Abstract
Simple Summary
Regional differences in infection rates of Babesia parasites affecting dogs in Taiwan were correlated with the presence of the local tick population. A positive correlation was found between pathogenic Babesia gibsoni infections in dogs and the tick Haemaphysalis hystricis in northern Taiwan. A second, less pathogenic species, Babesia vogeli, was more equally distributed, coinciding with the occurrence of Rhipicephalus sanguineus, a tick that is present throughout Taiwan. These findings provide the basis for advice for owners regarding outdoor activities with their dogs and local veterinarians with a regional diagnosis of babesiosis in Taiwan.
Abstract
The objective of our study was to survey Babesia infection rates by PCR and tick species on stray dogs to correlate the distribution of Babesia with the distribution of ticks infesting dogs in Taiwan. Three hundred eighty-eight blood samples and 3037 ticks were collected from 388 roaming, and free-ranging owned dogs at residential sites in Taiwan between January 2015 and December 2017. The prevalence of B. gibsoni and B. vogeli was 15.7% (61/388) and 9.5% (37/388), respectively. Most positive B. gibsoni dogs were found in the northern part of the country 56/61 (91.8%), whereas a few were found in the middle 5/61 (8.2%). Babesia vogeli infection rates were 10%, 3.6%, and 18.2% in the northern, central, and southern regions, respectively. Five species of ticks were found: Rhipicephalus sanguineus (throughout Taiwan), Rhipicephalus haemaphysaloides (in the north), Haemaphysalis hystricis (in the north and middle of Taiwan), and Amblyomma testidunarium and Ixodes ovatus (both in the north). None of the dogs in the south were infected with B gibsoni, which correlated with the absence of H. hystricis, a tick recently identified as the local vector for B gibsoni. Babesia vogeli was more equally distributed, coinciding with R. sanguineus, a tick that is present throughout Taiwan. Anaemia was detected in 86.9% of infected dogs; among these dogs, approximately 19.7% showed severe anaemia (HCT < 20). These findings provide useful advice for owners regarding outdoor activities with their dogs and local veterinarians with a regional differential diagnosis of babesiosis in Taiwan.
Keywords: Babesia, dogs, ticks, Rhipicephalus sanguineus, Haemaphysalis hystricis, Taiwan
1. Introduction
Ticks are hematophagous arthropods that parasitize vertebrates worldwide, including livestock, wildlife, and humans. Ticks play their vector roles in the transmission of a variety of pathogens. The ongoing geographic expansion of tick species, possibly driven by climatic and environmental changes, has drawn global attention [1]. However, mapping ticks and tick-borne diseases in companion animals requires further studies. For instance, babesiosis is one of the most important tick-borne infections of dogs with a worldwide distribution. Babesia gibsoni is the predominant tick-borne protozoan blood parasite throughout the oriental region, causing life-threatening hemolytic anaemia [2,3,4,5]. In a global distribution survey of Babesia species, Eastern Asia (Japan and South Korea) and Southeastern Asia (Singapore) showed a higher positive rate than other continents, especially B. gibsoni [6]. A B. gibsoni molecular detecting rate of 6.3% was also reported in stray dogs from Thailand [7]. Taiwan is in a subtropical region with a warm and humid climate all year round. Ticks are common ectoparasites on dogs. Therefore, tick-borne diseases in dogs have always been an important clinical topic. Canine babesiosis, including B. gibsoni and B. vogeli has been reported in dogs in Taiwan, but information on the epidemiology of the disease is limited. Several studies have focused on the prevalence of Babesia species in dogs in Taiwan. Chou et al. [8] collected 360 stray dogs from shelters in southern Yun-Chia-Nan and showed that the infection rate of Babesia species was 8.1%. Yang et al. [9] tested 265 dogs from 51 animal hospitals, and 28 dogs (10.6%) were positive for Babesia species, including B. gibsoni (26/28) and B. vogeli (2/28). Clinically, B. gibsoni cause more severe clinical symptoms and haematological abnormalities than B. vogeli in Taiwan.
Concerning ticks, the Asian longhorned tick, Haemaphysalis longicornis, is the primary vector tick in Asia for Babesia gibsoni. However, direct transmission from dog to dog during fighting and biting or blood transfusion has also been reported [10]. H. longicornis has been confirmed as an important vector of B. gibsoni in Japan, Korea, and China [11,12,13,14,15]. However, the tick has not been found until now in Taiwan. Earlier studies have reported that B. gibsoni was detected by molecular methods in different stages of the tick Rhipicephalus sanguineus [16]. Recently, molecular evidence for the transovarial passage of B. gibsoni has been reported in Haemaphysalis hystricis ticks from Taiwan [17]. Rhipicephalus sanguineus is the most common tick found on dogs in Taiwan [18], which was confirmed to play a major role in B. vogeli transmission [19]. Other species in relatively small numbers have also been reported on dogs in northern Taiwan, including Rhipicephalus haemaphysaloides, Haemaphysalis hystricis, Ixodes ovatus, Haemaphysalis formosensis, and Haemaphysalis lagrangei [18].
