Abstract
Aims
Frailty in older patients with stage B heart failure with preserved ejection fraction (HFpEF) has not been fully explored. We evaluated the prevalence and prognostic significance of frailty in older patients diagnosed with stage B HFpEF.
Methods
Our prospective cohort study included inpatients aged ≥65 years who were followed up for 3 years. Stage B HFpEF was defined as cardiac structural or functional abnormalities with a left ventricular ejection fraction (LVEF) ≥ 50% without signs or symptoms. Frailty was assessed using the Fried phenotype. The primary outcome was 3‐year all‐cause mortality or readmission.
Results
Overall, 520 older inpatients diagnosed with stage B HFpEF [mean ± standard deviation age: 75.5 ± 6.25 years, male: 222 (42.7%)] were included in the study. Of these, 145 (27.9%) were frail. Frail patients were older (78.5 ± 6.23 vs. 74.3 ± 6.22 years, P < 0.001), with a lower body mass index (24.6 ± 3.60 vs. 25.7 ± 3.27 kg/m2, P = 0.001), higher level of N‐terminal pro‐B‐type natriuretic peptide [279 (interquartile range: 112.4, 596) vs. 140 (67.1, 266) pg/mL, P < 0.001], longer timed up‐and‐go test result (19.9 ± 9.71 vs. 13.3 ± 5.08 s, P < 0.001), and poorer performance in the short physical performance battery (4.1 ± 3.26 vs. 8.2 ± 2.62, P < 0.001), basic activities of daily living (BADL, 4.7 ± 1.71 vs. 5.7 ± 0.57, P < 0.001), and instrumental activities of daily living (IADL, 4.4 ± 2.73 vs. 7.4 ± 1.33, P < 0.001). Frail patients were more likely to have a Mini‐Mental State Examination (MMSE) score <24 (55.9% vs. 28.8%, P < 0.001) and take more than five medications (64.1% vs. 47.2%, P = 0.001). Frail patients had a higher incidence of all‐cause mortality or readmission (62.8% vs. 47.7%, P = 0.002), all‐cause readmission (56.6% vs. 45.9%, P = 0.029), and readmission for non‐heart failure (55.2% vs. 41.3%, P = 0.004) during the 3‐year follow‐up, with a 1.53‐fold (95%CI 1.11–2.11, P = 0.009) higher risk of all‐cause mortality or readmission, a 1.52‐fold (95%CI 1.09–2.11, P = 0.014) higher risk of all‐cause readmission, and a 1.70‐fold (95%CI 1.21–2.38, P = 0.002) higher risk of readmission for non‐clinical heart failure, adjusted for sex, age, polypharmacy, Athens Insomnia Scale, MMSE, LVEF, BADL, and IADL.
Conclusions
Frailty is common in elderly patients with stage B HFpEF. Physical frailty, particularly low physical activity, can independently predict the long‐term prognosis in these patients.
Keywords: Frailty, Stage B heart failure, Heart failure with preserved ejection fraction, Prognosis
Introduction
Heart failure (HF) is a clinical syndrome consisting of cardinal symptoms that may be accompanied by signs, which is caused by a structural and/or functional abnormality of the heart, resulting in elevated intracardiac pressure and/or inadequate cardiac output at rest and/or during exercise. 1 In recent years, with the ageing population worldwide, the incidence of HF, particularly HF with preserved ejection fraction (HFpEF), has increased and is becoming a global health problem, with poor quality of life, frequent hospital readmissions, high mortality, and high healthcare costs. 2 Frailty is a clinical syndrome characterized by weakness, decreased endurance, and slow performance, 3 which becomes more prevalent with age. 4
HF and frailty are both associated with multisystem dysfunction; therefore, frailty has gradually gained considerable attention in the field of HF. Numerous studies have shown that in clinical HF (stage C) or refractory HF (stage D) patients, frailty is common and significantly increases the risk of mortality and readmission. 4 Our previous studies have demonstrated that frailty, irrespective of the assessment method used, is highly prevalent and could independently predict poor outcomes among older patients with stage B HF (SBHF). 5 , 6 Additionally, the risk of HFpEF increases considerably with age, 7 and frailty could predict mortality and rehospitalisation in elderly patients with HFpEF. 8 Interestingly, a recent study showed that stage B HFpEF appears to represent a transitional state from a healthy heart to HF and exercise may delay this transition. 9 However, frailty in older patients with stage B HFpEF, which is a clinically reversible stage, has not yet been explored.
We aimed to evaluate the prevalence and prognostic significance of frailty in older patients with stage B HFpEF and hoped to provide a new way to improve outcomes in this population. Therefore, we conducted this study to estimate the prevalence and predictive value of frailty in older patients with stage B HFpEF.
Methods
Study design
The present study was a subgroup analysis of our previous prospective observational cohort study on frailty among elderly Chinese patients. We collected associated data from the previous cohort 10 and performed annual telephonic follow‐up for 3 years to collect information about mortality and readmissions in order to explore the prevalence and prognostic value of frailty in older patients with stage B HFpEF.
Participants
Our previous study recruited 1000 elderly inpatients from 10 wards, including medical and surgical departments, in a tertiary referral hospital in Beijing, China, from September 2018 to February 2019. We included inpatients ≥65 years old and excluded inpatients who refused to provide informed consent or were unable to complete the assessment procedure. 10 In our study, 480 patients were excluded due to a lack of echocardiographic data or missing criteria for stage B HFpEF. Finally, we enrolled 520 patients and followed them for 3 years (Figure 1 ). This study complied with the principles outlined in the Declaration of Helsinki. The research protocol was approved by our institutional ethics committee (approval no. 2018BJYYEC‐121‐02), and written informed consent was obtained from each patient, relative, or legal guardian.
Figure 1.
