Abstract
Interstitial lung abnormalities (ILA) share many features with idiopathic pulmonary fibrosis (IPF), however it is not known if ILA are associated with decreased mean telomere length (MTL).
Telomere length was measured with quantitative polymerase chain reaction in COPDGene and AGES-Reykjavik, and southern blot analysis was used in the Framingham Heart Study (FHS). Logistic and linear regression were used to assess the association between ILA and MTL; Cox proportional hazards models were used to assess the association between MTL and mortality.
In all three cohorts ILA were associated with decreased MTL. In COPDGene and AGES-Reykjavik, after adjustment there was greater than two-fold increase in the odds of ILA when comparing the shortest quartile of telomere length to the longest quartile (odds ratio [OR]=2.2, 95% confidence interval [CI] 1.5-3.4, P=0.0001 and OR=2.6, 95% CI 1.4-4.9, P=0.003), respectively. In the FHS, those with ILA had shorter telomeres compared to those without ILA (−767bp, 95% CI 76-1584bp, P=0.03). Although decreased MTL was associated with chronic obstructive pulmonary disease (OR=1.3, 95% confidence interval [CI] 1.1-1.6, P=0.01) in COPDGene the effect estimate was less than that noted with ILA. There was no consistent association between MTL and risk of death, when comparing the shortest quartile of telomere length (hazard ratio [HR]=0.82, 95% CI 0.4-1.7, P=0.6 and HR=1.2, 95% CI 0.6-2.2, P=0.5) in COPDGene and AGES-Reykjavik respectively.
ILA are associated with decreased mean telomere length when compared to those without ILA.
Introduction
There is growing evidence of the similarities between specific patterns of interstitial lung abnormalities (ILA) and pulmonary fibrosis (PF). These similarities include decrements in diffusion capacity for carbon monoxide (DLCO) and total lung capacity (TLC) (1, 2), decreased exercise capacity (3, 4), imaging progression (5–7), forced vital capacity (FVC) decline (5) and an increased risk of death (8). There is also evidence that some forms of ILA and idiopathic pulmonary fibrosis (IPF) have overlapping genetic risk loci (9, 10) including an increased prevalence of the rs35705950 MUC5B promoter polymorphism. (1, 9–15) While IPF has been associated with reduced telomere length, (16, 17) and reduced telomere length has recently been associated with early stages of PF in high-risk relatives of patients with PF, (13, 14) less is known about the associations between telomere length and ILA at the population level.
We hypothesized research participants with ILA would have relative reductions in their measures of telomere length. To test this hypothesis, we evaluated the associations between ILA and measures of relative telomere length in the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPD) (COPDGene) and Age Gene/Environment Susceptibility Reykjavik (AGES-Reykjavik) cohorts measured by quantitative polymerase chain reaction (qPCR), and telomere length measured by Southern blot in the Framingham Heart Study (FHS). Based on these results we evaluated the associations between telomere length and ILA subtypes in COPDGene and AGES-Reykjavik, and quantitative assessments of interstitial features in COPDGene. To provide context on the effect estimates for these associations we provide a comparison of the associations between measures of telomere length and COPD in COPDGene. Finally, based on prior associations between reduced telomere length and mortality among IPF patients(17), we evaluated the associations between measures of telomere length and mortality among those with ILA.
METHODS
Study Populations
Protocols for participant enrolment in COPDGene, AGES-Reykjavik, and the FHS have been previously reported (18, 19). Briefly, COPDGene is a multicentre, longitudinal study of smokers designed to determine the epidemiologic and genetic risk factors for chronic obstructive pulmonary disease (COPD) (19). The AGES-Reykjavik study is a cohort derived from the Reykjavik Study, which was established in 1967 and includes men and women that were born in Reykjavik, Iceland from 1907 to 1935 who are followed by the Icelandic Heart Association (18). The FHS is a longitudinal study that began in 1948 and was originally designed to identify epidemiologic risk factors for cardiovascular disease; the current investigation includes the offspring cohort (20), and participants were included in this analysis if they had a chest CT from the FHS Multidetector Computed Tomography 2 study and telomere length measured by Southern blot. Participants in COPDGene and the FHS were not selected for telomere length measurement on the basis of ILA status, while AGES-Reykjavik participants with ILA were oversampled to provide adequate comparison. This study was approved by the Icelandic Bioethics Committee (VSN: 00-063) and the institutional review board of the Brigham and Women’s Hospital.
