Abstract
Background:
Patients undergoing remission-induction intensive chemotherapy for acute leukemia are at high risk for life-threatening invasive fungal infections (IFIs). Primary antifungal prophylaxis with posaconazole has been shown to reduce the incidence of IFI compared to fluconazole, but real-life data are limited and the effect on mortality remains unclear.
Methods:
This retrospective cohort study compared fluconazole and posaconazole as primary prophylaxis in real-life practice over a 10-year period, in a Canadian hospital.
Results:
A total of 299 episodes were included (fluconazole, n = 98; posaconazole, n = 201), of which 68% were first inductions. The underlying hematologic malignancy was acute myeloid leukemia or myelodysplastic syndrome in 88% of episodes and acute lymphoblastic leukemia in 9%. Overall, 20 cases of IFI occurred (aspergillosis, n = 17; candidiasis, n = 3) and 14 were considered as breakthrough IFI. IFI incidence was significantly lower in the posaconazole group (3.5% versus 13.2%; p = 0.001). Empirical or targeted antifungal therapy was also reduced in the posaconazole cohort. Mortality was similar in both groups.
Conclusions:
In a real-life setting in Canada, primary posaconazole prophylaxis reduces the incidence of IFI during remission-induction chemotherapy, compared to fluconazole.
Keywords: breakthrough, fluconazole, invasive fungal infections, leukaemia, posaconazole, prophylaxis
Abstract
Historique :
Les patients soumis à une chimiothérapie intensive visant à induire la rémission d’une leucémie aiguë sont très vulnérables à des infections fongiques invasives (IFI) au potentiel mortel. Il est démontré qu’une prophylaxie antifongique primaire au posaconazole réduit l’incidence d’IFI davantage que le fluconazole, mais les données sur le terrain sont limitées et l’effet de ce médicament sur la mortalité demeure nébuleux.
Méthodologie :
La présente étude de cohorte rétrospective a comparé le fluconazole au posaconazole comme prophylaxie primaire sur une période de dix ans dans un hôpital canadien.
Résultats :
Au total, 299 épisodes ont été inclus (fluconazole, n = 98; posaconazole, n = 201), dont 68 % étaient des premières occurrences. Dans 88 % des épisodes, la leucémie myéloïde était le cancer hématologique sous-jacent, et dans 9 % des cas, il s’agissait plutôt d’une leucémie aiguë lymphoblastique. Dans l’ensemble, 20 cas d’IFI ont été observés (aspergillose, n = 17; candidose, n = 3) et 14 étaient considérés comme des IFI qui avaient percé malgré une médication. L’incidence d’IFI était beaucoup plus faible dans le groupe prenant du posaconazole (3,5 % par rapport à 13,2 %; p = 0,001). Le traitement antifongique empirique ou ciblé était également limité dans cette cohorte. La mortalité était semblable dans les deux groupes.
Conclusions :
Sur le terrain au Canada, la prophylaxie primaire au posaconazole réduit l’incidence d’IFI davantage que le fluconazole pendant une chimiothérapie visant à induire une rémission.
Mots-clés : fluconazole, infections fongiques invasives, leucémie, percée, posaconazole, prophylaxie
Introduction
Patients undergoing remission-induction chemotherapy for acute leukemia (AL) are at high risk of invasive fungal infections (IFIs), including invasive candidiasis (IC) and invasive aspergillosis (IA). Timely diagnosis of these infections is challenging and they carry a significant burden of morbidity and mortality (1,2). Primary antifungal prophylaxis is therefore an attractive strategy, particularly with mould-active agents such as posaconazole. In a pivotal trial, posaconazole was shown to reduce the incidence of IFI and IA, and to improve overall survival among patients receiving remission-induction chemotherapy for de novo or first-relapsed acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS), compared with fluconazole or itraconazole (3). However, efficacy data of posaconazole in the broader AL population, namely in acute lymphoblastic leukemia (ALL) patients and multiple-relapsed patients, remain limited (4–6). Also, subsequent prospective and retrospective studies evaluating this strategy have yielded inconsistent results with regard to the impact on mortality (7–13).
At our center, a previous study established the incidence of IA at ∼9% in a cohort of adult AL (AML and ALL) patients treated with remission-induction chemotherapy between 2008 and 2010 (14). Based on this finding and the relative risk reduction reported by Cornely et al (3), the number needed to treat to prevent one case of IA was estimated at 13. A mould-active prophylaxis was deemed beneficial in this group. Posaconazole was then gradually implemented as the first-line agent for primary antifungal prophylaxis in all AL and MDS patients undergoing induction-remission chemotherapy.
In the present study, we sought to evaluate the efficacy and clinical utility of posaconazole prophylaxis in AL/MDS patients undergoing remission-induction chemotherapy, in a real-life setting. We conducted a retrospective cohort study to examine the incidence of IFI, overall mortality and other clinical outcomes (eg, occurrence of fever, occurrence of pulmonary infiltrates), as well as health resources utilization (eg, diagnostic procedures, antimicrobial therapy), among patients receiving posaconazole compared with fluconazole.