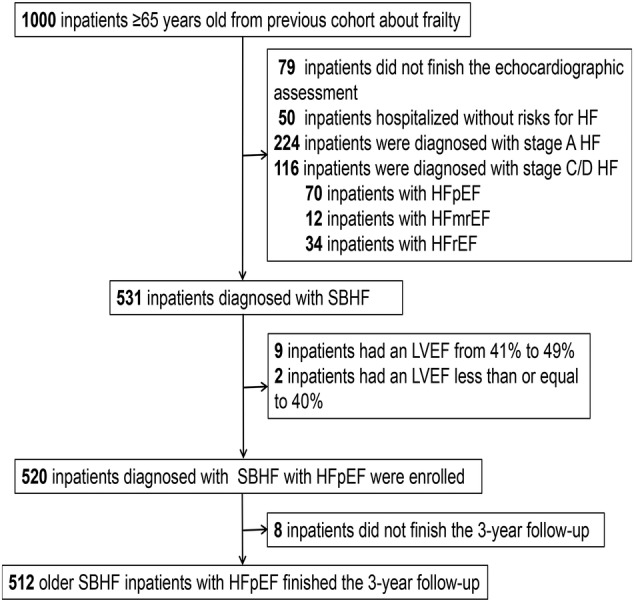
Flowchart of elderly inpatients with stage B HFpEF. HF, heart failure; HFmrEF, HF with mildly reduced ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; LVEF, left ventricular ejection fraction; SBHF, stage B HF.
Information collection
We collected demographic and baseline clinical data, including age, sex, smoking habit, drinking habit, body mass index (BMI), risk factors for HF (hypertension, coronary heart disease, diabetes mellitus, atrial fibrillation or atrial flutter), reasons for SBHF [left ventricular (LV) hypertrophy, LV enlargement, diastolic dysfunction, and valvular heart disease], chronic kidney disease, history of stroke, osteoporosis, sleep apnoea–hypopnoea syndrome, chronic respiratory disease, dyslipidaemia, history of surgery, polypharmacy (≥5 medications), LV ejection fraction (LVEF), and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) level. For the multidomain assessment of frailty, we collected information regarding social, physical, cognitive, and emotional components. All investigators underwent survey training before the start of patient recruitment, and the information collection and assessment of frailty were completed during hospitalisation. The study data were managed using the Research Electronic Data Capture (REDCap) software.
Definition of stage B HFpEF
SBHF refers to patients without current or prior symptoms or signs of HF but with at least one line of evidence of structural heart disease, abnormal cardiac function, or elevated natriuretic peptide levels or cardiac troponin levels. 11 Structural heart disease includes LV hypertrophy, cardiac chamber enlargement, ventricular wall motion abnormality, myocardial tissue abnormality, and valvular heart disease. Abnormal cardiac function includes reduced left or right ventricular systolic function, evidence of increased filling pressure, and abnormal diastolic dysfunction. The Atherosclerosis Risk in Communities study defined SBHF in the healthy population aged 67–91 years, which has been widely used. 12 Additionally, the Doppler Echocardiographic Measurements in Normal Chinese Adults study defined SBHF in the healthy yellow‐race population aged ≥65 years. 13 Considering the influence of age and race, we combined these two criteria to diagnose SBHF (Table 1 ). HFpEF was defined as LVEF ≥50% based on echocardiography. 14
Table 1.
Diagnostic criteria of stage B heart failure
| Male patients | Female patients | |
|---|---|---|
| Valvular heart disease | Moderate or greater stenosis or regurgitation in aortic or mitral valve | |
| Cardiac chamber enlargement | LVEDV/BSA > 60.2 mL/m2 | LVEDV/BSA > 51.9 mL/m2 |
| LV hypertrophy | LV mass/height2.7 > 45 g/m2 | LV mass/height2.7 > 41.5 g/m2 |
| Diastolic dysfunction | Septal e′ < 4.3 cm/s | Septal e′ < 4.1 cm/s |
| Septal E/e′ ratio < 14.8 | Septal E/e′ ratio < 17.4 | |
| LAAPD > 39.2 mm (aged 65–69 years) LAAPD > 40.3 mm (aged ≥70 years) | LAAPD > 38.3 mm (aged 65–69 years) LAAPD > 38.6 mm (aged ≥70 years) | |
| Systolic dysfunction | LVEF < 54.6% (aged 65–69 years) LVEF < 53% (aged ≥70 years) | LVEF < 54.5% (aged 65–69 years) LVEF < 53.5% (aged ≥70 years) |
BSA, body surface area; LAAPD, left atrial anteroposterior diameter; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction.
Assessment of frailty
Frailty was assessed using the Fried phenotype, which consists of five criteria: unintentional weight loss, exhaustion, low grip strength, slow walking speed, and low activity. Patients with a score ≥3 were diagnosed with frailty. 3
The social components of frailty included residence, the nature of employment, and marital status. Other assessments of physical frailty included the timed up‐and‐go test (TUG), short physical performance battery (SPPB), basic activities of daily living (BADL), instrumental activities of daily living (IADL), and history of falls in the past year. Cognitive and emotional statuses were assessed using the Mini‐Mental State Examination (MMSE) and the Hospital Anxiety and Depression Scale (HADS). We also assessed sleep disturbances using the Athens Insomnia Scale (AIS) to explore the relationship between insomnia and frailty.
Endpoints
The primary endpoint was the 3‐year all‐cause mortality or readmission rate. The secondary endpoints were all‐cause readmission, readmission for HF, and readmission for non‐ HF. Annual telephonic follow‐up was performed for 3 years after enrolment to confirm the occurrence of the endpoint events.
Statistical analysis
The Shapiro–Wilk test and quantile–quantile plots were used to evaluate the normal distribution of continuous variables. For baseline characteristics and multidimensional frailty evaluation, percentages, means ± standard deviations, and medians (interquartile ranges) were used to describe categorical variables, normally distributed continuous variables, and non‐normally distributed continuous variables, respectively, and the chi‐square test, two‐sample t‐test, and Mann–Whitney U test, respectively, were used to detect differences between the two groups (frail vs. non‐frail).
We used the Kaplan–Meier (log‐rank test) method to estimate survival without endpoint events. Multivariate Cox proportional‐hazard models were used to describe the association between possible prognostic factors and endpoint events, controlling for potential confounders. For the five components of the Fried phenotype, similar methods were performed to analyse the endpoint events. Univariate analysis was performed on clinical characteristics, including sex, age, smoking or drinking history, BMI, the nature of employment, marital status, hypertension, coronary artery disease, atrial fibrillation or atrial flutter, diabetes, chronic kidney disease, history of stroke, osteoporosis, sleep apnoea–hypopnoea syndrome, chronic respiratory disease, dyslipidaemia, cancer, history of surgery, polypharmacy, NT‐proBNP, and LVEF, as well as frailty components, including frailty assessed by the Fried phenotype, muscle weakness, slow gait, physical exhaustion, low physical activity, unintentional weight loss, BADL, IADL, falls in the past year, AIS, MMSE, and HADS. Potential confounders were partly selected based on univariate analysis, including age, frailty, polypharmacy, BADL, and IADL, which had P‐values <0.05 (Table 2 ), and partly on their clinical relevance, including LVEF, MMSE, AIS and sex. The results of the Cox proportional‐hazard models are expressed as hazard ratios and 95% confidence intervals (CIs).
