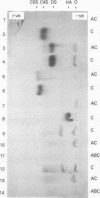
Abstract
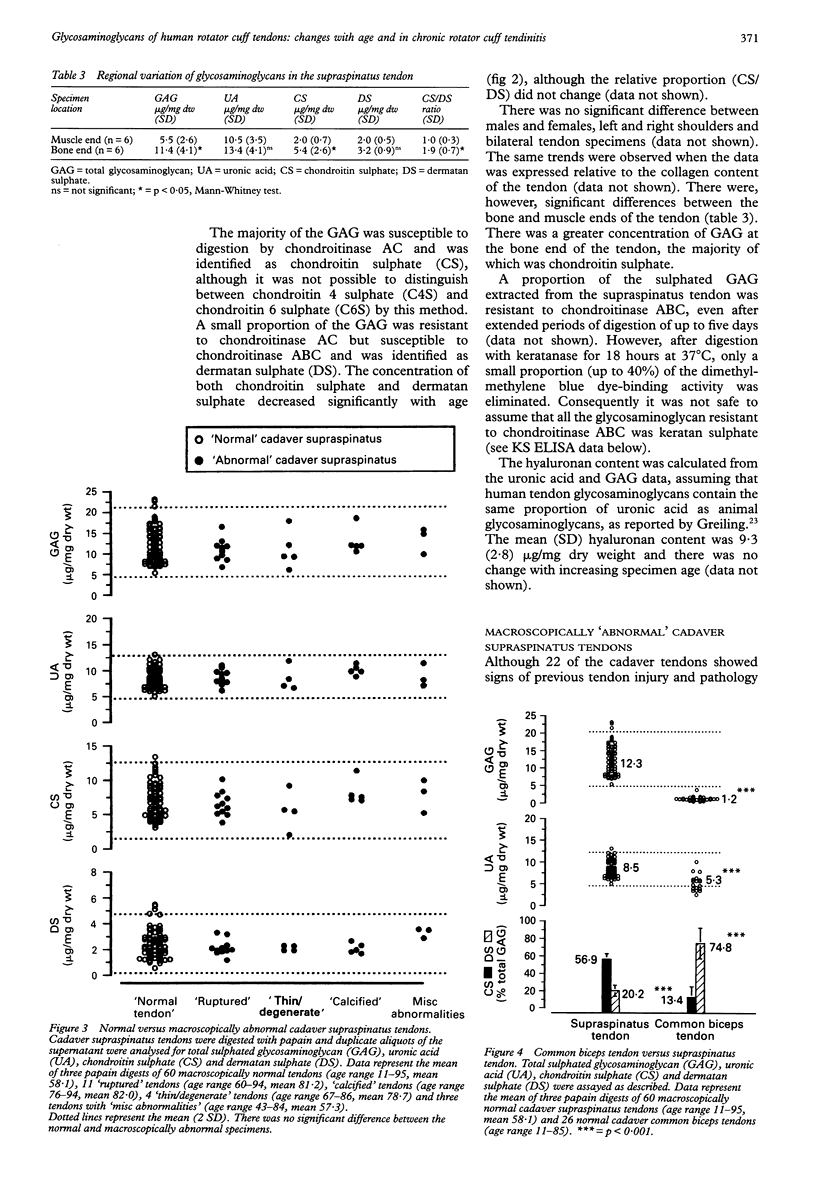
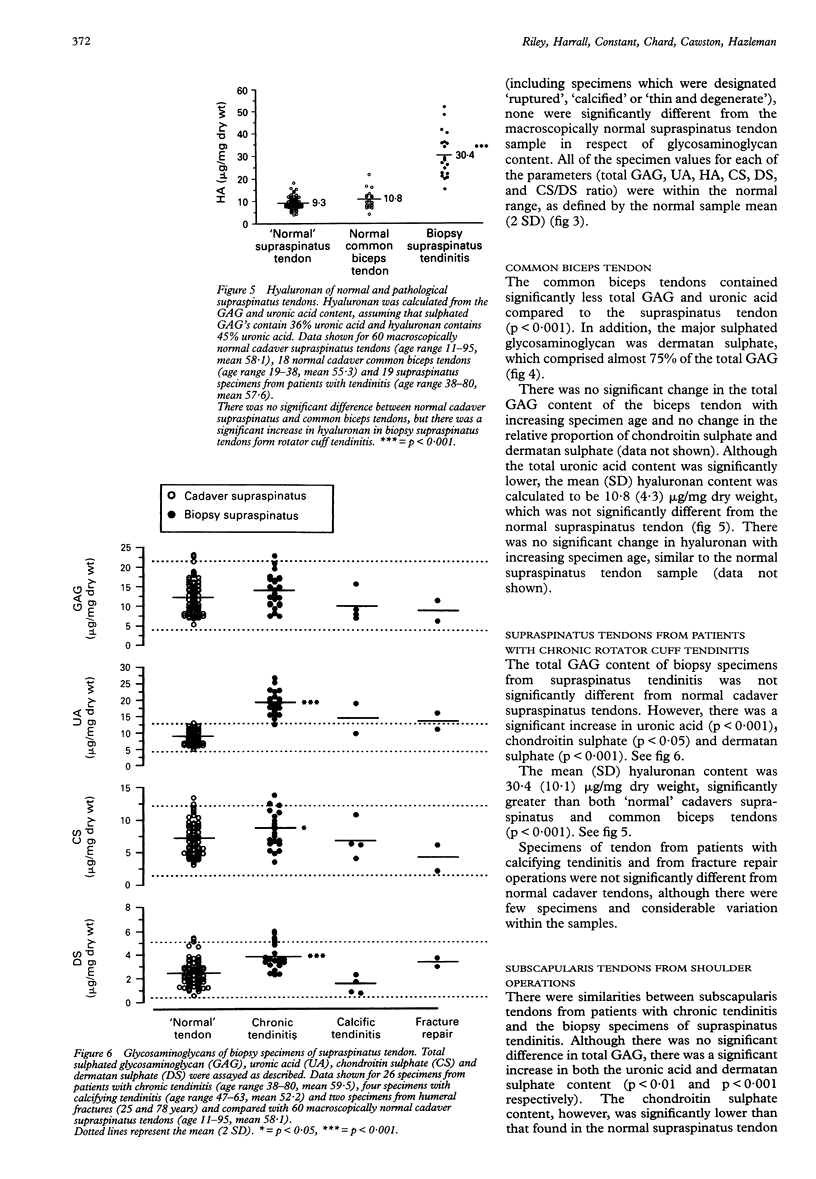
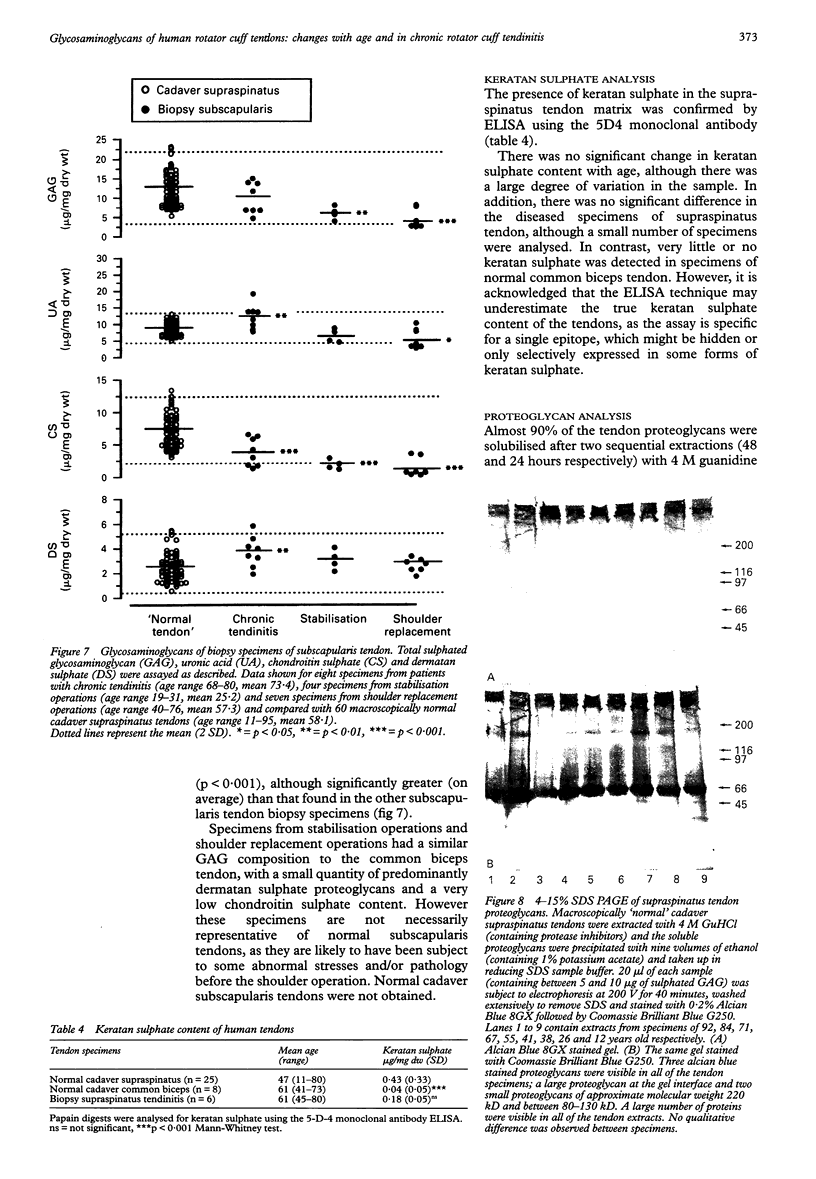
OBJECTIVES--To analyse the glycosaminoglycans of the adult human rotator cuff tendon matrix, to characterise changes in the glycosaminoglycan composition with age and in chronic rotator cuff tendinitis. METHODS--Rotator cuff (supraspinatus) tendons (n = 84) and common biceps tendons (n = 26) were obtained from cadavers with no history of tendon pathology (age range 11-95 years). Biopsies of rotator cuff tendons (supraspinatus and subscapularis tendons, n = 53) were obtained during open shoulder surgery to repair shoulder lesions (age range 38-80 years). Glycosaminoglycans were extracted by papain digestion and analysed by cellulose acetate electrophoresis, the carbazole assay for uronic acid and the dimethylmethylene blue dye-binding assay for sulphated glycosaminoglycans. Some digests were analysed for keratan sulphate by 5D4 monoclonal antibody ELISA. Soluble proteoglycans were extracted in 4M guanidine hydrochloride and analysed by 4-15% SDS PAGE. RESULTS--The mean (SD) sulphated glycosaminoglycan (GAG) content of the normal cadaver supraspinatus tendon was 12.3 (4.3) micrograms/mg dry weight, between three and ten times greater than in the common biceps tendon [1.2 (0.6) micrograms/mg dry weight]. The major GAG was chondroitin sulphate [6.9 (2.6) micrograms/mg dry weight], with a smaller proportion of dermatan sulphate [2.5 (1.2) micrograms/mg dry weight]. In contrast, the common biceps tendon contained predominantly dermatan sulphate [0.8 (0.2) microgram/mg dry weight] with less chondroitin sulphate [0.2 (0.2) microgram/mg dry weight]. There was no difference in the concentration of hyaluronan in these tendons [9.3 (2.8) micrograms/mg dry weight and 10.8 (4.3) micrograms/mg