Abstract
Background:
Neuroinflammation is ubiquitous in acute stroke and worsens outcome. However, the precise timing of the inflammatory response is unknown, hindering the design of acute anti-inflammatory therapeutic interventions. We sought to identify the onset of the neuroinflammatory cascade using a mobile stroke unit (MSU).
Methods:
The study is a proof-of-concept, cohort investigation of ultra-early blood- and extracellular vesicle (EV)-derived markers of neuroinflammation and outcome in acute stroke. Blood was obtained, prehospital, on an MSU. Outcomes were biomarker concentrations, modified Rankin Scale score, and NIH Stroke Scale score.
Results:
Forty-one adults were analyzed, including 15 patients treated on the MSU between August 2021 and April 2022, and 26 healthy controls to establish biomarker reference levels. Median patient age was 74 (range 36–97) years, 60% were female, and 80% white. Ten (67%) were diagnosed as stroke, with 8 (53%) confirmed and 2 likely TIA or stroke averted by thrombolysis; 5 were stroke mimics. For strokes, median initial NIHSS score was 11 (range 4–19) and 6 (75%) received tPA. Blood was obtained a median of 58 (range 36–133) minutes after symptom onset. Within 36 minutes after stroke, plasma interleukin-6 (IL-6), neurofilament light chain (NfL), ubiquitin C-terminal hydrolase L1 (UCH-L1) and glial fibrillary acidic protein (GFAP) were elevated by as much as 10 times normal. In EVs, matrix metalloproteinase-9 (MMP-9), chemokine (C-X-C motif) ligand 4 (CXCL4), C-reactive protein (CRP), IL-6, osteopontin (OPN) and platelet and endothelial cell adhesion molecule 1 (PECAM1) were elevated. Inflammatory markers increased rapidly in the first two hours and continued rising for 24 hours.
Conclusions:
The neuroinflammatory cascade was found to be activated within 36 to 133 minutes after stroke and progresses rapidly. This is earlier than observed previously in humans and suggests injury from neuroinflammation occurs faster than had been surmised. These findings could inform development of acute immunomodulatory stroke therapies and lead to new diagnostic tools and improved outcomes.
Introduction
Stroke is a leading cause of death and disability, affecting more than 12 million individuals worldwide each year.1 Neuroinflammation is a ubiquitous sequelae, and has a bimodal effect on outcomes, inducing secondary injury early after ischemic brain insult or hemorrhage and beneficial neural protection and repair, hours or days later.2 Acute clinical stroke treatment presently focuses primarily on restoration of cerebral circulation, or mitigation of intracranial bleeding. The post-stroke inflammatory cascade remains largely untreated. Glial fibrillary astrocytic protein (GFAP), ubiquitin C-terminal hydrolase (UCH-L1),3–6 and a constellation of other cytokines, chemokines and enzymes implicated in neuroinflammation are being studied in stroke, including interleukins IL-6, IL-17, tumor necrosis factor alpha (TNFα),7, 8 the enzyme matrix metalloproteinase-9 (MMP-9), and chemokine (C-X-C motif) ligand 4 (CXCL4). Prevailing data suggest the inflammatory response occurs many hours after stroke.9 However, inflammatory factors have only been studied in the hours and days following acute stroke onset in humans, typically after the 3–4.5-hour window for stroke thrombolysis.10 We sought to help fill this gap in knowledge earlier than previously possible by obtaining blood concurrent with brain imaging and neurological exam in the field, utilizing a prehospital treatment paradigm, the mobile stroke unit (MSU). We hypothesized that the neuroinflammatory cascade begins within minutes after the onset of stroke symptoms, representing a possible target for ultra-early intervention.
