released ventricles of rat. Endocrinology. 1989. Oct;125(4):2066–74. 10.1210/endo-125-4-2066 [DOI] [PubMed] [Google Scholar]
- Christ M, Mueller C. Use of natriuretic peptide assay in dyspnea. Dtsch Arztebl Int. 2008. Feb;105(6):95–100. 10.3238/arztebl.2008.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aburaya M, Minamino N, Hino J, Kangawa K, Matsuo H. Distribution and molecular forms of brain natriuretic peptide in the central nervous system, heart and peripheral tissue of rat. Biochem Biophys Res Commun. 1989. Dec;165(2):880–7. 10.1016/s0006-291x(89)80047-6 [DOI] [PubMed] [Google Scholar]
- Mitaka C, Hirata Y, Nagura T, Tsunoda Y, Itoh M, Amaha K. Increased plasma concentrations of brain natriuretic peptide in patients with acute lung injury. J Crit Care. 1997. Jun;12(2):66–71. 10.1016/s0883-9441(97)90003-4 [DOI] [PubMed] [Google Scholar]
- Clerico A, Recchia FA, Passino C, Emdin M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am J Physiol Heart Circ Physiol. 2006. Jan;290(1):H17–29. 10.1152/ajpheart.00684.2005 [DOI] [PubMed] [Google Scholar]
- Gerbes AL, Dagnino L, Nguyen T, Nemer M. Transcription of brain natriuretic peptide and atrial natriuretic peptide genes in human tissues. J Clin Endocrinol Metab. 1994. Jun;78(6):1307–11. 10.1210/jcem.78.6.8200930 [DOI] [PubMed] [Google Scholar]
- Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005. Mar;330(7492):625. 10.1136/bmj.330.7492.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheval L, Bakouh N, Walter C, Tembely D, Morla L, Escher G, et al. ANP-stimulated Na+ secretion in the collecting duct prevents Na+ retention in the renal adaptation to acid load. Am J Physiol Renal Physiol. 2019. Aug;317(2):F435–43. 10.1152/ajprenal.00059.2019 [DOI] [PubMed] [Google Scholar]
- Yokota N, Bruneau BG, Kuroski de Bold ML, de Bold AJ. Atrial natriuretic factor significantly contributes to the mineralocorticoid escape phenomenon. Evidence for a guanylate cyclase-mediated pathway. J Clin Invest. 1994. Nov;94(5):1938–46. 10.1172/JCI117544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, et al. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci USA. 1999. Jun;96(13):7403–8. 10.1073/pnas.96.13.7403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerico A, Del Ry S, Maffei S, Prontera C, Emdin M, Giannessi D. The circulating levels of cardiac natriuretic hormones in healthy adults: effects of age and sex. Clin Chem Lab Med. 2002. Apr;40(4):371–7. 10.1515/CCLM.2002.060 [DOI] [PubMed] [Google Scholar]
- Witthaut R, Busch C, Fraunberger P, Walli A, Seidel D, Pilz G, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med. 2003. Oct;29(10):1696–702. 10.1007/s00134-003-1910-0 [DOI] [PubMed] [Google Scholar]
- Harada M, Saito Y, Kuwahara K, Ogawa E, Ishikawa M, Nakagawa O, et al. Interaction of myocytes and nonmyocytes is necessary for mechanical stretch to induce ANP/BNP production in cardiocyte culture. J Cardiovasc Pharmacol. 1998;31 Suppl 1:S357–9. 10.1097/00005344-199800001-00100 [DOI] [PubMed] [Google Scholar]
- Hopkins WE, Chen Z, Fukagawa NK, Hall C, Knot HJ, LeWinter MM. Increased atrial and brain natriuretic peptides in adults with cyanotic congenital heart disease: enhanced understanding of the relationship between hypoxia and natriuretic peptide secretion. Circulation. 2004. Jun;109(23):2872–7. 10.1161/01.CIR.0000129305.25115.80 [DOI] [PubMed] [Google Scholar]