Telomere Length Measurements
In COPDGene and the AGES-Reykjavik studies, mean telomere length was measured using genomic DNA that was extracted from peripheral blood leukocytes. The measurements were done using a modified, high throughput version of a quantitative real time polymerase chain reaction-based telomere assay, as previously described (21). The mean relative telomere length (MTL) was then calculated as a ratio of telomere repeat copy number to a single gene copy number, each sample was run in triplicate and the value reported is the mean of those values. The mean telomere length is then reported as the exponentiated ratio of telomere repeat copy number to a single gene copy number corrected for a reference sample. The coefficient of variation for the triplicate assays was 6.4%. Given the large number of samples assayed and the time required to complete the assays, to account for potential batch effect, z-scores were calculated for the exponentiated relative telomere length by dividing by the mean per 384 well plate. Telomere lengths were measured using Southern blot analysis in the FHS as previously described (22, 23); these measurements were performed from 1995 to 1998, approximately 13 years prior to the ILA assessment. The mean telomere length measurements in COPDGene and AGES-Reykjavik were performed on samples that were collected at the time of the chest CT imaging. The difference in timing of the sample measurements was based on availability in each of the cohorts.
ILA Evaluation
First, the chest computed tomography (CT) scans were evaluated for ILA using a sequential reading method, as previously described (1, 2, 24) by up to three readers (radiologists and pulmonologists on a Canon Medical Inc., Japan workstation), who were blind to all participant specific information. ILA was initially defined as nondependent changes affecting greater than 5% of any lung zone. These abnormalities include ground glass, reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, traction bronchiectasis, or honeycombing. Chest CT scans with either focal or unilateral ground glass attenuation, focal or unilateral reticulation, or patchy ground glass abnormalities (bilateral or unilateral), or changes that affected less than 5% of the lung were indeterminate for ILA (1, 2). The definition of ILA for this manuscript utilizes the updated definition of ILA adopted by the Fleischner society(25). This definition excludes those with ILA with centrilobular nodules alone, based on imaging, genetic, and longitudinal outcome data, demonstrating this ILA subset should be viewed as a distinct phenotype(6, 11). As a result, individuals with only centrilobular nodules were considered to be indeterminate and excluded from the analyses. Further ILA subtyping (e.g. the identification of definite fibrosis (1)), was performed by a consensus of at least three readers as previously described (2, 11).
Identification of Interstitial Features
The objective identification of interstitial features on chest CT scans in COPDGene has been previously described in detail (26, 27). Briefly, a previously trained, local histogram based, machine learning classifier was used to measure the percentage of total lung volume occupied by interstitial features (reticulation, honeycombing, centrilobular nodules, linear scar, nodular opacities, linear scar, and ground glass), emphysema and normal tissue. Using this method, every part of the lung tissue was then classified as interstitial, normal, or emphysema, and the total volumes were summed, and then able to be expressed as a percentage of the total lung volume.
Statistical Analysis
In various analyses as indicated, MTL was analyzed as a continuous variable, and was divided into quartiles (and deciles), where comparisons were made to the quartile (or decile) with the longest telomere length. Analyses for the association between MTL and ILA were performed using logistic regression. The multivariable analyses were adjusted for age, sex, body-mass index, pack-years of smoking, current smoking status, and race in COPDGene. For analyses assessing the relationship between telomere length and ILA in the FHS, generalized estimating equations to account for familial correlation were used as previously described (28). Logistic regression was also used to assess the association between MTL and COPD. To evaluate the association MTL and mortality among those with ILA, only data from COPDGene and AGES-Reykjavik were used; the FHS subset lacked adequate statistical power for proper assessment due to the small sample size. Cox proportional hazards models were used to assess the association between MTL and time-to-mortality. All variables were assessed, and none violated the proportional hazards assumption. Reported P-values were two-sided and those less than 0.05 were considered statistically significant. SAS version 9.4 (SAS Institute Inc, Cary, NC) and R version 3.6.2 were used for the analyses.
RESULTS
ILA characterization and MTLs were available in 4,452 participants in COPDGene and in 498 participants from AGES-Reykjavik. ILA characterization and telomere length were available on 439 participants from the FHS. In this subset from COPDGene, 240 (5%) had ILA, 2,606 (59%) did not have ILA, and 1,503 (34%) were indeterminate for ILA status; 103 (2%) were excluded for having ILA characterized by centrilobular abnormalities only. In AGES-Reykjavik, 163 had ILA (33%), 243 (49%) did not have ILA, 88 (18%) were indeterminate for ILA, and 4 (1%) were excluded for having only centrilobular abnormalities. In FHS, 44 (10%) had ILA, and 204 (46%) did not have ILA, 191 (44%) were indeterminate for ILA; no participants had only centrilobular abnormalities on chest CT. Baseline characteristics of participants stratified by cohort and ILA status are included in Table 1, and baseline characteristics by quartile of telomere length can be found in online supplementary table 1. As has been previously demonstrated, participants with ILA were older and had increased tobacco exposure, and in the AGES-Reykjavik cohort participants with ILA were more likely to be men.
Table 1.
