Abstract
Aims
Hyperkalaemia is observed frequently in heart failure (HF) patients and is associated with an impaired prognosis and underuse of mineralocorticoid receptor antagonists (MRAs). However, the effects of serum potassium on prescription of the full guideline recommended daily dose of 50 mg in real‐world daily practice are unknown. Therefore, we investigated serum potassium and its association with the prescribed MRA dose in a large cohort of chronic HF patients.
Methods and results
A total of 5346 patients with chronic HF with a left ventricular ejection fraction ≤40% from 34 Dutch outpatient HF clinics between 2013 and 2016 were analysed on serum potassium and MRA (spironolactone and eplenerone) dose. Data were stratified by potassium as a serum potassium level <4.0, 4.0 to 5.0 or >5.0 mmol/L. Multivariable logistic regression models were used to assess the association between serum potassium and MRA dose and to adjust for potential confounders. Mean serum potassium was 4.4 ± 0.5 mmol/L and hyperkalaemia (serum potassium >5.0 mmol/L) was present in 399 patients (7.5%). MRA was used in 3091 patients (58.1%). Patients with hyperkalaemia significantly less often received ≥100% of the target dose (50 mg) compared with patients with a serum potassium between 4.0–5.0 mmol/L and <4.0 mmol/L (7.7% vs. 9.5% vs. 13.6% respectively, P = 0.0078). In the multivariable regression analyses, patients with hyperkalaemia were significantly less likely to receive ≥100% of the target dose compared with patients with serum potassium 4.0–5.0 mmol/L (OR 0.38, 95% CI 0.15–0.97, P = 0.044). Additionally, a one unit increase in serum potassium was significantly associated with a lower odds of receiving ≥100% of the target dose (OR 0.69, 95% CI 0.49–0.98, P = 0.036).
Conclusions
In this large registry of real‐world chronic HF patients, both an increase in serum potassium and hyperkalaemia were associated with a lower odds of receiving the guideline‐recommended MRA dose.
Keywords: Heart failure, Heart failure with reduced ejection fraction, Hyperkalaemia, Mineralocorticoid receptor antagonists, Renin‐angiotensin‐aldosterone system inhibitors, Guidelines
Background
Hyperkalaemia, generally defined as a serum potassium level >5.0 mmol/L, 1 is frequently observed in heart failure (HF) patients, with reported incidences between 3.1% and 16.6%. 2 , 3 , 4 , 5 , 6 , 7 Hyperkalaemia is potentially a life‐threatening condition and is associated with impaired prognosis, especially in HF patients. 4 , 6 , 8 , 9 , 10 Although renin‐angiotensin‐aldosterone system inhibitors (RAASi) are among the cornerstone therapies for HF patients with a reduced ejection fraction (HFrEF), 11 their use is also an independent risk factor for hyperkalaemia. 12 , 13 , 14 , 15 , 16 The implementation of guideline‐directed medical therapy (GDMT) in HF patients is suboptimal and seems especially challenging for mineralocorticoid receptor antagonists (MRAs), 10 , 17 , 18 , 19 , 20 which might be due to the increased risk of hyperkalaemia. 21 Hyperkalaemia has been associated with MRA discontinuation and dose reduction 22 , 23 and, in addition, was found to be a predictor of receiving <50% of the target dose. 7 Because achieving the full target dose is the aim in clinical practice, it is interesting to know the association between serum potassium and prescription of MRA at the guideline recommended daily target dose, especially considering the level 1A recommendation of MRAs and the introduction of potassium binding drugs. However, real‐world data on the extent of hyperkalaemia in clinical practice and in relation to MRA use and dose are lacking but important to improve guideline implementation and very relevant for the perspective and future of potassium binding drugs.
Aims
This study aimed to investigate the distribution of serum potassium in a large cohort of chronic HF patients and the association between serum potassium, including hyperkalaemia, and the prescribed MRA (spironolactone and eplerenone) dose relative to the guideline recommended daily target dose of 50 mg.