Table 2.
Patient characteristics and the outcome of all‐cause death or readmission: results of the univariate analysis
| With endpoint events n = 270 | Without endpoint events n = 242 | P value | |
|---|---|---|---|
| Male sex | 112 (41.5) | 105 (43.4) | 0.914 |
| Age, years | 76.1 ± 6.30 | 74.8 ± 6.63 | 0.027 |
| Smoking history | 73 (27.0) | 62 (25.6) | 0.515 |
| Drinking history | 52 (19.3) | 50 (20.7) | 0.551 |
| BMI, kg/m2 | 25.5 ± 3.51 | 25.2 ± 3.29 | 0.581 |
| Manual worker | 96 (35.6) | 91 (37.6) | 0.691 |
| Married | 206 (76.3) | 175 (72.3) | 0.227 |
| Hypertension | 214 (79.3) | 186 (76.9) | 0.614 |
| Coronary artery disease | 157 (58.1) | 124 (51.2) | 0.192 |
| Atrial fibrillation or atrial flutter | 44 (16.3) | 31 (12.8) | 0.163 |
| Diabetes | 98 (36.3) | 74 (30.6) | 0.203 |
| Chronic kidney disease | 45 (16.7) | 28 (11.6) | 0.179 |
| History of stroke | 62 (23.0) | 56 (23.1) | 0.915 |
| Osteoporosis | 32 (11.9) | 27 (11.2) | 0.565 |
| Sleep apnoea–hypopnoea syndrome | 9 (3.3) | 4 (1.7) | 0.186 |
| Chronic respiratory disease | 16 (5.9) | 15 (6.2) | 0.525 |
| Dyslipidaemia | 147 (54.4) | 138 (57.0) | 0.306 |
| Cancer | 27 (10.0) | 18 (7.4) | 0.109 |
| History of surgery | 186 (68.9) | 152 (62.8) | 0.202 |
| Polypharmacy | 155 (57.4) | 112 (46.3) | 0.036 |
| Frail | 91 (33.7) | 52 (21.5) | 0.001 |
| Muscle weakness | 123 (45.6) | 88 (36.4) | 0.031 |
| Slow gait | 166 (61.5) | 83 (34.3) | 0.022 |
| Physical exhaustion | 140 (51.9) | 104 (43.0) | 0.037 |
| Low physical activity | 83 (30.7) | 53 (21.9) | 0.004 |
| Unintentional weight loss | 31 (11.5) | 28 (11.6) | 0.906 |
| BADL | 5.3 ± 1.18 | 5.5 ± 0.99 | 0.048 |
| IADL | 6.5 ± 2.35 | 6.7 ± 2.19 | <0.001 |
| Falls in the past year | 78 (28.9) | 63 (26.0) | 0.739 |
| AIS > 4 | 77 (28.5) | 66 (27.3) | 0.953 |
| MMSE < 24 | 100 (37.0) | 87 (36.0) | 0.897 |
| HADS ≥ 8 | 22 (8.1) | 12 (5.0) | 0.088 |
| NT‐proBNP, pg/mL | 172 (90.4,387) | 127 (65.2,292) | 0.083 |
| LVEF, % | 63.3 ± 4.37 | 63.5 ± 4.34 | 0.496 |
Values are shown as mean ± standard deviation, n (%), or median (interquartile range). Data were analysed through log‐rank test for categorical data and univariate Cox proportional hazard models for continuous data.
AIS, Athens Insomnia Scale; BADL, basic activities of daily living; BMI, body mass index; HADS, Hospital Anxiety and Depression Scale; IADL, instrumental activities of daily living; LVEF, left ventricular ejection fraction; MMSE, Mini‐Mental State Examination; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
The proportion of missing data was 27.5% (143/520) for NT‐proBNP, 10% (52/520) for TUG, 5.6% (29/520) for falls in the past year, and 10% (52/520) for slow gait. No other data were missing.
SPSS Statistics 22 (IBM Corp., Armonk, NY, USA) was used to perform the statistical analyses. All statistical tests were two‐tailed, and a P‐value <0.05 was considered statistically significant.
Results
Patient characteristics
Overall, 1000 patients were screened, and 520 elderly [mean age: 75.5 ± 6.52 years, male: 222 (42.7%)] patients with stage B HFpEF were included in the final analysis. Of these, 301 (57.9%) patients were hospitalized in the cardiology department, for coronary heart disease (36.9%, 192/520), cardiac arrhythmia (14.0%, 73/520), hypertension (5.4%, 28/520), syncope (1.0%, 5/520), and peripheral artery disease (0.6%, 3/520). The remaining 219 patients were hospitalised in the orthopaedics (14.4%, 75/520), neurology (8.5%, 44/520), respiratory (7.9%, 41/520), general surgery (3.1%, 16/520), geriatric (2.5%, 13/520), urology (2.5%, 13/520), rehabilitation (1.5%, 8/520), traditional Chinese medicine (1.3%, 7/520), and cardiac surgery (0.4%, 2/520) departments. The comparison of baseline data between the cardiology and non‐cardiology departments is summarized in the Supporting Information (Table S1 ). Frailty was diagnosed in 145 (27.9%) patients. Table 3 shows the baseline characteristics of the patients. Frail patients with stage B HFpEF were older, with a lower BMI, higher level of NT‐proBNP, and larger proportion of chronic kidney disease, history of stroke, and osteoporosis and were more likely to take more than five medications. However, the type of stage B HFpEF was less often LV enlargement in frail patients.
Table 3.