Baseline characteristics of participants stratified by ILA status*.
| COPDGene | P-value | AGES-Reykjavik | P-value | Framingham Heart Study | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| No ILA (n=2606) | ILA (n=240) | No ILA (n=243) | ILA (n=163) | No ILA (n=204) | ILA (n=44) | ||||
|
| |||||||||
| Age, years† | 58 ± 9 | 63 ± 10 | <0.0001 | 75 ± 5 | 78 ± 6 | <0.0001 | 66 ± 7 | 75 ± 8 | <0.0001 |
|
| |||||||||
| Sex, no. female (%) | 1221 (47) | 102 (43) | 0.20 | 132 (54) | 67 (41) | 0.01 | 117 (57) | 23 (52) | 0.54 |
|
| |||||||||
| Body Mass Index† | 29 ± 6 | 30 ± 7 | 0.01 | 27 ± 4 | 27 ± 4 | 0.96 | 29 ± 5 | 29 ± 6 | 0.71 |
|
| |||||||||
| Race, no. white (%) | 1886 (72) | 171 (71) | 0.71 | -- | -- | -- | -- | -- | -- |
|
| |||||||||
| Pack Years Smoking, median, IQR‡ | 37 (25, 50) | 42 (33, 60) | <0.0001 | 0 (0, 22) | 14 (0, 30) | <0.0001 | 5 (0, 18) | 12 (1, 26) | 0.01 |
|
| |||||||||
| Smoking Status, no (%) | 0.28 | <0.0001 | 0.55 | ||||||
| Current | 1401 (54) | 138 (58) | 27 (11) | 28 (17) | 6 (3) | 2 (5) | |||
| Former | 1205 (46) | 102 (42) | 101 (42) | 98 (60) | 131 (64) | 31 (70) | |||
| Never | -- | -- | 115 (47) | 37 (23) | 67 (33) | 11 (25) | |||
ILA is interstitial lung abnormalities; AGES is the Age Gene/Environment Susceptibility – Reykjavik Study
values are means ± standard deviation
IQR is interquartile rang
Interstitial Abnormalities and Telomere Length
In the COPDGene and AGES-Reykjavik cohorts, after adjusting for covariates, measures of mean telomere length (MTL) were associated with ILA status (Table 2). For example, in COPDGene, after adjusting for age, sex, body-mass index, pack-years of smoking, current smoking status, race, and GOLD stage of COPD, participants in the shortest quartile of MTL had a 2.2-fold increase in their odds to have ILA (odds ratio [OR]=2.2, 95% confidence interval [CI] 1.5-3.4, P=0.0001) compared to those in the longest quartile of MTL (Table 2). In AGES-Reykjavik, after adjusting for age, sex, body-mass index, pack-years of smoking, and current smoking status, participants in the shortest quartile of mean telomere length (MTL) had a 2.6-fold increase in their odds to have ILA (OR=2.6, 95% CI 1.4-4.9, P=0.003) compared to those in the longest quartile of MTL (Table 2). Similar results were noted in analyses between ILA and continuous measures of MTL (Table 2).
Table 2.
Association between telomere length and interstitial lung abnormalities*.
| qPCR‡ Measurement | Continuous Telomere Length | Shortest Quartile of Telomere Length (Q1) † | ||
|---|---|---|---|---|
| Odds Ratio§ 95% Confidence Interval | P-value | Odds Ratio 95% Confidence Interval | P-value | |
| AGES-Reykjavik | 15.4 (3.8-62.5) | 0.0001 | 2.7 (1.4-5.1) | 0.002 |
| COPDGene | 4.2 (2.1-8.5) | <0.0001 | 2.2 (1.5-3.4) | 0.0001 |
| Southern Blot Measurement | Length Difference 95% Confidence Interval | P-value | ||
| Framingham Heart Study | 767bp (76-1584) | 0.03 | -- | -- |
AGES is Age Gene/Environment Susceptibility
Comparison is to the longest quartile of telomere length
qPCR is quantitative polymerase chain reaction
Analyses are adjusted for age, sex, body-mass-index, pack-year smoking, current smoking status, and in COPDGene also adjusted for race
Comparably, in the FHS, after adjusting for age, sex, body-mass index, pack-years of smoking, and current smoking status, participants with ILA had decreased measures of telomere length (767base pair[bp], 95% CI 76-1584bp, P=0.03) compared to those without ILA (Table 2)
Interstitial Lung Abnormality Subtypes and Telomere Length
Then we examined whether there were differences in MTL between different subtypes, or radiologic patterns of ILA. First, the relationship between definite fibrosis (evidence of parenchymal architectural distortion) and MTL was explored. In COPDGene, when compared to those without ILA, both fibrotic and non-fibrotic ILA were associated with decreased (shorter) MTL (OR=11.6, 95% CI 2.7-49.7, P=0.009 and OR=1.7, 95% CI 1.1-2.6, P=0.02), respectively, for the comparison of the shortest quartile of MTL to the longest quartile. Amongst those with ILA, ILA with definite fibrosis was associated with decreased MTL compared to those with ILA without fibrosis (OR=6.8, 95% CI 1.4-33.3, P=0.02 – for the comparison of the shortest quartile of MTL to the longest quartile). In AGES-Reykjavik there was no difference in MTL between those with ILA with and without evidence of definite fibrosis (OR=1.2, 95% CI 0.4-3.3, P=0.80). Similar results were seen when evaluating the association between MTL and consistency with a UIP pattern (see online supplementary material). Additional analyses limited to those with centrilobular abnormalities on chest CT in COPDGene are available in online supplementary table 2.
