Abstract
Lately, there has been increased interest in the development of phytochemical alternatives for the prevention and treatment of type 2 diabetes, the alternatives that are able to reduce or prevent glucose absorption by inhibiting digestive enzymes. In this context, this study aims to analyze the inhibitory α-amylase and α-glucosidase activities of Artemisia abrotanum and Symphytum officinale polyphenolic compound-rich extracts obtained by membrane technologies (micro- and ultrafiltration). Polyphenols and flavones content, HPLC-MS polyphenolic compounds profiling, antioxidant activity, and cytotoxic potential of these herbs were determined. Major phenolic acid compounds were chlorogenic acid, ellagic acid, caffeic acid, and rosmarinic acid. The flavone content was higher in the case of A. abrotanum extracts, and the major compounds were rutin and umbelliferone. The polyphenolic-rich extract of A. abrotanum had the highest quantities of polyphenols, 977.75 µg/mL, and flavones, 552.85 µg/mL, as well as a pronounced α-amylase inhibitory activity (IC50 1881.21 ± 1.8 mg/mL), a value close to acarbose inhibitory activity (IC50 1110.25 ± 8.82 mg/mL) that was used as the control for both enzymes. The α-glucosidase inhibitory activity was higher for both herb extracts, more pronounced for S. officinale polyphenolic-rich extract (IC50 291.56 ± 2.1 mg/mL), a value higher than that of acarbose (IC50 372.35 ± 3.2 mg/mL). These plants show potential as a complementary therapy for type 2 diabetes management.
Keywords: Artemisia abrotanum, Symphytum officinale polyphenolic compounds-rich extracts, bioactive compounds, antidiabetic potential
1. Introduction
Usage of nutraceuticals in the treatment of chronic diseases is encouraged in developed countries due to the side effects of synthetic drugs. The treatment based on natural remedies seems to offer more opportunities in the treatment of such conditions. Medicinal herbs were used widely because they contain biologically active compounds with a synergistic effect and have reduced side effects and lower costs compared to allopathic drugs [1,2].
Diabetes mellitus is a serious chronic endocrine condition with multiple etiologies and increasing incidence due to obesity and aging. Over time, this condition leads to severe complications, such as retinopathy, neuropathy, nephropathy, atherosclerosis, and vascular complications that increase cardiovascular risk up to heart attack [3,4,5]. Type 1 diabetes, which is insulin-dependent, is an autoimmune disease caused by the destruction of pancreatic β-cells, which secrete little or no insulin. In non-insulin-dependent type 2 diabetes, the pancreas secretes insulin, but it is not used efficiently in the cells. About 90–95% of diabetes cases are type 2 [6].
There are many oral drugs used to treat type 2 diabetes, for example, metformin [7] is the most commonly used; then, there are drugs in the sulfonylurea, thiazolidinedione, and α-glucosidase inhibitor classes [8]. However, they present a series of adverse side effects, such as liver and cholecystic lesions, diarrhea, nausea, vomiting, lactic acidosis [9], hypoglycemia, weight gain, skin reactions, porphyria [10], and gastrointestinal disorders [11].
A therapeutic alternative for diabetes treatment consists of the reduction of post-prandial hyperglycemia, which can be realized by delaying glucose absorption via α-glucosidase and α-amylase inhibiting [12]. The α-amylase hydrolyzes starch in maltose and glucose, while α-glucosidase hydrolyses maltose and sucrose with the release of glucose. Inhibiting these enzymes, carbohydrate digestion and glucose absorption are delayed and therefore, postprandial hyperglycemia is reduced [3,13]. Many attempts were made to find new and effective natural alternative inhibitors to these enzymes and to produce food additives or compounds used against diabetes [14]. Many studies have highlighted those bioactive compounds, especially polyphenols, which have the ability to inhibit these enzymes. For this reason, in this work, we studied the α-amylase and α-glucosidase inhibitory capacity of polyphenolic compound-rich extracts from Artemisia abrotanum and Symphytum officinale. These plants were chosen for this study because they are widespread in Europe and not at all studied from the point of view of inhibiting these enzymes.
Artemisia abrotanum (southernwood) (family Asteraceae) is a medicinal herb used as an antiseptic, anti-inflammatory astringent, antipyretic, tonic, and for the treatment of respiratory diseases. It has also been used against cancer, cough, fever, and tumors [15]. It also shows antibacterial, antifungal, and antiparasitic effects [16].
Symphytum officinale (comfrey) (family Boraginaceae) is a herb used in folk medicine. S. officinale leaves and the roots were used in the treatment of gastrointestinal tract ulcerations, bones, and respiratory diseases [17,18,19]. S. officinale contains allantoin, carotene, hydroxycinnamic acid, and essential oils. Comfrey also contains hepatotoxic pyrrolizidine alkaloids, so its long-term consumption is not recommended [20,21].
Special attention is paid to the extraction and concentration of polyphenolic compounds since they are thermolabile and prone to chemical changes. The membrane technologies—microfiltration, ultrafiltration, and nanofiltration—are some of the most modern and effective methods of separation and concentration of bioactive compounds from plant extracts. Processing is performed at mild temperatures, maintaining the original characteristics of the products processed, with minimal loss of vitamins, polyphenols, proteins, and other thermolabile compounds [22,23,24]. Recent studies demonstrated the efficiency of ultrafiltration and nanofiltration for the concentration of bioactive compounds, such as anthocyanins, flavonoids, oligosaccharides, and phenolic compounds from vegetable extracts [25,26,27].
2. Materials and Methods
2.1. Materials
All reagents have been purchased from Fluka (Buchs, Switzerland), Roth (Carl Roth GmbH, Karlsruhe, Germany), and Sigma (Sigma–Aldrich, Schnelldorf, Germany).
2.2. Plant Material
Artemisia abrotanum was harvested from Rodna, Bistrita-Nasaud district was identified by Sorana Hentea and deposited at Cluj Herbarium (voucher specimen nr. 210.240). The leaves of Symphytum officinale were collected from Valea Morii, Feleac, Cluj district, identified by Sorana Hentea, and deposited at Cluj Herbarium (voucher specimen nr. 657369).
2.3. Obtaining and Concentrating Extracts
The ethanol extracts were obtained as follows: 10 g of the dry plant (aerial parts) was added to 100 mL of 50% (v/v) EtOH, were mixed, then were sonicated for 1 h at 25 °C, and finally, filtered through a Whatman filter (125 mm, Blue–Slow).
First, the extracts were micro-filtrated through a Millipore membrane (0.45 µm pores) for purification. Then, the extracts were concentrated by ultrafiltration through Millipore membranes with a cut-off of 1000 Da. The concentration ratio (ratio of permeate volume to concentrate) was 3:1. For microfiltration and ultrafiltration, a KMS Laboratory Cell CF-1 installation was used.
2.4. Phytochemical Characterization
2.4.1. Polyphenolic Compound Content Determination
Polyphenolic compound content determination was done using Folin–Ciocalteu method [28]. Thus, 2.5 mL extract and 2.5 mL Folin–Ciocalteu reagent were mixed, shaken, and filtered; then, 1 mL of the filtrate was added to 9.5 mL sodium carbonate 20% and absorbance reading at 660 nm after 2 min. The polyphenolic compound content in the extracts was determined based on a gallic acid calibration curve.
