Abstract
Aims
The aim was to describe the prevalence, characteristics, and outcome of patients with acute myocardial infarction (MI) developing left ventricular (LV) systolic dysfunction or pulmonary congestion by applying different criteria to define the population.
Methods and results
In patients with MI included in the Swedish web‐system for enhancement and development of evidence‐based care in heart disease (SWEDEHEART) registry, four different sets of criteria were applied, creating four not mutually exclusive subsets of patients: patients with MI and ejection fraction (EF) < 50% and/or pulmonary congestion (subset 1); EF < 40% and/or pulmonary congestion (subset 2); EF < 40% and/or pulmonary congestion and at least one high‐risk feature (subset 3, PARADISE‐MI like); and EF < 50% and no diabetes mellitus (subset 4, DAPA‐MI like). Subsets 1, 2, 3, and 4 constituted 31.6%, 15.0%, 12.8%, and 22.8% of all patients with MI (n = 87 177), respectively. The age and prevalence of different co‐morbidities varied between subsets. For median age, 70 to 77, for diabetes mellitus, 22 to 33%; for chronic kidney disease, 22 to 38%, for prior MI, 17 to 21%, for atrial fibrillation, 7 to 14%, and for ST‐elevations, 38 to 50%. The cumulative incidence of death or heart failure hospitalization at 3 years was 17.4% (95% CI: 17.1–17.7%) in all MIs; 26.9% (26.3–27.4%) in subset 1; 37.6% (36.7–38.5%) in subset 2; 41.8% (40.7–42.8%) in subset 3; and 22.6% (22.0–23.2%) in subset 4.
Conclusions
Depending on the definition, LV systolic dysfunction or pulmonary congestion is present in 13–32% of all patients with MI and is associated with a two to three times higher risk of subsequent death or HF admission. There is a need to optimize management and improve outcomes for this high‐risk population.
Keywords: Myocardial infarction, Prevalence, Mortality, Heart failure, Left ventricular dysfunction
Introduction
Although the prognosis after acute myocardial infarction (MI) has improved significantly in the last years, patients who develop heart failure (HF) or left ventricular (LV) systolic dysfunction after acute MI are still a vulnerable population with a poor outcome. 1 , 2 , 3 The risk and trends in developing HF after an index MI have previously been studied. 4 , 5 , 6 However, there is a lack of studies describing a more contemporary and representative population of post‐MI patients with different degrees of LV systolic dysfunction and/or pulmonary congestion.
New treatment options for patients with chronic HF have recently been found effective. Sacubitril/valsartan (ARNI) was superior to the angiotensin‐converting enzyme (ACE) inhibitor enalapril in reducing the risks of death and hospitalization for HF. Sodium‐glucose co‐transporter 2 (SGLT‐2) inhibition has been shown to reduce cardiovascular death or worsening of HF in patients with established HF and reduced ejection fraction (EF). 7 , 8 These studies enrolled patients with chronic HF; the population with post‐MI HF has not been thoroughly studied. The recently reported Prospective ARNI vs. ACE Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After MI (PARADISE‐MI) compared sacubitril/valsartan with ramipril in a high‐risk post‐MI population with impaired LVEF (≤40%) or signs of pulmonary congestion, 9 whereas the Dapagliflozin Effects on Cardiovascular Events in Patients With an Acute Heart Attack (DAPA‐MI) is an ongoing randomized clinical trial comparing dapagliflozin with placebo in a post‐MI population with impaired LVEF (<50%) (NCT04564742). Knowledge about prevalence, patient characteristics, and outcome, when different criteria to define the population with LV systolic dysfunction and/or pulmonary congestion after MI is applied, is important for the understanding of the consequences of implementing new treatment strategies in clinical practice.
The purpose of this study was to describe the size (as a proportion of the total post‐MI population), patient characteristics, and long‐term outcome of a real‐world cohort of patients discharged alive after an acute MI during the years 2011–2018 applying different criteria to define the population with LV systolic dysfunction and/or pulmonary congestion. We wanted to describe a high‐risk population like the one in the PARADISE‐MI trial and a population with lower risk, like the DAPA‐MI trial population.
Methods
Since 1995, the SWEDEHEART registry has been an established national quality register including all Swedish hospitals that provide acute coronary care (n = 72). Almost all patients admitted to the hospital with an acute MI are continuously enrolled. More than 100 variables are collected prospectively. The database has been described elsewhere. 10 Each hospital is monitored every second year with a 95–96% agreement between the registry and the electronic health records.
Study population
The main population consisted of all patients, at least 18 years of age and Swedish residents, discharged alive after a spontaneous (type 1) MI (according to the European Society of Cardiology, American College of Cardiology, and American Heart Association consensus documents 10 ) registered in SWEDEHEART between 2011 and 2018. Patients with a history of chronic HF or LV systolic dysfunction prior to index MI and those undergoing coronary artery by‐pass grafting during initial hospitalization were excluded. From the main population, four subsets were derived by using different criteria for LV systolic impairment during the hospital stay as well as the presence or not of pulmonary congestion, diabetes mellitus, and other high‐risk features. Note that a case could meet criteria for more than one subset and thus be included in two or more subsets.
Subset 1: Evidence of any LV systolic dysfunction (EF < 50% assessed by echocardiography) and/or pulmonary congestion supported by clinical assessment (Killip class II or higher) requiring iv treatment (at least one of diuretics, vasodilators, vasopressors, and/or inotropes).
Subset 2: Evidence of at least moderate LV systolic dysfunction (EF < 40%) as assessed by echocardiography and/or pulmonary congestion (same definition as in subset 1).
Subset 3 (PARADISE‐MI like): Evidence of at least moderate LV systolic dysfunction (EF < 40% as assessed by echocardiography) and/or pulmonary congestion (same definition as in subset 1) and at least one of eight high‐risk factors, associated with a worse outcome (used as entry criteria to PARADISE‐MI to increase the event rate in the trial) (age ≥ 70 years, eGFR < 60 mL/min/1.73 m2, diabetes mellitus, documented history of prior MI, atrial fibrillation as noted by ECG associated with index MI, EF < 30% associated with index MI, Killip class III or IV associated with index MI requiring intravenous treatment, ST‐elevation myocardial infarction (STEMI) without reperfusion therapy within the first 24 h after presentation).