Quantitative Measure of Interstitial Features and Mean Telomere Length
Next, we assessed whether a quantitative measure of interstitial features on chest CT, that has been previously been associated with the MUC5B promoter polymorphism in COPDGene(27), was associated with telomere length. After adjustment, the shortest quartile of mean telomere length was associated with an increase of 0.67% interstitial features (standard error [SE] 0.20, P=0.001), when compared to the quartile with the longest telomere length. There was also a significant association when the mean telomere length was evaluated continuously, per standard deviation decrease in MTL, there was a 1.13% (SE=0.33, P=0.0007) increase in the percentage of interstitial features seen on chest CT (see Figure 1). When narrowed to those participants with ILA on chest CT, there was an increase in the effect size of this association. Amongst those with ILA, there was a 2% increase in the amount of interstitial features present on chest CT, in the shortest quartile of telomere length compared to longest quartile. We then assessed which components of the interstitial features were associated with decreased telomere length. After adjustment, the shortest quartile of mean telomere length was associated with an increase in the following features, linear scar, reticular markings, and subpleural line (see online supplementary table 3).
Figure 1.
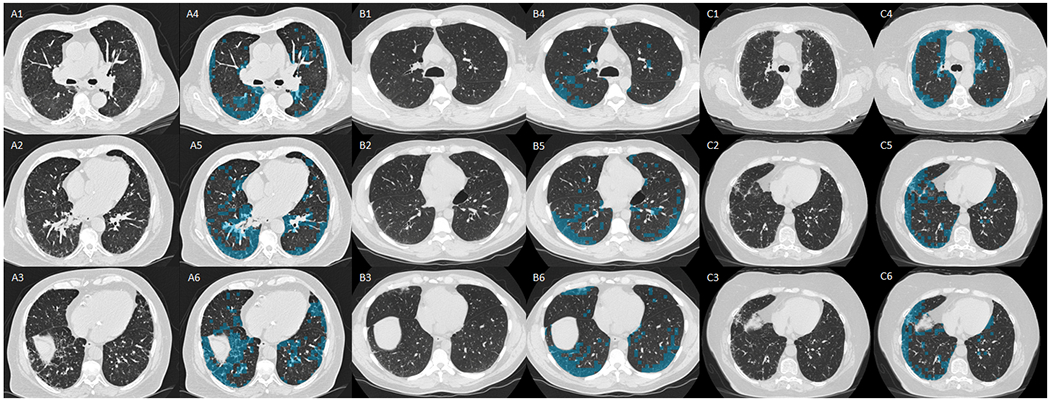
Three panels of six axial computed tomography (CT) images, from three participants (A, B, and C) from COPDGene, with interstitial lung abnormalities (ILA). In all panels (A, B, and C) 1, 2, and 3 are axial images at level of the carina (1), right inferior pulmonary vein (2), and base (3). Images 4, 5, and 6, are the same axial images but with the overlay of the local histogram used to detect interstitial features, blue represents areas of interstitial features.
COPD and Mean Telomere Length
Next, to provide a comparison to the results for ILA, and to follow-up on prior reports of association between decreased telomere length and measures of COPD(29), we analyzed the associations between COPD and MTL in COPDGene. Although there was evidence for an association between MTL and COPD in COPDGene, the effect estimate was smaller than that noted with ILA. A diagnosis of COPD (as defined by spirometry, Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II and higher), was associated with the lowest quartile (Q1) of MTL (odds ratio =1.3, 95% confidence interval 1.1-1.6, P=0.01); similar results were seen when those with ILA were removed from the analysis (OR=1.3, 95% CI 1.1-1.6, P=0.01).