Methods
The study is a prospective, proof-of-concept, cohort investigation of ultra-early blood- and extracellular vesicle (EV)-derived markers of neuroinflammation, cerebral insult and outcome in acute stroke. The research was approved by the University of Colorado (UC) IRB. Written informed consent was obtained from each study patient, or a legally authorized representative. The study included adult patients treated acutely for suspected stroke on the UC MSU, and subsequently admitted for acute care to the UC Hospital. Outcome was assessed at the time of acute hospitalization discharge. Outcome measures included the final clinical diagnosis, mRS and NIHSS scores. Blood was obtained on the MSU and 24 hours later, and plasma was analyzed with enzyme-linked and electrochemiluminescense immunoassays, and heat-shock protein isolation of vesicles. For the main statistical analyses, subjects were dichotomized into those with confirmed stroke vs. healthy adult controls. Patient biomarker levels were compared with clinical reference standards and with median and individual values for study control patients, and for grouped plasma of healthy adult controls in EV analyses. Data were analyzed with SPSS, version 28.0 (Armonk, NY, USA). This manuscript follows the STROBE reporting guideline.11 The data that support the findings of this study are available from the corresponding author upon reasonable request. Additional methods, including characteristics of controls, see Supplemental Materials)
Results
The study included 41 adult individuals. Between August 2021 and April 2022, 15 patients with acute stroke symptoms treated on the University of Colorado MSU were enrolled, and ultra-early blood samples were obtained. Median age was 74 years (range 36–97), 60% were female, and 80% white. Ten patients (67%) were diagnosed as stroke, with eight (53%) confirmed by clinical neurological examination and/or neuroimaging (Table 1, Table S1). The study included 26 healthy adults, and one pooled group of controls, for plasma and extracellular vesicle analyses.
Table 1.
Baseline demographic and clinical characteristics of patients treated on mobile stroke unit
