Abstract
A 44‐year‐old woman who was quarantined for 5 days after the diagnosis of coronavirus disease of 2019 (COVID‐19) was transferred to our hospital with the complaint of chest pain. The patient was unvaccinated. Electrocardiography revealed ST elevation in the lateral leads. Echocardiographic biventricular dysfunction with oedematous wall thickening was identified. Cardiac enzyme levels were elevated; however, C‐reactive protein (CRP) levels, and the coronary angiogram were normal. The patient required mechanical circulatory support to stabilize haemodynamics and was treated with remdesivir, baricitinib, and intravenous methylprednisolone. She recovered after 13 days of mechanical support. Serial cardiac magnetic resonance imaging revealed acute myocardial oedema and subsequent fibrosis. An endomyocardial biopsy on admission showed mild interstitial inflammatory infiltrates with endomyocardial fibrous thickening and mild interstitial fibrosis of the myocardium. Normal CRP levels suggested minor involvement of interleukin (IL)‐6, supporting the efficacy of baricitinib.
Keywords: Cardiac magnetic resonance, COVID‐19, C‐reactive protein, Fulminant myocarditis
Introduction
Myocarditis is an uncommon complication of coronavirus disease of 2019 (COVID‐19), occurring in 3.6% of patients undergoing autopsy 1 and 0.24% of hospitalized patients. 2 Although several randomized trials have attempted to explore effective medications for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infections (Supporting information, Table S1 ), optimal treatments specific for myocarditis have not been fully elucidated.
Case report
A 44‐year‐old woman with respiratory symptoms for 5 days and positive SARS‐CoV‐2 antigen test results was referred to our hospital because of worsening chest pain. She was not vaccinated for SARS‐CoV‐2 and did not have a significant past medical history. Vital signs were as follows: temperature, 36.1°C; blood pressure, 107/85 mmHg; heart rate, 107 bpm; and SpO2, 97% at room temperature. Laboratory data showed elevated levels of creatine kinase (244 U/L, >153 U/L), creatine kinase‐myocardial band (20.7 ng/mL, >3.3 ng/mL), and cardiac troponin I (4,475 pg/mL, >24 pg/mL). C‐reactive protein (CRP) was not elevated (0.07 mg/dL, <0.14 mg/dL). An electrocardiogram showed sinus rhythm with low voltage in limb leads, ST‐segment elevation in V5,6, poor R progression in V2,3, and