Subset 4 (DAPA‐MI like): Evidence of any LV systolic dysfunction (EF < 50%) as assessed by echocardiography and not previously known diabetes mellitus.
The study was approved by the regional ethics committee (Dnr: 2019‐04277) and complied with the Declaration of Helsinki.
Clinical characteristics and diagnoses
Baseline characteristics, in‐hospital course data (including examinations, interventions, and complications), discharge medications, and diagnoses were obtained from SWEDEHEART. The study database was merged with data from the National Patient Registry (NPR) regarding the history of diabetes mellitus, renal failure, MI, stroke, peripheral artery disease, HF, chronic pulmonary disease, dementia, and cancer. The NPR includes discharge diagnoses for all patients hospitalized in Sweden since 1987. Details regarding ICD‐codes are listed in Table S1 . To estimate the glomerular filtrate rate (eGFR) the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) equation was used. eGFR was calculated in mL/min/1.73m2 and dichotomized at <60 mL/min/1.73 m2 (normal/impaired renal function).
Outcomes
The primary outcome was the time to the first occurrence of all‐cause mortality or HF hospitalization (defined as a diagnosis of HF registered in the patient registry). Secondary outcomes were time to all‐cause mortality, first HF admission, non‐fatal MI admission (‘blanking’ the first 30 days due to inability to separate index event from any new event during this period), and non‐fatal stroke admission. We also followed the total number of HF admissions up to three events. Data regarding readmissions were retrieved from the NPR. Heart failure hospitalization was defined as an inpatient stay where HF was registered as the primary diagnosis, whereas the occurrence of MI and stroke as either primary or secondary diagnoses were considered an episode of the respective diagnosis. Mortality data were obtained from the Swedish population registry.
Statistical analyses
Descriptive analyses for patient demographics and characteristics at baseline are shown as numbers and proportions (95% CI) for categorical variables, and median (IQR) and mean (95% CI) for continuous variables. The 95% CIs are shown in Tables S2 and S3 . The number of patients with missing data is listed in Tables S4 and S5 . Bar charts are used to illustrate differences between groups. The incidence of time‐dependent outcomes was illustrated using Kaplan–Meier curves. The log‐rank test was used to test for differences in outcome between independent groups. Outcomes are also given as incident rates per 100 person‐years with 95% CI and as cumulative incidence with 95% CI at 3 years of follow‐up. When analysing the risk of HF, MI, and stroke hospitalization alone, patients were censored for death. There was no adjustment for competing risk. A multivariable Cox regression analysis was used to examine the independent association between high‐risk factors (age ≥ 70, eGFR < 60, diabetes mellitus, prior MI, atrial fibrillation, EF < 30%, Killip III–IV, and STEMI without reperfusion therapy), number of high‐risk factors, and death or readmission because of HF in subset 2. Because of a low number of missing values, we performed complete case analyses. As a sensitivity analysis, we also performed a multivariable Cox regression analysis in which missing values were replaced by multiple imputations resulting in five complete data sets. A multivariable Cox regression analysis was also used to examine the association between the presence of pulmonary rales, different degrees of LV systolic dysfunction, and 3 year risk of death and HF readmission, adjusting for high‐risk factors in all MI patients. All analyses were performed with IBM SPSS version 25 (IBM, Armonk, NY).
Results
Study population
Out of 136 161 admissions with acute MI between 1 January 2011 and 20 May 2018, 105 334 individuals were fulfilling the inclusion criteria. Out of these, 12 772 had a previous diagnosis of HF, and 5385 underwent CABG during hospitalization, leaving 87 177 to be included in the present analyses (Figure S1 ).
Out of those, 27 568 (31.2%) had evidence of any LV systolic dysfunction (EF < 50%) and/or pulmonary congestion (subset 1). If evidence of LV systolic dysfunction was defined as EF < 40% (subset 2), the number was 13 038 (15.0%). Out of these, 11 175 (85.7%) (12.8% of all individuals with MI) had at least one high‐risk factor (subset 3). A total of 19 906 (22.8%) patients had any LV systolic dysfunction (EF < 50%) and no previously known diabetes mellitus (subset 4). The sizes and relations between the subsets and the total population are presented in a Venn diagram (Figure S2 ). The figure demonstrates the (expected) overlap between groups.
Subsets 1 and 2
Using all MI patients as a reference, those with EF < 50% (and/or pulmonary congestion) (subset 1) and those with EF < 40% (and/or pulmonary congestion) (subset 2) were older with the highest age in subset 2. In addition, the proportion of patients with diabetes mellitus and chronic kidney disease was higher in subsets 1 and 2. At the same time, the proportion of other risk factors, such as BMI and smoking, did not differ (Table 1 ). Regarding other co‐morbidities, there were small differences, with a somewhat higher proportion with previous stroke, peripheral artery disease, and chronic obstructive pulmonary disease in subsets 1 and 2, but no differences regarding previous myocardial infarction, dementia, and cancer. Those with the worst systolic LV dysfunction (subset 2) had less often chest pain as their main complaint, more often atrial fibrillation, and more often Killip class 2 or more at presentation.
Table 1.
