Abstract
This review provides an overview of literature addressing progressive massive fibrosis (PMF) from September 2009 to the present. Advances are described in understanding its pathophysiology, epidemiology of the occurrence of PMF and related conditions, the impact of PMF on pulmonary function, advances in imaging of PMF, and factors affecting progression of pneumoconiosis in dust-exposed workers to PMF. Basic advances in understanding the etiology of PMF are impeded by the lack of a well-accepted animal model for human PMF. Recent studies evaluating lung tissue samples and epidemiologic investigations support an important role for the silica component of coal mine dust in causing coal workers’ pneumoconiosis and PMF in contemporary coal miners in the United States and for silica in causing silicosis and PMF in artificial stone workers throughout the world. Development of PMF is associated with substantial decline in pulmonary function relative to no disease or small opacity pneumoconiosis. In recent reports, computed tomography has had greater sensitivity for detecting PMF than chest x-ray. Magnetic resonance imaging shows promise in differentiating between PMF and lung cancer. Although PMF develops in dust-exposed workers without previously identified small opacity pneumoconiosis, the presence of small opacity pneumoconiosis increases the risk for progression to PMF, as does heavier dust exposure. Recent literature does not document any effective new treatments for PMF and new therapies to prevent and treat PMF are an important need.
Keywords: Progressive massive fibrosis, Coal workers’ pneumoconiosis, Silicosis, Chest imaging, Pulmonary function, Antifibrotic
1. Introduction/Background
Progressive massive fibrosis (PMF) is the most severe form of the dust-induced lung diseases chronic silicosis and coal worker’s pneumoconiosis (CWP) (Castranova & Vallyathan, 2000). Milder forms of these diseases often first present on chest radiographs as small (<one cm) opacities in the lungs, which may be rounded with an upper zone predominance or irregular with lower zone predominance (Laney & Petsonk, 2012; Laney & Weissman, 2014; Petsonk, Rose, & Cohen, 2013). Nodules develop as inflammatory and fibrotic reactions to inhaled dust. As fibrosis progresses, the small nodules may coalesce into larger masses of fibrotic tissue, resulting in PMF, also referred to as conglomerate or complicated disease (Fig. 1). PMF is defined radiographically by the formation of large (diameter ≥ one cm) opacities. In the case of silicosis, sometimes a larger diameter (≥ two cm) has been used as a threshold for pathologically defining fibrotic masses as PMF. Extensive fibrosis, emphysema formation, and cavitation and destruction of lung parenchyma including blood vessels and bronchioles can result in significant impairment of pulmonary function. On chest radiography, PMF may be confused with carcinoma, tuberculosis, or abnormalities caused by bacterial infections (Castranova & Vallyathan, 2000). Important risk factors for developing PMF include high levels of cumulative respirable coal mine dust or respirable crystalline silica inhalation, the presence of small opacity disease, and history of tuberculosis (Attfield et al., 2022; Ng & Chan, 1991). Because the presence of PMF is associated with a substantial worsening in morbidity and mortality, efforts to control exposures to coal mine dust are strongly focused on preventing PMF (Attfield et al., 2022). PMF is most often caused by CWP and silicosis and thus PMF caused by those diseases is the focus of this review. However, a small number of cases of PMF have been also described in pulmonary talcosis (Gibbs et al., 1992) and kaolin pneumoconiosis (Edenfield Edenfieid, 1960). Also, large conglomerate masses resembling PMF can occur in sarcoidosis (Criado et al., 2010).
Fig. 1.
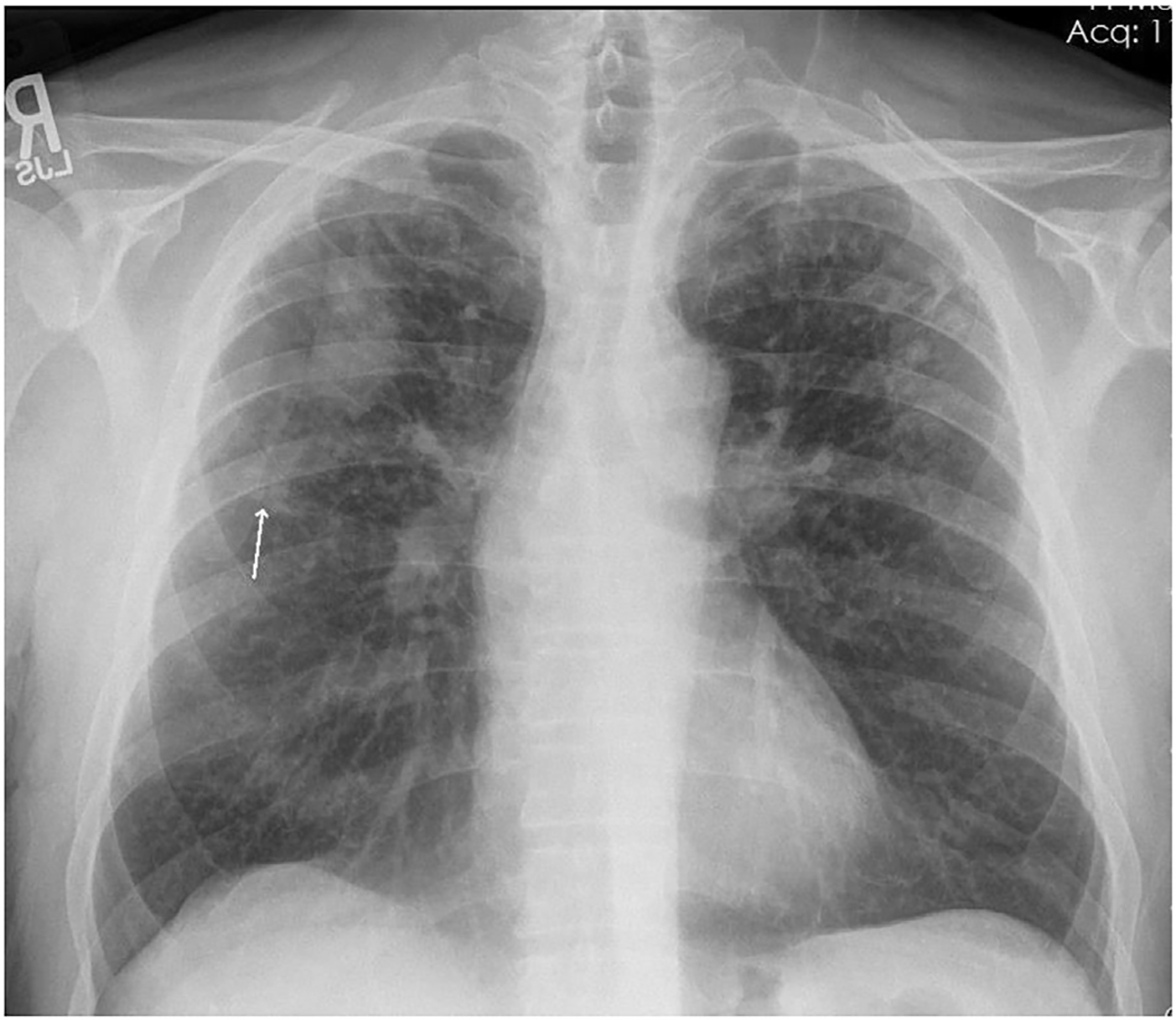
Chest radiograph of individual with PMF. Well-defined large opacity with flat margin laterally in the right upper zone. Background of small opacities present bilaterally in the upper and middle zones with minimal involvement of the right lower zone, and no involvement of the left lower zone. Arrow shows coalescence of small opacities, a precursor to formation of a large opacity, in the right middle zone (arrow). Source: CDC-NIOSH.
This overview provides an update on recent original scientific literature addressing PMF. Reports published in the period from September 2009 through May 2022 were identified by searching PubMed and are summarized to provide an update on recent advances in understanding PMF, including its pathophysiology, epidemiology of occurrence, impact on pulmonary function tests, radiologic imaging, and risk of progression from no or small opacity disease to PMF.
2. Approach to overview of the recent literature
The review was conducted as an overview of the literature (Grant & Booth, 2009). References were identified by searching PubMed (https://pubmed.ncbi.nlm.nih.gov/?term=) for the term “progressive massive fibrosis.” Inclusion criteria for references were that they were peer reviewed journal articles listed in PubMed that contained the term “progressive massive fibrosis” in fields searched by PubMed, addressed pulmonary disease caused by mineral dust, contained original data, and were written in English. Individual case reports were excluded. The author read titles and abstracts of all articles identified through the PubMed search to identify those meeting the inclusion and exclusion criteria. Frequent reasons for exclusion of references were that they addressed fibrosis in organs other than the lung or that they were reviews without original data. Final selections for inclusion were made based on more detailed review and examination of full-length articles. The period searched was September 2009 until May 30, 2022. A total of 54 references were identified that met inclusion criteria and were selected for inclusion in this overview (Table 1). Findings from the 54 recent references are summarized below. Based on the topics addressed by the references, they are grouped into the following categories: pathophysiology of PMF, epidemiology of occurrence of PMF and associated conditions in a range of work and environmental settings, impact of PMF on pulmonary function, imaging of PMF and radiographic progression to PMF over time. In some cases, additional references not identified by the PubMed search are cited in the narrative to provide background.
