Abstract
Rationale:
Clinically relevant pain is associated with functional impairment and behavioral depression, including depression of social behavior. Moreover, recovery of function is a major goal in pain treatment. We used a recently developed model of operant responding for social interaction in rats to evaluate the vulnerability of social behavior to an experimental pain manipulation and the sensitivity of pain-depressed social behavior to treatment with clinically effective analgesics.
Methods:
Sprague-Dawley male and female rats were trained to lever press for social access to another rat, and responding was evaluated after treatment with (a) intraperitoneal injection of dilute lactic acid (IP acid; 0.18–5.6%) administered alone as a visceral noxious stimulus, (b) the mu-opioid receptor (MOR) agonist morphine (0.32–10 mg/kg) or nonsteroidal anti-inflammatory drug (NSAID) ketoprofen (10 mg/kg) administered alone, or (c) morphine or ketoprofen administered before IP acid. For comparison, the same treatments were evaluated in separate rats trained to lever press for food delivery.
Results:
Both IP acid alone and morphine alone more potently decreased responding maintained by social interaction than by food, whereas ketoprofen did not affect responding for either reinforcer. In general, analgesics were most effective to rescue operant responding when relatively low IP acid concentrations produced significant but submaximal behavioral depression; however, morphine was not effective to rescue responding for social interaction.
Conclusions:
Operant responding maintained by social interaction was more sensitive to pain-related disruption and less responsive to opioid analgesic rescue than food-maintained operant responding. Social behavior may be especially vulnerable to depression by pain states.
Keywords: pain-depressed behavior, social self-administration, morphine, ketoprofen, pain, analgesia
INTRODUCTION
Clinically relevant pain is associated with functional impairment and behavioral depression, and a major goal in pain treatment is recovery of function (Dworkin et al. 2005). Preclinical research on the expression and treatment of pain-related functional impairment in laboratory animals has been investigated by a range of different approaches that generally involve three steps (Negus 2013; 2019; Negus et al. 2006). First, a baseline rate is established for some unconditioned behavior (e.g. feeding, locomotion, or burrowing in rats) or operant conditioned behavior (e.g. lever pressing for food or rewarding electrical brain stimulation). Second, an experimental pain state is introduced, and the degree to which the pain model disrupts ongoing behavior is assessed. Lastly, candidate treatments can be introduced to evaluate their effectiveness to alleviate pain-related behavioral depression and restore behavior to baseline rates. To date, this work has yielded two major findings. First, both unconditioned and operant-conditioned behaviors are most reliably depressed by acute inflammatory stimuli, such as intraperitoneal injection of dilute acid, intraplantar injection of complete Freund’s adjuvant, or paw incision; common models of mononeuropathy or polyneuropathy often have little or no effect on these types of behavior (Andrews et al. 2012; Cone et al. 2018; Ewan and Martin 2014; Kandasamy et al. 2016; Kwilasz and Negus 2012; Legakis et al. 2019; Matson et al. 2007; Negus et al. 2010; Okun et al. 2016; Pereira Do Carmo et al. 2009). Second, pain-related behavioral depression can usually be prevented or reversed by clinically effective analgesic drugs like cyclooxygenase inhibitors/nonsteroidal anti-inflammatory drugs (COX inhibitors/NSAIDs) or mu opioid receptor (MOR) agonists but not by many classes of drugs that do not function as analgesics in humans despite producing other behavioral effects (e.g. GABAA receptor positive allosteric modulators or centrally acting kappa opioid receptor agonists) (Andrews et al. 2012; Cone et al. 2018; Kandasamy et al. 2017; Kwilasz and Negus 2012; Legakis et al. 2020; Martin et al. 2004; Matson et al. 2007; Miller et al. 2015; Negus et al. 2010; Pereira Do Carmo et al. 2009).
Social behavior is one domain of behavior that can be impaired by pain states (Closs et al. 2009; Dawu et al. 2019; Restoux et al. 2020; Shega et al. 2012). For example, the Brief Pain Inventory is an instrument commonly used to query the degree to which a pain state interferes with mood and behavior, and “relations with other people” is one item assessed by this instrument, along with other items that include “general activity,” “walking ability,” and “normal work” (Cleeland and Ryan 1994; Dworkin et al. 2005; Jumbo et al. 2021). Despite the importance of social behavior in mental health and the clear clinical evidence that pain states can disrupt social behavior, relatively few preclinical studies in laboratory animals have attempted to directly compare either the impact of experimental pain states on social vs. other types of behavior or the effectiveness of treatments to restore social vs. other types of behavior (Liu et al. 2015; Parent et al. 2012; Sheahan et al. 2017; Urban et al. 2011). These studies have generally reported little effect of preclinical pain models on metrics of social behavior in rodents. Additionally, the impact of pain treatments may be especially relevant given that some classes of analgesics, such as opioids, can directly affect behavior in general and social behavior in particular (Panksepp et al. 1980; Vanderschuren et al. 2016).
Accordingly, the goal of the present study was to examine the effects of an experimental pain model delivered alone or after treatment with clinically effective analgesics in a recently developed model of operant social behavior in rats (Venniro and Shaham 2020; Venniro et al. 2018). Specifically, rats were initially trained to engage in operant responding reinforced by brief access to social interaction to a former cagemate. After initial parametric studies to confirm that social interaction functioned as a reinforcer, rats were tested with (a) intraperitoneal injection of dilute lactic acid (IP acid) administered alone as a visceral noxious stimulus, (b) the MOR agonist morphine or the COX1/2 inhibitor/NSAID ketoprofen administered alone, or (c) morphine or ketoprofen administered before IP acid. A parallel series of manipulations was evaluated in rats trained to respond for food reinforcement to evaluate the generalization of effects across operant behaviors maintained under similar schedules of reinforcement but by different reinforcers. Our results provide evidence for pain-related depression of operant social behavior in rats and provide a foundation for future studies to examine mechanisms and treatments for pain-related depression of social behavior.
METHODS
Subjects
Studies were conducted in a total of 30 male and female Sprague Dawley Rats (Harlen, Frederick, MD, USA). Studies of operant social interaction (referred to herein as “social self-administration”) were conducted in two cohorts of 12 rats reach, and each cohort consisted of six male and six female rats. These rats were initially housed in same-sex pairs to establish inter-animal familiarity during the first two weeks after arrival in the laboratory, then separated and individually housed for the remainder of the study with free access to food (Teklad standard diet - 19% protein; Envigo) in the home cage. Studies of food-maintained responding were conducted in a single cohort of three male and three female rats housed individually from the outset. For initial training and demand-curve determination (see below), food access in these rats was limited to 15±3 g/day delivered approximately 1 h after behavioral sessions. Subsequently, these rats also had free access to food in the home cage for all remaining studies. All subjects were maintained in an AAALAC-accredited temperature- and humidity-controlled housing facility on a 12 h light/12 h dark cycle (lights on at 6:00 a.m.) with free access to water. Protocols adhered to federal guidelines for the care and use of laboratory animals (National Research Council, 2011) and were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Drugs
Lactic acid was purchased from Sigma, diluted in sterile water, and injected IP. Morphine sulfate and naltrexone HCl were kindly provided by the National Institute on Drug Abuse Drug Supply System, and both were dissolved in saline for SC injection. Ketoprofen was purchased as a commercially available 100 mg/ml solution (Zoetis) and diluted in saline for SC injection. All injections were administered in a volume of 1.0 ml/kg.
Procedures for Social Self-Administration
Apparatus.
Behavioral sessions were conducted in operant-conditioning chambers (Med Associates) modified for social self-administration and choice procedures (Venniro et al. 2019; Venniro and Shaham 2020). Each chamber contained a large compartment (32 × 25.4 cm2 floor space; 33 cm high) and an adjacent smaller compartment (18.4 × 17.1 cm2 floor space; 33 cm high) separated by a wall containing an arched aperture (9.2 cm base and 10.5 cm peak height) that could be covered or revealed by a motor-driven guillotine door. The aperture was partially occluded by a grid of aluminum bars with open space sufficient to allow social interaction by facial contact while preventing rats from moving between compartments. The larger compartment was equipped with a response lever located 5 cm to the right of the aperture, a white stimulus light above the lever, and a white house light located on the opposite wall near the ceiling. Control of stimulus delivery and collection of data were accomplished with a computer, interface, and custom software (Med Associates).
Behavioral and pharmacological procedures.