Baseline demographics and characteristics on admission
| All MI‐patients (n = 87 177) | Subset 1 (EF < 50% or pulm congest) (n = 27 568) | Subset 2 (EF < 40% or pulm congest) (n = 13 038) | Subset 3 (PARADISE‐MI like) (n = 11 175) | Subset 4 (DAPA‐MI like) (n = 19 906) | |
|---|---|---|---|---|---|
| Demography | |||||
| Age, median (IQR) | 70 (61–79) | 72 (63–80) | 74 (65–83) | 77 (70–84) | 71 (62–80) |
| Male, n (%) | 58 584 (67) | 18 714 (68) | 8474 (65) | 7038 (63) | 13 734 (69) |
| Risk factors | |||||
| BMI, median (IQR) | 27 (24–30) | 26 (24–29) | 26 (26–29) | 26 (24–29) | 26 (24–49) |
| Current smoking, n (%) | 19 262 (22) | 6236 (23) | 2797 (21) | 2028 (18) | 4770 (24) |
| Hypertension, n (%) | 48 394 (56) | 15 295 (55) | 7640 (59) | 7073 (63) | 9568 (48) |
| Hyperlipidaemia, n (%) | 22 282 (26) | 6672 (24) | 3345 (26) | 3194 (29) | 3546 (18) |
| Diabetes mell, n (%) | 19 225 (22) | 6771 (25) | 3630 (28) | 3630 (33) | 0 (0) |
| eGFR < 60 mL/min/1.73 m2, n (%) | 18 269 (22) | 7050 (26) | 4104 (32) | 4104 (38) | 4416 (22) |
| Prev cardiovascular dis | |||||
| Myocard infarction, n (%) | 14 871 (17) | 4500 (16) | 2314 (18) | 2314 (21) | 2635 (13) |
| PCI, n (%) | 10 614 (12) | 2819 (10) | 1296 (10) | 1277 (11) | 1662 (8) |
| CABG, n (%) | 4925 (6) | 1571 (6) | 736 (6) | 731 (7) | 864 (4) |
| Stroke, n (%) | 6206 (7) | 2279 (8) | 1309 (10) | 1272 (11) | 1312 (7) |
| Periphery art dis, n (%) | 3536 (4) | 1315 (5) | 791 (6) | 759 (7) | 727 (4) |
| Previous other disease | |||||
| COPD, n (%) | 4981 (6) | 1824 (7) | 998 (8) | 909 (8) | 1258 (6) |
| Dementia, n (%) | 538 (1) | 195 (1) | 124 (1) | 123 (1) | 120 (1) |
| Cancer, n (%) | 2386 (3) | 824 (3) | 406 (3) | 388 (3) | 487 (2) |
| Presentation | |||||
| CPR before hospital, n (%) | 1524 (2) | 826 (3) | 453 (3) | 343 (3) | 664 (3) |
| Chest pain, n (%) | 76 524 (88) | 22 615 (82) | 9679 (74) | 8020 (72) | 16 879 (85) |
| Sinus, n (%) | 79 144 (91) | 24 157 (88) | 10 990 (84) | 9161 (82) | 17 641 (89) |
| AF/AFL, n (%) | 5753 (7) | 2476 (9) | 1539 (12) | 1539 (14) | 1634 (8) |
| STEMI, n (%) | 33 381 (38) | 14 789 (54) | 6880 (53) | 5564 (50) | 11 492 (58) |
| Heart rate | 77 (66–90) | 82 (70–98) | 88 (74–104) | 89 (74–105) | 80 (68–95) |
| Systolic blood pressure, median (IQR) | 150 (130–170) | 145 (127–165) | 140 (124–160) | 140 (124–160) | 144 (126–163) |
| Killip class | |||||
| I, n (%) | 79 444 (93) | 22 565 (83) | 8851 (69) | 7235 (66) | 17 437 (89) |
| II, n (%) | 4886 (6) | 3509 (13) | 3146 (25) | 2935 (27) | 1574 (8) |
| III, n (%) | 604 (1) | 578 (2) | 570 (4) | 570 (5) | 232 (1) |
| IV, n (%) | 632 (1) | 372 (1) | 241 (2) | 232 (2) | 264 (1) |
| Killip ≥ III requiring iv treatment, n (%) | 918 (1) | 827 (3) | 744 (6) | 744 (7) | 406 (2) |
Coronary angiography (83% vs. 88–89%) and subsequent PCI (73% vs. 77–79%) were performed less often in subset 2 than in subset 1 and all MI patients (Table 2 ). In all patients, in subset 1 and in subset 2, iv‐diuretics was used in 14%, 31%, and 50% and iv inotropic drugs in 2%, 5%, and 9%, respectively. Although the percentage of reinfarction during the hospital stay where similar in the different subsets, there were differences in cardiac arrest in‐hospital (2%, 4%, and 5%), cardiogenic shock (1%, 3%, and 4%), and episodes of atrial fibrillation (10%, 16%, and 21%), with the highest proportion in subset 2. Compared with all MI patients, patients in subset 2 were prescribed less aspirin (91% vs. 95%), P2Y12‐receptor blockade (88% vs. 92%) and statins (88% vs. 92%) and more beta‐blockers (93% vs. 88%), ACEI/ARB (88% vs. 81%), MRA (21% vs. 6%), and diuretics (45% vs. 21%).
Table 2.
