Abstract
Background
Eosinophilic myocarditis (EM) secondary to eosinophilic granulomatosis with polyangiitis (EGPA) is a rare disease, for which cardiac magnetic resonance imaging (CMRI) is a useful non-invasive modality for diagnosis. We present a case of EM in a patient who recently recovered from COVID-19 and discuss the role of CMRI and endomyocardial biopsy (EMB) to differentiate between COVID-19–associated myocarditis and EM.
Case summary
A 20-year-old Hispanic male with a history of sinusitis and asthma, and who recently recovered from COVID-19, presented to the emergency room with pleuritic chest pain, dyspnoea on exertion, and cough. His presentation labs were pertinent for leucocytosis, eosinophilia, elevated troponin, and elevated erythrocyte sedimentation rate and C-reactive protein. The electrocardiogram showed sinus tachycardia. Echocardiogram showed an ejection fraction of 40%. The patient was admitted, and on day 2 of admission, he underwent CMRI which showed findings of EM and mural thrombi. On hospital day 3, the patient underwent right heart catheterization and EMB which confirmed EM. The patient was treated with steroids and mepolizumab. He was discharged on hospital day 7 and continued outpatient heart failure treatment.
Discussion
This is a unique case of EM and heart failure with reduced ejection fraction as a presentation of EGPA, in a patient who recently recovered from COVID-19. In this case, CMRI and EMB were critical to identify the cause of myocarditis and helped in the optimal management of this patient.
Keywords: Case report, Eosinophilic myocarditis, COVID-associated myocarditis, Cardiac magnetic resonance imaging, Vasculitis
Learning points.
Cardiac magnetic resonance imaging is a potent, non-invasive diagnostic tool in the assessment of myocarditis; eosinophilic myocarditis (EM) shows a patchy or diffuse non-ischaemic sub-endocardial pattern of late gadolinium enhancement (LGE), but can also less frequently demonstrate mid-wall and subepicardial LGE.
Endomyocardial biopsy is definitive for the diagnosis of EM and shows inflammatory infiltrate with nests of eosinophils.
COVID-19 may precipitate previously silent EM, especially in patients with underlying conditions that predispose to eosinophilia, such as eosinophilic granulomatosis with polyangiitis.
Primary specialties involved other than cardiology
Radiology
Introduction
Eosinophilic myocarditis (EM) is a rare myocardial disease, characterized by eosinophilic infiltration of the myocardium. Cardiac damage from eosinophilia can manifest in myocarditis, which can clinically range from mild dyspnoea to symptoms of acute coronary syndrome, restrictive cardiomyopathy, malignant arrhythmias, and life-threatening acute fulminant myocarditis.1–3 Causes of eosinophilia which can trigger EM include hypersensitivity reactions, eosinophilic granulomatosis with polyangiitis (EGPA), and less frequent aetiologies such as infection, clonal myeloid disorders, and hematologic malignancies.1
Timeline
| Day | Events |
|---|---|
| 1 month prior to admission | Positive PCR for COVID-19. Recovers after 1 day of symptoms. |
| Day 0 | Presented to urgent care with a 3-day history of substernal pleuritic chest pain and shortness of breath. Labs significant for leucocytosis with eosinophilic predominance, elevated troponin (peak of 2582 pg/mL), and NT-proBNP (1849 pg/mL). Patient admitted. |
| Day 1 | Transthoracic echocardiogram performed. Infectious work-up including blood cultures for fungal and viral pathogens and respiratory pathogen panel. Started on cefepime and azithromycin for infection concerns based on chest CT. |
| Day 2 | Elevated IgE at 1535 IU/mL (normal <380 IU/mL). Cardiac MRI completed, concerning for myocarditis with mural thrombi. Left ventricle ejection fraction ∼35%. |
| Day 3 | Right heart catheterization (revealed mild pulmonary hypertension) and endomyocardial biopsy performed. Started on high-dose steroids and mepolizumab based on meeting 5/6 American College of Rheumatology criteria for EGPA. Started on angiotensin II receptor blocker (ARB) losartan for afterload reduction. |
| Day 4 | Biopsy revealed eosinophilic myocarditis, confirming MRI findings. |
| Day 5 | Intermittent sinus tachycardia. Denies chest discomfort or dyspnoea. |
| Day 7 | Patient asymptomatic, vital signs normalized. Discharged home with instructions to continue to losartan, mepolizumab, and prednisone taper. |