Behavioral sessions were conducted with the rat pairs established during the first two weeks of housing in the laboratory (i.e. three male pairs and three female pairs). During behavioral sessions, one rat in each pair (the responder rat) was placed in the larger compartment, and the other rat (the reinforcer rat) was placed in the smaller compartment. Each behavioral session began with onset of the lever light and house light. Completion of the response requirement on the lever turned off the lever light, opened the guillotine door for the designated door-open time, and permitted social interaction via the aperture between the compartments. At the conclusion of the door-open time, the door closed, the lever light was turned back on, and responding could again open the door.
Training began one week after pairs were separated for individual housing, and studies were conducted in two cohorts, each consisting of three male and female responder rats with their associated same-sex reinforcer rats. The first cohort was used for all parametric studies, and the second cohort was used for a follow-up study described below. For the first cohort, initial training sessions lasted 1 h, each response produced door opening under a fixed-ratio 1 (FR 1) schedule of reinforcement, and the door-open time was 30 s. These parameters remained in place until rats emitted ≥20 reinforced responses for two consecutive days, and the number of reinforced responses on each day differed from the mean across days by ≤15%. One female failed to reach these criteria within 18 days, so the responder and reinforcer rat for this pair were switched and the new responder rat was trained until the criteria were met.
Once all responder rats met the criteria for training, two studies were conducted to manipulate parameters of the social stimulus. First, the function of social interaction as a reinforcer was evaluated by comparing responses per session when a lever press produced one of four different consequences: (1) door opening with the reinforcer rat present in the small chamber, (2) no door opening but reinforcer rat present, (3) door opening but the reinforcer rat absent, and (4) no door opening and reinforcer rat absent. Each type of outcome was assessed during a one-week experiment with baseline conditions in place on Monday and Friday (i.e. responding produced door opening and the reinforcer rat was present) and the designated test condition in place for three consecutive days on Tuesday, Wednesday, and Thursday. The order of test conditions was randomized across rats using a Latin-Square design, and all rats were tested with all conditions. In the course of this experiment, it became apparent that rats completed most of their response requirements during the first 30 min of the 1h session, so all subsequent studies were conducted with 30-min sessions and the reinforcer rat present.
The second parametric study evaluated the effects of manipulating the door-open time and the response requirement. Door-open times of 15 s, 30 s, and 60 s were each examined for one week (Monday through Friday), and the FR requirement was 1, 2, 4, 8, and 16 on successive days of that week. The order of door-open times was randomized across rats according to a Latin-square design, and all rats were tested with all door-open times. All subsequent studies used a FR 1 response requirement and 30-s door-open time.
Once these parametric studies had been completed, responding was next evaluated after three different categories of treatments intended to assess pain- and analgesia-related changes in responding for social reinforcement: (a) effects of intraperitoneal lactic acid (IP acid) administered alone as a noxious visceral stimulus, (b) effects of the clinically effective analgesics morphine and ketoprofen administered alone, and (c) effects of IP acid after pretreatment with either morphine or ketoprofen. For studies with IP acid alone, rats were treated with vehicle (sterile water) or a range of IP acid concentrations (0.18–1.8%) administered 10 min before each session. For studies with morphine and ketoprofen alone, rats received a subcutaneous (SC) injection of vehicle (saline), morphine (0.32–10 mg/kg), or ketoprofen (10 mg/kg) 30 min before each session. Lastly, for studies with morphine or ketoprofen as a pretreatment to IP acid, rats received saline vehicle, morphine (0.1–3.2 mg/kg), or ketoprofen (10 mg/kg) SC 20 min before IP acid (1.0%), and sessions commenced 10 min after the acid injection. Morphine was tested across a range of doses up to those that alleviated pain-related behavioral depression in other studies (Altarifi et al. 2015) and that produced rate-decreasing effects on their own in the present study. Ketoprofen was tested at a single high dose shown to be in the antinociceptive dose range in previous studies (Leitl et al. 2014; Negus et al. 2012), and higher doses were not tested to avoid risk of stomach ulceration (Cabre et al. 1998; Scepovic and Radmanovic 1984). Within each category, treatment order was randomized across rats using a Latin-square design, and all rats received all treatments. Tests were generally conducted on Tuesdays and Fridays, with standard training sessions conducted on other weekdays.
By the conclusion of the experiments described above, all rats had grown, and it was determined that the smaller reinforcer-rat compartment would not accommodate further growth of reinforcer rats. Accordingly, a second cohort of rats was trained to conduct a follow-up study that evaluated effectiveness of morphine and ketoprofen to alleviate depression of operant responding produced by a lower concentration of 0.56% IP acid. For this second cohort, training was similar to that in the first cohort with the exception that training sessions lasted 30 min instead of 60 min, and the criteria for training were (a) ≥10 reinforced responses for two consecutive days and (b) the number of reinforced responses on each day were within 15% of the mean across those days. One male failed to reach these criteria within 15 days, so the responder and reinforcer rat for this pair were switched and the new responder rat was trained until the criteria were met. Once all rats met the training criteria, preliminary studies of extinction and IP acid-induced behavioral depression were conducted to assess whether (a) access to the reinforcer rat functioned as a reinforcer in the second group, and (b) 0.56% IP acid was sufficient to significantly depress responding in the second group. Extinction was assessed during a one-week experiment. On Monday and Friday, baseline conditions were in place (i.e. responding under an FR 1 reinforcement schedule produced 30-s door opening with the reinforcer rat present). On Tuesday, Wednesday, and Thursday, responding did not open the door and the reinforcer rat was absent. Acid-induced depression of responding was assessed the following week by testing rats with IP water or IP 0.56% IP acid 10 min before sessions on Tuesday and Friday, with normal training sessions on other weekdays. The order of treatments was counterbalanced across rats. Once these preliminary studies were completed and both significant extinction and significant IP acid-induced depression of responding had been confirmed, then morphine and ketoprofen were tested for their effectiveness to block 0.56% IP acid-induced behavioral depression. The rats received morphine (0.1–3.2 mg/kg) or ketoprofen (10 mg/kg) SC 20 min before IP acid (0.56%), and sessions commenced 10 min after the acid injection. As in the first cohort, treatment order was randomized across rats using a Latin-square design, all rats received all treatments, and tests were conducted on Tuesdays and Fridays, with training sessions conducted on other weekdays.
Procedures for Food-Maintained Responding
Apparatus.
Studies were conducted in sound-attenuating boxes containing modular acrylic and metal test chambers (29.2 × 30.5 × 24.1 cm; Med Associates). Each chamber contained a response lever, three stimulus lights (red, yellow, and green) centered above the lever, a white house light, and a pellet dispenser that delivered 45 mg grain pellets (BioServ; Product #F0165) to an aperture beside the lever. Control of stimulus delivery and collection of data were accomplished with a computer, interface, and custom software (Med Associates).
Behavioral and pharmacological procedures.
Behavioral sessions were designed to mimic key features of the social self-administration procedure. Thus, 30-min behavioral sessions were conducted every weekday and began with onset of the lever light and house light. Each response produced a single food pellet under an FR 1 reinforcement schedule and initiated a 30-s timeout (TO) when the lever light was off and responding had no scheduled consequences (FR 1:TO 30” schedule). Training began one week after rats arrived in the laboratory. For the first four days, food pellets were available under the FR 1 schedule with no time out. Subsequently, the terminal FR 1:TO 30” reinforcement schedule was in effect for the remainder of the study (except when the FR requirement was manipulated; see below).