Treatment, examination findings, complications, and medication at discharge
| All MI‐patients (n = 87 177) | Subset 1 (EF<50% or pulm congest) (n = 27 568) | Subset 2 (EF<40% or pulm congest) (n = 13 038) | Subset 3 (PARADISE‐MI like) (n = 11 175) | Subset 4 (DAPA‐MI like) (n = 19 906) | |
|---|---|---|---|---|---|
| Assessment | |||||
| Coronary angiography, n (%) | 77 370 (89) | 24 195 (88) | 10 760 (83) | 8925 (80) | |
| LV‐function assessment, n (%) | 73 734 (85) | 27 042 (98) | 12 513 (96) | 10 665 (96) | 19 906 (100) |
| LV‐EF | |||||
| EF > 50, n (%) | 46 990 (64) | 801 (3) | 801 (6) | 745 (7) | 0 (0) |
| EF 40–49, n (%) | 15 260 (21) | 15 260 (56) | 730 (6) | 679 (6) | 11 830 (59) |
| EF 30–39, n (%) | 8207 (11) | 8207 (30) | 8207 (66) | 6470 (61) | 6142 (31) |
| EF < 30, n (%) | 2753 (4) | 2753 (10) | 2753 (22) | 2753 (26) | 1934 (10) |
| Treatment | |||||
| Reperfusion in STEMI, n (%) | 28 939 (87) | 12 689 (86) | 5658 (82) | 4342 (78) | 9990 (87) |
| PCI, n (%) | 67 507 (77) | 21 792 (79) | 9512 (73) | 7786 (70) | 16 350 (82) |
| iv diuretics, n (%) | 11 969 (14) | 8556 (31) | 6456 (50) | 5992 (54) | 5072 (25) |
| iv inotropi, n (%) | 2118 (2) | 1502 (5) | 1108 (9) | 1010 (9) | 1028 (5) |
| Complications | |||||
| Reinfarction, n (%) | 475 (1) | 230 (1) | 126 (1) | 112 (1) | 163 (1) |
| Severe bleeding, n (%) | 709 (1) | 351 (1) | 219 (2) | 215 (2) | 220 (1) |
| Cardiac arrest, n (%) | 1856 (2) | 1032 (4) | 593 (5) | 495 (4) | 788 (4) |
| Cardiac shock, n (%) | 1219 (1) | 788 (3) | 534 (4) | 500 (5) | 550 (3) |
| AF at any time of hospitalization, n (%) | 9045 (10) | 4320 (16) | 2678 (21) | 2678 (24) | 2910 (15) |
| Medication at discharge | |||||
| Aspirin, n (%) | 82 358 (94) | 25 574 (93) | 11 808 (91) | 9993 (89) | 18 608 (93) |
| P2Y12‐receptor blocker, n (%) | 80 109 (92) | 25 158 (91) | 11 455 (88) | 9652 (86) | 18 340 (93) |
| Statins, n (%) | 80 436 (92) | 25 220 (92) | 11 447 (88) | 9630 (86) | 18 436 (93) |
| Beta‐blockers, n (%) | 77 004 (88) | 25 423 (92) | 12 065 (93) | 10 285 (92) | 18 425 (93) |
| ACEI, n (%) | 53 729 (62) | 19 357 (70) | 9030 (69) | 7457 (67) | 14 649 (74) |
| ARB, n (%) | 17 372 (20) | 5257 (19) | 2485 (19) | 2271 (20) | 3269 (16) |
| ARNI, n (%) | 11 (0) | 6 (0) | 5 (0) | 5 (0) | 5 (0) |
| ACEI or ARB | 70 507 (81) | 24 444 (89) | 11 437 (88) | 9658 (87) | 17 820 (90) |
| Diuretics, n (%) | 17 910 (21) | 8634 (31) | 5856 (45) | 5537 (50) | 5158 (26) |
| MRA, n (%) | 4542 (6) | 3201 (13) | 2326 (21) | 2079 (22) | 2173 (11) |
Subsets 3 and 4
As expected, subset 3 was older (76 vs. 73) and had more often hypertension (63% vs. 59%), diabetes mellitus (33% vs. 28%), CKD (38% vs. 32%), and prior MI (21% vs. 18%) (Table 1 ). Subsets 3 and 2 were similar regarding presentation, whereas subset 3 less often underwent coronary angiography (80% vs. 83% and PCI (70% vs. 73%) (Table 2 ). There were no major differences regarding in‐hospital complications and medication at discharge.
When comparing subset 1 with those with EF < 50% and no diabetes mellitus (subset 4), the latter had less often hypertension (48% vs. 55%), hyperlipidaemia (18% vs. 24%), chronic kidney disease (22% vs. 26%), previous MI (13% vs. 16%), and more seldom Killip class 2 or worse (10% vs. 16%) (Table 1 ) and accordingly less often treated with iv diuretics (25% vs. 31%) (Table 2 ).
Long‐term outcomes
For the primary outcome, all‐cause death or readmission because of HF, the incidence rate for all MI‐patients was 5.6 (95% CI: 5.5–5.7) per 100 person‐years, and the cumulative incidence at 3 years 17.4% (95% CI: 17.1–17.7%) (Figure 1 ). For subset 1, the incidence rate was higher than for all MI patients (9.6 (95% CI: 9.4–9.9) per 100 person‐years) with a cumulative incidence of 26.9% (95% CI: 26.3–27.4%). For subset 2, the incidence rate was almost 3 times higher (15.4 (95% CI: 15.0–15.8) per 100 person‐years), and the cumulative incidence was more than twice as high at 3 years 37.6% (95% CI: 36.7–38.5%). For subset 3, the incidence rate was even higher (17.8 (95% CI: 17.3–18.3) per 100 person‐years) with a cumulative incidence of 41.8% (95% CI: 40.7–42.8), whereas for subset 4 the incidence rate was 7.8 (95% CI: 7.6–8.0) per 100 person‐years with a cumulative incidence of 22.6% (95% CI: 22.0–23.2). When the composites of the primary endpoint were considered separately, both endpoints contributed to the difference between the subsets. In the order of increasing risk, going from all MI patients to subset 4, 1, 2, and 3, the cumulative incidence of all‐cause death at 3 years went from 13.8%, 16.2%, 19.6%, 27.1% to 30.5% (Figure 2 A ), and the cumulative incidence of re‐admission because of HF at 3 years went from 6.5%, 10.7%, 12.9%, 19.7% to 22.0% (Figure 2 B ).
Figure 1.