Materials and Methods
Study design and population
This is a retrospective cohort study including all adult patients who have received fluconazole or posaconazole as primary antifungal prophylaxis during intensive remission-induction chemotherapy for acute leukemia, between 2008 and 2017 inclusively (10-year period). Such regimens included but were not limited to: 7 + 3, 4 + 3 (high-dose cytarabine and anthracycline), FLAG-Ida, CALGB 9111 (Larson regimen 15), high-dose etoposide and cyclophosphamide (16), PETHEMA LPA99 and LPA2005 induction (all-trans-retinoic acid and idarubicin (17,18)), NOVE (mitoxantrone and etoposide (19), HiDAC, and Hyper CVAD. As per local protocols, antifungal prophylaxis was planned for the complete duration of neutropenia. Patients were identified using the hospital’s central pharmacy database following a three-step process. First, all patients who had received remission-induction chemotherapy were identified. Next, these patients were screened for the prescription of at least one dose of either fluconazole or posaconazole. Finally, the patients were included upon confirmation that fluconazole or posaconazole was used as the first-intended primary antifungal prophylaxis agent. Patients were excluded if medical charts were not available for review. An episode was defined as the time period between the first dose of antifungal prophylaxis agent and day 100, new chemotherapy treatment (reinduction or consolidation), hematopoietic stem cell transplantation, death, or last day of contact, whichever came first. Patients could be included for more than one chemotherapy episode, in cases of reinductions. Patients who switched antifungal during an episode were analyzed according to the agent that was initially intended (intent-to-treat). Demographic and clinical data were collected by chart review using a standard case report form. The primary outcome was the incidence of probable/proven IFI among included episodes. The main secondary outcome was overall survival across included episodes. Other secondary outcomes included the occurrence of fever or pulmonary infiltrates, as well as health care resource utilization, all of which were restricted to the neutropenic period within included episodes.
Definitions
IFIs were classified according to the 2020 revision of the consensus definitions by the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSG-ERC) (20), except for the galactomannan (GM) assay positivity criteria. As per the manufacturer’s recommendation and in accordance with the Canadian federal health agency approbation, any galactomannan index equal to or greater than 0.5 from either serum or bronchoalveolar lavage fluid (BALF) was considered positive (Platelia Aspergillus Ag, Bio-Rad Laboratories Inc., Hercules, CA, USA). Breakthrough IFI (bIFI) was defined following recently proposed definitions (2019) by the MSG-ERC and the European Confederation of Medical Mycology (21). Accordingly, the period of bIFI was considered to begin when significant exposure to antifungal drugs was achieved, after 3 and 5 days of therapy for posaconazole and fluconazole, respectively. The bIFI period extended until 8 hours after the last dose of posaconazole oral suspension and until 24 hours after the last dose of fluconazole or posaconazole tablets. Persistent fever was defined as three or more consecutive days with at least one body temperature measured at 38.0°C or higher. Neutropenia was defined as an absolute neutrophil count <500 cells/μL.
Statistical analyses
The t-test and Fisher exact test were used to calculate p-values for continuous and categorical data, respectively. A p-value ≤0.05 was considered statistically significant. Incidence of IFI and all-cause mortality were assessed using survival analysis and Kaplan–Meier curves. Log-rank test was used to compare groups. Time to event was considered as the number of days between the date of first exposure to fluconazole or posaconazole and the outcome, within an episode. Cox hazards modelling was used with exposure to fluconazole or posaconazole considered as the main effect. Multivariate models were built up sequentially with clinically relevant covariates. All survival analyses were conducted using Stata 16 (StataCorp LLC, College Station, TX, USA). Other calculations and figures were produced using Prism 9 (GraphPad Software, San Diego, CA, USA).
Results
Cohort characteristics
A total of 299 episodes (fluconazole group, n = 98; posaconazole group, n = 201) were included, which occurred among 233 different patients. Forty-three patients accounted for two episodes each and 10 patients had more than two episodes (up to four). The transition from fluconazole to posaconazole took place mostly in 2010–2011 (Figure 1). The underlying hematologic disorder was AML or MDS for 264 episodes, while ALL and other types of acute leukemias accounted for 26 and 9 episodes, respectively. Sixty-six (22%) episodes were second inductions, while 30 (10%) episodes were a third induction or higher. Table 1 shows the basic characteristics of both cohorts. The groups were similar with regard to age (mean) and sex, although the posaconazole group comprised more patients older than 50 years. The duration of follow-up was also similar in both groups. There was a higher proportion of patients with AML/MDS in the posaconazole group. This was expected since posaconazole was initially used as a primary agent exclusively for AML/MDS patients at our center, but was later gradually expanded to other AL. In addition, the posaconazole group had a higher proportion of reinductions, as well as a longer duration of profound neutropenia (absolute neutrophil count <100 cells/μL).
Figure 1:
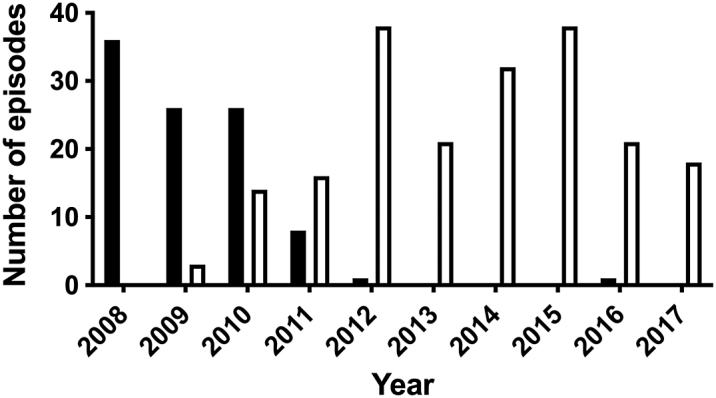
Distribution of fluconazole (black bars, n = 98) and posaconazole (white bars, n = 201) episodes over the study period
Table 1:
Basic demographic and clinical characteristics of patients for all episodes*
| Overall (n = 299) | Fluconazole (n = 98) | Posaconazole (n = 201) | p-value* | |
|---|---|---|---|---|
| Age, mean (SD) | 49.7 (13.3) | 47.9 (13.7) | 50.51 (13.0) | 0.109 |
| Age > 50 y, no. (%) | 161 (53.9) | 44 (44.9) | 117 (58.2) | 0.030 |
| Female, no. (%) | 132 (44.2) | 42 (42.9) | 90 (44.8) | 0.754 |
| AML/MDS, no. (%) | 264 (88.3) | 78 (79.6) | 186 (92.5) | 0.002 |
| First induction, no. (%) | 203 (67.9) | 80 (81.6) | 123 (61.2) | <0.001 |
| Previous HSCT, no. (%) | 27 (9.0) | 5 (5.1) | 22 (10.9) | 0.132 |
| Neutropenia <500† (days), mean (SD) | 20.1 (10.9) | 19.8 (11.2) | 20.2 (10.8) | 0.787 |
| Neutropenia <100† (days), mean (SD) | 10.9 (7.6) | 8.7 (5.2) | 12.0 (8.3) | <0.001 |
| Follow-up (days), mean (SD) | 48.6 (25.7) | 48.4 (25.7) | 48.7 (25.8) | 0.904 |
Statistically significant values (<0.05) are shown in bold
Patients could be included for more than one episode
Absolute neutrophil count (cells/μL)
AML = Acute myelogenous leukemia; HSCT = Hematopoietic stem cell transplantation; MDS = Myelodysplastic syndrome
Antifungal prophylaxis characteristics
Among posaconazole episodes, 103 and 98 courses were administered as a solution and tablets, respectively. On average, exposure to the primary intended antifungal agent was one day longer for posaconazole episodes, but the difference was not statistically significant (Table 2). However, early discontinuation of the primary intended agent was significantly more frequent for fluconazole episodes, mostly as a result of empirical or targeted antifungal therapy initiation. Conversely, toxicity or intolerance was proportionally more important among posaconazole episodes as a cause for early discontinuation. Among 41 episodes terminated early because of toxicity or intolerance, the main cause identified by treating teams was the elevation of liver enzymes in both groups (fluconazole, 5/7; posaconazole, 23/34), while rash (4/34) and gastrointestinal disturbances (3/34) were other commonly reported causes among posaconazole episodes.