Canine babesiosis has attracted considerable attention due to the social impact of companion animal diseases. Several drugs and drug combinations have been described for treating acute canine babesiosis, e.g., B. gibsoni; however, they cannot eliminate the parasite pathogen leading to an asymptomatic carrier that may relapse and transmit the infection, even producing a drug-resistant strain [4]. Therefore, a correct diagnosis before treatment is crucial. According to previous studies [5,20], B. gibsoni-infected dogs had various clinical signs, such as fever, vomiting, diarrhoea, pale mucous membranes, splenomegaly, and icterus. The most common hematologic abnormalities were anaemia, thrombocytopenia, hyperglobulinemia, and hyperbilirubinemia [20]. In contrast, B. vogeli-naturally infected dogs generally showed asymptomatic or mild clinical signs [21,22], and a similar finding was also reported in Wang et al. [23]. The clinicopathological abnormalities were mild, except in young dogs and in adults/old dogs with predisposing factors such as splenectomy or immunocompromised condition, which can cause fever, anorexia, malaise, weight loss, regenerative anaemia, thrombocytopenia, and decreased white blood cell counts [21,22,23]. Two of three experimentally splenectomized dogs developed severe life-threatening infections [23].
Since most clinical B. gibsoni-infected cases occur in northern Taiwan, and veterinarians in the south rarely see infected dogs, we set out to investigate why. A greater understanding of which Babesia species are detected in their region may help practitioners choose appropriate testing and treatment. This study aimed to correlate the prevalence of canine Babesia species with the local geographical distribution of ticks infesting dogs and provide complete blood counts in B. gibsoni -infected stray dogs in Taiwan.
2. Material and Methods
2.1. Animal and Tick Collections
During neutering procedures, surplus blood samples and ticks were collected from 388 stray dogs at various residential sites in Taiwan between January 2015 and December 2017. The prevalence of B. gibsoni and B. vogeli was determined by PCR, whereas all ticks were identified under a stereomicroscope. All residential sites of dogs were recorded. Complete blood cell counts were determined by using an automated blood analyzer (Exigo veterinary haematological system, Boulevard Medical AB, Spånga, Sweden). A blood smear was performed after blood collection and stained by Liu’s stain (Baso, Team Medical-Tech Co., Ltd., New Taipei City, Taiwan). Platelet and differential white blood cell counts were confirmed microscopically. Direct observation of Babesia spp. on the blood smear was also performed microscopically. The severity of anaemia was classified and defined as mild (30% ≤ hematocrit (HCT) < 37%), moderate (20% ≤ HCT < 30%), severe (13% ≤ HCT < 20%), or very severe (HCT < 13%).
2.2. Multiplex-Nested PCR Amplification of the B. gibsoni 18S rRNA Gene
DNA of each dog blood sample was extracted with a genomic DNA minikit (Geneaid Biotech, Taiwan) according to the manufacturer’s instructions and stored at −20 °C until processing. All samples were tested with the PCR method published by Lin et al. [4].
The first round of PCR mixture consisted of 3 mL of 10 × Taq buffer, 0.5 mL of each primer (5′-CTACCACATCTAAGGAAGGC-3′ and 5′-TGCTTTCGCAGTAGTTCGTC-3′), 1 mL dNTPs (2.5 mM), 0.5 mL Taq DNA polymerase (GeneTeks BioScience, Inc., Taipei), and 22.5 mL of 0.1% water. First-round amplification was done as follows: three minutes of preheating at 94 °C, 35 cycles of denaturation at 94 °C for 20 s, annealing at 63 °C for 20 s and extension at 72 °C for 35 s, and the final extension at 72 °C for 5 min. The multiplex-nested PCR was performed on the first PCR product by using nested primers (5′-TGCTTTCGCAGTAGTTCGTC-3′ (Babesia. spp.), 5′-GTTGAATTTCTGCGTTGCCC-3′ (B. gibsoni), and 5′-AGTTGCCATTCGTTTGG-3′ (B. vogeli)). The second-round PCR amplification was performed as follows: three minutes of preheating at 94 °C, 35 cycles of denaturation at 94 °C for 20 s, annealing at 63 °C for 20 s, extension at 72 °C for 20 s, and a final extension at 72 °C for 5 min.
The first-round PCR yielded the expected products for Babesia spp., which was 490 bp in length. The second-round PCR yielded the expected products for B. vogeli and B. gibsoni, 249 and 268 bp in length. Positive and negative controls were added in each amplification step.