| Patient Characteristics | All Patients (N=15) |
Confirmed Stroke (N=8) |
Stroke Mimic* (N=7) |
|---|---|---|---|
| Demographics | |||
| Age (years at stroke symptom onset) | 74 (36–97) | 67 (36–86) | 74 (40–97) |
| Sex (female) | 9 (60) | 3 (37) | 6 (86) |
| Race | |||
| White | 12 (80) | 6 (75) | 6 (86) |
| Black | 2 (13) | 1 (12) | 1 (14) |
| Hispanic | 1 (7) | 1 (12) | 0 (0) |
| Pre-morbid characteristics | |||
| Modified Rankin Scale score | |||
| 0. No symptoms | 9 (60) | 6 (75) | 3 (43) |
| 1. No significant disability | 3 (20) | 2 (25) | 1 (14) |
| 2. Slight disability | 0 (0) | 0 (0) | 0 (0) |
| 3. Moderate disability | 1 (7) | 0 (0) | 1 (14) |
| 4. Moderately severe disability | 2 (13) | 0 (0) | 2 (29) |
| 5. Severe disability | 0 (0) | 0 (0) | 0 (0) |
| Previous stroke | 4 (27) | 3 (37) | 1 (14) |
| Acute Clinical characteristics | |||
| MSU NIHSS score | 6 (1–22) | 11 (4–22) | 6 (1–7) |
| 0–4. Minor | 5 (33) | 2 (25) | 3 (43) |
| 5–15. Moderate | 8 (53) | 4 (50) | 4 (57) |
| 16–20. Moderate to severe | 0 (0) | 0 (0) | 0 (0) |
| 21–42. Severe | 2 (13) | 2 (25) | 0 (0) |
| ED NIHSS score | 5 (0–29) | 7 (1–29) | 2 (0–11) |
| 0–4. Minor | 7 (47) | 2 (25) | 5 (71) |
| 5–15. Moderate | 5 (33) | 3 (37) | 2 (29) |
| 16–20. Moderate to severe | 1 (7) | 1 (12) | 0 (0) |
| 21–42. Severe | 2 (13) | 2 (25) | 0 (0) |
| Δ NIHSS score (MSU to ED) | -1 (−4 to +4) | +0.5 (−4 to+5) | -1 (−4 to +2) |
| Improved | 8 (53) | 3(37) | 5 (71) |
| Unchanged | 1 (7) | 1(12) | 0 (0) |
| Worsened | 6 (40) | 4 (50) | 2 (29) |
| Etiology | |||
| Confirmed Acute stroke | 8 (53) | 8 (100) | N/A |
| Ischemic | 6 (40) | 6 (40) | N/A |
| Hemorrhagic | 2 (13) | 2 (13) | N/A |
| Unconfirmed stroke or mimic | 7 (47) | N/A | 7 (100) |
| TIA or stroke aborted by tPA | 2 (13) | N/A | 2 (29) |
| Seizure | 2 (13) | N/A | 2 (29) |
| Pulmonary embolism | 1 (7) | N/A | 1 (14) |
| COPD exacerbation | 1 (7) | N/A | 1 (14) |
| EtOH withdrawal | 1 (7) | N/A | 1 (14) |
| Acute Treatment | |||
| Time from symptom onset to MSU stroke treatment (mins)† | 69 (35–835) | 58 (36–133) | 405 (35–835) |
| Thrombolysis – IV tPA‡ (on MSU) | 5 (40) | 4 (50) | 1 (14) |
| Thrombolysis – IV tPA‡ (ED) | 2 (7) | 2 (25) | 0 (0) |
