Abstract
Background: Tocilizumab is a monoclonal antibody proposed to manage cytokine release syndrome (CRS) associated with severe COVID-19. Previously published reports have shown that tocilizumab may improve the clinical outcomes of critically ill patients admitted to the ICU. However, no precise data about the role of other medical therapeutics concurrently used for COVID-19 on this outcome have been published. Objectives: We aimed to compare the overall outcome of critically ill COVID-19 patients admitted to the ICU who received tocilizumab with the outcome of matched patients who did not receive tocilizumab while controlling for other confounders, including medical therapeutics for critically ill patients admitted to ICUs. Methods: A prospective, observational, multicenter cohort study was conducted among critically ill COVID-19 patients admitted to the ICU of 14 hospitals in Saudi Arabia between 1 March 2020, and October 31, 2020. Propensity-score matching was utilized to compare patients who received tocilizumab to patients who did not. In addition, the log-rank test was used to compare the 28 day hospital survival of patients who received tocilizumab with those who did not. Then, a multivariate logistic regression analysis of the matched groups was performed to evaluate the impact of the remaining concurrent medical therapeutics that could not be excluded via matching 28 day hospital survival rates. The primary outcome measure was patients’ overall 28 day hospital survival, and the secondary outcomes were ICU length of stay and ICU survival to hospital discharge. Results: A total of 1470 unmatched patients were included, of whom 426 received tocilizumab. The total number of propensity-matched patients was 1278. Overall, 28 day hospital survival revealed a significant difference between the unmatched non-tocilizumab group (586; 56.1%) and the tocilizumab group (269; 63.1%) (p-value = 0.016), and this difference increased even more in the propensity-matched analysis between the non-tocilizumab group (466.7; 54.6%) and the tocilizumab group (269; 63.1%) (p-value = 0.005). The matching model successfully matched the two groups’ common medical therapeutics used to treat COVID-19. Two medical therapeutics remained significantly different, favoring the tocilizumab group. A multivariate logistic regression was performed for the 28 day hospital survival in the propensity-matched patients. It showed that neither steroids (OR: 1.07 (95% CI: 0.75–1.53)) (p = 0.697) nor favipiravir (OR: 1.08 (95% CI: 0.61–1.9)) (p = 0.799) remained as a predictor for an increase in 28 day survival. Conclusion: The tocilizumab treatment in critically ill COVID-19 patients admitted to the ICU improved the overall 28 day hospital survival, which might not be influenced by the concurrent use of other COVID-19 medical therapeutics, although further research is needed to confirm this.
Keywords: tocilizumab, COVID-19, outcome, propensity-matching
1. Introduction
Tocilizumab is a monoclonal antibody directed against the pro-inflammatory cytokine interleukin 6 (IL-6), resulting in the blockage of IL-6 signaling and reduced inflammatory mediators. It has been proposed to manage cytokine release syndrome (CRS) associated with severe coronavirus disease (COVID-19) after being satisfactorily investigated for treatment-related outcomes, including efficacy [1,2]. Previously published reports have shown that tocilizumab may improve the clinical outcomes of critically ill patients admitted to intensive care units (ICUs). However, although the international Randomized Embedded Multifactorial Adaptive Platform for Community Acquired Pneumonia (REMAP-CAP) trial showed favorable outcomes with IL-6 antagonists [3], the COVACTA trial showed no statistically significant difference in clinical outcomes with tocilizumab [4]. Due to these contradictory findings, precise data about the impact of other medical therapeutics concurrently used to treat COVID-19 alongside tocilizumab are unavailable [5,6,7].
Therefore, we aimed to compare the overall outcome of critically ill COVID-19 patients admitted to the ICU who received tocilizumab with the outcome of matched patients who did not receive tocilizumab while controlling for other confounders, including medical therapeutics for critically ill patients admitted to ICUs, using propensity-score matching and other statistical analysis tools.
2. Methods
2.1. Study Design
This was a prospective, observational, multicenter cohort study conducted in 14 hospitals in Saudi Arabia. We included critically ill COVID-19 patients admitted to the ICU between 1 March 2020 and 31 October 2020. The data included in this study were obtained from the Saudi COVID-19 platform [8], and institutional review board (IRB) approval was obtained from the Saudi Ministry of Health’s Central IRB on 20 February, 2020, with the code number [20-80E] and from the ethical boards of each participating center. Informed consent was obtained from all subjects and/or their legal guardian(s). Propensity-matching analysis was utilized to compare patients who received tocilizumab to other patients who did not. In addition, we used multivariable logistic regression on the propensity-matched patients to evaluate these therapeutics’ impact on survival. The study adhered to the Standards for Reporting Diagnostic Accuracy Studies (STARD) guidelines (http://www.stard-statement.org/ accessed on 3 February 2023), the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines https://www.strobe-statement.org/ accessed on 3 February 2023), and the Helsinki Declaration of 1975.
2.2. Settings
The participating ICUs were located in accredited tertiary hospitals. The multidisciplinary treatment team adhered to Saudi Arabia’s Ministry of Health’s (and other internationally published) protocols and guidelines [9]. In addition, during the study period, non-ICU physicians joined the critical care team under the supervision of intensivists after receiving basic ICU management training.
2.3. Patients
2.3.1. Inclusion Criteria
All critically ill COVID-19 patients admitted to the ICU between 1 March 2020, and 31 October 2020 were included in the study. In each included patient, COVID-19 infection was confirmed by detecting SARS-CoV-2 using real-time polymerase chain reaction (RT-PCR) using nasopharyngeal swabs or tracheal aspirate specimens;
All included patients were adults (aged ≥ 18 years).
2.3.2. Exclusion Criteria
Patients with a Do-Not-Resuscitate (DNR) order on file;
Patients with a diagnosed active, intractable terminal malignancy;
Bedbound patients who were diagnosed as being in a vegetative state not conducive to treatment;
Patients who were adults <18 years old or children.