2.3. Phylogenetic Analysis of Babesia gibsoni from Seven Samples with Different Anaemic Severity
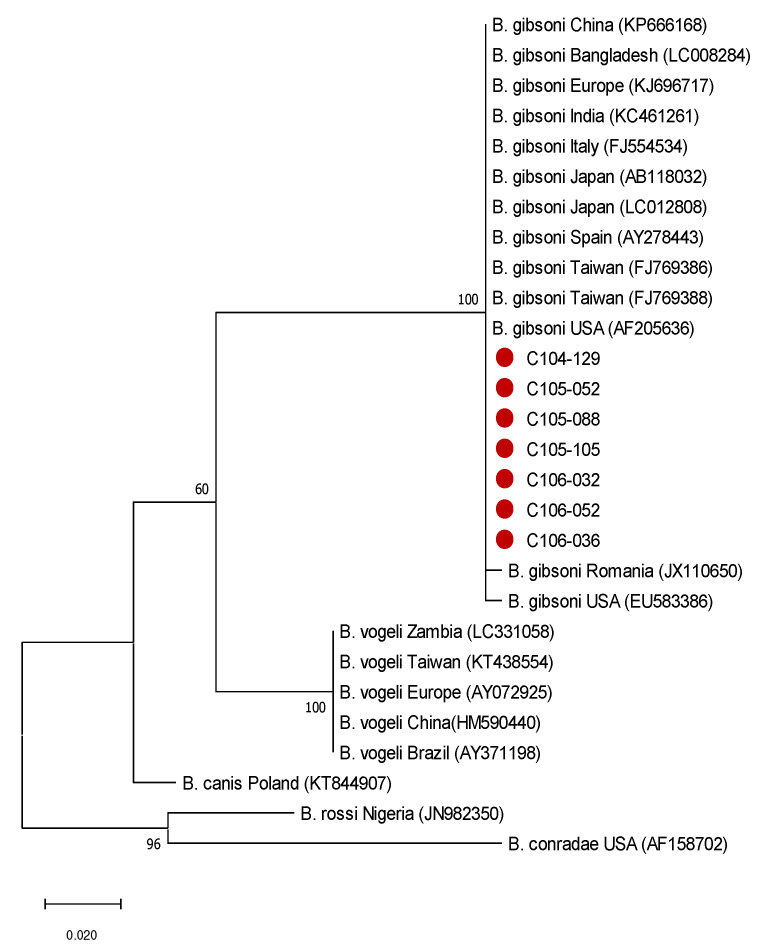
To analyze possible genetic fragments’ associated virulence, a total of seven samples with different anaemic severity were selected from B. gibsoni positive samples for sequencing, including two samples without anaemia (both from Taipei), one sample with mild anaemia (from Yilan), two samples with moderate anaemia (one sample from Taipei and the other from Nantou), and two severe anaemia samples (one from Nantou and the other from Taoyuan). The seven B. gibsoni-positive samples of 18s rRNA fragments were PCR-amplified by using primers 455-479F and 793-772R, described previously in Birkenheuer et al. [24]. The nucleotide sequences of target fragments were determined by using an ABI 3730XL genetic analyzer from GENOMICS (New Taipei City, Taiwan). The partial genomic sequence of seven 18srRNA of B. gibsoni was aligned with the other 21 18srRNA of Babesia spp. sequences available in the GenBank database, i.e., B. gibsoni: KP666168 (China), LC008284 (Bangladesh), KJ696717 (Europe), KC461261 (India), FJ554534 (Italy), AB118032 (Japan), LC012808 (Japan), AY278443 (Spain), FJ769386 (Taiwan), FJ769388 (Taiwan), AF205636 (USA), EU583386 (USA); B. vogeli: LC331058 (Zambia), KT438554 (Taiwan), AY072925 (Europe), HM590440 (China), AY371198 (Brazil); Babesia canis: KT844907 (Poland); Babesia rossi JN982350 (Nigeria); Babesia conradae AF158702 (USA). A phylogenetic analysis of partial genome sequences was performed by using the maximum-likelihood method in MEGA 10.0 software [25]. The Kimura two-parameter model analysed the genetic distance values of inter- and intraspecies variations of the Babesia species [26].
2.4. Statistical Analysis
Data obtained were analyzed by using IBM SPSS statistic version 26 (IBM Corp, Armonk, NY, USA). The results of categorical variables are expressed as percentages and presented as tables and, after that, subjected to Person’s chi-square analysis. The odds and relative risk ratios at a 95% confidence interval were used to assess the risk factors, and values of p < 0.05 were considered significant.