2.4.2. Flavonoid Content Determination
Flavonoid content determination was achieved using the spectrophotometric method described by [29]. Thus, 5 mL of sample and 7.5 mL of MeOH were mixed and then filtered. Afterward, 1 mL filtrate was mixed with 1 mL sodium acetate solution (10%); 0.6 mL of aluminum chloride hexahydrate solution (2.5%) and 0.5 mL MeOH were added. The absorbance reading was performed at 430 nm after 15 min. The flavonoid content was determined by a rutin calibration curve.
2.4.3. HPLC Analysis
The chromatographic analysis has been realized with an HPLC SHIMADZU system (C18 Nucleosil 3.5, 4.6 × 50 mm Zorbax column). An MS detector, LCMS-2010 detector (liquid chromatograph mass spectrometer), with an ESI interface and negative ionization mode, was coupled to the system. All reagents used have been of analytical purity. Ethanolic stock solutions, 1 mg/mL of ellagic acid, rutin, caffeic acid, quercetin, gallic acid, rosmarinic acid, chlorogenic acid, apigenin, quercetin 3-β-D-glucoside, luteolin, kaempferol, and umbelliferone were used as standards. The mobile phase consisted of water (solvent A) and acetonitrile (solvent B), adjusted to pH 3.0 with formic acid. Separation of phenolic compounds was achieved using binary gradient elution as follows: between 0.01–20 min; the initial 5% solvent B was increased to 30% and from 20.01–40 min maintained at 30%; then, at 50% between 40.01–50 min, returning to 5% between 50.01–52 min and held at 5% for 52.01–70 min. The flow rate was 0.1 mL/min between 0–5, 15.01–35, and 60.01–70 min, and 0.2 mL/min from 5.01–15 and 35.01–60 min. We used Alecu’s HPLC method to analyze polyphenols [30]. The mobile phase consisted of water (solvent A) and acetonitrile (solvent B), adjusted to pH 3.0 with formic acid. Separation of phenolic compounds was achieved using binary gradient elution as follows: between 0.01–20 min, the initial 5% solvent B was increased to 30%, from 20.01–40 min maintained at 30%, then at 50% between 40.01–50 min, returning to 5% between 50.01–52 min and held at 5%, 52.01–70 min. The flow rate was 0.1 mL/min between 0–5, 15.01–35, and 60.01–70 min, and 0.2 mL/min from 5.01–15 and 35.01–60 min. For qualitative analysis, we used acquisition mode, SCAN of MS, [M-H]−: 50-700, and UV scanning spectra. Ethanolic stock solutions, 1mg/mL of umbelliferone (Sigma, H24003), gallic acid (Fluka, 48630), caffeic acid (Sigma, C0625), apigenin (Fluka, 10798), luteolin (Sigma, 72511), kaempferol (Sigma, 60010), ellagic acid (Fluka, 45140), quercetin (Sigma, Q4951), chlorogenic acid (Sigma, C3878), rosmarinic acid (Sigma, 536954), quercetin 3-β-D-glucoside (Sigma, 17793), and rutin (Sigma, 78095) were used as external standards for quantitative analysis. Using the SIM (selected ion monitoring) mode ([M-H]-: 161, 169, 179, 269, 285, 301, 353, 359, 463, and 609), the corresponding peaks of the polyphenols compound fragment ions were obtained, and were in perfect accordance to the data resulted from PDA chromatogram.
2.5. Antioxidant Assays
The antioxidant activity has been analyzed through two methods.
2.5.1. DPPH Radical Scavenging Activity
Briefly, 100 μL extract having different concentrations (3 mg/mL, 1.5 mg/mL, 0.75 mg/mL, 0.3 mg/mL) was mixed with 1 mL 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution (0.25 mM) and 1.9 mL methanol [31]. The extracts’ scavenging activity was calculated through the reading of absorbance at 517 nm after 3 min and was determined by the formula:
| radical scavenging activity (%) = [(AB − AA)/AB] × 100, |
where AB = control absorbance, and AA = sample absorbance.
The IC50 (extract concentration which produces 50% radical scavenging activity) values were determined by linear regression analysis. Significant statistical differences were considered p < 0.05.
2.5.2. Reducing Power Activity (Iron (III) to Iron (II) Reduction)
Reducing power has been analyzed using Berker’s method [32]. Thus, a 0.1 mL sample with 2.5 mL of sodium phosphate buffer (200 mM/L, pH 6.6) and 2.5 mL potassium ferricyanide (1%) was mixed. The mixture was vigorously agitated, then heated for 20 min at 50 °C; then, 2.5 mL trichloroacetic acid (10% w/v) was added after incubation. Finally, into the mixture, 2.5 mL deionized water and 0.5 mL ferric chloride (0.1%) were added. The absorbance has been read at 700 nm. The EC50 (the concentration corresponding to an absorbance of 0.5) values were calculated through linear regression analysis. Significant statistical differences were considered p < 0.05.
2.6. Enzymes Inhibition Activity
The Artemisia abrotanum and Symphytum officinale extracts were analyzed for the α-amylase and α-glucosidase inhibition activity.
2.6.1. α-Amylase Inhibition
The α-amylase inhibition assay was performed with modified Ranilla’s method [33]. Thus, 100 μL of the sample was mixed with 250 μL α-amylase from hog pancreas (EC 3.2.1.1) (0.5 mg/mL) in sodium phosphate buffer (0.02 M, pH 6.9) and was incubated at 37 °C for 20 min; then, 250 μL starch solution (1%) was added; then, the mixture has been reincubated at 37 °C, 30 min. After that, 500 μL dinitrosalicylic acid (DNS) was added; then, the reaction mixture was kept in the boiling bath for another 5 min. Then, 5 mL of distilled water was added to the mixture. Finally, the absorbance reading was performed at 540 nm using a UV-visible spectrophotometer (Jasco-V630). As a positive control, acarbose was used. The calculations were done by the formula:
IC50 (concentration of extract, which produces 50% enzyme inhibition) values have been determined through the linear regression analysis. Significant statistical differences were considered as p < 0.05.
2.6.2. α-Glucosidase Inhibition
The α-glucosidase inhibitory activity was realized by Queiroz et al. method with minor modification [34]. Briefly, 120 μL α-glucosidase (EC 3.2.1.20) (0.5 U/mL) was mixed with 720 μL sodium phosphate buffer (0.1 M, pH 6.9) with 60 µL extract and heated at 37 °C for 15 min. Then, a 120 μL p-nitrophenyl-α-D-glucopyranoside (5 mM/L) solution was added. The mixture was heated at 37 °C for 15 min. Finally, the absorbance reading was performed at 405 nm. The positive control was acarbose. The calculations were done by the formula:
IC50 values were determined by the linear regression analysis. Significant statistical differences were considered as p < 0.05.
2.7. Testing Extracts’ Cytotoxic Activity In Vitro
The cytotoxic activity of A. abrotanum and S. officinale extracts was analyzed on NCTC clone 929—a mouse fibroblast cell line. These were procured from the European Collection of cell Culture (Sigma–Aldrich, St. Louis, MI, USA). All materials used for the culture experiments were obtained from Sigma–Aldrich (SUA): PSN (100 mg/mL penicillin, 100 mg/L streptomycin, and 500 mg/L neomycin), MEM (Minimum Essential Medium), Giemsa, and Neutral Red from Merck (Germany).