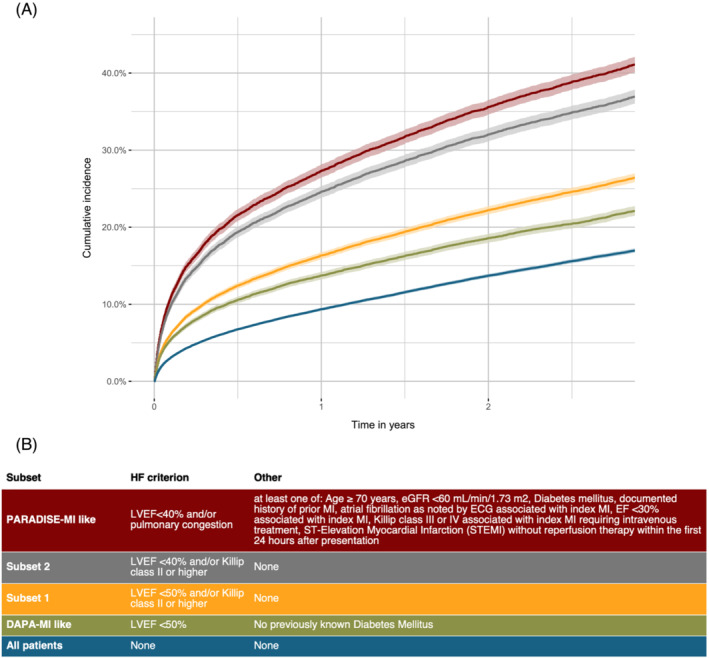
A Kaplan–Meier curve showing time to the combined endpoint of all‐cause death or readmission for HF (primary diagnosis). Groups: subset 1: EF < 50% a/o pulmonary congestion; subset 2: EF < 40% a/o pulmonary congestion; subset 3/PARADISE‐MI like: EF < 40% a/o pulmonary congestion and high‐risk; subset 4/DAPA‐MI like: EF < 50% and no diabetes mellitus.
Figure 2.
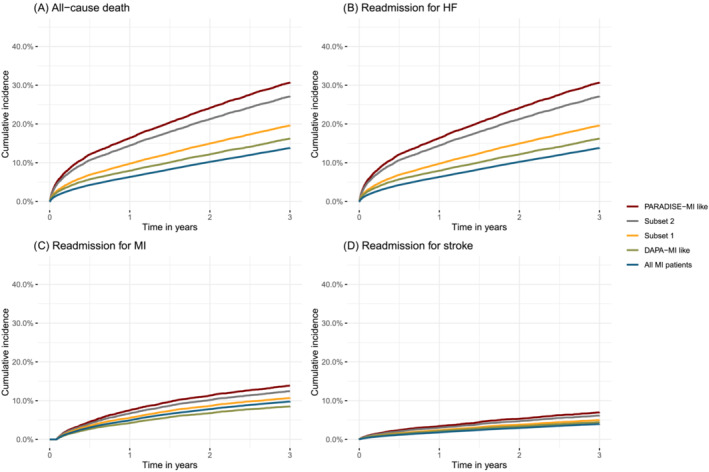
Kaplan–Meier curves showing time to all‐cause death (A), readmission for HF (B), readmission for MI (C), and stroke (D). Groups: subset 1: EF < 50% a/o pulmonary congestion, subset 2: EF < 40% a/o pulmonary congestion, subset 3/PARADISE‐MI like: EF < 40% a/o pulmonary congestion and high‐risk, subset 4/DAPA‐MI like: EF < 50% and no diabetes mellitus.
Regarding the risk of subsequent ischemic events, i.e., MI and stroke, differences in outcome between the populations were small. Going from all MI patients to subsets 1, 2, 3 and 4, the cumulative incidence of MI at 3 years was 9.8%, 10.7%, 12.4%, 13.9%, and 8.5% (Figure 2 C ) and the cumulative incidence of re‐admission because of stroke at 3 years 4.0%, 5.0%, 6.2% to 7.0% and 4.5% (Figure 2 D ). Comparisons regarding outcome in patients included and not included in each subset are presented in Figure S3a–t .
When comparing all MI patients with subsets 1, 2, and 3, the proportion of patients with at least two readmissions because of HF increased from 5.8%, 11.9%, 18.3%, and 20.3%, and the proportion with at least thre readmissions because of HF increased from 3.2%, 6.6%, 10.2%, and 11.4% (Figure S4 ). Subset 4 had a lower risk than others with LV systolic dysfunction, with at least two admissions because of HF in 10.0% and at least three admissions in 5.5%.
High‐risk factors for readmission for HF and death
The relationship between each high‐risk factor used to define the high‐risk population (subset 3) and the occurrence of all‐cause death or HF admission after acute MI are presented as Kaplan–Meier curves (Figure S5a–h ). All high‐risk factors were independently associated with the primary outcome (Table 3 ). The associations were similar when the same analysis was performed after multiple imputations (Table S6 ). Twenty‐seven per cent had two high‐risk factors, and only 1% had six high‐risk factors or more (Figure 3 A ). The cumulative incidence of all‐cause death or readmission because of HF was higher with more high‐risk factors (HR (95% CI):1.48 (1.44–1.51) for every additional high‐risk factor) (Figure 3 B ). When examining the association between the presence of pulmonary rales, different degrees of LV systolic dysfunction, and 3 year risk of death and HF readmission, adjusting for high‐risk factors in all MI patients, both pulmonary rales (HR (95% CI):1.85 (1.73–1.96), and decreasing EF (40–49%: HR (95% CI) 1.70 (1.61–1.79), 30–39%: 2.65 (2.51–2.80), <30%: 4.26 (3.98–4.57)) were associated higher event rates.
Table 3.
Association between high‐risk factors and primary outcome in MI patients with EF < 40% and/or pulmonary congestion
| Univariable | Multivariable a | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Demography | ||
| Age ≥ 70 | 2.34 (2.18–2.52) | 1.80 (1.66–1.95) |
| Female gender | 1.28 (1.20–1.36) | ‐ ‐ |
| Risk factors | ||
| Diabetes mellitus | 1.46 (1.37–1.55) | 1.35 (1.26–1.44) |
| eGFR < 60 | 2.10 (1.98–2.23) | 1.62 (1.52–1.74) |
| Prev cardiovascular disease | ||
| Prior MI | 1.38 (1.29–1.49) | 1.16 (1.07–1.25) |
| In‐hospital course | ||
| Killip ≥ 3 and iv treatment | 1.65 (1.48–1.84) | 1.34 (1.19–1.51) |
| No reperfusion in STEMI | 1.42 (1.29–1.55) | 1.34 (1.21–1.48) |
| Atrial fibrillation | 1.64 (1.54–1.76) | 1.35 (1.26–1.45) |
| LV‐EF < 30% | 1.82 (1.70–1.94) | 1.69 (1.58–1.81) |
In the multivariable analysis, there were 1083 (8.3%) patients excluded because of missing data.