3. Results
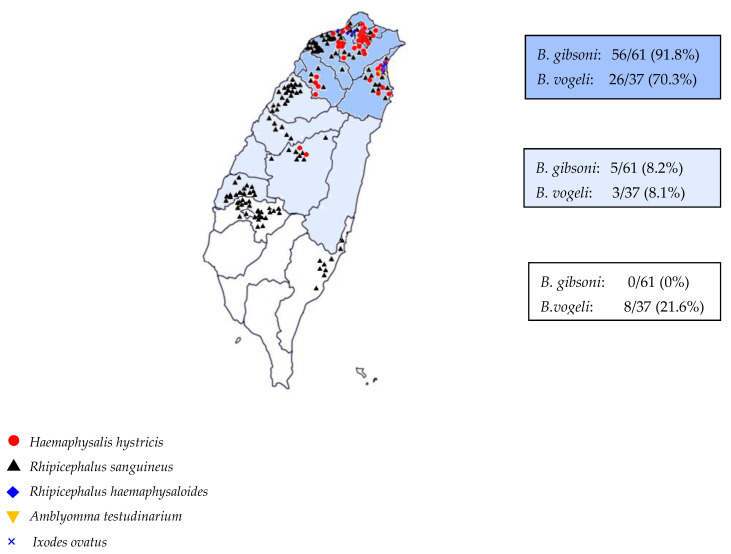
A total of 3037 ticks (2742 adults, 293 nymphs and two larvae) were collected from 388 dogs at different residential locations in Taiwan. An average of 7.8 ticks was distributed over 261 dogs in North Taiwan, 83 in the middle and 44 in the south. Five species of ticks were found: Rhipicephalus sanguineus (2576/3037, 84.8%), Rhipicephalus haemaphysaloides (19/3037, 0.6%), Haemaphysalis hystricis (409/3037, 13.5%), Amblyomma testudinarium (15/3037, 0.5%), and Ixodes ovatus (18/3037, 0.6%). R. sanguineus was the predominant species on 307 of 388 dogs (79.1%), distributed throughout Taiwan. H. hystricis (56/388, 14.4%) was mainly distributed north of Hsinchu, east to Yilan Luodong, a small part in Puli in the central part, but not found in the south of the country. R. haemaphysaloides was found in the northern and central Miaoli regions. I. ovatus was only found in Taipei, and A. testudinarium on dogs in Taipei and Yilan. Sixteen dogs (4.1%) were coinfested with two species of ticks, and six dogs (1.5%) were coinfested with three different species of ticks. Seventy-seven dogs (19.8%) were infested with H. hystricis ticks: 96.1% (74 dogs) were from the north, whereas only 3.9% (3 dogs) were from the middle of Taiwan (Table 1). R. sanguineus was located on flat land at low altitudes (less than 500 m). The temperatures during collection were between 17.2 and 28.5 °C, whereas H. hystricis was mostly found in low-to-middle-altitude mountainous areas, with a temperature of 19.9 to 25.1 °C. I. ovatus was located from near sea level land to mountainous areas with an altitude of 500 to 600 m, A. testudinarium was found in areas from near sea level land to 100 m above sea level, with an average temperature of 22.1 °C during collection.
Table 1.
The distribution of tick species collected from 388 dogs in northern, middle, and southern regions of Taiwan.
| Total | R. sanguineus a | H. hystricis b | R. haemaphysaloides c | I. ovatus d |
R. sanguineus a + H. hystricis b |
R. sanguineus a + H. hystricis b + A. testudinarium e |
H. hystrici b + A. testudinarium e |
R. sanguineus a + R. haemaphysaloides c |
R. sanguineus a + H. hystricis b + I. ovatus d |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Northern | 261 | 185 | 53 | 0 | 1 | 12 | 5 | 3 | 1 | 1 |
| (70.9%) | (20.3%) | (0%) | (0.4%) | (4.6%) | (1.9%) | (1.1%) | (0.4%) | (0.4%) | ||
| Central | 83 | 78 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| (94%) | (3.6%) | (1.2%) | (0%) | (0%) | (0%) | (0%) | (1.2%) | (0%) | ||
| Southern | 44 | 44 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (100%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) |
a Rhipicephalus sanguineus; b Haemaphysalis hystricis; c Rhipicephalus haemaphysaloides; d I. ovatus; e Amblyomma testudinarium.
The prevalence of B. gibsoni and B. vogeli infection in dogs was 15.7% (61/388) and 9.5% (37/388), respectively. The regional infection rate in Taiwan’s northern, middle and southern parts were 21.6, 6 and 0%, respectively. Most positive B. gibsoni dogs were found in the country’s north 56/61 (91.8%), whereas a few were found in the middle 5/61 (8.2%). None of the dogs in the south was infected with B gibsoni. Babesia vogeli infection rates were 10, 3.6, and 18.2% in northern, middle, and southern regions, respectively. The infection of B. gibsoni in north Taiwan was significantly higher than in the middle and south (p < 0.001). Among the 61 dogs that were B. gibsoni PCR positive, only 21 (21/61, 34.4%) had B. gibsoni parasites in blood smears; only 2 (2/37, 5.4%) samples had B. vogeli parasites in blood smears among the 37 B. vogeli PCR positive dogs.
B. gibsoni infection was positively correlated with H. hystricis, the relative risk was 6.042 and negatively correlated with R. sanguineus (Table 2). Finally, the correlation between Babesia species infecting dogs and the distribution of vector ticks in Taiwan is shown in Figure 1.
Table 2.
(a) The infection rate of Babesia spp. in dogs infested with H. hystricis and other tick species. (b) The infection rate of Babesia spp. in dogs infested with R. sanguineus and other tick species.
| (a) | |||||
|---|---|---|---|---|---|
| Dogs Infested with Tick Species | p-Value | Relative Risk (95% CI) |
Odds Ratio (95% CI) |
||
| H. hystricis | Others | ||||
| B. gibsoni | <0.001 | 6.042 (3.93–9.28) |
12.23 (6.2–24.15) |
||
| + | 27 | 22 | |||
| − | 29 | 289 | |||
| B. vogeli | 0.326 | ||||
| + | 3 | 31 | |||
| − | 53 | 270 | |||
| (b) | |||||
| Dogs Infested with Tick Species | p-Value |
Relative Risk
(95% CI) |
Odds Ratio
(95% CI) |
||
| R. sanguineus | Others | ||||
| B. gibsoni | <0.001 | 0.456 (0.33–0.64) |
0.076 (0.04–0.15) |
||
| + | 21 | 30 | |||
| − | 287 | 31 | |||
| B. vogeli | 0.481 | ||||
| + | 31 | 4 | |||
| − | 276 | 57 | |||
Figure 1.