Mean Telomere Length and Mortality
Finally, we evaluated the association between telomere length and the risk of death among those with ILA in the COPDGene and AGES-Reykjavik cohorts. Although most associations between MTL and mortality among those with ILA in the COPDGene and AGES-Reykjavik cohort were not statistically significant, positive associations were found in some analyses in AGES-Reykjavik. For example, in both the COPDGene and AGES-Reykjavik cohorts there was no evidence for an association between the lowest quartile of MTL and risk of death among those with ILA (HR=0.82, 95% CI 0.4-1.7, P=0.6) and (HR=1.2, 95% CI 0.6-2.2, P=0.5), respectively. While there was no evidence for an increased risk of death among those with ILA in the lowest 10th percentile of MTL in COPDGene (HR=1.3, 95% CI 0.9-1.8, P=0.14), there was evidence for an increased risk of death among those with ILA in the lowest 10th percentile of MTL in AGES-Reykjavik (HR=2.0, 95% CI 1.2-3.4, P=0.007). The evidence for an association between an increased risk of death among those with ILA in the lowest 10th percentile of MTL in AGES-Reykjavik was even greater when ILA was limited to those with probable UIP and UIP patterns (HR=3.5, 95% CI 1.7-7.4, P=0.0009), however there was not a positive association among those with ILA limited to those with probable UIP and UIP patterns in COPDGene (HR=1.2, 95% CI 0.8-1.9, P=0.3).
DISCUSSION
Our study presents the first comprehensive assessment of telomere length and interstitial lung abnormalities in the general population and in large populations of smokers and demonstrates several important findings. First, in three cohorts, including two general population samples, and with different types of measures, we demonstrate that ILA is associated with reduced telomere length. These findings imply that reduced telomere length, or processes strongly correlated with reduced telomere length, may be associated with the early developmental stages of pulmonary fibrosis. In addition, while we present evidence that there may be some correlation between COPD and measures of reduced telomere length, this association is weaker than the association with ILA. Finally, our varying findings of association with reduced telomere length and mortality among those with ILA suggests that this correlation may be more limited to those with the shortest telomere lengths, those more advanced imaging findings (e.g. patients with IPF) (17), and those with additional risk factors for an increased rate of mortality (e.g. advanced age).
Our findings add to a growing body of evidence that measures of reduced telomere length overall contribute not only to presence (16, 17) and morbidity (17) of advanced stages of PF, and early stages of PF in high-risk relatives (13, 14) but also likely play a role at increasing the risk of early undetected stages of PF in smokers and in the general population. However, it is important to note that reduced telomere length has also been associated with various forms of interstitial lung disease (ILD) including chronic hypersensitivity pneumonitis, interstitial pneumonia with autoimmune features, connective tissue disease associated ILD, and unclassifiable patterns, among others (30, 31). The spectrum of fibrotic lung disease associated with reduced telomere length, may help to explain the consistent associations we demonstrate with ILA, which encompass a variety imaging features/patterns, and likely represent disorders not limited to IPF (9).
Telomeres are regions of repetitive nucleotide sequences at the end of chromosomes that protect against the loss of genetic information during cell division, they shorten over time with repeated cell divisions, and once they reach a critical length cellular senescence is induced, and ultimately to apoptosis or cell cycle arrest (29, 32, 33). I n adults the effect of telomere length shortening most commonly affects low turnover tissues, including the lung, and it has also been shown the telomeres shorten during the aging of human fibroblasts, (34) it is possible that telomerase mutations accelerate this shortening and subsequent senescence in these cells,, potentially explaining why PF is the most frequent manifestation of telomerase associated disease (35). Future studies should look for evidence of the induction of biologic processes implicated by reduced telomere length in those with early developing stages of this disease.
The associations between telomere length and ILA and COPD deserve further consideration. Our findings are consistent with prior studies that have demonstrated associations between decreased telomere length and COPD (36, 37). While confirming these prior associations, it is important to note that the size of this effect is much less than that seen with ILA (odds of COPD are increased by 30% in the quartile with the shortest telomere length compared to a 120-170% increase in the odds of ILA).
It has been previously reported that there are clusters of rare TERT mutations in a small number (3 of 292) of research participants with severe emphysema (29). In addition to the genetic association, it has been shown that participants with COPD have decreased telomere length compared to controls (36, 37). In contrast, in a study of Mendelian randomization using polygenic risk scores, it was demonstrated that IPF was associated with mutations in telomere regulating genes, but COPD was not(38). Further work is needed to determine if these findings are generalizable across the COPD spectrum or if there are severe COPD phenotypes that may have different genetic associations as has been previously demonstrated (29).
Our study has several limitations. First, although we present associations between reduced telomere length and ILA in multiple cohorts, and with different types of measures, many of our findings of association are with MTL which limits our ability to determine if specific thresholds of reduced telomere length contribute to our findings in some cohorts. Second, the small numbers of participants with telomere length measurements in the FHS limit our ability to evaluate the association between telomere length and important outcomes, including risk of death. In addition, the length of time between telomere measurement and ILA assessment in the FHS, may limit our ability to evaluate outcomes. Third, our findings of association with reduced telomere length and mortality were inconsistent across populations. Although we speculate that these differences may be due to the distinct characteristics of the underlying populations (such as increased age in the AGES-Reykjavik cohort); we can’t rule out the possibility that they are due to technical limitations in telomere length measurement, or missing data in the AGES-Reykjavik cohort, or that some of our positive findings between ILA and mortality are spurious. Additionally, despite the consistency of the association between MTL and ILA, the oversampling of ILA cases in the AGES-Reykjavik cohort may have led to bias in our results. Finally, although our analyses are adjusted for covariates including age and smoking history, we can’t rule out the possibility that some other unmeasured confounding variable could be contributing to our findings.
