Abstract
Aims
Prognosis for ST‐segment elevation myocardial infarction (STEMI) is worse when heart failure is present on admission. Understanding clinical practice in different health systems can identify areas for quality improvement initiatives to improve outcomes. In the absence of international comparison studies, we aimed to compare treatments and in‐hospital outcomes of patients admitted with ST elevation myocardial infarction (STEMI) by heart failure status in two healthcare‐wide cohorts.
Methods and results
We used two nationwide databases to capture admissions with STEMI in the United Kingdom (Myocardial ischemia National Audit Project, MINAP) and Japan (Japanese Registry of All Cardiac and Vascular Diseases‐Diagnostic Procedure Combination, JROAD‐DPC) between 2012 and 2017. Participants were stratified using the HF Killip classification into three groups; Killip 1: no congestive heart failure, Killip 2–3: congestive heart failure, Killip 4: cardiogenic shock. We calculated crude rate and case mix standardized risk ratios (CSRR) for use of treatments and in‐hospital death. Patients were younger in the United Kingdom (65.4 [13.6] vs. 69.1 [13.0] years) and more likely to have co‐morbidities in the United Kingdom except for diabetes and hypertension. Japan had a higher percentage of heart failure and cardiogenic shock patients among STEMI during admission than that in the United Kingdom. Primary percutaneous coronary intervention (pPCI) rates were lower in the United Kingdom compared with Japan, especially for patients presenting with Killip 2–3 class heart failure (pPCI use in patients with Killip 1, 2–3, 4: Japan, 86.2%, 81.7%, 78.7%; United Kingdom, 79.6%, 58.2% and 79.9%). In contrast, beta‐blocker use was consistently lower in Japan than in the United Kingdom (61.4% vs. 90.2%) across Killip classifications and length of hospital stay longer (17.0 [9.7] vs. 5.0 [7.4] days). The crude rate of in‐hospital mortality increased with increasing Killip class group. Both the crude rate and CSRR was higher in the United Kingdom compared with Japan for Killip 2–3 (15.8% vs. 6.4%, CSRR 1.80 95% CI 1.73–1.87, P < 0.001), and similar for Killip 4 (36.9% vs. 36.3%, CSRR 1.11 95% CI 1.08–1.13, P < 0.001).
Conclusions
Important differences in the care and outcomes for STEMI with heart failure exist between the United Kingdom and Japan. Specifically, in the United Kingdom, there was a lower rate of pPCI, and in Japan, fewer patients were prescribed beta blockers and hospital length of stay was longer. This international comparison can inform targeted quality improvement programmes to narrow the outcome gap between health systems.
Keywords: ST elevation myocardial infarction, Heart failure, Medications, Mortality
Introduction
Heart failure (HF) complicating acute myocardial infarction (AMI) during hospitalization is a critical life‐threatening condition 1 , 2 , 3 and influences treatment decisions. 4 , 5 Uptake of evidence‐based treatments for STEMI vary between countries 6 and may vary by heart failure status. An international comparison of STEMI care by heart failure status may enable benchmarking of care delivery, informative learning and the development of quality improvement initiatives.
Previous research for STEMI care and outcomes across different health systems has not been stratified by heart failure status, 7 has been based on selected samples of hospital patients reported in voluntary registries, 6 , 7 or has not attempted to standardize the mortality of patients in one country by the casemix in another. 6
The United Kingdom and Japan have nationwide clinical database for admissions with myocardial infarction, representing the public healthcare of the National Health Service (NHS) in the United Kingdom and the universal health insurance system in Japan. 8 , 9 In the absence of previous international comparisons, we used healthcare‐wide clinical cohorts from the United Kingdom and Japan to compare the quality of care provision and in‐hospital outcomes for patients with STEMI by heart failure presentation. Furthermore, we assessed the contribution of clinical care to in‐hospital outcomes across the severity of heart failure presentation.
Methods
Study design
We analysed nationwide data from two independent healthcare systems in the United Kingdom (Myocardial ischemia National Audit Project, MINAP) and Japan (Japanese Registry of All Cardiac and Vascular Diseases‐Diagnostic Procedure Combination, JROAD‐DPC). MINAP is a comprehensive registry of admissions with acute coronary syndrome at acute NHS hospitals in England and Wales and is mandated by the UK Department of Health. 8 JROAD‐DPC was created by combining the Japan Circulation Society national survey of clinical activity at each Japanese institution and the Diagnosis Procedure Combination, which is a mixed case‐patient classification system launched by the Ministry of Health, Labour and Welfare of Japan that contains the information for each patient (i.e. patient demographics and several disease‐specific data). An attending physician is responsible for clinical data entry for each patient. 9 , 10
Eligible patients
We included patients admitted with ST‐segment elevation MI (STEMI) between 1 April 2012 and 31 March 2017. In JROAD‐DPC, STEMI were identified using International Classification of Diseases (ICD10) codes (I210, I211, I212, I213, I220, I221, or I228). 11 For MINAP and JROAD‐DPC, the diagnosis was determined by local clinicians. For both cohorts, patients aged <30 years or those with missing data for Killip class were excluded. Heart failure status during hospitalization was defined as Killip 1: no congestive heart failure, Killip 2–3: congestive heart failure, Killip 4: cardiogenic shock.
Casemix variables
We defined 11 casemix variables from data that could be evaluated in both databases and have been used in other research [age, sex, smoking history, diabetes mellitus (DM), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), history of ischaemic heart disease (IHD), history of cerebrovascular disease, peripheral vascular disease, Killip class, and cardiac arrest]. See Table S1 for a comparison of these variables by country.
Care
Hospital procedures and treatments included echocardiography, primary percutaneous coronary intervention (pPCI), any revascularization [coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention other than pPCI], and medications [aspirin, P2Y12 inhibitors, angiotensin‐converting enzyme inhibitor/angiotensin receptor blockers (ACE/ARB), beta‐blockers, loop diuretics, mineralocorticoid receptor antagonists (MRA) and statins]. The definitions of the variables are shown in Table S1 . In JROAD‐DPC, we defined pPCI as a percutaneous coronary intervention procedure performed on the day of or the day following admission. 12
Outcomes
The primary outcome was death from any cause during hospitalization. We also calculated the length of hospital stay and use of the guideline‐recommended treatments (during hospitalization, and only for survivors to hospital discharge). Outcomes were compared between patients by Killip class groups in the two countries. For each country, we quantified the association between care and in‐hospital mortality.
Statistical methods
Statistical analysis was performed for each dataset separately due to the respective data use policies. Continuous variables are presented as the mean ± standard deviation (SD) for normally distributed variables and as medians (interquartile range, IQR) for non‐normally distributed variables. Categorical data are presented as frequencies and percentages.