| 1-week follow-up after discharge | The patient reports feeling well. Heart failure guideline–directed medical therapy started, including adding beta-blocker, and aldosterone antagonist to the ARB. |
| 6-week follow-up after discharge | Tolerated up-titration of heart failure medications. Anticoagulation continued with plan to reassess in 3 months. NT-proBNP persistently elevated at 1224 pg/mL, but downtrending. Continues mepolizumab. |
| 6-month follow-up | Continue outpatient heart failure treatment with ARB, beta-blocker, and aldosterone antagonist, tolerating well. NT-proBNP continues to downtrend, 330 pg/mL. Continues mepolizumab. Anticoagulation discontinued. |
EGPA is an autoimmune vasculitis that affects small- to medium-sized vessels. Previously known as Churg–Strauss syndrome, it is a multisystem disorder characterized by obstructive airway disease, allergic rhinitis, and peripheral blood eosinophilia.4,5 Cardiac manifestations of EGPA include myocarditis, myocardial infarction, pericarditis, pericardial effusion, valvular heart disease, and acute heart failure.6
In the era of the COVID-19 pandemic, literature has emerged correlating COVID-19 infection to clinically suspected cases of myocarditis as well as an association between COVID-19 vaccines to rare instances of myocarditis.7,8 Cardiac magnetic resonance imaging (CMRI) has emerged as a useful non-invasive modality to identify patterns of abnormalities which can allow for separation of COVID-19–associated myocarditis from non-COVID causes of myocarditis, especially with histologically diagnosis of COVID-related myocarditis being difficult to prove.9,10
In this unique case report, we detail a presentation of a 20-year-old male, who recently recovered from COVID-19 infection, presented with subacute onset pleuritic chest pain, dyspnoea, and cough, and was diagnosed with EM and underlying EGPA, following a work-up with CMRI and endomyocardial biopsy (EMB). We aim to describe imaging features of EM on CMRI and how to distinguish it from COVID-19–related myocarditis.
Case presentation
A 20-year-old Hispanic male with a past medical history of chronic sinusitis and asthma presented to the emergency room with 3 days of worsening pleuritic chest pain, dyspnoea on exertion, and cough. He had COVID-19 infection 1 month prior to the current symptom onset.
Upon presentation, the patient was febrile to 100.4 F, blood pressure of 103/74 mmHg, heart rate of 124 b.p.m, and oxygen saturation of 92% on room air. Physical exam was notable for decreased breath sounds and tachycardia with a regular rhythm. There were no physical examination signs to suggest fluid overload. Laboratory investigation revealed a complete blood count significant for leucocytosis of 21.8 × 109/L (normal range: 4–11 × 109/L), and an eosinophil count of 10 227, equivalent to 47% of the differential (normal range: 0–4%). C-reactive protein was elevated to 12.8 mg/dL (normal: < 0.8 mg/dL), and the erythrocyte sedimentation rate was elevated at 50 mm/Hr (normal 0–15 mm/Hr). High-sensitivity troponin T was elevated at 1495 ng/L, with a peak of 1819 ng/L (normal <15 ng/L), and NT-proBNP was elevated at 1849 pg/mL (normal 0–93 pg/mL). Electrocardiogram (ECG) showed sinus tachycardia but was negative for signs of ischemia (see Supplementary material online, Figure S1).
The patient’s pleuritic chest pain, dyspnoea on exertion, cough, fever, significant troponin elevation, elevated inflammatory markers, and absence of acute ischaemic changes on ECG raised concern for myocarditis in the setting of suspected infection, including COVID-19–related myocarditis; lower on the differential was myocardial infarction with non-obstructive coronary arteries (MINOCA). Also in the differential was EGPA given history of asthma and sinusitis, and infection, given patient’s chronic steroid use and underlying immunosuppression. Infectious work-up included respiratory viral panel, which was negative (including for COVID-19). The patient also tested negative for human immunodeficiency virus, histoplasma, Blastomyces, acid-fast bacilli, ascaris, Farmer’s lung, trichinella, Toxocara, Trypanosoma, Strongyloides, Aspergillus galactomannan, and coccidioidomycosis. On hospital day 2, immunology testing revealed an elevated immunoglobulin E (IgE) at 1535 IU/mL (normal <380 IU/mL), with normal IgA, IgG, IgM titres, and negative antinuclear antibody (ANA) and anti-neutrophilic cytoplasmic antibody (ANCA).