Methods
For this study, data from the CHECK‐HF registry were used. The design and methods of the CHECK‐HF registry have been described in detail elsewhere. 24 In short, this cross‐sectional registry consisted of 10 910 chronic HF patients from 34 Dutch participating centres with data collected between 2013 and 2016. All patients were diagnosed with HF and were treated according to the 2012 ESC guideline for the diagnosis and treatment of acute and chronic HF. 25 Detailed information was collected on patient characteristics and HF drug prescriptions. The study was conducted in accordance with the Declaration of Helsinki, and ethical approval was provided for anonymously analysing existing patient data by the medical ethical committee of the Maastricht University Medical Center. For this analysis, patients with a left ventricular ejection fraction (LVEF) ≤ 40% were selected (N = 6256) (HFrEF). 11 Moreover, patients with missing information on serum potassium were excluded (N = 910), and a total of 5346 patients with an LVEF ≤40% were analysed in this study. Data were stratified by serum potassium categories <4.0, 4.0–5.0, and >5.0 mmol/L. For the MRA analyses, only spironolactone and eplerenone were included. A daily MRA dose of 50 mg in real‐world was considered 100% of the guideline‐recommended dose for both spironolactone and eplerenone, which is in line with the current ESC HF guideline. 11 The proportion of patients treated with an MRA was compared between the different potassium categories. Furthermore, the prescribed dose in categories of <50% (equal to <25 mg), 50%–99% (equal to 25–49 mg), and ≥100% (equal to ≥50 mg) of the guideline recommended target dose (≥50 mg) was compared by potassium category. Multivariable logistic regression analyses with the MRA dose categories as outcome were performed to further assess the association between serum potassium and MRA dose as odds ratios (ORs) with corresponding 95% confidence intervals (CIs). Serum potassium was included in the models either as continuous or categorical variable (<4.0, 4.0–5.0, and >5.0 mmol/L). For the latter, the 4.0–5.0 mmol/L serum potassium category was considered the reference group. In the multivariable models, the association between serum potassium and MRA dose was adjusted for age, sex, body mass index (BMI), systolic blood pressure (SBP), New York Heart Association (NYHA) class, QRS time, estimated glomerular filtration rate (eGFR), diabetes mellitus type 2 (DM2), chronic obstructive pulmonary disease (COPD), diuretics use, angiotensin‐converting enzyme inhibitor (ACEi) use, and angiotensin receptor blocker (ARB) use.
Results
Of all 5346 patients, 941 patients (17.6%) had a serum potassium <4.0 mmol/L, 4006 patients (74.9%) had a serum potassium 4.0–5.0 mmol/L, and 399 patients (7.5%) had a serum potassium >5.0 mmol/L. Mean serum potassium in the overall cohort was 4.36 ± 0.49 mmol/L (Figure 1 ). The baseline characteristics by serum potassium category are presented in Table 1 . Patients with a serum potassium >5.0 mmol/L were older, more often male, had worse renal function, were less often treated with diuretics, and more often treated with ACEi. A total of 3091 patients (58.1%) were treated with an MRA, of whom 3075 (99.5%) had complete data on the prescribed dose. Serum potassium in patients with <50%, 50%–99%, and ≥100% of MRA target dose was 4.41 ± 0.47, 4.35 ± 0.49, 4.26 ± 0.51 (P < 0.001), respectively (Figure 2 ). The MRA dose overall and by serum potassium category is presented in Table 2 . There were no significant differences in MRA use between patients with hyperkalaemia as compared with patients with serum potassium between 4.0–5.0 mmol/L and <4.0 mmol/L (55.8% vs. 58.2% vs. 58.6%, respectively). However, patients with hyperkalaemia significantly less often received ≥100% of the target dose compared with patients with a serum potassium between 4.0–5.0 mmol/L and <4.0 mmol/L (7.7% vs. 9.5% vs. 13.6% respectively, P = 0.0078).
Figure 1.
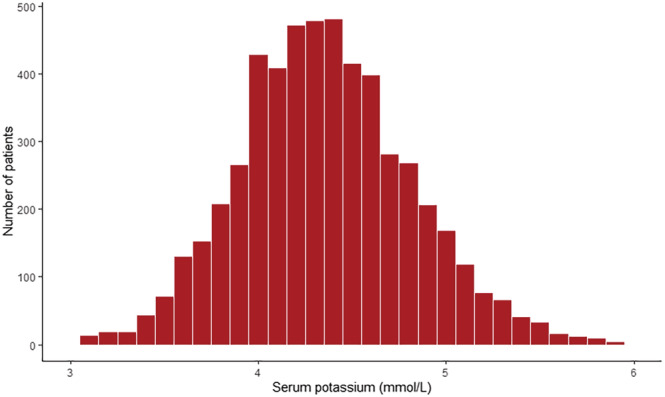
Serum potassium distribution in the overall cohort
Table 1.
