Abstract
Aims
We investigated titration patterns of angiotensin‐converting enzyme inhibitors (ACEis)/angiotensin receptor blockers (ARBs) and beta‐blockers, quality of life (QoL) over 6 months, and associated 1 year outcome [all‐cause mortality/heart failure (HF) hospitalization] in a real‐world population with HF with reduced ejection fraction (HFrEF).
Methods and results
Participants with HFrEF (left ventricular ejection fraction <40%) from a prospective multi‐centre study were examined for use and dose [relative to guideline‐recommended maintenance dose (GRD)] of ACEis/ARBs and beta‐blockers at baseline and 6 months. ‘Stay low’ was defined as <50% GRD at both time points, ‘stay high’ as ≥50% GRD, and ‘up‐titrate’ and ‘down‐titrate’ as dose trajectories. Among 1110 patients (mean age 63 ± 13 years, 16% women, 26% New York Heart Association Class III/IV), 714 (64%) were multi‐ethnic Asians from Singapore and 396 were from New Zealand (mainly European ethnicity). Baseline use of either ACEis/ARBs or beta‐blockers was high (87%). Loop diuretic was prescribed in >80% of patients, mineralocorticoid receptor antagonist in about half of patients, and statins in >90% of patients. At baseline, only 11% and 9% received 100% GRD for each drug class, respectively, with about half (47%) achieving ≥50% GRD for ACEis/ARBs or beta‐blockers. At 6 months, a large majority remained in the ‘stay low’ category, one third remained in ‘stay high’, whereas 10–16% up‐titrated and 4–6% down‐titrated. Patients with lower (vs. higher) N‐terminal pro‐beta‐type natriuretic peptide levels were more likely to be up‐titrated or be in ‘stay high’ for ACEis/ARBs and beta‐blockers (P = 0.002). Ischaemic aetiology, prior HF hospitalization, and enrolment in Singapore (vs. New Zealand) were independently associated with higher odds of ‘staying low’ (all P < 0.005) for prescribed doses of ACEis/ARBs and beta‐blockers. Adjusted for inverse probability weighting, ≥100% GRD for ACEis/ARBs [hazard ratio (HR) = 0.42; 95% confidence interval (CI) 0.24–0.73] and ≥50% GRD for beta‐blockers (HR = 0.58; 95% CI 0.37–0.90) (vs. Nil) were associated with lower hazards for 1 year composite outcome. Country of enrolment did not modify the associations of dose categories with 1 year composite outcome. Higher medication doses were associated with greater improvements in QoL.
Conclusions
Although HF medication use at baseline was high, most patients did not have these medications up‐titrated over 6 months. Multiple clinical factors were associated with changes in medication dosages. Further research is urgently needed to investigate the causes of lack of up‐titration of HF therapy (and its frequency), which could inform strategies for timely up‐titration of HF therapy based on clinical and biochemical parameters.
Keywords: Medication dose, Heart failure, Reduced ejection fraction, Renin–angiotensin system inhibitors, Beta‐blockers
Introduction
Guideline‐directed medical therapy (GDMT) is the cornerstone in managing heart failure (HF) with reduced ejection fraction (HFrEF), proven to improve survival and patient health‐related quality of life (QoL). 1 , 2 For maximal benefits, international guidelines recommend that each HF medication be titrated to guideline‐recommended target doses (GRD), as tolerated. 1 , 2 Unfortunately, under‐dosing of GDMT occurs in majority of HFrEF cases. 3 , 4 , 5 , 6 , 7 With the ever‐growing burden of HF, even in non‐elderly persons, 8 , 9 guideline‐directed dose optimization is crucial in improving clinical outcomes and reining in the huge costs of frequent hospitalization. There is thus an urgent need to characterize the gaps in longitudinal titration patterns of evidence‐based medications in real‐world HF populations. 5 , 6
Studies from Asia have shown prescription of particularly low dosages of GDMT among HFrEF patients, 4 , 7 not explained by contraindications to up‐titration. Asian patients are generally younger, smaller, and leaner than patients of White ethnicity. 4 , 7 , 8 Therefore, there is a common perception that Asian patients might do with lower doses. 10 , 11 , 12 However, data relating to any sustained differences between Asian and Western populations in longitudinal titration patterns of evidence‐based anti‐HF medications are sparse.
Therefore, this study aimed to investigate titration patterns of angiotensin‐converting enzyme inhibitors (ACEis)/angiotensin receptor blockers (ARBs) and beta‐blockers (as the ‘backbone’ of HFrEF therapy), change in the QoL over 6 months, and associated 1 year composite outcome of all‐cause mortality/HF hospitalization in real‐world populations with HFrEF in Singapore (SG) and New Zealand (NZ). As a secondary objective, we examined if the country of enrolment modified the association of medication doses with 1 year composite outcome. A better understanding of the factors influencing prescribing patterns could provide opportunities to optimize medications.
Methodology
Study design and study population
The design and study population had been previously described. 13 , 14 A prospective longitudinal multi‐centre study of patients with HF was conducted in parallel in NZ (four centres) and SG (six centres) according to a common protocol. Patient enrolment commenced from March 2010 through August 2014. The inclusion and exclusion criteria had been previously described. 8 , 13 Patients enrolled were adults aged ≥18 years with a clinical diagnosis of HF admitted to hospital for a primary diagnosis of HF or attending an outpatient clinic in the hospital for management of HF within 6 months of an episode of decompensated HF. All patients had signs and symptoms of HF and objective evidence based on echocardiography in accordance to the European Society of Cardiology 2012 guidelines, 15 confirmed by the attending physician and verified by the site investigator. Only patients with HFrEF [defined as left ventricular ejection fraction (LVEF) <40%] were included for dose analysis for this study.
Exclusion criteria included severe valvular heart disease, transient acute pulmonary oedema in the context of primary acute coronary syndrome, end‐stage renal failure, specific HF subgroups (including constrictive pericarditis, congenital heart disease, hypertrophic cardiomyopathy, cardiac amyloid, and chemotherapy‐associated cardiomyopathy), isolated right HF, life‐threatening comorbidity with life expectancy <1 year, and inability to provide consent. Transient causes of reduced ejection fraction (EF) due to acute myocarditis or takotsubo cardiomyopathy were not specifically examined.
Patients were followed up prospectively for a 1 year composite outcome of all‐cause mortality or HF hospitalization.
Ethics approvals were obtained from each participating centre's local institutional review committee, and all participating subjects gave informed consent. This study conforms to the ethical guidelines as laid down in the Declaration of Helsinki.
Data collection and medications
Patients were assessed upon enrolment (baseline visit) and visits at 6 weeks and 6 months and by telephone calls at 1 and 2 years. Demographics, clinical signs and symptoms on physical examination, New York Heart Association (NYHA) functional status, serum biochemistry, medical history, 12‐lead electrocardiography, medications (including doses prescribed), prior interventions, and QoL measurements were recorded. Information on HF history (including aetiology and duration of HF) and prior use of implantable cardioverter defibrillator (ICD) or pacemaker was also collected. Comprehensive two‐dimensional echocardiography was also performed on all eligible patients, using standardized machines at study sites.
Health‐related QoL was assessed at baseline and 6 week and 6 month visits using the Minnesota Living with Heart Failure Questionnaire (MLHFQ). 16 The MLHFQ is a self‐report questionnaire comprising 21 items, which assesses how HF affects the physical and emotional dimensions of well‐being. 17 These dimensions are combined into a total score that reflects a global assessment of well‐being. The MLHFQ score, computed by the summation of the scores to all the questions, ranges from 0 (which equates to no impairment as a consequence of HF) to 105 for maximum impairment. Lower MLHFQ scores therefore correlate with better QoL. Absolute values of MLHFQ or change in total and domain scores at 6 months from baseline were assessed.
Use and dose (calculated as per cent of GRD, Supporting Information, Table S1 ) of ACEis/ARBs and beta‐blockers at baseline and 6 months were examined. Doses were grouped into the following categories: 0%, 1–24%, 25–49%, 50–99%, and ≥100%. Use of other medications: mineralocorticoid receptor antagonist (MRA), loop diuretics, and statins were captured as binary variables (yes/no). As patient enrolment occurred before market availability of ivabradine, angiotensin receptor neprilysin inhibitor (ARNi), and sodium–glucose cotransporter‐2 inhibitors (SGLT2is), these medications were not reported. For transition across dose categories (between baseline and 6 months), ‘stay low’ was defined as prescription of <50% GRD at both visits, ‘stay high’ as ≥50% GRD at both visits, and ‘up‐titrate’ and ‘down‐titrate’ if transition occurred from <50% to ≥50% GRD in the former and vice versa for the latter. Patients were considered successfully up‐titrated when ≥50% recommended target doses for ACEis/ARBs or beta‐blockers were achieved after up‐titration. 4 In cases where medication doses at 6 months are missing, medication doses at 6 weeks were used to replace or carry forward to 6 months. However, up‐titration of medications might be underestimated using this method because up‐titration would usually be done within 3 months, not within 6 weeks. One‐year medication doses were not used to replace 6 months' missing data in view of the bias associated with telephone follow‐up and excess elapsed duration from the early phase when up‐titration would have customarily been completed.