For casemix standardization, we modelled in‐hospital mortality for each procedure and medication in each Killip group (Total, Killip 1, Killip 2–3, and Killip 4) for the two countries with the 11 casemix variables. We then applied the UK model to the Japanese participants to estimate the casemix standardized risk ratio (CSRR) of observed in‐hospital mortality. We also created casemix adjusted models using the UK model for each care (including procedures and prescriptions), which we then applied to Japanese subjects to estimate the CSRR of each care. The association between hospital procedures and treatments and in‐hospital death was analysed using a multivariate logistic regression model adjusted for casemix. For model construction, missing casemix variables were imputed using multiple imputation with chained equations (MICE) (Table S1 ). For the calculation of the CSRR of aspirin for survivors with Killip 4 categories, we did not apply MICE because it was not possible to adapt the model. Statistical significance was set at < 0.05. Analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA) and STATA 16 (StataCorp, College Station, TX, USA).
Ethics statement
MINAP data were fully anonymized, and as such, ethical approval was not required under NHS research governance arrangements as NICOR, which includes the MINAP database (Ref: NIGB: ECC 1–06 (d)/2011), has support, under section 251 of the NHS Act 2006, to use patient information for medical research without consent. JROAD‐DPC data were approved by the institutional review board of the National Cerebral and Cardiovascular Center (No: R20103). This study was conducted in compliance with the principles of the Declaration of Helsinki and is reported according to the STROBE guideline.
Results
Baseline characteristics
Overall, 326 577 patients with STEMI were admitted between 1 April 2012 and 31 March 2017, with 156 199 from the United Kingdom and 170 458 from Japan. After exclusions, the analytical cohort comprised 109 719 patients in MINAP and 161 911 in JROAD‐DPC (Figure S1 ). Patients were younger in the United Kingdom [65.4. (13.6) vs. 69.1 (13.0) years] and more likely to have previous IHD, CKD and COPD. In contrast, DM was more frequent in Japan than in the United Kingdom (Table 1 ). Compared with MINAP, a greater proportion of patients were classified as Killip class 2 or greater in JROAD‐DPC. Patients in the United Kingdom with Killip classes 2–3, compared with patients with Killip class 1 or 4, were older, more likely to be women, and had a higher prevalence of comorbidities in the United Kingdom (Table 1 ). The direction of adjusted associations between each case mix and in hospital mortality were mostly similar in the United Kingdom and Japan (Figure S2 ).
Table 1.
Baseline characteristics in STEMI patients stratified with heart failure status by Killip classification between the MINAP and JROAD‐DPC
| Total (Killip class 1–4) | Killip class 1 (no congestive heart failure) | Killip class 2–3 (congestive heart failure) | Killip class 4 (cardiogenic shock) | |||||
|---|---|---|---|---|---|---|---|---|
| Group | MINAP (UK; N = 109 719) | JROAD‐DPC (Japan; N = 161 911) | MINAP (UK; N = 87 191) | JROAD‐DPC (Japan; N = 79 983) | MINAP (UK; N = 15 136) | JROAD‐DPC (Japan; N = 59 620) | MINAP (UK; N = 7392) | JROAD‐DPC (Japan; N = 22 308) |
| Mean age (SD) | 65.4 (13.6) | 69.1 (13.0) | 63.9 (13.3) | 67.2 (12.9) | 72.2 (13.2) | 70.7 (12.9) | 68.3 (13.2) | 71.9 (12.8) |
| Sex (woman) (%) | 31 381 (28.6) | 42 256 (26.1) | 23 308 (26.7) | 18 801 (23.5) | 5665 (37.4) | 16 782 (28.2) | 2408 (32.6) | 6673 (29.9) |
| Smoker (current/ex.) (%) | 37 824 (63.5) | 96 092 (59.4) | 31 704 (38.2) | 49 782 (62.3) | 4116 (28.8) | 33 417 (56.3) | 2124 (33.8) | 12 759 (57.2) |
| Previous IHD (%) | 29 975 (27.3) | 25 446 (15.7) | 22 268 (25.5) | 18 603 (17.0) | 5257 (34.7) | 9305 (15.6) | 2450 (33.1) | 2553 (11.4) |
| Hypertension (%) | 44 275 (43.6) | 103 898 (64.2) | 34 111 (42.0) | 50 490 (70.6) | 7196 (52.1) | 38 235 (64.1) | 2968 (45.3) | 9173 (41.1) |
| Diabetes (%) | 18 677 (17.4) | 62 944 (38.9) | 13 659 (15.9) | 28 045 (35.1) | 3550 (23.8) | 24 124 (40.5) | 1468 (21.2) | 10 775 (48.3) |
| Chronic renal failure (%) | 3388 (3.4) | 4605 (2.8) | 1973 (2.4) | 1635 (2.0) | 1055 (7.7) | 2044 (3.4) | 360 (5.6) | 926 (4.1) |
| Cerebrovascular disease (%) | 5616 (5.6) | 6785 (4.2) | 3746 (4.6) | 2960 (3.7) | 1388 (10.1) | 2763 (4.6) | 482 (7.4) | 1062 (4.8) |
| Peripheral vascular disease (%) | 3175 (3.1) | 6125 (3.8) | 2086 (2.6) | 3013 (3.8) | 710 (5.2) | 2366 (4.0) | 379 (5.8) | 746 (3.3) |
| COPD (%) | 12 611 (12.5) | 3874 (2.4) | 9212 (11.4) | 1851 (2.3) | 2514 (18.2) | 1605 (2.7) | 885 (13.6) | 418 (1.9) |
| Killip class 1 | 87 191 (79.5) | 79 983 (49.4) | 87 191 (100) | 79 983 (100) | ‐ | ‐ | ‐ | |
| Killip class 2 | 10 783 (9.8) | 45 682 (28.2) | ‐ | ‐ | 10 783 (71.2) | 45 682 (76.6) | ‐ | |
| Killip class 3 | 4353 (4.0) | 13 938 (8.6) | ‐ | ‐ | 4353 (28.8) | 13 938 (23.4) | ‐ | |
| Killip class 4 | 7392 (6.7) | 22 308 (13.8) | ‐ | ‐ | 7392 (100.0) | 22 308 (100) | ||
| Cardiac arrest | 13 855 (12.7) | 14 744 (9.1) | 8031 (9.3) | 2678 (3.4) | 2539 (16.9) | 4084 (6.9) | 3285 (44.9) | 7982 (35.8) |
Abbreviations: UK, United Kingdom; BMI, body mass index; IHD, ischaemic heart disease; COPD, chronic obstructive pulmonary disease.
Care
Compared with Japan, the use of pPCI in the United Kingdom was lower (crude rate 76.7% vs. 83.5%, CSRR: 0.800, 95% CI 0.797–0.803). The magnitude of the difference was greatest for patients with Killip classes 2–3 (crude rate 58.2% vs. 81.7%, CSRR 0.672, 95% CI 0.667–0.678) (Table 2 and Figure 1 ). The use of echocardiography during hospitalization was also lower in the United Kingdom in each Killip class group (crude [%]: Killip 1: 75.9 [United Kingdom] vs. 92.6% [Japan]; Killip 2–3: 77.9 [United Kingdom] vs. 93.3% [Japan]; and Killip 4: 72.1 [United Kingdom] vs. 85.1% [Japan]) (Table 2 ). Of pharmacotherapies prescribed during hospitalization, beta‐blocker use for Killip 1 was lower in Japan compared with in the United Kingdom (crude rate 92.5% vs. 61.8%, CSRR 1.489, 95% CI 1.480–1.497) (Table 2 and Figure 1 ). The prescription of MRA for Killip 2–3 was also 1.12‐fold lower in Japan than the United Kingdom, reaching less than a quarter of eligible patients (Table 2 and Figure 1 ).