Table 2:
Antifungal prophylaxis characteristics
| Overall (n = 299) | Fluconazole (n = 98) | Posaconazole (n = 201) | p-value | |
|---|---|---|---|---|
| Duration of antifungal prophylaxis (days), mean (SD) | 16.4 (9.6) | 15.7 (9.3) | 16.8 (9.8) | 0.367 |
| Early discontinuation of first intended antifungal prophylaxis agent, no. (%) | 145 (48.5) | 58 (59.2) | 87 (43.3) | 0.014 |
| Reason for early discontinuation of first intended antifungal prophylaxis agent, no. (%*) | <0.001 § | |||
| Empirical or targeted antifungal therapy | 79 (54.5) | 44 (75.9) | 35 (40.2) | |
| Toxicity/intolerance† | 41 (28.3) | 7 (12.1) | 34 (39.1) | |
| Other‡/unknown | 25 (17.2) | 7 (12.1) | 18 (20.7) |
Using number of episodes with early discontinuation as the denominator
Including but not limited to liver function tests elevation, gastrointestinal disturbances and rash
Including but not limited to drug interactions, absorption impairment and severe mucositis preventing oral drug administration
Chi-square test for observed and expected frequencies
Invasive fungal infections
A total of 42 IFIs occurred during the study period (7 proven, 13 probable, 22 possible). Incidence of proven/probable IFI was significantly higher among fluconazole versus posaconazole episodes with 13 (13.2%) and 7 (3.5%) cases, respectively (p = 0.001, Fisher exact test). Among seven proven/probable cases associated with posaconazole, no difference was seen with regard to the drug formulation in use (solution, 3/103; tablet, 4/98; p = 0.7158, Fisher exact test). Survival analysis for proven/probable IFI (Figure 2A) showed a statistically significant difference (p = 0.0017, log-rank test) and revealed a curve separation within 30 days of prophylaxis, which was sustained up to the end of follow-up. A risk factor analysis was conducted (Table 3) and identified the use of posaconazole as the only variable associated with IFI (protective effect). The association remained after accounting for previously known risk factors in multivariate modelling (adjusted hazard ratio [aHR], 0.22; 95% CI, 0.08 to 0.59).
Figure 2:
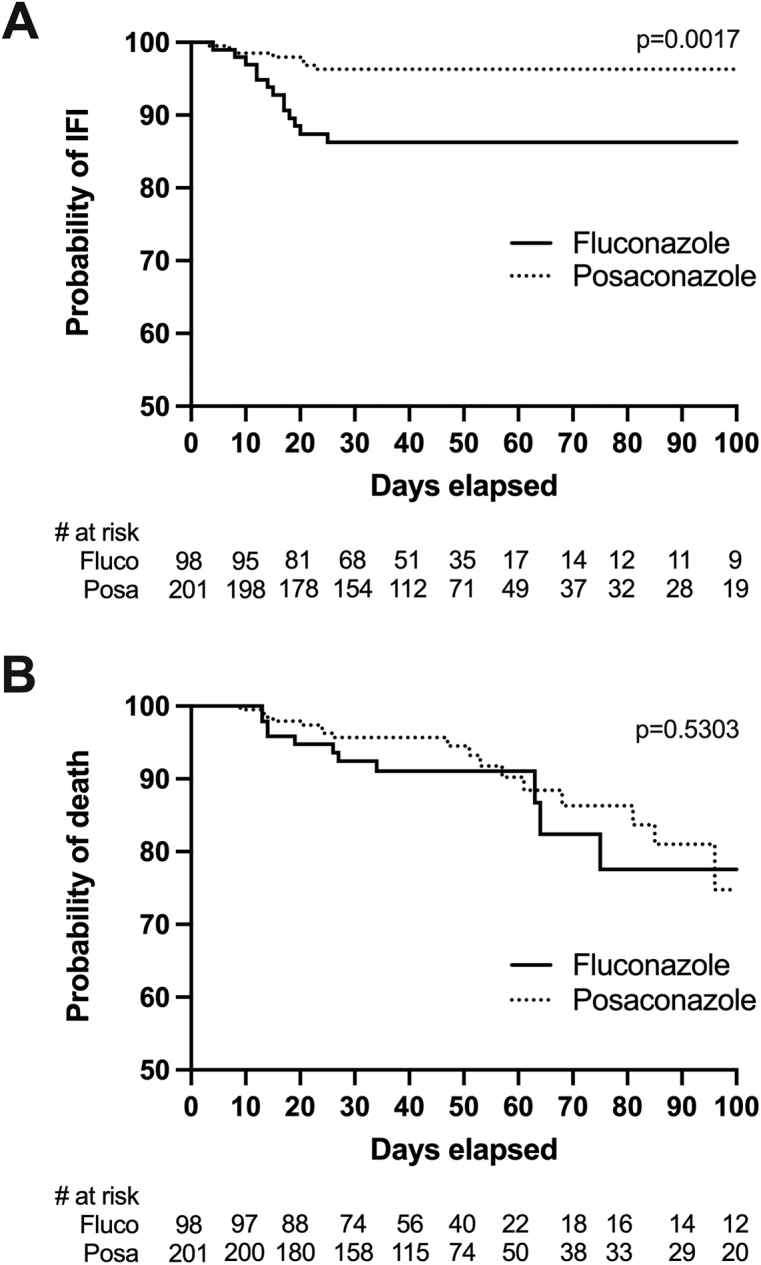
Kaplan–Meier survival estimates for the occurrence of probable and proven IFI (A) and all-cause mortality (B), according to antifungal prophylactic agent
IFI = Invasive fungal infection
Table 3:
Cox regression risk factor analysis for the occurrence of probable and proven IFI
| Factor | Crude HR (95% CI) | p-value (cHR) | Adjusted HR (95% CI) | p-value (aHR) |