Outcomes
The primary outcome was a composite outcome of all‐cause mortality or HF hospitalization at 1 year. Health‐related QoL measured at 6 months and number of hospitalization days (LOS) for first HF hospitalization from baseline were secondary outcomes.
Statistical analysis
Baseline characteristics of patients stratified into five dose categories of each therapeutic class at baseline (none, 1–<25%, 25–<50%, 50–<100%, and ≥100% of GRD) were described and compared. The binning of dose categories reflected the predominance of low doses of medications prescribed. Patients were further characterized based on their dose trajectories (transition groups) over the 6 months. Standard descriptive statistics, including, as appropriate, mean ± standard deviation (SD) and median plus 25th–75th percentiles or numbers and percentages, were used to characterize the study populations and subgroups. Distribution of all continuous variables was checked at start of the analysis. For variables that have skewed non‐Gaussian distribution, non‐parametric tests were used and log‐transformation was applied in multivariable regression analyses. We tested differences between groups using the one‐way analysis of variance or Kruskal–Wallis test (for continuous variables) or the χ 2 test (for categorical variables).
Multivariable logistic regression models were used to examine the association of independent baseline variables with ‘stay low’ and separately ‘up‐titrate’ in the dose transition. Recognizing that the study was an observational non‐randomized study, we were careful to adjust for treatment indication bias in outcome analyses. All analyses for the associations of ACEi/ARB and beta‐blocker treatment with outcomes were inversely weighted for the probability of achieving ≥50% GRD. 4 These weights were calculated by the mean probability per patient predicted by a penalized logistic model fitted to five imputed datasets [implemented for missing data in the MICE package using the R statistical program (Version 4.0.4)]. For the penalized logistic regression analysis used to predict successful treatment (of ≥50% GRD at 6 months), we included a comprehensive list of clinical variables (Supporting Information, Table S4 ).
Univariable Cox proportional hazard models inversely weighted for the probability of achieving ≥50% GRD and multivariable Cox models were used to examine the association of dose categories (0%, 1–<50%, 50–<100%, and ≥100%) achieved at 6 months, by medication class with outcomes. The association of dose transition categories (‘stay low’, ‘stay high’, ‘up‐titrate’, and ‘down‐titrate’) at 6 months by therapeutic class with outcomes was similarly examined. We tested the proportionality of hazard assumptions, and they were valid. Patients who were missing information on medications (ACEi/ARB or beta‐blocker) at baseline were excluded from the analysis. Furthermore, those lost to follow‐up or who died before the 6 month visit were excluded from dose transition and survival analysis (Supporting Information, Figure S1 ).
Linear regression models were used to examine the association of independent variables with QoL (and change in QoL) scores at 6 months. For all analyses, reported P values were two‐sided and significant at 5% level. All analyses were performed using Stata (Version 14) (College Station, TX, USA).
Results
Among 1110 patients with HFrEF (mean age 63 ± 13 years, 16% women, 26% NYHA Class III/IV), 714 (64%) were multi‐ethnic Asians from SG and 396 were from NZ (mainly NZ European ethnicity). Patients from SG (compared with those from NZ) were younger and less frequently women, had lower body mass index (BMI), and were less likely to have comorbid atrial fibrillation and be in NYHA Class III/IV. In contrast, they were more likely to have ischaemic aetiology, hypertension, anaemia, prior HF hospitalization, and notably twice as high prevalence of diabetes (all P < 0.05, Supporting Information, Table S2 ). Symptom burden was consistent regardless of country, with similar proportions manifesting orthopnoea, peripheral oedema, paroxysmal nocturnal dyspnoea, and dyspnoea at rest.
Ischaemic aetiology was predominant, in 60% of the patients with HFrEF. Of the non‐ischaemic subgroup, ‘dilated/idiopathic’ accounted for about half (48%), followed by hypertensive and arrhythmias. Cardiac devices [ICD, cardiac resynchronization therapy defibrillator (CRT‐D), and pacemaker] were implanted in only 129 patients (11.6% of the cohort), ICD or CRT‐D in 94 patients, and pacemaker in 35 patients.
Tables 1 and 2 showed the baseline characteristics of patients from the total cohort in the five different baseline dose categories (none, 1–24%, 25–49%, 50–99%, and ≥100%) for ACEis/ARBs and beta‐blockers, respectively. Lower doses (<50% GRD) of ACEis/ARBs (vs. higher dose categories) were more likely prescribed among those who were older, who had lower BMI or body surface area (BSA) and lower LVEF but higher heart rate, with underlying ischaemic aetiology and anaemia, who were enrolled as inpatients, and of Asian descent (Table 1 ). These characteristics were similar to those in lower dose categories for beta‐blockers (Table 2 ).
Table 1.
Baseline characteristics of patients in the different baseline RASi (angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers) dose categories
| Factor | No RASi at baseline | RASi 1–24% GRD | RASi 25–49% GRD | RASi 50–99% GRD | RASi ≥ 100% GRD | P value |
|---|---|---|---|---|---|---|
| N | 150 | 376 | 256 | 199 | 122 | |
| Demographics | ||||||
| Age (years), mean (SD) | 66.0 (13.4) | 63.2 (12.7) | 60.2 (13.2) | 62.4 (12.4) | 62.3 (12.4) | <0.001 |
| Women | 22 (14.7%) | 56 (14.9%) | 45 (17.6%) | 33 (16.6%) | 21 (17.2%) | 0.88 |
| Enrolled in SG (vs. NZ) | 105 (70.0%) | 260 (69.1%) | 196 (76.6%) | 105 (52.8%) | 44 (36.1%) | <0.001 |
| Enrolled as inpatient | 108 (72.0%) | 244 (64.9%) | 144 (56.3%) | 122 (61.3%) | 70 (57.4%) | 0.014 |
| Clinical exam/signs and symptoms | ||||||
| NYHA Class III/IV (vs. I/II) | 53 (37.1%) | 101 (27.4%) | 42 (16.5%) | 51 (25.8%) | 30 (25.0%) | <0.001 |
| BMI (kg/m2), mean (SD) | 25.7 (4.5) | 25.5 (5.2) | 26.6 (5.2) | 27.5 (6.2) | 29.8 (7.1) | <0.001 |
| BSA (m2), mean (SD) | 1.77 (0.21) | 1.80 (0.22) | 1.81 (0.21) | 1.87 (0.26) | 1.98 (0.28) | <0.001 |
| Systolic BP (mmHg), mean (SD) | 118.1 (19.6) | 114.0 (18.4) | 120.3 (19.1) | 123.5 (21.8) | 124.0 (22.7) | <0.001 |
| Diastolic BP (mmHg), mean (SD) | 69.3 (12.0) | 69.2 (11.6) | 71.7 (12.3) | 74.7 (13.9) | 72.4 (13.6) | <0.001 |
| Heart rate (b.p.m.), mean (SD) | 77.9 (15.6) | 77.8 (14.1) | 77.5 (14.0) | 75.4 (15.3) | 72.1 (12.1) | 0.001 |
| LVEF (%), median (IQR) | 25 (20, 33) | 25 (20, 30) | 26 (20, 32) | 28 (22, 34) | 29 (25, 35) | <0.001 |
| Ischaemic aetiology | 96 (64.0%) | 232 (61.7%) | 155 (60.5%) | 114 (57.3%) | 61 (50.0%) | 0.13 |