| Mechanical thrombectomy | 2 (13) | 2 (25) | 0 (0) |
| Hospital course | |||
| Hospital LOS (days) | 4 (3–6) | 4 (4–6) | 4 (1–6) |
Data are N (%), median (range). ED, emergency department; NIHSS, National Institutes of Health Stroke Scale; MSU, mobile stroke unit; tPA, tissue plasminogen activator; COPD, chronic obstructive pulmonary disease; EtOH, ethanol; LOS, length of stay.
Includes one patient with suspected TIA, and one patient with a possible ischemic stroke aborted by tPA.
Time of blood draw on MSU.
Alteplase
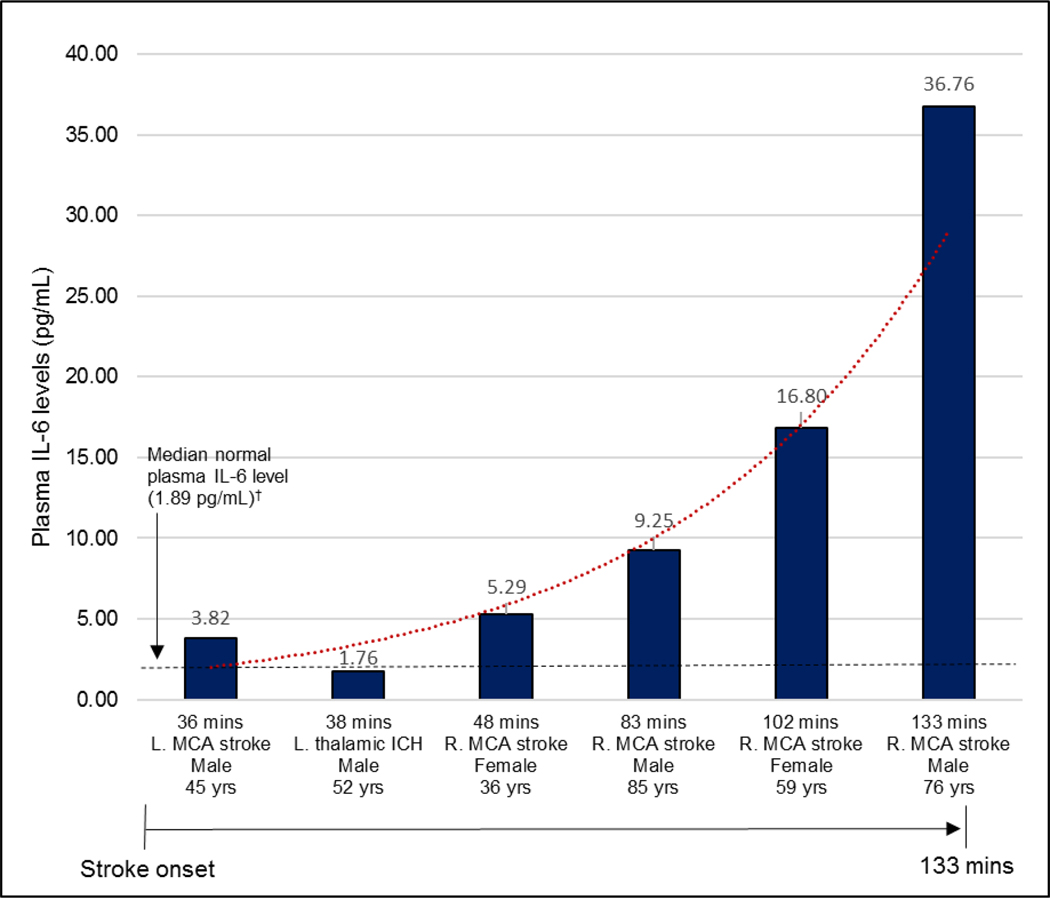
Peripheral venous blood was obtained at the patients’ homes at a median of 58 (range 36–133) minutes after stroke symptom onset for all 8 confirmed stroke cases. Blood samples were obtained prior to administration of tPA, and was drawn between 11:11 a.m and 6:43 p.m., on all days of the week except Wednesday.
Plasma Neuroinflammatory Factor Levels
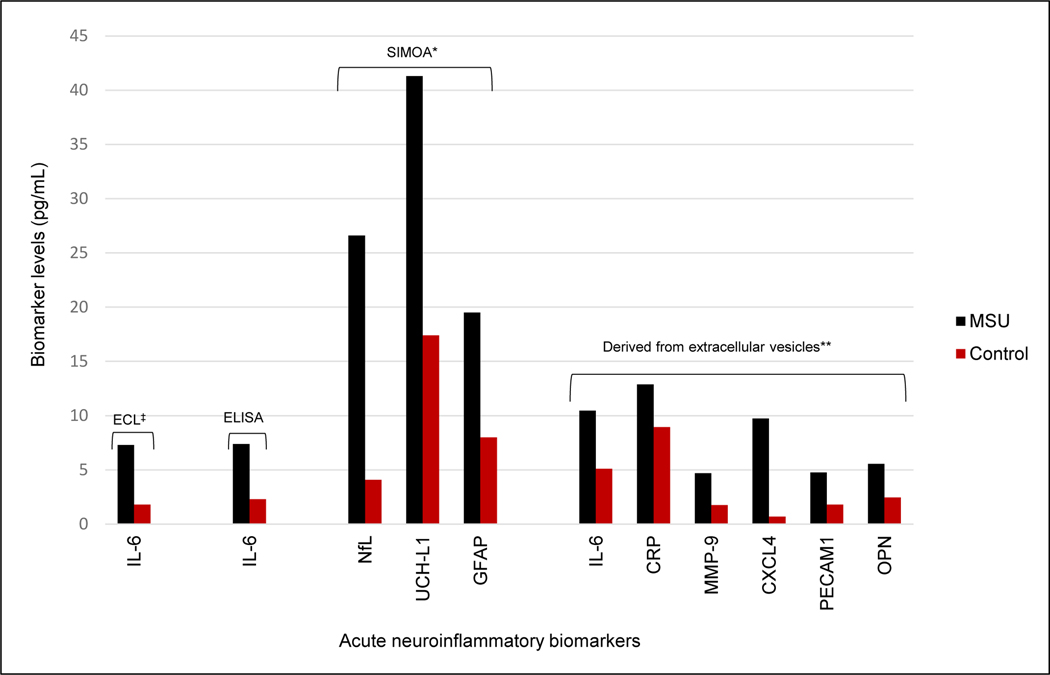
Among patients with confirmed stroke, plasma concentrations of IL-6, neurofilament light chain (NfL), UCH-L1 and GFAP were abnormally elevated above reference or healthy control levels by 36 minutes after stroke symptom onset, and in blood samples taken during the first 133 minutes after stroke (Figs. 1, 2)(Figs. S1–S3). These biomarkers were as much as 10 times normal levels (Tables S2–S6). Plasma concentrations of pro-inflammatory cytokines IL-17A, IL-1β, TNFα and interferon gamma (IFN-γ), were in the normal range during this time period (Table S3). Median plasma IL-6 concentration, measure by electrochemiluminescence, was about four times higher in confirmed stroke patients (7.27 pg/mL, range 1.76–36.76), compared with a median 1.89 pg/mL (range 0.3–5.0) for healthy adults.12, 13 Follow-up blood samples were obtained at 24 hours during acute hospitalization for six (75%) of confirmed stroke patients. Plasma IL-6 levels increased between 1.7-fold and 10.2-fold in all patients during this 24-hour time period (Fig. S1). Plasma IL-6 was also elevated when assessed with enzyme-linked immunoassay (ELISA). Plasma proteins NfL, UCH-L1 and GFAP were quantified with single molecule array (SIMOA) in 6 confirmed stroke patients [4 acute ischemic stroke (AIS), 2 intracerebral hemorrhage (ICH)], and 21 adult healthy controls. All were elevated in stroke patients compared to controls, both in the minutes after stroke onset and at 24 hours (Tables S5–S6).