Table 1.
References on PMF included in review.
| Subject area / reference | Type of study | Comment |
|---|---|---|
|
| ||
| Occurrence of PMF and Related Conditions in Work and Environmental Settings | ||
| Coal Mining | ||
| Almberg et al., 2018 | Observational, no control | Reports findings among those filing for benefits from the U.S. Black Lung Benefits Program for the period 1970–2016 |
| Blackley et al., 2014 | Observational, no control | Reports findings from U.S. coal miners participating in a health screening program, 2005–2012 |
| Blackley et al., 2016 | Observational, no control | Reports a cluster of coal miners with PMF1 cared for in a clinic in eastern Kentucky |
| Blackley, Halldin, & Laney, 2018 | Observational, no control | Reports findings from national surveillance for pneumoconiosis in U.S. coal miners, 1970–2017 |
| Blackley, Reynolds, Short, et al., 2018 | Observational, no control | Reports a cluster of coal miners with PMF cared for in 3 clinics in western Virginia |
| Graber et al., 2017 | Observational, no control | Reports findings among those filing for benefits from the U.S. Black Lung Benefits Program for the period 2001 to 2013 |
| Halldin et al., 2015 | Observational, no control | Describes 8 surface coal miners with PMF identified in U.S. screening program, 2010–2011 |
| Hall et al., 2020 | Observational, no control | Reports findings among U.S. surface coal miners participating in national surveillance, 2014–2019 |
| Hall et al., 2022 | Observational, no control | Reports findings among American Indian/Alaska native participants in national surveillance, 2014–2019 |
| Laney & Attfield, 2010 | Observational, no control | Reports findings among U.S. coal miners participating in national surveillance, 1970–2009 |
| Laney, Petsonk, & Attfield, 2010 | Observational, no control | Reports findings among U.S. coal miners participating in national surveillance, 1980–2008 |
| Laney, Wolfe, Petsonk et al., 2012 | Observational, no control | Reports findings among U.S. surface coal miners screened for pneumoconiosis, 2010–2011 |
| Laney, Petsonk, Hale, et al., 2012 | Observational, no control | Reports findings among U.S. coal miners participating in health screening, 2005–2009 |
| Reynolds et al., 2018 | Observational, no control | Reports results of interviews to identify hazards experienced by19 former U.S. coal miners with PMF |
| Tomaskova et al., 2012 | Observational, cohort study with comparison to general male population | Former coal miners in the Czech Republic without coal workers’ pneumoconiosis or with varying degrees of radiological severity of coal workers’ pneumoconiosis |
| Vallyathan et al., 2011 | Observational, no control | Reports results of lung histopathology from coal miners’ autopsies submitted to U.S. government program, 1971–1996 |
| Wade et al., 2011 | Observational, no control | Reports findings among coal miners filing for compensation from the West Virginia State Occupational Pneumoconiosis Board, 2000–2009 |
| Settings Associated with Silicosis | ||
| Bakan et al. 2012 | Observational, follow up of clinical cohort | Describes features of silicosis in 32 Turkish denim sandblasters followed for a median of 29 months (range 3–101 months). |
| Govindagoudar et al., 2021 | Observational, no control | Reports results of radiographic screening of stone crushing workers in India |
| Hua et al., 2022 | Observational, no control | Describes features of registrants in an international registry of engineered stone workers with silicosis |
| Hoyet al., 2017 | Observational, no control | Case series of silicosis in 7 Australian workers engaged in workwith artificial stone |
| Kramer et al., 2012 | Observational, no control | Describes 25 artificial stone workers with silicosis who were referred to a lung transplantation program in Israel |
| Lopes et al., 2012 | Observational, cross-sectional comparison of sandblasters and stone carvers | Describes and compares features of silicosis in 25 Brazilian shipyard sandblasters and 16 Brazilian stone carvers |
| Nandi et al., 2021 | Observational, no control | Describes silicosis in sandstone mine workers in Rajasthan, India. |
| Nicol et al., 2015 | Observational, no control | Describes 6 cases of silicosis among stonemasons <40 years old in the UK |
| Reilly et al., 2018 | Observational, no control | Describes cases of silicosis reported to the state of Michigan, 1988–2016 |
| Siribaddana et al., 2016 | Observational, no control | Describes silicosis in employees of a quartz processing facility in Sri Lanka |
| Spalgais et al., 2015 | Observational, no control | Describes 6 cases of nonoccupational anthracosis and silicosis among homemakers in the Ladakh region of Jammu and Kashmir, India |
| Wu et al., 2020 | Observational, cross-sectional comparison of artificial and natural stone workers | Describes and compares features of silicosis in China in18 artificial stone workers and 63 natural stone workers who had worked in jade processing or in mines |
| Progression to PMF | ||
| Almberg et al., 2020 | Observational, no control (cohort) | Reports findings among those filing for benefits from the U.S. Black Lung Benefits Program who had more than one chest x-ray in the period 2000–2013 |
| Laney et al., 2017 | Observational, no control (cohort) | Reports findings among U.S. coal miners first found to have PMF during national surveillance conducted 2000–2016 who had at least one prior chest x-ray |
| León-Jiménez et al., 2020 | Observational, no control (cohort) | Reports findings among 106 Spanish artificial stone workers diagnosed with silicosis from 2009 to 2018 with subsequent clinical follow up for a mean of 4 years |
| Association Between PMF and Pulmonary Function | ||
| Grubstein et al., 2016 | Observational, no control | Describes findings in marble workers exposed to artificial stone dust referred to an outpatient pulmonary clinic in Israel, 1997–2015 |
| Kurth et al., 2020 | Observational, no control | Describes findings in U.S. coal miners participating in health screening, 2005–2016 |
| Rosenman et al., 2010 | Observational, no control | Describes findings in individuals with silicosis reported to the Michigan Silicosis Registry, 1985–2002. |
| Wang et al., 2013 | Observational, no control | Reports findings among U.S. coal miners participating in health screening, 2005–2009 |
| Imaging of PMF | ||
| Choi et al., 2020 | Retrospective evaluation of patient data | Retrospective evaluation of PET2/CT3 findings from 49 patients with pneumoconiosis being evaluated for suspected lung cancer |
| Chung et al., 2010 | Retrospective evaluation of patient data | Retrospective evaluation of FDG4/PET findings from 9 patients with PMF |
| Friedman et al., 2021 | Observational, no control | Describes findings of physicians classifying chest x-rays in U.S. Black Lung Benefits proceedings that were filed 2000–2013 |
| Guneyli et al., 2021 | Retrospective evaluation of patient data | Describes magnetic resonance imaging findings in 60 male patients with histories of working in underground coal mines who were evaluated due to suspicion of lung cancer after computed tomography. |
| Halldin et al., 2020a | Observational, no control | Describes findings on chest radiographs showing PMF obtained from U.S. coal miners participating in periodic health surveillance from 2000 to 2015 |
| Hekimoğlu et al., 2010 | Prospective evaluation of patient data | Describes findings from 20 coal workers with PMF evaluated with computed tomography and magnetic resonance imaging |
| Jones et al., 2020 | Observational, no control | Describes and provides review of radiologic findings encountered during Australian outbreak of silicosis in artificial stone workers and provides examples from several individual cases |
| Ogihara et al., 2018 | Retrospective evaluation of clinical data | Retrospective evaluation of 28 patients with known pneumoconiosis undergoing magnetic resonance imaging because of concern for possible lung cancer |
| Ozmen et al., 2010 | Observational, no control | Describes chest computed tomography in 60 consecutive patients with histories of exposure to silica during denim sandblasting presenting for care at a clinic in Turkey |
| Sari et al., 2022 | Observational, no control | Describes chest computed tomography in 90 patients with PMF primarily after coal mine dust or silica exposure in Turkey |
| Takahashi et al., 2018 | Observational, cross-sectional comparison 2 occupational groups. | Describes and compares radiologic findings in 66 patients with arc welders’ pneumoconiosis and 33 patients with silicosis seen in Japanese clinics |
| Zhang et al., 2017 | Retrospective evaluation of clinical data | Compares magnetic resonance imaging findings from 25 patients with coal workers’ pneumoconiosis and PMF and 34 patients with lung-tumor like lesions (21 had lung cancer) |
| Mechanisms of PMF Development | ||
| Cohen et al., 2016 | Observational, no control | Reports histopathology and lung particle analysis from 13 coal miners with rapidly progressive pneumoconiosis |
| Cohen et al., 2022 | Observational, with comparison group | Reports histopathology and lung particle analysis from 85 coal miners with PMF, compares coal miners born before and after 1930 |
| García-Núñez et al., 2022 | Observational, with comparison group | Reports and compares blood inflammation biomarkers in 53 artificial stone workers with small opacity silicosis, 38 with PMF, and 22 healthy male volunteers |
| Komai et al., 2019 | Animal model | Describes animal model of silicosis induced by intratracheal instillation of silica in F344 rats |
| Lee et al., 2015 | Observational, cross-sectional comparison of subgroups | Describes findings in 106 male retired Korean coal miners, with results compared between those without pneumoconiosis, those with small opacity pneumoconiosis, and those with large opacities (PMF) |
| León-Jiménez et al., 2021 | Observational, no comparison group | Describes histopathologic and mineralogic analyses of lung biopsies from seven engineered stone workers with silicosis, four of which developed PMF during follow up |
Progressive massive fibrosis.