Cell Viability
NCTC has been grown in MEM with PSN and 10% fetal bovine serum (FBS). The NCTC line was inoculated at 4 × 104 cells/mL density, then incubated in the wet atmosphere (5% CO2, 24 h) at 37 °C to allow the adherence of the cells. The untreated cells (Mc) were used as the control, and cells cultured in 100 µM H2O2 were used as the positive control. Then, the culture medium was changed by the same medium, with several ex-tract concentrations (50, 100, 500, and 1000 µg/mL). The cells were cultured in standard conditions (24, 48, and 72 h).
Neutral Red Test
The cell viability was analyzed by Borenfreund’s Neutral Red (NR) method [35]. The cellular line of fibroblast cultures (NCTC) was used to test cytotoxicity. After removal from the culture medium, the cells were washed with phosphate buffer. Then, 0.5 mL of freshly prepared NR solution (10 µg/mL) was added to each well and incubated at 37 °C for 3 h. Subsequently, the NR solution was removed and fixed with a 1% formol solution for 3 min. The plates were stirred at room temperature for 15 min and then transferred to a microplate reader (Tecan Microplate Reader). Spectrophotometric determinations were performed at 540 nm. All experiments were done in triplicate.
The viability of cells has been determined by the equation:
| % cell viability = (As/Ac) × 100, |
where Ac = cellular line control, and As = the cellular line with various extracts. Significant statistical differences were considered p < 0.05.
Light Microscopy
The NCTC culture cell was fixed in methanol and then Giemsa dye-marked. Cells were observed 72 h after the addition of extracts. The images were taken with a Zeiss AxioStar Plus microscope. Untreated cells (Mc) were used as a control, and cells cultured in the presence of curcumin (1 mM) were used as a control.
2.8. Statistical Analysis
The tests have been done in triplicate. For the statistical analysis, the software Microsoft Office Excel 2007 was used, and the standard deviation (STDV) was less than 10%. Statistical analysis was performed using Student’s t-test, and the values were considered significant when p < 0.05. The test was used to evaluate the relationship between antioxidant activity and biological compound content (polyphenols, flavonoids) as well as the relationship between enzyme inhibition activity and biological compound content.
3. Results and Discussions
3.1. Polyphenols and Flavonoid Content Determination
The content of total polyphenols was in the tested samples between 667.58–977.75 μg/mL and was similar for the two plants, slightly higher in the case of Artemisia abrotanum extracts. The highest content of polyphenols, 977.75 μg/mL, was determined in the concentrated extract of A. abrotanum. Regarding the flavonoid content, the A. abrotanum extracts showed significantly higher amounts than the S. officinale extracts. The highest flavonoid content—552.85 μg/mL was determined in the same concentrated extract of A. abrotanum. The results obtained are shown in Table 1.
Table 1.
The active biological polyphenolic compound content in the analyzed extracts.
| Sample | Polyphenols (GAEμg/mL) |
Flavonoids (RE μg/mL) |
|
|---|---|---|---|
| A. abrotanum | MF | 723.32 ± 25.32 | 403.51 ± 12.59 |
| extracts | concentrate | 977.75 ± 31.67 | 552.85 ± 15.36 |
| S. officinale | MF | 667.58 ± 17.64 | 84.53 ± 3.2 |
| extracts | concentrate | 896.95 ± 27.21 | 103.21 ± 5.16 |
Values are expressed as mean ± SD (n = 3); GAE: gallic acid equivalent; RE: rutin equivalent.
A. abrotanum extracts showed higher amounts of polyphenols and especially flavonoids than S. officinale extracts; the concentrates were enriched in polyphenols and flavonoids compared to the initial extracts (microfiltration); A. abrotanum concentrate showed the highest concentration of polyphenols and flavonoids.
More biological activities are attributed to phenolic compounds, such as antioxidant activity, antimicrobial anti-inflammatory, anticancer, and coronary protective activities [36,37]. Recently, it was found that phenolics can contribute to type 2 diabetes treatment because they play a role in amylase inhibition [38]. Numerous studies showed that antioxidant activity is related to compounds, such as polyphenols, flavonoids, isoflavonoids, flavonoids, catechins, and vitamins (ascorbic acid, α-tocopherol, β-carotene) [39].
3.2. HPLC Analysis
The HPLC-PDA-MS method was applied to the identification of polyphenolic compounds analytes by retention time method, SCAN mode of MS, and UV spectra, and the results obtained regarding the concentrations of the compound from the tested plant extracts are given in Table 2 and Figure 1. Good linearity of the method with the studied concentration range (0.1–50 µg/mL) was observed.
Table 2.
The polyphenolic compound content found in the plant extracts by HPLC.
| Compound | A. abrotanum Polyphenolic Compounds-Rich Extract, µg/mL | S. officinale Polyphenolic Compounds-Rich Extract, μg/mL | ||
|---|---|---|---|---|
| MF | Conc. | MF | Conc. | |
| Rutin | 9.39 ± 0.23 | 10.57 ± 0.6 | - | 1.06 ± 0.06 |
| Luteolin | 1.90 ± 0.09 | 4.35 ± 0.08 | - | 1.69 ± 0.05 |
| Quercetin | 3.17 ± 0.02 | 5.36 ± 0.27 | 1.87 ± 0.04 | 3.42 ± 0.07 |
| Quercetin 3-β-D-glucoside | 0.29 ± 0.01 | 1.07 ± 0.06 | - | - |
| Kaempferol | - | 1.33 ± 0.08 | 1.96 ± 0.07 | 6.79 ± 0.11 |
| Gallic acid | - | 2.83 ± 0.06 | - | - |
| Caffeic acid | 0.58 ± 0.02 | 1.43 ± 0.05 | 7.73 ± 0.23 | 9.42 ± 0.24 |
| Apigenin | 1.95 ± 0.09 | 3.84 ±0.16 | - | 0.71 ± 0.02 |
| Umbelliferone | 9.59 ± 0.03 | 17.03 ± 0.92 | 3.68 ± 0.12 | 4.28 ± 0.13 |
| Chlorogenic acid | 85.49 ± 2.12 | 103.47 ± 7.21 | 1.45 ± 0.08 | 2.30 ± 0.05 |
| Ellagic acid | - | - | 37.41 ± 1.23 | 43.79 ± 1.89 |
| Rosmarinic acid | - | - | 120.83 ± 1.25 | 136.14 ± 5.16 |
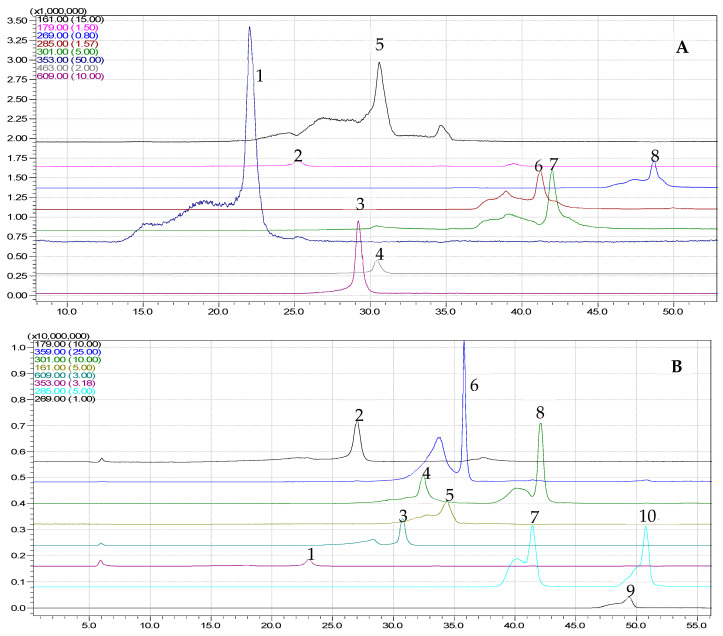
Figure 1.