2.4. Data Collection
The data were collected manually in the CRF and entered into the electronic database Research Electronic Data Capture (REDCap, Vanderbilt University, Nashville, TN, USA) [10]. Next, it was validated using secondary sources. The gathered data included patient demographics, comorbidities, signs and symptoms of COVID-19 illness, laboratory abnormalities, mechanical ventilator (MV) utilization, supplementary therapies, drugs, complications, and outcomes. The arterial oxygen partial pressure to fractional inspired oxygen ratio (PaO2:FiO2) was calculated for each spontaneously breathing patient by converting O2 flow to an estimated FiO2 [11]. An immunocompromised state was defined as solid organ malignancy, leukemia, current steroid use (prednisone >7 mg daily for >2 weeks), post-organ transplantation, or rheumatological disease with immunomodulator treatment (such as azathioprine, methotrexate, infliximab, mycophenolate mofetil, or others). Infection was defined as a positive culture in the blood or tracheal aspirate in compliance with the 2018 Declaration of Helsinki.
2.5. Outcomes
2.5.1. The Primary Outcome
The overall 28 day hospital survival of patients who received tocilizumab versus matched patients who did not receive tocilizumab.
2.5.2. The Secondary Outcomes
The secondary outcomes include the length of ICU stay and survival to ICU discharge.
3. Statistical Analysis
Continuous variables were calculated as medians with interquartile ranges (IQR) of 25–75%, and categorical variables were calculated as frequencies and percentages. Demographics, baseline clinical features, co-interventions, and outcome variables were compared between patients who received tocilizumab in the ICU and those who did not. Student’s t-tests or Wilcoxon rank-sum tests were used for continuous variables. For categorical variables, the chi-square test or the Fisher’s exact test was used. The log-rank test was used to compare the 28 day hospital survival of patients who received tocilizumab with those who did not.
Propensity-score matching was used in a systematic, stepwise manner [11,12]. The initial step was to address some variables’ missing values. Based on Rubin’s taxonomy [13], we assumed that missing variables were missing at random (MAR). Multiple imputations were applied using the mice package in R and the classification and regression trees (CART) method with five runs of imputation and five iterations at each run (a total of 25 complete datasets) [14,15]. A new imputed full dataset was extracted and used to calculate the propensity score using the MatchIt package in R [16,17]. The propensity scores for receiving tocilizumab for all patients in our cohort were calculated, and the “full matching” method results in a ratio of 1:2 (matching one patient receiving tocilizumab to two patients with closely similar characteristics who did not receive tocilizumab), with the following confounders: age in years, body mass index (BMI) in kg/m2, gender, diabetes (DM), hypertension (HTN), chronic obstructive pulmonary disease (COPD) or asthma, chronic kidney disease (CKD), need for intubation within the first 48 h from ICU admission, oxygen saturation <90%, C-reactive protein level (CRP), ferritin level, white blood count neutrophil to lymphocyte ratio (WBC N/L) at ICU, sequential organ failure (SOFA) score [8], and PaO2:FiO2 (Table 1).
Table 1.
Characteristics, demographics, and combined interventions of tocilizumab and non-tocilizumab patients.
| Variable | Unmatched Patients (n = 1470) | Propensity-Score-Matched Patients (n = 1278) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 1470) |
No Tocilizumab (n = 1044) |
Tocilizumab (n = 426) |
p-Value | SMD Unadjusted |
Overall (n = 1278) |
No Tocilizumab (n = 852) |
Tocilizumab (n = 426) |
p-Value | SMD Adjusted |
|
| Age (years) median (IQR) | 56 (45–66) | 55 (45–66) | 57 (47.5–67) | 0.031 | 0.105 | 56 (46–67) | 56 (45–67) | 57 (47–67) | 0.186 | 0.052 |
| Gender male, n (%) | 382 (26.0) | 249 (23.9) | 133 (31.2) | 0.008 | −0.068 | 336 (26.3) | 203 (23.8) | 133 (31.2) | 0.006 | −0.561 |
| 1085 (73.8) | 792 (75.9) | 293 (68.8) | 942 (73.7) | 649 (76.2) | 293 (68.8) | |||||
| BMI, n (%) <30 |
761 (51.8) | 571 (54.7) | 191 (44.8) | <0.001 | 0.293 | 686 (53.7) | 495 (58.1) | 191 (44.8) | <0.001 | 0.168 |
| >30 | 608 (41.4) | 374 (35.8) | 235 (55.2) | 592 (46.3) | 357 (41.9) | 235 (55.2) | ||||
| DM, n (%) | 770 (52.4) | 531 (50.9) | 244 (57.3) | 0.002 | 0.048 | 695 (54.4) | 451 (52.9) | 244 (57.3) | 0.159 | 0.008 |