Positron emission tomography.
Computedtomography.
18-Fluorine-labeled 2-deoxy-2-fluoro-d-glucose.
3. Pathophysiology
Relatively little is known about the specific mechanisms that underlie progression from small opacities of silicosis and CWP to PMF. This is at least partly due to a lack of a generally recognized rodent model for human PMF (Green, Vallyathan, & Hahn, 2007). In contrast, basic mechanisms for cellular damage and development of a fibrotic pulmonary response to inhaled respirable crystalline silica has been extensively studied (Adamcakova & Mokra, 2021; Hamilton Jr, Thakur, & Holian, 2008; Sayan & Mossman, 2015). Likely cellular targets of inhaled crystalline silica particles include alveolar macrophages and respiratory epithelial cells. Crystalline silica particles can interact with cell-surface scavenger receptors on alveolar macrophages and activate a multiprotein complex called the nucleotide-binding oligomerization domain (NOD-like) receptor (NLR), pyrin domain-containing 3 (NLRP3) inflammasome. In turn, NLRP3 activates caspase-1, leading to conversion of cytokines such as interleukin (IL)-1β and IL-18 from inactive into active forms. It also causes extracellular release of growth factors and alarmins such as basic fibroblast growth factor (bFGF) and high mobility group protein B1 (HMGB1) (Peeters et al., 2014). Crystalline silica particles can also generate free radicals that are directly cytotoxic to cellular targets, another likely trigger for fibrogenic factor release (Castranova & Vallyathan, 2000).
Single nucleotide polymorphisms were evaluated as risk factors for PMF in coal miners (Yucesoy et al., 2008). A combination of three single nucleotide polymorphisms in the genes for vascular endothelial growth factor (VEGF), intercellular cell adhesion molecule (ICAM-1) and IL-6 was associated with significantly increased risk for PMF, whereas individual polymorphisms were not associated with increased risk, suggesting the importance of multiple cytokines and growth factors in PMF.
Several papers identified in the literature search addressed pathophysiology of PMF. Komai et al. described a model of silicosis induced in F344 female rats by intratracheal instillation of 10 mg Min-U-Sil-5 (Komai et al., 2019). Rats were sacrificed at one, three, six, and 12 months after instillation. There was time-dependent progressive fibrosis in the lungs, with formation of silicotic nodules, PMF, and diffuse interstitial fibrosis. Western blotting, immunohistochemistry, light microscopy, and electron microscopy findings suggested that enhanced TGF-β expression and epithelial-mesenchymal transition of hyperplastic type II epithelial cells are involved in the development of progressive massive pulmonary fibrosis during silicosis.
Lee et al. evaluated levels of serum interleukin-8 (IL-8), macrophage inflammatory protein-1α (MIP-α), and intercellular adhesion molecule-1 (ICAM-1) in relation to presence or absence of PMF in retired coal miners (Lee, Shin, & Choi, 2015). Twenty-seven did not have radiographic pneumoconiosis, 59 had simple (small opacity) pneumoconiosis, and 20 had PMF. IL-8 levels were higher in those with PMF than those with no pneumoconiosis (p < 0.001) or simple CWP (P = 0.003). Also, levels of ICAM-1 were higher in those with PMF than those with no pneumoconiosis (p = 0.001) and tended to be higher than in those with simple pneumoconiosis (p = 0.054). In another study evaluating serum markers in PMF, Garcia-Nunez et al. studied 22 healthy male volunteers without exposure to silica dust, 53 male former engineered stone workers with small opacity silicosis, and 38 male former engineered stone workers with PMF (García-Núñez et al., 2022). Out of many potential blood biomarkers, lactate dehydrogenase (LDH), angiotensin-converting enzyme (ACE), and fibrinogen were the ones that showed increases from controls to small opacity disease to PMF. However, only LDH was significantly different between small opacity disease and PMF.
Several reports evaluating human lung pathology identified the importance of crystalline silica in rapidly progressive pneumoconiosis including PMF among coal miners in the United States. Coal mine dust contains many components including coal, crystalline silica, silicates, and other materials. A study from 2016 reviewed lung pathology from 13 contemporary coal miners with rapidly progressive pneumoconiosis. Many had features of accelerated silicosis and mixed dust lesions. Twelve had PMF and 11 had silicosis. Polarized light microscopy demonstrated large amounts of birefringent mineral dust particles consistent with silica and silicates in the lungs (Cohen et al., 2016). A study from 2022 evaluated lung pathology findings from 23 contemporary coal miners and 62 historical controls with PMF (Cohen et al., 2022). Contemporary coal miners were more likely to have silica-type PMF and mineral dust-associated alveolar proteinosis, a finding associated with acute silicosis, than historical controls. They were less likely to have coal-type PMF and coal macules than historical controls. Contemporary coal miners had a greater concentration of silica particles and percent of particles that contained silica in their lungs, as well as a greater percent of particles that contained aluminum silicates, than historical controls. Also, the concentration of particles containing silica was greater in the presence of silica-type PMF, mineral dust-associated alveolar proteinosis, silicotic nodules, or immature silicotic nodules. These findings support the importance of respirable crystalline silica as a cause of pneumoconiosis, including PMF, in contemporary coal miners in the United States. This is in marked contrast to an earlier study that evaluated lungs obtained from coal miners’ autopsies from 1971 through 1996. It found significant decreases in findings of CWP, silicosis, and PMF in miners who had worked after promulgation of stricter dust standards in 1969 (Vallyathan et al., 2011).
Leon-Jimenez et al. evaluated mineral content in artificial stone countertop material and in the lungs of seven Spanish artificial stone workers (León-Jiménez et al., 2021). Artificial stone countertop material was largely composed of crystalline silica, with variable proportions of quartz and cristobalite depending on the sample. Lung biopsy samples evaluated using scanning electron microscopy and energy dispersive x-ray spectroscopy showed higher elemental proportions of silicon and aluminum at the center of silicotic nodules with decreasing levels at the periphery. Silicon and aluminum concentrations correlated with each other. At presentation, one of the seven stone workers had PMF. After follow-up for an average of 8 years, four had PMF. Nodule silicon content was significantly correlated with radiographic progression rate per year. A trend was also noted for aluminum content, but it did not reach statistical significance.
Thus, themes from recent literature on the pathophysiology of PMF include the importance of crystalline silica as a cause of PMF in contemporary coal miners from the United States and in silica-exposed artificial stone workers. In addition, the presence of PMF is associated with blood markers of inflammation and injury with the potential to be useful as biomarkers of disease.
4. Epidemiology of occurrence of PMF and related conditions in work and environmental settings
Many of the reports identified in the recent literature describe issues such as burden and risk factors for PMF in various coal mine dust- and silica-exposed populations. Examples include contemporary coal miners affected by an outbreak of CWP in the United States and workers in many countries exposed to respirable crystalline silica such as sandblasters and artificial stone workers.
Recent reports describe increases in the burden of PMF among contemporary coal miners in the United States. Almberg et al. found that from January 1, 1970 until December 31, 2016, there were 4679 unique PMF cases among claimants filing for benefits from the Black Lung Benefits Program (Almberg et al., 2018). Two thousand four hundred seventy-four were identified on or after 1996, when the annual proportion of claimants with PMF started to significantly increase. Most claimants had worked in central Appalachia. Graber et al. evaluated Black Lung Benefits claimants from 2001 to 2013 and noted that prevalence of advanced disease was higher among those who started working as coal miners after 1970, as well as in claimants who were aged 56 years and younger or 80 years and older (Graber et al., 2017).