Chromatograms obtained for A. abrotanum polyphenolic compound-rich extract (A) and S. officinale polyphenolic compound-rich extract (B) by HPLC-MS ((A), 1-chlorogenic acid, 2-caffeic acid, 3-rutin, 4-quercetin 3-β-D-glucoside, 5-umbelliferone, 6-luteolin, 7-quercetin, 8-apigenin peaks, respectively, (B), 1-chlorogenic acid, 2-caffeic acid, 3-rutin, 4-ellagic acid, 5-umbelliferone, 6-rosmarinic acid, 7-luteolin, 8-quercetin, 9-apigenin, and 10-kaempferol peaks).
The results obtained regarding the tested plant extracts are given in Table 2.
From Table 2, it can be observed that the polyphenolic compounds are concentrated by ultrafiltration. A total of 12 compounds were identified and quantified in both extracts. The A. abrotanum polyphenolic compound-rich extract had higher quantities of rutin (10.57 µg/mL), umbelliferone (17.03 µg/mL), and chlorogenic acid (103.47 µg/mL), while S. officinale polyphenolic compound-rich extract had higher quantities of caffeic acid (9.42 µg/mL), ellagic acid (43.79 µg/mL), and rosmarinic acid (136.140 µg/mL). As shown in other studies, chlorogenic acid and rutin have also been identified as major constituents in Artemisia ethanolic extract from other species [40,41]. Although there are few studies on the polyphenolic compounds profile of S. officinale extracts, rosmarinic and caffeic acid were also found to be major polyphenols by Sowa et al. [42], Trifan et al. [43], and the presence of caffeic acid was also reported by Nastić et al. [44].
3.3. Antioxidant Activity Determination
For the assessment of the antioxidant potential of extracts, a single assay method is not sufficient. Therefore, for antioxidant activity evaluation of the studied herbal extracts, we used two different methods, one used organic radical producers DPPH, and the other used metal ion for oxidation (Fe).
The antioxidant activity of microfiltrate and polyphenolic compound-rich extract from both plants was evaluated using two assays, reducing power and DPPH. The results obtained are shown in Table 3.
Table 3.
Antioxidant activity of extracts.
| Samples | Reducing Power Activity EC50 (mg/mL) |
DPPH Radical Scavenging Activity IC50 (µg/mL) |
|
|---|---|---|---|
| A. abrotanum | MF | 521.21 ± 4.8 * | 15.33 ± 1.23 * |
| extracts | concentrate | 92.14 ± 3.2 * | 9.31 ± 0.81 * |
| 1570.32 ± 6.5 * | 17.02 ± 0.89 * | ||
| S. officinale extracts | MF | 1091.13 ± 5.7 * | 13.06 ± 0.61 * |
| Ascorbic acid | concentrate | 1110.15 ± 4.8 | 11.76 ± 0.69 |
The data represent the means ± SD of triplicate samples of three independent experiments. * p < 0.05, reducing power compared with the polyphenols content from the extract. * p < 0.05, reducing power compared with the flavonoid content from the extract. * p < 0.05, radical scavenging activity compared with the polyphenols content from the extract. * p < 0.05, radical scavenging activity compared with the flavonoid content from the extract.
It can be observed from Table 3 that the A. abrotanum extract had higher antioxidant activity than S. officinale by both analysis methods. The A. abrotanum extract had a more reducing power activity, EC50 92.14 mg/mL, than the ascorbic acid, EC50 1110.15 mg/mL (used as control), while S. officinale extract had similar reducing power with the control.
Considering the DPPH radical scavenging activity, both plant extracts showed smaller activity values than ascorbic acid does, except the A. abrotanum concentrate (IC50—9.31 mg/mL) had higher activity than the control activity—IC50—11.76 μg/mL. Moreover, the highest content of polyphenols and flavonoids was obtained for A. abrotanum concentrate.
DPPH analysis is one of the best-known, accurate, and frequently employed methods for evaluating antioxidant activity. The effect of antioxidants on DPPH is thought to be due to their hydrogen-donating ability.
Reducing power is also widely used in evaluating the antioxidant activity of plant polyphenols. The reducing power is generally associated with the presence of reductants, which exert antioxidant action by breaking the free radical chains by donating a hydrogen atom.
An antioxidant is a compound that exhibits the capacity to transfer electron and/or hydrogen atoms during free radical neutralization [45,46]. The antioxidant ability of phenolic compounds depends on the number of hydroxyl groups in the ring structure and their arrangements.
A good correlation (p < 0.05) was observed between the content of polyphenols and flavonoids and the radical scavenging activity and the reducing power activity of the analyzed extracts for both studied herbs. Other studies have also indicated significant correlations between the polyphenolic compound content and antioxidant capacity, indicating that polyphenols were the main constituents in the tested medicinal plants [47]. The antioxidant properties of polyphenols and flavonoids derive from their redox properties, metal ion chelating activity, and singlet oxygen scavenging ability [48]. Therefore, the composition of the extracts could lead to a synergistic and even stronger antioxidant capacity.
Polyphenols, through their antioxidant effect, can have an important role in diseases generated by oxidative stress. This is due to the disturbance of the equilibrium between the appearance of free radicals and the body’s ability to eliminate them. The appearance of many ailments, such as diabetes mellitus, cancer, Alzheimer’s, etc., relates to these radicals that can be scavenged by antioxidants [31,49].