| Shortness of breath on exertion | 114 (76.5%) | 265 (70.7%) | 155 (60.5%) | 147 (73.9%) | 83 (68.0%) | 0.004 |
| Shortness of breath at rest | 31 (20.8%) | 45 (12.0%) | 28 (10.9%) | 23 (11.6%) | 13 (10.7%) | 0.034 |
| Reduction in exercise tolerance | 116 (77.9%) | 252 (67.0%) | 149 (58.2%) | 146 (73.4%) | 86 (70.5%) | <0.001 |
| Orthopnoea | 52 (34.7%) | 106 (28.2%) | 61 (23.8%) | 53 (26.6%) | 29 (23.8%) | 0.16 |
| Paroxysmal nocturnal dyspnoea | 36 (24.2%) | 59 (15.7%) | 48 (18.8%) | 38 (19.1%) | 17 (14.0%) | 0.15 |
| Peripheral oedema | 71 (47.3%) | 121 (32.4%) | 87 (34.0%) | 63 (31.7%) | 48 (39.7%) | 0.011 |
| Laboratory values | ||||||
| NT‐proBNP (ng/L), median (IQR) | 4119 (2139, 8306) | 2564 (1350, 5140) | 2149 (980, 4587) | 2353 (1178, 4863) | 1752 (749, 3890) | <0.001 |
| Serum potassium (mmol/L), median (IQR) | 4.1 (3.7, 4.4) | 4.1 (3.8, 4.4) | 4.0 (3.7, 4.5) | 4.1 (3.8, 4.4) | 4.1 (3.8, 4.6) | 0.36 |
| eGFR (mL/min/1.73 m2), median (IQR) | 49.0 (29.2, 69.2) | 61.2 (47.2, 77.7) | 62.1 (48.5, 77.6) | 58.2 (44.8, 71.6) | 56.9 (42.8, 74.9) | <0.001 |
| Medical history | ||||||
| Hypertension | 101 (67.8%) | 197 (53.7%) | 155 (61.5%) | 141 (72.3%) | 87 (71.9%) | <0.001 |
| Diabetes | 85 (56.7%) | 148 (39.8%) | 132 (51.6%) | 88 (44.2%) | 54 (44.3%) | 0.003 |
| Charlson comorbidity index, mean (SD) | 2.8 (1.7) | 2.4 (1.5) | 2.3 (1.3) | 2.5 (1.5) | 2.6 (1.7) | 0.004 |
| Atrial fibrillation | 61 (40.7%) | 119 (31.6%) | 61 (23.8%) | 66 (33.2%) | 46 (37.7%) | 0.005 |
| Anaemia | 83 (57.6%) | 125 (37.0%) | 76 (34.9%) | 56 (30.9%) | 30 (26.3%) | <0.001 |
| Prior hospitalization for heart failure | 97 (15.2%) | 203 (31.8%) | 168 (26.3%) | 106 (16.6%) | 65 (10.2%) | 0.006 |
| Medications at baseline | ||||||
| Beta‐blockers | 121 (81.2%) | 329 (88.2%) | 227 (88.7%) | 176 (89.8%) | 114 (94.2%) | 0.019 |
| Beta‐dose (%GRD), median (IQR) | 25 (13, 50) | 25 (13, 26) | 25 (13, 50) | 25 (25, 50) | 50 (25, 75) | <0.001 |
| MRA | 55 (36.7%) | 191 (50.8%) | 150 (58.6%) | 100 (50.3%) | 64 (52.5%) | 0.001 |
| Loop diuretic | 135 (90.0%) | 354 (94.1%) | 238 (93.0%) | 189 (95.0%) | 117 (95.9%) | 0.25 |
| Statin | 100 (66.7%) | 270 (71.8%) | 199 (77.7%) | 146 (73.4%) | 94 (77.0%) | 0.12 |
| Outcomes | ||||||
| Composite outcome at 1 year | 72 (48.0%) | 121 (32.2%) | 74 (28.9%) | 78 (39.2%) | 35 (28.7%) | <0.001 |
%GRD, per cent guideline‐recommended dose; BMI, body mass index; BP, blood pressure; BSA, body surface area; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐beta‐type natriuretic peptide; NYHA, New York Heart Association; NZ, New Zealand; RASi, renin–angiotensin system inhibitor; SD, standard deviation; SG, Singapore.
Table 2.
Baseline characteristics of patients in the different baseline beta‐blocker dose categories
| Factor | No BB at baseline | BB dose 1–24% GRD | BB dose 25–49% GRD | BB dose 50–99% GRD | BB dose ≥100% GRD | P value |
|---|---|---|---|---|---|---|
| N | 134 | 288 | 334 | 244 | 101 | |
| Demographics | ||||||
| Age (years), mean (SD) | 66.1 (14.7) | 63.1 (12.7) | 61.5 (12.8) | 62.3 (12.6) | 60.4 (12.2) | 0.003 |
| Women | 23 (17.2%) | 50 (17.4%) | 53 (15.9%) | 37 (15.2%) | 15 (14.9%) | 0.95 |
| Enrolled in SG (vs. NZ) | 74 (55.2%) | 211 (73.3%) | 230 (68.9%) | 141 (57.8%) | 56 (55.4%) | <0.001 |
| Enrolled as inpatient | 95 (70.9%) | 187 (64.9%) | 207 (62.0%) | 142 (58.2%) | 58 (57.4%) | 0.098 |
| Clinical exam/signs and symptoms | ||||||
| NYHA Class III/IV (vs. I/II) | 46 (34.8%) | 78 (28.0%) | 75 (22.7%) | 50 (20.8%) | 29 (28.7%) | 0.021 |
| BMI (kg/m2), mean (SD) | 26.6 (6.8) | 26.1 (5.5) | 26.2 (5.5) | 27.4 (5.4) | 27.6 (5.7) | 0.015 |
| BSA (m2), mean (SD) | 1.81 (0.25) | 1.79 (0.23) | 1.82 (0.23) | 1.87 (0.23) | 1.90 (0.25) | <0.001 |
| Systolic BP (mmHg), mean (SD) | 116.5 (19.3) | 116.0 (20.2) | 118.1 (19.2) | 122.7 (20.4) | 123.3 (22.3) | <0.001 |
| Diastolic BP (mmHg), mean (SD) | 69.9 (11.7) | 69.4 (12.8) | 71.0 (12.4) | 73.2 (12.6) | 73.7 (13.4) | 0.002 |
| Heart rate (b.p.m.), mean (SD) | 79.5 (14.7) | 76.9 (13.8) | 76.3 (13.8) | 76.4 (15.1) | 74.6 (15.4) | 0.11 |
| LVEF (%), median (IQR) | 25 (19, 32) | 25 (20, 31) | 25 (20, 30) | 28 (22, 35) | 28 (23, 34) | <0.001 |
| Ischaemic aetiology | 77 (57.5%) | 179 (62.2%) | 203 (60.8%) | 134 (54.9%) | 61 (60.4%) | 0.49 |
| Shortness of breath on exertion | 108 (80.6%) | 199 (69.1%) | 219 (65.6%) | 168 (69.4%) | 69 (68.3%) | 0.036 |
| Shortness of breath at rest | 25 (18.7%) | 36 (12.5%) | 32 (9.6%) | 35 (14.5%) | 11 (10.9%) | 0.083 |
| Reduction in exercise tolerance | 104 (77.6%) | 204 (70.8%) | 210 (62.9%) | 165 (67.9%) | 64 (63.4%) | 0.019 |
| Orthopnoea | 49 (36.6%) | 87 (30.2%) | 81 (24.3%) | 54 (22.1%) | 31 (30.7%) | 0.015 |
| Paroxysmal nocturnal dyspnoea | 36 (26.9%) | 63 (21.9%) | 45 (13.5%) | 39 (16.0%) | 15 (14.9%) | 0.003 |
| Peripheral oedema | 63 (47.0%) | 90 (31.5%) | 106 (31.7%) | 96 (39.7%) | 32 (31.7%) | 0.006 |
| Laboratory values | ||||||
| NT‐proBNP (ng/L), median (IQR) | 2988 (1596, 6558) | 2460 (1283, 5502) | 2536 (1059, 5316) | 2404 (1306, 4483) | 1895 (926, 4448) | 0.058 |
| Serum potassium (mmol/L), median (IQR) | 4.0 (3.7, 4.4) | 4.1 (3.7, 4.4) | 4.1 (3.8, 4.5) | 4.1 (3.8, 4.4) | 4.2 (3.9, 4.5) | 0.12 |
| eGFR (mL/min/1.73 m2), median (IQR) | 53.5 (40.2, 71.9) | 62.1 (46.3, 75.2) | 58.7 (44.9, 77.1) | 58.1 (44.1, 76.1) | 52.5 (42.1, 68.7) | 0.035 |
| Medical history | ||||||
| Hypertension | 81 (61.8%) | 164 (58.2%) | 210 (64.0%) | 155 (64.9%) | 70 (69.3%) | 0.27 |
| Diabetes | 55 (41.4%) | 129 (44.9%) | 149 (44.7%) | 117 (48.3%) | 53 (52.5%) | 0.44 |
| Charlson comorbidity index, mean (SD) | 2.4 (1.5) | 2.4 (1.4) | 2.4 (1.5) | 2.5 (1.5) | 2.9 (1.7) | 0.048 |
| Atrial fibrillation | 47 (35.1%) | 72 (25.0%) | 90 (26.9%) | 103 (42.2%) | 37 (36.6%) | <0.001 |
| Anaemia | 55 (44.7%) | 102 (39.1%) | 112 (37.8%) | 72 (32.4%) | 27 (30.0%) | 0.11 |
| Prior hospitalization for heart failure | 72 (11.3%) | 161 (25.3%) | 192 (30.1%) | 147 (23.1%) | 65 (10.2%) | 0.444 |
| Medications at baseline | ||||||
| ACEi/ARB | 100 (78.1%) | 238 (82.6%) | 303 (90.7%) | 213 (87.3%) | 92 (91.1%) | 0.001 |
| ACEi/ARB dose (%GRD), median (IQR) | 25 (13, 50) | 19 (13, 33) | 25 (13, 50) | 33 (20, 67) | 50 (25, 100) | <0.001 |
| MRA | 54 (40.3%) | 153 (53.1%) | 178 (53.3%) | 118 (48.4%) | 56 (55.4%) | 0.066 |
| Loop diuretic | 120 (89.6%) | 274 (95.1%) | 309 (92.5%) | 227 (93.0%) | 96 (95.0%) | 0.26 |
| Statin | 80 (59.7%) | 216 (75.0%) | 253 (75.7%) | 180 (73.8%) | 78 (77.2%) | 0.005 |
| Outcomes | ||||||
| Composite outcome at 1 year | 68 (50.7%) | 107 (37.2%) | 90 (26.9%) | 81 (33.2%) | 33 (32.7%) | <0.001 |
%GRD, per cent guideline‐recommended dose; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta‐blocker; BMI, body mass index; BP, blood pressure; BSA, body surface area; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐beta‐type natriuretic peptide; NYHA, New York Heart Association; NZ, New Zealand; SD, standard deviation; SG, Singapore.
