Abstract
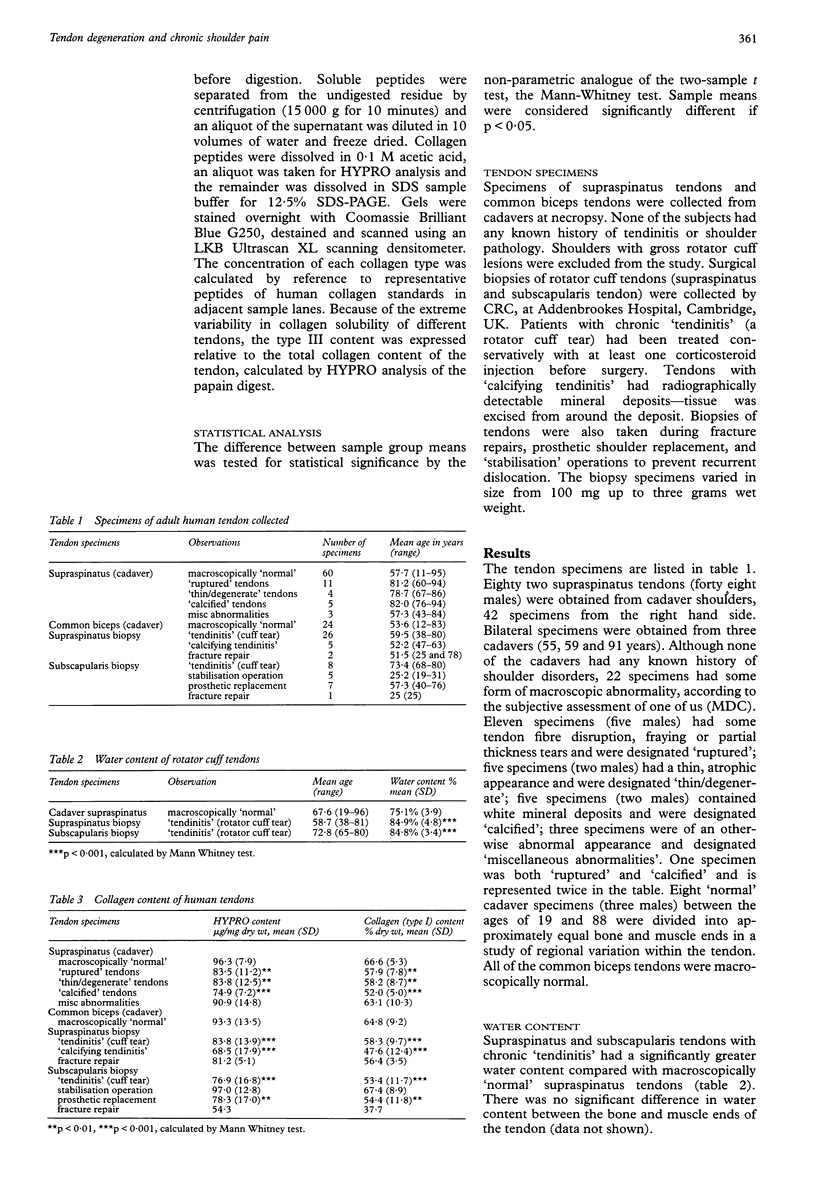
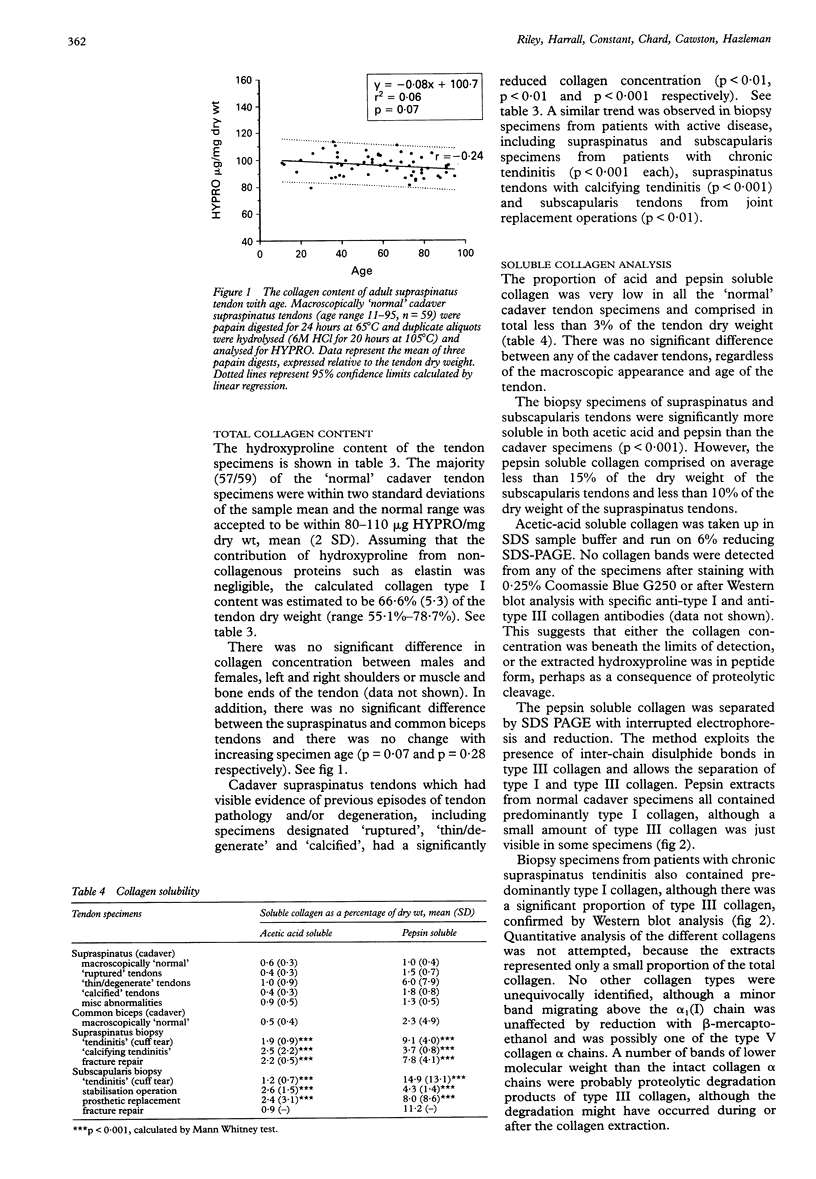
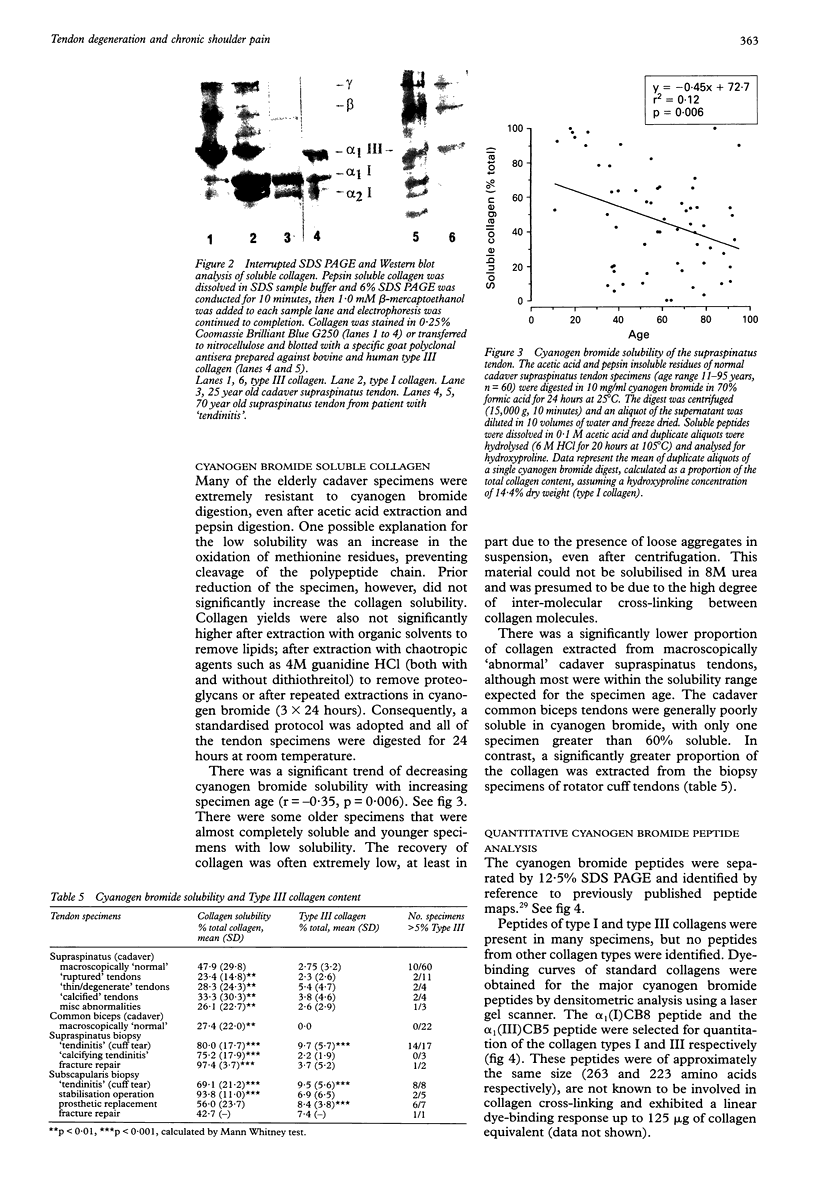
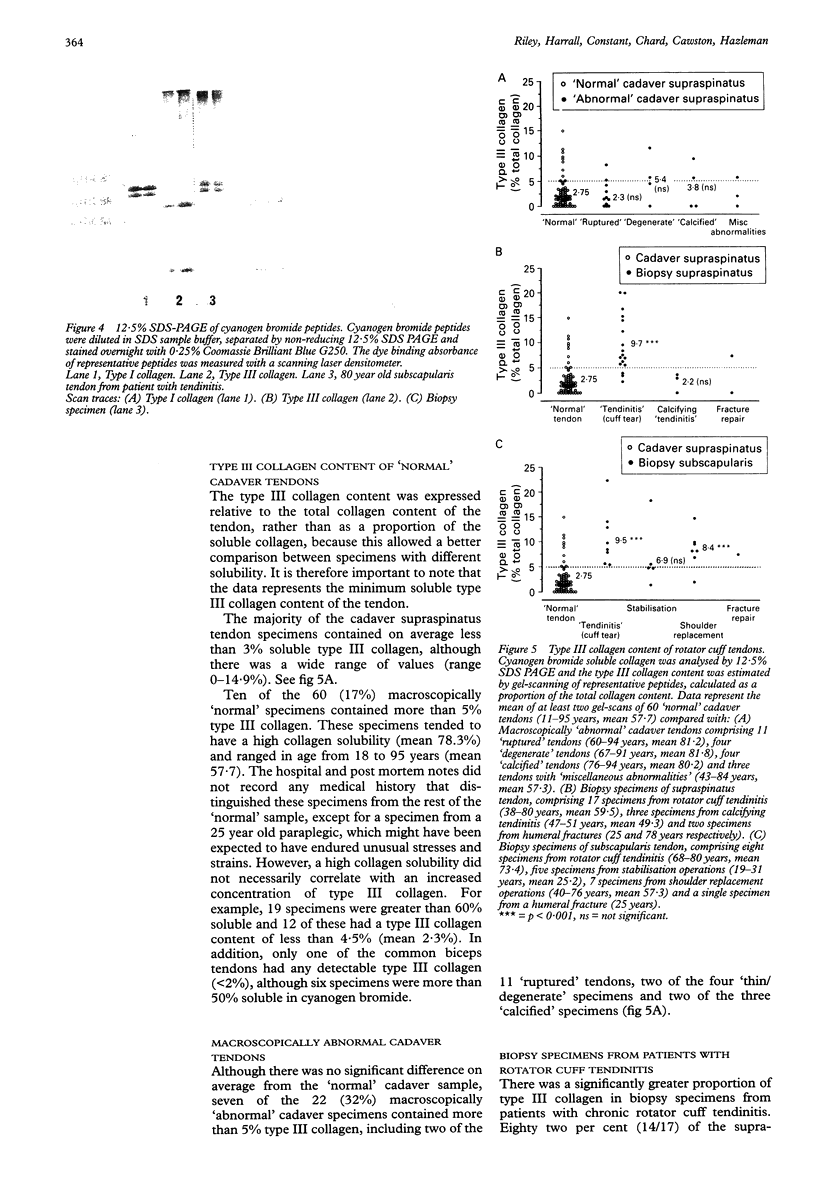
OBJECTIVES--To analyse the collagen composition of normal adult human supraspinatus tendon and to compare with: (1) a flexor tendon (the common biceps tendon) which is rarely involved in any degenerative pathology; (2) degenerate tendons from patients with chronic rotator cuff tendinitis. METHODS--Total collagen content, collagen solubility and collagen type were investigated by hydroxyproline analysis, acetic acid and pepsin digestion, cyanogen bromide peptide analysis, SDS-PAGE and Western blotting. RESULTS--The collagen content of the normal cadaver supraspinatus tendons (n = 60) was 96.3 micrograms HYPRO/mg dry weight (range 79.3-113.3) and there was no significant change across the age range 11 to 95 years. There was no significant difference from the common biceps tendon [93.3 (13.5) micrograms HYPRO/mg dry weight, n = 24]. Although extremely insoluble in both acetic acid and pepsin, much of the collagen was soluble after cyanogen bromide digestion [mean 47.9% (29.8)]. Seventeen per cent (10/60) of the 'normal' cadaver supraspinatus tendon sample contained more than 5% type III collagen, although none of the common biceps tendons had significant amounts. Degenerate supraspinatus and subscapularis tendons had a reduced collagen content [83.8 (13.9) micrograms/mg dry weight and 76.9 (16.8) micrograms/mg dry wt respectively) and were