Table 2.
Management during hospitalisation and outcome for all patients and prescription for survivors to discharge from hospital in STEMI according to heart failure status between MINAP and JROAD‐DPC
| Total (Killip class 1–4) | Killip class 1 (no congestive heart failure) | Killip class 2–3 (congestive heart failure) | Killip class 4 (cardiogenic shock) | |||||
|---|---|---|---|---|---|---|---|---|
| Group | MINAP (UK) N = 109 719) | JROAD‐DPC (Japan) N = 161 911) | MINAP (UK) N = 87 191) | JROAD‐DPC (Japan) N = 79 983) | MINAP (UK) N = 15 136) | JROAD‐DPC (Japan) N = 59 620) | MINAP (UK) N = 7392) | JROAD‐DPC (Japan) N = 22 308) |
| In‐hospital care | ||||||||
| pPCI (%) | 83 821 (76.7) | 135 214 (83.5) | 69 168 (79.6) | 68 950 (86.2) | 8768 (58.2) | 48 700 (81.7) | 5885 (79.9) | 17 564 (78.7) |
| Revascularization other than pPCI (including CABG surgery) (%) | 8295 (8.1) | 10 607 (6.6) | 6712 (8.1) | 4152 (5.2) | 1306 (10.1) | 4996 (8.4) | 277 (4.0) | 1459 (6.5) |
| Aspirin (%) | 106 906 (98.9) | 150 772 (93.3) | 85 670 (99.3) | 76 099 (95.3) | 14 448 (97.9) | 56 385 (94.7) | 6788 (96.1) | 18 288 (82.2) |
| P2Y12 inhibitor (%) | 99 640 (92.8) | 144 947 (89.7) | 80 633 (94.2) | 73 865 (92.6) | 13 317 (90.0) | 53 500 (89.9) | 5690 (81.6) | 17 552 (79.0) |
| ACE‐I/ARB (%) | 67 556 (71.5) | 112 952 (69.9) | 55 343 (73.5) | 59 023 (73.9) | 8854 (67.7) | 42 505 (71.4) | 3359 (55.2) | 11 424 (51.3) |
| Beta‐blocker (%) | 94 842 (90.2) | 99 395 (61.4) | 78 043 (92.5) | 49 440 (61.8) | 12 377 (86.2) | 38 843 (65.2) | 4422 (68.3) | 11112 (49.8) |
| Furosemide (%) | 18 199 (19.7) | 56 837 (35.2) | 8687 (11.8) | 16 794 (21.0) | 7475 (57.3) | 27 410 (46.0) | 2037 (34.0) | 12 633 (56.8) |
| MRA (%) | 13 986 (14.9) | 26 937 (16.7) | 9214 (12.4) | 7465 (9.4) | 3785 (28.5) | 13 851 (23.4) | 987 (16.2) | 5529 (24.9) |
| Echocardiography (%) | 77 890 (75.9) | 148 549 (91.8) | 62 112 (75.9) | 73 936 (92.6) | 10 955 (77.9) | 55 367 (93.3) | 4823 (72.1) | 18 892 (85.1) |
| Outcomes | ||||||||
| In‐hospital death (%) | 8095 (7.4) | 13 262 (8.2) | 2986 (3.4) | 1383 (1.7) | 2385 (15.8) | 3785 (6.4) | 2724 (36.9) | 8094 (36.3) |
| Length of hospital stay (days), mean (SD) | 5.0 (7.4) | 17.0 (14.4) | 4.3 (6.2) | 14.6 (9.7) | 8.3 (10.2) | 19.0 (15.1) | 6.7 (10.6) | 20.9 (22.4) |
| Prescription for survivors to discharge from hospital | MINAP (UK) N = 101 624) | JROAD‐DPC (Japan) N = 148 649) | MINAP (UK) N = 84 205) | JROAD‐DPC (Japan) N = 78 600) | MINAP (UK) N = 12 751) | JROAD‐DPC (Japan) N = 55 835) | MINAP (UK) N = 4668) | JROAD‐DPC (Japan) N = 14 214) |
| Aspirin (%) | 100 269 (99.5) | 143 592 (96.7) | 83 239 (99.5) | 75 892 (96.7) | 12 458 (99.0) | 54 050 (96.9) | 4572 (99.2) | 13 650 (96.1) |
| P2Y12 inhibitor (%) | 94 498 (94.7) | 137 974 (92.9) | 78 674 (95.0) | 73 501 (93.8) | 11 669 (93.0) | 51 370 (92.5) | 4155 (93.1) | 12 999 (91.8) |
| Statins (%) | 90 590 (90.8) | 125 352 (84.4) | 75 946 (91.6) | 67 584 (86.1) | 11 082 (88.2) | 46 408 (83.2) | 3562 (82.1) | 11 360 (80.0) |
| ACE‐I/ARB (%) | 65 615 (74.7) | 112 254 (75.6) | 54 540 (74.9) | 59 959 (76.4) | 8119 (73.2) | 42 033 (75.4) | 2956 (75.5) | 10 262 (72.3) |
| Beta‐blocker (%) | 92 066 (93.4) | 98 104 (66.0) | 76 864 (93.9) | 49 895 (63.5) | 11 309 (91.5) | 38 237 (68.5) | 3893 (89.6) | 9963 (70.3) |
| MRA (%) | 13 563 (15.6) | 25 475 (17.2) | 9102 (12.7) | 7430 (9.5) | 3568 (31.7) | 13 268 (23.8) | 893 (22.7) | 4777 (33.7) |
Abbreviations: UK, United Kingdom; ACE‐I/ARB, angiotensin angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist; pPCI, primary percutaneous coronary intervention; GABG, coronary artery bypass grafting.
Figure 1.