QT prolongation (Figure 1 ). Echocardiography showed biventricular oedematous wall thickening with systolic dysfunction and a moderate amount of pericardial effusion (Videos S1 and S2). Coronary angiography revealed no evidence of stenosis. An endomyocardial biopsy of the right ventricular septum was performed on admission. Quantitative testing of SARS‐CoV‐2 by reverse transcription‐polymerase chain reaction was positive upon admission.
Figure 1.
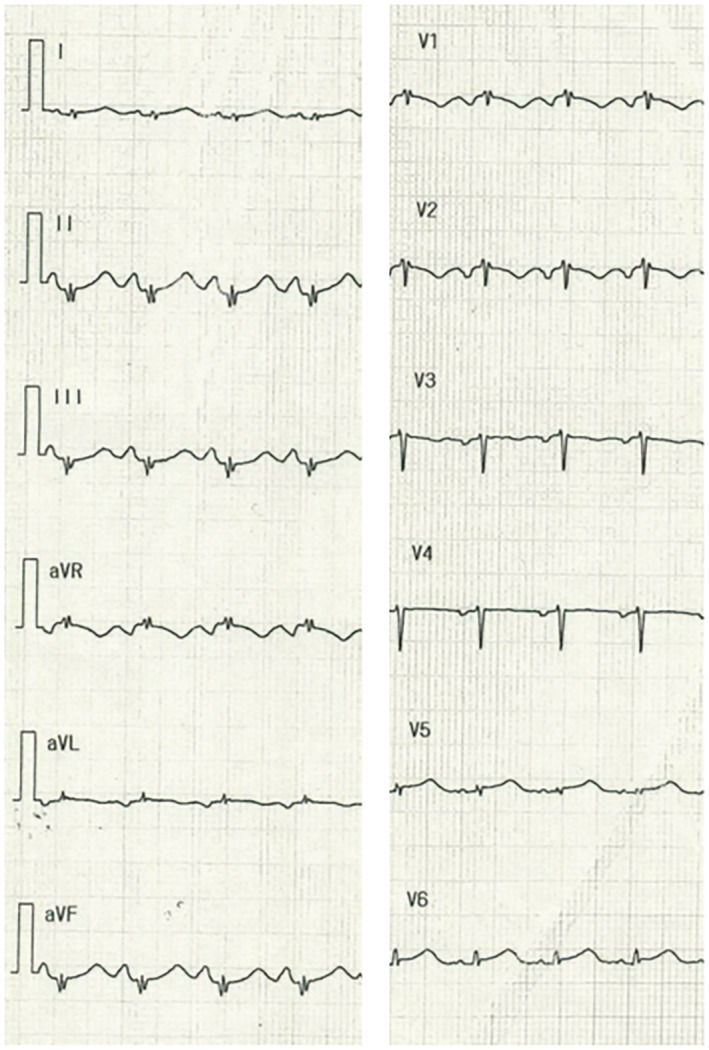
Electrocardiogram. Electrocardiography revealed sinus tachycardia, low voltages in limb leads, ST‐segment elevations in V5,6, poor R progression in V2,3, and QT prolongation.
The hospital course during the acute phase is shown in Figure 2 . The patient was diagnosed with cardiac tamponade due to perimyocarditis and treated with pericardiocentesis on Day 2. After that, she was treated with remdesivir (200 mg on Day 1, followed by 100 mg daily for up to nine additional days) and intravenous corticosteroids (methylprednisolone, 1000 mg daily for 3 days). Since pericardiocentesis improved haemodynamic status only transiently and cardiogenic shock worsened after that, the patient required inotropes with mechanical circulatory support devices [veno‐arterial extracorporeal membrane oxygenation and a percutaneous left ventricular assist device (Impella CP, Abiomed, MA, USA)] on Day 3. Since her haemodynamics remained deteriorated, the second round of intravenous corticosteroids (a similar dose to the first round) and baricitinib (4 mg on Days 6 and 7, followed by 2 mg on Days 8 and 10 due to acute kidney injury) were started on Day 6. After these medications, her pulse pressure and biventricular function recovered (Videos S3 and S4 ), leading to the removal of mechanical circulatory support on Day 13. The CRP levels remained normal during the acute phase of hospitalization. The patient was discharged after the rehabilitation on hospital Day 99. The peak troponin I level was 34 961 pg/mL on Day 4, and it decreased over time. Echocardiographic biventricular dysfunction recovered at discharge (Videos S5 and S6 ). Reverse transcription‐polymerase chain reaction results were negative on Day 48.
Figure 2.
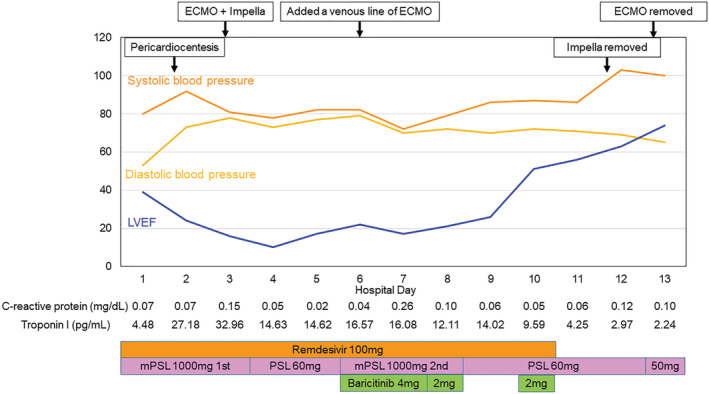
Hospital course in an acute phase. ECMO, extracorporeal membrane oxygenation; LVEF, left ventricular ejection fraction; mPSL, methylprednisolone; PSL, prednisolone.