dry weight respectively] and there was no significant change of hyaluronan with age. Keratan sulphate was a small but significant component of the supraspinatus tendon [0.43 (0.33) microgram/mg dry weight, n = 25], whereas there was little or none in the common biceps tendon [0.04 (0.05) microgram/mg dry weight, n = 8] and there was no significant change across the age range. In the supraspinatus tendon, there was a significant decrease in total glycosaminoglycan, chondroitin sulphate and dermatan sulphate with age (p < 0.001), whether expressed relative to the tendon dry weight or total collagen content, and no change in the relative proportion of the different GAG types. There was, however, a large degree of variation within the samples. Supraspinatus tendons from patients with chronic tendinitis had a significantly increased concentration of hyaluronan [30.4 (10.1) micrograms/mg dry weight, p < 0.001], chondroitin sulphate [8.4 (1.8) micrograms/mg dry weight, p < 0.05] and dermatan sulphate [3.8 (1.1) micrograms/mg dry weight, p < 0.001] compared with normal cadaver supraspinatus tendons, although the keratan sulphate content was not significantly different [0.18 (0.05) microgram/mg dry weight]. CONCLUSIONS--The normal supraspinatus tendon has the proteoglycan/glycosaminoglycan of tendon fibrocartilage, which it is suggested is an adaptation to mechanical forces (tension, compression and shear) which act on the rotator cuff tendons in the shoulder, although other factors such as reduced vascularity, low oxygen tension and the influence of local growth factors may also be important. This functional adaptation may have important consequences for the structural strength of the supraspinatus tendon and to influence the ability of the tendon to repair after injury. The glycosaminoglycan composition of tendon specimens from patients with chronic tendinitis is consistent with acute inflammation and new matrix proteoglycan synthesis, even in relatively old tendon specimens and after at least one injection of corticosteroid.