Baseline and clinical characteristics
| Overall | Frail | Non‐frail | P values | |
|---|---|---|---|---|
| n = 520 | n = 145 (27.9%) | n = 375 (72.1%) | ||
| Demographics | ||||
| Age, years | 75.5 ± 6.52 | 78.5 ± 6.32 | 74.3 ± 6.22 | <0.001 |
| Male sex | 222 (42.7) | 62 (42.8) | 160 (42.7) | 0.985 |
| Smoking history | 140 (26.9) | 38 (26.2) | 102 (27.2) | 0.819 |
| Drinking history | 34 (6.5) | 7 (4.8) | 27 (7.2) | 0.326 |
| BMI, kg/m2 | 25.4 ± 3.40 | 24.6 ± 3.60 | 25.7 ± 3.27 | 0.001 |
| Risk factors for HF | ||||
| Hypertension | 405 (77.9) | 114 (78.6) | 291 (77.6) | 0.801 |
| Coronary artery disease | 283 (54.4) | 78 (53.8) | 205 (54.7) | 0.858 |
| Diabetes | 174 (33.5) | 55 (37.9) | 119 (31.7) | 0.179 |
| Atrial fibrillation or atrial flutter | 75 (14.4) | 27 (18.6) | 48 (12.8) | 0.090 |
| Reasons for SBHF | ||||
| LV hypertrophy | 265 (51.0) | 73 (50.3) | 192 (51.2) | 0.839 |
| LV enlargement | 314 (60.4) | 76 (52.4) | 238 (63.5) | 0.017 |
| Diastolic dysfunction | 178 (34.2) | 55 (37.9) | 123 (32.8) | 0.976 |
| Valvular heart disease | 35 (6.7) | 12 (8.3) | 23 (6.1) | 0.382 |
| Other co‐morbidities | ||||
| Chronic kidney disease | 74 (14.2) | 28 (19.3) | 46 (12.3) | 0.039 |
| History of stroke | 121 (23.3) | 53 (36.6) | 68 (18.1) | <0.001 |
| Osteoporosis | 62 (11.9) | 28 (19.3) | 34 (9.1) | 0.001 |
| Sleep apnoea–hypopnoea syndrome | 13 (2.5) | 5 (3.4) | 8 (2.1) | 0.389 |
| Chronic respiratory disease | 31 (6.0) | 8 (5.5) | 23 (6.1) | 0.790 |
| Dyslipidaemia | 289 (55.6) | 79 (54.5) | 210 (56.0) | 0.755 |
| Cancer | 46 (8.8) | 13 (9.0) | 33 (8.8) | 0.952 |
| History of surgery | 343 (66.0) | 102 (70.3) | 241 (64.3) | 0.190 |
| ≥5 medications | 270 (51.9) | 93 (64.1) | 177 (47.2) | 0.001 |
| NT‐proBNP, pg/mL | 158 (78.6, 341) | 279 (112.4, 596) | 140 (67.1, 266) | <0.001 |
| LVEF, % | 63.4 ± 4.34 | 63.3 ± 4.42 | 63.4 ± 4.31 | 0.393 |
Values are shown as the mean ± standard deviation, n (%), or median (interquartile range). Data were analysed using the t‐test, Mann–Whitney U test, and chi‐square test for normally distributed continuous data, non‐normally distributed continuous data, and categorical data, respectively.
BMI, body mass index; HF, heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SBHF, stage‐B heart failure.
Multidimensional frailty evaluation
Compared with non‐frail patients, frail patients with stage B HFpEF showed poor performance in the physical components, with a longer TUG test, slower walking speed, and lower handgrip strength, physical activity, and SPPB and ADL scores. Frail patients were also more likely to experience exhaustion and unintentional weight loss. For the cognitive and emotional components, frail patients had lower MMSE and HADS scores than non‐frail patients. However, the perception of social support was similar in both groups (Table 4 ).
Table 4.
Frailty components
| Overall | Frail | Non‐frail | P values | |
|---|---|---|---|---|
| n = 520 | n = 145 (27.9%) | n = 375 (72.1%) | ||
| Social components | ||||
| Residence, urban | 501 (96.3) | 141 (97.2) | 360 (96.0) | 0.499 |
| Manual worker | 192 (36.9) | 59 (40.7) | 133 (35.5) | 0.268 |
| Married | 389 (74.8) | 100 (69.0) | 289 (77.1) | 0.056 |
| Physical components | ||||
| TUG, s | 14.8 ± 6.92 | 19.9 ± 9.71 | 13.3 ± 5.08 | <0.001 |
| SPPB | 7.1 ± 3.37 | 4.1 ± 3.26 | 8.2 ± 2.62 | <0.001 |
| BADL | 5.4 ± 1.10 | 4.7 ± 1.71 | 5.7 ± 0.57 | <0.001 |
| IADL | 6.6 ± 2.28 | 4.4 ± 2.73 | 7.4 ± 1.33 | <0.001 |
| Falls in the past year | 31 (6.0) | 11 (7.6) | 20 (5.3) | 0.277 |
| Muscle weakness | 214 (41.2) | 116 (80.0) | 98 (26.1) | <0.001 |
| Slow gait | 203 (39.0) | 78 (53.8) | 125 (33.3) | <0.001 |
| Unintentional weight loss | 61 (11.7) | 38 (26.2) | 23 (6.1) | <0.001 |
| Low physical activity | 138 (26.5) | 95 (65.5) | 43 (11.5) | <0.001 |
| Physical exhaustion | 248 (47.7) | 124 (85.5) | 124 (33.1) | <0.001 |
| Cognitive component | ||||
| MMSE < 24 | 189 (36.3) | 81 (55.9) | 108 (28.8) | <0.001 |
| Emotion | ||||
| HADS ≥ 8 | 34 (6.5) | 15 (10.3) | 19 (5.1) | 0.029 |
| Sleep disturbance | ||||
| AIS > 4 | 145 (27.9) | 40 (27.6) | 105 (28.0) | 0.925 |
Values are shown as mean ± standard deviation or n (%). Data were analysed using the t‐test, Mann–Whitney U test, and chi‐square test for normally distributed continuous data, non‐normally distributed continuous data and categorical data, respectively.
AIS, Athens Insomnia Scale; BADL, basic activities of daily living; HADS, Hospital Anxiety and Depression Scale; IADL, instrumental activities of daily living; MMSE, Mini‐Mental State Examination; SPPB, short physical performance battery; TUG, timed up‐and‐go test.