Baseline characteristics by serum potassium category
| Missing (N, %) | Total (N = 5346) | Serum potassium <4.0 mmol/L (N = 941) | Serum potassium 4.0–5.0 mmol/L (N = 4006) | Serum potassium >5.0 mmol/L (N = 399) | P‐value for difference | |
|---|---|---|---|---|---|---|
| Age | 7 (0.1) | 72.2 ± 11.6 | 70.8 ± 12.2 | 72.4 ± 11.5 | 73.26 ± 10.76 | <0.001 |
| Sex (female) | 23 (0.4) | 1862 (35.0) | 372 (39.7) | 1358 (34.1) | 132 (33.1) | 0.0039 |
| BMI | 356 (6.7) | 27.3 ± 5.1 | 27.4 ± 5.3 | 27.3 ± 5.0 | 27.1 ± 5.1 | 0.59 |
| SBP | 47 (0.9) | 123.6 ± 19.7 | 123.2 ± 20.2 | 123.7 ± 19.5 | 123.0 ± 19.6 | 0.63 |
| DBP | 41 (0.8) | 70.6 ± 11.0 | 71.4 ± 11.5 | 70.6 ± 10.8 | 69.1 ± 11.6 | 0.0022 |
| HR | 57 (1.1) | 71.6 ± 13.7 | 73.0 ± 13.8 | 71.2 ± 13.7 | 72.0 ± 13.4 | 0.0014 |
| LVEF | 928 (17.4) | 30.3 ± 9.5 | 29.6 ± 9.3 | 30.3 ± 9.5 | 31.5 ± 9.9 | 0.012 |
| NYHA | <0.001 | |||||
| Class I | 63 (1.2) | 612 (11.6) | 106 (11.3) | 460 (11.6) | 46 (11.7) | |
| Class II | 3162 (59.9) | 520 (55.6) | 2416 (61.1) | 226 (57.5) | ||
| Class III | 1397 (26.4) | 288 (30.8) | 1008 (25.5) | 101 (25.7) | ||
| Class IV | 112 (2.1) | 21 (2.2) | 71 (1.8) | 20 (5.1) | ||
| Atrial fibrillation | 61 (1.1) | 1279 (24.2) | 225 (24.2) | 958 (24.2) | 96 (24.3) | 1.00 |
| LBBB | 0 (0.0) | 949 (17.8) | 172 (18.3) | 705 (17.6) | 72 (18.0) | 0.88 |
| QRS | 859 (16.1) | 128.1 ± 34.0 | 130.2 ± 35.2 | 127.8 ± 33.71 | 126.3 ± 34.5 | 0.12 |
| Primary aetiology | 0.19 | |||||
| Ischaemic | 0 (0.0) | 2616 (48.9) | 436 (46.3) | 1978 (49.4) | 202 (50.6) | |
| Non‐ischaemic | 2730 (51.1) | 505 (53.7) | 2028 (50.62) | 197 (49.4) | ||
| Hypertension | 611 (11.4) | 1879 (39.7) | 327 (39.4) | 1404 (39.6) | 148 (40.9) | 0.88 |
| Diabetes type 2 | 611 (11.4) | 1234 (26.1) | 191 (23.0) | 920 (26.0) | 123 (34.0) | <0.001 |
| COPD | 611 (11.4) | 886 (18.7) | 152 (18.3) | 668 (18.9) | 66 (18.2) | 0.90 |
| OSAS | 611 (11.4) | 295 (6.2) | 62 (7.5) | 211 (6.0) | 22 (6.1) | 0.27 |
| Thyroid disease | 611 (11.4) | 340 (7.2) | 60 (7.2) | 257 (7.3) | 23 (6.4) | 0.82 |
| eGFR | 771 (14.4) | 61.0 ± 24.8 | 65.1 ± 24.3 | 61.1 ± 24.4 | 51.0 ± 27.2 | <0.001 |
| eGFR | <0.001 | |||||
| <30 | 476 (10.4) | 68 (8.6) | 323 (9.4) | 85 (23.9) | ||
| 30–44 | 872 (19.1) | 110 (13.9) | 671 (19.6) | 91 (25.6) | ||
| 45–60 | 972 (21.2) | 157 (19.9) | 749 (21.8) | 66 (18.6) | ||
| ≥ 60 | 2255 (49.3) | 455 (57.6) | 1687 (49.2) | 113 (31.8) | ||
| Diuretics | 24 (0.4) | 4554 (85.2) | 839 (89.4) | 3368 (84.5) | 329 (83.1) | <0.001 |
| ACEi | 24 (0.4) | 3043 (57.2) | 504 (53.7) | 2299 (57.7) | 240 (60.6) | 0.030 |
| ARB | 24 (0.4) | 1389 (26.1) | 229 (24.4) | 1055 (26.5) | 105 (26.5) | 0.42 |
| Beta‐blocker | 24 (0.4) | 4293 (80.7) | 729 (77.6) | 3238 (81.2) | 326 (82.3) | 0.030 |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; LBBB, left bundle branch block; COPD, chronic obstructive pulmonary disease; OSAS, obstructive sleep apnoea syndrome; eGFR, estimated glomerular filtration rate; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Figure 2.