|---|---|---|---|---|
| Posaconazole versus fluconazole | 0.26 (0.10 to 0.64) | 0.004 | 0.22 (0.08 to 0.59) | 0.002 |
| Age groups >50 versus <50 y | 2.04 (0.78 to 5.32) | 0.143 | 2.44 (0.89 to 6.67) | 0.081 |
| Female versus male | 1.02 (0.45 to 2.47) | 0.960 | 0.95 (0.38 to 2.39) | 0.918 |
| AML/MDS vs. ALL and others | 0.62 (0.22 to 1.69) | 0.347 | 0.57 (0.19 to 1.65) | 0.297 |
| First induction versus others | 2.06 (0.69 to 6.17) | 0.195 | 1.43 (0.44 to 4.72) | 0.553 |
| Previous HSCT versus none | 0.52 (0.07 to 3.87) | 0.521 | 0.81 (0.09 to 7.03) | 0.847 |
| Duration of neutropenia <100* (d) | 1.01 (0.97 to 1.06) | 0.594 | 1.03 (0.98 to 1.08) | 0.193 |
Statistically significant values (<0.05) are shown in bold
Absolute neutrophil count (cells/μL)
ALL = Acute lymphoblastic leukemia; AML = Acute myelogenous leukemia; HR = Hazard ratio; HSCT = Hematopoietic stem cell transplantation; IFI = Invasive fungal infection; MDS = Myelodysplastic syndrome
Detailed clinical and microbiological data for 20 proven/probable IFIs are presented in Table 4. There were 17 cases of IA and 3 cases of IC. For four IA cases, a positive culture allowed speciation (2 Aspergillus fumigatus, 1 Aspergillus terreus, 1 Aspergillus glaucus complex), while 13 cases were microbiologically documented solely by the mean of galactomannan detection. Candida species were C. glabrata (n = 2) and C. tropicalis (n = 1). Overall, 14 proven/probable IFIs were deemed as breakthrough infections, with 11 and 3 cases occurring during fluconazole and posaconazole therapy, respectively. Among 11 fluconazole-associated bIFI cases, nine were IA and two were IC (one C. glabrata and one C. tropicalis). Antifungal susceptibility testing results were not available for these two Candida sp isolates. Posaconazole-associated bIFI included one IC (C. glabrata) and two probable IA (one A. glaucus complex, one galactomannan only). The C. glabrata isolate displayed high minimal inhibitory concentrations (MIC) for both fluconazole (≥128 mg/L) and voriconazole (4 mg/L). Antifungal susceptibility testing for posaconazole was not performed. The posaconazole MIC for the A. glaucus isolate was low (0.008 mg/L). A. glaucus invasive infections are extremely rare (22,23). We cannot rule out that this species represents bystander respiratory tract colonization, with a different uncultured filamentous mould causing lung and liver lesions. Therapeutic drug monitoring was not performed in any of the 20 proven/probable IFI patients.