Three‐year mortality and readmission
During the follow‐up period, 270 (51.9%) patients experienced all‐cause mortality or readmission, including 91 with frailty and 179 without frailty (62.8% vs. 47.7%, P = 0.002). The mean survival time was 692 ± 39.3 days and 806 ± 23.7 days in the frailty and non‐frailty groups, respectively (P = 0.001 in the log‐rank test; Figure 2 A ). Univariate analysis showed that patients with mortality or readmission were older (76.1 ± 6.30 vs. 74.8 ± 6.63 years, P = 0.027; Table 2 ) and had poorer performance in BADL (5.3 ± 1.18 vs. 5.5 ± 0.99, P = 0.048) and IADL (6.5 ± 2.35 vs. 6.7 ± 2.19, P < 0.001). Furthermore, a larger proportion of frail patients exhibited polypharmacy (57.4% vs. 46.3%, P = 0.036), muscle weakness (45.6% vs. 36.4%, P = 0.031), slow gait (43.0% vs. 34.3%, P = 0.022), physical exhaustion (51.9% vs. 43.0%, P = 0.037), and low physical activity (30.7% vs. 21.9%, P = 0.004). Multivariate Cox analysis showed that frail individuals had a 1.53‐fold increase in the risk of 3‐year all‐cause mortality or readmission (95%CI 1.11–2.11, P = 0.009; Figure 3 A ), adjusted for sex, age, polypharmacy, AIS score, MMSE score, LVEF, BADL score, and IADL score. Among the five Fried phenotype components, low physical activity was associated with a higher adjusted risk of 3‐year all‐cause mortality or readmission (Figure 4 ).
Figure 2.
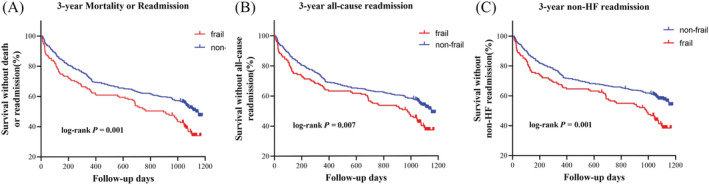
Kaplan–Meier survival curves by frailty in older patients with stage B HFpEF. Frailty was defined by the Fried phenotype. Data of 3‐year mortality or readmission (A), 3‐year all‐cause readmission (B), and 3‐year non‐HF‐related readmission (C) were analysed using the log‐rank test. HF, heart failure; HFpEF, HF with preserved ejection fraction.
Figure 3.
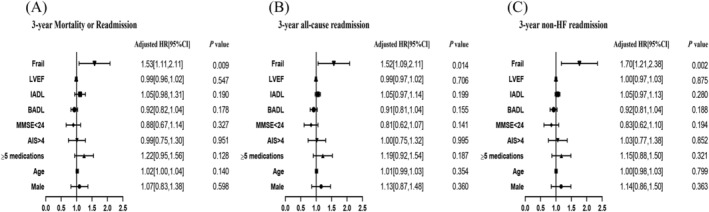
Multivariable Cox proportional‐hazard models of frailty and residence, adjusted for LVEF, MMSE, AIS, BADL, IADL, polypharmacy, age, and male sex. Frailty was diagnosed based on the Fried phenotype. The events were defined as 3‐year mortality or readmission (A), 3‐year all‐cause readmission (B), and 3‐year non‐HF‐related readmission (C). 95% CI, 95% confidence interval; AIS, Athens Insomnia Scale; BADL, basic activities of daily living; HF, heart failure; HR, hazard ratio; IADL, instrumental activities of daily living; LVEF, left ventricular ejection fraction; MMSE, Mini‐Mental State Examination.
Figure 4.
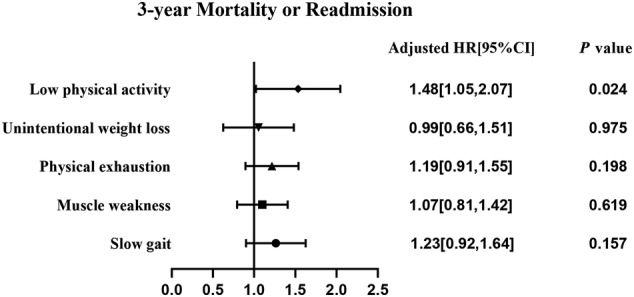
Multivariable Cox proportional‐hazard models of the five frailty components, adjusted for LVEF, MMSE, AIS, BADL, IADL, polypharmacy, age, and male sex. Frailty was diagnosed based on the Fried phenotype. 95% CI, 95% confidence interval; AIS, Athens Insomnia Scale; BADL, basic activities of daily living; HF, heart failure; HR, hazard ratio; IADL, instrumental activities of daily living; LVEF, left ventricular ejection fraction; MMSE, Mini‐Mental State Examination.
Further analysis showed that during the 3‐year follow‐up, 254 (48.8%) patients experienced all‐cause readmission, including 82 with frailty and 172 without frailty (56.6% vs. 45.9%, P = 0.029). The mean survival time was 719 ± 40.4 days and 809 ± 24.0 days in the frailty and non‐frailty groups, respectively (P = 0.007 in the log‐rank test; Figure 2 B ). Frail individuals had a 1.52‐fold increase in the risk of 3‐year all‐cause readmission (95%CI 1.09–2.11, P = 0.014; Figure 3 B ), adjusted for sex, age, polypharmacy, AIS score, MMSE score, LVEF, BADL score, and IADL score. Upon evaluation of the reasons for readmission we discovered that 19 (3.7%) patients were rehospitalized for clinical HF, including two with frailty and 17 without frailty (1.4% vs. 4.5%, P = 0.086). The mean survival time was 1175 ± 4.3 days and 1167 ± 4.5 days in the frailty and non‐frailty groups, respectively (P = 0.127 in the log‐rank test). However, 235 (45.2%) patients experienced non‐HF‐related readmission, including 80 with frailty and 155 without frailty (55.2% vs. 41.3%, P = 0.004). The mean survival time was 733 ± 40.2 days and 842 ± 23.6 days in the frailty and non‐frailty groups, respectively (P = 0.001 in the log‐rank test; Figure 2 C ). Furthermore, frail individuals had a 1.70‐fold increase in the risk of 3‐year non‐HF‐related readmission (95%CI 1.21–2.38, P = 0.002; Figure 3 C ), adjusted for sex, age, polypharmacy, AIS score, MMSE score, LVEF, BADL score, and IADL score.
Discussion
Our study is the first to assess the prevalence and prognostic value of frailty in older inpatients with stage B HFpEF. We found that frailty was common in this population and frailty could predict all‐cause 3‐year mortality or readmission independently, with physical components playing an important role.