Other reports from the United States describe the burden of CWP and PMF among diverse coal mining populations including participants in national coal workers’ health surveillance (Blackley et al., 2018; Blackley, Crum, Halldin, Storey, & Laney, 2016; Hall et al., 2022), coal miners with PMF as patients in clinics in Kentucky (Blackley et al., 2016) and western Virginia (Blackley, Halldin, & Laney, 2018), and coal miners who had compensation claims approved by the West Virginia State Occupational Pneumoconiosis Board (Wade, Petsonk, Young, & Mogri, 2011). These reports show PMF among coal miners to be a significant and increasing problem, with Blackley et al., 2016 showing that as of 2015, >5 % of underground coal miners from central Appalachia with mining tenure of 25 years or more who participated in a national health surveillance program had PMF (Blackley et al., 2016). A report focusing on American Indian and Alaska Native coal miners, primarily in the western United States, who participated in surveillance from 2014 to 2019 found that of those with at least 10 years’ tenure, 3 % had radiographic findings of pneumoconiosis and 0.3% findings of PMF (Hall et al., 2022). Three reports showed that pneumoconiosis, including PMF, also occurred in U.S. surface coal miners, particularly those engaged in activities such as drilling, which is associated with respirable crystalline silica exposure (Hall, Halldin, Blackley, & Laney, 2020; Halldin et al., 2015; Laney, Wolfe, Petsonk and Halldin, 2012).
Several reports seek to explain underlying causes of recent increases in CWP, including PMF, in the United States. In one report, investigators interviewed 19 Appalachian former coal miners with PMF to identify potential issues that might have affected these miners. Many of the miners reported having to cut through rock to extract coal. They also reported other issues such as working downwind of dust-generating equipment, nonadherence to mine ventilation plans and dust controls, and improper sampling for respirable coal mine dust. They reported continuing to work even after developing respiratory illness (Reynolds et al., 2018). Several studies found that working in small mines, which likely had less safety resources and sophistication than large mines, was a risk factor for CWP, including PMF (Blackley, Halldin, Wang, & Laney, 2014; Laney & Attfield, 2010; (Laney, Petsonk, Hale, Wolfe and Attfield, 2012)). One study implicated increased exposure to respirable crystalline silica in coal mine dust as a cause for increased PMF in central Appalachian coal miners (Laney, Petsonk and Attfield, 2010). It found increases in both r-type opacities, a radiographic finding associated with silicosis, and PMF in Central Appalachian coal miners after 1999 compared to earlier years. Finally, several studies already discussed in the section on pathophysiology used lung pathology data to identify the importance of crystalline silica as a component of the complex mixture of materials found in coal mine dust in causing pneumoconiosis and PMF (Cohen et al., 2016; Cohen et al., 2022).
PMF in silica-exposed workers has also received much attention in the recent literature. Two reports addressed the hazards of sandblasting. One report compared Brazilian sandblasters to Brazilian stone masons. Sandblasters were more likely to have PMF and had more severely impaired exercise tolerance (Lopes et al., 2012). Another report described 32 Turkish men with silicosis due to work as denim sandblasters. Their mean age at diagnosis was 31.5 years. They had worked as denim sandblasters for a mean of 66.4 h per week for a median 28.5 months. At diagnosis, 11 had PMF and over the period of follow up, 6 died from PMF (Bakan et al., 2011).
Silicosis, including PMF, in artificial (engineered) stone workers is an emerging international issue. Artificial stone is an engineered product that contains a high percentage of crushed quartz bound together with polymeric resins and pigments (León-Jiménez et al., 2021). Respirable dust can be generated from artificial stone by actions such as grinding, cutting, and drilling the material. An Israeli case series described 25 artificial stone workers with silicosis who were referred for lung transplantation evaluation from January 1997 to December 2010 (Kramer et al., 2012). All had engaged in dry cutting of artificial stone. Two had PMF. Ten underwent lung transplantation and three died without lung transplantation. An Australian case series describes seven cases in workers who did dry cutting of artificial stone in small kitchen and bathroom benchtop fabrication businesses (Hoy et al., 2018). Six of seven patients had PMF. The median duration of exposure prior to symptoms was seven years. One report compared artificial stone silicosis (18 patients) to natural stone-associated silicosis (63 patients) in Chinese workers who developed silicosis (Wu, Xue, Yu, & Ye, 2020). A high proportion of those with artificial stone-associated silicosis had PMF (14/18 patients). Artificial stone-associated silicosis had shorter latency from onset of exposure to silicosis (6.4 vs. 29.3 years), more rapid radiological progression, accelerated decline in lung function and high mortality (27.8% vs. 0.0% during a 6 to 12 month period of follow up).
A multinational registry of 169 artificial stone workers with silicosis from Israel, Spain, Australia, and the United States provides much information about the clinical features of silicosis in this population (Hua et al., 2022). PMF was present in 55 (32.5%) of these workers, with 10 (18.5%) reporting that they had worked in the artificial stone industry for 10 years or less. Among 154 artificial stone workers with data available, 33 (21.4%) were diagnosed with an autoimmune disease or had abnormal autoimmune serology. Diagnosed autoimmune diseases including rheumatoid arthritis (nine workers) systemic sclerosis (seven workers) and psoriasis/psoriatic arthritis (six workers). It was not reported if presence of PMF was associated with the diagnosis of an autoimmune disease or abnormal autoimmune serology.
Silicosis and PMF were also described in other settings. Respirable crystalline silica exposure causing PMF was described in six stonemasons presenting to two specialist respiratory clinics in Edinburgh, Scotland from 2007 to 2013 (Nicol, McFarlane, Hirani, & Reid, 2015). It is not stated if they were exposed to artificial stone, but all were asymptomatic or minimally symptomatic and identified by radiographic surveillance. Three had already progressed to PMF at the time of referral, showing the importance of dust control and organized health surveillance to identify cases prior to progression to advanced disease. Silicosis and PMF were also identified among workers in Sri Lanka processing quartz for use in products such as microchips (Siribaddana et al., 2016), and in India among sandstone mine workers (Nandi, Dhatrak, & Sarkar, 2021) and stone crushing workers (Govindagoudar et al., 2021).
A report from the United States describes all cases of silicosis reported to the state of Michigan from 1988 to 2016 (Reilly, Timmer, & Rosenman, 2018). A total of 1048 cases were reported. Numbers of reported cases declined to 136 during the period 2008–2016. The primary industries where cases were employed included manufacturing (874 cases), construction (98 cases) and mining (47 cases). Within manufacturing, 706 cases worked in foundries. The cases identified were severe. 22% had PMF and 38% had small opacity profusion 2 or 3. African American men aged 40 and older had a 5-fold greater cumulative incidence rate than white men.
One report described a series of six nonoccupational cases of anthracosis and silicosis (anthracosilicosis) in Kashmir, India (Spalgais, Gothi, Jaiswal, & Gupta, 2015). All the patients were women and were homemakers with an average age of 56 years. On average, they had been symptomatic for four years. Chest computed tomography (CT) documented PMF and calcified mediastinal lymph nodes in four of six cases. Bronchoscopy documented anthracosis with narrowing and distortion of the bronchus in five of six cases. Silicosis was attributed to regional dust storms with high levels of silica and particles small enough to be inhaled. Anthracosis was attributed large amounts of time spent in poorly ventilated indoor spaces where biomass burning was used for heating and cooking.
A report sought to examine the relationship between CWP, including with PMF, and risk for incident lung cancer in Czech coal miners (Tomaskova, Jirak, Splichalova, & Urban, 2012). It found that compared to the general male population of the Czech Republic, risk for incident lung cancer was approximately doubled in miners with CWP and was approximately quadrupled in the 95 miners with PMF (nine cases observed; 2.1 expected). There was no increased risk for lung cancer in ex-miners without CWP. The study’s findings are weakened by issues including lack of information about smoking in 51% of the miners with lung cancer and so do not establish a causal relationship between coal mining and lung cancer.
Several themes emerge from recently published epidemiological studies. Respirable crystalline silica has played an important role in the mixed dust pneumoconiosis including PMF that has emerged over the past several decades among coal miners in the United States. Artificial stone work is an important internationally emerging source of crystalline silica exposure, silicosis, and PMF, and workers can present with advanced disease after less than ten years of exposure. In addition, silica exposure and PMF continue to occur in traditionally recognized settings such as sandblasting, foundry work, and stone crushing operations.
5. PMF and pulmonary function
Recent literature has evaluated the association of PMF with various measures of pulmonary function. Several have examined this relationship in coal miners. Wang et al. evaluated relationships between chest x-ray and spirometry findings in U.S. coal miners (Wang, Beeckman-Wagner BeeckmanWagner, Wolfe, Syamlal, & Petsonk, 2013). Based on multivariate analyses adjusted for age, body mass index, underground tenure, and cigarette smoking, they found that coal miners with simple CWP had a 1.8-fold greater risk of suffering abnormal lung function than miners without CWP and that miners with PMF had a 3.7-fold greater risk of lung-function impairment than miners without PMF. Kurth et al. described the association between presence of PMF and airflow obstruction in never-smoking coal miners (Kurth, Laney, Blackley, & Halldin, 2020). Prevalence of airflow obstruction among never-smoking coal miners including those without radiographic evidence of pneumoconiosis was 7.7%, among those with evidence of pneumoconiosis 16.4%, and among those with large opacities consistent with PMF 32.3%.