more soluble in acetic acid, pepsin and cyanogen bromide (p < 0.001). Eighty two per cent (14/17) of supraspinatus tendons and 100% (8/8) of subscapularis tendons from patients with tendinitis contained more than 5% type III collagen. CONCLUSIONS--The changes in collagen composition in rotator cuff tendinitis are consistent with new matrix synthesis, tissue remodelling and wound healing, in an attempt to repair the tendon defect, even in old and degenerate tendons. An increase in type III collagen in some 'normal' cadaver supraspinatus tendons is evidence that changes in collagen synthesis and turnover may precede tendon rupture. These changes may be the result of repeated minor injury and microscopic fibre damage or a consequence of local factors such as reduced vascular perfusion, tissue hypoxia, altered mechanical forces and the influence of cytokines. These collagenous changes may accumulate with age and substantially weaken the tendon structure, predisposing the tendon to rotator cuff tendinitis and eventual tendon rupture.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M., Deyl Z. Degenerated annulus fibrosus of the intervertebral disc contains collagen type II. Ann Rheum Dis. 1984 Apr;43(2):258–263. doi: 10.1136/ard.43.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145–153. doi: 10.3109/03009747409097141. [DOI] [PubMed] [Google Scholar]
- Bazin S., Le Lous M., Duance V. C., Sims T. J., Bailey A. J., Gabbiani G., D'Andiran G., Pizzolato G., Browski A., Nicoletis C. Biochemistry and histology of the connective tissue of Dupuytren's disease lesions. Eur J Clin Invest. 1980 Feb;10(1):9–16. doi: 10.1111/j.1365-2362.1980.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Berry H., Fernandes L., Bloom B., Clark R. J., Hamilton E. B. Clinical study comparing acupuncture, physiotherapy, injection and oral anti-inflammatory therapy in shoulder-cuff lesions. Curr Med Res Opin. 1980;7(2):121–126. doi: 10.1185/03007998009112038. [DOI] [PubMed] [Google Scholar]
- Binder A. I., Bulgen D. Y., Hazleman B. L., Roberts S. Frozen shoulder: a long-term prospective study. Ann Rheum Dis. 1984 Jun;43(3):361–364. doi: 10.1136/ard.43.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgen D. Y., Binder A. I., Hazleman B. L., Dutton J., Roberts S. Frozen shoulder: prospective clinical study with an evaluation of three treatment regimens. Ann Rheum Dis. 1984 Jun;43(3):353–360. doi: 10.1136/ard.43.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson R. E., Nimni M. E. Collagen types. Molecular structure and tissue distribution. Clin Orthop Relat Res. 1992 Sep;(282):250–272. [PubMed] [Google Scholar]
- Chard M. D., Cawston T. E., Riley G. P., Gresham G. A., Hazleman B. L. Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis. 1994 Jan;53(1):30–34. doi: 10.1136/ard.53.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofield R. H. Rotator cuff disease of the shoulder. J Bone Joint Surg Am. 1985 Jul;67(6):974–979. [PubMed] [Google Scholar]