At baseline, treatment with ACEis/ARBs and beta‐blockers was high (86–88%), with no significant difference among those with or without prior HF hospitalization. Loop diuretic was prescribed in >80% of patients, MRA in about half of patients, and statins in >90% of patients. Target (100%) GRD was prescribed in only 11% and 9% for ACEis/ARBs and beta‐blockers, respectively, in the combined cohort, with about half (47%) achieving ≥50% GRD for ACEis/ARBs or beta‐blockers. Median dose at baseline for ACEis/ARBs was 25% (vs. 40% GRD) in Asian compared with NZ patients (P < 0.001). No such difference was observed for beta‐blockers.
Distribution of dose categories in the total cohort at baseline and 6 months and in stratified groups [with and without prior HF hospitalization, inpatients and outpatients, and by country of enrolment (NZ/SG)] for ACEis/ARBs and beta‐blockers is shown in Figure 1 A,C and B,D , respectively. Patients with no prior HF hospitalization and those enrolled from NZ were more likely to achieve doses of ≥50% GRD for ACEis/ARBs at 6 months.
Figure 1.
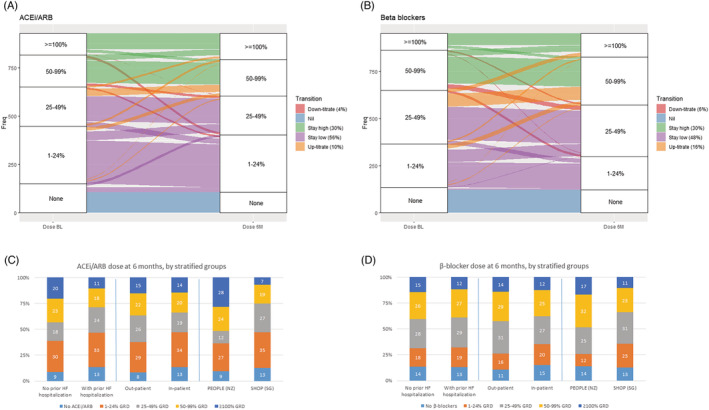
Longitudinal titration patterns and dose trajectories over 6 months for patients treated with (A) angiotensin‐converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) and (B) beta‐blockers. Dose category distribution at 6 months by stratified groups of patients with vs. no prior heart failure (HF) hospitalization, inpatients vs. outpatients, and enrolment from New Zealand (NZ) vs. from Singapore (SG) for (C) ACEis/ARBs and (D) beta‐blockers. GRD, guideline‐recommended dose.
Medication dose trajectories over 6 months from baseline
Over the 6 month period, for ACEis/ARBs, successful up‐titration occurred only in 10% of patients and down‐titration in 4% of patients with no change observed in 86% of patients [‘stay low’ (n = 439, 56%) and ‘stay high’ (n = 236, 30%)] (Figure 1 A ). However, more patients were up‐titrated for beta‐blockers (16% vs. 10% for ACEis/ARBs, Figure 1 B ). Patients with lower N‐terminal pro‐beta‐type natriuretic peptide (NT‐proBNP) levels were more likely to be up‐titrated or be in ‘stay high’ for ACEis/ARBs and beta‐blockers; in contrast, those who transited to ‘down‐titrate’ or remained in ‘stay low’ had the highest NT‐proBNP levels (Tables 3 and 4 ). The patients enrolled at the time of a hospitalization (as compared with outpatients) were more likely to have subsequent up‐titration of beta‐blockers (P = 0.04); however, for ACEis/ARBs, attempts to up‐titrate were observed in some but doses were curtailed by 6 months. Notably, patients from SG were more likely to be in ‘stay low’ compared with NZ patients (P < 0.001) (Tables 3 and 4 ).
Table 3.
Baseline characteristics of dose trajectories for RASi (angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers) groups at 6 months
| Factor | Stay low | Up‐titrate | Down‐titrate | Stay high | P value |
|---|---|---|---|---|---|
| N | 439 | 82 | 34 | 236 | |
| Demographics | |||||
| Age (years), mean (SD) | 61.9 (13.2) | 61.1 (12.9) | 64.0 (10.6) | 61.1 (11.9) | 0.6 |
| Women | 70 (15.9%) | 12 (14.6%) | 8 (23.5%) | 37 (15.7%) | 0.67 |
| Enrolled in SG (vs. NZ) | 328 (74.7%) | 41 (50.0%) | 20 (58.8%) | 106 (44.9%) | <0.001 |
| Enrolled as inpatient | 260 (59.2%) | 50 (61.0%) | 22 (64.7%) | 139 (58.9%) | 0.92 |
| History of prior HF hospitalization | 273 (62.2%) | 32 (39.0%) | 18 (52.9%) | 121 (51.3%) | <0.001 |
| Clinical exam/signs and symptoms | |||||
| NYHA Class III/IV (vs. I/II) | 99 (22.8%) | 14 (17.1%) | 10 (29.4%) | 54 (23.2%) | 0.5 |
| BMI (kg/m2), mean (SD) | 26.1 (5.1) | 27.2 (5.5) | 26.0 (4.9) | 28.9 (6.6) | <0.001 |
| BSA (m2), mean (SD) | 1.79 (0.22) | 1.89 (0.22) | 1.82 (0.24) | 1.94 (0.26) | <0.001 |
| Systolic BP (mmHg), mean (SD) | 116.2 (18.1) | 124.0 (22.4) | 123.8 (22.6) | 123.7 (21.5) | <0.001 |
| Diastolic BP (mmHg), mean (SD) | 70.1 (11.5) | 75.4 (14.6) | 72.3 (12.4) | 74.6 (14.3) | <0.001 |
| Heart rate (b.p.m.), mean (SD) | 77.7 (13.5) | 73.6 (14.6) | 76.4 (16.7) | 74.4 (13.7) | 0.007 |
| LVEF (%), median (IQR) | 25 (20, 30) | 25 (23, 31) | 30 (25, 34) | 28 (22, 34) | <0.001 |
| Ischaemic aetiology | 277 (63.1%) | 35 (42.7%) | 21 (61.8%) | 118 (50.0%) | <0.001 |
| Shortness of breath on exertion | 281 (64.2%) | 51 (62.2%) | 27 (79.4%) | 165 (69.9%) | 0.14 |
| Shortness of breath at rest | 62 (14.2%) | 4 (4.9%) | 4 (11.8%) | 28 (11.9%) | 0.13 |
| Reduction in exercise tolerance | 271 (61.7%) | 52 (63.4%) | 25 (73.5%) | 165 (69.9%) | 0.13 |
| Orthopnoea | 107 (24.4%) | 17 (20.7%) | 10 (29.4%) | 56 (23.7%) | 0.78 |
| Paroxysmal nocturnal dyspnoea | 72 (16.4%) | 14 (17.1%) | 10 (29.4%) | 33 (14.0%) | 0.16 |
| Peripheral oedema | 136 (31.2%) | 24 (29.3%) | 14 (41.2%) | 81 (34.5%) | 0.51 |
| Laboratory values | |||||
| NT‐proBNP (ng/L), median (IQR) | 2314 (1223, 4507) | 1745 (980, 4689) | 3310 (1608, 7295) | 1651 (851, 3946) | 0.002 |
| Potassium (mmol/L), median (IQR) | 4.1 (3.7, 4.5) | 4.0 (3.8, 4.3) | 4.1 (3.8, 4.5) | 4.1 (3.8, 4.5) | 0.48 |
| eGFR (mL/min/1.73 m2), median (IQR) | 59.2 (47.0, 76.8) | 67.4 (54.1, 83.4) | 59.3 (40.8, 75.2) | 58.2 (46.9, 72.7) | 0.067 |
| Medical history | |||||
| Hypertension | 239 (55.6%) | 42 (51.9%) | 28 (82.4%) | 168 (72.1%) | <0.001 |
| Diabetes | 202 (46.2%) | 30 (36.6%) | 13 (38.2%) | 109 (46.2%) | 0.34 |
| Atrial fibrillation | 131 (29.8%) | 24 (29.3%) | 12 (35.3%) | 78 (33.1%) | 0.77 |
| Anaemia | 142 (37.2%) | 24 (30.8%) | 14 (43.8%) | 56 (25.9%) | 0.022 |
| Charlson comorbidity index, mean (SD) | 2.3 (1.3) | 2.1 (1.4) | 2.6 (1.7) | 2.5 (1.6) | 0.078 |
| Medications at baseline | |||||
| Beta‐blockers at baseline | 387 (88.6%) | 74 (90.2%) | 32 (94.1%) | 213 (91.0%) | 0.61 |
| Beta‐blocker dose (%GRD) at baseline, median (IQR) | 25 (13, 50) | 25 (13, 50) | 50 (25, 63) | 50 (25, 63) | <0.001 |
| MRA at baseline | 239 (54.4%) | 39 (47.6%) | 21 (61.8%) | 123 (52.1%) | 0.49 |
| Loop diuretic at baseline | 403 (91.8%) | 74 (90.2%) | 34 (100.0%) | 222 (94.1%) | 0.21 |
| Statin at baseline | 329 (74.9%) | 55 (67.1%) | 25 (73.5%) | 175 (74.2%) | 0.53 |
| Outcomes | |||||
| Composite outcome at 1 year | 131 (29.8%) | 16 (19.5%) | 15 (44.1%) | 66 (28.0%) | 0.054 |
%GRD, per cent guideline‐recommended dose; BMI, body mass index; BP, blood pressure; BSA, body surface area; eGFR, estimated glomerular filtration rate; HF, heart failure; IQR, interquartile range; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐beta‐type natriuretic peptide; NYHA, New York Heart Association; NZ, New Zealand; RASi, renin–angiotensin system inhibitor; SD, standard deviation; SG, Singapore.