Several reports have evaluated pulmonary function in those with PMF due to respirable crystalline silica. Rosenman et al. evaluated spirometry findings relative to chest x-ray findings among 526 individuals with silicosis listed in the Michigan state silicosis registry (Rosenman, Reilly, & Gardiner, 2010). In multivariate analyses controlling for age, duration of silica exposure, and cigarette-smoking status, odds ratios for mixed obstruction/restriction and any spirometry abnormality were significantly higher in those with PMF compared to those with small opacity profusion category one.
Grubstein et al. evaluated relationships between chest CT findings and pulmonary function test results in marble workers exposed to artificial stone dust and diagnosed as having silicosis (Grubstein et al., 2016). A chest CT score based on presence and severity of nodular profusion and PMF was calculated. Statistically significant inverse relationships were found between chest CT score and various pulmonary function results including forced expired volume in one second (FEV1) (r = −0.54, P < 0.0001), total lung capacity (r = −0.4, P < 0.0001), and single breath diffusion capacity for carbon monoxide (DLCO)(r = −0.6, P < 0.0001). Sari et al. evaluated relationships between CT findings and spirometry in 90 patients presenting with PMF to an occupational disease clinic in Turkey (Sarı, Gökçek, Koyuncu, & Şimşek, 2022). Thirty-five of the patients were coal miners, and the rest were in occupations associated with silica exposure. Increasing PMF size was significantly associated with decreases in FEV1 and forced vital capacity (FVC).
Overall, recently published literature continues to indicate that presence of PMF is associated with worsening of pulmonary function as measured by spirometry relative to pneumoconiosis of lesser severity. Recently published papers identified in this review did not specifically address the impact specifically of PMF on other types of pulmonary function tests, although diffusing capacity for carbon monoxide worsened with worsening CT score in individuals with silicosis (Grubstein et al., 2016).
6. Imaging of PMF
Recent literature addresses various aspects of chest imaging in relation to PMF. Reports addressed the radiographic appearance of PMF imaged using chest x-rays and chest CT. They also addressed the ability to differentiate between PMF and lung cancer using fluorodeoxyglucose (FDG) positron emission tomography (PET) and magnetic resonance imaging (MRI).
As background, it is important to be aware of the International Labour Office (ILO) system for classifying findings of pneumoconiosis in chest-rays (ILO, 2011). The ILO classification system is used widely and provides a standardized approach to describing the presence and severity of findings of pneumoconiosis relative to appearances on standard films provided by ILO. First, film quality is graded. Then, the presence and frequency (profusion) of small opacities is graded. Small opacities are described based on their shape and size. Small round opacities are described as p (up to 1.5 mm diameter), q (1.5–3 mm diameter), or r (3–10 mm diameter). Irregular small opacities are classified by width as s, t, or u, using the same width thresholds as for small rounded opacities. Profusion of small opacities is expressed on a four-point major category scale ranging from zero to three. Each major profusion category has three minor categories, expressed as two numbers with a slash between them, resulting in a 12-point scale ranging from 0/− to 3/+. Large opacities such as those defining PMF are defined as any opacity greater than one cm. These are classified as category A (one or more large opacities with a combined dimension not exceeding five cm), category B (one or more large opacities with a combined dimension exceeding five cm but not exceeding the area of the right upper lung zone), or category C (one or more large opacities with a combined dimension exceeding the area of the right upper lung zone). Pleural abnormalities are also classified. Other abnormal features of the chest radiograph can be noted on a check list and comments can be provided. A classification system for chest CT scans paralleling the ILO classification system for chest x-rays has also been developed. It is called the International Classification of High-Resolution Computed Tomography for Occupational and Environmental Respiratory Diseases (Suganuma et al., 2009).
Several recent reports describe the appearances of PMF in radiographic chest imaging. Halldin et al. described the appearances of PMF on chest radiographs obtained in coal miners participating in periodic health surveillance in the United States from 2000 to 2015 (Halldin, Blackley, Markle, Cohen, & Laney, 2020). Two hundred four radiographs showing PMF were identified. Large opacities were noted primarily in the right upper lung zone (41%) and left upper lung zone (28%). However, 31% were in middle or lower lung zones. Unilateral large opacities were described in 34%, of which 82% were on the right. The median small opacity profusion category for radiographs with PMF according to the ILO system was 2/1.
Sari et al. described CT findings in 90 patients presenting with PMF to an occupational disease clinic in Turkey (Sarı et al., 2022). PMF was identified bilaterally in 90% of cases, with 88.5% of the unilateral lesions in the right upper lobe. In 95.6% of cases, PMF was solely in the upper lobes. Only 4.4% of cases had PMF lesions located in the middle and lower lobes. Potential reasons offered to explain upper lobe distribution were increased particle deposition and lesser lymphatic drainage. Of 171 PMF lesions, shape was irregular in 78.3%, spherical in 15.2% and discoid in 6.4%. Enlarged lymph nodes were found in 83.3% of cases and lymph node calcification in 63% of cases. In 86% of cases, band structures extended between the PMF lesion and adjacent pleura. In 80% of cases, invagination in the adjacent lung parenchyma was observed. ILO classification of chest x-rays identified a background of small opacities in all 90 cases. Predominant shapes of small opacities were q (43.3%) and r (40%).
Jones et al. described silicosis in Australian artificial stone workers (Jones, Pasricha, Heinze, & Mac Donald, 2020). Features of accelerated disease, including ground-glass opacities and PMF, were prominent radiographic features. Takahashi et al. described CT findings in arcwelder’s pneumoconiosis vs. silicosis (Takahashi et al., 2018). Well-defined rounded opacities (p < 0.0001), PMF (p < 0.0001), and mediastinal lymphadenopathy (p < 0.0001) were more common in silicosis. CT identified more cases with large opacities than chest x-ray. Among 33 silicosis cases, large opacities were identified in 14 by CT and in 6 by chest-ray. Large opacities were also identified in 4 of 66 patients with arc welders’ pneumoconiosis by CT and in 3 of 66 by chest x-ray. Another study described in detail below in the section below on progression to PMF also showed greater sensitivity of CT for detection of PMF (León-Jiménez et al., 2020). Ozmen et al. described chest CT findings in denim sandblasters (Ozmen et al., 2010). Nodules were present in all cases with centrilobular type as the commonest (63.6%). PMF was present in 11.4% of cases and nodular profusion score correlated with the presence of PMF.
Friedman et al. found associations between those who employed physicians to classify x-rays for presence and severity of pneumoconiosis for compensation proceedings and the physicians’ findings of PMF (Friedman, De, Almberg, & Cohen, 2021). There were statistically significant differences, with those hired by defendants less likely and those hired by claimants more likely to classify PMF. Thus, a limitation to determination of PMF in contested proceedings is the potential for introduction of bias, perhaps because adversaries hire experts who are likely to support their adversarial positions.
Two papers described the utility of FDG/PET in differentiating between PMF and lung cancer. Choi et al. evaluated various metrics from FDG/PET and CT in making this differentiation (Choi, Park, Yoo, Kim, & Kim, 2020). The FDG/PET metric was maximum standardized uptake value (SUVmax) and the CT metrics evaluated included parameters such as size, shape and density. Masses identified to be due to lung cancer had higher SUVmax, longer short-axis diameter, smaller ratio of long to short axis diameter, and lower density (measured as Hounsfield units). The authors concluded that assessment of FDG/PET and CT features of masses may have a role in differentiating lung cancer and PMF in those with pneumoconiosis. In contrast, Chung et al. found that FDG/PET identified PMF lesions as hypermetabolic and thus might have only a limited role in the diagnosis of PMF, particularly if lung cancer or granulomatous disease were diagnostic considerations (Chung, Lee, Kim, Kim, & Kim, 2010).
Four papers addressed the potential role of MRI in evaluating PMF. Hekimoğlu et al. compared volumetric interpolated breath-hold examination (VIBE) and half-Fourier-acquisition single-shot turbo spinecho (HASTE) fast MRI to chest CT (Hekimoğlu et al., 2010). They found that a contrast-enhanced VIBE protocol provided the best imaging of PMF as assessed by semi-quantitative rating of image quality and presence of artifacts by 5 radiologists and could be an alternative to CT. Zhang et al. described the potential clinical utility of MRI in differentiating PMF from lung cancer (Zhang et al., 2017). The authors concluded that PMF lesions of pneumoconiosis can be differentiated from lung cancer tumors by evaluating T2-weighted images and spectral presaturation with inversion recovery (fat suppression) images (SPIR). T2-weighted images and SPIR showed slightly low, equal, and uneven equal-low signals relative to chest wall muscle in 97.4% and 94.9% of PMF cases, respectively. In contrast, for lung cancer, high signals on T2-weighted images and SPIR relative to chest wall muscle occurred in 95.2% of cases. The authors concluded that MRI was potentially useful for identifying PMF. Ogihara et al. found that T2-weighted images differentiated between PMF and lung cancer, with low signal intensity in PMF and high signal intensity in lung cancer (Ogihara et al., 2018). The investigators concluded that when differentiation between PMF and lung cancer is difficult by chest CT, MRI with T2-weighted images might help to distinguish between the two. Guneyli et al. found that several MRI-related parameters distinguished between PMF and lung cancer (Guneyli et al., 2021). On T1-weighted spin-echo, T2-weighted spin-echo, and single-shot turbo spin echo most PMF lesions were predominantly iso- or hypointense. However, most malignant lesions predominantly showed high signal intensity.