Table 4:
Detailed clinical and microbiological characteristics of probable and proven IFI (n = 20)
| Underlying disease | First induction | Antifungal agent | IFI type | Species | Microbiological diagnostic methods* | EORTC/MSG-ERC classification | bIFI | IFI-related mortality |
|---|---|---|---|---|---|---|---|---|
| AML | Yes | Fluco | IA | NA | GM (BAL, 3.5) | Probable | Yes | No |
| ALL | Yes | Fluco | IA | A. fumigatus | Culture (skin biopsy); GM (serum, >6.4) | Proven | Yes | Yes |
| AML | Yes | Fluco | IC | C. glabrata | Culture (blood) | Proven | Yes | No |
| AML | Yes | Fluco | IA | NA | GM (BAL, 1.9) | Probable | Yes | No |
| BPL | No | Fluco | IA | NA | GM (BAL, 7.0) | Probable | Yes | No |
| AML | Yes | Fluco | IA | A. terreus | HP (lung biopsy); culture (sputum); GM (serum, >3.0; BAL, 4.2) |
Proven | Yes | No |
| ALL | Yes | Fluco | IC | C. tropicalis | Culture (blood) | Proven | Yes | Yes |
| AML | Yes | Fluco | IA | NA | HP (autopsy); GM (BAL, >3.0) | Proven | Yes | Yes |
| AML | Yes | Fluco | IA | NA | GM (serum, 4.5) | Probable | Yes | No |
| AML | Yes | Fluco | IA | NA | GM (BAL, 5.0) | Probable | No | No |
| AML | Yes | Fluco | IA | NA | GM (BAL, 0.52) | Probable | Yes | No |
| AML | Yes | Fluco | IA | NA | GM (BAL, 1.0) | Probable | Yes | No |
| AML | No | Fluco | IA | NA | GM (serum, >5.9; BAL, 3.0) | Probable | No | No |
| AML | Yes | Posa | IA | A. fumigatus | Culture (BAL), GM (BAL, 0.97) | Probable | No | Yes |
| AML | Yes | Posa | IA | NA | GM (BAL, 0.93) | Probable | No | No |
| AML | Yes | Posa | IC | C. glabrata | Culture (blood) | Proven | Yes | No |
| AML | No | Posa | IA | NA | GM (BAL, 4.7) | Probable | No | No |
| AML | Yes | Posa | IA | A. glaucus † | HP (liver); culture (BAL); GM (BAL, 1.7) | Proven | Yes | No |
| AML | Yes | Posa | IA | NA | GM (BAL, 2.2) | Probable | No | No |
| AML | No | Posa | IA | NA | GM (serum, 3.1; BAL, >3.0) | Probable | Yes | No |
Galactomannan values are shown for each positive specimen; when multiple samples are positive during the same episode, only the highest value is shown
A. glaucus may have represented bystander respiratory tract colonization
ALL = Acute lymphoblastic leukemia; AML = Acute myeloid leukemia; BAL = Bronchoalveolar lavage; bIFI = Breakthrough invasive fungal infection; BPL = Biphenotypic leukemia; IA = Invasive aspergillosis; IC = Invasive candidiasis; IFI = Invasive fungal infection; GM = Galactomannan; HP = Histopathology
Of note, two probable IA cases (one each in the fluconazole and the posaconazole group) displayed BAL galactomannan values between 0.5 and 1.0, without a positive culture, and hence would have been classified as possible cases according to the most recent EORTC/MSG-ERC microbiological criteria. However, the difference in IFI incidence remained significant after omitting these cases (fluconazole, 12/98 versus posaconazole, 6/201; p = 0.003, Fisher exact test). To account for potential biases associated with the inclusion of patients for more than one episode, a separate analysis was also conducted considering only the first episode for each patient (n = 233) (see Supplementary Material). The basic characteristics of these cohorts are shown in Table S1. The incidence of proven/probable IFI was still significantly lower in the posaconazole group, regardless of the galactomannan definition used (Table S2).
Mortality and other clinical outcomes
All-cause mortality was similar in both cohorts (Figure 2B). Death was attributed to IFI in 4 of 20 (20%) proven/probable cases (Table 4), including three IA and one IC. There was no significant difference in IFI-related mortality between fluconazole (3/98, 3.1%) and posaconazole (1/201, 0.5%) cohorts (p = 0.1047). Neutropenic fever characteristics were also similar between fluconazole and posaconazole cohorts, including occurrence of fever (80.6% versus 81.6%, p = 0.8750) or refractory fever (37.8% versus 43.8%, p = 0.3821), and mean duration of fever (6.0 versus 5.7 days, p = 0.6694). Similarly, there was no difference with regard to lung infiltrates, whether considering any type (46.9% versus 51.2%, p = 0.5383) or nodules only (11.2% versus 12.4%, p = 0.8512).
Utilization of health care resources
Utilization of diagnostic-oriented resources was equivalent in both cohorts, except for computed tomography (CT) scans, which were performed more frequently in the posaconazole group (Table 5). Antibacterial agents consumption was the same in both groups. In contrast, there was a significant reduction in the number of episodes where antifungal therapy (empirical or targeted) was used among patients receiving posaconazole prophylaxis.
Table 5:
Comparison of resources utilization among the two groups
| Resources | Overall (n = 299) | Fluconazole (n = 98) | Posaconazole (n = 201) | p-value |
|---|---|---|---|---|
| Imaging studies,* no. (%) | ||||
| Chest X-ray | 265 (88.6) | 84 (85.7) | 181 (90.0) | 0.332 |
| Computarized tomography scan | 135 (45.2) | 32 (32.7) | 103 (51.2) | 0.003 |
| Invasive diagnostic procedures,* no. (%) | ||||
| Bronchoscopy | 57 (19.1) | 18 (18.4) | 39 (19.4) | 0.877 |
| Biopsy† | 10 (3.3) | 5 (5.1) | 5 (2.5) | 0.305 |
| Antimicrobial therapy‡ | ||||
| Antibacterial,* no. (%) | 233 (77.9) | 70 (71.4) | 163 (81.1) | 0.074 |
| Duration, mean (SD) | 15.4 (9.7) | 14.1 (9.1) | 15.9 (9.9) | 0.187 |
| Antifungal,* no. (%) | 96 (32.1) | 47 (48.0) | 49 (24.4) | <0.001 |
| Duration, mean (SD) | 11.2 (10.8) | 10.9 (10.5) | 11.4 (11.1) | 0.838 |
Number of episodes with at least one occurrence
All sites except skin biopsies
Empirical or targeted (excluding prophylaxis)
Discussion
In this real-life assessment of posaconazole prophylaxis among neutropenic patients undergoing remission-induction chemotherapy for acute leukemia, we showed a significant reduction of IFI (probable and proven), compared with fluconazole. To our knowledge, the present study represents the second-largest comparison between those two antifungal agents (12) since the pivotal randomized-controlled trial by Cornely et al (3). It is also the first report from a Canadian center. In our experience presented herein, posaconazole prophylaxis resulted in a 9.7% absolute decrease in the incidence of IFI (3.5% versus 13.2%) and a four-fold risk reduction after adjusting for confounding variables (aHR = 0.22). The magnitude of the effect is in line with previous studies that have yielded 2%–25% absolute incidence reduction. Also, in agreement with other reports and expected with a mould-active agent, IFI reduction was mostly attributable to a decrease in IA cases. However, we did not observe a survival benefit in this cohort, as both all-cause and IFI-related mortality were similar in fluconazole and posaconazole episodes. This finding contrasts with the aforementioned landmark study (3), where time to death at day 100 was lower in the posaconazole group, compared to fluconazole or itraconazole. Interestingly, the effect on mortality was not confirmed in a second randomized-controlled trial (open-label), conducted in China (7). Only one observational study demonstrated a better fungal-free survival with posaconazole compared to fluconazole, although all-cause mortality was similar in both groups (12). Dissimilarities between study designs and patient care may explain such differences across studies. Lack of power is also likely at play, as the difference was borderline significant in the landmark study and all subsequent studies, including our own, had smaller sample sizes (3,7–13). In support of the latter possibility, it is worth mentioning that a network meta-analysis has shown posaconazole to be associated with a reduction of both all-cause and IFI-attributable mortality (24).