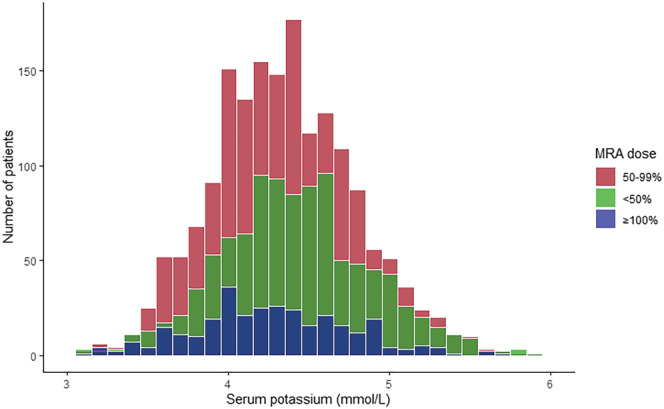
Serum potassium distribution by MRA dose. MRA, mineralocorticoid receptor antagonist
Table 2.
MRA dose by serum potassium category
| Missing (N, %) | Total (N = 5346) | Serum potassium <4.0 mmol/L (N = 941) | Serum potassium 4.0–5.0 mmol/L (N = 4006) | Serum potassium >5.0 mmol/L (N = 399) | P‐value for difference | |
|---|---|---|---|---|---|---|
| MRA use | 24 (0.4) | 3091 (58.1) | 550 (58.6) | 2320 (58.2) | 221 (55.8) | 0.62 |
| MRA dose | ||||||
| <50% (<25 mg) | 16 (0.5) | 1018 (33.1) | 159 (28.9) | 770 (33.4) | 89 (40.3) | 0.0082 |
| 50%–99% (25–49 mg) | 1745 (56.7) | 316 (57.5) | 1314 (57.0) | 115 (52.0) | 0.34 | |
| ≥100% (≥50 mg) | 312 (10.1) | 75 (13.6) | 220 (9.5) | 17 (7.7) | 0.0078 |
Abbreviation: MRA, mineralocorticoid receptor antagonist.
The results of the logistic regression analyses are summarized in Table 3 . In the multivariable models, a one unit increase in serum potassium was associated with both a lower odds of receiving ≥100% of the target dose (OR 0.69, 95% CI 0.49–0.98, P = 0.036) and a higher odds of receiving <50% of the target dose (OR 1.47, 95% CI 1.18–1.83, P < 0.001). Furthermore, patients with hyperkalaemia were significantly less likely to receive ≥100% of the target dose (OR 0.38, 95% CI 0.15–0.97, P = 0.044) and significantly more likely to receive <50% of the target dose (OR 1.55, 95% CI 1.07–2.26, P = 0.021).
Table 3.
Logistic regression analysis results
| Univariable model (N = 3075) | Multivariable model (N = 1799) | |||||
|---|---|---|---|---|---|---|
| Serum potassium | OR | 95% CI | P‐value | OR | 95% CI | P‐value |
| MRA dose <50% (<25 mg) | ||||||
| Continuous | 1.35 | 1.15–1.57 | <0.001 | 1.47 | 1.18–1.83 | <0.001 |
| Categorical | ||||||
| <4.0 | 0.81 | 0.66–0.99 | 0.043 | 0.80 | 0.60–1.07 | 0.13 |
| 4.0–5.0 | Reference | Reference | Reference | Reference | Reference | Reference |
| >5.0 | 1.34 | 1.01–1.78 | 0.041 | 1.55 | 1.07–2.26 | 0.021 |
| MRA dose 50%–99% (25–49 mg) | ||||||
| Continuous | 0.90 | 0.78–1.04 | 0.17 | 0.82 | 0.67–1.01 | 0.064 |
| Categorical | ||||||
| <4.0 | 1.02 | 0.84–1.23 | 0.86 | 1.14 | 0.88–1.47 | 0.32 |
| 4.0–5.0 | Reference | Reference | Reference | Reference | Reference | Reference |
| >5.0 | 0.82 | 0.62–1.08 | 0.15 | 0.81 | 0.56–1.17 | 0.27 |
| MRA dose ≥100% (≥50 mg) | ||||||
| Continuous | 0.62 | 0.48–0.80 | <0.001 | 0.69 | 0.49–0.98 | 0.036 |
| Categorical | ||||||
| <4.0 | 1.50 | 1.13–1.98 | 0.0049 | 1.07 | 0.71–1.60 | 0.75 |
| 4.0–5.0 | Reference | Reference | Reference | Reference | Reference | Reference |
| >5.0 | 0.79 | 0.47–1.32 | 0.367 | 0.38 | 0.15–0.97 | 0.044 |
Note: Multivariable models were adjusted for age, sex, body mass Index (BMI), systolic blood pressure (SBP), New York Heart Association (NYHA) class, QRS time, estimated glomerular filtration rate (eGFR), diabetes mellitus type 2 (DM2), chronic obstructive pulmonary disease (COPD), diuretics use, angiotensin‐converting enzyme inhibitors (ACEi) use, and angiotensin receptor blocker (ARB) use.