- Gopi V, Subramanian V, Manivasagam S, Vellaichamy E. Angiotensin II down-regulates natriuretic peptide receptor-A expression and guanylyl cyclase activity in H9c2 (2-1) cardiac myoblast cells: role of ROS and NF-κB. Mol Cell Biochem. 2015. Nov;409(1-2):67–79. 10.1007/s11010-015-2513-0 [DOI] [PubMed] [Google Scholar]
- Abbey-Hosch SE, Cody AN, Potter LR. Sphingosine-1-phosphate inhibits C-type natriuretic peptide activation of guanylyl cyclase B (GC-B/NPR-B). Hypertension. 2004. May;43(5):1103–9. 10.1161/01.hyp.0000124668.80811.d3 [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava MB, Trachte GJ. Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacol Rev. 1993. Dec;45(4):455–97. [PubMed] [Google Scholar]
- Bennett BD, Bennett GL, Vitangcol RV, Jewett JR, Burnier J, Henzel W, et al. Extracellular domain-IgG fusion proteins for three human natriuretic peptide receptors. Hormone pharmacology and application to solid phase screening of synthetic peptide antisera. J Biol Chem. 1991. Dec;266(34):23060–7. 10.1016/S0021-9258(18)54463-X [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Identification of the G protein-activating domain of the natriuretic peptide clearance receptor (NPR-C). J Biol Chem. 1999. Jun;274(25):17587–92. 10.1074/jbc.274.25.17587 [DOI] [PubMed] [Google Scholar]
- Kuwahara K. The natriuretic peptide system in heart failure: diagnostic and therapeutic implications. Pharmacol Ther. 2021. Nov;227:107863. 10.1016/j.pharmthera.2021.107863 [DOI] [PubMed] [Google Scholar]
- Rose RA, Giles WR, Giles WR. Natriuretic peptide C receptor signalling in the heart and vasculature. J Physiol. 2008. Jan;586(2):353–66. 10.1113/jphysiol.2007.144253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egom EE. Natriuretic Peptide Clearance Receptor (NPR-C) Pathway as a Novel Therapeutic Target in Obesity-Related Heart Failure With Preserved Ejection Fraction (HFpEF). Front Physiol. 2021. May;12:674254. 10.3389/fphys.2021.674254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egom EE. Pulmonary Arterial Hypertension Due to NPR-C Mutation: A Novel Paradigm for Normal and Pathologic Remodeling? Int J Mol Sci. 2019. Jun;20(12):3063. 10.3390/ijms20123063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzani R, Spannella F, Giulietti F, Balietti P, Cocci G, Bordicchia M. Cardiac Natriuretic Peptides, Hypertension and Cardiovascular Risk. High Blood Press Cardiovasc Prev. 2017. Jun;24(2):115–26. 10.1007/s40292-017-0196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egom EE. BNP and Heart Failure: Preclinical and Clinical Trial Data. J Cardiovasc Transl Res. 2015. Apr;8(3):149–57. 10.1007/s12265-015-9619-3 [DOI] [PubMed] [Google Scholar]
- Hayoz D, Drexler H, Munzel T, Hornig B, Zeiher AM, Just H, et al. Flow-mediated arterial dilation is abnormal in congestive heart failure. Circulation. 1993;87:92–6. [Google Scholar]
- Atlas SA, Volpe M, Sosa RE, Laragh JH, Camargo MJ, Maack T. Effects of atrial natriuretic factor on blood pressure and the renin-angiotensin-aldosterone system. Fed Proc. 1986. Jun;45(7):2115–21. [PubMed] [Google Scholar]
- Guo LJ, Alli AA, Eaton DC, Bao HF. ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling. Am J Physiol Renal Physiol. 2013. Apr;304(7):F930–7. 10.1152/ajprenal.00638.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger JP, Burnett JC Jr, Romero JC, Opgenorth TJ, Salazar J, Joyce M. Elevated levels of atrial natriuretic peptide during aldosterone escape. Am J Physiol. 1987. May;252(5 Pt 2):R878–82. 10.1152/ajpregu.1987.252.5.R878 [DOI] [PubMed] [Google Scholar]
- Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006. Feb;69(2):318–28. 10.1016/j.cardiores.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Matsumura T, Kugiyama K, Sugiyama S, Ohgushi M, Amanaka K, Suzuki M, et al. Neutral endopeptidase 24.11 in neutrophils modulates protective effects of natriuretic peptides against neutrophils-induced endothelial cytotoxity. J Clin Invest. 1996. May;97(10):2192–203. 10.1172/JCI118660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla F, Garcia-Dorado D, Agulló L, Barrabés JA, Inserte J, Escalona N, et al. Intravenous administration of the natriuretic peptide urodilatin at low doses during coronary reperfusion limits infarct size in anesthetized pigs. Cardiovasc Res. 2001. Aug;51(3):592–600. 10.1016/s0008-6363(01)00242-5 [DOI] [PubMed] [Google Scholar]
- Hempel A, Friedrich M, Schlüter KD, Forssmann WG, Kuhn M, Piper HM. ANP protects against reoxygenation-induced hypercontracture in adult cardiomyocytes. Am J Physiol. 1997. Jul;273(1 Pt 2):H244–9. 10.1152/ajpheart.1997.273.1.H244 [DOI] [PubMed] [Google Scholar]
- Sarzani R, Dessì-Fulgheri P, Paci VM, Espinosa E, Rappelli A. Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest. 1996. Oct;19(9):581–5. 10.1007/BF03349021 [DOI] [PubMed] [Google Scholar]
- Sengenès C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000. Jul;14(10):1345–51. 10.1096/fasebj.14.10.1345 [DOI] [PubMed] [Google Scholar]
- Gower WR Jr, Premaratne S, McCuen RW, Arimura A, McAfee Q, Schubert ML. Gastric atrial natriuretic peptide regulates endocrine secretion in antrum and fundus of human and rat stomach. Am J Physiol Gastrointest Liver Physiol. 2003. Apr;284(4):G638–45. 10.1152/ajpgi.00427.2002 [DOI] [PubMed] [Google Scholar]
- Schlueter N, de Sterke A, Willmes DM, Spranger J, Jordan J, Birkenfeld AL. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol Ther. 2014. Oct;144(1):12–27. 10.1016/j.pharmthera.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Jaubert J, Jaubert F, Martin N, Washburn LL, Lee BK, Eicher EM, et al. Three new allelic mouse mutations that cause skeletal overgrowth involve the natriuretic peptide receptor C gene (Npr3). Proc Natl Acad Sci USA. 1999. Aug;96(18):10278–83. 10.1073/pnas.96.18.10278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthast R, Abbey-Hosch SE, Antos LK, Marchant JS, Kuhn M, Potter LR. Calcium-dependent dephosphorylation mediates the hyperosmotic and lysophosphatidic acid-dependent inhibition of natriuretic peptide receptor-B/guanylyl cyclase-B. J Biol Chem. 2004. Nov;279(47):48513–9. 10.1074/jbc.m408247200 [DOI] [PubMed] [Google Scholar]
- Abbey SE, Potter LR. Vasopressin-dependent inhibition of the C-type natriuretic peptide receptor, NPR-B/GC-B, requires elevated intracellular calcium concentrations. J Biol Chem. 2002. Nov;277(45):42423–30. 10.1074/jbc.m206686200 [DOI] [PubMed] [Google Scholar]
- Chrisman TD, Garbers DL. Reciprocal antagonism coordinates C-type natriuretic peptide and mitogen-signaling pathways in fibroblasts. J Biol Chem. 1999. Feb;274(7):4293–9. 10.1074/jbc.274.7.4293 [DOI] [PubMed] [Google Scholar]
- Clerico A, Iervasi G, Del Chicca MG, Emdin M, Maffei S, Nannipieri M, et al. Circulating levels of cardiac natriuretic peptides (ANP and BNP) measured by highly sensitive and specific immunoradiometric assays in normal subjects and in patients with different degrees of heart failure. J Endocrinol Invest. 1998. Mar;21(3):170–9. 10.1007/BF03347297 [DOI] [PubMed] [Google Scholar]
- Cowie MR, Struthers AD, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997. Nov;350(9088):1349–53. 10.1016/S0140-6736(97)06031-5 [DOI] [PubMed] [Google Scholar]