In addition to reducing IFI, we sought to determine whether broad-spectrum antifungal prophylaxis would decrease neutropenic fever and associated diagnostic and therapeutic procedures. In our cohort, posaconazole had no impact on either occurrence or duration of fever. Consistent with this lack of effect on fever, diagnostic procedures and antibacterial agents utilization were not reduced. On the contrary, CT scans were more often used in the posaconazole group, which may have been a consequence of standard practice evolution in the management of febrile neutropenia over the study period. However, the use of empirical and targeted antifungal therapy was lower among posaconazole patients. It confirms, in a real-life context, that physicians are confident in posaconazole efficacy and act accordingly during the management of neutropenic fever. This reduction is critical, as it mitigates cost differences between both strategies and significantly contributes to the cost-effectiveness of posaconazole prophylaxis (25).
Antifungal prophylaxis during ALL chemotherapy is not as clearly defined as for AML. Large cohort studies have reported IA incidence around 3%–4%, a figure considered as moderately high, albeit slightly lower than what is observed during AML remission induction chemotherapy (1,26). However, recent studies have observed a much higher burden, especially among older adults (27–30). Some centers have published favourable outcomes with posaconazole prophylaxis among ALL patients (4–6). In our experience reported in a precursor study, IA incidence was identical among ALL and AML patients, although numbers were too small to allow statistically meaningful comparisons. Based on these data and in keeping with current guidelines, we opted for using posaconazole prophylaxis in all AL types for remission-induction chemotherapy regimens, except when toxicities and interactions were prohibitive, namely with paediatric-inspired intensive chemotherapy for ALL in adolescents/young adults or chemotherapy combined with tyrosine-kinase inhibitors for Philadelphia chromosome-positive ALL. Patients with multiple relapses represent another subgroup for whom specific antifungal prophylaxis data is lacking. These patients are generally considered at very high risk of IFI, yet they were excluded from pivotal randomized-controlled trials, likely due to extremely poor prognosis competing with trial outcomes. We chose to include this subgroup of patients in our posaconazole prophylaxis strategy. As such, our study provides real-life efficacy data in a broader AL population.
The emergence of resistant organisms, pharmacokinetics failure, and profound immunosuppression have all been associated with bIFI (31), although the relative contribution of such phenomena is undefined and the cause remains elusive in many cases. In this cohort, neither therapeutic drug monitoring nor antimicrobial susceptibility testing data were available (except for one susceptible A. glaucus isolate); hence, a specific cause for prophylactic failure could not be ascertained. Of note, fluconazole-associated bIFI was predominantly caused by intrinsically fluconazole-resistant Aspergillus spp The dominance of non-albicans Candida species (C. tropicalis and C. glabrata) is in line with previous reports on azole-prophylaxis bIFI. C. glabrata has a known propensity for developing azole resistance and global collections have demonstrated 3.5% and 8% of isolates being non-susceptible to posaconazole (32) and fluconazole (33), respectively. Posaconazole-resistant C. tropicalis emergence was also documented recently (34). While azole-resistance in Aspergillus spp is emerging globally, recent studies showed that it remained extremely rare in our geographic area (<1%) (35,36). More data are needed to better understand the biological mechanisms driving bIFI.
This work has several limitations. First, because of the observational and retrospective nature of the study, patient characteristics in both antifungal arms were not identical. This was further influenced by the fact that cohorts were not entirely contemporaneous, but only partly overlapping, which has likely resulted in practice differences across treatment arms. Such differences were at least partly alleviated by using multivariate modelling. Second, the population included was more heterogeneous than some comparable studies (including multiple relapsed disease and ALL), which may negatively affect internal validity, but may increase external validity to a wider group of acute leukemia patients.
Conclusions
In this large Canadian-based real-life evaluation of posaconazole for primary antifungal during remission-induction chemotherapy for acute leukemia, we found that the agent resulted in a significant reduction of IFI compared to fluconazole, but had no effect on mortality or neutropenic fever. Posaconazole was also associated with a reduction in empirical antifungal therapy. Those findings emerged from a broad acute leukemia population including both MDS/AML and ALL patients, as well as patients with multiple relapses, but more data are still needed for these specific groups. As posaconazole prophylaxis is growing Worldwide, defining the epidemiology, risk factors and optimal therapy for posaconazole-bIFI remains a research priority.
Funding Statement
Supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Canada Inc (grant #53575). The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Canada Inc.
Acknowledgements:
The authors are grateful to Sylvie Bélanger and Lyse Desjardins for their contribution to chart review and data collection.