Figure 1.
Ultra-early neuroinflammatory factor levels in acute stroke patients†
ECL, electrochemiluminescence immunoassay; ELISA, enzyme-linked immunosorbent assay; SIMOA, single molecule array; HSP, heat shock protein; MSU, mobile stroke unit; IL-6, interleukin 6; NfL, neurofilament light chain; UCH-L1, ubiquitin C-terminal hydrolase L1; GFAP, glial fibrillary astrocytic protein; CRP, C-reactive protein; MMP-9, matrix metalloproteinase 9; CXCL4, C-X-C motif ligand 4; PECAM-1, platelet endothelial cell adhesion molecule-1; OPN, osteopontin.
†All values are median plasma neuroinflammatory factor concentrations, for stroke patients and controls.
‡For ECL, the reference is 1.89 pg/mL (Todd J., Simpson P., Estis J., Torres V., Wub A. H. Reference range and short- and long-term biological variation of interleukin (IL)-6, IL-17A and tissue necrosis factor-alpha using high sensitivity assays. Cytokine. Dec 2013;64(3):660–665.)
*Values for SIMOA results are reduced by a factor of 10 for scale.
**For extracellular vesicle analyses, levels are expressed as arbitrary units of pixel density.
Figure 2.
Plasma IL-6 levels in first 133 minutes after stroke onset*
MCA, middle cerebral artery; ICH, intracerebral hemorrhage.
*Plasma IL-6 levels measured by electrochemiluminescence immunoassay.
†Todd J., Simpson P., Estis J., Torres V., Wub A. H. Reference range and short- and long-term biological variation of interleukin (IL)-6, IL-17A and tissue necrosis factor-alpha using high sensitivity assays. Cytokine. Dec 2013;64(3):660-665.
Extracellular Vesicle-derived Neuroinflammatory Factor Levels
Among inflammatory factors with known association with stroke, CRP, MMP-9, CXCL4, PECAM1, IL-6 and OPN were higher for confirmed stroke patients in the minutes after symptom onset, than for healthy controls, both in terms of medians, and in individual patient values in 97% of cases (Table S7, Figs. S4–S5) (Additional results, see Supplemental Materials, Fig. S6, Tables S8–S11).