Initial training under the terminal schedule continued until rats earned ≥20 pellets for two consecutive days, and the number of reinforced responses on each day differed from the mean across days by ≤15%. Subsequently, pellet delivery was withheld for three consecutive days to evaluate extinction of operant responding. After resumption of pellet delivery and recovery of baseline reinforcement rates, the following series of experiments was conducted to parallel the studies conducted with social self-administration. First, the FR parameter was increased across values of 1, 2, 4, 8, and 16 on successive days of a single week; the 30-s timeout after each pellet delivery remained in effect. Second, food restriction was terminated, and rats were provided free access to food in the home cage for the remainder of the study. After one week of free access to food, the manipulation of FR values was repeated. Third, responding was evaluated after three different categories of treatments intended to assess pain- and analgesia-related changes in food-maintained responding: (a) effects of IP acid administered alone, (b) effects of morphine and ketoprofen administered alone, and (c) effects IP acid after pretreatment with either morphine or ketoprofen. For studies with IP acid alone, rats were treated with vehicle (sterile water) or a range of IP acid concentrations (0.18–1.8%) administered 10 min before each session. For studies with morphine and ketoprofen alone, rats received SC injection of vehicle (saline), morphine (0.32–10 mg/kg), or ketoprofen (10 mg/kg) 30 min before each session. For studies with morphine or ketoprofen as a pretreatment to IP acid, rats received morphine (0.1–3.2 mg/kg) or ketoprofen (10 mg/kg) SC 20 min before IP acid (1.8%), and sessions commenced 10 min after the acid injection. Treatment order for each manipulation was randomized across rats using a Latin-square design, and tests were conducted on Tuesdays and Fridays, with training sessions conducted on other weekdays. Because 1.8% IP acid produced a significant but only partial decrease in food-maintained responding, rats were subsequently treated once a week with higher concentration of 3.2% IP acid administered alone or after pretreatment with 1.0 mg/kg morphine or 10 mg/kg ketoprofen SC and then with an even higher concentration of 5.6% IP acid administered alone or after pretreatment with 1.0 mg/kg morphine or 10 mg/kg ketoprofen SC. Treatments were again randomized using a Latin-square design within each acid concentration, and testing frequency with high acid concentrations was reduced to once-per-week to minimize any carry-over effects.
Data Analysis
The primary dependent measure was the total number of response requirements completed and reinforcers delivered during each behavioral session. These data were averaged across rats and compared across conditions using a t-test, one-way ANOVA, or two-way ANOVA with the Geisser-Greenhouse correction for sphericity as appropriate. A significant ANOVA was followed by the Dunnett post hoc test. All analyses were conducted using Prism 9 (Graphpad Software), and the criterion for significance was p<0.05 for all analyses.
In addition to these analyses of raw data, the data from studies of parametric manipulations, and from studies of IP acid and morphine alone, were also transformed within each rat to a percent of an appropriate baseline using the equation % Baseline = (# reinforcers per session/baseline) * 100. These data were then used for two other analyses. First, in the experiments that manipulated response requirement, data were plotted as demand curves to relate log Response Requirement on the X axis to log % FR 1 Reinforcers Earned on the Y axis. This normalization of the x-axis was similar to a previous approach (Hursh and Winger 1995; Winger et al. 2006). All curves were subsequently analyzed as described previously to quantify reinforcing effectiveness as the “Essential Value” (Hursh 2014; Hursh and Silberberg 2008; Legakis et al. 2019; Townsend et al. 2019). Measures of essential value across experimental conditions were considered to be different if 95% confidence limits did not overlap.
Second, data from studies of IP acid alone and morphine alone were expressed as % Vehicle and used to determine EC50 and ED50 values (i.e. the effective concentration of IP acid or the effective dose of morphine to decrease reinforcement to 50% of vehicle control levels). EC50 and ED50 values and their 95% confidence limits were determined by linear regression using the linear portion of the IP acid concentration-effect curve or morphine dose-effect curve. EC50 and ED50 values for IP acid and morphine to decrease social- vs. food-maintained responding were considered to be different if 95% confidence limits did not overlap.
Both male and female rats were included in the present study to comply with National Institutes of Health mandate to include both sexes in preclinical research designs (Miller et al. 2017). However, the study was not intended to investigate sex differences, it was not powered to detect sex differences, and no sex differences were detected with the number of rats that are included. Accordingly, data analysis shows results collapsed across sex, although individual data are shown in selected figures to illustrate effects in males and females.
RESULTS
Parametric Assessment of Social Interaction as an Operant Reinforcer
Social Self-Administration.
Figure 1 shows that access to social interaction functioned as a reinforcer. Thus, Figure 1A shows that responding decreased when social interaction was prevented by removing response-contingent door opening and/or the reinforcer rat. Two-way ANOVA indicated a significant effect of social-access condition on reinforcement rate, with main effects of both reinforcer-rat status [present or absent, F(1,5)=11.15, p=0.021] and response-contingent door opening [yes or no F(1,5)=6.86, p=0.047]. The interaction was not significant, but planned comparisons with the control condition (+/+, response-contingent door opening and reinforcer rat present) indicated that absence of the reinforcer rat significantly decreased responding regardless of whether responding opened the door (p=0.025) or not (p=0.005). Mean response rates also decreased when the door did not open but the reinforcer was present; however, this decrease did not reach the criterion for significance (p=0.116).
Figure 1. Social access functions as an effective reinforcer in rats.
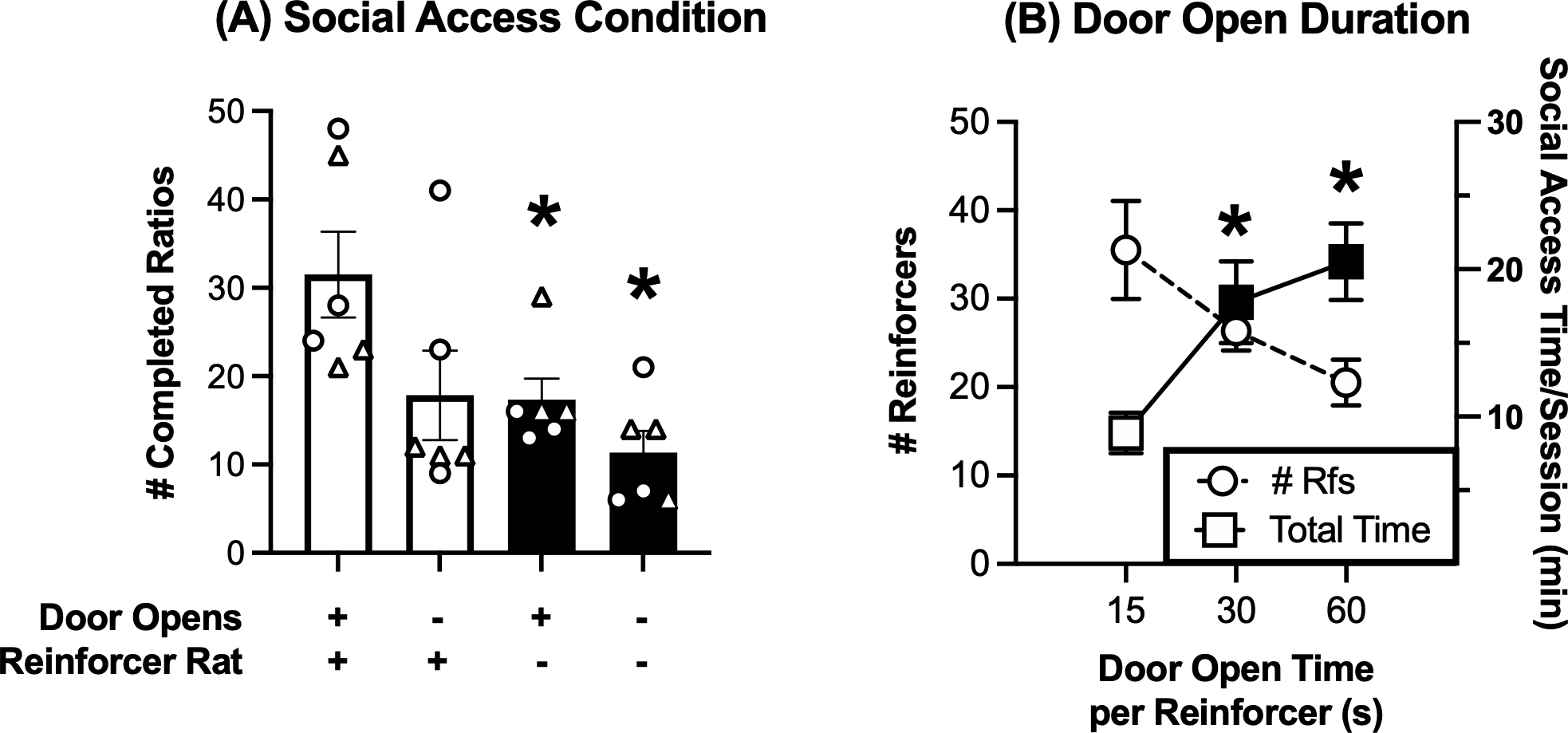
(A) Panel A shows the effect of manipulating the social access conditions. Abscissa: Social-access condition with or without door opening and/or reinforcer rat. Ordinate: number of completed ratios per 1-h session. Bars show mean ± SEM for 6 rats, and points show individual data (triangles for females, circles for males). Filled bars with asterisks on the right indicate different from the “+/+ Door Open and Reinforcer Rat Present” condition on the left, p<0.05. (B) Panel B shows the effect of manipulating the door-open duration when the reinforcer rat was present. Abscissa: Door open time in seconds. Left Ordinate: number of reinforcers per 1-h session. Right Ordinate: total time of access to social interaction via open door during a 30-min session in min. All symbols show mean ± SEM for 6 rats, and filled symbols with asterisks indicate different from 15-s door-open time, p<0.05
Figure 1B shows the effect of manipulating door-open time on both the reinforcement rate and the total amount of time the door was open under the FR 1 response requirement on the first day of demand-curve determinations. There was a trend for reinforcement rate to decrease as duration of door-open time increased, but this effect did not reach the criterion for statistical significance [F(1.16, 5.81)=4.97, p=0.066]. Similarly, Table 1 shows that 95% confidence limits for reinforcement rate overlapped for all door-open times. However, there was a significant effect of door-open time on the total duration of social access during each 30-min session [F(1.12, 5.58)=8.03, p=0.031]. The 30-s and 60-s door-open times maintained longer total durations of social access than the 15-s door-open time.