Frailty is an age‐related clinical condition characterized by an accumulation and increased susceptibility of sudden, disproportionate functional decline following stressor events. 15 The functional decline associated with frailty is gradual and incessant and may be exacerbated by acute illness. 16 However, frailty is also potentially reversible, and its associated functional decline is a potentially preventable disability. 17 Therefore, Dent et al. advocated that effective strategies for the management of frailty, including frailty screening, case identification, and individually tailored interventions, are important in clinical practice. 15 Thus, the hospital stays of inpatients with stage B HFpEF, is a critical period during which frailty can be identified before more serious cardiovascular events occur and during which integrated management, including lifestyle interventions, proper nutrition, and increased activity levels and social engagement, can be systematically developed to improve long‐term outcomes. These aspects provide a rationale for frailty assessment during patient hospitalization.
We found that, among older inpatients with stage B HFpEF, the prevalence of frailty was 27.9%, which was higher than that reported among SBHF inpatients (24.6%) in a previous study. 5 Although several studies have shown that frailty is common in patients with HF, the reported prevalence varies widely. A meta‐analysis showed that the prevalence of frailty in elderly patients with HF ranged from 15% to 89%, which may be related to the different populations and criteria for frailty among studies. Nevertheless, the prevalence of frailty is significantly higher in patients with HF than in patients without HF (3%). 18 Many studies have shown that frailty commonly coexists with HF with reduced ejection fraction (HFrEF); however, HFpEF is the most common type of HF in the elderly, and the burden of frailty in HFpEF is similar to or higher than that in HFrEF. 19 , 20 , 21 Compared with healthy people, lower physical activity, high co‐morbidity burden, ageing, and hospitalizations may contribute to the higher prevalence of frailty among elderly patients with SBHF. 19 , 22 Furthermore, advanced age and a higher co‐morbidity burden may explain the high prevalence of frailty among elderly patients with stage B HFpEF. 20 , 21
Patients with stage B HFpEF and frailty in our study were more likely to be older and have a lower BMI, higher NT‐proBNP level, and higher risk of polypharmacy, renal dysfunction, osteoporosis, and stroke, as shown in previous studies. 3 , 4 , 21 , 23 , 24 The relationship between these factors and frailty may include age‐related sarcopenia or anorexia, 3 malnutrition, 23 chronic inflammation, and downregulation of multiple endocrine factors. 25 Our findings indicate an urgent need to identify these risk factors for frailty in older patients with stage B HFpEF.
We also found that older patients with stage B HFpEF and frailty had a lower of physical activity and a higher proportion of cognitive impairment and were more likely to be depressed. Recently, an increasing number of studies introduced the ‘multidomain’ concept into the assessment of frailty in patients with HF. Not only physical frailty but also social frailty, cognitive dysfunction, and mood disorders were found to be common and related to short‐term outcomes in inpatients with HF. 26 However, these factors are often ignored and not comprehensively addressed in daily clinical practice. A meta‐analysis of 26 studies showed that the prevalence of frailty diagnosed using physical frailty measures was lower than that diagnosed using multidimensional frailty measures (42.9% vs. 47.4%) 27 in patients with HF. These results also suggest that more attention should be paid to the comprehensive assessment of older frail patients with stage B HFpEF.
Consistent with other studies, our study showed that frailty could predict 3‐year all‐cause mortality or readmission independently in older patients with stage B HFpEF. A previous study demonstrated that, in older patients with acute decompensated HF, physical frailty assessed by the SPPB was an independent predictor of higher readmission, mortality, and longer hospital stay. 28 The predictive value of frailty was confirmed in the FRAIL‐HF cohort, 29 and another study also showed that frailty may be predictive of mortality and rehospitalization in patients with HFpEF. 8 Taken together, frailty assessment could help identify older patients with HF who are at higher risk of poor outcomes at each stage of the disease. Numerous experimental studies have explored the mechanism of association between frailty and poor outcome. They found that frailty may lead to overactivated inflammation and cardiac overload, which could contribute to ventricular remodelling, myocardial injury, and further cardiac dysfunction. 30 We observed that certain diseases, such as atrial fibrillation, were not independently predictive of long‐term outcomes in stage B HFpEF, which was inconsistent with previous observations in previous studies. 31 , 32 This difference has several possible explanations. First, as we recruited participants from 10 departments, including cardiology and non‐cardiology departments, the proportion of patients with baseline atrial fibrillation was relatively low. Second, the follow‐up period may have been insufficient, possibly leading to an underestimation of the predictive power of atrial fibrillation for outcomes in older patients with stage B HFpEF.
We further explored the reasons for readmission and found that frailty could predict 3‐year non‐HF‐related readmission independently in older patients with stage B HFpEF, but not HF readmission. Frailty is a geriatric syndrome caused by multisystem impairment involving upregulation of inflammatory pathways, endocrine dysregulation, and sarcopenia. 33 In our study, frail participants were more likely to have multiple complications such as renal dysfunction, osteoporosis, and stroke than non‐frail participants. HFpEF is common in elderly patients, and a recent study revealed that HFpEF is frequently characterised by systemic inflammation. 34 Braunwald recently demonstrated that HFpEF is frequently characterized by one or more co‐morbidities, such as diabetes, obesity, metabolic syndrome, and chronic kidney disease. 35 Sze et al. analysed patients with HFrEF, and revealed that frail patients were more likely to be readmitted for non‐cardiovascular causes than for cardiovascular causes. 36 A longitudinal analysis of 86 000 individuals demonstrated that, after long time follow‐up, the non‐cardiovascular mortality rate was higher in the patients with HF aged ≥80 years. 37 Therefore, we postulate that patients with stage B HFpEF have a particularly high burden of co‐morbidities, which leads to a high risk of all‐cause readmission or non‐HF‐related readmission. Taken together, in addition to preventing the progression of cardiovascular diseases in elderly patients with stage B HFpEF, more attention should be paid to the management of non‐cardiovascular diseases such as physical dysfunction, cognitive impairment, and malnutrition.
Regarding the predictive value of the five Fried phenotype frailty components, a previous study showed that only unintentional weight loss could independently predict mortality or readmission in SBHF inpatients within a 6‐month follow‐up. 5 However, when we extended the length of follow‐up to 3 years, the results suggested that a low level of physical activity was the only component predictive of poor outcomes in patients with stage B HFpEF, which was consistent with a previous study in patients with clinical HF. 29 A low degree of physical activity has been identified as a component that is strongly associated with cardiovascular disease, 38 which may partly explain the association between a low degree of physical activity and poor outcomes in elderly patients with stage B HFpEF. Additionally, several studies have shown that supervised exercise training can improve the quality of life of patients with HF. 39 Hieda et al. recently demonstrated that exercise training could protect patients with stage B HFpEF against poor outcomes by improving fitness and reversing LV myocardial stiffening. 9 These findings indicate the importance of physical activity in frail patients and that more interventions are needed to improve exercise capacity in older patients with stage B HFpEF.