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson S. O. Matrix metabolism and healing in the flexor tendon. Experimental studies on rabbit tendon. Scand J Plast Reconstr Surg Hand Surg Suppl. 1991;23:1–51. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Chard M. D., Cawston T. E., Riley G. P., Gresham G. A., Hazleman B. L. Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis. 1994 Jan;53(1):30–34. doi: 10.1136/ard.53.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard M. D., Sattelle L. M., Hazleman B. L. The long-term outcome of rotator cuff tendinitis--a review study. Br J Rheumatol. 1988 Oct;27(5):385–389. doi: 10.1093/rheumatology/27.5.385. [DOI] [PubMed] [Google Scholar]
- Evanko S. P., Vogel K. G. Ultrastructure and proteoglycan composition in the developing fibrocartilaginous region of bovine tendon. Matrix. 1990 Dec;10(6):420–436. doi: 10.1016/s0934-8832(11)80150-2. [DOI] [PubMed] [Google Scholar]
- Fearnley M. E., Vadasz I. Factors influencing the response of lesions of the rotator cuff of the shoulder to local steroid injection. Ann Phys Med. 1969 May;10(2):53–63. doi: 10.1093/rheumatology/10.2.53. [DOI] [PubMed] [Google Scholar]
- Gillard G. C., Reilly H. C., Bell-Booth P. G., Flint M. H. The influence of mechanical forces on the glycosaminoglycan content of the rabbit flexor digitorum profundus tendon. Connect Tissue Res. 1979;7(1):37–46. doi: 10.3109/03008207909152351. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Ippolito E., Natali P. G., Postacchini F., Accinni L., De Martino C. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. J Bone Joint Surg Am. 1980;62(4):583–598. [PubMed] [Google Scholar]