Distribution of tick species and the prevalence of Babesia spp. in the northern, middle and southern regions of Taiwan.
The severities of anaemia for 388 dogs were 40.2% (156/388), 37.9% (68/388), 17.5% (68/388) and 4.4% (17/388) for nonanaemia, mild, moderate, and severe, respectively. None of the dogs had very severe anaemia (HCT < 13%). Of the 61 Babesia gibsoni-positive dogs, the average of hematocrit was 28.7 ± 9.1 (95% CI, 26.3–31.0), and the severities of anaemia were 13.1 (8/61), 29.5 (18/61), 37.7 (23/61), and 19.7% (12/61) for nonanaemia, mild, moderate, and severe, respectively. A total of 86.9% of infected dogs were affected by anaemia; among these dogs, approximately 19.7% showed severe anaemia (HCT < 20) (Table 3). None of the B. gibsoni-infected dogs were coinfected with other pathogens. Of the 37 B. vogeli-positive dogs, the average of hematocrit was 34.64 ± 5.5 (95% CI, 20.7–47.7). Five of them were coinfected with other pathogens (tested by PCR methods, three with Anaplasma platys; one with Ehrlichia canis; and one with Candidatus Mycoplasma haematoparvum). Excluding the five coinfected dogs, the average hematocrit was 35.29 ± 5.13 (95% CI, 24.4–47.7), and the severities of anaemia were 37.5% (12/32), 46.9% (15/32), and 15.6% (5/32) for nonanaemia, mild, and moderate, respectively.
Table 3.
Hematological variables recorded in 61 B. gibsoni infected dogs.
| Item | Total | Nonanemia | Mild | Moderate | Severe | Reference | |
|---|---|---|---|---|---|---|---|
| Number of dogs | 61 | 8 (13.1%) | 18 (29.5%) | 23 (37.7%) | 12 (19.7%) | ||
| Red blood cell count | M/μL | 4.62 ± 1.28 | 6.67 ± 0.85 | 5.20 ± 0.56 | 4.02 ± 0.58 | 2.62 ± 0.40 | 5.5–8.5 |
| Hematocrit | % | 28.7 ± 9.1 | 44.6 ± 7.4 | 33.4 ± 2.2 | 25.2 ± 2.9 | 17.14 ± 2.15 | 37–55 |
| Hemoglobin | g/dL | 10.38 ± 2.80 | 14.66 ± 2.15 | 11.81 ± 1.24 | 9.00 ± 1.15 | 6.13 ± 0.80 | 12.0–18.0 |
| Mean corpuscular volume | fL | 62.9 ± 3.94 | 64.3 ± 4.38 | 62.2 ± 2.07 | 62.5 ± 4.44 | 65.87 ± 5.84 | 60–72 |
| Mean corpuscular hemoglobin | Pg | 22.6 ± 1.38 | 21.9 ± 1.68 | 22.6 ± 0.94 | 22.6 ± 1.57 | 23.62 ± 1.52 | 19.5–25.5 |
| Mean corpuscular hemoglobin concentration | g/dL | 35.9 ± 1.51 | 34.2 ± 1.07 | 36.4 ± 1.41 | 36.0 ± 1.50 | 35.82 ± 1.34 | 32–38.5 |
| Red cell distribution width | % | 17.4 ± 1.67 | 17.7 ± 2.36 | 18.3 ± 5.35 | 17.5 ± 2.00 | 17.94 ± 1.39 | 12.0–17.5 |
| White blood cell count | /μL | 13,150 ± 5434 | 16,847 ± 6765 | 12,394 ± 5016 | 12,939 ± 4986.9 | 12,000 ± 4889 | 6000–17,000 |
| Neutrophil | /μL | 8967 ± 4878 | 11,292 ± 6525 | 8384 ± 4385 | 8869 ± 4636 | 8581 ± 4167 | 2950–11,640 |
| Lymphocyte | /μL | 2277 ± 1307 | 2809 ± 2705 | 2027 ± 893 | 2236 ± 993 | 1208 ± 790 | 1050–5100 |
| Monocyte | /μL | 1435 ± 955 | 2379 ± 1350 | 1539 ± 945 | 1191 ± 631 | 851 ± 562 | 160–1120 |
| Eosinophil | /μL | 399 ± 538 | 373 ± 465 | 424 ± 603 | 473 ± 582 | 132 ± 179 | 60–1230 |
| Platelets | K/μL | 169 ± 94 | 158 ± 68.0 | 185 ± 102.3 | 195 ± 106 | 155 ± 225 | 200–500 |
Phylogenetic tree analysis is shown in Figure 2. The sequence of the seven samples showed 99–100% similarity by comparing with 13 B. gibsoni strains from the NCBI gene bank, 89% to B. canis, 89% to B. conradae, 86% to B. vogeli, and 86% to B. rossi.