- Krüger S, Graf J, Merx MW, Stickel T, Kunz D, Hanrath P, et al. Brain natriuretic peptide kinetics during dynamic exercise in patients with chronic heart failure. Int J Cardiol. 2004. May;95(1):49–54. 10.1016/j.ijcard.2003.04.021 [DOI] [PubMed] [Google Scholar]
- Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365–71. 10.1016/j.carpath.2011.11.007 [DOI] [PubMed] [Google Scholar]
- Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess. 2009. Jul;13(32):1–207. 10.3310/hta13320 [DOI] [PubMed] [Google Scholar]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. American College of Cardiology Foundation. American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013. Oct;62(16):e147–239. 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, et al. Writing Committee. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021. Feb;77(6):772–810. 10.1016/j.jacc.2020.11.022 [DOI] [PubMed] [Google Scholar]
- Emdin M, Clerico A, Clemenza F, Galvani M, Latini R, Masson S, et al. Italian Association of Hospital Cardiologists. Italian Society of Cardiology. Italian Federation of Cardiology. Italian Society of Clinical Chemistry and Molecular Biology. Italian Society of Laboratory Medicine. Italian Society of Emergency Medicine. Recommendations for the clinical use of cardiac natriuretic peptides. Ital Heart J. 2005. May;6(5):430–46. [PubMed] [Google Scholar]
- Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JG, Kozhuharov N, et al. Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019. Jun;21(6):715–31. 10.1002/ejhf.1494 [DOI] [PubMed] [Google Scholar]
- Tung RH, Camargo CA Jr, Krauser D, Anwaruddin S, Baggish A, Chen A, et al. Amino-terminal pro-brain natriuretic peptide for the diagnosis of acute heart failure in patients with previous obstructive airway disease. Ann Emerg Med. 2006. Jul;48(1):66–74. 10.1016/j.annemergmed.2005.12.022 [DOI] [PubMed] [Google Scholar]
- Parmley WW. Pathophysiology and current therapy of congestive heart failure. J Am Coll Cardiol. 1989. Mar;13(4):771–85. 10.1016/0735-1097(89)90215-5 [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003. Jun;107(23):2920–5. 10.1161/01.CIR.0000072767.89944.6E [DOI] [PubMed] [Google Scholar]
- Therkelsen SK, Groenning BA, Kjaer A, Svendsen JH, Boje Jensen G. ANP and BNP in atrial fibrillation before and after cardioversion—and their relationship to cardiac volume and function. Int J Cardiol. 2008. Jul;127(3):396–9. 10.1016/j.ijcard.2007.04.059 [DOI] [PubMed] [Google Scholar]
- Reinmann M, Meyer P. B-Type Natriuretic Peptide and Obesity in Heart Failure: A Mysterious but Important Association in Clinical Practice. Cardiovasc Med. 2020;•••: 10.4414/cvm.2020.02095 [DOI] [Google Scholar]
- Takemura G, Takatsu Y, Doyama K, Itoh H, Saito Y, Koshiji M, et al. Expression of atrial and brain natriuretic peptides and their genes in hearts of patients with cardiac amyloidosis. Journal of the American College of Cardiology. 1998; 31: 754–765. doi: / 10.1016/s0735-1097(98)00045-x [DOI] [PubMed]
- Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit Care. 2002. Dec;6(6):500–8. 10.1186/cc1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo JR, Zagler A, Carrillo-Jimenez R, Hennekens CH. Brain natriuretic peptide: a potential marker for mortality in septic shock. Int J Infect Dis. 2004. Sep;8(5):271–4. 10.1016/j.ijid.2003.12.007 [DOI] [PubMed] [Google Scholar]