Abbreviations: MRA, mineralocorticoid receptor antagonist; OR, odds ratio; 95% CI, 95% confidence interval.
Discussion
In this large cohort of real‐world chronic HF patients, hyperkalaemia was present in 7.5% of the patients, which is similar to earlier studies. 3 , 4 , 5 , 6 , 8 Interestingly, the use of MRA overall was not associated with serum potassium and hyperkalaemia, but both hyperkalaemia and a one unit increase in serum potassium were significantly associated with a lower odds of receiving ≥100% and a higher odds of receiving <50% of the guideline‐recommended target dose.
Earlier studies found hyperkalaemia to be associated with non‐use and discontinuation of MRAs, 10 , 21 and a higher odds of receiving <50% of the guideline‐recommended MRA target dose. 7 To the best of our knowledge, serum potassium has not been studied in relation to MRA treatment at the guideline‐recommended dose. Compared with the earlier research, our study contained more recent data and also explored the association with ≥100% of the target dose and showed that hyperkalaemia was associated with a 62% lower odds of receiving the guideline‐recommended dose. Furthermore, our study showed that an increase in serum potassium itself was significantly associated with a lower odds of receiving ≥100% of the target dose, which is important information in light of the introduction of potassium binding drugs. The current study therefore adds significantly to current literature and indicates that there is room for further improvement of MRA treatment in this subgroup of HFrEF patients.
Maintaining normal serum potassium levels is important but can be especially challenging with MRA use. Although the guideline‐recommended target dose for MRAs is 50 mg, a large proportion of patients in the two landmark trials, EMPHASIS‐HF and RALES, did not achieve this dose, but nevertheless, a clear benefit from MRAs was shown. 26 , 27 It therefore may deserve consideration to lower the dose rather than to discontinue the MRA completely, as benefit is still likely to be present even at a lower dose. Two potassium binding drugs, sodium zirconium cyclosilicate (ZS‐9) and patiromer, have been shown effective in maintaining normal serum potassium levels and decreasing recurrent episodes of hyperkalemia. 28 , 29 The recently completed DIAMOND trial reported that the use of patiromer was associated with significantly lower serum potassium, fewer hyperkalaemia episodes, concurrent use of high doses of MRAs, and overall higher RAASi use in patients with HFrEF and RAASi‐related hyperkalaemia. 30 Unfortunately, although initially planned to do so, the DIAMOND trial did not completely answer the question whether optimizing RAASi therapy in combination with patiromer improves outcomes. 31 , 32 Potassium binding drugs have been recommended to maintain normal serum potassium levels during RAASi therapy in two recent expert consensus documents 33 , 34 but are not included in the current guideline. 11 The future of potassium binding drugs in clinical practice is unclear, especially considering the limited observed recurrent hyperkalaemia and high number needed to treat. 35
Study limitations
This study has a number of limitations to consider. Due to the cross‐section nature of the data, it was not possible to assess any clinical responses to lowering the dose or discontinuing the MRA in patient with hyperkalaemia. However, despite being a cross‐sectional study, our findings are relevant and important as they provide insight in the association between serum potassium and MRA dose in a large real‐world population. Moreover, the data collection was performed between 2013 and 2016, which was a different period in terms of GDMT with the later introduction of the angiotensin receptor–neprilysin inhibitor (ARNI) and sodium–glucose co‐transporter 2 inhibitor (SGLT2i). 11 Nevertheless, we believe the results of this study are still relevant, considering the MRA was the main focus of this study and the recommended target dose for the MRA has remained unchanged.