| HTN, n (%) | 676 (46.0) | 457 (43.8) | 225 (52.8) | <0.001 | 0.058 | 627 (49.1) | 402 (47.2) | 225 (52.8) | 0.066 | 0.009 |
| IHD, n (%) | 184 (12.5) | 125 (12.0) | 64 (13.8) | <0.001 | 183 (14.3) | 119 (14.0) | 64 (15.0) | 0.672 | ||
| CKD, n (%) | 123 (8.4) | 76 (7.3) | 47 (11.0) | 0.432 | 0.047 | 117 (9.1) | 72 (8.4) | 45 (10.5) | 0.551 | 0.007 |
| BA or COPD, n (%) | 149 (10.1) | 84 (8.0) | 66 (15.5) | <0.001 | 0.061 | 152 (11.9) | 86 (10.1) | 66 (15.5) | 0.007 | 0.048 |
| Immune def, n (%) | 72 (4.9) | 27 (2.6) | 45 (10.6) | <0.001 | 72 (5.6) | 27 (3.2) | 45 (10.6) | <0.001 | ||
| Admitted to the hospital already intubated, n (%) | 128 (8.7) | 98 (9.4) | 30 (7.0) | 0.167 | 109 (8.5) | 79 (9.3) | 30 (7.0) | 0.215 | ||
| SOFA, median (IQR) | 5 (3–8) | 5 (2–8) | 5 (3–7) | 0.144 | −0.125 | 5 (3–8) | 5 (2–8) | 5 (3–7) | 0.2 | −0.049 |
| ROX, median (IQR) | 4 (3–6) | 4 (3–5) | 4 (3–6) | 0.422 | 4 (3–6) | 4 (3–7) | 4 (3–6) | 0.532 | ||
| Mean arterial pressure, n (%) <65 | 135 (9.2) | 92 (8.8) | 43 (10.1) | 0.241 | 126 (9.9) | 83 (9.7) | 43 (10.1) | 0.921 | ||
| >65 (mmHg) | 1296 (88.2) | 920 (88.1) | 383 (89.9) | 1152 (90.1) | 769 (90.3) | 383 (89.9) | ||||
| Need for vasopressors in the first 5 days of ICU, n (%) | 395 (26.9) | 288 (27.6) | 107 (25.1) | 0.366 | 355 (27.8) | 248 (29.1) | 107 (25.1) | 0.151 | ||
| PO2:FiO2 ratio (mmHg) (ICU admission) median (IQR) | 101 (70.5–163) | 105 (72.2–171) | 94.8 (66.9–138) | <0.001 | −0.610 | 97.5 (68.4–148) | 98.6 (69.1–151) | 94.8 (66.9–138) | 0.265 | −0.054 |
| Intubation within the first 48 hrs of ICU admission n% | 594 (40.4) | 443 (42.4) | 151 (35.4) | 0.016 | −0.069 | 509 (39.8) | 358 (42.0) | 151 (35.4) | 0.028 | −0.075 |
| Laboratory data on ICU admission: WBC (109/L), mean (±SD) |
10.66 (6.29) | 11.03 (6.04) | 9.83 (6.77) | 0.001 | 10.65 (6.29) | 11.04 (6.02) | 9.83 (6.77) | 0.002 | ||
| WBC NL ratio, mean (±SD) | 10.30 (8.68) | 10.17 (8.33) | 10.47 (9.14) | 0.465 | 0.012 | 10.44 (8.64) | 10.43 (8.38) | 10.47 (9.14) | 0.937 | −0.076 |
| Creatinine (mmol), mean (±SD) | 139.58 (166.59) | 149.16 (180.7) | 118.69 (130.27) | 0.002 | 134.89 (157.1) | 143.22 (169.47) | 118.69 (130.27) |
0.007 | ||
| CRP (mg/L), mean (±SD) | 121.82 (98.78) | 118.18 (100.9) | 125.65 (93.31) | 0.17 | 0.048 | 124.20 (99.25) | 123.47 (102.13) | 125.65 (93.31) | 0.712 | 0.059 |
| D-dimer (mg/L), mean (±SD) | 1.50 (1.07) | 1.58 (1.12) | 1.88 (1.15) | 0.005 | 1.77 (1.14) | 1.54 (1.07) | 1.88 (1.15) | <0.001 | ||
| Ferritin (mcg/L), mean (±SD) | 1006 (781.10) | 985.64 (788.61) | 1012.91 (775.00) | 0.362 | −0.007 | 1052.1(802.5) | 1071.70 (815.68) | 1012.91 (775.00) | 0.217 | −0.047 |
| IMV, n (%) | 778 (52.9) | 562 (53.8) | 217 (50.9) | 0.302 | 677 (52.9) | 460 (54.0) | 217 (50.9) | 0.294 | ||
| ECMO, n (%) | 71 (4.8) | 43 (4.1) | 28 (6.6) | 0.116 | 66 (5.2) | 38 (4.5) | 28 (6.6) | 0.14 | ||
| Steroids, n (%) | 1085 (73.8) | 694 (66.5) | 359 (84.3) | <0.001 | 977 (76.4) | 618 (72.5) | 359 (84.3) | 0.001 | ||
| Convalescent plasma, n (%) | 53 (3.6) | 7 (0.7) | 46 (10.8) | <0.001 | 54 (4.2) | 7 (0.7) | 46 (10.8) | <0.001 | ||
| Chloroquine, n (%) | 429 (29.2) | 288 (27.6) | 141 (33.1) | 0.001 | 384 (30.0) | 241 (28.3) | 141 (33.1) | 0.061 | ||
| Favipiravir, n (%) | 316 (21.5) | 152 (14.6) | 164 (38.5) | <0.001 | 302 (23.6) | 136 (16.0) | 164 (38.5) | <0.001 | ||
| Ramdesivir, n (%) | 13 (0.9) | 7 (0.7) | 6 (1.4) | 0.001 | 12 (0.9) | 6 (0.7) | 6 (1.4) | 0.356 | ||
| Ribavirin, n (%) | 241 (16.4) | 160 (15.3) | 83 (19.5) | 0.001 | 219 (17.1) | 136 (16.0) | 83 (19.5) | 0.135 | ||
| Interferon, n (%) | 152 (10.3) | 97 (9.3) | 55 (12.9) | 0.001 | 141 (11.0) | 85 (10.0) | 55 (12.9) | 0.107 | ||
| IVIG, n (%) | 51 (3.5) | 39 (3.7) | 12 (2.8) | 0.001 | 46 (3.6) | 34 (4.0) | 12 (2.8) | 0.367 | ||
SMD: standard mean difference; BMI: Body mass index; DM: Diabetes mellitus; HTN: Hypertension; IHD: Ischemic heart disease (def: known based on a coronary angiogram, cardiac CT, non-invasive diagnosis, or previous clinical diagnosis); CKD: Chronic kidney disease (def: GFR <60 mL/min per 1.73 m2); BA: Bronchial asthma; COPD: Chronic obstructive pulmonary disease, immunocompromised: (def: solid organ malignancy, leukemia, on steroids (prednisone >7 mg daily for >two weeks), post organ transplant at any time); SOFA: Sequential organ failure; ROX: Respiratory rate-oxygenation; PO2/FiO2: Partial oxygen pressure to fraction inspired oxygen ratio; WBC: White blood cells; ECMO: Extracorporeal membrane oxygenation; CRP: C-reactive protein; IMV: Invasive Mechanical Ventilation; and IVIG: Immunoglobulin therapy.