Discussion
Nine inflammatory factors were fully analyzed, including 6 that reflect neurotoxicity during the hyper-acute phase of stroke (IL-6, NfL, GFAP, CRP, MMP-9, CXCL4) and 3 believed to be involved in neuroprotection later in the acute stroke course and recovery (UCH-L1, OPN, PECAM1). Blood samples for all confirmed stroke patients in this report were obtained less than 133 minutes after stroke onset. To our knowledge, this represents the earliest the neuroinflammatory cascade has been evaluated in humans following stroke. Furthermore, these results provide a unique snapshot of the neuroinflammatory response in a naturally progressing stroke, before it has been interrupted by treatment. Because inflammatory factors arise from different body systems, the study attempted to localize neuroinflammatory factors in or on EVs arising from stressed cells displaying surface heat shock proteins on EVs. This analysis found elevated levels of CRP, MMP-9, CXCL4, PECAM1, IL-6 and OPN, confirming the presence of an active inflammatory process and further refining findings from peripheral blood.
Among the cytokines evaluated in our study, IL-6 was elevated. Previous studies have reported that IL-6 is detected only four or more hours after stroke.9, 14, 15 Our results demonstrated IL-6 was elevated as early as 36 minutes after stroke. Because IL-6 is a systemic cytokine, elevated concentration at stroke onset could represent an acute infection or chronic comorbidity. However, rapidly increasing IL-6 during the hyper-acute (≤2 hours) and acute stage up to 24 hours after stroke suggests an active response to an acute event, and points to the ischemic or hemorrhagic insult. This is bolstered by the lack of significant premorbid disability in any of the stroke patients in this sample (mRS≤1). IL-6 is of particular interest in stroke as it is measurable from a peripheral blood sample at the site of initial stroke evaluation with point-of-care devices that produce a result in as little as 20 minutes.16–18 The study results demonstrated increasing inflammatory factor levels in all stroke patients for whom a second blood sample was obtained, during the first 24 hours. This change arguably demonstrates an active and increasing inflammatory response which is attributable to the acute stroke, rather than a chronic condition (Additional discussion, see Supplemental Materials).
Limitations
The primary limitation of this report is the small patient sample size and heterogeneity. Larger numbers of patients are needed for more complete characterization of the post-stroke neuroinflammatory cascade and development of diagnostic tools. The present sample size of patients enrolled is not sufficient for inferential univariate or multivariable analyses. The results are therefore reported here with descriptive comparisons for relevance to rapidly advancing developments in stroke research and clinical care. Changes in biomarkers occurring more than 24 hours after stroke were not evaluated and would be a potentially valuable component of future research. Finally, stroke onset time for the study was predicated on reports of symptom onset from patients or observers, and is subject to errors of recall.
Conclusion
The study found the inflammatory cascade is activated as early as 36 minutes after stroke, and progresses rapidly. This is earlier than observed previously in humans, and suggests injury from neuroinflammation may occur faster than had been surmised. The findings may inform development of immunomodulatory therapies for acute stroke, better describe the stroke trajectory and estimate its onset, leading to improved care and outcomes in this devastating neurological disease.
Supplementary Material
Sources of Funding
This research was sponsored by the National Institute of Neurological Disorders and Stroke (NINDS), NIH 2674-12.
Disclosures
Dr. Kowalski reports research grant funding from the National Institutes of Health (NIH), and the University of Colorado School of Medicine, Department of Neurosurgery. Dr. Deighan reports employment by NanoFCM. Dr. Monte reports research grant funding from the NIH. Dr. Grotta reportes compensation from Frazier Ltd., for consultant services. Dr. Lewis reports employment by vivitide. Dr. Neumann reports research funding from the NIH.