- Cataliotti A, Malatino LS, Jougasaki M, Zoccali C, Castellino P, Giacone G, et al. Circulating natriuretic peptide concentrations in patients with end-stage renal disease: role of brain natriuretic peptide as a biomarker for ventricular remodeling. Mayo Clin Proc. 2001. Nov;76(11):1111–9. 10.4065/76.11.1111 [DOI] [PubMed] [Google Scholar]
- Mark PB, Stewart GA, Gansevoort RT, Petrie CJ, McDonagh TA, Dargie HJ, et al. Diagnostic potential of circulating natriuretic peptides in chronic kidney disease. Nephrol Dial Transplant. 2006. Feb;21(2):402–10. 10.1093/ndt/gfi187 [DOI] [PubMed] [Google Scholar]
- Sayama H, Nakamura Y, Saito N, Kinoshita M. Why is the concentration of plasma brain natriuretic peptide in elderly inpatients greater than normal? Coron Artery Dis. 1999. Oct;10(7):537–40. 10.1097/00019501-199910000-00016 [DOI] [PubMed] [Google Scholar]
- Kukrety SP, Parekh JD, Bailey KL. Chronic obstructive pulmonary disease and the hallmarks of aging. Lung India. 2018;35(4):321–7. 10.4103/lungindia.lungindia_266_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNee W. Is Chronic Obstructive Pulmonary Disease an Accelerated Aging Disease? Ann Am Thorac Soc. 2016. Dec;13 Suppl 5:S429–37. 10.1513/AnnalsATS.201602-124AW [DOI] [PubMed] [Google Scholar]
- Chen HH, Burnett JC Jr. Natriuretic peptides in the pathophysiology of congestive heart failure. Curr Cardiol Rep. 2000. May;2(3):198–205. 10.1007/s11886-000-0069-3 [DOI] [PubMed] [Google Scholar]
- Cargill RI, Lipworth BJ. Atrial natriuretic peptide and brain natriuretic peptide in cor pulmonale. Hemodynamic and endocrine effects. Chest. 1996. Nov;110(5):1220–5. 10.1378/chest.110.5.1220 [DOI] [PubMed] [Google Scholar]
- Koulouri S, Acherman RJ, Wong PC, Chan LS, Lewis AB. Utility of B-type natriuretic peptide in differentiating congestive heart failure from lung disease in pediatric patients with respiratory distress. Pediatr Cardiol. 2004;25(4):341–6. 10.1007/s00246-003-0578-0 [DOI] [PubMed] [Google Scholar]
- Ahmed AM, Mohamed NA, Abdelhamid EM, Taha RI, Abo-Hashish MM. N-terminal pro-brain natriuretic peptide as a biomarker for differentiating cardiac and pulmonary disease in term neonates with respiratory distress. J Saudi Heart Assoc. 2020. Apr;32(1):65–70. 10.37616/2212-5043.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Song W, Wang L, Zeng Q, Zhao Z, Feng N, et al. NT-pro BNP in AECOPD-PH: old biomarker, new insights-based on a large retrospective case-controlled study. Respir Res. 2021. Dec;22(1):321. 10.1186/s12931-021-01917-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins NM, Khosla A, Virani SA, McMurray JJ, FitzGerald JM. B-type natriuretic peptides in chronic obstructive pulmonary disease: a systematic review. BMC Pulm Med. 2017. Jan;17(1):11. 10.1186/s12890-016-0345-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz RP, Farber HW, Badesch DB, Elliott CG, Frost AE, McGoon MD, et al. Baseline and serial brain natriuretic peptide level predicts 5-year overall survival in patients with pulmonary arterial hypertension: data from the REVEAL registry. Chest. 2018. Jul;154(1):126–35. 10.1016/j.chest.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin KM, Rubin LJ, Channick R, Di Scala L, Gaine S, Galiè N, et al. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation. 2019. May;139(21):2440–50. 10.1161/CIRCULATIONAHA.118.039360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrish M, Nannaka VB, Cano EJ, Bajantri B, Diaz-Fuentes G. Significance of NT-pro-BNP in acute exacerbation of COPD patients without underlying left ventricular dysfunction. Int J Chron Obstruct Pulmon Dis. 2017. Apr;12:1183–9. 10.2147/COPD.S134953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Barretto AC, Carlo CH, Cardoso JN, Ochiai ME, Lima MV, Curiati MC, et al. Role of BNP levels on the prognosis of decompensated advanced heart failure. Arq Bras Cardiol. 2013. Mar;100(3):281–7. 10.5935/abc.20130066 [DOI] [PubMed] [Google Scholar]