Figure 3.
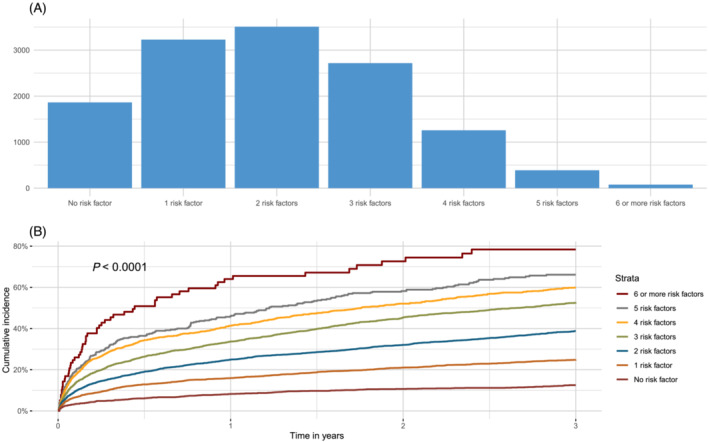
(A) Distribution of risk factors in patients with LVEF <40% and/or pulmonary congestion. (B) Cumulative incidence of all‐cause death or readmission for HF stratified by number of risk factors.
Discussion
In patients with acute MI and no previous history of HF, almost one out of three developed LV systolic dysfunction (defined as EF < 50%) and/or pulmonary congestion during initial care. When defining LV systolic dysfunction as EF < 40%, about 15% of all MI patients had LV systolic dysfunction or pulmonary congestion. The prognosis in the latter group was poor with twice as high mortality and three times higher risk of readmission because of HF at 3 years compared with all MI patients. High‐risk factors used to select subjects for the PARADISE‐MI trial were present in a majority (86%, 12.8% of all MIs) and all factors were independent predictors of all‐cause death or readmission because of HF. There was almost a 50% increase in relative risk for every added factor. The subset with EF < 50% and no previously known diabetes mellitus, that is, those who would be eligible for the DAPA‐MI trial, constituted 23% of all MI patients and had an overall higher risk than all MI‐patients but a lower risk compared with those EF < 50% and/or pulmonary congestion during initial care.
This is a large observational study, examining the risk of a real‐world cohort of post MI patients with evidence of LV systolic dysfunction or pulmonary congestion with nationwide coverage of patients. Sweden is one of few countries with a continuous national quality of care and outcome registry for acute myocardial infarction in which all hospitals participate, 9 enabling accurate assessment of trends and risks. Furthermore, Sweden has a population, health care system, and a prevalence of cardiovascular disease resembling many developed countries. These factors increase the generalizability of our findings. Indeed, when the SWEDEHEART registry has been compared with other continuous national registries including patients with MI, baseline characteristics, given treatments and outcomes have been similar. 11 , 12 , 13 , 14 , 15
The PARADISE‐MI trial was recently reported and failed to show a significant difference between sacubitril/valsartan and ramipril regarding the centrally adjudicated primary endpoint of cardiovascular death, HF hospitalization, or outpatient development of HF. 9 There was, however, a significant reduction of investigator‐reported primary endpoints and total (first and recurrent) number of primary events in the sacubitril/valsartan arm, which may indicate a beneficial effect. When comparing baseline characteristics in PARADISE‐MI like patients in the present study with the actual PARADISE‐MI trial population, 16 there are both differences and similarities. Notably, in the PARADISE‐MI trial, the mean age at inclusion was 64 years, which is in line with other similar clinical trials, 17 , 18 , 19 , 20 , 21 but 12 years younger than the mean age of the PARADISE‐MI like patients in our study. Interestingly, there was a significant interaction between age and the effect of sacubitril/valsartan in the PARADISE‐MI trial with a significant reduction of the primary endpoint in patients ≥65 years of age (HR (95% CI): 0.76 (0.63–0.92)). Thus, it can be speculated whether the PARADISE‐MI trial would have shown positive results for the primary endpoint if a more real‐world population had been enrolled. Perhaps because of the age difference, there were also fewer females (24% vs. 37%) and patients with chronic kidney disease (24% vs. 38%) in the PARADISE‐MI trial compared with the corresponding population in our study. There were also fewer patients with previous MI (17% vs. 21%) and stroke (5% vs. 11%). There were no major differences regarding the proportion of patients with prior revascularization, hypertension, atrial fibrillation, EF < 30%, but a higher proportion of patients with diabetes mellitus (42% vs. 33%). Altogether, this is well in line with previous comparisons between trial‐ and real‐world populations, 22 indicating the importance of follow‐up data from registries when any findings from trials are implemented.
A possible new treatment option is SGLT‐2 inhibition, currently being tested in the DAPA‐MI trial. In the present study, the population potentially eligible for the DAPA‐MI trial, had less often hypertension, hyperlipidaemia, chronic kidney disease, and prior coronary artery disease but were similar to the all‐MI population with regard to age, sex, and other co‐morbidities. The DAPA‐MI like patients had a higher prevalence of ST‐elevations (58%) and the risk of subsequent death or readmission because of heart failure was 30–40% higher than for the all‐MI population but lower than for patients with EF < 50% and/or signs of pulmonary congestion. Thus, the DAPA‐MI trial will cover a broad population regarding age, co‐morbidities, and subsequent outcome.