Overall, recent literature continues to advance the understanding of chest imaging in PMF. Large opacities primarily occur in the upper lobes, with a greater upper lobe predominance reported in a study using CT imaging (Sarı et al., 2022) than in a study using chest x-rays (Halldin et al., 2020). Bilateral PMF was identified in 90% of cases by CT (Sarı et al., 2022) and in 66% of cases by chest x-ray (Halldin et al., 2020). Perhaps these differences occurred because CT is more sensitive in detecting PMF than chest x-ray (León-Jiménez et al., 2020; Takahashi et al., 2018). Unilateral PMF occurs most frequently in the right upper lobe and PMF is generally accompanied by concurrent small opacities. CT demonstrates that PMF often involves adjacent pleura (Sarı et al., 2022). Pure PMF appears as hypermetabolic on FDG/PET imaging, which cannot differentiate on its own between PMF, cancer, and granulomatous inflammation such as from tuberculosis. MRI shows promise in identifying PMF and in differentiating it from lung cancer.
7. Progression to PMF
Several recently published papers have sought to describe the frequency and risk factors for radiographic progression to PMF in CWP and silicosis. A study examined progression from no radiographic findings of pneumoconiosis or findings of small opacity pneumoconiosis to PMF in coal miners who had filed as claimants for black lung benefits in the United States (Almberg, Friedman, Rose, Go, & Cohen, 2020). Findings were based on chest x-rays classified using the standardized ILO classification system. Out of 37,548 coal miners identified as claimants from 2000 to 2013, 3351 had records of chest x-rays at least two years apart. At the time of last chest x-ray, 18/1786 (1%) who were Category 0 at baseline (no pneumoconiosis) progressed to PMF; and of those with small opacities at baseline, 54/1384 (3.9%) of Category 1, 29/158 (18.4%) of Category 2, and 10/23 (43.5%) of Category 3 progressed to PMF. Of the 111 miners who progressed to PMF, 52 (46.8%) did so in 5 years or less and 19 (17.1%) did so in 2 years. Younger age at time of first chest x-ray obtained for claims and higher small opacity profusion on first chest x-ray were significantly associated with progression to PMF.
Another study examining progression to PMF in coal miners used data from a national program of health surveillance offering periodic chest x-rays classified according to the ILO system to active coal miners in the United States (Laney, Blackley, & Halldin, 2017). Participation in the program is voluntary and many miners do not have chest x-rays at the planned interval of about every 5 years. However, the investigators identified 192 miners with PMF first identified between January 2000 and October 2016 who had at least one prior radiograph. 84% had worked in Kentucky, West Virginia, or Virginia and 88% had worked only underground. Mean time to progress from a normal radiograph to PMF in the program was 20.7 years and 17% progressed from normal radiograph to PMF in <10 years. The interval to documentation of progression was shorter in those who had more surveillance chest radiographs during their careers.
One report addressed progression to PMF in silicosis among 106 Spanish artificial stone workers diagnosed from 2009 to 2018 with subsequent clinical follow up (León-Jiménez et al., 2020). Data are reported for both chest x-rays classified using the ILO system and chest CT scans classified using the standardized International Classification of High Resolution Computed Tomography for Occupational and Environmental Respiratory Diseases system. All patients stopped working in the stone industry after they were diagnosed. At diagnosis, evaluation with chest x-ray identified 82/106 (77.4%) with small opacity disease and 5/106 (4.7%) with PMF. After a mean follow-up of 4 years, the number with PMF had increased to 31, despite 6 patients being lost to follow up. In contrast, evaluation at the time of diagnosis with chest CT identified more patients with small opacity disease, 99/106 (93.4%), and more with PMF, 7/106 (6.6%). After a mean period of follow-up of 4 years, despite removal from additional respirable crystalline silica exposure, the number of workers with PMF identified by chest CT had increased to 40/106 (37.7%). In multivariate analysis, lower baseline FVC (p < 0.01) and longer duration of working with artificial stone (p = 0.06) were associated with increased risk for progression to PMF.
These recently published papers provide much useful information about risk factors for progression to PMF. Presence of small opacities is an important risk factor for progression to PMF, with risk increasing at higher small opacity profusion (Almberg et al., 2020). Among coal miners in the United States, working in the central Appalachian region where exposure to crystalline silica has been implicated as an important in causing pneumoconiosis and presenting for compensation at a younger age which may be a marker for heavier exposures to coal mine dust are risk factors for progressing to PMF (Almberg et al., 2020; Laney et al., 2017). Among artificial stone workers with silicosis, lower baseline FEV1 and longer duration of exposure were risk factors for progression to PMF (León-Jiménez et al., 2020).
8. Conclusion
Severe pneumoconiosis with PMF continues to be an important problem throughout the world. The reports identified in this overview of the literature provide advances in understanding the pathophysiology of PMF, epidemiology of the occurrence of PMF and related conditions, the impact of PMF on pulmonary function, advances in imaging of PMF, and factors affecting progression of pneumoconiosis in dust-exposed workers to PMF.
Recent literature on the pathophysiology of PMF supports the importance of crystalline silica as a cause of PMF in contemporary coal miners from the United States and in silica-exposed artificial stone workers. In addition, the presence of PMF is associated with blood markers of inflammation and injury with the potential to be useful as biomarkers of disease. There has been relatively little recent progress in identifying basic mechanisms specific to progression from small opacity/nodular CWP and silicosis to PMF. The lack of a generally accepted rodent model for PMF that parallels the features of human disease is an important barrier to progress. This is also an important barrier to pre-clinical identification and testing of therapeutic agents aimed at preventing progression of CWP and silicosis to PMF.
Recently published epidemiological studies also support the role of respirable crystalline silica as a component of coal mine dust that causes a mixed dust pneumoconiosis including PMF that has appeared in recent decades as an emerging disease among coal miners in the United States. Recent epidemiological studies have also documented silicosis in artificial stone workers as an important emerging cause of PMF, with some workers presenting with advanced disease after considerably <10 years of exposure. In addition, silica exposure and PMF continue to occur in traditionally recognized settings such as sandblasting, foundry work, and stone crushing operations.
Consistent with older studies, recent literature continues to show that development of PMF is associated with a substantial decline in pulmonary function relative to no disease or small opacity pneumoconiosis. Older studies also show that development of PMF is associated with increased morbidity and mortality (Attfield et al., 2022).
In recent reports, computed tomography has had greater sensitivity for detecting PMF than chest x-ray. Recent studies show that PMF occurs with an upper zone predominance and most often occurs bilaterally, although it can occur in the mid or lower lung zones and unilaterally. Unilateral PMF occurs predominantly in the right upper zone. By CT, PMF often involves the pleura. Magnetic resonance imaging shows promise in differentiating between PMF and lung cancer. On its own, FDG/PET cannot make this differentiation.
PMF can develop in dust-exposed workers without previously identified small opacity pneumoconiosis. However, the presence of small opacity pneumoconiosis increases the risk for progression to PMF, as does heavier dust exposure. Recent literature does not document any effective new treatments to prevent PMF in dust-exposed workers and new therapies to prevent PMF in high-risk individuals and to treat it once present are important needs.
Acknowledgments
The author thanks Ms. Kathy Boyce for her excellent help in obtaining references for review. This review was funded as part of the author’s official duties as a U.S. government employee.
Footnotes
Declaration of Competing Interest
The author is a full time employee of the U.S. government and declares that there are no conflicts of interest.