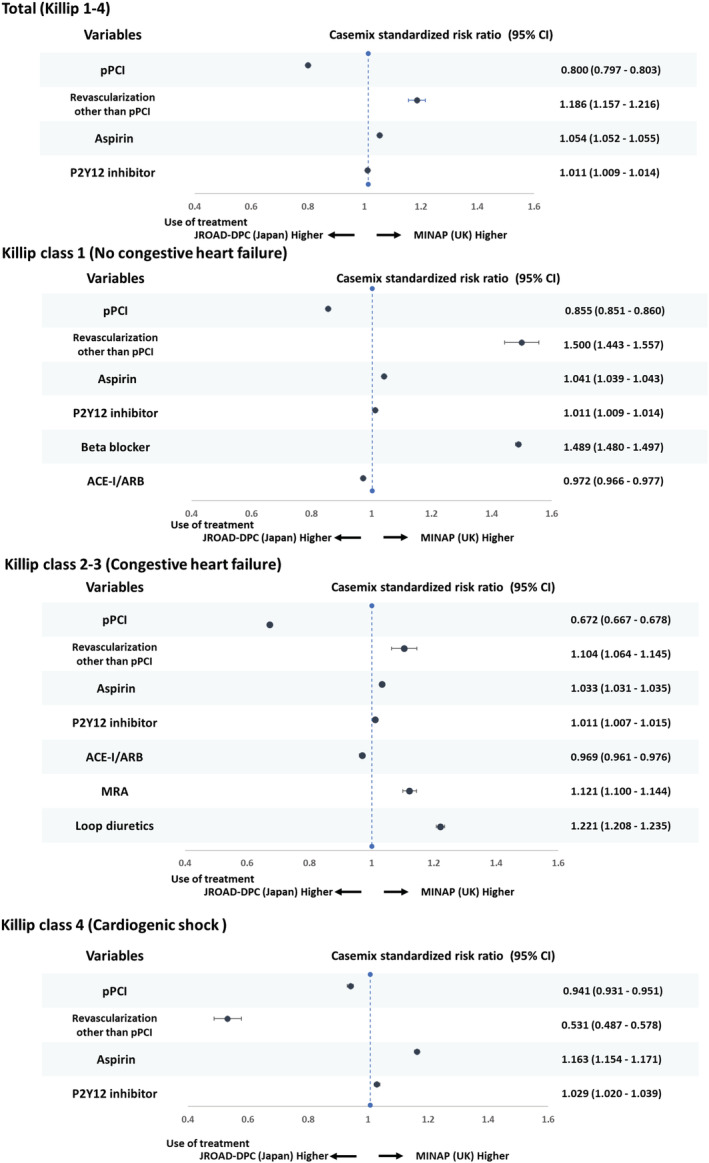
Forrest plot of casemix standardized risk ratio for use of treatment in patient with STEMI according to heart failure status between the MINAP and JROAD‐DPC. STEMI, ST‐elevation myocardial infarction; pPCI, primary percutaneous coronary intervention; UK, United Kingdom; ACE‐I/ARB, angiotensin angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist; CI, confidence interval.
Among patients that survived to the time of hospital discharge, antiplatelet (aspirin and P2Y12 inhibitor) prescription rates were high and similar in the United Kingdom and Japan (Table 2 ). For ACE/ARB, prescription rates were similar according to Killip class group in both countries (Killip 1: 74.9% [United Kingdom] vs. 76.4% [Japan], CSRR 0.957, 95% CI 0.951–0.962; Killip 2–3 73.2 [United Kingdom] vs. 75.4% [Japan], 0.969, 95% CI 0.963–0.976; Killip 4: 75.5 [United Kingdom] vs. 72.3% [Japan], 1.034 95% CI 1.019–1.048) (Figure 2 ). For MRA, the prescription rates varied by Killip class group and, in general, were infrequently used (Killip 1: 12.7% [United Kingdom] vs. 9.5% [Japan], CSRR 1.145, 95% CI 1.111–1.179; Killip 2–3 31.7% [United Kingdom] vs. 23.8% [Japan], 1.171, 95% CI 1.149–1.196; Killip 4: 22.7% [United Kingdom] vs. 33.7% [Japan], CSRR 0.602, 95% CI 0.578–0.626). The use of statins and beta‐blockers was lower in Japan than the United Kingdom (Table 2 ) and for beta‐blockers was particularly evident in all Killip class groups (Killip 1: 93.9 [United Kingdom] vs. 63.5% [Japan], CSRR 1.470, 95% CI 1.461–1.478; Killip 2–3 91.5 [United Kingdom] vs. 68.5% [Japan], CSRR 1.351, 95% CI 1.343–1.360; Killip 4: 89.6 [United Kingdom] vs. 70.3% [Japan], CSRR 1.291, 95% CI 1.275–1.306) (Table 2 and Figure 3 ).
Figure 2.
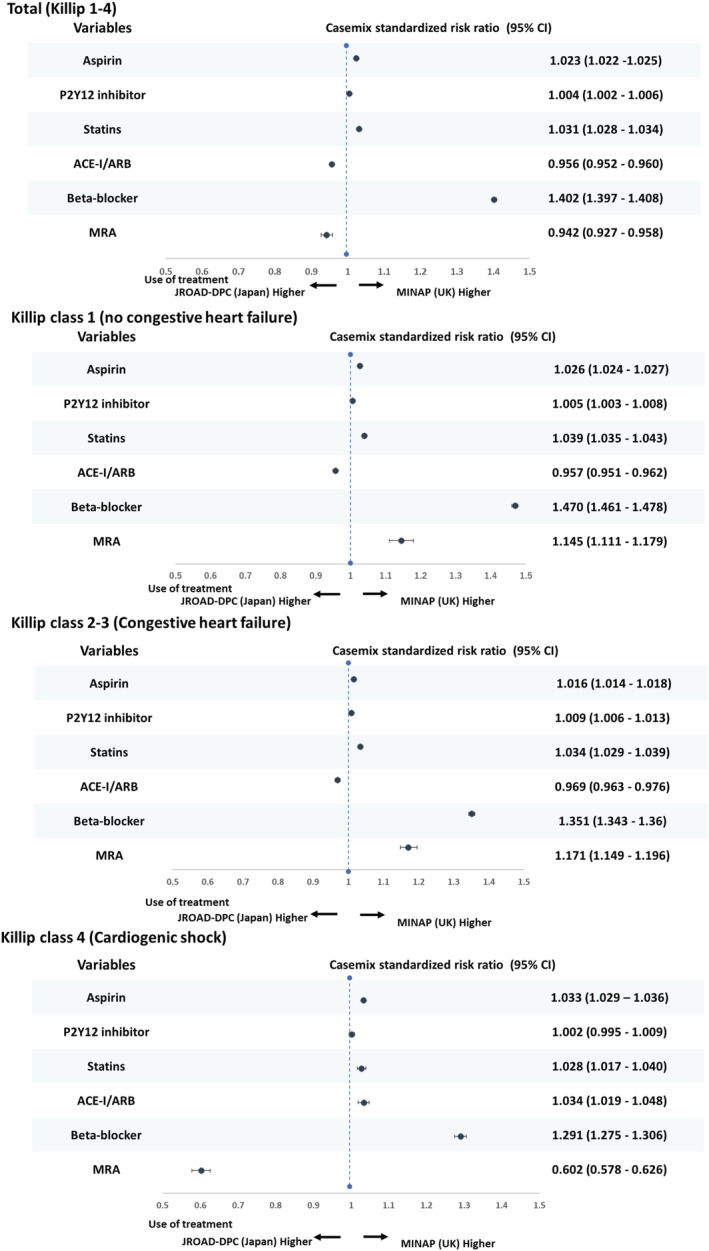
Casemix standardized risk ratio for prescription in STEMI survivor due to heart failure status between the MINAP and JROAD‐DPC. STEMI, ST‐elevation myocardial infarction; UK, United Kingdom; ACE‐I/ARB, angiotensin angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist; CI, confidence interval.
Figure 3.