Table 1. Relative effects of FR increases, IP acid, and SC morphine on social- vs. food-maintained responding.
For social self-administration, demand curves were determined for three door-open times (15, 30, and 60 sec), but effects of IP acid and analgesics were determined only for the 30-sec door open time. For food-maintained responding, the status of food restriction or free feeding is specified, and effects of IP and analgesics were determined only under free-feeding conditions. All data show mean values (95% CL) from 6 rats (3 female, 3 male).
| Demand Curves |
||||
|---|---|---|---|---|
| Reinforcement | FR 1 Baseline | Essential | IP Acid | Morphine |
| Conditions | # Reinforcers | Value | EC50 (%) | ED50 (mg/kg) |
| Social | ||||
| 15 s Door Open | 35.5 (24.6–46.4) | 5.1 (4.4–5.8) | ND | ND |
| 30 s Door Open | 26.3 (21.9–30.7) | 5.6 (4.9–6.3) | 0.42 (0.36–0.48) | 1.06 (0.70–1.63) |
| 60 s Door Open | 20.5 (15.4–25.6) | 7.0 (5.3–8.7) | ND | ND |
| Food | ||||
| Food Restricted | 53.2 (49.3–57.0)* | 15.2 (9.1–21.4)* | ND | ND |
| Free Fed | 53.2 (47.0–59.3)* | 10.8 (9.1–12.5)* | 2.40* (1.99–2.91) | 5.26* (4.05–6.68) |
Asterisk indicates that values for food reinforcement are significantly higher than all values for social reinforcement as determined by non-overlapping confidence limits.
ND-Not Determined. Individual α values were transformed to “essential value” such that a larger number reflects greater reinforcing effectiveness using the following equation (Hursh 2014):
Figure 2A shows demand curves for the different door-open times, with reinforcement rate expressed as a percentage of the FR 1 baseline rates of responding. Increasing FR values decreased reinforcement rates, and essential values derived from these demand curves are shown in Table 1. These normalized demand curves were similar to each other, and although derived essential value measures of reinforcer effectiveness trended toward higher values for longer door-open times, this difference did not meet the criterion for significance.
Figure 2. Social reinforcement has higher elasticity of demand than food reinforcement.
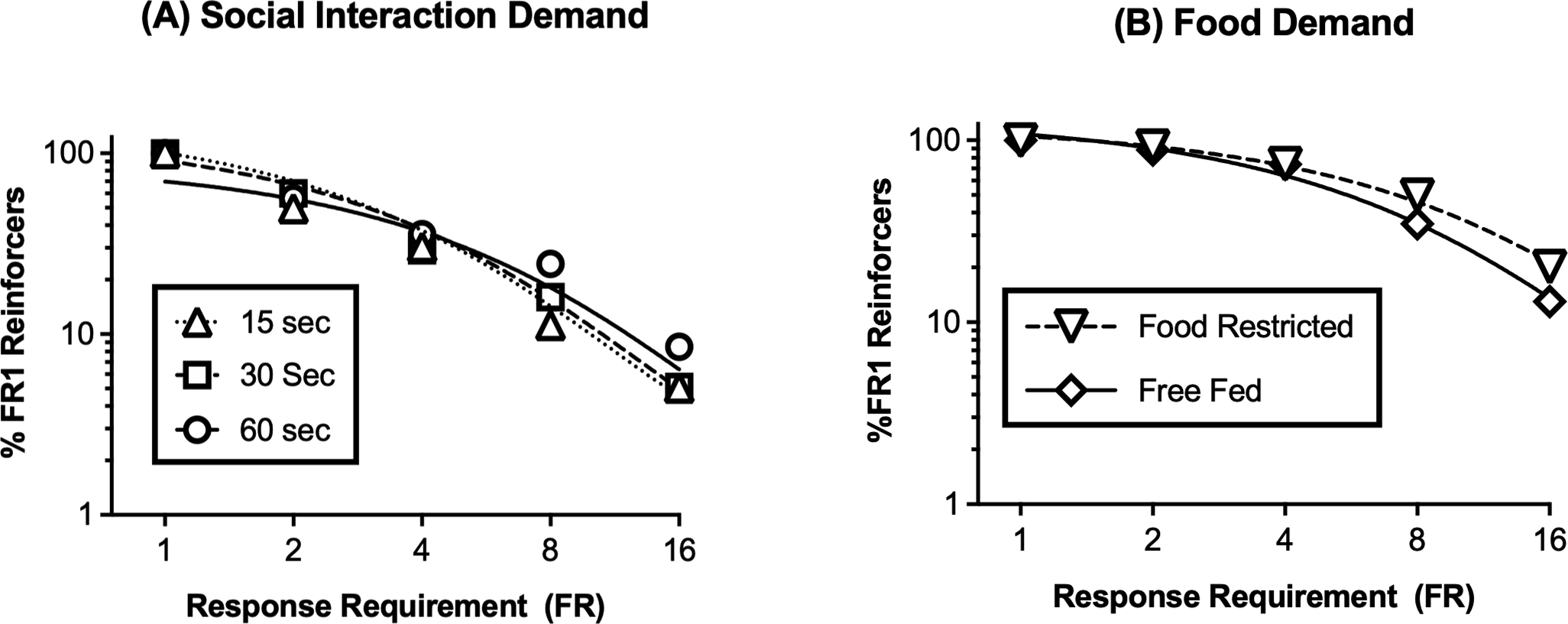
(A) Demand curves for social reinforcement are similar across different door-open times. Abscissa: Response requirement (log scale). Ordinate: % FR 1 number of reinforcers per 30-min session (log scale). (B) Demand curves for food reinforcement are similar in the presence or absence of food restriction. Abscissa: Response requirement (log scale). Ordinate: % FR 1 number of reinforcers per session (log scale). Table 1 shows FR 1 reinforcement rate for each condition and essential values determined from each demand curve. All data show mean ± SEM for 6 rats.
Food-Maintained Responding.
Food pellets also functioned as a reinforcer. During initial training and testing, rats were food restricted, responding was established under the FR 1:TO 30” schedule, and the number of response requirements completed per 30-min session decreased significantly from a mean (95% CL) pre-extinction level of 41.3 (32.6–50.0) on the last day of training to 20.7 (18.6–22.8) by the third day of extinction (t(5)=6.257, p=0.002). Responding for food reinforcement was then reestablished under the FR 1:TO 30” schedule for one week, and food restriction continued during determination of the initial demand curve shown in Figure 2B. Table 1 shows that both the number of baseline reinforcers earned under the FR 1:TO 30” schedule and the essential value determined from the normalized demand curve were significantly higher for food than for social reinforcement. In an effort to reduce food demand to a level more comparable to that of social reinforcement, food restriction was terminated, rats had free access to food in the home cage for one week while responding, and the demand curve was then redetermined under free-feeding conditions. Free feeding did not significantly alter food demand, and both FR 1 reinforcers and essential value remained significantly higher for food than for social reinforcement (Figure 2B, Table 1).
Effects of IP acid, morphine, or ketoprofen alone
Social Self-Administration.
Mean (95%CL) baseline reinforcement rates maintained by 30-s door open times under an FR 1 schedule were 27.3 (21–33.7) after IP water and 23.3 (18.9–27.8) after SC saline as the vehicle controls for studies with IP acid alone and analgesics alone, respectively. Figure 3A shows that both IP acid alone [F(2.124, 10.62)=35.16, p<0.001] and morphine alone [F(2.989, 14.94)=23.10, p<0.001] decreased rates of social self-administration. The IP acid EC50 and morphine ED50 values are shown in Table 1. In contrast to morphine, 10 mg/kg ketoprofen did not affect rates of social reinforcement.