In conclusion, our study demonstrates that reduced telomere length is associated with ILA. In addition, our study also provides evidence for the differential association between telomere length and interstitial abnormalities, and telomere length and measures of COPD. Future studies are needed to help determine the role of telomere length and if the progression of early stages of PF, and whether efforts to intervene in this pathway could help prevent progression to more advanced stages of fibrotic lung disease.
Supplementary Material
Acknowledgements:
The COPDGene project was supported by Award Number U01 HL089897 and Award Number U01 HL089856 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. COPDGene is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
Support:
Dr. Putman is supported by NIH grant K08 HL140087. Dr. Sanders is supported by NIH grant T32 HL007633. Dr. Ash is supported by NIH grant K08 HL145118. Dr. Gudmundsson is supported by project grant 141513-051 from the Icelandic Research Fund and Landspitali Scientific Fund A-2019-029 and A-2018-024. Dr. Nishino is supported by NIH grant R01 CA203636. Dr. Qiao is supported by NIH grant K01 HL129039. Dr. San Jose Estepar is supported by NIH grants R21HL140422 and R01HL149877. Dr. Rosas is supported by NIH grants U01 HL133232 and R01 HL130974. Dr. Washko is supported by NIH grants R01 HL116473 and R01 HL122464. The Age, Gene/Environment Susceptibility-Reykjavik Study was supported by NIH contracts N01-AG-1-2100 and HHSN27120120022C, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). Dr. Gudnason is supported by NIA grant: 27120120022C and project grant 141513-051 from the Icelandic Research Fund. Dr. DeMeo is supported by NIH grant P01 HL132825. Dr. Hatabu is supported by NIH grant 5U01CA209414-03. Dr. Cho is supported by NIH grants HL137927, HL147148, HL149861, and HL135142. Dr. Hunninghake and this work were supported by NIH grants R01 HL111024, R01 HL130974, R01 135142, and project grant 141513-051 from the Icelandic Research Fund. The Framingham Heart Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (Contract numbers N01-HC-25195, HHSN268201500001I and 75N92019D00031). The COPDGene study is supported by NIH grants U01 HL089897 and U01 HL089856.
References
- 1.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, Nishino M, Araki T, Zazueta OE, Kurugol S, Ross JC, San Jose Estepar R, Murphy E, Steele MP, Loyd JE, Schwarz MI, Fingerlin TE, Rosas IO, Washko GR, O’Connor GT, Schwartz DA. MUC5B promoter polymorphism and interstitial lung abnormalities. The New England journal of medicine 2013; 368: 2192–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Lynch DA, Brehm JM, Andriole KP, Diaz AA, Khorasani R, D’Aco K, Sciurba FC, Silverman EK, Hatabu H, Rosas IO, Investigators CO. Lung volumes and emphysema in smokers with interstitial lung abnormalities. The New England journal of medicine 2011; 364: 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle TJ, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Divo MJ, Celli BR, Sciurba FC, Silverman EK, Hatabu H, Rosas IO, Hunninghake GM, Investigators CO. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med 2012; 185: 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelsson GT, Putman RK, Miller ER, Araki T, Sigurdsson S, Gudmundsson EF, Eiriksdottir G, Siggeirsdottir K, Aspelund T, Launer LJ, Harris TB, Hatabu H, Gudnason V, Hunninghake GM, Gudmundsson G. Interstitial lung abnormalities and physical function. ERJ Open Res 2018; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, Nishino M, Zazueta OE, Kurugol S, Ross JC, San Jose Estepar R, Schwartz DA, Rosas IO, Washko GR, O’Connor GT, Hunninghake GM. Development and Progression of Interstitial Lung Abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putman RK, Gudmundsson G, Axelsson GT, Hida T, Honda O, Araki T, Yanagawa M, Nishino M, Miller ER, Eiriksdottir G, Gudmundsson EF, Tomiyama N, Honda H, Rosas IO, Washko GR, Cho MH, Schwartz DA, Gudnason V, Hatabu H, Hunninghake GM. Imaging Patterns are Associated with Interstitial Lung Abnormality Progression and Mortality. Am J Respir Crit Care Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hino T, Lee KS, Han J, Hata A, Ishigami K, Hatabu H. Spectrum of Pulmonary Fibrosis from Interstitial Lung Abnormality to Usual Interstitial Pneumonia: Importance of Identification and Quantification of Traction Bronchiectasis in Patient Management. Korean J Radiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH, El-Chemaly S, Coxson HO, Celli BR, Fernandez IE, Zazueta OE, Ross JC, Harmouche R, Estepar RS, Diaz AA, Sigurdsson S, Gudmundsson EF, Eiriksdottir G, Aspelund T, Budoff MJ, Kinney GL, Hokanson JE, Williams MC, Murchison JT, MacNee W, Hoffmann U, O’Donnell CJ, Launer LJ, Harrris TB, Gudnason V, Silverman EK, O’Connor GT, Washko GR, Rosas IO, Hunninghake GM, Evaluation of CLtIPSEI, Investigators CO. Association Between Interstitial Lung Abnormalities and All-Cause Mortality. JAMA 2016; 315: 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobbs BD, Putman RK, Araki T, Nishino M, Gudmundsson G, Gudnason V, Eiriksdottir G, Zilhao Nogueira NR, Dupuis J, Xu H, O’Connor GT, Manichaikul A, Nguyen J, Podolanczuk AJ, Madahar P, Rotter JI, Lederer DJ, Barr RG, Rich SS, Ampleford EJ, Ortega VE, Peters SP, O’Neal WK, Newell JD Jr., Bleecker ER, Meyers DA, Allen RJ, Oldham JM, Ma SF, Noth I, Jenkins RG, Maher TM, Hubbard RB, Wain LV, Fingerlin TE, Schwartz DA, Washko GR, Rosas IO, Silverman EK, Hatabu H, Cho MH, Hunninghake GM, Copdgene Investigators EISRG, Consortium UI. Overlap of Genetic Risk Between Interstitial Lung Abnormalities and Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen RJ, Guillen-Guio B, Oldham JM, Ma SF, Dressen A, Paynton ML, Kraven LM, Obeidat M, Li X, Ng M, Braybrooke R, Molina-Molina M, Hobbs BD, Putman RK, Sakornsakolpat P, Booth HL, Fahy WA, Hart SP, Hill MR, Hirani N, Hubbard RB, McAnulty RJ, Millar AB, Navaratnam V, Oballa E, Parfrey H, Saini G, Whyte MKB, Zhang Y, Kaminski N, Adegunsoye A, Strek ME, Neighbors M, Sheng XR, Gudmundsson G, Gudnason V, Hatabu H, Lederer DJ, Manichaikul A, Newell JD Jr., O’Connor GT, Ortega VE, Xu H, Fingerlin TE, Bosse Y, Hao K, Joubert P, Nickle DC, Sin DD, Timens W, Furniss D, Morris AP, Zondervan KT, Hall IP, Sayers I, Tobin MD, Maher TM, Cho MH, Hunninghake GM, Schwartz DA, Yaspan BL, Molyneaux PL, Flores C, Noth I, Jenkins RG, Wain LV. Genome-Wide Association Study of Susceptibility to Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2020; 201: 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putman RK, Gudmundsson G, Araki T, Nishino M, Sigurdsson S, Gudmundsson EF, Eiriksdottir G, Aspelund T, Ross JC, San Jose Estepar R, Miller ER, Yamada Y, Yanagawa M, Tomiyama N, Launer LJ, Harris TB, El-Chemaly S, Raby BA, Cho MH, Rosas IO, Washko GR, Schwartz DA, Silverman EK, Gudnason V, Hatabu H, Hunninghake GM. The MUC5B promoter polymorphism is associated with specific interstitial lung abnormality subtypes. The European respiratory journal 2017; 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathai SK, Humphries S, Kropski JA, Blackwell TS, Powers J, Walts AD, Markin C, Woodward J, Chung JH, Brown KK, Steele MP, Loyd JE, Schwarz MI, Fingerlin T, Yang IV, Lynch DA, Schwartz DA. MUC5B variant is associated with visually and quantitatively detected preclinical pulmonary fibrosis. Thorax 2019; 74: 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salisbury ML, Hewlett JC, Ding G, Markin CR, Douglas K, Mason W, Guttentag A, Phillips JA 3rd, Cogan JD, Reiss S, Mitchell DB, Wu P, Young LR, Lancaster LH, Loyd JE, Humphries SM, Lynch DA, Kropski JA, Blackwell TS. Development and Progression of Radiologic Abnormalities in Individuals at Risk for Familial ILD. Am J Respir Crit Care Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunninghake GM, Quesada-Arias LD, Carmichael NE, Martinez Manzano JM, Poli De Frias S, Alvarez Baumgartner M, DiGianni L, Gampala-Sagar SN, Leone DA, Gulati S, El-Chemaly S, Goldberg HJ, Putman RK, Hatabu H, Raby BA, Rosas IO. Interstitial Lung Disease in Relatives of Patients with Pulmonary Fibrosis. Am J Respir Crit Care Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. A common MUC5B promoter polymorphism and pulmonary fibrosis. The New England journal of medicine 2011; 364: 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton CA, Batra K, Torrealba J, Kozlitina J, Glazer CS, Aravena C, Meyer K, Raghu G, Collard HR, Garcia CK. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. The European respiratory journal 2016; 48: 1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuart BD, Lee JS, Kozlitina J, Noth I, Devine MS, Glazer CS, Torres F, Kaza V, Girod CE, Jones KD, Elicker BM, Ma SF, Vij R, Collard HR, Wolters PJ, Garcia CK. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med 2014; 2: 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007; 165: 1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. Copd 2010; 7: 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB Sr., Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007; 165: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 21.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 2007; 16: 815–819. [DOI] [PubMed] [Google Scholar]