- Duance V. C., Restall D. J., Beard H., Bourne F. J., Bailey A. J. The location of three collagen types in skeletal muscle. FEBS Lett. 1977 Jul 15;79(2):248–252. doi: 10.1016/0014-5793(77)80797-7. [DOI] [PubMed] [Google Scholar]
- ELLIOTT D. H. STRUCTURE AND FUNCTION OF MAMMALIAN TENDON. Biol Rev Camb Philos Soc. 1965 Aug;40:392–421. doi: 10.1111/j.1469-185x.1965.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Fearnley M. E., Vadasz I. Factors influencing the response of lesions of the rotator cuff of the shoulder to local steroid injection. Ann Phys Med. 1969 May;10(2):53–63. doi: 10.1093/rheumatology/10.2.53. [DOI] [PubMed] [Google Scholar]
- Hanson A. N., Bentley J. P. Quantitation of type I to type III collagen ratios in small samples of human tendon, blood vessels, and atherosclerotic plaque. Anal Biochem. 1983 Apr 1;130(1):32–40. doi: 10.1016/0003-2697(83)90646-2. [DOI] [PubMed] [Google Scholar]
- Hansson H. A., Dahlin L. B., Lundborg G., Löwenadler B., Paleus S., Skottner A. Transiently increased insulin-like growth factor I immunoreactivity in tendons after vibration trauma. An immunohistochemical study on rats. Scand J Plast Reconstr Surg Hand Surg. 1988;22(1):1–6. doi: 10.3109/02844318809097928. [DOI] [PubMed] [Google Scholar]
- Keene D. R., Sakai L. Y., Bächinger H. P., Burgeson R. E. Type III collagen can be present on banded collagen fibrils regardless of fibril diameter. J Cell Biol. 1987 Nov;105(5):2393–2402. doi: 10.1083/jcb.105.5.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. J. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am J Physiol. 1987 Jan;252(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1987.252.1.C1. [DOI] [PubMed] [Google Scholar]