Figure 3. Social reinforcement is more sensitive than food reinforcement to treatment with IP acid alone or morphine alone.
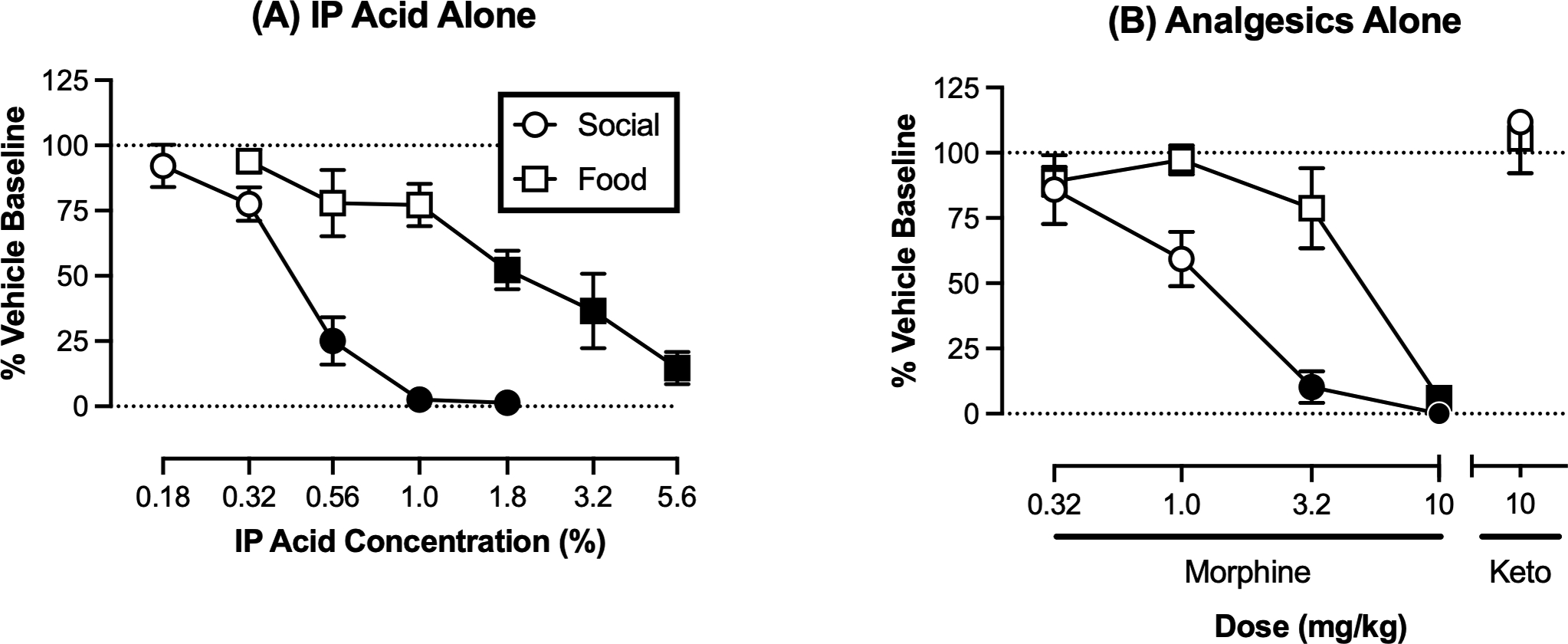
(A) IP acid is more potent to decrease social- vs. food self-administration. Abscissa: concentration of lactic acid administered IP in a volume of 1.0 ml/kg. Ordinate: number of reinforcers per 30-min session expressed as a percent of the vehicle (water) baseline. (B) Morphine was more potent to decrease social- vs. food self-administration, whereas ketoprofen did not affect responding for either reinforcer. Abscissa: morphine or ketoprofen (Keto) dose in mg/kg. Ordinate: number of reinforcers per session expressed as a percent of the vehicle (saline) baseline. Raw vehicle baseline data are reported in text, and Table 1 shows EC50/ED50 values determined from each concentration- or dose-effect curve. Filled points indicate values significantly different from vehicle baseline as determined by analysis of the raw data using one-way ANOVA followed by Dunnett’s post hoc test, p<0.05. All points show mean ± SEM from 6 rats.
Food Reinforcement.
Mean (95%CL) baseline reinforcement rates maintained by food delivery were 53.8 (52.0–55.7) after IP water and 50.8 (45.4–56.3) after SC saline as the vehicle controls for studies with IP acid alone and analgesics alone, respectively. Figure 3B shows that both IP acid alone [F(2.967, 14.84)=15.59, p<0.001] and morphine alone [F(1.512, 7.560)=25.05, p<0.001] decreased rates of food-maintained responding. IP acid EC50 and morphine ED50 values are shown in Table 1, and both were less potent to reduce responding maintained by food than by social interaction. Ketoprofen (10 mg/kg) did not alter food-maintained responding.
Effects of morphine or ketoprofen pretreatment to IP acid.
Social Reinforcement.
Figure 4A and 4B shows the effectiveness of morphine and ketoprofen to alleviate pain-related depression of social self-administration by IP acid. When responding was depressed by 1% IP acid in the first cohort of rats, there was not a significant effect of treatment [F(1.732,8.660)=3.431, p=0.0842]. Thus, neither morphine nor ketoprofen significantly alleviated 1% IP acid-induced depression of social self-administration. A second cohort of rats was trained to evaluate morphine and ketoprofen effects on behavioral depression produced by a lower concentration of 0.56% IP acid. The second group showed both (a) significant extinction [paired t-test of baseline vs. extinction conditions: t(5)=19.42, p<0.0001] and (b) significant depression of responding after pretreatment with 0.56% IP acid [paired t-test of IP water vs. IP acid conditions: t(5)=7.069, df=5, p<0.001)]. In this cohort, there was a significant effect of analgesic treatment [F(1.956, 9.781)=4.707, p=0.038]. Morphine still failed to alleviate IP acid induced depression of social self-administration, but relative to IP acid alone, 3.2 mg/kg morphine significantly decreased reinforcement rate. In contrast to morphine, 10 mg/kg ketoprofen significantly increased reinforcement rate, indicative of antinociception.
Figure 4. Ketoprofen, but not morphine, rescues pain-related depression of social reinforcement.