Non‐contrast cardiac magnetic resonance (CMR) imaging was performed using a 3.0‐T scanner (GE Healthcare, IL, USA) on Days 64 and 93 during hospitalization for acute renal dysfunction, and contrast CMR imaging was performed on Day 240 after discharge (Figure 3 ). CMR on Day 64 showed diffuse oedematous wall thickening with high signal intensity in T2‐weighted images, which recovered on Days 99 and 240. Native T1‐mapping using a modified Look–Locker inversion recovery sequence showed an increased value in the inferolateral mid‐myocardial wall on Day 64, which decreased over time. Late‐gadolinium enhancement (LGE) was pronounced in the basal to the apical inferolateral mid‐myocardial wall, corresponding to the regions with the highest native T1 value. In addition, the extracellular volume was elevated, particularly in the inferolateral wall (40.6%).
Figure 3.
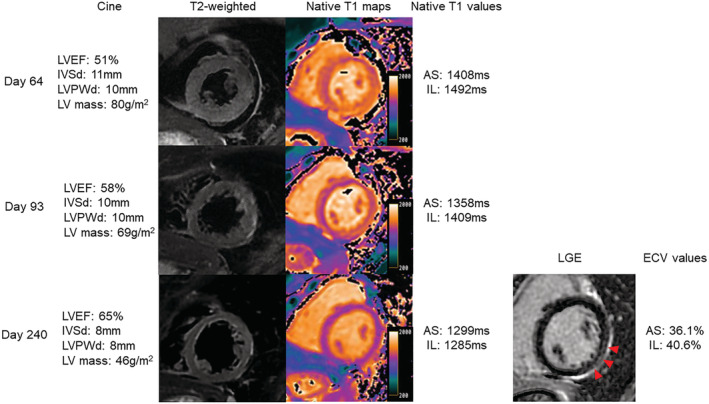
Serial cardiac magnetic resonance imaging. LVEF and LV mass, measured using cine imaging, recovered over time. Oedematous wall thickening with high signal intensity on T2‐weighted images was observed on Day 64 and subsequently recovered. Native T1 values in the anteroseptal and inferolateral walls were high on Day 64 and decreased over time. LGE was observed in the basal‐to‐apical inferolateral mid‐myocardial wall (red arrowheads), corresponding to the regions with the highest native T1 value. The ECV was high in the anteroseptal and inferolateral walls. AS, anteroseptal wall; ECV, extracellular volume; IL, inferolateral wall; LGE, late gadolinium enhancement; IVSd, interventricular septal thickness in diastole; LVEF, left ventricular ejection fraction; LVPWd, left ventricular posterior wall thickness in diastole.
Endomyocardial biopsy specimens showed mild interstitial inflammatory infiltrates predominantly composed of macrophages admixed with a few T‐cells without adjacency to cardiomyocyte necrosis. The absence of eosinophils, moderate endomyocardial fibrous thickening, and mild interstitial fibrosis of the myocardium were also observed. The presence of platelets in obstructive microthrombi within the lumen of microvessels was confirmed by immunohistochemical reactivity for CD61. Furthermore, immunohistochemical reactivity for tenascin‐C (TN‐C) was observed in the endocardium and partially in the interstitium (Figure 4 ).
Figure 4.
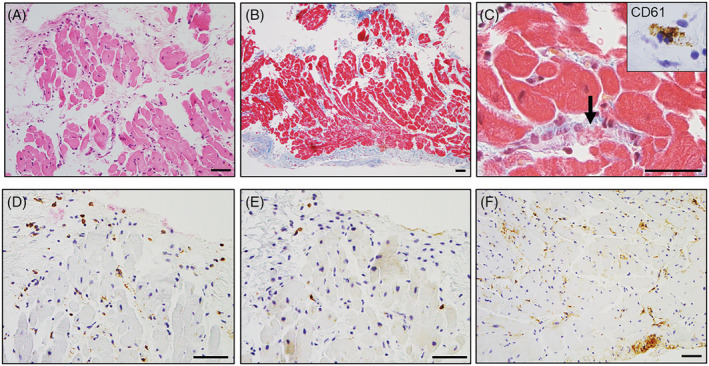
Histopathological findings of endomyocardial biopsy samples. An endomyocardial biopsy showing mild inflammation on haematoxylin and eosin staining (A). Moderate endomyocardial thickening and mild interstitial fibrosis on Masson's trichrome staining (B). High‐power view of Masson's trichrome staining showing a microthrombus with fibrin and platelets within the arteriole (C, arrow). Immunohistochemical features of myocarditis: CD61 (C, inset), CD68 (D), CD3 (E), and tenascin‐C (F). CD61‐positive platelets were observed in obstructive microthrombi in the microvessels. CD68‐positive macrophages and a few CD3‐positive T‐cells infiltrated sparsely in the interstitium without necrosis of the adjacent cardiomyocytes. Tenascin‐C was distributed in the endocardium and partially located in the interstitium. Scale bars: 50 μm.