Table 4.
Baseline characteristics of dose trajectories for beta‐blockers at 6 months
| Factor | Stay low | Up‐titrate | Down‐titrate | Stay high | P value |
|---|---|---|---|---|---|
| N | 382 | 125 | 46 | 242 | |
| Demographics | |||||
| Age (years), mean (SD) | 63.1 (12.8) | 58.9 (12.2) | 63.3 (13.9) | 61.4 (12.0) | 0.009 |
| Women | 62 (16.2%) | 19 (15.2%) | 4 (8.7%) | 38 (15.7%) | 0.61 |
| Enrolled in SG (vs. NZ) | 274 (71.7%) | 65 (52.0%) | 24 (52.2%) | 128 (52.9%) | <0.001 |
| Enrolled as inpatient | 227 (59.4%) | 86 (68.8%) | 33 (71.7%) | 130 (53.7%) | 0.014 |
| History of prior HF hospitalization | 223 (58.4%) | 59 (47.2%) | 29 (63.0%) | 145 (59.9%) | 0.082 |
| Clinical exam/signs and symptoms | |||||
| NYHA class | 80 (21.3%) | 34 (27.4%) | 12 (26.1%) | 56 (23.4%) | 0.52 |
| BMI (kg/m2), mean (SD) | 25.6 (5.2) | 28.2 (6.0) | 27.0 (6.2) | 28.0 (5.4) | <0.001 |
| BSA (m2), mean (SD) | 1.78 (0.23) | 1.91 (0.25) | 1.87 (0.25) | 1.90 (0.22) | <0.001 |
| Systolic BP (mmHg), mean (SD) | 115.6 (18.5) | 120.7 (21.4) | 121.3 (23.4) | 124.0 (20.7) | <0.001 |
| Diastolic BP (mmHg), mean (SD) | 69.9 (12.1) | 73.3 (14.3) | 70.7 (11.8) | 74.5 (13.3) | <0.001 |
| Heart rate (b.p.m.), mean (SD) | 75.2 (13.1) | 79.2 (14.1) | 73.5 (15.2) | 76.7 (15.1) | 0.022 |
| LVEF (%), median (IQR) | 25 (20, 30) | 25 (21, 30) | 28 (25, 35) | 28 (23, 35) | 0.001 |
| Ischaemic aetiology | 232 (60.7%) | 64 (51.2%) | 24 (52.2%) | 133 (55.0%) | 0.19 |
| Shortness of breath at rest | 40 (10.5%) | 16 (12.8%) | 8 (17.4%) | 34 (14.1%) | 0.39 |
| Reduction in exercise tolerance | 239 (62.6%) | 88 (70.4%) | 33 (71.7%) | 153 (63.2%) | 0.29 |
| Orthopnoea | 92 (24.1%) | 34 (27.2%) | 11 (23.9%) | 53 (21.9%) | 0.73 |
| Paroxysmal nocturnal dyspnoea | 62 (16.2%) | 26 (21.0%) | 5 (10.9%) | 37 (15.3%) | 0.37 |
| Peripheral oedema | 118 (31.1%) | 34 (27.2%) | 25 (55.6%) | 77 (32.0%) | 0.005 |
| Laboratory values | |||||
| NT‐proBNP (ng/L), median (IQR) | 2439 (1122, 5193) | 2041 (1040, 4263) | 2495 (1562, 6500) | 1947 (1040, 4078) | 0.073 |
| Potassium (mmol/L), median (IQR) | 4.1 (3.7, 4.4) | 4.1 (3.7, 4.4) | 4.0 (3.8, 4.5) | 4.2 (3.8, 4.5) | 0.7 |
| eGFR (mL/min/1.73 m2), median (IQR) | 59.9 (47.0, 76.0) | 61.5 (48.0, 75.4) | 58.9 (41.1, 71.4) | 56.8 (44.6, 73.9) | 0.36 |
| Shortness of breath on exertion | 246 (64.4%) | 86 (68.8%) | 37 (80.4%) | 158 (65.6%) | 0.16 |
| Medical history | |||||
| Hypertension | 214 (56.6%) | 80 (66.1%) | 28 (62.2%) | 155 (64.9%) | 0.12 |
| Diabetes | 163 (42.7%) | 60 (48.4%) | 18 (40.0%) | 119 (49.2%) | 0.32 |
| Atrial fibrillation | 99 (25.9%) | 38 (30.4%) | 22 (47.8%) | 99 (40.9%) | <0.001 |
| Anaemia | 124 (36.4%) | 44 (37.9%) | 22 (50.0%) | 58 (27.0%) | 0.011 |
| Charlson comorbidity index, mean (SD) | 2.3 (1.4) | 2.4 (1.5) | 2.6 (1.8) | 2.6 (1.5) | 0.2 |
| Medications at baseline | |||||
| ACEi/ARB at baseline | 331 (86.6%) | 117 (93.6%) | 40 (87.0%) | 217 (89.7%) | 0.18 |
| ACEi/ARB dose (%GRD) at baseline, median (IQR) | 20 (13, 38) | 25 (13, 50) | 38 (20, 100) | 50 (25, 100) | <0.001 |
| MRA at baseline | 211 (55.2%) | 60 (48.0%) | 22 (47.8%) | 129 (53.3%) | 0.47 |
| Loop diuretic at baseline | 357 (93.5%) | 114 (91.2%) | 43 (93.5%) | 228 (94.2%) | 0.75 |
| Statin at baseline | 283 (74.1%) | 89 (71.2%) | 28 (60.9%) | 185 (76.4%) | 0.15 |
| Outcomes | |||||
| Composite outcome at 1 year | 114 (29.8%) | 21 (16.8%) | 18 (39.1%) | 73 (30.2%) | 0.009 |
%GRD, per cent guideline‐recommended dose; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; BSA, body surface area; eGFR, estimated glomerular filtration rate; HF, heart failure; IQR, interquartile range; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐beta‐type natriuretic peptide; NYHA, New York Heart Association; NZ, New Zealand; SD, standard deviation; SG, Singapore.
Higher heart rate, ischaemic aetiology, prior HF hospitalization, enrolment in SG (vs. NZ), higher baseline NT‐proBNP, and anaemia were independently associated with higher odds of ‘staying low’ (all P < 0.005), whereas history of hypertension, higher BMI, and higher LVEF were associated with lower adjusted odds of ‘staying low’ with respect to ACEi/ARB dose. For beta‐blockers, increasing age, ischaemic aetiology, prior HF hospitalization, and enrolment in SG (vs. NZ) were associated with higher odds of ‘staying low’, whereas comorbid atrial fibrillation, history of hypertension, and higher BMI were associated with lower odds of ‘staying low’ (Supporting Information, Table S3 ). Enrolment in SG (vs. NZ) was the strongest independent predictor of ‘staying low’ for both ACEi/ARB [odds ratio (OR) = 3.56; 95% confidence interval (CI) 2.54–4.98] and beta‐blocker doses (OR = 3.13; 95% CI 2.25–4.34) prescribed at 6 months even after adjusting for age, BMI, systolic blood pressure (SBP), heart rate, and prior hospitalization.
Outcomes
Crude 1 year composite outcome (of all‐cause mortality or HF hospitalization) occurred in 34.7% (385 events) of the overall cohort. The crude 1 year composite outcome was similar between patients enrolled from SG and NZ (P = 0.272), as was HF hospitalization alone (P = 0.089). Mean LOS did not differ between countries (5.18 ± 4.7 vs. 5.55 ± 13.5 days, P = 0.384); 81 patients (7%) died within 6 months and were thus excluded from the 1 year time‐to‐event analysis of dose and transition categories at 6 months.