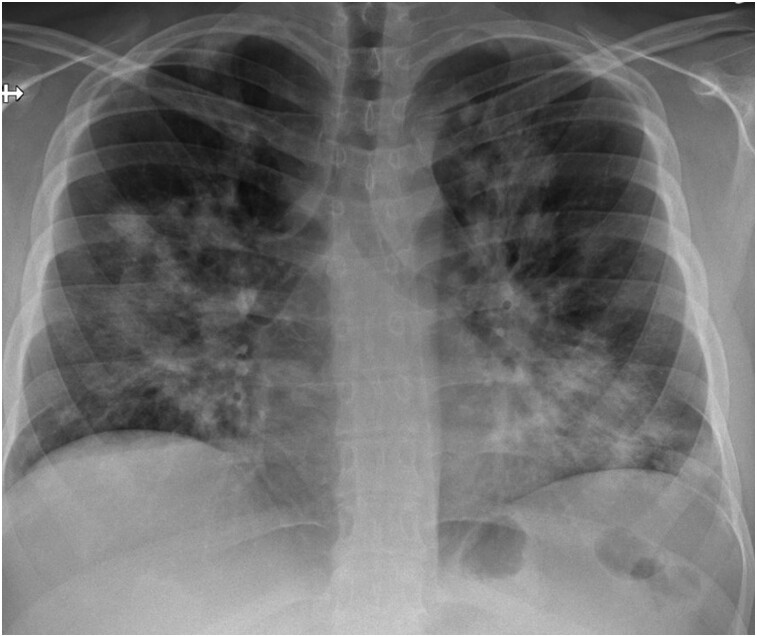
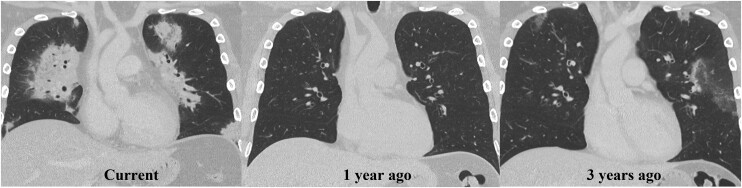
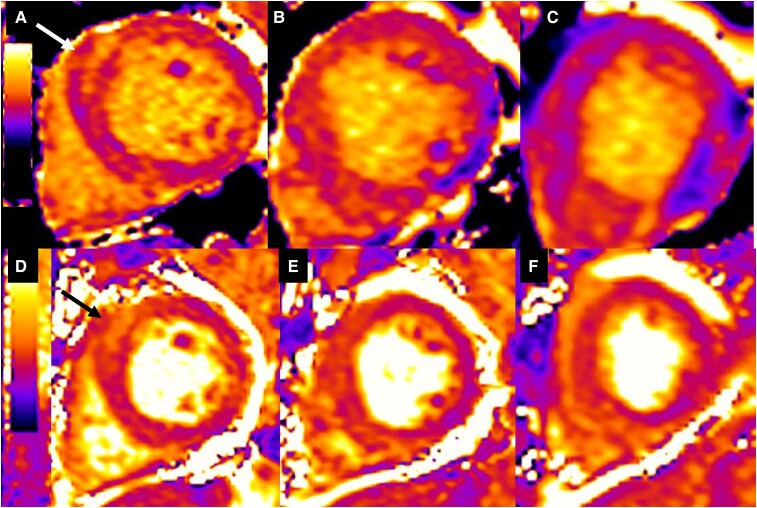
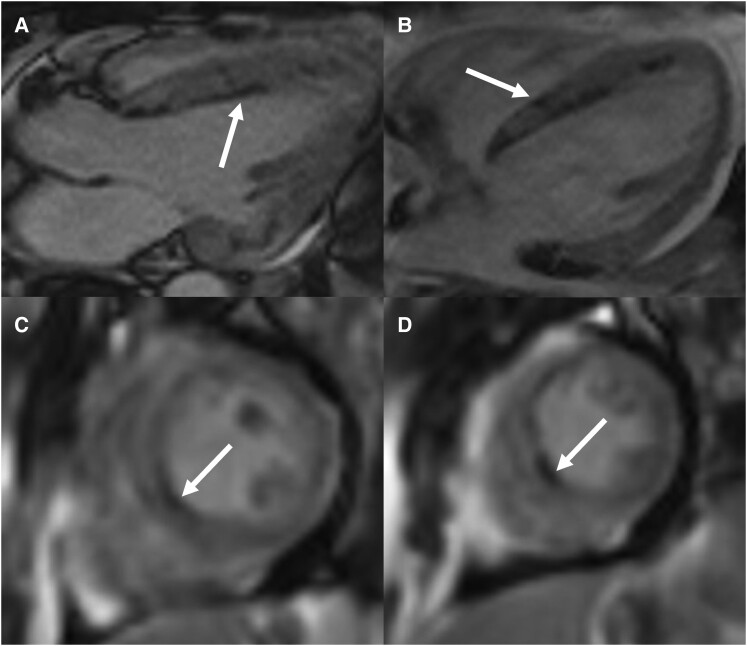
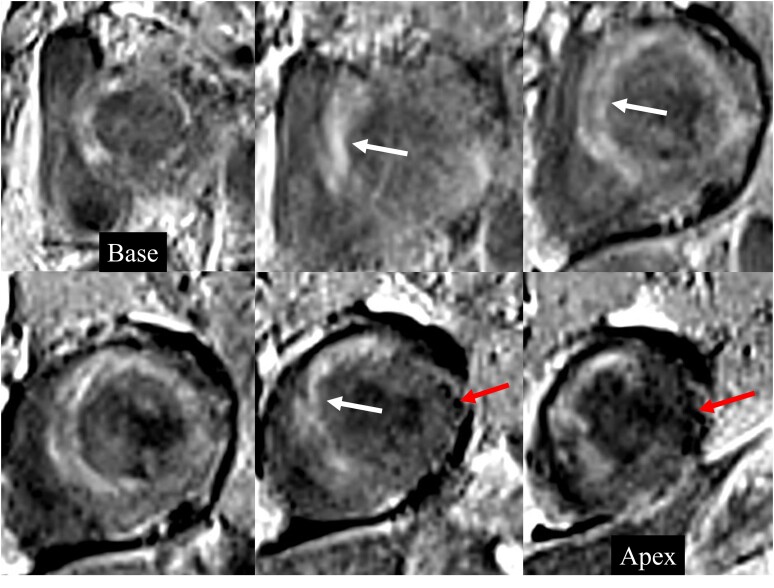
Imaging work-up included chest radiograph, computed tomography (CT) of the chest, transthoracic echocardiography (TTE), and CMRI during inpatient admission. Chest radiograph revealed patchy parenchymal opacification in both lungs predominantly in the perihilar regions (Figure 1). CT chest with contrast showed multiple areas of consolidation with surrounding ground-glass opacification in the bilateral upper lobes and the left lower lobe (Figure 2). Findings raised concern for multifocal atypical infection. Of note, a CT chest performed 3 years prior due to persistent cough not resolving on antibiotics showed left upper lobe airspace opacities (Figure 2). Transthoracic echocardiography demonstrated an ejection fraction of 40% with hypokinesis of the anteroseptal wall and a trivial pericardial effusion. Echocardiogram findings and NYHA Class III symptoms were supportive of heart failure with reduced ejection fraction (HFrEF). Clinical suspicion of myocarditis in the setting of peripheral eosinophilia motivated investigation with CMRI to try differentiating between COVID-19–associated myocarditis (due to recent history) and EM. This differentiation was important since management is different for the two entities. High-dose steroids are required for EM while patients with COVID-19 myocarditis are managed conservatively with supportive treatment. CMRI findings were notable for elevated native T1 myocardial relaxation time, predominantly in the basal and mid-anterior septum, with diffuse oedema on T2 mapping (Figure 3), elevated extracellular volume (ECV) fraction of 50% (see Supplementary material online, Figure S2), multiple sub-endocardial perfusion defects on high inversion time images suggesting mural thrombi (Figure 4, Supplementary material online, Videos S1 and S2), and diffuse heterogeneous near transmural patchy areas of late gadolinium enhancement (LGE), sparing the mid- to distal lateral wall (Figures 5, 6AandB). Left ventricle systolic function was moderately depressed at 35% with near akinesia of basal anteroseptal and mid-inferior septal walls (see Supplementary material online, Video S3). Feature tracking myocardial strain showed significantly deranged circumferential and radial strain predominantly along the septum and anterior wall (see Supplementary material online, Figure S3). The constellation of imaging findings in the setting of peripheral eosinophilia and elevated IgE favoured the diagnosis of eosinophilic myocarditis.