Conclusions
Our real‐world data in a large cohort of HF patients with measured serum potassium are unique and show that both hyperkalaemia and an increase in serum potassium are associated with receiving a lower odds of receiving the full target MRA dose, which adds to the literature that higher serum potassium levels may also be an impediment for reaching the full MRA target dose.
Conflict of interest
None declared.
Funding
This work was supported by Servier, the Netherlands, who funded the inclusion of data and software program. The steering committee (H.P.B.L.R., G.C.M.L., and J.J.B.) received no funding for this project. This analysis was initiated by the authors and was designed, conducted, interpreted, and reported independently of the sponsor. The current study had no other funding source or any with a participating role in outcome assessment or writing of the manuscript.
Clephas, P. R. D. , Radhoe, S. P. , Linssen, G. C. M. , Langerveld, J. , Plomp, J. , Smits, J. P. P. , Nagelsmit, M. J. , Rocca, H.‐P. B.‐L. , and Brugts, J. J. (2023) Serum potassium level and mineralocorticoid receptor antagonist dose in a large cohort of chronic heart failure patients. ESC Heart Failure, 10: 1481–1487. 10.1002/ehf2.14285.
Pascal R. D. Clephas and Sumant P. Radhoe contributed equally.
References
- 1. Fudim M, Grodin JL, Mentz RJ. Hyperkalemia in heart failure: probably not O"K". J Am Heart Assoc. 2018; 7: e009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tromp J, Ter Maaten JM, Damman K, O'Connor CM, Metra M, Dittrich HC, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, van der Wal MH, Jaarsma T, van Veldhuisen DJ, Hillege HL, Voors AA, van der Meer P. Serum potassium levels and outcome in acute heart failure (data from the PROTECT and COACH trials). Am J Cardiol. 2017; 119: 290–296. [DOI] [PubMed] [Google Scholar]
- 3. Beusekamp JC, Tromp J, van der Wal HH, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Rossignol P, Zannad F, Voors AA, van der Meer P. Potassium and the use of renin‐angiotensin‐aldosterone system inhibitors in heart failure with reduced ejection fraction: data from BIOSTAT‐CHF. Eur J Heart Fail. 2018; 20: 923–930. [DOI] [PubMed] [Google Scholar]
- 4. Cooper LB, Benson L, Mentz RJ, Savarese G, DeVore AD, Carrero JJ, Dahlstrom U, Anker SD, Lainscak M, Hernandez AF, Pitt B, Lund LH. Association between potassium level and outcomes in heart failure with reduced ejection fraction: a cohort study from the Swedish heart failure registry. Eur J Heart Fail. 2020; 22: 1390–1398. [DOI] [PubMed] [Google Scholar]
- 5. Crespo‐Leiro MG, Barge‐Caballero E, Segovia‐Cubero J, Gonzalez‐Costello J, Lopez‐Fernandez S, Garcia‐Pinilla JM, Almenar‐Bonet L, de Juan‐Baguda J, Roig‐Minguell E, Bayes‐Genis A, Sanz‐Julve M, Lambert‐Rodriguez JL, Lara‐Padron A, Perez‐Ruiz JM, Fernandez‐Vivancos Marquina C, de la Fuente‐Galan L, Varela‐Roman A, Torres‐Calvo F, Andres‐Novales J, Escudero‐Gonzalez A, Pascual‐Figal DA, Ridocci‐Soriano F, Sahuquillo‐Martinez A, Bierge‐Valero D, Epelde‐Gonzalo F, Gallego‐Page JC, Dalmau Gonzalez‐Gallarza R, Bover‐Freire R, Quiles‐Granado J, Maggioni AP, Lund LH, Muniz J, Delgado‐Jimenez J. Hyperkalemia in heart failure patients in Spain and its impact on guidelines and recommendations: ESC‐EORP‐HFA heart failure long‐term registry. Rev Esp Cardiol (Engl Ed). 2020; 73: 313–323. [DOI] [PubMed] [Google Scholar]
- 6. Aldahl M, Jensen AC, Davidsen L, Eriksen MA, Moller Hansen S, Nielsen BJ, Krogager ML, Kober L, Torp‐Pedersen C, Sogaard P. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J. 2017; 38: 2890–2896. [DOI] [PubMed] [Google Scholar]
- 7. Rossignol P, Lainscak M, Crespo‐Leiro MG, Laroche C, Piepoli MF, Filippatos G, Rosano GMC, Savarese G, Anker SD, Seferovic PM, Ruschitzka F, Coats AJS, Mebazaa A, McDonagh T, Sahuquillo A, Penco M, Maggioni AP, Lund LH. Heart failure long‐term registry Investigators G. unravelling the interplay between hyperkalaemia, renin‐angiotensin‐aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC‐HFA‐EORP heart failure long‐term registry. Eur J Heart Fail. 2020; 22: 1378–1389. [DOI] [PubMed] [Google Scholar]