Number of 1 year crude composite events decreased with increasing ACEi/ARB doses prescribed at 6 months: Nil treatment (40%), 1–<50% (30.6%), 50–<100% (29.7%), and ≥100% GRD (18.5%). Through the 6 month titration follow‐up, patients who were in the ‘up‐titrate’ or ‘stay high’ dose trajectory groups had better outcomes (19.5% and 27.0% event rates, respectively) compared with those who were in the ‘stay low’ (29.5% event rate) or ‘down‐titrate’ dose trajectory groups (44.1% event rate, P = 0.05). The corresponding crude composite events for beta‐blocker dose categories at 6 months were Nil treatment (41.8%), 1–<50% (30.7%), 50–<100% (24.8%), and ≥100% GRD (25.4%), P < 0.001, respectively. Similar observations were seen for beta‐blocker dose trajectory groups as for ACEi/ARB treatment.
Figures 2 and 3 showed the hazard ratios for ACEi/ARB and beta‐blocker dose categories at 6 months and dose trajectory groups, respectively. Notably, in Cox analysis weighted by the inverse probability of successful treatment, there appeared to be a ‘threshold,’ that is, 50% or more GRD for ACEis/ARBs for a trend in reduction in the hazard ratio (HR, as compared with no treatment). In contrast, for beta‐blockers, any dose (even for low dose 1–<50%) was associated with a trend of lower adjusted hazards of a 1 year composite outcome, though not all statistically significant (Figure 2 ). There is a consistent trend observed, in that as compared with those in the ‘stay low’ group, those who were in the ‘up‐titrate’ or ‘stay high’ groups for both ACEis/ARBs and beta‐blockers had improvement in outcomes, whereas those were in the ‘down‐titrate’ group had worse outcomes at 1 year (Figure 3 ). Country of enrolment did not modify the associations of dose categories [ACEis/ARBs (P interaction = 0.208); beta‐blockers (P interaction = 0.631)] nor dose trajectory groups [ACEis/ARBs (P interaction = 0.796); beta‐blockers (P interaction = 0.695)] with 1 year composite outcome.
Figure 2.
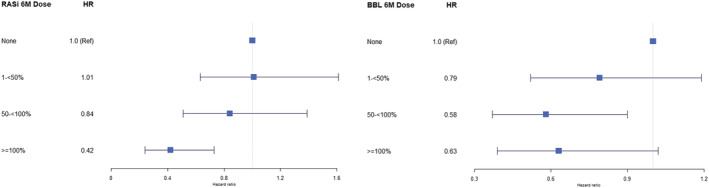
Association of medication dose categories at 6 months with 1 year composite outcome (inversely weighted for probability of achieving ≥50% guideline‐recommended dose). BBL, beta‐blocker; HR, hazard ratio; RASi, renin–angiotensin system inhibitor.
Figure 3.
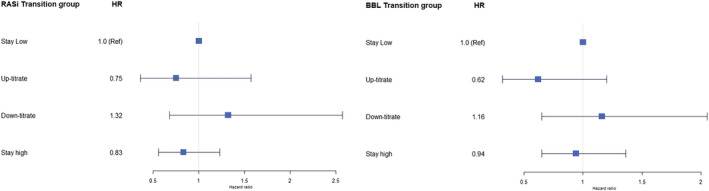
Association of medication transition groups with 1 year composite outcome (using multivariable‐adjusted Cox proportional hazard model). BBL, beta‐blocker; HR, hazard ratio; RASi, renin–angiotensin system inhibitor.
Increasing dose categories of ACEis/ARBs (at 6 months) were associated with a gradual decline in hospitalization days for HF (within 1 year), with the lowest mean LOS at doses [75–99% and ≥100%; 3.38 ± 2.2 vs. 8.32 ± 36.3 (for doses <25%), P < 0.001]. For transition groups, patients who ‘up‐titrate’ (3.45 ± 2.6 days) or ‘stay high’ (4.17 ± 4.6 days) had the shortest mean LOS compared with other transition groups, especially the ‘down‐titrate’ group that had the longest mean LOS (20.8 ± 76.3; P < 0.001) for ACEis/ARBs. Similar findings were observed for beta‐blockers (P < 0.001).
Quality of life
In 767 patients with QoL assessed at baseline and 6 months, there was overall improvement in the total QoL score (mean improvement 13 points). Among patients treated with ACEis/ARBs, improvement in total overall QoL score was highest (with a mean of 18 points) among those who achieved ≥100% GRD, compared with 9–13 points of improvement in lower dose categories (P = 0.070). Interestingly, for beta‐blocker dose categories, mean improvement (of 11–14 points) in overall score across all (including 1–<50% GRD) dose categories achieved was observed, with no significant difference between them (P = 0.866). No significant difference in change in physical limitation domain score across dose categories was observed for ACEis/ARBs (5–9 points; P = 0.147) or beta‐blockers (5–7 points; P = 0.744). There were also no significant differences in changes in total QoL scores across transition dose categories for ACEis/ARBs (P = 0.136) or beta‐blockers (P = 0.151), although those in the ‘up‐titrate’ groups had the greatest improvement. Differences persisted even on multivariable adjustment.
Discussion
Among a real‐world cohort of patients with HF and LVEF of <40% enrolled from SG and NZ, who were followed for longitudinal titration of ACEis/ARBs and beta‐blockers, a large majority did not receive GRD of the two foundational therapies for HFrEF. Despite high baseline medication use, only 1 in 10 patients were initiated or up‐titrated on the dose of ACEis/ARBs over 6 months, and <1 in 5 patients were initiated or up‐titrated on the dose of beta‐blockers. More than 80% had stable dosing, with two distinctive large clusters at two ends of the spectrum: stable ‘stay low’ sub‐target doses (dominated by Asian patients from SG) and ‘stay high’ (mainly patients from NZ). The best improvement in patient health‐related QoL and least risk of the composite outcome of 1 year all‐cause mortality or HF hospitalization were observed among those who achieved target GRD for ACEis/ARBs. Interestingly, for beta‐blockers, all dose categories (including small doses) improved overall QoL and primary composite outcome. Country of enrolment did not modify the associations of dose categories or dose transition groups of ACEis/ARBs and beta‐blockers with a primary composite outcome at 1 year.
Multiple factors were associated with titration patterns for ACEis/ARBs and beta‐blockers, reflecting the complex web of clinical, biological, and socio‐economic determinants of treatment. In general, patients with a higher comorbidity burden, greater age, chronic kidney disease (CKD), anaemia, history of prior HF hospitalization, and/or higher heart rate had a lower likelihood of receiving medication doses ≥50% GRD. In contrast, higher SBP, higher BMI, lower NT‐proBNP levels, and/or absence of prior HF hospitalization were associated with a higher likelihood of achieving higher doses of ACEis/ARBs and beta‐blockers. Of note, we observed that patients (regardless of country of enrolment) who started on low medication dosages were more likely to remain in ‘stay low’ group over the 6 months.
So far, few studies, for example, the US CHAMP‐HF (Change the Management of Patients with Heart Failure registry), 5 QUALIFY (QUality of Adherence to guideline recommendations for LIFe‐saving treatment in heart failure surveY), 6 and IMPROVE‐HF (Improve the Use of Evidence‐Based Heart Failure Therapies in the Outpatient Setting) 18 programme, have reported longitudinal titration of medical therapy among patients with HFrEF, with a paucity of data from Asia‐Pacific. In CHAMP‐HF 19 and QUALIFY, 6 findings were largely similar to our own: only a small proportion of patients were up‐titrated during the follow‐up period. Furthermore, patient characteristics associated with higher doses achieved or up‐titrated were similar, that is, younger patients with no/lower comorbidities, higher blood pressure (BP), and higher BMI. In contrast, older and sicker patients with high comorbidity burden were less likely to be up‐titrated. Our current study extends previous findings: firstly, in examining the change in patient health‐related QoL associated with dose categories of ACEis/ARBs and beta‐blockers, and secondly, in comparing the longitudinal titration patterns in an Asian sub‐cohort (from SG) with NZ (mainly White patients) using identical protocols.