Figure 1.
Frontal chest radiograph shows patchy parenchymal opacification in both lungs predominantly in perihilar regions.
Figure 2.
Migratory pulmonary opacities. Coronal CT chest images from the current study show bilateral consolidative airspace disease with surrounding ground-glass opacity most prominent along the hila and along the bronchovascular tree in the left lower lobe. CT chest from 1 year ago was normal. CT chest from 3 years ago showed ground-glass opacities in the left upper lobe. These findings are consistent with transient pulmonary opacities.
Figure 3.
Native T1 mapping short-axis slices at base (A), mid- (B), and apex (C) level show heterogeneous diffuse myocardial oedema with predominant increase at the basal anterior wall and basal anteroseptum (arrow) wall (elevated global native T1 time at 1170 ms). T2 mapping short-axis slices at base (D), mid- (C), and apex (F) level show heterogeneous diffuse myocardial oedema with a predominant increase at the basal anteroseptum (arrow) wall (elevated T2 time at 70 ms).
Figure 4.
High TI images: three-chamber (A) and four-chamber (B) cine images acquired immediately after contrast administration show multiple patchy areas of perfusion defect along the endocardial and epicardial borders of the septum (arrows). High inversion time (600 ms) post-contrast short-axis image slices at mid-cavity (C) and distal cavity (D) show that these perfusion defects are persistent, suggesting intramural thrombi.
Figure 5.
Late gadolinium–enhanced images. Short-axis LGE images from base to apex show diffuse heterogeneous near transmural patchy areas of enhancement (white arrows) involving the entire heart with sparing of mid- to distal lateral wall (red arrows).
Figure 6.
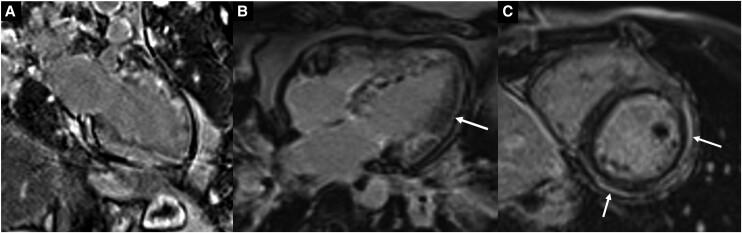
Late gadolinium–enhanced images of a two-chamber (A) and four-chamber (B) view showing diffuse heterogeneous near transmural patchy areas of enhancement involving the entire heart with sparing of mid- to distal lateral wall (arrow) in this patient with eosinophilic myocarditis and comparison LGE short-axis image (C) showing subepicardial enhancement (arrows) in the lateral and inferior walls in a separate patient with COVID-19–associated myocarditis.
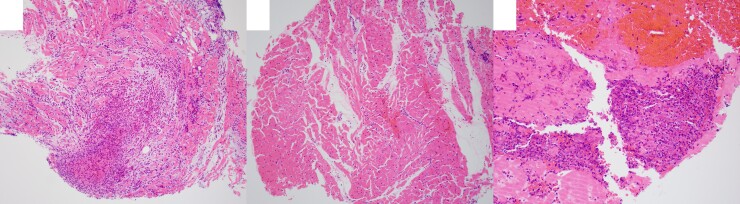
To further confirm EM, on hospital day 3, the patient underwent right heart catheterization using an echocardiogram and fluoroscopic guidance that yielded borderline elevated left and right-sided filling pressures with mildly elevated pulmonary artery pressures (see Supplementary material online, Figure S4). Due to the presence of multiple thrombi on the left ventricular side of the septum, only right EMB was performed to reduce the risk of embolization. Pathology showed inflammatory infiltrates composed of numerous eosinophils with extensive degranulation and patchy, multifocally extensive myocyte damage/necrosis associated with inflammatory infiltrates and three of the five specimens showed eosinophil-rich thrombus (Figure 7). Findings were concordant with MRI and supportive of eosinophilic myocarditis.