The initial analysis of the original dataset was repeated on the propensity-matched dataset (PS dataset). In addition, we used multivariable logistic regression on the propensity-matched patients to evaluate the therapeutics’ impact on survival. All statistical analyses were performed using R software version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). All statistical tests were two-sided and deemed significant when the p-values were <0.05.
4. Results
4.1. Patient Characteristics
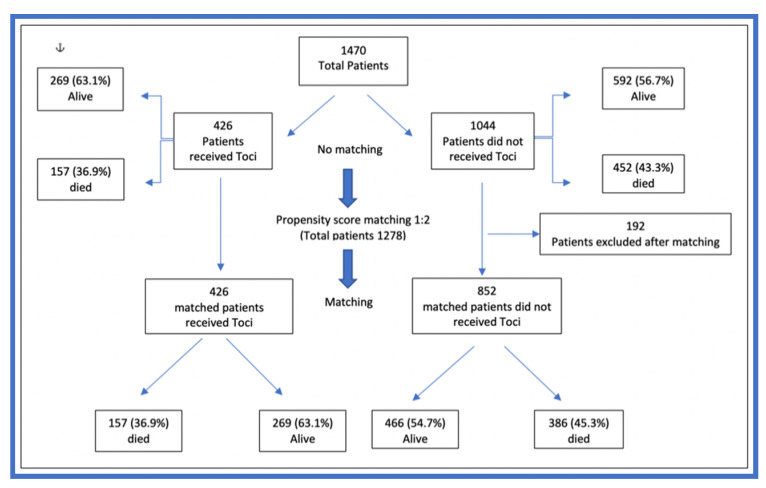
Table 1 shows that the total number of unmatched patients was 1470, and 426 patients received tocilizumab. The patients in the tocilizumab group were significantly older than those in the non-tocilizumab group (57 years (IQR 47.5–67) vs. 55 years (IQR 45–66), respectively, p = 0.031). The total number of patients with propensity-matching was 1278 (Figure 1). After propensity-matching, age was no longer significantly different between the tocilizumab and non-tocilizumab groups (57 years (IQR 47–67) vs. 56 years (IQR 46–67), respectively, p < 0.186).
Figure 1.
The flowchart of patients’ recruitment and propensity-matching.
4.2. Medical Therapeutics Combined with Tocilizumab
While the model successfully matched common medical therapeutics seen in COVID-19 between the two groups, two medical therapeutics remained significantly different in favor of the tocilizumab group in the unmatched and propensity-matching analyses. In the non-matched analysis, steroids were given to 694 (66.5%) patients in the non-tocilizumab group and 359 (84.3%) patients in the tocilizumab group (p < 0.001), while after propensity matching, they were found to be received by 618 (72.5%) patients in the non-tocilizumab group and 359 (84.3%) patients in the tocilizumab group (p = 0.001). Similarly, favipiravir was given to 152 (14.6%) patients in the non-tocilizumab group and 164 (38.5%) patients in the tocilizumab group (p < 0.001) in the non-matching analysis, while after the propensity-matching analysis, 136 (16%) patients in the non-tocilizumab group and 164 (38.5%) patients in the tocilizumab group (p < 0.001) received it. (Table 1).
5. Outcome
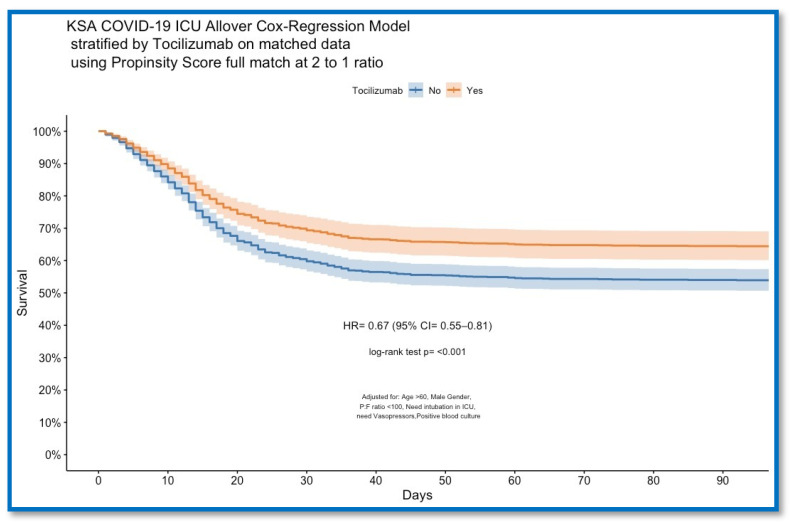
Overall, 28 day hospital survival showed a significant difference in survival between the unmatched non-tocilizumab (586, 56.1%) and tocilizumab (269, 63.1%) groups (p = 0.016), and in the propensity-matched analysis between the non-tocilizumab (466, 54.6%) and tocilizumab (269, 63.1%) groups (p = 0.005). (Table 2). The log-rank test showed a significant difference in overall 28 day hospital survival with increasing ICU days between the propensity-matched tocilizumab and non-tocilizumab groups (p < 0.001), as is shown in Figure 2. Multivariable logistic regression analysis in the full propensity-matched patients that includes tocilizumab as an independent factor showed (OR: 1.38 (95% CI: 0.75–1.53)) (p = 0.0332) remained a predictor for 28 day survival and showed also that neither steroids nor favipiravir remained predictors for survival (OR: 1.07 (95% CI: 0.75–1.53)) (p = 0.697) and (OR: 1.08 (95% CI: 0.61–1.9)) (p = 0.799), respectively, (Table 3).