Figure 2.
Phylogenetic tree of B. gibsoni. Phylogenetic relationships based on the 18S rRNA gene of Babesia isolates were constructed by the maximum likelihood method based on the Kimura 2-parameter model with 1000 bootstrapping replicates to determine every internal node. The red circles represent the Babesia gibsoni strains in this study.
4. Discussion
Babesia spp. were surveyed in 2012 from 360 stray dogs in the Yun-Chia-Nan area (southern Taiwan), with a positivity rate of 8.1%. However, the species of Babesia was not determined [8]. In 2022, Yang et al. [9] reported an increasing positive rate of 10.6%, of which 92.8% was B. gibsoni. In our study, the infection rate of Babesia spp. was generally higher, indicating that infection rates significantly increased over time. The reason may be that the sample group included stray dogs, which are more exposed to ticks, or the ongoing geographic expansion of tick species may increase the infection rate of tick-borne pathogens. In recent years, carrying domestic dogs for outdoor activities has also been increasing in Taiwan; the higher chance of natural exposure to ticks, the higher the infection rate of babesiosis.
Over 90% of positive B. gibsoni dogs were found in northern Taiwan, whereas only a few were found in the middle part, and none were in the south. Compared with the distribution of H. hystricis, it can be seen that most of the H. hystricis are distributed in the north, and only a small number is found in the central Nantou. None of the H. hystricis was found on dogs in the southern area. H. hystrics is a three-host tick that feeds on many mammalian species, including rodents, dogs, cattle, buffaloes, porcupines, birds, and even humans [27]. In the first survey of hard ticks infesting dogs in northern Taiwan, R. sanguineus (92.5%) was the dominant species, followed by H. hystricis (4.6%), R. haemaphysaloides (2.3%), I. ovatus (0.54%), H. lagrangei (0.04%), and H. formosensis (0.01%) [18]. In our study, we also found Rhipicephalus sanguineus (84.8%), Rhipicephalus haemaphysaloides (0.6%), Haemaphysalis hystricis (13.5%), and Ixodes ovatus (0.6%) (Table 1). The area where H. hystricis was collected in our study was not only in northern Taiwan, but also a small part in the middle of Taiwan, consistent with an earlier record in Puli [28]. Moreover, Amblyomma testudinarium was first found on dogs. The areas where H. hystricis are found is usually a slightly higher latitude and a lower temperature, which is more suitable for this tick species.
None of the dogs in the south of Taiwan was infected with B. gibsoni, which correlated with the absence of H. hystricis. In our study, B. gibsoni infection was positively associated with H. hystricis, with a relative risk was 6.042 (Table 2). This correlation also indirectly confirms that H. hystricis is the local vector of B. gibsoni in Taiwan and supports the earlier molecular evidence for the transovarial passage of B. gibsoni in H. hystricis ticks [17].
The infection rate of Babesia vogeli varied according to the region of Taiwan (Figure 1). The vector tick, R. sanguineus is an indoor tick (endophilic), and all stages prefer the same species of a host (monotropic) and a three-host tick. It prefers a warm and humid environment and parasitizes dogs [29]. R. sanguineus is the most common tick species infecting dogs in Taiwan and is distributed throughout Taiwan, and is a proven vector for B. vogeli in Taiwan [30,31]. R. sanguineus has been collected from dogs in Taipei, Taichung, Nantou, Kaohsiung, Taitung, and Hualien. The results of R. sanguineus distribution in this study were like those previously reported. The distribution of ticks was also consistent with the prevalence of B. vogeli. Interestingly, B. gibsoni has also been detected in R. sanguineus ticks by PCR [16]. Therefore, R. sanguineus is considered a vector of B. gibsoni in other world regions. However, since all B. gibsoni-positive specimens of adult and nymphal R. sanguineus ticks were collected from pet clinics in various districts of Taipei city in northern Taiwan, the tick may have fed on infected dog blood, explaining the positive PCR result.
Anaemia and thrombocytopenia were the primary haematological abnormalities in the Babesia gibsoni-infected dogs, consistent with previous reports [5,20]. The average hematocrit was 28.7%. About 86.9% of the dogs had anaemia, similar to Liu’s [20] survey of 60 client-owned dogs. However, the severe anaemia in this study was only approximately 20%, much less than in Liu’s study. The possible reason is that all the dogs in this study are stray dogs. If they are very anaemic (PCV < 13), they may die in the wild or have been rescued for further therapy. Our samples were collected during the neutering procedure, and the dogs showed no clinical signs (e.g., anorexia, lethargy, or inactivity) at that time. Mild anaemia was also found in B. vogeli-infected dogs in our study. The average hematocrit of B. vogeli-positive dogs was 34.64%, similar to a report about 33 naturally infected dogs in Thailand (the median of hematocrit was 35%) [22]. Even though some dogs were anaemic, there were no reported deaths at the time of surgery and within a few days after surgery.
The phylogenetic analysis of the gene fragment in this study was 100% similar to Taiwan-reported strains in the past. No new pathogenic strain was found in the study. Furthermore, there was no difference between all samples with different severities of anaemia. It is likely that the gene fragments were not associated with virulence.