Nonstandard Abbreviations and Acronyms
- CRP
C-reactive protein
- CXCL4
chemokine (C-X-C motif) ligand 4
- ECL
electrochemiluminescence immunoassay
- ELISA
enzyme-linked immunoassay
- EV
Extracellular vesicle
- GFAP
glial fibrillary acidic protein
- IFN-γ
interferon gamma
- IL-6
interleukin-6
- MMP-9
matrix metalloproteinase-9
- MSU
mobile stroke unit
- NfL
neurofilament light chain
- OPN
osteopontin
- PECAM1
platelet and endothelial cell adhesion molecule 1
- SIMOA
single molecule array
- TNFα
tumor necrosis factor alpha
- UCH-L1
ubiquitin C-terminal hydrolase
References
- 1.Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, Abbasifard M, Abbasi-Kangevari M, Abd-Allah F, Abedi V, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the global burden of disease study 2019. The Lancet Neurology. 2021;20:795–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambertsen KL, Finsen B, Clausen BH. Post-stroke inflammation—target or tool for therapy? Acta Neuropathologica. 2018;137:693–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jickling GC, Sharp FR. Biomarker panels in ischemic stroke. Stroke. 2015;46:915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustamante A, Penalba A, Orset C, Azurmendi L, Llombart V, Simats A, Pecharroman E, Ventura O, Ribó M, Vivien D, et al. Blood biomarkers to differentiate ischemic and hemorrhagic strokes. Neurology. 2021;96:e1928–e1939 [DOI] [PubMed] [Google Scholar]
- 5.Luger S, Jæger HS, Dixon J, Bohmann FO, Schaefer J, Richieri SP, Larsen K, Hov MR, Bache KG, Foerch C. Diagnostic accuracy of glial fibrillary acidic protein and ubiquitin carboxy-terminal hydrolase-l1 serum concentrations for differentiating acute intracerebral hemorrhage from ischemic stroke. Neurocrit Care. 2020;33:39–48 [DOI] [PubMed] [Google Scholar]
- 6.Ren C, Kobeissy F, Alawieh A, Li N, Li N, Zibara K, Zoltewicz S, Guingab-Cagmat J, Larner SF, Ding Y, et al. Assessment of serum uch-l1 and gfap in acute stroke patients. Scientific reports. 2016;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muhammad S, Chaudhry SR, Kahlert UD, Niemelä M, Hänggi D. Brain immune interactions—novel emerging options to treat acute ischemic brain injury. Cells. 2021;10:2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, et al. Pivotal role of cerebral interleukin-17–producing γδt cells in the delayed phase of ischemic brain injury. Nature Medicine. 2009;15:946–950 [DOI] [PubMed] [Google Scholar]
- 9.Ceulemans A-G, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. The dual role of the neuroinflammatory response after ischemic stroke: Modulatory effects of hypothermia. Journal of Neuroinflammation. 2010;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagonnier M, Donnan GA, Davis SM, Dewey HM, Howells DW. Acute stroke biomarkers: Are we there yet? Front Neurol. 2021;12:619721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strobe reporting guideline.2022 [Google Scholar]
- 12.Todd J, Simpson P, Estis J, Torres V, Wub AH. Reference range and short- and long-term biological variation of interleukin (il)-6, il-17a and tissue necrosis factor-alpha using high sensitivity assays. Cytokine. 2013;64:660–665 [DOI] [PubMed] [Google Scholar]
- 13.Mayo clinic laboratories plasma interleukin-6 clinical information.2022 [Google Scholar]
- 14.Nilupul Perera M, Ma HK, Arakawa S, Howells DW, Markus R, Rowe CC, Donnan GA. Inflammation following stroke. Journal of Clinical Neuroscience. 2006;13:1–8 [DOI] [PubMed] [Google Scholar]
- 15.Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: Pathophysiology and role of inflammatory mediators. FEBS Journal. 2009;276:13–26 [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Chu D, Kalantar‐Zadeh K, George J, Young HA, Liu G. Cytokines: From clinical significance to quantification. Advanced Science. 2021;8:2004433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: A step toward the early treatment of acute intra-amniotic inflammation/infection. The Journal of Maternal-Fetal & Neonatal Medicine. 2015;29:360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer SK, Williams K, Wang L, Capio E, Briman M. Development of an il-6 point-of-care assay: Utility for real-time monitoring and management of cytokine release syndrome and sepsis. Bioanalysis. 2019;11:1777–1785 [DOI] [PubMed] [Google Scholar]