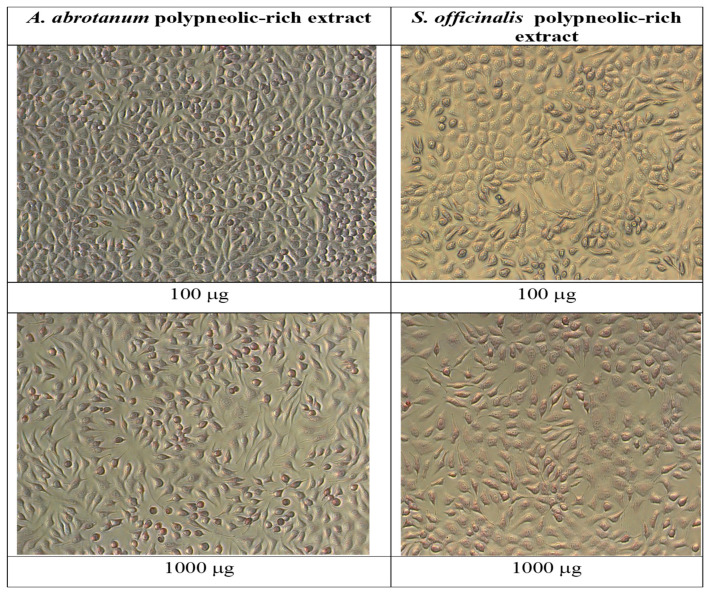
3.4. Testing the Cytotoxic Activity of the Extracts
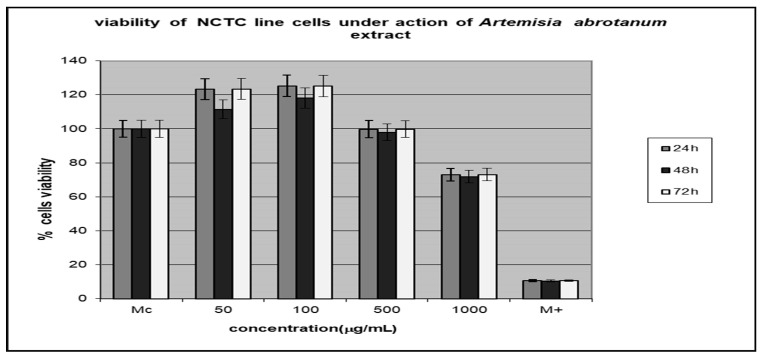
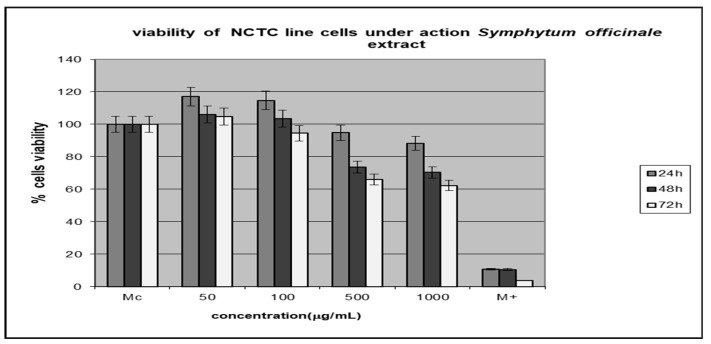
In order to increase the reliability of the obtained results and to avoid underestimating the toxicity of plant extracts, the cytotoxic effect of the A. abrotanum and S. officinale extracts were examined using NCTC cells. The extract cytotoxicity has been analyzed by testing the cellular viability in percent, and the results are presented in Figure 2 and Figure 3. The data represent the means ± SD of triplicate samples of three independent experiments.
Figure 2.
The viability of NCTC cell lines under the action of A. abrotanum extracts.
Figure 3.
The viability of NCTC cell lines under action S. officinale extracts.
The A. abrotanum polyphenolic compound-rich extract was tested in the concentration range of 50–100 µg/mL, and it showed a non-cytotoxic effect at 500 µg/mL and a little cytotoxic effect at 1000µg/mL.
The S. officinale polyphenolic compound-rich extract had a non-cytotoxic effect in the concentration range of 50–100 µg/mL and was slightly cytotoxic starting with 500 µg/mL.
The extracts’ cytotoxicity has been dependent both on the extract concentration and the time interval, and it has been higher than 40% in the case of S. officinale tested at the highest concentration and longest time of action.
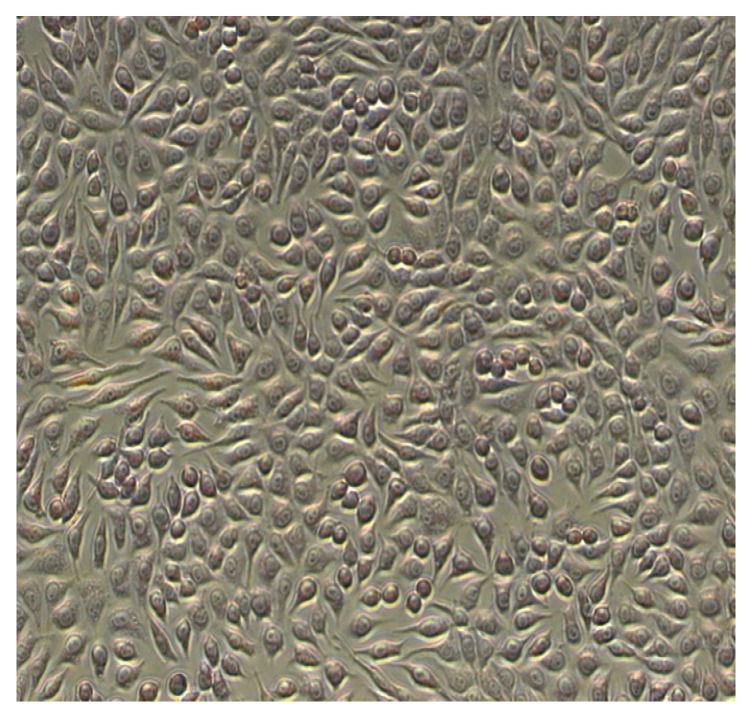
Morphological Characterization of Cell Lines
The cytotoxic effect of the studied polyphenolic compound-rich extracts in two different doses, 100 µg/mL and 1000 µg/mL, for 72h was shown in the photomicrographs.
The NCTC cell line presents fibroblast-type morphology—the cells have a spindle shape with a large spherical nucleus, fine cytoplasmic granulation, and two–three nucleoli. At 72 h, the cell line was confluent. (Figure 4).
Figure 4.
NCTC control, 72 h.
The NCTC cells exposed to A. abrotanum polyphenolic compound-rich extract were similar to the cellular line, did not present any morphological modifications, and the cellular density was comparable to the control. The culture was dense, the cells covering the whole well surface in the concentration range of 50–500 µg/mL, with a weak decrease at concentrations higher than 1000 µg/mL (Figure 5).
Figure 5.
Normal NCTC cell line.
The structure of the NCTC cells exposed to S. officinale polyphenolic compound-rich extract had morphological modifications compared to the control, which became obvious at 1000 µg/mL (Figure 4).
3.5. α-Amylase and α-Glucosidase Inhibition Activity
Recently, more and more natural sources have been investigated to find remedies to lower glucose production and absorption in the intestine [50]. An effective method of controlling diabetes is to inhibit α-amylase and α-glucosidase activities. These enzymes are involved in the starch cleavage in sucrose and maltose, with glucose release, hence decreasing so glucose absorption [51]. In this study, the potential enzyme inhibitors of two herbs from Romania—A. abrotanum and S. officinale—were investigated; these herbs have not been researched before from this point of view. Data obtained regarding the α-amylase and α-glucosidase inhibition activity by the analyzed extracts are shown in Table 4.
Table 4.
The inhibition activity of α-amylase and α-glucosidase of analyzed extracts.
| Samples | α-Amylase Inhibition IC50 (mg/mL) |
α-Glucosidase Inhibition IC50 (mg/mL) |
|
|---|---|---|---|
| A. abrotanum extracts | MF | 2110.12 ± 9.2 * | 1450.32 ± 2.7 * |
| concentrate | 1881.21 ± 1.8 * | 1171.16 ± 6.5 * | |
| S. officinale extracts | MF | 28,270.35 ± 16.7 * | 413.02 ± 4.2 * |
| concentrate | 24,812.02 ± 12.9 * | 291.56 ± 2.1 * | |
| acarbose | 1110.25 ± 8.82 | 372.35 ± 3.2 | |
| quercetin | 351.25 ± 3.1 | 72.52 ± 1.5 | |
| chlorogenic acid | 192.31 ± 2.3 | 51.21 ± 1.8 | |
| rosmarinic acid | 95.63 ± 2.5 | 20.31 ± 0.9 | |
| kaempferol | 401.32 ± 3.9 | 62.36 ± 2.3 |
* p < 0.05 the α-amylase inhibition activity compared with polyphenols and flavonoid contents. * p < 0.05 the α-glucosidase inhibition activity compared with polyphenols and flavonoid contents.