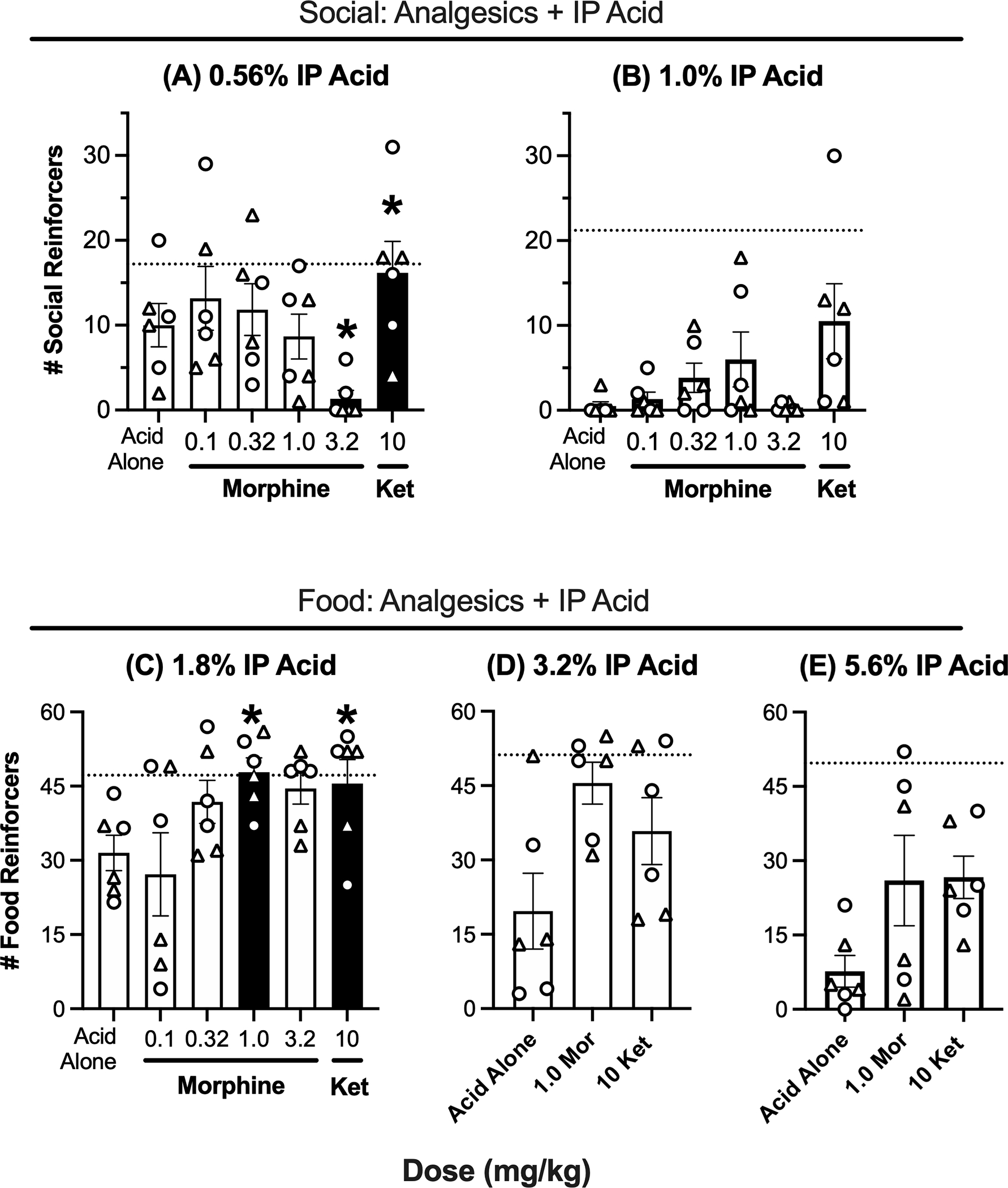
Effects of morphine or ketoprofen on social self-administration suppressed by (A) 0.56% IP acid or (B) 1.0% IP acid. Effects of morphine or ketoprofen on food reinforcement suppressed by (C)1.8, (D) 3.2, or (E) 5.6% IP acid. Abscissae: Dose of morphine or ketoprofen in mg/kg. Ordinates: Number of food reinforcers per 30-min session. All bars show mean ± SEM from 6 rats, and points show individual data (triangles for females, circles for males). Filled bars with asterisks indicate different from “Acid Alone” (p<0.05), and dotted line indicates baseline reinforcement rates before studies with each IP acid concentration.
Food-Maintained Responding.
Figure 4C–E shows the effectiveness of morphine and ketoprofen to alleviate IP acid-induced depression of food-maintained responding. When food-maintained responding was depressed by 1.8% IP acid, one-way ANOVA indicated a significant effect of treatment [F(1.926, 9.630)=4.765, p=0.037], and both 1.0 mg/kg morphine and 10 mg/kg ketoprofen produced significant antinociception. To minimize the number of tests with higher acid concentrations, only 1.0 mg/kg morphine and 10 mg/kg ketoprofen were tested with 3.2% and 5.6% IP acid. At 3.2% IP acid, there was a significant effect of treatment [F(1.650, 8.251)=5.336, p=0.037] with a trend toward antinociception with both analgesics; however, Dunnett’s post hoc test indicated that neither morphine (p=0.068) nor ketoprofen (p=0.076) met the criterion for significant antinociception. At 5.6% IP acid, neither morphine nor ketoprofen produced significant antinociception [F(1.505, 7.525)=2.828, p=0.128].
DISCUSSION
Reinforcing Effects of Access to Social Interaction.
The present results confirm that transient social access to a familiar conspecific can function as an operant reinforcer in individually housed rats (Venniro et al. 2020; Venniro et al. 2019; Venniro and Shaham 2020; Venniro et al. 2018). Specifically, responding extinguished when response-contingent social access was removed, responding recovered when social access was reinstated, and a reduction in the duration of access time from 30 to 15 s significantly reduced the total time of social access during self-administration sessions. A point of methodological importance with the apparatus used here is that significant extinction required absence of the reinforcer rat. Thus, even when responding did not open the door to allow social interaction, the reinforcer rat may have been a source of noncontingent stimuli (e.g. auditory or olfactory stimuli) that supplemented programmed discriminative stimuli to set the occasion for operant responding.
Food delivery also served as an operant reinforcer under a reinforcement schedule similar to that used for social self-administration; however, three findings suggested that social interaction with a peer functioned as a weaker reinforcer than food delivery. First, rats earned significantly fewer social reinforcers than food reinforcers during comparable 30-min sessions. Second, demand-curve analysis indicated that responding for social interaction was more sensitive than food-maintained responding to increases in the response requirement. Lastly, as will be discussed in more detail below, responding for social interaction was more sensitive to disruption by treatment with either IP acid or morphine. The weaker reinforcing efficacy of social interaction was observed even though rats were nominally deprived of social interaction (by virtue of being individually housed) and even when rats responding for food had free access to food in their home cage. Nonetheless, the relative reinforcing effectiveness of social interaction and food should be narrowly interpreted in consideration of the specific parameters of the procedures used here, because factors other than reinforcer type can influence overall reinforcing effectiveness. For example, social interaction was constrained by the grid that separated the two compartments of the operant conditioning chamber, and opportunities for more extensive social interaction may function as stronger reinforcers (Trezza et al. 2011) Conversely, only one food-reinforcer magnitude was tested (a single 45-mg pellet), and smaller magnitudes would be expected to function as weaker reinforcers (Hodos 1961).
Effects of IP acid and analgesics alone.
The present results extend the range of conditions under which IP acid can serve as an acute, visceral noxious stimulus to produce pain-related behavioral depression in laboratory animals. For example, IP acid also produces concentration-dependent decreases in operant responding for food and electrical brain stimulation in rats (Cone et al. 2018; Pereira Do Carmo et al. 2009; Reiner et al. 2021). However, IP acid was more potent to decrease responding for social interaction than for food or electrical brain stimulation. For example, 1.0% IP acid nearly eliminated responding for social interaction while having little effect on responding for food in this study or for electrical brain stimulation reported previously (Pereira Do Carmo et al. 2009). This finding also agrees with a previous report of reduced social interaction in rats in a model of more chronic inflammatory pain (28 days after intraplantar injection of complete Freund’s adjuvant) (Parent et al. 2012) despite a more transient effect of this treatment on food-maintained operant responding (Negus et al. 2020). On the other hand, the present findings in rats contrast with previous reports that experimental inflammatory and neuropathic pain states often have little effect on metrics of unconditioned social behavior in mice (Liu et al. 2015; Sheahan et al. 2017; Urban et al. 2011).
The present results are also consistent with previous studies showing that morphine and other MOR agonists dose-dependently decrease operant responding maintained by various reinforcer types including food (Cone et al. 2018; Hiranita et al. 2019; Leander 1980; Picker et al. 1992). As with IP acid, morphine was more potent to decrease responding for social interaction than for food in this study or in previous studies in rats (Hiranita et al. 2019; Leander 1980; Picker et al. 1992). MORs may play a role in mediating some forms of social behavior, and MOR agonists like morphine can increase social behavior in rats under some circumstances (Panksepp et al. 1980; Trezza et al. 2011; Vanderschuren et al. 2016). Additionally, MOR agonists can also increase rates of operant responding under some circumstances (e.g. when responding is maintained by low frequencies or intensities of electrical brain stimulation) (Negus and Miller 2014; Negus and Moerke 2019). However, there was no evidence here to suggest that morphine increased operant responding for social interaction. The present results with ketoprofen are consistent with previous findings that ketoprofen has little effect on operant responding in rats (Leitl and Negus 2016).
Effect of analgesics on IP acid-induced behavioral depression.
Analgesic effectiveness to alleviate IP acid-induced depression of operant responding was influenced by the type of reinforcing stimulus, the magnitude of pain-related behavioral depression, and the analgesic mechanism of action. In general, ketoprofen and morphine were most effective to rescue partial behavioral depression produced by relatively low noxious stimulus intensities, and effectiveness decreased at higher noxious stimulus intensities that produced greater levels of behavioral depression. This pattern was independent of absolute noxious stimulus intensity. For example, 1.8% IP acid produced only a partial depression of food-maintained operant responding, and both morphine and ketoprofen alleviated this effect. However, social self-administration was more sensitive to IP acid effects, a lower concentration of 1.0% IP acid nearly eliminated responding, and neither morphine nor ketoprofen rescued this level of behavioral depression despite the lower IP acid concentration. Ketoprofen did however block a lower level of behavioral depression produced by a lower IP acid concentration in rats responding for social interaction. Taken together, these findings are consistent with previous studies showing that antinociceptive potency and/or effectiveness of test drugs decreases as noxious stimulus intensity increases (Altarifi et al. 2015; Garner et al. 2021; Morgan and Picker 1996). The present results add the nuance that both sensitivity to pain-related behavioral depression and analgesic effectiveness to alleviate that behavioral depression can vary across behavioral endpoints.