- Khanam SS, Son JW, Lee JW, Youn YJ, Yoon J, Lee SH, et al. Prognostic value of short-term follow-up BNP in hospitalized patients with heart failure. BMC Cardiovasc Disord. 2017. Aug;17(1):215. 10.1186/s12872-017-0632-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida PL, Don-Wauchope AC, Oremus M, McKelvie R, Ali U, Hill SA, et al. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev. 2014. Aug;19(4):453–70. 10.1007/s10741-014-9442-y [DOI] [PubMed] [Google Scholar]
- Burke MA, Cotts WG. Interpretation of B-type natriuretic peptide in cardiac disease and other comorbid conditions. Heart Fail Rev. 2007. Mar;12(1):23–36. 10.1007/s10741-007-9002-9 [DOI] [PubMed] [Google Scholar]
- Moe GW, Howlett J, Januzzi JL, Zowall H, Canadian Multicenter Improved Management of Patients With Congestive Heart Failure (IMPROVE-CHF) Study Investigators. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007. Jun;115(24):3103–10. 10.1161/CIRCULATIONAHA.106.666255 [DOI] [PubMed] [Google Scholar]
- Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, et al. American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association [Erratum in: Circulation. 2017 Nov 7;136] [19] [:e345]. Circulation. 2017. May;135(22):e1054–91. 10.1161/CIR.0000000000000490 [DOI] [PubMed] [Google Scholar]
- Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008. Sep;10(9):824–39. 10.1016/j.ejheart.2008.07.014 [DOI] [PubMed] [Google Scholar]
- de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003. Jul;362(9380):316–22. 10.1016/S0140-6736(03)13976-1 [DOI] [PubMed] [Google Scholar]
- Arram EO, Saleh M, Hammad E. Evaluation of B-type natriuretic peptide in patients with community-acquired pneumonia. Egypt J Chest Dis Tuberc. 2013;62(2):293–300. 10.1016/j.ejcdt.2013.05.003 [DOI] [Google Scholar]
- Li J, Ye H, Zhao L. B-type natriuretic peptide in predicting the severity of community-acquired pneumonia. World J Emerg Med. 2015;6(2):131–6. 10.5847/wjem.j.1920-8642.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T, Ogawa K, Yamaki K, Hara M, Takagi K. Plasma concentrations of atrial, brain, and C-type natriuretic peptides and endothelin-1 in patients with chronic respiratory diseases. Chest. 1996. Aug;110(2):462–8. 10.1378/chest.110.2.462 [DOI] [PubMed] [Google Scholar]
- Yap LB, Mukerjee D, Timms PM, Ashrafian H, Coghlan JG. Natriuretic peptides, respiratory disease, and the right heart. Chest. 2004. Oct;126(4):1330–6. 10.1378/chest.126.4.1330 [DOI] [PubMed] [Google Scholar]
- Weinfeld MS, Chertow GM, Stevenson LW. Aggravated renal dysfunction during intensive therapy for advanced chronic heart failure. Am Heart J. 1999. Aug;138(2 Pt 1):285–90. 10.1016/s0002-8703(99)70113-4 [DOI] [PubMed] [Google Scholar]
- Ma KK, Ogawa T, de Bold AJ. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J Mol Cell Cardiol. 2004. Apr;36(4):505–13. 10.1016/j.yjmcc.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020. Jul;5(7):802–10. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino S, Cacia M, Leo I, Polimeni A, Sabatino J, Spaccarotella CA, et al. B-Type Natriuretic Peptide as Biomarker of COVID-19 Disease Severity-A Meta-Analysis. J Clin Med. 2020. Sep;9(9):2957. 10.3390/jcm9092957 [DOI] [PMC free article] [PubMed] [Google Scholar]