Finally, this first report of B. gibsoni infection in stray dogs in Taiwan is limited by the relatively small number of dogs collected in the south, which may have caused some deviations in the detection rate of pathogens. Therefore, additional dogs are being monitored for this purpose.
5. Conclusions
More than 90% of B. gibsoni positive dogs were found in northern Taiwan, the hotspot of H. hystricis. The geographical prevalence of canine Babesia species was consistent with the distribution of the local vector ticks. The results provide helpful travel advice for dog owners and assist veterinarians with the differential diagnosis of canine babesiosis in Taiwan.
Author Contributions
Conceptualization: B.-L.S. and J.-C.F.; methodology: P.-C.L.; formal analysis: P.-C.L.; writing—original draft preparation: B.-L.S.; writing—review and editing: F.J.; resources: B.-L.S.; supervision: B.-L.S. All authors critically revised the manuscript for intellectual content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the samples were not collected for this study but were surplus samples obtained during neutering activities.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included in the current paper.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors did not receive any support, funding or grant from any organization to prepare and submit this manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sonenshine D.E. Range expansion of tick disease vectors in North America: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health. 2018;15:478. doi: 10.3390/ijerph15030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkenheuer A.J., Levy M.G., Breitschwerdt E.B. Efficacy of combined atovaquone and azithromycin for therapy of chronic Babesia gibsoni (Asian genotype) infections in dogs. J. Vet. Intern. Med. 2004;18:494–498. doi: 10.1111/j.1939-1676.2004.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 3.Jefferies R., Ryan U., Jardine J., Broughton D., Robertson I., Irwin P. Blood, bull terriers and babesiosis: Further evidence for direct transmission of Babesia gibsoni in dogs. Aust. Vet. J. 2007;85:459–463. doi: 10.1111/j.1751-0813.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 4.Lin E.C., Chueh L.L., Lin C.N., Hsieh L.E., Su B.L. The therapeutic efficacy of two anti-babesial strategies against Babesia gibsoni. Vet. Parasitol. 2012;186:159–164. doi: 10.1016/j.vetpar.2011.11.073. [DOI] [PubMed] [Google Scholar]
- 5.Liu P.C., Su B.L. Causes of canine anemia in Taiwan: A five-year retrospective survey. Taiwan Vet. J. 2015;41:31–37. doi: 10.1142/S1682648515500031. [DOI] [Google Scholar]
- 6.Birkenheuer A.J., Buch J., Beall M.J., Braff J., Chandrashekar R. Global distribution of canine Babesia species identified by a commercial diagnostic laboratory. Vet. Parasitol. Reg. Stud. Rep. 2022;22:100471. doi: 10.1016/j.vprsr.2020.100471. [DOI] [PubMed] [Google Scholar]
- 7.Do T., Ngasaman R., Saechan V., Pitaksakulrat O., Liu M., Xuan X., Inpankaew T. First Molecular Detection of Babesia gibsoni in Stray Dogs from Thailand. Pathogens. 2021;10:639. doi: 10.3390/pathogens10060639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou S.J., Wu J.T., Liao P.J., Huang H.C., Wang K.T., Chang H.Y., Hsieh Y.C., Lee C.C., Wang J.H., Chan K.W. Epidemiological Survey of Tick-borne Disease of Stray Dogs in Yun-Chia-Nan Areas in Taiwan. Taiwan Vet. J. 2012;38:276–282. [Google Scholar]
- 9.Yang W.Y., Reynolds C., Mestek A., Huang G.C., Lee C.J., Wang S.L. A molecular and serological survey in Taiwan to determine the true risk of babesiosis in dogs not receiving regular tick prevention. Vet. Parasitol. Reg. Stud. Rep. 2022;27:100670. doi: 10.1016/j.vprsr.2021.100670. [DOI] [PubMed] [Google Scholar]
- 10.Birkenheuer A.J., Levy M.G., Stebbins M., Poore M., Breitschwerdt E. Serosurvey of anti-Babesia antibodies in stray dogs and American pit bull terriers and American staffordshire terriers from North Carolina. J. Am. Anim. Hosp. Assoc. 2003;39:551–557. doi: 10.5326/0390551. [DOI] [PubMed] [Google Scholar]
- 11.Ikadai H., Tanaka H., Shibahara N., Matsuu A., Uechi M., Itoh N., Oshiro S., Kudo N., Igarashi I., Oyamada T. Molecular evidence of infections with Babesia gibsoni parasites in Japan and evaluation of the diagnostic potential of a loop-mediated isothermal amplification method. J. Clin. Microbiol. 2004;42:2465–2469. doi: 10.1128/JCM.42.6.2465-2469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyama T., Sakata Y., Shimada Y., Ogino S., Watanabe M., Itamoto K., Okuda M., Verdida R.A., Xuan X., Inokuma H. Epidemiological survey of Babesia gibsoni infection in dogs in eastern Japan. J. Vet. Med. Sci. 2005;67:467–471. doi: 10.1292/jvms.67.467. [DOI] [PubMed] [Google Scholar]