Discussion
We report the case of a patient with COVID‐19‐associated fulminant myocarditis and normal CRP levels during the acute phase. The patient required mechanical circulatory support and recovered after treatment with remdesivir, baricitinib, and intravenous methylprednisolone. CMR findings in the subacute phase, including T2‐high images, elevated native T1 values, and global wall motion abnormalities, met the updated Lake–Louise criteria for diagnosing acute myocarditis. 2 , 3
In the chronic phase, the elevation of native‐T1 value with corresponding LGE in the mid‐myocardial inferolateral wall was observed, suggesting extensive myocardial fibrosis. 4 The involvement of the inferolateral mid‐myocardial wall, seen in this case, was commonly observed in viral myocarditis. 3
In the present case, despite mild lymphocytic infiltration in the myocardium without necrosis of adjacent myocytes, immune activation, or a reaction induced by inflammatory damage was recognized because of positive immunostaining for TN‐C. TN‐C reactivity occurs in response to tissue repair after inflammation based on the hypothesis that TN‐C regulates the pro‐inflammatory phenotype of macrophages. 5 These findings led to the hypothesis that the pathogenesis of COVID‐19‐associated myocarditis would be different from that of usual acute lymphocytic myocarditis. Indeed, increasing evidence suggests that direct cytotoxic effects caused by SARS‐CoV‐2 invasion in cardiomyocytes may be associated with cardiac dysfunction. 1 When SARS‐CoV‐2 invades the cardiomyocytes via angiotensin‐converting enzyme 2 (ACE2), angiotensin‐(1‐7)/MAS‐mediated cardioprotective effects are impaired due to abrogation of ACE2 catalytic activity, 6 thereby elevating the level of angiotensin II, which in turn aggravating its cardiotoxic effects. 6 Also, invasion of SARS‐CoV‐2 could trigger programmed cardiomyocyte death, such as apoptosis and pyroptosis, leading to irreversible cardiac damage without massive lymphocyte infiltration. The serum CRP values in the present case were consistently normal, which was not observed in any other reported cases of COVID‐19‐associated fulminant myocarditis (Table S2 ). The administration of high‐dose corticosteroids on Day 1 may have lowered the serum CRP levels. However, in this case, the CRP level was extremely low, given that a recent multicentre study reported the median peak CRP level in 51 COVID‐19‐associated acute myocarditis cases was 20.7 (5.2–33.6) mg/dL. 2 As CRP is produced in hepatocytes in response to interleukin (IL)‐6‐mediated signalling, 7 we speculated that the inflammatory response in the present case might be mainly mediated through IL‐6‐independent mechanisms. The Janus kinase (JAK)‐STAT pathway is associated with various immune responses that are mediated through multiple cytokines, including IL‐2/6/12/15/23 and interferons. 8 Thus, it would be reasonable to assume that the administration of baricitinib, a JAK inhibitor, instead of tocilizumab, an anti‐IL‐6 receptor monoclonal antibody, was effective as an immunosuppressive agent in this case. This study has several limitations. First, we did not evaluate the presence of SARS‐CoV‐2 in the cardiomyocytes. Second, we could not determine the effectiveness of baricitinib because of the multiple medications. Furthermore, we did not measure IL‐6 levels.