Despite the geographical differences between SG and NZ (as two highly developed countries), physicians' prescribing patterns at baseline were similar in regard to the high uptake of both therapeutic groups and median dose of beta‐blockers (at baseline). However, inter‐country differences in the longitudinal trajectories of drug prescription were observed over the 6 months. We cannot fully explain this finding based on measured variables. Although all sites are tertiary care hospitals and similar protocols are used for enrolment, between‐country differences cannot be excluded. Other plausible explanations for the variation in dose trajectories include heterogeneity in patient factors, for example, severity of HF, high comorbidity burden, prior HF hospitalization, frailty, and side effects experienced by patients limiting the up‐titration of the medications. Previous studies 20 , 21 undertaken in Asian ethnicities had reported prevalence of persistent cough with ACEi, even in those who discontinued their ACEi. Curtailment of doses observed in some patients after initial attempted up‐titration during the 6 months suggests that intolerance could be a contributing factor. Higher BMI or BSA in general was associated with higher medication doses prescribed; however, patients' risk factor profiles [SBP, heart rate, estimated glomerular filtration rate (eGFR) or CKD, and anaemia] are also likely to have influenced HF dosage patterns, as observed in this study and a previous multi‐ethnic study. 22 Nonetheless, review of baseline characteristics of patients who remained in the ‘stay low’ groups showed generally robust SBP (median >115 mmHg), heart rate (median >75 b.p.m.), and kidney function (median eGFR ~60 mL/min/1.73 m2), suggesting that many patients may have tolerated higher doses of ACEi/ARB or beta‐blocker had they been prescribed. Such findings may be consistent with previously reported examples of clinical inertia in medical therapy for HFrEF and potential overemphasis on perceived risk of intolerance rather than actual intolerance limiting guideline‐directed medication titration. Although landmark clinical trials suggest broad and consistent efficacy and safety of GDMT across patient subsets, it remains to be seen whether individualization in sequencing, timing, and dosing may facilitate better overall implementation in real‐world practice. Regardless, management of this heterogeneous and complex syndrome requires assessment not only limited to the presenting clinical features but also extended to the nuances of aetiology, comorbidities, frailty, and precipitants of decompensation. 23 Up‐titration of GDMT should be undertaken in a timely fashion, subject to tolerability, in contrast to our observation of a large proportion of patients who have received persistently low dosages over time. Importantly, rapid up‐titration of GDMT (under close follow‐up and monitoring) had been shown to improve patients' outcomes and QoL within 180 days in the recent STRONG‐HF study. 24 Although data are mixed, a biomarker (e.g. NT‐proBNP)‐guided approach 25 may hold promise as a strategy to improve implementation and up‐titration of GDMT.
Strengths and limitations
The prospective design with identical parallel protocols in NZ and SG and the nationwide mortality/hospital admission registers for complete follow‐up and low rate of missing data are strengths of this study. As an observational real‐world cohort, it is not an interventional study and causality cannot be established. Patients' compliance, adherence, and persistence to oral HF therapies were not examined. Robust statistical analytical methods were used, and we corrected for indication bias; unfortunately, there remains potential for residual bias from unmeasured factors. The study cannot fully account for heterogeneity in ethnicity, geographical area, healthcare systems, individual physician practices, patient compliance, and individual characteristics. Furthermore, causes of death or cardiovascular (CV) mortality could not be examined. Reasons for not achieving target doses (including whether titration had not been attempted and been attempted but complicated by side effects, and other reasons, e.g. frailty) were not recorded, and the impact of socio‐economic status on medication doses was not examined. However, as ACEis/ARBs and HF beta‐blockers were on standard formulary listings of all the hospitals (investigation sites), these medications are cheap and readily available. Although healthcare systems in NZ and SG might differ, in the latter, government subsidies of up to 80% of the total charges coupled with layers of healthcare financing aid are available to ensure that citizens are not denied access to healthcare. Newer therapies, such as ARNi and SGLT2i, have not been examined, which might provide opportunities to optimize treatment in patients using a wider array of therapeutic options. Until more data are available to guide implementation, physicians should continue to adhere to clinical guidelines including achievement of target doses of ACEis/ARBs and beta‐blockers—the ‘backbone’ of HF therapy for improved outcomes among patients with HFrEF.
Conclusions
Although HF medication use at baseline was high, doses seldom met guideline‐endorsed targets and most patients in SG and NZ did not have these medications up‐titrated over 6 months. Multiple clinical factors were associated with changes in medication dosages. Higher doses of ACEis/ARBs and beta‐blockers were associated with better patient‐related QoL and 1 year composite outcomes. Future research on other concomitant drugs, sex and age group differences, and their impact on HF therapies are warranted. Further research is urgently needed to investigate the causes of lack of up‐titration of HF therapy (and its frequency), which could inform strategies for timely up‐titration of HF therapy based on clinical and biochemical parameters.
Clinical implications and competencies in medical knowledge
Higher doses of ACEis/ARBs and beta‐blockers are associated with better outcomes, including patient health‐related QoL.
Up‐titration of GDMT in patients with HFrEF should be undertaken in a timely fashion, subject to tolerability, in contrast to our observation of a large proportion of patients who have received persistently low dosages over time.
A biomarker (e.g. NT‐proBNP)‐guided approach may hold promise as a strategy to improve implementation and up‐titration of GDMT.
Translational outlook
Multiple clinical factors were associated with changes in medication dosages. Further research is needed to understand the factors influencing prescribing patterns and opportunities to optimize medications in HFrEF.
Conflict of interest
R.N.D. reports research support from New Zealand Heart Foundation, Health Research Council of New Zealand, Auckland Medical Research Foundation, TM Hosking Trust (New Zealand), and Roche Diagnostics during the conduct of the study. C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from AstraZeneca, Bayer, Boston Scientific, and Roche Diagnostics; has served as consultant or on the Advisory Board/Steering Committee/Executive Committee for Actelion, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., Us2.ai, Janssen Research & Development LLC, Medscape, Merck, Novartis, Novo Nordisk, Radcliffe Group Ltd., Roche Diagnostics, Sanofi, and WebMD Global LLC; and serves as co‐founder and non‐executive director of Us2.ai. A.M.R. has received research support from Boston Scientific, Bayer, AstraZeneca, Medtronic, Roche Diagnostics, Abbott Laboratories, Thermo Fisher, and Critical Diagnostics and has consulted for Bayer, Novartis, Merck, AstraZeneca, and Roche Diagnostics. J.T. received speaker or consultancy fees from Roche Diagnostics, Daiichi Sankyo, Boehringer Ingelheim, and Us2.ai and is supported by the National University of Singapore start‐up grant and a ministry of education tier 1 grant. K.P. has received senior fellowship support from the New Zealand Heart Foundation and research support from the Health Research Council of New Zealand for unrelated projects. The other authors have nothing to declare.
Funding
This work was supported by the National Medical Research Council of Singapore (Grant Number R‐172‐003‐219‐511), Health Research Council of New Zealand, A*STAR‐NZ HRC (Grant Number JGC 10_027), Clinician Scientist Award (C.S.P.L.), New Zealand Heart Foundation Project Grant, TM Hosking Trust (Auckland), Waikato Medical Research Foundation, HRC Programme Grant, New Zealand Heart Foundation Chair in Cardiovascular Studies (A.M.R.), New Zealand Heart Foundation Chair of Heart Health (R.N.D.), and Auckland Medical Research Foundation.
Supporting information
Figure S1. Flow diagram of patients' recruitment, inclusion or exclusion into the current study.
Table S1. Guideline‐recommended doses of ACE inhibitors, angiotensin receptor blockers, β‐blockers and mineralocorticoid receptor antagonists
Table S2. Baseline characteristics of patients from New Zealand (NZ) and Singapore (SG) at baseline, among patients with HFrEF (LVEF <40%)
Table S3. Associations of baseline characteristics with “Stay low” on RAS inhibitors and β‐blockers
Table S4. Clinical variables for penalized logistic regression analysis to predict successful treatment (≥50% GRD) at 6 months for RAS inhibitors and β‐blockers
Acknowledgements
We thank the clinical and research teams from the participating hospitals: New Zealand—University of Auckland and Auckland City Hospital (M. Copley, N. Earle, M. Fish, J. Hannah, G. Quill, M. Matthews, H. McGrinder, and M. ter Bals); Middlemore Hospital (M. Oldfield and L. M. Pearce); Christchurch Heart Institute, University of Otago, Christchurch (L. Skelton, B. Neame, and S. Rose); and Waikato Hospital (R. Fisher, F. Bartley, and A. Silverstone); Singapore—National University Hospital (C. S. P. Lam, A. M. Richards, L. H. Ling, R. C. C. Wong, P. Chai, K. K. Poh, C. F. Xu, and N. F. M. Fadzillah); National University of Singapore (T. P. Ng, S. P. Chan, L. L. Gong, J. B. Lunaria, H. M. Hazli, T. Kwan, J. P. C. Chong, and J. Y. X. Ng); Tan Tock Seng Hospital (D. P. S. Yeo, S. Y. Loh, and F. F. Yin); Khoo Teck Puat Hospital (H. Y. Ong, B. Jeevith, and L. Chen); Singapore General Hospital (F. R. Jaufeeraly and J. H. Chen); National Heart Centre Singapore (D. K. L. Sim and G. S. H. Lee); and Changi General Hospital (G. K. T. Leong and W. Esa).
Teng, T.‐H. K. , Tay, W. T. , Ouwerkerk, W. , Tromp, J. , Richards, A. M. , Gamble, G. , Greene, S. J. , Yiu, K.‐H. , Poppe, K. , Ling, L. H. , Lund, M. , Sim, D. , Devlin, G. , Loh, S. Y. , Troughton, R. , Ren, Q. , Jaufeerally, F. , Lee, S. G. S. , Tan, R. S. , Soon, D. K. N. , Leong, G. , Ong, H. Y. , Yeo, D. P. S. , Lam, C. S. P. , and Doughty, R. N. (2023) Titration of medications and outcomes in multi‐ethnic heart failure cohorts (with reduced ejection fraction) from Singapore and New Zealand. ESC Heart Failure, 10: 1280–1293. 10.1002/ehf2.14275.