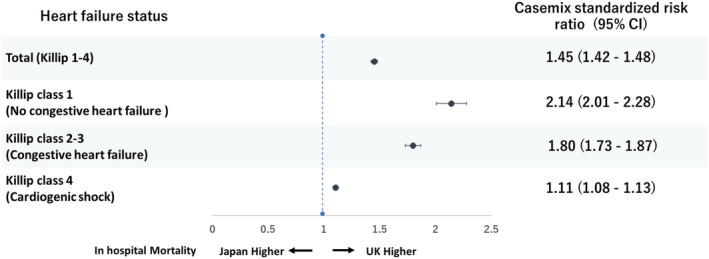
Forrest plot of casemix standardized risk ratio for in‐hospital mortality in patient with STEMI according to heart failure status between the MINAP and JROAD‐DPC. STEMI, ST‐elevation myocardial infarction; UK, United Kingdom; CI, confidence interval.
Length of hospital stay due to heart failure status
Overall, the length of hospital stay was longer in Japan than in the United Kingdom (17.0 [9.7] vs. 5.0 [7.4] days), and this was evident for each Killip class group (Killip 1: 14.6 [9.7] vs. 4.3 [6.2] days, Killip 2–3: 19.0 [15.1] vs. 8.3 [10.2] days, Killip 4: 20.9 [22.9] vs. 6.7 [10.6] days).
Crude and standardized mortality by heart failure status
The in‐hospital mortality rate was 7.4% in the United Kingdom and 8.2% in Japan. The crude rates and standardized risk of in‐hospital mortality increased with increasing Killip class group and was higher in the United Kingdom compared with Japan for Killip 1 (3.4% vs. 1.7%, CSRR 2.14 95% CI 2.01–2.28) and Killip 2–3 (15.8% vs. 6.4%, 1.80 95% CI 1.73–1.87), and similar for Killip 4 (36.9% vs. 36.3%, 1.11 95% CI 1.08–1.13) (Table 2 and Figure 3 ).
Association of care and in‐hospital mortality
Most treatments were inversely associated with in‐hospital mortality across the Killip class groups, and the strength and direction of casemix‐adjusted associations were similar in both the United Kingdom and Japan except for revascularization (CABG and PCI other than pPCI) and the use of loop diuretics. Revascularization (CABG and PCI other than pPCI) was inversely associated with in‐hospital mortality in Killip 2–3 in both countries but otherwise showed a varying relationship by country and Killip class group. The use of loop diuretics was associated with better outcomes in the United Kingdom, but not in Japan (Figure 4 ).
Figure 4.
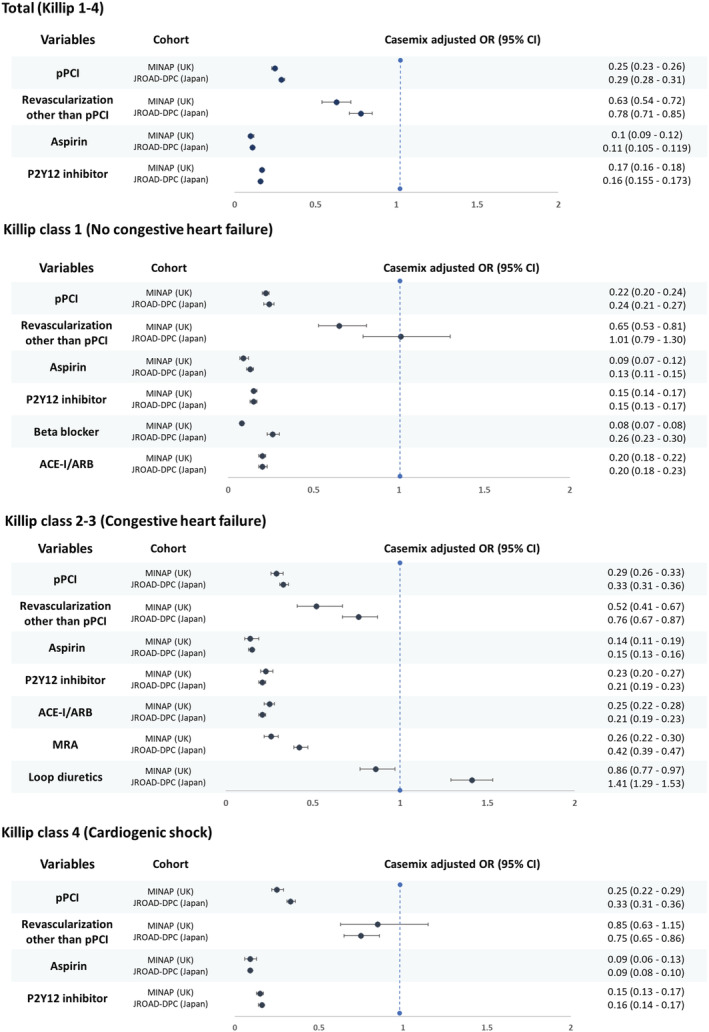
Forest plot of casemix adjusted odds ratios from multivariate models for the association of hospital treatment with in‐hospital mortality according heart failure status among STEMI patients between the MINAP and JROAD‐DPC. STEMI, ST‐elevation myocardial infarction; pPCI, primary percutaneous coronary intervention; UK, United Kingdom; ACE‐I/ARB, angiotensin angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist; OR, odds ratio; CI, confidence interval.
Discussion
Using nationwide cohorts in the United Kingdom and Japan, we compared the treatments and in‐hospital outcomes of 271 630 patients admitted with STEMI according to Killip class. We found that in‐hospital mortality for STEMI when associated with Killip class 2–3 heart failure was up to five‐fold higher than for patients with STEMI and no heart failure. Although in‐hospital mortality was higher in the United Kingdom than Japan in Killip class 2–3, the in‐hospital mortality rate for patients presenting with STEMI and cardiogenic shock was close to 40% in both countries. The use of pPCI was lower in the United Kingdom than Japan, particularly in patients with Killip class 2–3. In Japan, the length of hospital stay was more than three‐fold longer and there was more frequent use of echocardiography, whereas beta‐blockers were consistently used less frequently irrespective of the heart failure presentation. In the United Kingdom and Japan, most treatments were inversely associated with in‐hospital mortality, suggesting that the delivery of guideline‐recommended care offers the opportunity to improve outcomes in this high‐risk group of patients.
Heart failure complicating acute myocardial infarction is common and is associated with significant in‐hospital mortality even in the era of pPCI. 13 Moreover, among those who survive the acute phase, more than a quarter of patients will develop heart failure within 5 years. 14 Although newer pharmacotherapies or devices for heart failure may help reduce early mortality from STEMI complicated by heart failure, 15 it is necessary to understand the current provision of care to this population and optimize outcomes. There is an absence of comparative analyses across different healthcare systems investigating management and outcomes of STEMI complicated by heart failure, and this represents an important missed opportunity to improve health systems and prevent deaths.