The A. abrotanum extracts have stronger α-amylase inhibitory activity than the S. officinale extracts, particularly the concentrated extract—IC50 = 1881.21 ± 1.8 mg/mL and close to the value of acarbose used as control. Regarding the α-glucosidase inhibitory activity, both extracts have high activity, close to acarbose activity (IC50 = 372.35 ± 3.2 mg/mL) used as control, namely IC50 = 291.56 ± 2.1 mg/mL for S. officinale extract and IC50 = 1171.16 ± 6.5 mg/mL for A. abrotanum extract, respectively. The concentrated extracts had higher activities than the extracts obtained by microfiltration. Moreover, a good correlation was noticed between the polyphenols and flavonoid content and the α-amylase and α-glucosidase inhibitory activity of both tested herbs. In conclusion, the tested extracts had a strong α-glucosidase inhibitory activity and a weaker α-amylase inhibitory activity in agreement with the literature data [51,52].
The previous studies had assigned the high α-amylase and α-glucosidase inhibitory effect of the extracts to the high content of polyphenols and flavonoids [53].
Chlorogenic acid, present in high quantities in A. abrotanum and in many other plants, has antioxidant, antidiabetic, and other pharmacological activities. Some previous studies showed hypoglycemic and hypolipidemic effects of chlorogenic acid and rutin, specifically in their possible application in the prevention and treatment of diabetes mellitus [53,54,55].
Rosmarinic acid, the main compound in S. officinalis, is a potent antioxidant compound. Rosmarinic acid-rich extract from herbal plants has shown significant anti-diabetic effects in diabetes-induced animal models in vivo and insulin-like effects in insulin target cells in vitro. Recent studies indicated that rosmarinic acid acts through various mechanisms, such as inhibition of α-amylase and α-glucosidase, suppression of insulin-resistant HepG2 cells through activation of AMPK phosphorylation, as well as acting as an antioxidant [56].
The flavonoids reduce the oxidative stress at the level of beta cells from pancreatic, thus reducing the risk of type 2 diabetes apparition [57,58]. Flavonoids, such as rutin, found in A. abrotanum extracts, quercetin, and kaempferol, found in both types of extracts, apigenin, and myricetin, have potential protective properties against cardiovascular disease and diabetes, and quercetin is a good α-amylase inhibitor [59]. Umbelliferone, found in A. abrotanum extracts, belongs to the coumarin family, a compound known to have strong analgesic, antirheumatic, and antipyretic properties [60]. Umbelliferone was isolated for the first time from a banana flower ethanolic extract (Musa sp.) and inhibited a-glucosidase—IC50: 7.08 ± 0.17 μg/mL (IC50 of acarbose: 9.68 ± 0.48 μg/mL), inhibited α-amylase enzyme—IC50: 32.26 ± 1.80 μg/mL (IC50 of acarbose: 29.71 ± 1.51 μg/mL), and showed radical scavenging activity—EC50_DPPH: 21.70 ± 0.37μg/mL (EC50 _BHA: 40.43 ± 0.81 μg/mL [61].
Studies showed that phenolic compounds present activity inhibitory of α-glucosidase, so more studies regarding α-amylase and α-glucosidase inhibitors have focused on the use of phenolic compounds [3,11,62].
The chlorogenic acid found in high amounts in A. abrotanum extracts, together with ellagic and rosmarinic acids found in high amounts in S. officinale extracts, showed high inhibitory activity of both enzymes, which is in agreement with the literature data [63,64,65]. The obtained experimental results recommend the two tested medicinal plants—A. abrotanum and S. officinale –as an approach to reducing post-prandial hyperglycemia.
4. Conclusions
The present study analyzed the chemical profile of Artemisia abrotanum and Symphytum officinale polyphenolic compounds-rich extracts obtained by membrane technologies (ultrafiltration) and their α-amylase and α-glucosidase inhibitory activity. This is the first scientific study regarding the effect of these plants in the inhibition of these enzymes.
According to the results of the present study, Artemisia abrotanum polyphenolic compounds-rich extracts showed high inhibitory activity of α-amylase and α-glucosidase, while Symphytum officinale polyphenolic compounds-rich extracts showed high α-glucosidase inhibitory activity and moderate α-amylase activity.
The obtained results showed the existence of a positive correlation between the amount of polyphenols and the antioxidant activity of the extracts, with the extracts enriched in polyphenols presenting the highest antioxidant activity through both tests used (DPPH and reducing power).
Our results regarding the correlation between chemical composition and anti-diabetic and antioxidant activities of the polyphenolic compound-rich extracts open opportunities for future research to develop nutraceuticals that possess both anti-diabetic and antioxidant activity and could be used in type 2 diabetes.
Author Contributions
E.N. and G.P. obtained, processed, and analyzed the extracts; G.P. conducted research; C.A. was implicated in the determination of phenolic compounds level by HPLC; S.A.-M.V.E. contributed to the manuscript language preparation; G.L.R. and G.P. made the final drafting work. All the authors have read and approved the final manuscript. The writing was realized by E.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Ministry of Research, Innovation, and Digitization: the Core Program (project 7N/23-02-0101/2023), and the project PFE 2/2021.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Patel D.K., Prasad S.K., Kumar R., Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012;2:320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irudayaraj S.S., Stalin A., Sunil C., Duraipandiyan V., Al-Dhabi N.A., Ignacimuthu S. Antioxidant, antilipidemic and antidiabetic effects of ficusin with their effects on GLUT4 translocation and PPARγ expression in type 2 diabetic rats. Chem. Biol. Interact. 2016;256:85–93. doi: 10.1016/j.cbi.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari M.R., Jong-Anurakkun N., Hong G., Kawabata J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.) Food Chem. 2008;106:247–252. doi: 10.1016/j.foodchem.2007.05.077. [DOI] [Google Scholar]
- 4.Ighodaro O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018;108:656–662. doi: 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 5.Yeung S., Soliternik J., Mazzola N. Nutritional supplements for the prevention of diabetes mellitus and its complications. J. Nutr. Intermed. Metab. 2018;14:16–21. doi: 10.1016/j.jnim.2018.07.003. [DOI] [Google Scholar]
- 6.Ghadge A.A., Kuvalekar A.A. Controversy of oral hypoglycemic agents in type 2 diabetes mellitus: Novel move towards combination therapies. Diabetes Metab. Syndr. 2017;11:S5–S13. doi: 10.1016/j.dsx.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M., Matthews D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson G., Hall G.M. Diabetes mellitus: New drugs for a new epidemic. Br. J. Anaesth. 2011;107:65–73. doi: 10.1093/bja/aer120. [DOI] [PubMed] [Google Scholar]