Contributors:
Conceptualization, M Laverdière, SF Dufresne, J Bergeron; Data Curation, SF Dufresne, S Beauchemin, CN Abou Chakra, B Vadnais; Formal Analysis, SF Dufresne, ML Laverdière, CN Abou Chakra; Funding Acquisition, M Laverdière, SF Dufresne; Investigation, SF Dufresne, S Beauchemin, M Laverdière, B Vadnais, P Bouchard, J Bergeron; Methodology, M Laverdière, SF Dufresne, CN Abou Chakra, B Vadnais, J Bergeron, AC Labbé; Project Administration: SF Dufresne, M Laverdière, S Beauchemin; Writing – Original Draft, SF Dufresne; Writing – Review & Editing, SF Dufresne, M Laverdière, S Beauchemin, CN Abou Chakra, B Vadnais, P Bouchard, J Bergeron, AC Labbé.
Ethics Approval:
The Institutional Review Board of CIUSSS-de-l’Est-de-l’Île-de-Montréal approved this study.
Informed Consent:
N/A
Registry and the Registration no. of the Study/Trial:
N/A
Data Accessibility:
The authors confirm that the data supporting the findings of this study are available within the article. Additional data are available from the corresponding author, S.F.D., upon reasonable request and with the permission of Merck Canada Inc.
Funding:
Supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Canada Inc (grant #53575). The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Canada Inc.
Disclosures:
SF Dufresne has received honoraria for consultancy and research funding from AVIR Pharma Inc. and Merck & Co.; J Bergeron has received consultancy honoraria from AVIR Pharma, Novartis, Abbvie, Astellas Pharma, Celgene/Bristol Myers Squibb, Pfizer, Amgen; B Vadnais has received compensation from AVIR Pharma Inc. as a speaker. The other authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
Supplemental Material
References
- 1.Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(8):1068–75. Medline: [PubMed] [Google Scholar]
- 2.Azie N, Neofytos D, Pfaller M, et al. The PATH (prospective antifungal therapy) Alliance(R) registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis. 2012;73(4):293–300. 10.1016/j.diagmicrobio.2012.06.012. Medline: [DOI] [PubMed] [Google Scholar]
- 3.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–59. 10.1056/NEJMoa061094. Medline: [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Xing Y, Chen L, et al. Fluconazole versus mould-active triazoles for primary antifungal prophylaxis in adult patients with acute lymphoblastic leukemia: clinical outcome and cost-effectiveness analysis. Int J Hematol. 2018;107(2):235–43. 10.1007/s12185-017-2342-x. Medline: [DOI] [PubMed] [Google Scholar]
- 5.Zhang T, Bai J, Huang M, et al. Posaconazole and fluconazole prophylaxis during induction therapy for pediatric acute lymphoblastic leukemia. J Microbiol Immunol Infect. 2021;54(6):1139–46. 10.1016/j.jmii.2020.07.008. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Xu XH, Zhang L, Cao XX, et al. Evaluation of the implementation rate of primary antifungal prophylaxis and the prognosis of invasive fungal disease in acute leukemia patients in China. J Infect Chemother. 2017;23(6):360–7. 10.1016/j.jiac.2017.02.011. Medline: [DOI] [PubMed] [Google Scholar]
- 7.Shen Y, Huang XJ, Wang JX, et al. Posaconazole vs. fluconazole as invasive fungal infection prophylaxis in China: a multicenter, randomized, open-label study. Int J Clin Pharmacol Therap. 2013;51(9):738–45. 10.5414/CP201880. Medline: [DOI] [PubMed] [Google Scholar]
- 8.Kung HC, Johnson MD, Drew RH, et al. Clinical effectiveness of posaconazole versus fluconazole as antifungal prophylaxis in hematology-oncology patients: a retrospective cohort study. Cancer Med. 2014;3(3):667–73. 10.1002/cam4.225. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlén T, Kalin M, Cederlund K, et al. Decreased invasive fungal disease but no impact on overall survival by posaconazole compared to fluconazole prophylaxis: a retrospective cohort study in patients receiving induction therapy for acute myeloid leukaemia/myelodysplastic syndromes. Eur J Haematol. 2016;96(2):175–80. 10.1111/ejh.12565. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal D, Seth T, Kumar R, et al. Efficacy of posaconazole prophylaxis in patients with acute myeloid leukemia undergoing iInduction chemotherapy: an observational study in resource limited settings. Indian J Hematol Blood Transfus. 2018;34(3):460–5. 10.1007/s12288-018-0916-2. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devanlay C, Tavernier-Tardy E, Bourmaud A, et al. Impact of fluconazole versus posaconazole prophylaxis on the incidence of fungal infections in patients receiving induction chemotherapy for acute myeloid leukemia. Biomed J. 2015;38(3):235–43. 10.4103/2319-4170.143491. Medline: [DOI] [PubMed] [Google Scholar]
- 12.Cho SY, Lee DG, Choi SM, et al. Posaconazole for primary antifungal prophylaxis in patients with acute myeloid leukaemia or myelodysplastic syndrome during remission induction chemotherapy: a single-centre retrospective study in Korea and clinical considerations. Mycoses. 2015;58(9):565–71. 10.1111/myc.12357. Medline: [DOI] [PubMed] [Google Scholar]
- 13.Hahn J, Stifel F, Reichle A, et al. Clinical experience with posaconazole prophylaxis—a retrospective analysis in a haematological unit. Mycoses. 2011;54(Suppl 1):12–6. 10.1111/j.1439-0507.2010.01980.x. Medline: [DOI] [PubMed] [Google Scholar]
- 14.Barkati S, Dufresne SF, Bélanger S, et al. Incidence of invasive aspergillosis in remission-induction chemotherapy for acute leukemia: a retrospective cohort study in a single Canadian tertiary care center. Can Med Assoc J Open. 2014;2(2):E86–93. 10.9778/cmajo.20130062. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson RA, Dodge RK, Linker CA, et al. A randomized controlled trial of filgrastim during remission induction and consolidation chemotherapy for adults with acute lymphoblastic leukemia: CALGB study 9111. Blood. 1998;92(5):1556–64. Medline: [PubMed] [Google Scholar]