In conclusion, we demonstrated the usefulness of CMR imaging for diagnosing COVID‐19‐induced fulminant myocarditis, even in its subacute phase. In addition, normal serum CRP levels might suggest the benefit of administrating baricitinib rather than tocilizumab in patients hospitalized due to COVID‐19‐associated myocarditis.
Conflict of interest
None declared.
Supporting information
Table S1. Randomized Controlled Trials Investigating Immunosuppressive Therapies Against COVID‐19
Table S2. Previous Case Reports of Fulminant Myocarditis Associated With COVID‐19 Infection
Video S1. Long‐axis echocardiographic view on admission
Video S2. Short‐axis echocardiographic view on admission
Video S3. Long‐axis echocardiographic view on day 13
Video S4. Short‐axis echocardiographic view on day 13
Video S5. Long‐axis echocardiographic view at discharge
Video S6. Short‐axis echocardiographic view at discharge
Usui, E. , Nagaoka, E. , Ikeda, H. , Ohmori, M. , Tao, S. , Yonetsu, T. , Maejima, Y. , Arai, H. , Amemiya, K. , Ikeda, Y. , and Sasano, T. (2023) Fulminant myocarditis with COVID‐19 infection having normal C‐reactive protein and serial magnetic resonance follow‐up. ESC Heart Failure, 10: 1426–1430. 10.1002/ehf2.14228.
References
- 1. Kawakami R, Sakamoto A, Kawai K, Gianatti A, Pellegrini D, Nasr A, Kutys B, Guo L, Cornelissen A, Mori M, Sato Y, Pescetelli I, Brivio M, Romero M, Guagliumi G, Virmani R, Finn AV. Pathological evidence for SARS‐CoV‐2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol 2021; 77: 314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ammirati E, Lupi L, Palazzini M, Hendren NS, Grodin JL, Cannistraci CV, Schmidt M, Hekimian G, Peretto G, Bochaton T, Hayek A, Piriou N, Leonardi S, Guida S, Turco A, Sala S, Uribarri A, Van De Heyning CM, Mapelli M, Campodonico J, Pedrotti P, Barrionuevo Sánchez MI, Ariza Sole A, Marini M, Matassini MV, Vourc HM, Cannatà A, Bromage DI, Briguglia D, Salamanca J, Diez‐Villanueva P, Lehtonen J, Huang F, Russel S, Soriano F, Turrini F, Cipriani M, Bramerio M, Di Pasquale M, Grosu A, Senni M, Farina D, Agostoni P, Rizzo S, De Gaspari M, Marzo F, Duran JM, Adler ED, Giannattasio C, Basso C, McDonagh T, Kerneis M, Combes A, Camici PG, De Lemos JA, Metra M. Prevalence, characteristics, and outcomes of COVID‐19‐associated acute myocarditis. Circulation 2022; 145: 1123–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferreira VM, Schulz‐Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J Am Coll Cardiol 2018; 72: 3158–3176. [DOI] [PubMed] [Google Scholar]
- 4. Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: A comprehensive review. J Cardiovasc Magn Reson 2016; 18: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imanaka‐Yoshida K. Tenascin‐C in heart diseases—the role of inflammation. Int J Mol Sci 2021; 22: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samavati L, Uhal BD. ACE2, much more than just a receptor for SARS‐COV‐2. Front Cell Infect Microbiol 2020; 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Libby P, Rocha VZ. All roads lead to IL‐6: a central hub of cardiometabolic signaling. Int J Cardiol 2018; 259: 213–215. [DOI] [PubMed] [Google Scholar]
- 8. O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK‐STAT pathway: Impact on human disease and therapeutic intervention. Annu Rev Med 2015; 66: 311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Randomized Controlled Trials Investigating Immunosuppressive Therapies Against COVID‐19
Table S2. Previous Case Reports of Fulminant Myocarditis Associated With COVID‐19 Infection
Video S1. Long‐axis echocardiographic view on admission
Video S2. Short‐axis echocardiographic view on admission
Video S3. Long‐axis echocardiographic view on day 13
Video S4. Short‐axis echocardiographic view on day 13
Video S5. Long‐axis echocardiographic view at discharge
Video S6. Short‐axis echocardiographic view at discharge