- Koob T. J., Clark P. E., Hernandez D. J., Thurmond F. A., Vogel K. G. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch Biochem Biophys. 1992 Oct;298(1):303–312. doi: 10.1016/0003-9861(92)90127-i. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Merrilees M. J., Tiang K. M., Scott L. Changes in collagen fibril diameters across artery walls including a correlation with glycosaminoglycan content. Connect Tissue Res. 1987;16(3):237–257. doi: 10.3109/03008208709006979. [DOI] [PubMed] [Google Scholar]
- Neer C. S., 2nd Impingement lesions. Clin Orthop Relat Res. 1983 Mar;(173):70–77. [PubMed] [Google Scholar]
- Riemersma D. J., De Bruyn P. Variations in cross-sectional area and composition of equine tendons with regard to their mechanical function. Res Vet Sci. 1986 Jul;41(1):7–13. [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Rothman R. H., Parke W. W. The vascular anatomy of the rotator cuff. Clin Orthop Relat Res. 1965 Jul-Aug;41:176–186. [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Scott J. E., Orford C. R., Hughes E. W. Proteoglycan-collagen arrangements in developing rat tail tendon. An electron microscopical and biochemical investigation. Biochem J. 1981 Jun 1;195(3):573–581. doi: 10.1042/bj1950573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vailas A. C., Pedrini V. A., Pedrini-Mille A., Holloszy J. O. Patellar tendon matrix changes associated with aging and voluntary exercise. J Appl Physiol (1985) 1985 May;58(5):1572–1576. doi: 10.1152/jappl.1985.58.5.1572. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Koob T. J. Structural specialization in tendons under compression. Int Rev Cytol. 1989;115:267–293. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Ordög A., Pogány G., Oláh J. Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. J Orthop Res. 1993 Jan;11(1):68–77. doi: 10.1002/jor.1100110109. [DOI] [PubMed] [Google Scholar]