- 9.Saadi T., Waterman M., Yassin H., Baruch Y. Metformin-induced mixed hepatocellu-lar and cholestatic hepatic injury: Case report and literature review. Int. J. Gen. Med. 2013;6:703–706. doi: 10.2147/IJGM.S49657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar A., Tiwari A., Bhasin P.S., Mitra M. Pharmacological and pharmaceutical pro-file of gliclazide: A review. J. Appl. Pharm. Sci. 2011;1:11–19. [Google Scholar]
- 11.Kim K.-T., Rioux L.-E., Turgeon S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry. 2014;98:27–33. doi: 10.1016/j.phytochem.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Souza P.M., Sales P.M., Simeoni L.A., Silva E.C., Silveira D., de Oliveira Magalhães P. Inhibitory activity of α-amylase and α-glucosidase by plant extracts from the Brazilian cerrado. Planta Med. 2012;78:393–399. doi: 10.1055/s-0031-1280404. [DOI] [PubMed] [Google Scholar]
- 13.Rhabasa-Lhoret J.L., Chiasson R. Alpha-glucosidase inhibitors. In: De-fronzo R.A., Ferrannini E., Keen H., Zimmet P., editors. International Textbook of Diabetes Mellitus. 3rd ed. Volume 1 John Wiley; New York, NY, USA: 2004. [Google Scholar]
- 14.Gao H., Huang Y.N., Gao B., Xu P.Y., Inagaki C., Kawabata J. α-Glucosidase inhibitory effect by the flower buds of Tussilago farfara L. Food Chem. 2008;106:1195–1201. doi: 10.1016/j.foodchem.2007.07.064. [DOI] [Google Scholar]
- 15.Taleghani A., Emami S.A., Tayarani-Najaran Z. Artemisia: A promising plant for the treatment of cancer. Bioorganic Med. Chem. 2020;28:115180. doi: 10.1016/j.bmc.2019.115180. [DOI] [PubMed] [Google Scholar]
- 16.Suresh J., Vasav R.A., Dhanya R., Ihsanullah M., Nayeemmullah M.K. Antimicrobial Activity of Artemisia abrotanum and Artemisia pallens. Int. J. Pharm. Phytochem. Res. 2010;3:18–21. [Google Scholar]
- 17.Trifan A., Skalicka-Woźniak K., Granica S., Czerwińska M.E., Kruk A., Marcourt L., Wolfender J.-L., Wolfram E., Esslinger N., Grubelnik A., et al. Symphytum officinale L.: Liquid-liquid chromatography isolation of caffeic acid oligomers and evaluation of their influence on pro-inflammatory cytokine release in LPS-stimulated neutrophils. J. Ethnopharmacol. 2020;262:113169. doi: 10.1016/j.jep.2020.113169. [DOI] [PubMed] [Google Scholar]
- 18.Frost R., O’Meara S., MacPherson H. The external use of comfrey: A practitioner survey. Complement. Ther. Clin. Pract. 2014;20:347–355. doi: 10.1016/j.ctcp.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Grube B., Grunwald J., Krug L., Staiger C. Efficacy of a comfrey root (Symphytioffic. radix) extract ointment in the treatment of patients with painful osteoarthritis of the knee: Results of a double-blind, randomised, bicenter, placebo-controlled trial. Phytomedicine. 2007;14:2–10. doi: 10.1016/j.phymed.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Public Statement on the Use of Herbal Medicinal Products Containing Toxic, Unsaturated Pyrrolizidine Alkaloids (PAs) HMPC; Valetta, Malta: 2014. [Google Scholar]
- 21.Oberlies N.H., Kim N.C., Brine D.R., Collins B.J., Handy R.W., Sparacino C.M., Wani M.C., Wall M.E. Analysis of herbal teas made from the leaves of comfrey (Symphytum officinale): Reduction of N-oxides results in order of magnitude increases in the measurable concentration of pyrrolizidine alkaloids. Public Health Nutr. 2004;7:919–924. doi: 10.1079/PHN2004624. [DOI] [PubMed] [Google Scholar]
- 22.Gerke I.B.B., Hamerski F., Scheer A.P., Silvab V.R. Clarification of crude extract of yerba mate (Ilex paraguariensis) by membrane processes: Analysis of fouling and loss of bioactive compounds. Food Bioprod. Process. 2017;102:204–212. doi: 10.1016/j.fbp.2016.12.008. [DOI] [Google Scholar]
- 23.Habert A.C., Borges C.P., Nobrega R. Processos de Separacão por Membranas. 1st ed. E-papers; Rio de Janeiro, Brazil: 2006. [Google Scholar]
- 24.Díaz-Reinoso B. Chapter 14—Concentration and purification of seaweed extracts using membrane technologies. In: Maria Dolores Torres M.D., Kraan S., Herminia Dominguez H., editors. Sustainable Seaweed Technologies, Cultivation, Biorefinery, and Applications. 1st ed. Elsevier; Oxford, UK: 2020. pp. 371–390. (Advances in Green and Sustainable Chemistry series). [Google Scholar]
- 25.Dushkova M., Mihalev K., Dinchev A., Vasilev K., Georgiev D., Terziyska M. Concentration of polyphenolic antioxidants in apple juice and extract using ultrafiltra-tion. Membranes. 2022;12:1032. doi: 10.3390/membranes12111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paun G., Neagu E., Moroeanu V., Albu C., Savin S., Radu G.L. Chemical and bioactivity evaluation of eryngium planum and cnicus benedictus polyphenolic-rich extracts. BioMed Res. Int. 2019;2019:3692605. doi: 10.1155/2019/3692605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uyttebroek M., Vandezande P., Van Dael M., Vloemans S., Noten B., Bongers B., Lemmens B. Concentration of phenolic compounds from apple pomace extracts by nanofiltration at lab and pilot scale with a technoeconomic assessment. J. Food. Process. Eng. 2018;41:e12629. doi: 10.1111/jfpe.12629. [DOI] [Google Scholar]
- 28.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Methods Enzymology. Volume 299. Academic Press; Cambridge, MA, USA: 1999. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent; pp. 152–178. [Google Scholar]
- 29.Lin J.-Y., Tang C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101:140–147. doi: 10.1016/j.foodchem.2006.01.014. [DOI] [Google Scholar]
- 30.Alecu A., Albu C., Litescu S.C., Eremia S.A.V., Radu G.L. Phenolic Anthocyanin Profile of Valea Calugareasca Red Wines by HPLC-PDA-MS, MALDI-TOF. Analysis. Food Analyt. Meth. 2016;9:300–310. doi: 10.1007/s12161-015-0197-4. [DOI] [Google Scholar]
- 31.Bondet V., Brand-Williams W., Berset C. Kinetics and mechanism of antioxidant activity using the DPPH free radical method. Leb. Wiss Technol. 1997;30:609–615. doi: 10.1006/fstl.1997.0240. [DOI] [Google Scholar]
- 32.Berker K., Guclu K., Tor I., Apak R. Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP) and ferricyanide reagents. Talanta. 2007;72:1157–1165. doi: 10.1016/j.talanta.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Ranilla L.G., Kwon Y.I., Apostolidis E., Shetty K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010;101:4676–4689. doi: 10.1016/j.biortech.2010.01.093. [DOI] [PubMed] [Google Scholar]
- 34.Queiroz D.P., Ferreira A.G., Lima A.S., Lima E.S., Lima M.D. Isolation and identification of α-glucosidase, α-amylase and lipase inhibitors from hortia longifolia. Int. J. Pharm. Pharm. Sci. 2013;5:336–339. [Google Scholar]
- 35.Borenfreund E., Puerner J.A. A simple quantitative procedure using monolayer cultures for cytotoxicity assays. J. Tiss Culture Meth. 1984;9:7–9. doi: 10.1007/BF01666038. [DOI] [Google Scholar]