- 19.Bartell PA, Nilsonne G, Lekander M, Åkerstedt T, Axelsson J, Ingre M. Diurnal variation of circulating interleukin-6 in humans: A meta-analysis. PLoS One. 2016;11:e0165799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davey M, Benzina S, Savoie M, Breault G, Ghosh A, Ouellette RJ. Affinity captured urinary extracellular vesicles provide mrna and mirna biomarkers for improved accuracy of prostate cancer detection: A pilot study. International journal of molecular sciences. 2020;21:8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths S, Cormier M, Clayton A, Doucette A. Differential proteome analysis of extracellular vesicles from breast cancer cell lines by chaperone affinity enrichment. Proteomes. 2017;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths SG, Ezrin A, Jackson E, Dewey L, Doucette AA. A robust strategy for proteomic identification of biomarkers of invasive phenotype complexed with extracellular heat shock proteins. Cell Stress and Chaperones. 2019;24:1197–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bijnsdorp IV, Maxouri O, Kardar A, Schelfhorst T, Piersma SR, Pham TV, Vis A, van Moorselaar RJ, Jimenez CR. Feasibility of urinary extracellular vesicle proteome profiling using a robust and simple, clinically applicable isolation method. Journal of Extracellular Vesicles. 2017;6:1313091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelhak A, Foschi M, Abu-Rumeileh S, Yue JK, D’Anna L, Huss A, Oeckl P, Ludolph AC, Kuhle J, Petzold A, et al. Blood gfap as an emerging biomarker in brain and spinal cord disorders. Nature Reviews Neurology. 2022;18:158–172 [DOI] [PubMed] [Google Scholar]
- 25.Amalia L Glial fibrillary acidic protein (gfap): Neuroinflammation biomarker in acute ischemic stroke. Journal of Inflammation Research. 2021;Volume 14:7501–7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foerch C, Niessner M, Back T, Bauerle M, De Marchis GM, Ferbert A, Grehl H, Hamann GF, Jacobs A, Kastrup A, et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clinical chemistry. 2012;58:237–245 [DOI] [PubMed] [Google Scholar]
- 27.Rozanski M, Waldschmidt C, Kunz A, Grittner U, Ebinger M, Wendt M, Winter B, Bollweg K, Villringer K, Fiebach JB, et al. Glial fibrillary acidic protein for prehospital diagnosis of intracerebral hemorrhage. Cerebrovascular Diseases. 2017;43:76–81 [DOI] [PubMed] [Google Scholar]
- 28.Uphaus T, Bittner S, Groschel S, Steffen F, Muthuraman M, Wasser K, Weber-Kruger M, Zipp F, Wachter R, Groschel K. Nfl (neurofilament light chain) levels as a predictive marker for long-term outcome after ischemic stroke. Stroke. 2019;50:3077–3084 [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Povysheva N, Rose ME, Mi Z, Banton JS, Li W, Chen F, Reay DP, Barrionuevo G, Zhang F, et al. Role of uchl1 in axonal injury and functional recovery after cerebral ischemia. Proc Natl Acad Sci U S A. 2019;116:4643–4650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhlmann CR, Librizzi L, Closhen D, Pflanzner T, Lessmann V, Pietrzik CU, de Curtis M, Luhmann HJ. Mechanisms of c-reactive protein-induced blood-brain barrier disruption. Stroke. 2009;40:1458–1466 [DOI] [PubMed] [Google Scholar]
- 31.Bustamante A, Simats A, Vilar-Bergua A, García-Berrocoso T, Montaner J. Blood/brain biomarkers of inflammation after stroke and their association with outcome: From c-reactive protein to damage-associated molecular patterns. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2016;13:671–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kollikowski AM, Pham M, März AG, Papp L, Nieswandt B, Stoll G, Schuhmann MK. Platelet activation and chemokine release are related to local neutrophil-dominant inflammation during hyperacute human stroke. Translational Stroke Research. 2021;13:364–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.