Table 2.
Complications and patient outcomes.
| Variable | Unmatched Patients (n = 1044) | Propensity-Score-Matched Patients (n = 1278) | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 1470) |
No Tocilizumab (n = 1044) |
Tocilizumab (n = 426) |
p-Value | Overall (n = 1278) |
No Tocilizumab (n = 852) |
Tocilizumab (n = 426) |
p-Value | |
| ICU length of stay, median (IQR) | 9 (5–16) | 8 (4–15) | 12 (7–21) | <0.001 | 10 (5–17) | 9 (4–15) | 12 (7–21) | <0.001 |
| Hospital length of stay, median (IQR) | 15 (9–24) | 14 (8–23) | 18 (12–30) | <0.001 | 15 (10–25) | 15 (9–23) | 18 (12–30) | <0.001 |
| MV duration (days) median (IQR) | 7 (0–14) | 6 (0–13) | 8 (0–15) | 0.538 | 3 (0–12) | 3 (0–11) | 3 (0–13) | 0.288 |
| DVT, n (%) | 33 (2.2) | 20 (1.9) | 14 (3.3) | 0.304 | 31(2.4) | 17 (2.0) | 14 (3.3) | 0.222 |
| PE, n (%) | 44 (3.0) | 33 (3.2) | 11 (2.6) | 0.241 | 39(3.1) | 28 (3.3) | 11 (2.6) | 0.605 |
| Pneumothorax, n (%) | 89 (6.1) | 57 (5.5) | 32 (7.5) | 0.114 | 84(6.6) | 52 (6.1) | 32 (7.5) | 0.402 |
| MI, n (%) | 64 (4.4) | 50 (4.8) | 16 (3.8) | 0.255 | 52(4.1) | 36 (4.2) | 16 (3.8) | 0.802 |
| Cardiac arrest, n (%) | 379(25.8) | 280 (26.8) | 99 (23.2) | 0.267 | 332 (26.0) | 232 (27.2) | 100 (23.5) | 0.169 |
| RRT, n (%) | 227(15.4) | 148 (14.2) | 79 (18.5) | 0.058 | 202 (15.8) | 123 (14.4) | 79 (18.5) | 0.069 |
| Stroke, n (%) | 32 (2.2) | 23 (2.2) | 9 (2.1) | 0.431 | 29 (2.3) | 20 (2.3) | 9 (2.1) | 0.947 |
| ICH, n (%); | 33 (2.2) | 23 (2.2) | 10 (2.3) | 0.986 | 31 (2.4) | 21 (2.5) | 10 (2.3) | 1 |
| ICU survival to discharge, n (%) | 868 (59.0) | 592 (56.7) | 276 (64.8) | 0.005 | 748 (58.5) | 472 (55.4) | 276 (64.8) | 0.002 |
| 28 day hospital overall survival, n (%) | 855 (58.2) | 586 (56.1) | 269 (63.1) | 0.016 | 735 (57.5) | 466 (54.7) | 269 (63.1) | 0.005 |
MV: Mechanical ventilation; DVT: Deep venous thrombosis; PE: Pulmonary embolism; MI: myocardial infarction; RRT: Renal replacement therapy; and ICH: intracerebral hemorrhage.
Figure 2.
COVID-19 ICU Cox regression model of overall 28 day survival stratified by tocilizumab on propensity-matched data with a 2:1 ratio.
Table 3.
Multivariate logistic regression for the overall 28 day hospital survival in the fully propensity-matched patients.
| Univariate | Multivariate | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Units | Odds Ratio |
CI.95 | p-Value | Odds Ratio |
CI.95 | p-Value | |||||||
| 1 | Age | >60 | Ref | Ref | ||||||||||
| <60 | 1.57 | [1.26;1.97] | <1 × 10−4 | S | 1.63 | [1.21;2.19] | 0.001175 | S | ||||||
| 2 | Gender | Male | Ref | Ref | ||||||||||
| Female | 1 | [0.78;1.28] | 0.9919 | NS | 1.8 | [0.82;3.96] | 0.141482 | NS | ||||||
| 3 | DM | Yes | Ref | Ref | ||||||||||
| No | 1.25 | [1.00;1.57] | 0.04802 | S | 0.9 | [0.64;1.24] | 0.510627 | NS | ||||||
| 4 | HTN | Yes | Ref | Ref | ||||||||||
| No | 1.48 | [1.18;1.85] | 0.000616 | S | 1.56 | [1.12;2.18] | 0.008437 | S | ||||||
| 5 | COPD | Yes | Ref | Ref | ||||||||||
| No | 0.8 | [0.57;1.12] | 0.1905 | NS | 0.73 | [0.48;1.11] | 0.136765 | NS | ||||||
| 6 | CKD | Yes | Ref | Ref | ||||||||||
| No | 1.28 | [0.89;1.85] | 0.1822 | NS | 0.92 | [0.59;1.44] | 0.710813 | NS | ||||||
| 7 | PaO2:FiO2. | <100 | Ref | |||||||||||
| >100 | 1.34 | [1.07;1.68] | 0.01072 | S | 1.41 | [1.07;1.85] | 0.01503 | S | ||||||
| 8 | IMV | Yes | Ref | Ref | ||||||||||
| No | 9.94 | [7.59;13.02] | <1 × 10−4 | S | 7.05 | [5.22;9.54] | <1 × 10−4 | S | ||||||
| 9 | Vasopressors | Yes | Ref | Ref | ||||||||||
| No | 4.99 | [3.81;6.52] | <1 × 10−4 | S | 2.68 | [1.97;3.65] | <1 × 10−4 | S | ||||||
| 10 | Tocilizumab | No | Ref | Ref | ||||||||||
| Yes | 1.36 | [1.07;1.73] | 0.01164 | S | 1.38 | [1.03;1.85] | 0.033253 | S | ||||||
| 11 | Steroids | Yes | Ref | Ref | ||||||||||
| No | 1.32 | [0.99;1.75] | 0.05849 | NS | 1.07 | [0.75;1.53] | 0.697434 | NS | ||||||
| 12 | Favipiravir | Yes | Ref | Ref | ||||||||||
| No | 0.99 | (0.78–1.28) | 0.99 | NS | 1.08 | (0.61–1.9) | 0.799 | NS | ||||||
| 13 | WBC.NLratio | >8.5 | Ref | Ref | ||||||||||
| ≤8.5 | 1.45 | [1.16;1.81] | 0.001215 | S | 1.08 | [0.82;1.43] | 0.573611 | NS | ||||||
| 14 | Ferritin | >1400 | Ref | Ref | ||||||||||
| ≤1400 | 1.48 | [1.16;1.89] | 0.001493 | S | 1.41 | [1.04;1.91] | 0.025717 | S | ||||||
| 15 | Ddimer | >1.5 | Ref | Ref | ||||||||||
| ≤1.5 | 1.81 | [1.45;2.27] | <1 × 10−4 | S | 1.45 | [1.10;1.91] | 0.008396 | S | ||||||