- 36.Liu R. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 37.Ali A., Cottrell J.J., Dunshea F.R. LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants. Metabolites. 2022;12:1016. doi: 10.3390/metabo12111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheplick S., Kwon Y., Bhowmik P., Shetty K. Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresource Technol. 2010;101:404–413. doi: 10.1016/j.biortech.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 39.Oboh G., Rocha J.B.T. Antioxidant in Foods: A New Challenge for Food Processors: Leading Edge Antioxidants Research. Nova Science Publishers Inc.; New York, NY, USA: 2007. [Google Scholar]
- 40.Minda D., Ghiulai R., Banciu C.D., Pavel I.Z., Danciu C., Racoviceanu R., Soica C., Budu O.D., Muntean D., Diaconeasa Z., et al. Phytochemical Pro-file, Antioxidant and Wound Healing Potential of Three Artemisia Species: In Vitro and In Ovo Evaluation. Appl. Sci. 2022;12:1359. doi: 10.3390/app12031359. [DOI] [Google Scholar]
- 41.Baiceanu E., Vlase L., Baiceanu A., Nanes M., Rusu D., Crisan G. New polyphenols identified in Artemisiae abrotani herba extract. Molecules. 2015;20:11063–11075. doi: 10.3390/molecules200611063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sowa I., Paduch R., Strzemski M., Zielińska S., Rydzik-Strzemska E., Sawicki J., Kocjan R., Polkowski J., Matkowski A., Latalski M., et al. Proliferative and antioxidant activity of Symphytum officinale root extract. Nat. Prod. Res. 2018;32:605–609. doi: 10.1080/14786419.2017.1326492. [DOI] [PubMed] [Google Scholar]
- 43.Trifan A., Zengin G., Sinan K.I., Wolfram E., Skalicka-Woźniak K., Luca S.V. LC-HRMS/MS phytochemical profiling of symphytum officinale L. and Anchusa ochroleuca M. bieb. (boraginaceae): Unveiling their multi-biological potential via an integrated approach. J. Pharm. Biomed. Anal. 2021;204:114283. doi: 10.1016/j.jpba.2021.114283. [DOI] [PubMed] [Google Scholar]
- 44.Nastić N., Borrás-Linares I., Lozano-Sánchez J., Švarc-Gajić J., Segura-Carretero A. Comparative Assessment of Phytochemical Profiles of Comfrey (Symphytum officinale L.) Root Extracts Obtained by Different Extraction Techniques. Molecules. 2020;25:837. doi: 10.3390/molecules25040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brewer M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011;10:221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- 46.Olszowy M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019;144:135–143. doi: 10.1016/j.plaphy.2019.09.039. [DOI] [PubMed] [Google Scholar]
- 47.Siddharthan S., Yi-Zhong C., Harold C., Mei S. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007;102:938–953. [Google Scholar]
- 48.Rice-Evans C.A., Miller N.J., Paganga G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 49.Zengina G., Sarikurkcub A., Aktumseka R., Ceylana O. Ceylan, A comprehensive study on phytochemical characterization of Haplophyl lummyrti folium Boiss. endemic to Turkey C, and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crop Prod. 2014;53:244–251. doi: 10.1016/j.indcrop.2013.12.043. [DOI] [Google Scholar]
- 50.Matsui T., Tanaka T., Tamura S., Toshima A., Miyata Y., Tamaya K., Miyata Y., Tanaka K., Matsumoto K. Alpha-glucosidase inhibitory profile of catechins and theafla-vins. J. Agric. Food Chem. 2007;55:99–105. doi: 10.1021/jf0627672. [DOI] [PubMed] [Google Scholar]
- 51.Shang Q., Xiang J.F., Tang Y.L. Screening α-glucosidase inhibitors from mulberry extracts via DOSY and relaxation-edited NMR. Talanta. 2012;97:362–367. doi: 10.1016/j.talanta.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 52.Rammohan S., Asmaw M.Z.B., Amirin S. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008;55:391–398. [PubMed] [Google Scholar]
- 53.Zuñiga L.Y., Aceves-de Aceves-de la Mora M.C., González-Ortiz M., Ramos-Núñez J.L., Martínez-Abundis E. Effect of chlorogenic acid administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance. J. Med. Food. 2018;21:469–473. doi: 10.1089/jmf.2017.0110. [DOI] [PubMed] [Google Scholar]
- 54.Jin S., Chang C., Zhang L., Liu Y., Huang X., Chen Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE. 2015;10:e120842. doi: 10.1371/journal.pone.0120842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunyadi A., Martins A., Hsieh T.J., Seres A., Zupkó I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE. 2012;7:e50619. doi: 10.1371/journal.pone.0050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ngo Y.L., Lau C.H., Chua L.S. Review on rosmarinic acid extraction, fractiona-tion and its anti-diabetic potential. Food Chem. Toxicol. 2018;121:687–700. doi: 10.1016/j.fct.2018.09.064. [DOI] [PubMed] [Google Scholar]
- 57.Oboh G., Akinyemi A.J., Ademiluyi A.O., Bello F.O. Inhibition of α-amylase and α-glucosidase activities by ethanolic extract of Amaranthus cruentus leaf as affected by blanching. Afr. J. Pharm. Pharmacol. 2013;7:1026–1032. doi: 10.5897/AJPP12.595. [DOI] [Google Scholar]
- 58.Ademiluyi A.O., Oboh G., Aragbaiye F.P., Oyeleye S.I., Ogunsuyi O.B. Antioxidant properties and in vitro a-amylase and a-glucosidase inhibitory properties of phenolics constituents from different varieties of Cor-chorus spp. J. Taibah Univ. Med. Sci. 2015;10:278–287. [Google Scholar]
- 59.Song Y., Manson J.E., Buring J.E., Sesso H.D., Liu S. Association of dietary flavo-noids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflam-mation in women: A prospective study and cross sectional analysis. J. Am. Coll. Nutr. 2005;24:376–384. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- 60.Molnar V., Garai J. Plant-derived anti-inflammatory compounds affect MIF tautomer-ase activity. Intern Immunopharmacol. 2005;5:849–856. doi: 10.1016/j.intimp.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 61.Shodehinde S.A., Oboh G. Antioxidant properties of aqueous extracts of unripe (Musa paradisiaca) on sodium nitroprusside induced lipid peroxidation in rat pancreas in vitro. Asian Pac. J. Trop. Biomed. 2013;3:449–457. doi: 10.1016/S2221-1691(13)60095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramu R., Shirahatti P., Zameer F., Ranganatha L.V., Nagendra M., Prasad N. Inhibitory effect of banana (Musa sp. var. Nanjangud rasa bale) flower extract and its constitu-ents Umbelliferone and Lupeol on α-glucosidase, aldose reductase and glycation at multi-ple stages. S. Afr. J. Bot. 2014;95:54–63. doi: 10.1016/j.sajb.2014.08.001. [DOI] [Google Scholar]
- 63.Zhang J.Z., Tiller C., Shen J.K., Wang C., Girouard G.S., Dennis D., Barrow C.J., Miao M.S., Ewart H.S. Antidiabetic properties of polysaccharide and polyphenolic en-riched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007;85:1116–1123. doi: 10.1139/Y07-105. [DOI] [PubMed] [Google Scholar]
- 64.Gülçin İ. Antioxidant activity of food constituents: An overview. Arch. Toxikol. 2012;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 65.Sales P.M., Souza P.M., Simeoni L.A., Silveira D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012;5:141–183. doi: 10.18433/J35S3K. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.