Our study has several limitations. First, the participants were enrolled from one hospital; therefore, the results may not be generalizable to populations in different geographical locations. Second, only hospitalized patients were included in our study, which may limit the generalizability of our findings to elderly patients within the community. Third, patients admitted to cardiology wards had different characteristics to those admitted to non‐cardiology wards in this study. However, we pooled these two groups of patients, which might have affected our analysis on causes of readmission. Fourth, some elderly patients refused to participate in the study, although they met the inclusion criteria, which may have increased the recruitment bias. Fifth, we screened outpatients with SBHF from a total of 1000 patients and subsequently selected patients with the LVEF ≥50% from patients with SBHF to define stage B HFpEF, which may be partly inconsistent with the latest definition of HFpEF due to such enrolment process. In addition, to the best of our knowledge, the diagnosis of HFpEF remains challenging, and there is no acknowledged criterion for SBHF and no gold standard measurement of frailty in older Chinese patients. Community or multicentre studies of larger samples of patients with a wide age range and lower degree selection bias are warranted in the future.
In conclusion, frailty was highly prevalent among older patients with stage B HFpEF. Frailty could independently predict 3‐year all‐cause mortality or readmission, all‐cause readmission, and non‐HF readmission. Among the different domains, a low degree of physical activity appeared to have the greatest predictive ability. Overall, physical frailty, particularly a low degree of physical activity, can strongly predict the long‐term prognosis in older patients with stage B HFpEF. These findings indicate the need to identify frailty in this population, and more attention should be focussed on long‐term regular interventions targeting frailty in this population.
Conflict of interest
Chen Meng, Ke Chai, Ying‐Ying Li, Yao Luo, Hua Wang, and Jie‐Fu Yang declare that they have no conflict of interest.
Funding
This work was supported by the Beijing Municipal Science and Technology Commission, China (D181100000218003), and the Capital's Funds for Health Improvement and Research (2022‐1‐4052).
Supporting information
Table S1. Baseline and clinical characteristics of patients in different wards.
Acknowledgements
We acknowledge Jiao Fengling, Du Pingjing, Sun Qingyun, Gao Junyan, Xu Li, Li Meilan, Shen Yueli, and Yi Lin for their assessment of the participants. We also wish to thank Zeng Min and He Wei for the patient follow‐up.
Meng, C. , Chai, K. , Li, Y.‐Y. , Luo, Y. , Wang, H. , and Yang, J.‐F. (2023) Prevalence and prognosis of frailty in older patients with stage B heart failure with preserved ejection fraction. ESC Heart Failure, 10: 1133–1143. 10.1002/ehf2.14274.
Hua Wang and Jie‐Fu Yang contributed equally to this article.
The work was performed in the Peking University Fifth School of Clinical Medicine, Beijing, China; Department of Cardiology, Beijing Hospital; National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China.
Contributor Information
Hua Wang, Email: wanghua2764@bjhmoh.cn.
Jie‐Fu Yang, Email: yangjiefu2011@126.com.
References
- 1. Mcdonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland J, Coats A, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam C, Lyon AR, Mcmurray J, Mebazaa A, Mindham R, Muneretto C, Francesco PM, Price S, Rosano G, Ruschitzka F, Kathrine SA. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam C, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, Mcburnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 4. Pandey A, Kitzman D, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, Nelson MB, Upadhya B, Chen H, Reeves GR. Frailty among older decompensated heart failure patients: prevalence, association with patient‐centered outcomes, and efficient detection methods. JACC Heart Fail. 2019; 7: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng PP, Yao SM, He W, Wan YH, Wang H, Yang JF. Frailty related all‐cause mortality or hospital readmission among adults aged 65 and older with stage‐B heart failure inpatients. BMC Geriatr. 2021; 21: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng PP, Yao SM, Shi J, Wan YH, Guo D, Cui LL, Sun N, Wang H, Yang JF. Prevalence and prognostic significance of frailty in gerontal inpatients with pre‐clinical heart failure: a subgroup analysis of a prospective observational cohort study in China. Front Cardiovasc Med. 2020; 7: 607439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017; 14: 591–602. [DOI] [PubMed] [Google Scholar]
- 8. Goyal P, Yum B, Navid P, Chen L, Kim DH, Roh J, Jaeger BC, Levitan EB. Frailty and post‐hospitalization outcomes in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2021; 148: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hieda M, Sarma S, Hearon CJ, Macnamara JP, Dias KA, Samels M, Palmer D, Livingston S, Morris M, Levine BD. One‐year committed exercise training reverses abnormal left ventricular myocardial stiffness in patients with stage b heart failure with preserved ejection fraction. Circulation. 2021; 144: 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang YD, Zhang YN, Li YM, Chen YH, Xu JY, Liu M, Li J, Ma Z, Qiao LL, Wang Z, Yang JF, Wang H. Identification of frailty and its risk factors in elderly hospitalized patients from different wards: a cross‐sectional study in China. Clin Interv Aging. 2019; 14: 2249–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bozkurt B, Coats A, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, Anker SD, Atherton J, Bohm M, Butler J, Drazner MH, Michael FG, Filippatos G, Fiuzat M, Fonarow GC, Gomez‐Mesa JE, Heidenreich P, Imamura T, Jankowska EA, Januzzi J, Khazanie P, Kinugawa K, Lam C, Matsue Y, Metra M, Ohtani T, Francesco PM, Ponikowski P, Rosano G, Sakata Y, Seferovic P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the universal definition of heart failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021; 23: 352–380. [DOI] [PubMed] [Google Scholar]