Our study found that the STEMI case mix varied between the United Kingdom and Japan. The prevalence of cardiovascular disease and comorbidities differs between Western and Asian countries due to differences in lifestyle and risk factor profile. 16 We report a high prevalence of ischaemic heart disease in the United Kingdom and a high prevalence of DM in Japan, consistent with other studies, although ours is the first to make a direct comparison between the two countries. 17 It is possible that these differences in baseline comorbidities may have impacted on treatment and in‐hospital mortality rates. 18 , 19
Both crude and casemix standardized analyses demonstrated underutilization of pPCI in the United Kingdom compared with Japan, especially in patients complicated with heart failure. Notably, the implementation rate of pPCI in the United Kingdom, compared with other categories in the United Kingdom and Killip 2–3 class heart failure in Japan, was over 20% lower in patients presenting with Killip class 2–3 heart failure. We found that use of pPCI was inversely associated with mortality and so it is possible that this difference contributed to international differences in patient outcomes. A couple of factors could explain the difference in care provided. First, in general, rates of pPCI for STEMI have been shown to lag in the United Kingdom compared with other high income countries with universal health coverage. 20 Second, patients presenting with Killip class 2–3 heart failure in the United Kingdom were older and more comorbid that patients presenting with Killip class 1 and 4 heart failure, which may have influenced individual‐level decision making on the feasibility, risks, and benefits of immediate invasive therapy and previous reports have shown that use of pPCI in routine practice is lower in older patients with STEMI. 21 However this is counter‐intuitive given that evidence suggests pPCI improves prognosis in this group, 22 and the National Institute for Health and Care Excellence (NICE) in the United Kingdom specifically recommends that eligibility for pPCI is assessed ‘irrespective of age’ and European Society of Cardiology (ESC) guidance stresses that there is ‘no upper age limit with respect to reperfusion, especially with pPCI’. 23 Our findings suggest that targeted policy initiatives could have impact to reduce this shortfall in provision of interventional therapy for patients presenting with STEMI and signs of heart failure in the United Kingdom.
We identified a low utilization of beta‐blockers across all Killip class categories in Japan compared with the United Kingdom. This could partly relate to international differences in guideline recommendation levels for beta‐blockers. The 2013 American Heart Association (AHA) guideline recommends the use of beta‐blockers within 24 h in Class 1 (level of evidence B), 5 and a survey in the United States indicated that the high use of beta‐blockers for AMI. 24 The ESC 2012 and 2017 guidelines for STEMI recommends beta‐blockers during hospitalization (IIa), 4 , 25 and in the United Kingdom, NICE guidance 2013 recommends a beta‐blocker as soon as possible after an MI. In Japan, the 2013 Japanese Circulation Society (JCS) guidelines recommended early hospital beta‐blocker (IIa) prescription in the absence of contraindications, 26 but in the 2018 iteration, it is IIb for secondary prevention purposes at discharge in the absence of evident heart failure. 27 Furthermore, racial differences in coronary artery vasomotor reactivity among ischaemic heart disease in Japan compared with Caucasians, which indicates an association between vasoconstriction and ischaemia, may precipitate avoidance of beta‐blocker use in myocardial infarction. 28
The use of evidence‐based pharmaceutical therapies on discharge showed a mixed pattern, with use of both beta‐blocker and statin lower in Japan, whereas prescriptions of ACE/ARB and MRA in both countries varied depending on heart failure category. These findings suggest the existence of selective practice patterns rather than an overall low prescription. Inconsistent prescribing practices across each health system may be limiting the benefit that can be derived by achieving guideline‐directed care and thus constitutes an appropriate target for quality improvement programmes.
Previous country‐level comparison using Organisation for Economic Cooperation and Development data suggests that Japan uses higher resources in health care than the United Kingdom. 29 A comparison study using individual data in patients with hospitalized acute heart failure showed consistent results, suggesting longer hospitalization and higher resource use in Japan than in the United Kingdom. 30 Our study with STEMI also suggests that the longer length of hospital stay in Japan, but the use of each recommended care showed a selective pattern. This may partially explain the complexity of the relationship between high resource use and outcomes shown by previous studies. 29 , 30
Healthcare systems are typically built on a national or supra‐regional basis. Therefore, there could be a certain bias in care that was not recognized when studying a single system. Our first comparative study of STEMI across heart failure categories revealed the existence of variation in care and outcomes between the United Kingdom and Japan and confirmed that international comparative studies provide a valuable opportunity for learning and benchmarking of care across different healthcare systems. To overcome the various difficulty of international comparison and enable well‐matched studies, the recent formulation of quality indicators in European Society of Cardiology 31 and the harmonization of data definitions and data collection systems by EuroHeart 32 will be one approach. It is essential to proceed with multifaceted efforts to identify the areas for improvement and take appropriate approaches to improve the healthcare system, based on consideration and respect to the diverse background and characteristics of each system.
We acknowledge our study limitations. First, each cohort was extracted from a different healthcare system with its own data definitions, mechanisms for participant entry, data recording, and quality control. Such differences could not be adjusted for, even following case mix standardization. Second, missing values in either cohort, particularly in the Killip class, can dissociate subjects and outcomes from actual conditions. Third, the definition of heart failure was reliant on the Killip classification; however, the sensitivity analysis showed that modification of the definition of heart failure did not affect the results (Supplementary Text and Table S3 ). Fourth, in using ICD10 codes, JROAD may have misclassified some patients with STEMI. However, a recent study showed selecting STEMI by ICD10 code had high sensitivity and specificity. 33 , 34 Fifth, our data do not include comparable information on emergency care prior to hospitalization. Though emergency medical care is provided to the entire population under the NHS system in the United Kingdom and the compulsory and universal health insurance system in Japan, there may be differences in the process of treatment pre‐hospital which could lead to differences in the distribution of disease severity between two cohorts. Sixth, our data do not include comparable data on rates of cardiac rehabilitation, which has been evidenced to improve outcomes after acute myocardial infarction. 35 Seventh, the prevalence of comorbidities may be affected by differences in screening practices in each healthcare system which, if the degree of deviation from the true prevalence differs between the two countries, could affect the case‐mix adjusted analysis. Eighth, our data do not include the reason why patients did not receive a treatment. Finally, we compared the use of procedures and medications in all patients regardless of contraindication or patient preference owing to the lack of this information.
Conclusions
We found variation in care and outcomes of patients with STEMI across heart failure categories between the United Kingdom and Japan. Specifically, in the United Kingdom, pPCI use was lower in patients with STEMI presenting with heart failure; in Japan, the use of beta‐blocker was lower. International comparison can inform targeted quality improvement programmes to narrow the outcome gap between health systems.
Conflict of interest
The following authors reports disclosures outside the submitted work. Y.M.A. reports grants and personal fees from Bayer outside the submitted work. S.Y. reports grants from Abbott Medical Japan, Amicus Therapeutics, Otsuka Pharmaceutical, Kowa Company, Sumitomo Dainippon Pharma, Roche Diagnostics, personal fee from Daiichi Sankyo and Bayer outside the submitted work. H.O. reports personal fee from Bayer, Novartis, Bristol‐Meyers Squibb, Daiichi Sankyo, Pfizer, Eisai, Behringer Ingelheim, Takeda Pharmaceutical, Towa Pharmaceutical, Toa Eiyo, Teijin, Abbott Medical Japan, Kowa Pharmaceuticals outside the submitted work. C.P.G. reports grants from British Heart Foundation, Horizon 2020, Bristol Myers Squibb, National Institute for Health Research and Abbott Diabetes, personal fees from AstraZeneca, Bristol Myers Squibb, Boston Scientific, Daiichi Sankyo, Organon, Bayer, Boehrinher‐Ingleheim, Chiesi, Menarini, Raisio Group, Zydus, Menarini, and Wondr Medical, Member in advisory board of Amgen, Bristol Myers Squibb, Chiesi, Menarini, Bayer, Boehrinher‐Ingleheim, Daiichi Sankyo and Novartis.