This study has some limitations. We did not separate individuals with LV systolic dysfunction from those with pulmonary congestion. However, previous studies have shown that signs of heart failure such as pulmonary congestion indicate a worse prognosis than having only reduced LV systolic dysfunction, and patients with both signs of heart failure and reduced LV systolic dysfunction have the worst prognosis. 5 In this study, we described and compared prevalence, characteristics, and prognosis using different criteria for patients with MI and subsequent LV systolic dysfunction and/or pulmonary congestion. Since a patient could be included in two or more populations, comparisons between independent groups were not possible. Echocardiography was not performed in 15% of all MIs during this period. The proportion of patients with reduced EF would have increased if more patients had undergone EF assessment. Although most assessments of EF are usually performed 2–3 days after admission, the exact time is not registered. LV adverse remodelling with an increase in LV end‐diastolic volume has previously been considered an important determinant of the prognosis of post‐MI patients and not captured in the present study. However, LV adverse remodelling is less common today and its prognostic value has been questioned. 23 Although SWEDEHEART registers almost all patients with MI, it misses some of the oldest patients. SWEDEHEART centres are monitored every second year with a 95–96% agreement between registry data and health record data. Still, the data quality cannot be of the same quality as in a well‐performed clinical trial and there is a risk of errors and misclassifications. The NPR has been validated showing a high validity for MI diagnoses. However, for the diagnosis of heart failure (ICD code I50) the positive predictive value is around 85%. 24 Another limitation is the lack of data on the doses of the heart failure treatment. In this study, we can present what treatment patients are prescribed at discharge but not if target doses are reached or not, as well as adherence to treatment. Finally, data are limited to 2011–2018. According to annual reports from SWEDEHEART, baseline characteristics and prognosis after MI have not changed significantly during the last 10 years. Hence, the present study still reflects current practice.
Conclusions
Depending on the definition, LV systolic dysfunction or pulmonary congestion is present in 13–32% of all MI patients without prior HF and is associated with a two to three times higher risk of subsequent death or readmission because of HF. The relative risk increases by about 50% with every additional risk factor. There is a need to optimize management and improve outcomes for this high‐risk population.
Conflict of interest
Dr. Hamilton, Prof. Jernberg and Dr Reitan reports institutional grants from Novartis, during the conduct of the study; Dr. Desta has nothing to disclose; Dr. Lundberg is an employee of Novartis Sweden AB; Dr. Alfredsson reports personal fees from Novartis Advisory board, during the conduct of the study; grants from Research grant from Astra‐Zeneca, personal fees from Lecture fees from Astra‐Zeneca, personal fees from Served on advisory board for Astra‐ Zeneca, personal fees from Lecture fees Boehringer Ingelheim, outside the submitted work; Dr. Christersson reports personal fees from Novartis, personal fees from Boehringer Ingelheim, personal fees from Pfizer, personal fees from Bristol Myers Squibb, personal fees from AstraZeneca, outside the submitted work; Dr. Erlinge has nothing to disclose. Dr. Kellerth has nothing to disclose. Dr. Lindmark has nothing to disclose. Dr. Omerovic reports other from Janssen, other from Bayer, other from AstraZeneca, outside the submitted work.
Supporting information
Table S1. Variables.
Table S2. Baseline demographics and characteristics on admission – incl 95%CIs.
Table S3. Treatment, examination findings, complications and medication at discharge for the total population and the subgroups – incl 95% CIs.
Table S4. Number of patients with missing value.
Table S5. Number of patients with missing value.
Table S6. Association between key risk factors and primary outcome (after multiple imputation).
Hamilton, E. , Desta, L. , Lundberg, A. , Alfredsson, J. , Christersson, C. , Erlinge, D. , Kellerth, T. , Lindmark, K. , Omerovic, E. , Reitan, C. , and Jernberg, T. (2023) Prevalence and prognostic impact of left ventricular systolic dysfunction or pulmonary congestion after acute myocardial infarction. ESC Heart Failure, 10: 1347–1357. 10.1002/ehf2.14301.
References
- 1. Jernberg T, Johanson P, Held C, Svennblad B, Lindbäck J, Wallentin L. Association between adoption of evidence‐based treatment and survival for patients with ST‐elevation myocardial infarction. JAMA. 2011; 305: 1677–1684. [DOI] [PubMed] [Google Scholar]
- 2. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, James S, Kellerth T, Lindahl B, Ravn‐Fischer A, Rydberg E, Yndigegn T, Jernberg T. Improved outcomes in patients with ST‐elevation myocardial infarction during the last 20 years are related to implementation of evidence‐based treatments: experiences from the SWEDEHEART registry 1995‐2014. Eur Heart J; 26: 2114–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szummer K, Wallentin L, Lindhagen L, Alfredsson J, Erlinge D, Held C, James S, Kellerth T, Lindahl B, Ravn‐Fischer A, Rydberg E, Yndigegn T, Jernberg T. Relations between implementation of new treatments and improved outcomes in patients with non‐ST‐elevation myocardial infarction during the last 20 years: experiences from SWEDEHEART registry 1995 to 2014. Eur Heart J. 2018; 39: 3766–3776. [DOI] [PubMed] [Google Scholar]
- 4. Desta L, Jernberg T, Löfman I, Hofman‐Bang C, Hagerman I, Spaak J, Persson H. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART registry (Swedish web‐system for enhancement and development of evidence‐based care in heart disease evaluated according to recommended therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC: Heart Fail. 2015; 3: 234–242. [DOI] [PubMed] [Google Scholar]