The present results are also consistent with previous evidence to suggest that both opioids and NSAIDs are effective to alleviate pain-related depression of food-maintained operant responding in rats (Cone et al. 2018; Martin et al. 2004); however, in the present study, only the NSAID ketoprofen was effective in rats responding for social interaction. Morphine was not effective even at a low IP acid concentration that produced only a partial decrease in responding. This resistance of pain-depressed social self-administration to opioid antinociception requires further study, but it may reflect the high sensitivity of social self-administration to rate-decreasing effects of morphine. In any assay of pain-depressed behavior, antinociception is manifested by an increase in responding, and expression of these increases may be opposed and obscured by direct rate-decreasing effects of a test drug (see Figure 5). Morphine had higher potency to decrease rates of responding maintained by social interaction than by food delivery, and as a result, morphine’s rate-decreasing effects were more likely to obscure rescue of behavior by analgesic morphine doses in rats responding for social interaction than for food. This hypothesis suggests that a stronger social reinforcer might be more resistant to rate-decreasing effects of morphine and might be more likely to reveal morphine antinociceptive rescue of pain-depressed responding.
Figure 5. Analgesic rescue of pain-depressed behavior reflects an interaction between analgesia and motor/cognitive impairment.
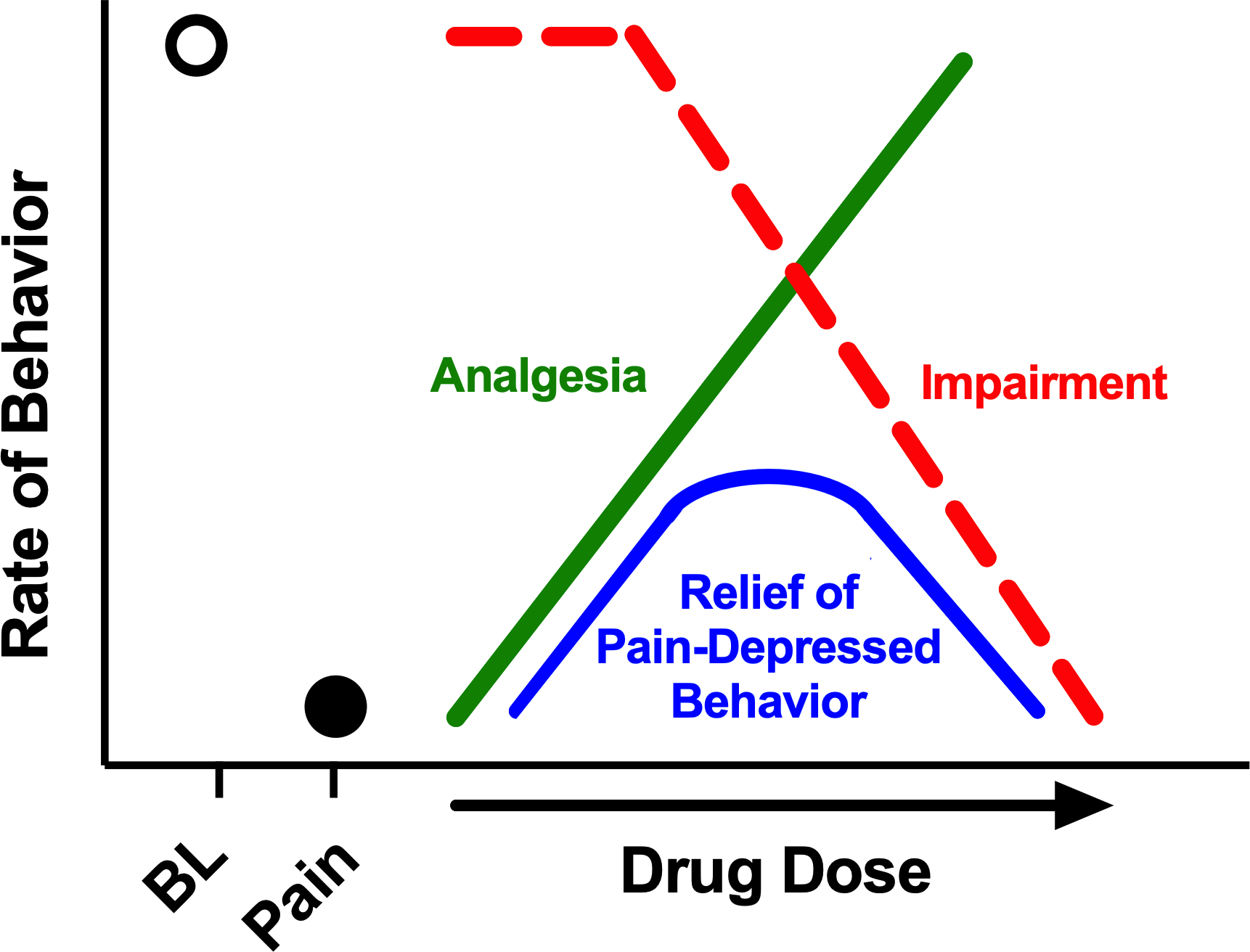
Under Baseline (BL) conditions, the target behavior occurs at a high rate, and experimental pain models (Pain) may reduce behavioral rates as a sign of pain-related behavioral depression. Drug-induced relief of pain-depressed behavior (blue) will depend on an interaction between analgesic effects (green), which increase behavioral rate, and motor/cognitive impairment effects (red), which decrease behavioral rate. As drug potency to produce impairment increases (i.e. left shifts in the impairment dose-effect curve), the potential for drug-induced relief of pain-depressed behavior decreases.
Summary.
The present results extend the range of conditions under which IP acid has been shown to produce pain-related behavioral depression in rodents. Additionally, these results show that pain-related depression and analgesic rescue of operant behavior can be influenced by interactions between the type of reinforcing stimulus, the intensity of noxious stimulus, and the magnitude of pain-related behavioral depression. We propose that this new operant social self-administration procedure can be used to model both a major pain-related human condition of disruption of social behavioral and the efficacy of potential antinociceptive medications in reversing this effect of pain.
Acknowledgements
This work was supported by NIDA-NIH grants P30-DA033934 and R00-DA047976 and by the Intramural Research Program of NIDA-NIH.