Disclaimer
The findings and conclusions in this report are those of the author (s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
References
- Adamcakova J, & Mokra D (2021). New insights into Pathomechanisms and treatment possibilities for lung silicosis. International Journal of Molecular Sciences 22(8), 4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almberg KS, Friedman LS, Rose CS, Go LH, & Cohen RA (2020). Progression of coal workers’ pneumoconiosis absent further exposure. Occupational and Environmental Medicine 77(11), 748–751. [DOI] [PubMed] [Google Scholar]
- Almberg KS, Halldin CN, Blackley DJ, Laney AS, Storey E, Rose CS, … Cohen RA (2018). Progressive massive fibrosis resurgence identified in US coal miners filing for black lung benefits, 1970–2016. Annals of the American Thoracic Society 15(12), 1420–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attfield M, Castranova V, Hale JM, Suarthana E, Thomas KC, & Wang ML (2022). Coal mine dust exposures and associated health outcomes; a review of information published since 1995. DHHS (NIOSH) Publication No. 2011–172. [Google Scholar]
- Bakan ND, Özkan G, Çamsari G, Gür A, Bayram M, Açikmeşe B, & Çetinkaya E (2011). Silicosis in denim sandblasters. Chest 140(5), 1300–1304. [DOI] [PubMed] [Google Scholar]
- Blackley DJ, Crum JB, Halldin CN, Storey E, & Laney AS (2016). Resurgence of progressive massive fibrosis in coal miners—Eastern Kentucky, 2016. Morbidity and Mortality Weekly Report 65(49), 1385–1389. [DOI] [PubMed] [Google Scholar]
- Blackley DJ, Halldin CN, & Laney AS (2018). Continued increase in prevalence of coal workers’ pneumoconiosis in the United States, 1970–2017. American Journal of Public Health 108(9), 1220–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackley DJ, Halldin CN, Wang ML, & Laney AS (2014). Small mine size is associated with lung function abnormality and pneumoconiosis among underground coal miners in Kentucky, Virginia and West Virginia. Occupational and Environmental Medicine 71(10), 690–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackley DJ, Reynolds LE, Short C, Carson R, Storey E, Halldin CN, & Laney AS (2018). Progressive massive fibrosis in coal miners from 3 clinics in Virginia. JAMA 319(5), 500–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castranova V, & Vallyathan V (2000). Silicosis and coal workers’ pneumoconiosis. Environmental Health Perspectives 108(Suppl. 4), 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EK, Park HL, Yoo IR, Kim SJ, & Kim YK (2020). The clinical value of F-18 FDG PET/CT in differentiating malignant from benign lesions in pneumoconiosis patients. European Radiology 30(1), 442–451. [DOI] [PubMed] [Google Scholar]
- Chung SY, Lee JH, Kim TH, Kim SJ, & Kim HJ (2010). Ryu YH. 18 F-FDG PET imaging of progressive massive fibrosis. Annals of Nuclear Medicine 24(1), 21–27. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Petsonk EL, Rose C, Young B, Regier M, Najmuddin A, … Green FH (2016). Lung pathology in US coal workers with rapidly progressive pneumoconiosis implicates silica and silicates. American Journal of Respiratory and Critical Care Medicine 193(6), 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Rose CS, Go LHT, Zell-Baran LM, Almberg KS, Sarver EA, … Green FHY (2022). Pathology and mineralogy demonstrate respirable crystalline silica is a major cause of severe pneumoconiosis in US coal miners. Annals of the American Thoracic Society. 10.1513/AnnalsATS.202109-1064OC Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado E, Sánchez M, Ramírez J, Arguis P, de Caralt TM, Perea RJ, & Xaubet A (2010). Pulmonary sarcoidosis: Typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics 30(6), 1567–1586. [DOI] [PubMed] [Google Scholar]
- Edenfieid RW (1960). A clinical and roentgenological study of kaolin workers. Archives of Environmental Health 1(5), 392–403. [DOI] [PubMed] [Google Scholar]
- Friedman LS, De S, Almberg KS, & Cohen RA (2021). Association between financial conflicts of interest and ILO classifications for black lung disease. Annals of the American Thoracic Society 18(10), 1634–1641. [DOI] [PubMed] [Google Scholar]
- García-Núñez A, Jiménez-Gómez G, Hidalgo-Molina A, Córdoba-Doña JA, León-Jiménez A, & Campos-Caro A (2022). Inflammatory indices obtained from routine blood tests show an inflammatory state associated with disease progression in engineered stone silicosis patients. Scientific Reports 12(1), 8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs AE, Pooley FD, Griffiths DM, Mitha R, Craighead JE, & Ruttner JR (1992). Talc pneumoconiosis: A pathologic and mineralogic study. Human Pathology 23(12), 1344–1354. [DOI] [PubMed] [Google Scholar]
- Govindagoudar MB, Singh PK, Chaudhry D, Chaudhary R, Sachdeva A, Dhankhar S, & Tyagi D (2021). Burden of Silicosis among Stone Crushing Workers in India. Occupational Medicine (Lond) Epub ahead of print34729596. [DOI] [PubMed] [Google Scholar]
- Graber JM, Harris G, Almberg KS, Rose CS, Petsonk EL, & Cohen RA (2017). Increasing severity of pneumoconiosis among younger former US coal miners working exclusively under modern dust-control regulations. Journal of Occupational and Environmental Medicine 59(6), e105–e111. [DOI] [PubMed] [Google Scholar]
- Grant MJ, & Booth A (2009). A typology of reviews: An analysis of 14 review types and associated methodologies. Health Information and Libraries Journal 26(2), 91–108. [DOI] [PubMed] [Google Scholar]
- Green FH, Vallyathan V, & Hahn FF (2007). Comparative pathology of environmental lung disease: An overview. Toxicologic Pathology 35(1), 136–147. [DOI] [PubMed] [Google Scholar]
- Grubstein A, Shtraichman O, Fireman E, Bachar GN, Noach-Ophir N, & Kramer MR (2016). Radiological evaluation of artificial stone silicosis outbreak: Emphasizing findings in lung transplant recipients. Journal of Computer Assisted Tomography 40 (6), 923–927. [DOI] [PubMed] [Google Scholar]
- Guneyli S, Tor M, Hassoy H, Aygun MS, Altinmakas E, Altintas SD, & Savas R (2021). Spin-echo and diffusion-weighted MRI in differentiation between progressive massive fibrosis and lung cancer. Diagnostic and Interventional Radiology 27(4), 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall NB, Halldin CN, Blackley DJ, & Laney AS (2020). Assessment of pneumoconiosis in surface coal miners after implementation of a national radiographic surveillance program, United States, 2014–2019. American Journal of Industrial Medicine 63 (12), 1104–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall NB, Nye MJ, Blackley DJ, Scott Laney A, Mazurek JM, & Halldin CN (2022). Respiratory health of American Indian and Alaska native coal miners participating in the coal Workers’ health surveillance program, 2014–2019. American Journal of Industrial Medicine 65(3), 162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldin CN, Blackley DJ, Markle T, Cohen RA, & Laney AS (2020). Patterns of progressive massive fibrosis on modern coal miner chest radiographs. Archives of Environmental & Occupational Health 75(3), 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldin CN, Reed WR, Joy MG, Colinet MJ, Rider MJ, Petsonk EL, … Laney AS (2015). Debilitating lung disease among surface coal miners with no underground mining tenure. Journal of Occupational and Environmental Medicine 57(1), 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RF Jr., Thakur SA, & Holian A (2008). Silica binding and toxicity in alveolar macrophages. Free Radical Biology & Medicine 44(7), 1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekimoğlu K, Sancak T, Tor M, Beşir H, Kalaycıoğlu B, & Gündoğdu S (2010). Fast MRI evaluation of pulmonary progressive massive fibrosis with VIBE and HASTE sequences: Comparison with CT. Diagnostic and Interventional Radiology 16(1), 30–37. [DOI] [PubMed] [Google Scholar]
- Hoy RF, Baird T, Hammerschlag G, Hart D, Johnson AR, King P, … Yates DH (2018). Artificial stone-associated silicosis: A rapidly emerging occupational lung disease. Occupational and Environmental Medicine 75(1), 3–5. [DOI] [PubMed] [Google Scholar]
- Hua JT, Zell-Baran L, Go LHT, Kramer MR, Van Bree JB, Chambers D, … Rose CS (2022). Demographic, exposure and clinical characteristics in a multinational registry of engineered stone workers with silicosis. Occupational and Environmental Medicine. 10.1136/oemed-2021-108190 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILO (2011). Guidelines for the Use of the ILO International Classification of Radiographs of Pneumoconioses. Revised edition 2011Geneva: International Labour Office; Available at https://www.ilo.org/global/topics/safety-and-health-at-work/resources-library/publications/WCMS_168260/lang-en/index.htm. [Google Scholar]
- Jones CM, Pasricha SS, Heinze SB, & Mac Donald S (2020). Silicosis in artificial stone workers: spectrum of radiological high-resolution CT chest findings. Journal of Medical Imaging and Radiation Oncology 64(2), 241–249. [DOI] [PubMed] [Google Scholar]
- Komai M, Mihira K, Shimada A, Miyamoto I, Ogihara K, Naya Y, … Takano H (2019). Pathological study on epithelial-mesenchymal transition in silicotic lung lesions in rat. Veterinary Sciences 6(3), 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MR, Blanc PD, Fireman E, Amital A, Guber A, Rhahman NA, & Shitrit D (2012). Artificial stone silicosis: Disease resurgence among artificial stone workers. Chest 142(2), 419–424. [DOI] [PubMed] [Google Scholar]