- 5. Desta L, Jernberg T, Spaak J, Hofman‐Bang C, Persson H. Heart failure with normal ejection fraction is uncommon in acute myocardial infarction settings but associated with poor outcomes: a study of 91 360 patients admitted with index myocardial infarction between 1998 and 2010. Eur J Heart Fail. 2016; 18: 46–53. [DOI] [PubMed] [Google Scholar]
- 6. Desta L, Jernberg T, Spaak J, Hofman‐Bang C, Persson H. Risk and predictors of readmission for heart failure following a myocardial infarction between 2004 and 2013: a Swedish nationwide observational study. Int J Cardiol. 2017; 248: 221–226. [DOI] [PubMed] [Google Scholar]
- 7. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. Kardiologiya. [DOI] [PubMed]
- 8. McMurray J, Solomon S, Inzucchi S, Køber L, Kosiborod M, Martinez F, Ponikowski P, Sabatine M, Anand I, Bělohlávek J, Böhm M, Chiang C, Chopra V, de Boer R, Desai A, Diez M, Drozdz J, Dukát A, Ge J, Howlett J, DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 9. Jering KS, Claggett B, Pfeffer MA, Granger C, Køber L, Lewis EF, Maggioni AP, Mann D, McMurray JJV, Rouleau JL, Solomon SD, Steg PG, van der Meer P, Wernsing M, Carter K, Guo W, Zhou Y, Lefkowitz M, Gong J, Wang Y, Merkely B, Macin SM, Shah U, Nicolau JC, Braunwald E. Prospective ARNI vs. ACE inhibitor trial to DetermIne superiority in reducing heart failure events after myocardial infarction (PARADISE‐MI): design and baseline characteristics. Eur J Heart Fail. 2021; 23: 1040–1048. [DOI] [PubMed] [Google Scholar]
- 10. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish web‐system for enhancement and development of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010; 96: 1617–1621. [DOI] [PubMed] [Google Scholar]
- 11. Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, Heuschmann P, Wallentin L, Deanfield J, Timmis A, Jernberg T, Hemingway H. Acute myocardial infarction: a comparison of short‐term survival in national outcome registries in Sweden and the UK. The Lancet. 2014; 383: 1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McNamara RL, Chung SC, Jernberg T, Holmes D, Roe M, Timmis A, James S, Deanfield J, Fonarow GC, Peterson ED, Jeppsson A, Hemingway H. International comparisons of the management of patients with non‐ST segment elevation acute myocardial infarction in the United Kingdom, Sweden, and the United States: the MINAP/NICOR, SWEDEHEART/RIKS‐HIA, and ACTION registry‐GWTG/NCDR registries. Int J Cardiol. 2014; 175: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rapsomaniki E, Thuresson M, Yang E, Blin P, Hunt P, Chung SC, Stogiannis D, Pujades‐Rodriguez M, Timmis A, Denaxas SC, Danchin N, Stokes M, Thomas‐Delecourt F, Emmas C, Hasvold P, Jennings E, Johansson S, Cohen DJ, Jernberg T, Moore N, Janzon M, Hemingway H. Using big data from health records from four countries to evaluate chronic disease outcomes: a study in 114 364 survivors ofmyocardial infarction. Eur Heart J Qual Care Clin Outcomes. 2016; 2: 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blöndal M, Ainla T, Eha J, Lõiveke P, Marandi T, Saar A, Veldre G, Edfors R, Lewinter C, Jernberg T, Jortveit J, Halvorsen S, Becker D, Csanádi Z, Ferenci T, Andréka P, Jánosi A. Comparison of management and outcomes of ST‐segment elevation myocardial infarction patients in Estonia, Hungary, Norway, and Sweden according to national ongoing registries. Eur Heart J Qual Care Clin Outcomes. 2022; 8: 307–314. [DOI] [PubMed] [Google Scholar]
- 15. Edfors R, Jernberg T, Lewinter C, Blöndal M, Eha J, Lõiveke P, Marandi T, Ainla T, Saar A, Veldre G, Ferenci T, Andréka P, Jánosi A, Jortveit J, Halvorsen S. Differences in characteristics, treatments and outcomes in patients with non‐ST‐elevation myocardial infarction: novel insights from four national European continuous real‐world registries. Eur Heart J Qual Care Clin Outcomes. 2021; 8: 429–436. [DOI] [PubMed] [Google Scholar]
- 16. Jering KS, Claggett B, Pfeffer MA, Granger C, Køber L, Lewis EF, Maggioni AP, Mann D, McMurray JJV, Rouleau JL, Solomon SD, Steg PG, Meer P, Wernsing M, Carter K, Guo W, Zhou Y, Lefkowitz M, Gong J, Wang Y, Merkely B, Macin SM, Shah U, Nicolau JC, Braunwald E. Prospective ARNI vs. ACE inhibitor trial to DetermIne superiority in reducing heart failure events after myocardial infarction (PARADISE‐MI): design and baseline characteristics methods and results. Eur J Heart Fail. 2021; 23: 1040–1048. [DOI] [PubMed] [Google Scholar]
- 17. Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high‐risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Lancet. 2002; 360: 752–760. [DOI] [PubMed] [Google Scholar]
- 18. Køber L, Torp‐Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbæk J, Cole DS, Auclert L, Pauly NC, Aliot E, Persson S, Camm AJ. A clinical trial of the angiotensin‐converting‐enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril cardiac evaluation (TRACE) study group {see comments}. N Engl J Med. 1995; 333: 1670–1676. [DOI] [PubMed] [Google Scholar]
- 19. Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992; 327: 669–677. [DOI] [PubMed] [Google Scholar]
- 20. Pfeffer MA, McMurray JJV, Velazquez EJ, Rouleau J‐L, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003; 349: 1893–1906. [DOI] [PubMed] [Google Scholar]
- 21. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators . Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Lancet. 1993; 342: 821–828. [PubMed] [Google Scholar]
- 22. Wallentin L. Different perspectives on outcomes in patients with non‐ST‐elevation myocardial infarction when observed in clinical trials and in real life. Eur Heart J; 29: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. del Buono MG, Garmendia CM, Seropian IM, Gonzalez G, Berrocal DH, Biondi‐Zoccai G, Trankle CR, Bucciarelli‐Ducci C, Thiele H, Lavie CJ, Crea F, Abbate A. Heart failure after ST‐elevation myocardial infarction: beyond left ventricular adverse remodeling. Curr Probl Cardiol. 2022: 101215. [DOI] [PubMed] [Google Scholar]
- 24. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Variables.
Table S2. Baseline demographics and characteristics on admission – incl 95%CIs.
Table S3. Treatment, examination findings, complications and medication at discharge for the total population and the subgroups – incl 95% CIs.
Table S4. Number of patients with missing value.
Table S5. Number of patients with missing value.
Table S6. Association between key risk factors and primary outcome (after multiple imputation).