- 8. Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, Bushinsky DA. Association of Serum Potassium with all‐cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017; 46: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferreira JP, Butler J, Rossignol P, Pitt B, Anker SD, Kosiborod M, Lund LH, Bakris GL, Weir MR, Zannad F. Abnormalities of potassium in heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020; 75: 2836–2850. [DOI] [PubMed] [Google Scholar]
- 10. Volterrani M, Perrone V, Sangiorgi D, Giacomini E, Iellamo F, Degli Esposti L, on the behalf of a LSG . Effects of hyperkalaemia and non‐adherence to renin‐angiotensin‐aldosterone system inhibitor therapy in patients with heart failure in Italy: a propensity‐matched study. Eur J Heart Fail. 2020; 22: 2049–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, Group ESCSD . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 12. Poggio R, Grancelli HO, Miriuka SG. Understanding the risk of hyperkalaemia in heart failure: role of aldosterone antagonism. Postgrad Med J. 2010; 86: 136–142. [DOI] [PubMed] [Google Scholar]
- 13. Michel A, Martin‐Perez M, Ruigomez A, Garcia Rodriguez LA. Risk factors for hyperkalaemia in a cohort of patients with newly diagnosed heart failure: a nested case‐control study in UK general practice. Eur J Heart Fail. 2015; 17: 205–213. [DOI] [PubMed] [Google Scholar]
- 14. Desai AS, Swedberg K, McMurray JJ, Granger CB, Yusuf S, Young JB, Dunlap ME, Solomon SD, Hainer JW, Olofsson B, Michelson EL, Pfeffer MA, Investigators CP. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM program. J Am Coll Cardiol. 2007; 50: 1959–1966. [DOI] [PubMed] [Google Scholar]
- 15. Vardeny O, Claggett B, Anand I, Rossignol P, Desai AS, Zannad F, Pitt B, Solomon SD. Randomized Aldactone evaluation study I. incidence, predictors, and outcomes related to hypo‐ and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014; 7: 573–579. [DOI] [PubMed] [Google Scholar]
- 16. Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, Shi H, Messig M, Vincent J, Girerd N, Bakris G, Pitt B, Zannad F. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in mild patients hospitalization and survival study in heart failure (EMPHASIS‐HF). Circ Heart Fail. 2014; 7: 51–58. [DOI] [PubMed] [Google Scholar]
- 17. Brunner‐La Rocca HP, Linssen GC, Smeele FJ, van Drimmelen AA, Schaafsma HJ, Westendorp PH, Rademaker PC, van de Kamp HJ, Hoes AW, Brugts JJ, Investigators C‐H. Contemporary drug treatment of chronic heart failure with reduced ejection fraction: the CHECK‐HF registry. JACC Heart Fail. 2019; 7: 13–21. [DOI] [PubMed] [Google Scholar]
- 18. Thorvaldsen T, Benson L, Dahlstrom U, Edner M, Lund LH. Use of evidence‐based therapy and survival in heart failure in Sweden 2003‐2012. Eur J Heart Fail. 2016; 18: 503–511. [DOI] [PubMed] [Google Scholar]
- 19. Zahir D, Bonde A, Madelaire C, Malmborg M, Butt JH, Fosbol E, Gislason G, Torp‐Pedersen C, Andersson C, Rossignol P, McMurray JJV, Kober L, Schou M. Temporal trends in initiation of mineralocorticoid receptor antagonists and risk of subsequent withdrawal in patients with heart failure: a nationwide study in Denmark from 2003‐2017. Eur J Heart Fail. 2022; 24: 539–547. [DOI] [PubMed] [Google Scholar]
- 20. Savarese G, Bodegard J, Norhammar A, Sartipy P, Thuresson M, Cowie MR, Fonarow GC, Vaduganathan M, Coats AJS. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden). Eur J Heart Fail. 2021; 23: 1499–1511. [DOI] [PubMed] [Google Scholar]
- 21. Trevisan M, Fu EL, Xu Y, Savarese G, Dekker FW, Lund LH, Clase CM, Sjolander A, Carrero JJ. Stopping mineralocorticoid receptor antagonists after hyperkalaemia: trial emulation in data from routine care. Eur J Heart Fail. 2021; 23: 1698–1707. [DOI] [PubMed] [Google Scholar]
- 22. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlstrom U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish heart failure registry. Eur J Heart Fail. 2018; 20: 1326–1334. [DOI] [PubMed] [Google Scholar]