- Kurth L, Laney AS, Blackley DJ, & Halldin CN (2020). Prevalence of spirometry-defined airflow obstruction in never-smoking working US coal miners by pneumoconiosis status. Occupational and Environmental Medicine 77(4), 265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laney AS, & Attfield MD (2010). Coal workers’ pneumoconiosis and progressive massive fibrosis are increasingly more prevalent among workers in small underground coal mines in the United States. Occupational and Environmental Medicine 67(6), 428–431. [DOI] [PubMed] [Google Scholar]
- Laney AS, Blackley DJ, & Halldin CN (2017). Radiographic disease progression in contemporary US coal miners with progressive massive fibrosis. Occupational and Environmental Medicine 74(7), 517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laney AS, & Petsonk EL (2012). Small pneumoconiotic opacities on U.S. coal worker surveillance chest radiographs are not predominantly in the upper lung zones. American Journal of Industrial Medicine 55(9), 793–798. [DOI] [PubMed] [Google Scholar]
- Laney AS, Petsonk EL, & Attfield MD (2010). Pneumoconiosis among underground bituminous coal miners in the United States: Is silicosis becoming more frequent? Occupational and Environmental Medicine 67(10), 652–656. [DOI] [PubMed] [Google Scholar]
- Laney AS, Petsonk EL, Hale JM, Wolfe AL, & Attfield MD (2012). Potential determinants of coal workers’ pneumoconiosis, advanced pneumoconiosis, and progressive massive fibrosis among underground coal miners in the United States, 2005–2009. American Journal of Public Health 102(S2), S279–S283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laney AS, & Weissman DN (2014). Respiratory diseases caused by coal mine dust. Journal of Occupational and Environmental Medicine 56(010), S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laney AS, Wolfe AL, Petsonk EL, & Halldin CN (2012). Pneumoconiosis and advanced occupational lung disease among surface coal miners–16 states, 2010–2011. MMWR. Morbidity and Mortality Weekly Report 61(23), 431–434. [PubMed] [Google Scholar]
- Lee JS, Shin JH, & Choi BS (2015). Serum levels of IL-8 and ICAM-1 as biomarkers for progressive massive fibrosis in coal workers’ pneumoconiosis. Journal of Korean Medical Science 30(2), 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Jiménez A, Hidalgo-Molina A, Conde-Sánchez MÁ, Pérez-Alonso A, Morales-Morales JM, García-Gámez EM, & Córdoba-Doña JA (2020). Artificial stone silicosis: Rapid progression following exposure cessation. Chest 158(3), 1060–1068. [DOI] [PubMed] [Google Scholar]
- León-Jiménez A, Mánuel JM, García-Rojo M, Pintado-Herrera MG, López-López JA, Hidalgo-Molina A, … Morales FM (2021). Compositional and structural analysis of engineered stones and inorganic particles in silicotic nodules of exposed workers. Particle and Fibre Toxicology 18(1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes AJ, Costa W, Mafort TT, de Sá FA, de Menezes SS, & Guimarães FS (2012). Silicosis in sandblasters of shipyard versus silicosis in stone carvers in Brazil: A comparison of imaging findings, lung function variables and cardiopulmonary exercise testing parameters. Revista Portuguesa de Pneumologia (English Edition) 18(6), 260–266. [DOI] [PubMed] [Google Scholar]
- Nandi SS, Dhatrak SV, & Sarkar K (2021). Silicosis, progressive massive fibrosis and silico-tuberculosis among workers with occupational exposure to silica dusts in sandstone mines of Rajasthan state: an urgent need for initiating national silicosis control programme in India. Journal of Family Medicine and Primary Care 10(2), 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TP, & Chan SL (1991). Factors associated with massive fibrosis in silicosis. Thorax 46(4), 229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol LM, McFarlane PA, Hirani N, & Reid PT (2015). Six cases of silicosis: Implications for health surveillance of stonemasons. Occupational Medicine 65(3), 220–225. [DOI] [PubMed] [Google Scholar]
- Ogihara Y, Ashizawa K, Hayashi H, Nagayasu T, Hayashi T, Honda S, & Uetani M (2018). Progressive massive fibrosis in patients with pneumoconiosis: Utility of MRI in differentiating from lung cancer. Acta Radiologica 59(1), 72–80. [DOI] [PubMed] [Google Scholar]
- Ozmen CA, Nazaroglu H, Yildiz T, Bayrak AH, Senturk S, Ates G, & Akyildiz L (2010). MDCT findings of denim-sandblasting-induced silicosis: A cross-sectional study. Environmental Health 9(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters PM, Eurlings IM, Perkins TN, Wouters EF, Schins RP, Borm PJ, … Albrecht C (2014). Silica-induced NLRP3 inflammasome activation in vitro and in rat lungs. Particle and Fibre Toxicology 11(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsonk EL, Rose C, & Cohen R (2013). Coal mine dust lung disease. New lessons from an old exposure. American Journal of Respiratory and Critical Care Medicine 187(11), 1178–1185. [DOI] [PubMed] [Google Scholar]
- Reilly MJ, Timmer SJ, & Rosenman KD (2018). The burden of silicosis in Michigan: 1988–2016. Annals of the American Thoracic Society 15(12), 1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LE, Blackley DJ, Colinet JF, Potts JD, Storey E, Short C, … Halldin CN (2018). Work practices and respiratory health status of Appalachian coal miners with progressive massive fibrosis. Journal of Occupational and Environmental Medicine 60(11), Article e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenman KD, Reilly MJ, & Gardiner J (2010). Results of spirometry among individuals in a silicosis registry. Journal of Occupational and Environmental Medicine 52(12), 1173–1178. [DOI] [PubMed] [Google Scholar]
- Sarı G, Gökçek A, Koyuncu A, & Şimşek C (2022). Computed tomography findings in progressive massive fibrosis: analyses of 90 cases. La Medicina del Lavoro 113(1), Article e2022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayan M, & Mossman BT (2015). The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Particle and Fibre Toxicology 13(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siribaddana AD, Wickramasekera K, Palipana WM, Peiris MD, Upul BK, Senevirathna KP, & Dassanayake DL (2016). A study on silicosis among employees of a silica processing factory in the Central Province of Sri Lanka. The Ceylon Medical Journal 61(1), 6–10. [DOI] [PubMed] [Google Scholar]
- Spalgais S, Gothi D, Jaiswal A, & Gupta K (2015). Nonoccupational anthracofibrosis/anthracosilicosis from Ladakh in Jammu and Kashmir, India: A case series. Indian Journal of Occupational and Environmental Medicine 19(3), 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma N, Kusaka Y, Hering KG, Vehmas T, Kraus T, Arakawa H, … Hosoda Y (2009). Reliability of the proposed international classification of high-resolution computed tomography for occupational and environmental respiratory diseases. Journal of Occupational Health 51(3), 210–222. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Nitta N, Kishimoto T, Ohtsuka Y, Honda S, & Ashizawa K (2018). Computed tomography findings of arc-welders’ pneumoconiosis: Comparison with silicosis. European Journal of Radiology 107, 98–104. [DOI] [PubMed] [Google Scholar]
- Tomaskova H, Jirak Z, Splichalova A, & Urban P (2012). Cancer incidence in Czech black coal miners in association with coalworkers’ pneumoconiosis. International Journal of Occupational Medicine and Environmental Health 25(2), 137–144. [DOI] [PubMed] [Google Scholar]
- Vallyathan V, Landsittel DP, Petsonk EL, Kahn J, Parker JE, Osiowy KT, & Green FH (2011). The influence of dust standards on the prevalence and severity of coal worker’s pneumoconiosis at autopsy in the United States of America. Archives of Pathology & Laboratory Medicine 135(12), 1550–1556. [DOI] [PubMed] [Google Scholar]
- Wade WA, Petsonk EL, Young B, & Mogri I (2011). Severe occupational pneumoconiosis among west Virginian coal miners: One hundred thirty-eight cases of progressive massive fibrosis compensated between 2000 and 2009. Chest 139(6), 1458–1462. [DOI] [PubMed] [Google Scholar]
- Wang ML, Beeckman-Wagner LA, Wolfe AL, Syamlal G, & Petsonk EL (2013). Lung-function impairment among US underground coal miners, 2005 to 2009: Geographic patterns and association with coal workers’ pneumoconiosis. Journal of Occupational and Environmental Medicine 55(7), 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Xue C, Yu S, & Ye Q (2020). Artificial stone-associated silicosis in China: A prospective comparison with natural stone-associated silicosis. Respirology 25(5), 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucesoy B, Johnson VJ, Kissling GE, Fluharty K, Kashon ML, Slaven J, … Luster MI (2008). Genetic susceptibility to progressive massive fibrosis in coal miners. The European Respiratory Journal 31(6), 1177–1182. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang C, Yan Q, Zhang T, Han Z, & Jiang G (2017). Diagnostic and clinical application value of magnetic resonance imaging (MRI) for progressive massive fibrosis of coal worker pneumoconiosis. Medicine (Baltimore) 96(20), Article e6890. [DOI] [PMC free article] [PubMed] [Google Scholar]