Tiew‐Hwa Katherine Teng and Wan Ting Tay are co‐primary authors.
Clinical trial registration: Australian New Zealand Clinical Trial Registry (ACTRN 12610000374066).
Contributor Information
Carolyn S.P. Lam, Email: carolyn.lam@duke-nus.edu.sg.
Rob N. Doughty, Email: r.doughty@auckland.ac.nz.
References
- 1. Writing Committee , Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, Motiwala SR, Oliveros E, Patterson JH, Walsh MN, Wasserman A, Yancy CW, Youmans QR. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021; 77: 772–810. [DOI] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 3. Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, ter Maaten JM, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Metra M, Zwinderman AH. Determinants and clinical outcome of uptitration of ACE‐inhibitors and beta‐blockers in patients with heart failure: a prospective European study. Eur Heart J. 2017; 38: 1883–1890. [DOI] [PubMed] [Google Scholar]
- 4. Ouwerkerk W, Teng TK, Tromp J, Tay WT, Cleland JG, van Veldhuisen DJ, Dickstein K, Ng LL, Lang CC, Anker SD, Zannad F, Hung CL, Sawhney JPS, Naik A, Shimizu W, Hagiwara N, Wander GS, Anand I, Richards AM, Voors AA, Lam CSP. Effects of combined renin–angiotensin–aldosterone system inhibitor and beta‐blocker treatment on outcomes in heart failure with reduced ejection fraction: insights from BIOSTAT‐CHF and ASIAN‐HF registries. Eur J Heart Fail. 2020; 22: 1472–1482. [DOI] [PubMed] [Google Scholar]
- 5. Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Butler J. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019; 73: 2365–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowie MR, Schope J, Wagenpfeil S, Tavazzi L, Böhm M, Ponikowski P, Anker SD, Filippatos GS, Komajda M, QUALIFY Investigators . Patient factors associated with titration of medical therapy in patients with heart failure with reduced ejection fraction: data from the QUALIFY international registry. ESC Heart Fail. 2021; 8: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teng THK, Tromp J, Tay WT, Anand I, Ouwerkerk W, Chopra V, Wander GS, Yap JJ, MacDonald M, Xu CF, Chia YM, Shimizu W, ASIAN‐HF Investigators , Richards AM, Voors A, Lam CS. Prescribing patterns of evidence‐based heart failure pharmacotherapy and outcomes in the ASIAN‐HF registry: a cohort study. Lancet Global Health. 2018; 6: e1008–e1018. [DOI] [PubMed] [Google Scholar]
- 8. Lam CS, Teng TK, Tay WT, Anand I, Zhang S, Shimizu W, Narasimhan C, Park SW, Yu CM, Ngarmukos T, Omar R, Reyes EB, Siswanto BB, Hung CL, Ling LH, Yap J, MacDonald M, Richards AM. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. 2016; 37: 3141–3153. [DOI] [PubMed] [Google Scholar]
- 9. Chan DZL, Kerr A, Grey C, Selak V, Lee MAW, Lund M, Poppe K, Doughty RN. Contrasting trends in heart failure incidence in younger and older New Zealanders, 2006–2018. Heart. 2022; 108: 300–306. [DOI] [PubMed] [Google Scholar]
- 10. Hori M, Sasayama S, Kitabatake A, Toyo‐oka T, Handa S, Yokoyama M, Matsuzaki M, Takeshita A, Origasa H, Matsui K, Hosoda S, MUCHA Investigators . Low‐dose carvedilol improves left ventricular function and reduces cardiovascular hospitalization in Japanese patients with chronic heart failure: the Multicenter Carvedilol Heart Failure Dose Assessment (MUCHA) trial. Am Heart J. 2004; 147: 324–330. [DOI] [PubMed] [Google Scholar]
- 11. Kitakaze M, Sarai N, Ando H, Sakamoto T, Nakajima H. Safety and tolerability of once‐daily controlled‐release carvedilol 10–80 mg in Japanese patients with chronic heart failure. Circ J. 2012; 76: 668–674. [DOI] [PubMed] [Google Scholar]
- 12. Okamoto H, Hori M, Matsuzaki M, Tsutsui H, Yamazaki T, Nagai R, Yoshikawa T, Fujio Y, Nonen S, Azuma J, Izumi T, Ohashi Y, Kitabatake A, J‐CHF Investigators . Minimal dose for effective clinical outcome and predictive factors for responsiveness to carvedilol: Japanese chronic heart failure (J‐CHF) study. Int J Cardiol. 2013; 164: 238–244. [DOI] [PubMed] [Google Scholar]
- 13. Santhanakrishnan R, Ng TP, Cameron VA, Gamble GD, Ling LH, Sim D, Leong GKT, Yeo PSD, Ong HY, Jaufeerally F, Wong RCC, Chai P, Low AF, Lund M, Devlin G, Troughton R, Richards AM, Doughty RN, Lam CSP. The Singapore Heart Failure Outcomes and Phenotypes (SHOP) study and Prospective Evaluation of Outcome in Patients with Heart Failure with Preserved Left Ventricular Ejection Fraction (PEOPLE) study: rationale and design. J Card Fail. 2013; 19: 156–162. [DOI] [PubMed] [Google Scholar]
- 14. Lam CSP, Gamble GD, Ling LH, Sim D, Leong KTG, Yeo PSD, Ong HY, Jaufeerally F, Ng TP, Cameron VA, Poppe K, Lund M, Devlin G, Troughton R, Richards AM, Doughty RN. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi‐ethnic cohort study. Eur Heart J. 2018; 39: 1770–1780. [DOI] [PubMed] [Google Scholar]
- 15. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 16. Stein G, Teng TK, Tay WT, Richards AM, Doughty R, Dong Y, Sim D, Yeo PSD, Jaufeerally F, Leong G, Soon D, Ling LH, Lam CSP. Ethnic differences in quality of life and its association with survival in patients with heart failure. Clin Cardiol. 2020; 43: 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double‐blind, placebo‐controlled trial of pimobendan. Am Heart J. 1992; 124: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 18. Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence‐based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence‐Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010; 122: 585–596. [DOI] [PubMed] [Google Scholar]
- 19. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol. 2018; 72: 351–366. [DOI] [PubMed] [Google Scholar]
- 20. Ng LP, Goh PS. Incidence of discontinuation of angiotensin‐converting enzyme inhibitors due to cough, in a primary healthcare centre in Singapore. Singapore Med J. 2014; 55: 146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woo KS, Nicholls MG. High prevalence of persistent cough with angiotensin converting enzyme inhibitors in Chinese. Br J Clin Pharmacol. 1995; 40: 141–144. [PMC free article] [PubMed] [Google Scholar]
- 22. Teng TK, Cooper L, Tay WT, Luo N, Sharma A, Whellan D, Anand I, Pina IL, Tromp J, Richards AM, Kraus WE, Connor CMO, Mentz RJ, Lam CSP. Association between body surface area and prescribed doses of guideline‐directed medications among international patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2020; 22: 754–758. [DOI] [PubMed] [Google Scholar]
- 23. Ahmad FA, Petrie MC, McMurray JJV, Lang NN. Personalized medicine and hospitalization for heart failure: if we understand it, we may be successful in treating it. Eur J Heart Fail. 2019; 21: 699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mebazaa A, Davison B, Chioncel O, Cohen‐Solal A, Diaz R, Filippatos G, Metra M, Ponikowski P, Sliwa K, Voors AA, Edwards C, Novosadova M, Takagi K, Damasceno A, Saidu H, Gayat E, Pang PS, Celutkiene J, Cotter G. Safety, tolerability and efficacy of up‐titration of guideline‐directed medical therapies for acute heart failure (STRONG‐HF): a multinational, open‐label, randomised, trial. Lancet. 2022; 400: 1938–1952. [DOI] [PubMed] [Google Scholar]
- 25. Ouwerkerk W, Zwinderman AH, Ng LL, Demissei B, Hillege HL, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, ter Maaten JM, Lang CC, van der Harst P, Filippatos G, Dickstein K, Cleland JG, Anker SD, Voors AA. Biomarker‐guided versus guideline‐based treatment of patients with heart failure: results from BIOSTAT‐CHF. J Am Coll Cardiol. 2018; 71: 386–398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow diagram of patients' recruitment, inclusion or exclusion into the current study.
Table S1. Guideline‐recommended doses of ACE inhibitors, angiotensin receptor blockers, β‐blockers and mineralocorticoid receptor antagonists
Table S2. Baseline characteristics of patients from New Zealand (NZ) and Singapore (SG) at baseline, among patients with HFrEF (LVEF <40%)
Table S3. Associations of baseline characteristics with “Stay low” on RAS inhibitors and β‐blockers
Table S4. Clinical variables for penalized logistic regression analysis to predict successful treatment (≥50% GRD) at 6 months for RAS inhibitors and β‐blockers