Footnotes
DECLARATIONS
On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
- Altarifi AA, Rice KC, Negus SS (2015) Effects of mu-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of mu-agonist efficacy and noxious stimulus intensity. J Pharmacol Exp Ther 352: 208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Pheby T, Huang W, Burgess G, Machin I, Rice AS (2012) Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain 16: 485–95. [DOI] [PubMed] [Google Scholar]
- Cabre F, Fernandez F, Zapatero MI, Arano A, Garcia ML, Mauleon D (1998) Intestinal ulcerogenic effect of S(+)-ketoprofen in the rat. J Clin Pharmacol 38: 27S–32S. [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM (1994) Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap 23: 129–38. [PubMed] [Google Scholar]
- Closs SJ, Staples V, Reid I, Bennett MI, Briggs M (2009) The impact of neuropathic pain on relationships. J Adv Nurs 65: 402–11. [DOI] [PubMed] [Google Scholar]
- Cone K, Lanpher J, Kinens A, Richard P, Couture S, Brackin R, Payne E, Harrington K, Rice KC, Stevenson GW (2018) Delta/mu opioid receptor interactions in operant conditioning assays of pain-depressed responding and drug-induced rate suppression: assessment of therapeutic index in male Sprague Dawley rats. Psychopharmacology (Berl) 235: 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawu W, Chao H, Kaiting L, Lan S, Xiaohua P, Dingqun B (2019) Pain correlates with social integration in individuals with traumatic spinal cord injury: A cross-sectional survey. J Rehabil Med 51: 506–512. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J, Immpact (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113: 9–19. [DOI] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ (2014) Differential suppression of intracranial self-stimulation, food-maintained operant responding, and open field activity by paw incision and spinal nerve ligation in rats. Anesth Analg 118: 854–62. [DOI] [PubMed] [Google Scholar]
- Garner JP, Marshall LS, Boyer NM, Alapatt V, Miller LL (2021) Effects of ketoprofen and morphine on pain-related depression of nestlet shredding in male and female mice. Front Pain Res In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Leon F, Felix JS, Restrepo LF, Reeves ME, Pennington AE, Obeng S, Avery BA, McCurdy CR, McMahon LR, Wilkerson JL (2019) The effects of mitragynine and morphine on schedule-controlled responding and antinociception in rats. Psychopharmacology (Berl) 236: 2725–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W (1961) Progressive ratio as a measure of reward strength. Science 134: 943–4. [DOI] [PubMed] [Google Scholar]
- Hursh S (2014) Behavioral economics and the analysis of consumption and choice. In: McSweeney FK, Murphy ES (eds) The Wiley Blackwell Handbook of Operant and Classical Conditioning. John Wiley and Sons, Chichester, West Sussex, UK, pp 275–305 [Google Scholar]
- Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychological Review 115: 186–198. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G (1995) Normalized demand for drugs and other reinforcers. J Exp Anal Behav 64: 373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumbo SU, MacDermid JC, Kalu ME, Packham TL, Athwal GS, Faber KJ (2021) Measurement Properties of the Brief Pain Inventory-Short Form (BPI-SF) and Revised Short McGill Pain Questionnaire Version-2 (SF-MPQ-2) in Pain-related Musculoskeletal Conditions: A Systematic Review. Clin J Pain 37: 454–474. [DOI] [PubMed] [Google Scholar]
- Kandasamy R, Calsbeek JJ, Morgan MM (2016) Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. J Neurosci Methods 263: 115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Calsbeek JJ, Morgan MM (2017) Analysis of inflammation-induced depression of home cage wheel running in rats reveals the difference between opioid antinociception and restoration of function. Behav Brain Res 317: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilasz AJ, Negus SS (2012) Dissociable effects of the cannabinoid receptor agonists Delta9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther 343: 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander JD (1980) Comparison of morphine, meperidine, anileridine, and alphaprodine on schedule-controlled responding and analgesia. Pharmacol Biochem Behav 12: 797–801. [DOI] [PubMed] [Google Scholar]
- Legakis LP, Diester CM, Townsend EA, Karim-Nejad L, Negus SS (2019) Comparison of chemotherapy effects on mechanical sensitivity and food-maintained operant responding in male and female rats. Behav Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis LP, Karim-Nejad L, Negus SS (2020) Effects of repeated treatment with monoamine-transporter-inhibitor antidepressants on pain-related depression of intracranial self-stimulation in rats. Psychopharmacology (Berl) 237: 2201–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Negus SS (2016) Pharmacological modulation of neuropathic pain-related depression of behavior: effects of morphine, ketoprofen, bupropion and [INCREMENT]9-tetrahydrocannabinol on formalin-induced depression of intracranial self-stimulation in rats. Behav Pharmacol 27: 364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA Jr., Banks ML, Negus SS (2014) Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous kappa-opioids. Neuropsychopharmacology 39: 614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang L, Yu J, Zhang YQ (2015) Persistent, comorbid pain and anxiety can be uncoupled in a mouse model. Physiol Behav 151: 55–63. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC (2004) Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology 101: 191–203. [DOI] [PubMed] [Google Scholar]
- Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN (2007) Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther 320: 194–201. [DOI] [PubMed] [Google Scholar]
- Miller LL, Altarifi AA, Negus SS (2015) Effects of repeated morphine on intracranial self-stimulation in male rats in the absence or presence of a noxious pain stimulus. Exp Clin Psychopharmacol 23: 405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, McCarthy MM, Sohrabji F, Schiebinger L, Wetherington CL, Makris S, Arnold AP, Einstein G, Miller VM, Sandberg K, Maier S, Cornelison TL, Clayton JA (2017) Considering sex as a biological variable in preclinical research. FASEB J 31: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Picker MJ (1996) Contribution of individual differences to discriminative stimulus, antinociceptive and rate-decreasing effects of opioids: importance of the drug’s relative intrinsic efficacy at the mu receptor. Behav Pharmacol 7: 261–284. [PubMed] [Google Scholar]
- Negus SS (2013) Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 42: 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS (2019) Core Outcome Measures in Preclinical Assessment of Candidate Analgesics. Pharmacol Rev 71: 225–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Marsh SA, Townsend EA (2020) Resistance of Food-Maintained Operant Responding to Mechanical Punishment in Rats: Further Evidence for Weak “Affective/Motivational Pain” in Rat Models of Inflammatory and Neuropathic Pain. Front Pharmacol 11: 615782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL (2014) Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev 66: 869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Moerke MJ (2019) Determinants of opioid abuse potential: Insights using intracranial self-stimulation. Peptides 112: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC (2010) Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 210: 149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC (2012) Effects of peripherally restricted kappa opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther 340: 501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D (2006) Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther 319: 507–14. [DOI] [PubMed] [Google Scholar]
- Okun A, McKinzie DL, Witkin JM, Remeniuk B, Husein O, Gleason SD, Oyarzo J, Navratilova E, McElroy B, Cowen S, Kennedy JD, Porreca F (2016) Hedonic and motivational responses to food reward are unchanged in rats with neuropathic pain. Pain 157: 2731–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG (1980) Endogenous opioids and social behavior. Neurosci Biobehav Rev 4: 473–87. [DOI] [PubMed] [Google Scholar]
- Parent AJ, Beaudet N, Beaudry H, Bergeron J, Berube P, Drolet G, Sarret P, Gendron L (2012) Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav Brain Res 229: 160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS (2009) Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain 144: 170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker MJ, Craft RM, Negus SS, Powell KR, Mattox SR, Jones SR, Hargrove BK, Dykstra LA (1992) Intermediate efficacy mu opioids: examination of their morphine-like stimulus effects and response rate-decreasing effects in morphine-tolerant rats. J Pharmacol Exp Ther 263: 668–81. [PubMed] [Google Scholar]
- Reiner DJ, Townsend EA, Orihuel J, Applebey SV, Claypool SM, Banks ML, Shaham Y, Negus SS (2021) Lack of effect of different pain-related manipulations on opioid self-administration, reinstatement of opioid seeking, and opioid choice in rats. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restoux LJ, Dasariraju SR, Ackerman IN, Van Doornum S, Romero L, Briggs AM (2020) Systematic Review of the Impact of Inflammatory Arthritis on Intimate Relationships and Sexual Function. Arthritis Care Res (Hoboken) 72: 41–62. [DOI] [PubMed] [Google Scholar]
- Scepovic Z, Radmanovic BZ (1984) Interaction between reserpine and non-steroidal anti-inflammatory agents in producing gastric ulcers in rats. Eur J Pharmacol 98: 445–8. [DOI] [PubMed] [Google Scholar]
- Sheahan TD, Siuda ER, Bruchas MR, Shepherd AJ, Mohapatra DP, Gereau RWt, Golden JP (2017) Inflammation and nerve injury minimally affect mouse voluntary behaviors proposed as indicators of pain. Neurobiol Pain 2: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shega JW, Andrew M, Hemmerich J, Cagney KA, Ersek M, Weiner DK, Dale W (2012) The relationship of pain and cognitive impairment with social vulnerability--an analysis of the Canadian Study of Health and Aging. Pain Med 13: 190–7. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Caine SB, Thomsen M, Banks ML (2019) Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 44: 2022–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Vanderschuren LJ (2011) Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci 1: 444–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI (2011) Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 152: 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Achterberg EJ, Trezza V (2016) The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev 70: 86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Russell TI, Ramsey LA, Richie CT, Lesscher HMB, Giovanetti SM, Messing RO, Shaham Y (2020) Abstinence-dependent dissociable central amygdala microcircuits control drug craving. Proc Natl Acad Sci U S A 117: 8126–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Russell TI, Zhang M, Shaham Y (2019) Operant Social Reward Decreases Incubation of Heroin Craving in Male and Female Rats. Biol Psychiatry 86: 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Shaham Y (2020) An operant social self-administration and choice model in rats. Nat Protoc 15: 1542–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, Morales M, Epstein DH, Shaham Y (2018) Volitional social interaction prevents drug addiction in rat models. Nat Neurosci 21: 1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Galuska CM, Hursh SR, Woods JH (2006) Relative reinforcing effects of cocaine, remifentanil, and their combination in rhesus monkeys. J Pharmacol Exp Ther 318: 223–9. [DOI] [PubMed] [Google Scholar]