- 16.Brown RA, Herzig RH, Wolff SN, et al. High-dose etoposide and cyclophosphamide without bone marrow transplantation for resistant hematologic malignancy. Blood. 1990;76(3):473–9. Medline: [PubMed] [Google Scholar]
- 17.Sanz MA, Martín G, González M, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103(4):1237–43. 10.1182/blood-2008-05-159632. Medline: [DOI] [PubMed] [Google Scholar]
- 18.Sanz MA, Montesinos P, Rayón C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137–46. 10.1182/blood-2003-07-2462. Medline: [DOI] [PubMed] [Google Scholar]
- 19.Knauf WU, Berdel WE, Ho AD, et al. Combination of mitoxantrone and etoposide in the treatment of myelodysplastic syndromes transformed into acute myeloid leukemia. Leuk Lymphoma. 1994;12(5–6):421–5. 10.3109/10428199409073783. Medline: [DOI] [PubMed] [Google Scholar]
- 20.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group education and research consortium. Clin Infect Dis. 2020;71(6):1367–76. 10.1093/cid/ciz1008. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornely OA, Hoenigl M, Lass-Flörl C, et al. Defining breakthrough invasive fungal infection-position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses. 2019;62(9):716–29. 10.1111/myc.12960. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sridhar H, Jayshree RS, Bapsy PP, et al. Invasive aspergillosis in cancer. Mycoses. 2002;45(9–10):358–63. 10.1046/j.1439-0507.2002.00803.x. Medline: [DOI] [PubMed] [Google Scholar]
- 23.Traboulsi RS, Kattar MM, Dbouni O, et al. Fatal brain infection caused by Aspergillus glaucus in an immunocompetent patient identified by sequencing of the ribosomal 18S-28S internal transcribed spacer. Eur J Clin Microbiol Infect Dis. 2007;26(10):747–50. 10.1007/s10096-007-0361-x. Medline: [DOI] [PubMed] [Google Scholar]
- 24.Zhao YJ, Khoo AL, Tan G, et al. Network meta-analysis and pharmacoeconomic evaluation of fluconazole, itraconazole, posaconazole, and voriconazole in invasive fungal infection prophylaxis. Antimicrob Agents Chemother. 2016;60(1):376–86. 10.1128/AAC.01985-15. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho SY, Lee DG, Choi JK, et al. Cost-benefit analysis of posaconazole versus fluconazole or itraconazole as a primary antifungal prophylaxis in high-risk hematologic patients: a propensity score-matched analysis. Clin Ther. 2015;37(9):2019–27. 10.1016/j.clinthera.2015.06.014. Medline: [DOI] [PubMed] [Google Scholar]
- 26.Mariette C, Tavernier E, Hocquet D, et al. Epidemiology of invasive fungal infections during induction therapy in adults with acute lymphoblastic leukemia: a GRAALL-2005 study. Leuk Lymphoma. 2017;58(3):586–93. 10.1080/10428194.2016.1204652. Medline: [DOI] [PubMed] [Google Scholar]
- 27.Cattaneo C, Gramegna D, Malagola M, et al. Invasive pulmonary aspergillosis in acute leukemia: a still frequent condition with a negative impact on the overall treatment outcome. Leuk Lymphoma. 2019;60(12):3044–50. 10.1080/10428194.2019.1613535. Medline: [DOI] [PubMed] [Google Scholar]
- 28.Daenen S, van der Holt B, Dekker AW, et al. Intensive chemotherapy to improve outcome in patients with acute lymphoblastic leukemia over the age of 40: a phase II study for efficacy and feasibility by HOVON. Leukemia. 2012;26(7):1726–9. 10.1038/leu.2012.53. Medline: [DOI] [PubMed] [Google Scholar]
- 29.Sive JI, Buck G, Fielding A, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. 2012;157(4):463–71. 10.1111/j.1365-2141.2012.09095.x. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh SM, Byun JM, Chang E, et al. Incidence of invasive fungal infection in acute lymphoblastic and acute myelogenous leukemia in the era of antimold prophylaxis. Sci Rep. 2021;11(1):22160. https://doi.org/10.1038/s41598-021-01716-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenks JD, Cornely OA, Chen SC, et al. Breakthrough invasive fungal infections: who is at risk? Mycoses. 2020;63(10):1021–32. 10.1111/myc.13148. Medline: [DOI] [PubMed] [Google Scholar]
- 32.Pfaller MA, Messer SA, Woosley LN, et al. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol. 2013;51(8):2571–81. 10.1128/JCM.00308-13. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaller MA, Diekema DJ, Turnidge JD, et al. Twenty years of the SENTRY antifungal surveillance program: results for Candida species from 1997–2016. Open Forum Infect Dis. 2019;6(Suppl 1):S79–94. 10.1093/ofid/ofy358. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desnos-Ollivier M, Lortholary O, Bretagne S, et al. Azoles susceptibility profiles of more than 9,000 clinical yeast isolates belonging to 40 common and rare species. Antimicrob Agents Chemother. 2021;65(6):e02615–20. 10.1128/AAC.02615-20. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parent-Michaud M, Dufresne PJ, Fournier E, et al. Prevalence and mechanisms of azole resistance in clinical isolates of Aspergillus section Fumigati species in a Canadian tertiary care centre, 2000 to 2013. J Antimicrob Chemother. 2020;75(4):849–58. 10.1093/jac/dkz534. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng MP, Lawandi A, Lee TC, et al. Triazole antifungal susceptibility patterns among Aspergillus species in Quebec, Canada. J Clin Microbiol. 2019;57(6):e00404–19. 10.1128/JCM.00404-19. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. Additional data are available from the corresponding author, S.F.D., upon reasonable request and with the permission of Merck Canada Inc.