- 12. Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kucharska‐Newton A, Sueta CA, Mosley TH, Wright JD, Coresh J, Heiss G, Folsom AR, Solomon SD. Heart failure stages among older adults in the community: the atherosclerosis risk in communities study. Circulation. 2017; 135: 224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao GH, Deng Y, Liu Y, Xu MJ, Zhang C, Deng YB, Ren WD, Li ZA, Tang H, Zhang QB, Mu YM, Fang LG, Zhang M, Yin LX, Zhang Y. Echocardiographic measurements in normal Chinese adults focusing on cardiac chambers and great arteries: a prospective, nationwide, and multicenter study. J Am Soc Echocardiogr. 2015; 28: 570–579. [DOI] [PubMed] [Google Scholar]
- 14. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DJ, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, Mcbride PE, Mcmurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 15. Dent E, Martin FC, Bergman H, Woo J, Romero‐Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019; 394: 1376–1386. [DOI] [PubMed] [Google Scholar]
- 16. Cohen‐Mansfield J, Skornick‐Bouchbinder M, Brill S. Trajectories of end of life: a systematic review. J Gerontol B Psychol Sci Soc Sci. 2018; 73: 564–572. [DOI] [PubMed] [Google Scholar]
- 17. Tse G, Gong M, Nunez J, Sanchis J, Li G, Ali‐Hasan‐Al‐Saegh S, Wong WT, Wong SH, Wu W, Bazoukis G, Yan GX, Lampropoulos K, Baranchuk AM, Tse LA, Xia Y, Liu T, Woo J. Frailty and mortality outcomes after percutaneous coronary intervention: a systematic review and meta‐analysis. J Am Med Dir Assoc. 2017; 18: 1091–1097. [DOI] [PubMed] [Google Scholar]
- 18. Yang X, Lupon J, Vidan MT, Ferguson C, Gastelurrutia P, Newton PJ, Macdonald PS, Bueno H, Bayes‐Genis A, Woo J, Fung E. Impact of frailty on mortality and hospitalization in chronic heart failure: a systematic review and meta‐analysis. J Am Heart Assoc. 2018; 7: e8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pandey A, Kitzman D, Reeves G. Frailty is intertwined with heart failure: mechanisms, prevalence, prognosis, assessment, and management. JACC Heart Fail. 2019; 7: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanders NA, Supiano MA, Lewis EF, Liu J, Claggett B, Pfeffer MA, Desai AS, Sweitzer NK, Solomon SD, Fang JC. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail. 2018; 20: 1570–1577. [DOI] [PubMed] [Google Scholar]
- 21. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of frailty in chronic heart failure. JACC Heart Fail. 2019; 7: 291–302. [DOI] [PubMed] [Google Scholar]
- 22. Gidding SS, Lloyd‐Jones D, Lima J, Ambale‐Venkatesh B, Shah SJ, Shah R, Lewis CE, Jacobs DJ, Allen NB. Prevalence of american heart association heart failure stages in black and white young and middle‐aged adults: the CARDIA study. Circ Heart Fail. 2019; 12: e5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol. 2017; 106: 533–541. [DOI] [PubMed] [Google Scholar]
- 24. Tom SE, Adachi JD, Anderson FJ, Boonen S, Chapurlat RD, Compston JE, Cooper C, Gehlbach SH, Greenspan SL, Hooven FH, Nieves JW, Pfeilschifter J, Roux C, Silverman S, Wyman A, Lacroix AZ. Frailty and fracture, disability, and falls: a multiple country study from the global longitudinal study of osteoporosis in women. J Am Geriatr Soc. 2013; 61: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fontana L, Addante F, Copetti M, Paroni G, Fontana A, Sancarlo D, Pellegrini F, Ferrucci L, Pilotto A. Identification of a metabolic signature for multidimensional impairment and mortality risk in hospitalized older patients. Aging Cell. 2013; 12: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsue Y, Kamiya K, Saito H, Saito K, Ogasahara Y, Maekawa E, Konishi M, Kitai T, Iwata K, Jujo K, Wada H, Kasai T, Nagamatsu H, Ozawa T, Izawa K, Yamamoto S, Aizawa N, Yonezawa R, Oka K, Momomura SI, Kagiyama N. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE‐HF cohort study. Eur J Heart Fail. 2020; 22: 2112–2119. [DOI] [PubMed] [Google Scholar]
- 27. Denfeld QE, Winters‐Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta‐analysis. Int J Cardiol. 2017; 236: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Volpato S, Cavalieri M, Guerra G, Sioulis F, Ranzini M, Maraldi C, Fellin R, Guralnik JM. Performance‐based functional assessment in older hospitalized patients: feasibility and clinical correlates. J Gerontol A Biol Sci Med Sci. 2008; 63: 1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vidan MT, Blaya‐Novakova V, Sanchez E, Ortiz J, Serra‐Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non‐dependent elderly patients with heart failure. Eur J Heart Fail. 2016; 18: 869–875. [DOI] [PubMed] [Google Scholar]
- 30. Soysal P, Arik F, Smith L, Jackson SE, Isik AT. Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol. 2020; 1216: 55–64. [DOI] [PubMed] [Google Scholar]
- 31. Nuzzi V, Cannata A, Manca P, Castrichini M, Barbati G, Aleksova A, Fabris E, Zecchin M, Merlo M, Boriani G, Sinagra G. Atrial fibrillation in dilated cardiomyopathy: outcome prediction from an observational registry. Int J Cardiol. 2021; 323: 140–147. [DOI] [PubMed] [Google Scholar]
- 32. Proietti M, Romiti GF, Raparelli V, Diemberger I, Boriani G, Dalla VL, Bellelli G, Marzetti E, Lip GY, Cesari M. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: a systematic review and meta‐analysis of 1,187,000 patients. Ageing Res Rev. 2022; 79: 101652. [DOI] [PubMed] [Google Scholar]
- 33. Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014; 63: 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paulus WJ, Zile MR. From systemic inflammation to myocardial fibrosis: the heart failure with preserved ejection fraction paradigm revisited. Circ Res. 2021; 128: 1451–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Braunwald E. Heart failure with preserved ejection fraction: a stepchild no more! Eur Heart J. 2021; 42: 3900–3901. [DOI] [PubMed] [Google Scholar]
- 36. Sze S, Pellicori P, Zhang J, Weston J, Squire IB, Clark AL. Effect of frailty on treatment, hospitalisation and death in patients with chronic heart failure. Clin Res Cardiol. 2021; 110: 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Conrad N, Judge A, Canoy D, Tran J, Pinho‐Gomes AC, Millett E, Salimi‐Khorshidi G, Cleland JG, Mcmurray J, Rahimi K. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86000 individuals. JAMA Cardiol. 2019; 4: 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S, Corti MC, Baggio G, Toffanello ED, Crepaldi G, Perissinotto E, Manzato E. Pre‐frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.a. Study J Am Coll Cardiol. 2015; 65: 976–983. [DOI] [PubMed] [Google Scholar]
- 39. Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta‐analysis of randomized control trials. Circ Heart Fail. 2015; 8: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline and clinical characteristics of patients in different wards.