- 22.Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A. Telomere length in the newborn. Pediatr Res 2002; 52: 377–381. [DOI] [PubMed] [Google Scholar]
- 23.Vasan RS, Demissie S, Kimura M, Cupples LA, Rifai N, White C, Wang TJ, Gardner JP, Cao X, Benjamin EJ, Levy D, Aviv A. Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: the Framingham Heart Study. Circulation 2008; 117: 1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu JF, Washko GR, Nakahira K, Hatabu H, Patel AS, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Diaz AA, Li HP, Qu JM, Himes BE, Come CE, D’Aco K, Martinez FJ, Han MK, Lynch DA, Crapo JD, Morse D, Ryter SW, Silverman EK, Rosas IO, Choi AM, Hunninghake GM, Investigators CO. Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation. Am J Respir Crit Care Med 2012; 185: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M, Verschakelen J, Nicholson AG, Beasley MB, Christiani DC, San Jose Estepar R, Seo JB, Johkoh T, Sverzellati N, Ryerson CJ, Graham Barr R, Goo JM, Austin JHM, Powell CA, Lee KS, Inoue Y, Lynch DA. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med 2020; 8: 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ash SY, Harmouche R, Ross JC, Diaz AA, Hunninghake GM, Putman RK, Onieva J, Martinez FJ, Choi AM, Lynch DA, Hatabu H, Rosas IO, Estepar RSJ, Washko GR. The Objective Identification and Quantification of Interstitial Lung Abnormalities in Smokers. Academic radiology 2017; 24: 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ash SY, Harmouche R, Putman RK, Ross JC, Diaz AA, Hunninghake GM, Onieva Onieva J, Martinez FJ, Choi AM, Lynch DA, Hatabu H, Rosas IO, San Jose Estepar R, Washko GR, Investigators CO. Clinical and Genetic Associations of Objectively Identified Interstitial Changes in Smokers. Chest 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uh HW, Wijk HJ, Houwing-Duistermaat JJ. Testing for genetic association taking into account phenotypic information of relatives. BMC Proc 2009; 3 Suppl 7: S123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanley SE, Chen JJ, Podlevsky JD, Alder JK, Hansel NN, Mathias RA, Qi X, Rafaels NM, Wise RA, Silverman EK, Barnes KC, Armanios M. Telomerase mutations in smokers with severe emphysema. J Clin Invest 2015; 125: 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley B, Newton CA, Arnould I, Elicker BM, Henry TS, Vittinghoff E, Golden JA, Jones KD, Batra K, Torrealba J, Garcia CK, Wolters PJ. The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: an observational cohort-control study. Lancet Respir Med 2017; 5: 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newton CA, Oldham JM, Ley B, Anand V, Adegunsoye A, Liu G, Batra K, Torrealba J, Kozlitina J, Glazer C, Strek ME, Wolters PJ, Noth I, Garcia CK. Telomere length and genetic variant associations with interstitial lung disease progression and survival. The European respiratory journal 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol 2006; 35: 1424–1429. [DOI] [PubMed] [Google Scholar]
- 33.Armanios M Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest 2013; 123: 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990; 345: 458–460. [DOI] [PubMed] [Google Scholar]
- 35.Armanios M Telomerase and idiopathic pulmonary fibrosis. Mutat Res 2012; 730: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cordoba-Lanus E, Cazorla-Rivero S, Espinoza-Jimenez A, de-Torres JP, Pajares MJ, Aguirre-Jaime A, Celli B, Casanova C. Telomere shortening and accelerated aging in COPD: findings from the BODE cohort. Respir Res 2017; 18: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rode L, Bojesen SE, Weischer M, Vestbo J, Nordestgaard BG. Short telomere length, lung function and chronic obstructive pulmonary disease in 46,396 individuals. Thorax 2013; 68: 429–435. [DOI] [PubMed] [Google Scholar]
- 38.Duckworth A, Gibbons MA, Allen RJ, Almond H, Beaumont RN, Wood AR, Lunnon K, Lindsay MA, Wain LV, Tyrrell J, Scotton CJ. Telomere length and risk of idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease: a mendelian randomisation study. Lancet Respir Med 2021; 9: 285–294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