- 23. Trevisan M, de Deco P, Xu H, Evans M, Lindholm B, Bellocco R, Barany P, Jernberg T, Lund LH, Carrero JJ. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail. 2018; 20: 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brugts JJ, Linssen GCM, Hoes AW, Brunner‐La Rocca HP, investigators C‐H. Real‐world heart failure management in 10,910 patients with chronic heart failure in the Netherlands: design and rationale of the chronic heart failure ESC guideline‐based cardiology practice quality project (CHECK‐HF) registry. Neth Heart J. 2018; 26: 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Guidelines ESCCfP. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J. 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 26. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone evaluation study Investigators. N Engl J Med. 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 27. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Group E‐HS . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011; 364: 11–21.21073363 [Google Scholar]
- 28. Anker SD, Kosiborod M, Zannad F, Pina IL, McCullough PA, Filippatos G, van der Meer P, Ponikowski P, Rasmussen HS, Lavin PT, Singh B, Yang A, Deedwania P. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS‐9) in heart failure patients: results from a phase 3 randomized, double‐blind, placebo‐controlled trial. Eur J Heart Fail. 2015; 17: 1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pitt B, Bakris GL, Bushinsky DA, Garza D, Mayo MR, Stasiv Y, Christ‐Schmidt H, Berman L, Weir MR. Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail. 2015; 17: 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butler J, Anker SD, Lund LH, Coats AJS, Filippatos G, Siddiqi TJ, Friede T, Fabien V, Kosiborod M, Metra M, Piña IL, Pinto F, Rossignol P, van der Meer P, Bahit C, Belohlavek J, Böhm M, Brugts JJ, Cleland JGF, Ezekowitz J, Bayes‐Genis A, Gotsman I, Goudev A, Khintibidze I, Lindenfeld J, Mentz RJ, Merkely B, Montes EC, Mullens W, Nicolau JC, Parkhomenko A, Ponikowski P, Seferovic PM, Senni M, Shlyakhto E, Cohen‐Solal A, Szecsödy P, Jensen K, Dorigotti F, Weir MR, Pitt B. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J. 2022; 43: 4362–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Butler J, Anker SD, Siddiqi TJ, Coats AJS, Dorigotti F, Filippatos G, Friede T, Göhring UM, Kosiborod MN, Lund LH, Metra M. Patiromer for the management of hyperkalaemia in patients receiving renin‐angiotensin‐aldosterone system inhibitors for heart failure: design and rationale of the DIAMOND trial. Eur J Heart Fail. 2022; 24: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Butler J, Anker SD, Lund LH, Coats AJS, Filippatos G, Siddiqi TJ, Friede T, Fabien V, Kosiborod M, Metra M, Pina IL, Pinto F, Rossignol P, van der Meer P, Bahit C, Belohlavek J, Bohm M, Brugts JJ, Cleland JGF, Ezekowitz J, Bayes‐Genis A, Gotsman I, Goudev A, Khintibidze I, Lindenfeld J, Mentz RJ, Merkely B, Montes EC, Mullens W, Nicolau JC, Parkhomenko A, Ponikowski P, Seferovic PM, Senni M, Shlyakhto E, Cohen‐Solal A, Szecsody P, Jensen K, Dorigotti F, Weir MR, Pitt B. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J. 2022; 43: 4362–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosano GMC, Tamargo J, Kjeldsen KP, Lainscak M, Agewall S, Anker SD, Ceconi C, Coats AJS, Drexel H, Filippatos G, Kaski JC, Lund L, Niessner A, Ponikowski P, Savarese G, Schmidt TA, Seferovic P, Wassmann S, Walther T, Lewis BS. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the working group on cardiovascular pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2018; 4: 180–188. [DOI] [PubMed] [Google Scholar]
- 34. Burton JO, Coats AJS, Kovesdy CP, Palmer BF, Pina IL, Rosano G, Sood MM, Zieroth S. An international Delphi consensus regarding best practice recommendations for hyperkalaemia across the cardiorenal spectrum. Eur J Heart Fail. 2022; 24: 1467–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Packer M. Potassium binders for patients with heart failure? The real enlightenment of the DIAMOND trial. Eur Heart J. 2022; 43: 4374–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]