Funding
This work was supported by JSPS KAKENHI Grant Number 20K08483 (K. N.) and Japanese Cardiovascular Research Foundation The Bayer Scholarship for Cardiovascular Research (K. N.). Research grant from The Japan Research Foundation for Healthy Aging (K. N.) and grant from Great Britain Sasakawa Foundation for collaboration project of the United Kingdom and Japan. The funders had no role in the design and conduct of the study; in collection, management, analysis, or interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Supporting information
Table S1. Definition of dataset's variables from the UK and Japan.
Table S2. The Extent of missingness (%) of casemix variables for multivariate models of this study.
Table S3. Sensitivity analysis according to different HF definitions.
Figure S1. Study flow chart.
Figure S2. Forest plot of odds ratios with 95% confidence intervals from multivariate models for the association of casemix with in‐hospital mortality among patients for casemix variables and in hospital death the UK and the Japan.
Text. Supplementary method.
Nakao, K. , Dafaalla, M. , Nakao, Y. M. , Wu, J. , Nadarajah, R. , Rashid, M. , Mohammad, H. , Sumita, Y. , Nakai, M. , Iwanaga, Y. , Miyamoto, Y. , Noguchi, T. , Yasuda, S. , Ogawa, H. , Mamas, M. A. , and Gale, C. P. (2023) Comparison of care and outcomes for myocardial infarction by heart failure status between United Kingdom and Japan. ESC Heart Failure, 10: 1372–1384. 10.1002/ehf2.14290.
Kazuhiro Nakao and Mohamed Dafaalla equally contributed to this work.
This work was performed at University of Leeds, University of Keele, and National Cerebral and Cardiovascular Center.
References
- 1. Auffret V, Leurent G, Gilard M, Hacot JP, Filippi E, Delaunay R, Rialan A, Rouault G, Druelles P, Castellant P, Coudert I, Boulanger B, Treuil J, Bot E, Bedossa M, Boulmier D, Le Guellec M, Donal E, Le Breton H. Incidence, timing, predictors and impact of acute heart failure complicating ST‐segment elevation myocardial infarction in patients treated by primary percutaneous coronary intervention. Int J Cardiol. 2016; 221: 433–442. [DOI] [PubMed] [Google Scholar]
- 2. Spencer FA, Meyer TE, Gore JM, Goldberg RJ. Heterogeneity in the management and outcomes of patients with acute myocardial infarction complicated by heart failure: the National Registry of myocardial infarction. Circulation. 2002; 105: 2605–2610. [DOI] [PubMed] [Google Scholar]
- 3. Wu AH, Parsons L, Every NR, Bates ER. Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the second National Registry of myocardial infarction (NRMI‐2). J Am Coll Cardiol. 2002; 40: 1389–1394. [DOI] [PubMed] [Google Scholar]
- 4. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, Group ESD . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018; 39: 119–177. [DOI] [PubMed] [Google Scholar]
- 5. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Philippides GJ, Jacobs AK, Halperin JL, Albert NM, Creager MA, DeMets D, Guyton RA, Kushner FG, Ohman EM, Stevenson W, Yancy CW. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013; 61: e179–e347. [DOI] [PubMed] [Google Scholar]
- 6. Zeymer U, Ludman P, Danchin N, Kala P, Laroche C, Sadeghi M, Caporale R, Shaheen SM, Legutko J, Iakobsishvili Z. Reperfusion therapies and in‐hospital outcomes for ST‐elevation myocardial infarction in Europe: the ACVC‐EAPCI EORP STEMI registry of the European Society of Cardiology. Eur Heart J. 2021; 42: 4536–4549. [DOI] [PubMed] [Google Scholar]
- 7. Eagle KA, Goodman SG, Avezum Á, Budaj A, Sullivan CM, López‐Sendón J, Investigators G. Practice variation and missed opportunities for reperfusion in ST‐segment‐elevation myocardial infarction: findings from the global registry of acute coronary events (GRACE). Lancet. 2002; 359: 373–377. [DOI] [PubMed] [Google Scholar]
- 8. Wilkinson C, Weston C, Timmis A, Quinn T, Keys A, Gale CP. The myocardial Ischaemia National Audit Project (MINAP). Eur Heart J Qual Care Clin Outcomes. 2020; 6: 19–22. [DOI] [PubMed] [Google Scholar]
- 9. Yasuda S, Nakao K, Nishimura K, Miyamoto Y, Sumita Y, Shishido T, Anzai T, Tsutsui H, Ito H, Komuro I, Saito Y, Ogawa H, Investigators otboJ . The current status of cardiovascular medicine in Japan ‐ analysis of a large number of health records from a Nationwide claim‐based database, JROAD‐DPC. Circ J. 2016; 80: 2327–2335. [DOI] [PubMed] [Google Scholar]
- 10. Nakao K, Yasuda S, Nishimura K, Noguchi T, Nakai M, Miyamoto Y, Sumita Y, Shishido T, Anzai T, Ito H, Tsutsui H, Saito Y, Komuro I, Ogawa H. Prescription rates of guideline‐directed medications are associated with in‐hospital mortality among Japanese patients with acute myocardial infarction: a report from JROAD ‐ DPC study. J Am Heart Assoc. 2019; 8: e009692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexandrescu R, Bottle A, Jarman B, Aylin P. Current ICD10 codes are insufficient to clearly distinguish acute myocardial infarction type: a descriptive study. BMC Health Serv Res. 2013; 13: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uemura S, Okamoto H, Nakai M, Nishimura K, Miyamoto Y, Yasuda S, Tanaka N, Kohsaka S, Kadota K, Saito Y, Tsutsui H, Komuro I, Ikari Y, Ogawa H, Nakamura M. Primary percutaneous coronary intervention in elderly patients with acute myocardial infarction ‐ an analysis from a Japanese Nationwide claim‐based database. Circ J. 2019; 83: 1229–1238. [DOI] [PubMed] [Google Scholar]
- 13. Itzahki Ben Zadok O, Ben‐Gal T, Abelow A, Shechter A, Zusman O, Iakobishvili Z, Cohen T, Shlomo N, Kornowski R, Eisen A. Temporal trends in the characteristics, management and outcomes of patients with acute coronary syndrome according to their Killip class. Am J Cardiol. 2019; 124: 1862–1868. [DOI] [PubMed] [Google Scholar]
- 14. Desta L, Jernberg T, Spaak J, Hofman‐Bang C, Persson H. Risk and predictors of readmission for heart failure following a myocardial infarction between 2004 and 2013: a Swedish nationwide observational study. Int J Cardiol. 2017; 248: 221–226. [DOI] [PubMed] [Google Scholar]