- 13.Yao D.W., Jiang J.Y., Yu Z.Z., Yao D.Q., Yang D.J., Zhao Y.B. Canine Babesiosis in China caused by Babesia gibsoni: A molecular approach. Iran. J. Parasitol. 2014;9:163–168. [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M.J., Yu D.H., Yoon J.S., Li Y.H., Lee J.H., Chae J.S., Park J. Epidemiologic and clinical surveys in dogs infected with Babesia gibsoni in South Korea. Vector Borne Zoonotic Diseases. 2009;9:681–686. doi: 10.1089/vbz.2008.0116. [DOI] [PubMed] [Google Scholar]
- 15.Iwakami S., Ichikawa Y., Inokuma H. Molecular survey of Babesia gibsoni using Haemaphysalis longicornis collected from dogs and cats in Japan. J. Vet. Med. Sci. 2014;76:1313–1316. doi: 10.1292/jvms.14-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao L.L., Liao H.T., Ho T.Y., Shih C.M. First detection and molecular identification of Babesia gibsoni from Rhipicephalus sanguineus ticks. Acta Trop. 2017;166:356–362. doi: 10.1016/j.actatropica.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Jongejan F., Su B.L., Yang H.J., Berger L., Bevers J., Liu P.C., Fang J.C., Cheng Y.W., Kraakman C., Plaxton N. Molecular evidence for the transovarial passage of Babesia gibsoni in Haemaphysalis hystricis (Acari: Ixodidae) 9cks from Taiwan: A novel vector for canine babesiosis. Parasites Vectors. 2018;11:134–142. doi: 10.1186/s13071-018-2722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao L.L., Hsieh C.K., Ho T.Y., Shih C.M. First zootiological survey of hard ticks (Acari: Ixodidae) infesting dogs in northern Taiwan. Exp. Appl. Acarol. 2019;77:105–115. doi: 10.1007/s10493-018-0328-x. [DOI] [PubMed] [Google Scholar]
- 19.Zahler M., Schein E., Rinder H., Gothe R. Characteristic genotypes discriminate between Babesia canis isolates of differing vector specigicity and pathogenicity to dogs. Parasitol. Res. 1998;84:544–548. doi: 10.1007/s004360050445. [DOI] [PubMed] [Google Scholar]
- 20.Liu P.C., Lin C.N., Su B.L. Clinical characteristics of naturally Babesia gibsoni infected dogs: A study of 60 dogs. Vet. Parasitol. Reg. Stud. Rep. 2022;28:100675. doi: 10.1016/j.vprsr.2021.100675. [DOI] [PubMed] [Google Scholar]
- 21.Salano-Gallego L., Trotta M., Carli E., Carcy B., Caldin M., Furlanello T. Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. Vet. Paratitol. 2008;157:211–221. doi: 10.1016/j.vetpar.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Rawangchue T., Sungpradit S. Clinicopathological and molecular profiles of Babesia vogeli infection and Ehrlichia canis coinfection. Vet. World. 2020;13:1294–1302. doi: 10.14202/vetworld.2020.1294-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Zhang J., Kelly P., Zheng X., Li M., You J., Huang K., Qiu H., Wang Y., Zhang R., et al. first description of pathogenicity of Babesia vogeli in experimentally infected dogs. Vet. Parasitol. 2018;253:1–7. doi: 10.1016/j.vetpar.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Birkenheuer A.J., Levy M.G., Breitschwerdt E.B. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian Genotype) and Babesia canis DNA in canine blood samples. J. Clin. Microbiol. 2003;41:4172–4177. doi: 10.1128/JCM.41.9.4172-4177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura K., Stecher G., Peterson D., Flipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura M. A simple method for estimating evolutionary rate base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguti N., Tipton V.J., Keegan H.L., Toshioka S. Ticks of Japan, Korea, and the Ryuku Islands. Brigham Young Univ. Sci. Bull. 1971;15:77–83. [Google Scholar]
- 28.Yuasa Y., Tsai Y.L., Chang C.C., Hsu T.H., Chou C.C. The prevalence of Anaplasma platys and a potential novel Anaplasma species exceed that of Ehrlichia canis in asymptomatic dogs and Rhipicephalus sanguineus in Taiwan. J. Vet. Med. Sci. 2017;17:2–24. doi: 10.1292/jvms.17-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasites Vectors. 2010;3:1–11. doi: 10.1186/1756-3305-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao L.L., Shih C.M. Molecular analysis of Rhipicephalus sanguineus (Acari: Ixodidae), an incriminated vector tick for Babesia vogeli in Taiwan. Exp. Appl. Acarol. 2016;70:469–481. doi: 10.1007/s10493-016-0094-6. [DOI] [PubMed] [Google Scholar]
- 31.Chao L.L., Yeh S.T., Hsieh C.K., Shih C.M. First detection and molecular identification of Babesia vogeli from Rhipicephalus sanguineus (Acari: Ixodidae) in Taiwan. Exp. Appl. Acarol. 2016;68:539–551. doi: 10.1007/s10493-015-0010-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the current paper.