- Macnab I. Rotator cuff tendinitis. Ann R Coll Surg Engl. 1973 Nov;53(5):271–287. [PMC free article] [PubMed] [Google Scholar]
- Mays P. K., Bishop J. E., Laurent G. J. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988 Nov 30;45(3):203–212. doi: 10.1016/0047-6374(88)90002-4. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Rhodes R. K. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Neer C. S., 2nd Impingement lesions. Clin Orthop Relat Res. 1983 Mar;(173):70–77. [PubMed] [Google Scholar]
- Ozaki J., Fujimoto S., Nakagawa Y., Masuhara K., Tamai S. Tears of the rotator cuff of the shoulder associated with pathological changes in the acromion. A study in cadavera. J Bone Joint Surg Am. 1988 Sep;70(8):1224–1230. [PubMed] [Google Scholar]
- Rothman R. H., Parke W. W. The vascular anatomy of the rotator cuff. Clin Orthop Relat Res. 1965 Jul-Aug;41:176–186. [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I. F., McCullagh K. G., Silver I. A. The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res. 1984;12(3-4):211–227. doi: 10.3109/03008208409013684. [DOI] [PubMed] [Google Scholar]
- van der Rest M., Garrone R. Collagen family of proteins. FASEB J. 1991 Oct;5(13):2814–2823. [PubMed] [Google Scholar]
- von der Mark K. Localization of collagen types in tissues. Int Rev Connect Tissue Res. 1981;9:265–324. doi: 10.1016/b978-0-12-363709-3.50012-7. [DOI] [PubMed] [Google Scholar]