| 16 | CRP | >150 | Ref | Ref | ||||||||||
| ≤150 | 1.12 | [0.89;1.40] | 0.3541 | NS | 0.86 | [0.64;1.14] | 0.286094 | NS | ||||||
| No | 2.47 | [1.92;3.17] | <1 × 10−4 | S | 1.38 | [1.02;1.87] | 0.036575 | S | ||||||
DM: Diabetes mellitus; HTN: Hypertension; CKD: Chronic kidney disease (def: GFR <60 mL/min per 1.73 m2); COPD: Chronic obstructive pulmonary disease; PO2/FiO2: Partial oxygen pressure to fraction inspired oxygen ratio; IMV: Invasive Mechanical Ventilation; WBC: White blood cells; and CRP: C-reactive protein.
6. Discussion
In this prospective, observational, multicenter cohort study, we found that tocilizumab use increased 28 day survival in critically ill COVID-19 patients; this finding was obtained from analysis of the matching of the dataset (Table 2) and confirmed by multivariable logistic regression (Table 3 and Table S1) and Cox regression (Figure 2). Nevertheless, this study found no effect of medical therapeutics commonly used in COVID-19 on the survival of tocilizumab use.
We used a propensity-score analysis to match the non-tocilizumab and tocilizumab groups to compare outcomes and successfully mitigate the influence of different medical therapeutics on COVID-19 when paired with tocilizumab except for steroids and favipiravir. Thus, we performed a multi-variable logistic regression for overall 28 day hospital survival in the fully propensity-matched patients to eliminate the effects of favipiravir and steroids on the tocilizumab outcome.
Numerous COVID-19 treatments have been evaluated separately for efficacy and have provided inconsistent results for various reasons. There is no consensus regarding their use, as multiple studies conducted in the past have given contradictory results [18,19,20,21,22,23]. Tocilizumab, a recombinant humanized anti-human IL-6 antagonist, is one of these therapeutics that has been extensively investigated, with increasing evidence of its use [22,23]. The drug was introduced in the early 2000s to treat autoimmune disorders such as refractory rheumatoid arthritis and systemic juvenile idiopathic arthritis [24]. Later on, in 2017, it was approved by the FDA for CRS treatment. During the COVID-19 pandemic, the drug significantly improved patient outcomes [25]. A large, randomized trial concluded that tocilizumab is an effective treatment for enhancing the survival outcomes of hospitalized COVID-19 patients with evidence of cytokine release [23]. In their study, Petrak R et al. found that each additional day of delay from admission to tocilizumab administration independently increased the odds of receiving mechanical ventilation by 21% (95% CI: (1.08–1.38), p = 0.002) [26]. However, studies have not evaluated combined therapies with tocilizumab. A study conducted by Zhao H et al. found that the combination of an antiviral drug (favipiravir) and an IL-6 receptor blocker (tocilizumab) can significantly reduce mortality in COVID-19 patients [27]. In this trial, we searched for other therapeutics that may contribute to the outcomes of tocilizumab patients. Next to steroids, favipiravir was the second therapeutic that may be associated with the outcomes of tocilizumab patients. Nevertheless, the multiple logistic regression did not retain this effect (OR = 2.46).
Mikulska et al. was an early study that provided a hint of the favorable outcome of the combined therapy of steroids with tocilizumab, in which the methylprednisolone + tocilizumab treatment arm demonstrated a superior outcome in averting death and intubation compared to single therapy arms [28]. A recently published systematic review and meta-analysis by Lim et al. showed that the concurrent use of a corticosteroid such as methylprednisolone was a significant influencer of tocilizumab efficacy in reducing mortality and improving survival [29]. This is especially visible in the excellent outcomes demonstrated by the CHIC study, which used 3–6 days of methylprednisolone in combination with tocilizumab in the late stages of the disease [30]. RECOVERY [23] and REMAP-CAP [3] have also confirmed the efficacy of combined therapy with corticosteroids and tocilizumab. Unlike previously mentioned cohorts, corticosteroids had no role in tocilizumab increasing 28 day survival in our cohort; this could be because the patients received corticosteroids in the late stage of the disease when they were already very sick.