- 15. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner‐La Rocca HP, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, Investigators E‐PT. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 16. Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, Nakamura Y, Okamura T. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008; 118: 2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan MA, Hashim MJ, Mustafa H, Baniyas MY, Al Suwaidi SKBM, AlKatheeri R, Alblooshi FMK, Almatrooshi MEAH, Alzaabi MEH, Al Darmaki RS, Lootah SNAH. Global epidemiology of ischemic heart disease: results from the global burden of disease study. Cureus. 2020; 12: e9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall M, Dondo TB, Yan AT, Mamas MA, Timmis AD, Deanfield JE, Jernberg T, Hemingway H, Fox KAA, Gale CP. Multimorbidity and survival for patients with acute myocardial infarction in England and Wales: latent class analysis of a nationwide population‐based cohort. PLoS Med. 2018; 15: e1002501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yadegarfar ME, Gale CP, Dondo TB, Wilkinson CG, Cowie MR, Hall M. Association of treatments for acute myocardial infarction and survival for seven common comorbidity states: a nationwide cohort study. BMC Med. 2020; 18: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, Heuschmann P, Wallentin L, Deanfield J, Timmis A, Jernberg T, Hemingway H. Acute myocardial infarction: a comparison of short‐term survival in national outcome registries in Sweden and the UK. Lancet. 2014; 383: 1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franken M, Nussbacher A, Liberman A, Wajngarten M. ST elevation myocardial infarction in the elderly. J Geriatr Cardiol. 2012; 9: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Boer SP, Westerhout CM, Simes RJ, Granger CB, Zijlstra F, Boersma E, Group P‐TC . Mortality and morbidity reduction by primary percutaneous coronary intervention is independent of the patient's age. JACC Cardiovasc Interv. 2010; 3: 324–331. [DOI] [PubMed] [Google Scholar]
- 23. Mills GB, Ratcovich H, Adams‐Hall J, Beska B, Kirkup E, Raharjo DE, Veerasamy M, Wilkinson C, Kunadian V. Is the contemporary care of the older persons with acute coronary syndrome evidence‐based? Eur Heart J Open. 2022; 2: oeab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xian Y, Pan W, Peterson ED, Heidenreich PA, Cannon CP, Hernandez AF, Friedman B, Holloway RG, Fonarow GC, Hospitals GSCa . Are quality improvements associated with the get with the guidelines‐coronary artery disease (GWTG‐CAD) program sustained over time? A longitudinal comparison of GWTG‐CAD hospitals versus non‐GWTG‐CAD hospitals. Am Heart J. 2010; 159: 207–214. [DOI] [PubMed] [Google Scholar]
- 25. Steg PG, James SK, Atar D, Badano LP, Blömstrom‐Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van’t Hof A, Widimsky P, Zahger D, (ESC) TFotmoS‐seamiotESoC . ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012; 33: 2569–2619. [DOI] [PubMed] [Google Scholar]
- 26. Kazuo K. Guidelines for the management of patients with ST‐elevation acute myocardial infarction (JCS 2013). http://saigaiin.sakura.ne.jp Accessed 09 Sep 2022.
- 27. Kimura K, Kimura T, Ishihara M, Nakagawa Y, Nakao K, Miyauchi K, Sakamoto T, Tsujita K, Hagiwara N, Miyazaki S, Ako J, Arai H, Ishii H, Origuchi H, Shimizu W, Takemura H, Tahara Y, Morino Y, Iino K, Itoh T, Iwanaga Y, Uchida K, Endo H, Kongoji K, Sakamoto K, Shiomi H, Shimohama T, Suzuki A, Takahashi J, Takeuchi I, Tanaka A, Tamura T, Nakashima T, Noguchi T, Fukamachi D, Mizuno T, Yamaguchi J, Yodogawa K, Kosuge M, Kohsaka S, Yoshino H, Yasuda S, Shimokawa H, Hirayama A, Akasaka T, Haze K, Ogawa H, Tsutsui H, Yamazaki T, Group JCSJW. JCS . Guideline on diagnosis and treatment of acute coronary syndrome. Circ J. 2018; 2019: 1085–1196. [DOI] [PubMed] [Google Scholar]
- 28. Beltrame JF, Sasayama S, Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol. 1999; 33: 1442–1452. [DOI] [PubMed] [Google Scholar]
- 29. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high‐income countries. JAMA. 2018; 319: 1024–1039. [DOI] [PubMed] [Google Scholar]
- 30. Sundaram V, Nagai T, Chiang CE, Reddy YNV, Chao TF, Zakeri R, Bloom C, Nakai M, Nishimura K, Hung CL, Miyamoto Y, Yasuda S, Banerjee A, Anzai T, Simon DI, Rajagopalan S, Cleland JGF, Sahadevan J, Quint JK. Hospitalization for heart failure in the United States, UK, Taiwan, and Japan: an international comparison of administrative health records on 413,385 individual patients. J Card Fail. 2022; 28: 353–366. [DOI] [PubMed] [Google Scholar]
- 31. Aktaa S, Polovina M, Rosano G, Abdin A, Anguita M, Lainscak M, Lund LH, McDonagh T, Metra M, Mindham R, Piepoli M, Störk S, Tokmakova MP, Seferović P, Gale CP, Coats AJS. European Society of Cardiology quality indicators for the care and outcomes of adults with heart failure. Developed by the working Group for Heart Failure Quality Indicators in collaboration with the heart failure Association of the European Society of cardiology. Eur J Heart Fail. 2022; 24: 132–142. [DOI] [PubMed] [Google Scholar]
- 32. Wallentin L, Gale CP, Maggioni A, Bardinet I, Casadei B. EuroHeart: European unified registries on heart care evaluation and randomized trials. Eur Heart J. 2019; 40: 2745–2749. [DOI] [PubMed] [Google Scholar]
- 33. Saavedra A, Morris RW, Tam CS, Killedar M, Ratwatte S, Huynh R, Yu C, Yuan DZ, Cretikos M, Gullick J, Vernon ST, Figtree GA, Morris J, Brieger D. Validation of acute myocardial infarction (AMI) in electronic medical records: the SPEED‐EXTRACT Study. medRxiv 2020:2020.2012.2008.20245720.
- 34. Patel AB, Quan H, Welsh RC, Deckert‐Sookram J, Tymchak W, Sookram S, Surdhar I, Kaul P. Validity and utility of ICD‐10 administrative health data for identifying ST‐ and non‐ST‐elevation myocardial infarction based on physician chart review. CMAJ Open. 2015; 3: E413–E418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta‐analysis. J Am Coll Cardiol. 2016; 67: 1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of dataset's variables from the UK and Japan.
Table S2. The Extent of missingness (%) of casemix variables for multivariate models of this study.
Table S3. Sensitivity analysis according to different HF definitions.
Figure S1. Study flow chart.
Figure S2. Forest plot of odds ratios with 95% confidence intervals from multivariate models for the association of casemix with in‐hospital mortality among patients for casemix variables and in hospital death the UK and the Japan.
Text. Supplementary method.