More than three years after the first cases of COVID-19 were reported, multiple treatment options have been investigated for short-term outcomes; evidence gaps exist for long-term outcomes and quality of life. The REMAP-CAP trial investigators most recently closed these gaps by evaluating six treatment classes for 4689 patients admitted to the intensive care unit with COVID-19 from March 2020 through June 2021 for long-term outcomes (180 days) and quality of life. One of the main findings is that IL-6 receptor antagonists like tocilizumab again showed a very high >99.9% posterior probability of superiority over a placebo (adjusted hazard ratio, 0.74 [95% credible interval CrI, 0.61–0.90]). In addition, this 180 day outcome provides reassurance that the early mortality benefit from IL-6 receptor antagonists did not result in longer-term adverse outcomes like late opportunistic infection and others [31].
The strength of the study is the prospective and multicenter design of the trial, which provides a relatively big cohort to be evaluated. In addition, the research was conducted according to a registered protocol with a propensity score matching design.
Limitations
The findings of the current study should be interpreted while considering its limitations. Firstly, prospective observational studies cannot draw cause-and-effect inferences due to known and unknown confounders. Secondly, misclassifying the data is possible as the data were collected from electronic health records in some hospitals and manually in others. Third, this cohort enrolled patients at a relatively early stage of the pandemic, when there were only a few small variations in the usual clinical management of COVID-19 patients in general and the indications for tocilizumab in particular that were independent of interleukin 6 levels, leaving confounders unaccounted for. Furthermore, the median time of tocilizumab administration was not collected, but it was administered within 48 h of ICU admission. Finally, our study focused on Saudi Arabian patients. However, the country’s population is diverse, and only 14 hospitals were included in the study, which may limit its applicability to other geographical areas.
7. Conclusions
The tocilizumab treatment in critically ill COVID-19 patients admitted to the ICU improved the overall 28 day hospital survival rate with favorable outcomes on the length of ICU stay and the survival to ICU discharge that might not be influenced by the concurrent use of other COVID-19 medical therapeutics, although further research is needed to confirm this.
Acknowledgments
The authors would like to thank the Saudi Critical Care Trials Group for their kind guidance and the Saudi COVID-19 working group for their great help with data sharing and collection, including Mohamed Ali Al-Odat, Huda A. Mhawish, Bobby Rose Marasigan, Armalene Cabuyadao Bautista, Katrina Baguisa, Zahra Alfrdan, Bernadette Daher, Mohammed H. Lhmdi, Waleed Alatreeby, Mahdi Othman, Ashraf Shawat, Ahmed Y Zakarya, Mohamed Abdulzaher, Saleh Samir Saleh Ashgar, Jumana Khalid Nabeeh Alansari, Mohammed Amin, Mohammed Rio, Duaa Jihad Zain Basrawi, Hussam Mahmoud Omar Baeissa, Bashayer Mohammed Alsaadi, Ibrahim Mohamed Ibrahim Nasr, Mahdi Alajaj, Ozaz Hamza, Saud A Alghamdi, Hamza S Ghazal, Omar S Elrabi, Sumaih A Alomran, Saleh S Alomari, Taghred M Shwaish, Afaf S Alotaibi, Omar Alrabi, Amani Alharbi, Mohammed Alzanbaqi, Fahad Alsallum, Mohammed Bashanfar, Mohammed Jastaniah, Hamdan Alguydi, Ahmed Fakhri, and Sheryl S. Lugue. Their willingness to give their time so generously is very much appreciated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12062301/s1, Table S1: Multivariable logistic regression analysis that includes all variables with significant differences after matching and included tocilizumab as a dependent factor.
Author Contributions
Design conception: A.E. and A.A.R. data collection: A.E., H.S., A.M., A.A. (Adnan Alghamdi); A.A. (Ali Altalaq); A.A.-O., M.S.A., M.H.A., A.S., A.K., Z.A.A.-A., G.A.A., W.T., S.A.A., F.F., A.A. (Abdulrahman Alharthy), J.A.A.-T., R.G.M., and the Saudi COVID-19 working group; data validation and cleaning, A.E., H.S. and H.N.M.; statistical analysis, model development, and validation, H.N.M.; acquisition, analysis, and interpretation of data, A.E., A.A.R., and H.N.M.; manuscript—drafting and writing, A.E. and A.A.R.; manuscript—editing and reviewing, Y.M.A., M.S.A., Z.A.A.-A., A.A.-O., A.A. (Abdulrahman Alharthy) and H.N.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethics committee name: Saudi Ministry of Health Central Institutional Review Board. Approval Code: [20-80E]. Approval Date: 20 February 2020.
Informed Consent Statement
Consent was waived as all data are anonymous.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., Tomelleri A., Baldissera E., Rovere-Querini P., Ruggeri A., et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: A single-center retrospective cohort study. Eur. J. Intern. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canziani L.M., Trovati S., Brunetta E., Testa A., De Santis M., Bombardieri EGuidelli G., Albano G., Folci M., Squadroni M., Beretta G.D. Interleukin-6 receptor blocking with intra-venous Tocilizumab in COVID-19 severe acute respiratory distress syndrome: A retrospective case-control survival analysis of 128 patients. J. Autoimmune. 2020;114:102511. doi: 10.1016/j.jaut.2020.102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Remap-Cap Investigators Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosas I.O., Brau N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., et al. Tocilizumab in Hospitalized Patients with Severe COVID-19 Pneumonia. N. Engl. J. Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M., et al. Efficacy of Tocilizumab in patients hospitalized with COVID-19. N. Engl. J. Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., Bruzzi P., Boni F., Braglia L., Turrà C., et al. Effect of Tocilizumab vs. standard care on clinical worsening in patients hospitalized with COVID-19 Pneumonia: A randomized clinical trial. JAMA Intern. Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P. Effect of Tocilizumab vs. usual care in adults hospitalized with COVID-19 and moderate or severe Pneumonia: A randomized clinical trial. JAMA Intern. Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhazmi A., Al-Omari A., Sallam H., Mufti H.N., Rabie A.A., Alshahrani M., Mady A., Alghamdi A., Altalaq A., Azzam M.H., et al. Machine learning decision tree algorithm role for predicting mortality in critically ill adult COVID-19 patients admitted to the ICU. J. Infect. Public Health. 2022;15:826–834. doi: 10.1016/j.jiph.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Disease Control. [(accessed on 1 February 2023)]; Available online: https://ncdc.gov.in/index1.php?lang=1&level=1&sublinkid=703&lid=550.
- 10.Read K., LaPolla F.W.Z. A new hat for librarians: Providing REDCap support to establish the library as a central data hub. J. Med. Libr. Assoc. 2018;106:120–126. doi: 10.5195/jmla.2018.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H., Reinhart C.K., Suter P., Thijs L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 12.Austin P. A tutorial and case study in propensity score analysis. An application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivar. Behav Res. 2011;46:119–151. doi: 10.1080/00273171.2011.540480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris H., Horst S.J. A brief guide to decisions at each step of the propensity score matching process. Pract. Assess. Res. Eval. 2016;21:4. [Google Scholar]
- 14.Leyrat C., Seaman S.R., White I.R., Douglas I., Smeeth L., Kim J., Resche-Rigon M., Carpenter J.R., Williamson E.J. Propensity score analysis with partially observed covariates: How should multiple imputations be used? Stat. Methods Med. Res. 2019;28:3–19. doi: 10.1177/0962280217713032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi J., Dekkers O.M., le Cessie S. A comparison of different methods to handle missing data in the context of propensity score analysis. Eur. J. Epidemiol. 2019;34:23–36. doi: 10.1007/s10654-018-0447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olmos A., Govindasamy P. Propensity scores: A practical introduction using R. J. Multidiscip. Eval. 2015;11:68–88. [Google Scholar]
- 17.Zhang Z., Kim H.J., Lonjon G., Zhu Y. Balance diagnostics after propensity score matching. Ann. Transl. Med. 2019;7:22–26. doi: 10.21037/atm.2018.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alkofide H., Almohaizeie A., Almuhaini S., Alotaibi B., Alkharfy K.M. Tocilizumab and Systemic Corticosteroids in the Management of Patients with COVID-19: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021;110:320–329. doi: 10.1016/j.ijid.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berardicurti O., Ruscitti P., Ursini F., D’Andrea S., Ciaffi J., Meliconi R., Iagnocco A., Cipriani P., Giacomelli R. Mortality in tocilizumab-treated patients with COVID-19: A systematic review and meta-analysis. Clin. Exp. Rheumatol. 2020;38:1247–1254. [PubMed] [Google Scholar]
- 20.Malgie J., Schoones J.W., Pijls B.G. Decreased Mortality in Coronavirus Disease 2019 Patients Treated with Tocilizumab: A Rapid Systematic Review and Meta-analysis of Observational Studies. Clin. Infect. Dis. 2021;72:742–749. doi: 10.1093/cid/ciaa1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan S.H., Lai C.C., Huang H.T., Chang S.P., Lu L.C., Hsueh P.R. Tocilizumab for severe COVID-19: A systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2020;56:106103. doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tleyjeh I.M., Kashour Z., Damlaj M., Riaz M., Tlayjeh H., Altannir M., Altannir Y., MAl-Tannir M., Tleyjeh R., Hassett L., et al. Efficacy and safety of tocilizumab in COVID-19 patients: A living systematic review and meta-analysis. Clin. Microbiol. Infect. 2021;27:215–227. doi: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomized, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Benedetti F., Brunner H.I., Ruperto N., Kenwright A., Wright S., Calvo I., Cuttica R., Ravelli A., Schneider R., Woo P., et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 2012;367:2385–2895. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- 25.Roche FDA Approves Roche’s Actemra/RoActemra (Tocilizumab) for Treating CAR T Cell-Induced Cytokine Release Syndrome. [(accessed on 7 August 2022)]. Available online: https://www.roche.com/media/releases/med-cor-2017-08-30.htm.htm.
- 26.Petrak R.M., Skorodin N.C., Van Hise N.W., Fliegelman R.M., Pinsky J., Didwania V., Anderson M., Diaz M., Shah K., Chundi V.V., et al. Tocilizumab as a Therapeutic Agent for Critically Ill Patients Infected with SARS-CoV-2. Clin. Transl. Sci. 2021;14:2146–2151. doi: 10.1111/cts.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H., Zhu C., Zhang C., Jiawen Li Wei M., Qin Y., Zhao H., Zhu Q., Zhang C., Li J., Wei M., et al. Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size. Biomed. Pharmacother. 2021;133:110825. doi: 10.1016/j.biopha.2020.110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikulska M., Nicolini L.A., Signori A., Biagio A.D., Sepulcri C., Russo C., Dettori S., Berruti M., Sormani M.P., Giacobbe D.R., et al. Tocilizumab and steroid treatment in patients with COVID-19 Pneumonia. PLoS ONE. 2020;15:e0237831. doi: 10.1371/journal.pone.0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim P.C., Wong K.L., Rajah R., Chong M.F., Chow T.S., Subramaniam S., Lee C.Y. Comparing the efficacy of tocilizumab with corticosteroid therapy in treating COVID-19 patients: A systematic review and meta-analysis. DARU J. Pharm. Sci. 2022;30:211–228. doi: 10.1007/s40199-021-00430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramiro S., Mostard R.L.M., Magro-Checa C., van Dongen C.M.P., Dormans T., Buijs J., Gronenschild M., De Kruif M.D., Van Haren E.H., Van Kraaij T., et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: Results of the CHIC study. Ann. Rheum. Dis. 2020;79:1143–1151. doi: 10.1136/annrheumdis-2020-218479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Writing Committee for the REMAP-CAP Investigators Long-term (180-Day) Outcomes in Critically Ill Patients With COVID-19 in the REMAP-CAP Randomized. Clin. Trial. JAMA. 2023;329:39–51. doi: 10.1001/jama.2022.23257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.