Figure 7.
EMB pathology showed patchy, multifocally extensive myocyte damage/necrosis associated with areas of eosinophil-rich thrombus and infiltrating eosinophils with extensive degranulation. Infiltrating eosinophils are represented by round/oval cells with primary purple granules, and secondary orange/red refractile granules, with increased cytoplasm and indented or bilobed nuclei.
The patient was also evaluated by allergy/immunology, haematology, pulmonology, and infectious disease teams. The patient met five of the six criteria from the American College of Rheumatology for EGPA diagnosis, resulting in 85% specificity and 99% sensitivity for diagnosis of EGPA.5 These included (i) asthma, (ii) > 10% eosinophils on CBC with differential, (iii) migratory or transient pulmonary opacities, (iv) paranasal sinus abnormalities, and (v) biopsy demonstrating vasculitis with accumulation of eosinophils.
During the inpatient stay, the patient was managed with high-dose steroids (prednisone 80 mg daily by mouth) and administration of one dose of mepolizumab, 300 mg subcutaneous injection, for treatment of myocarditis, and with antibiotics (cefepime and azithromycin) for suspected pneumonia during the initial hospital course. The patient was also started on warfarin 5 mg due to the mural thrombi observed on CMRI and given his high risk for thrombosis in the hyper-inflammatory state. For the heart failure symptoms, he was started on 12.5 mg losartan; a beta-blocker was initially deferred as the patient’s sinus tachycardia was assessed to be compensatory. The patient’s symptoms improved, and on hospital day 7, the patient was haemodynamically stable, ambulatory, and deemed safe for discharge, after which he continued outpatient treatment for heart failure, including losartan 12.5 mg, metoprolol succinate 25 mg, empagliflozin 10 mg daily, and spironolactone 12.5 mg daily. The patient was maintained on 80 mg prednisone by mouth for a total of 14 days, followed by a 3-week taper. The mepolizumab was maintained indefinitely, 300 mg subcutaneous injection every 4 weeks. Six weeks after discharge, the patient was tolerating up-titration of his heart failure medications (losartan 25 mg, metoprolol succinate 50 mg, empagliflozin 10 mg daily, and spironolactone 25 mg daily), continuing warfarin and mepolizumab, and laboratory results revealed persistently elevated, but down-trending NT-proBNP (722 pg/mL, normal 0–93 pg/mL). At 6 months following discharge, the patient was doing well with no significant complaints, and NT-prBNP had downtrended to 330 pg/mL. The patient’s anticoagulation was discontinued, but heart failure treatment with an angiotensin II receptor blocker (ARB), beta-blocker, and aldosterone antagonist was maintained, which the patient tolerated well. The transthoracic echocardiogram (TTE) noted an improvement of the ejection fraction to 45%. While there are no clear data on this patient population, shared decision-making between the heart failure physician and patient was made to continue guideline-directed medical therapy given his extensive scar burden, improvement in ejection fraction, and tolerability of medications.
Discussion
We present a unique case of EM presenting as HFrEF in the setting of EGPA, in a patient who recovered from COVID-19 infection. Although EGPA and its cardiac manifestations are well studied, this case highlights the importance of CMRI in distinguishing diagnoses of EM and COVID-19–associated myocarditis.
Eosinophilic infiltration into the myocardium can result in variable cardiac damage ranging from acute myocarditis to endomyocardial fibrosis. Definitive diagnosis of EM is made from EMB; however, this is invasive and prone to sampling error due to heterogeneous infiltration and has low sensitivity in non-acute settings.11,12 The current standard of non-invasive diagnosis is CMRI, which has utility in both acute and subacute and chronic settings, as it helps detect myocardial oedema and fibrosis.2 The updated Lake Louise main criteria for myocardial inflammation rely on non-ischaemic myocardial injury as demonstrated by a regional or global increase of native T1 or ECV, or regional LGE signal increase, and evidence of myocardial oedema suggested by visible myocardial oedema or regional or global increase of native T2, or increased T2 signal intensity ratio.13 Eosinophilic myocarditis, more specifically, tends to follow a patchy or diffuse non-ischaemic sub-endocardial pattern of LGE but can also less frequently demonstrate mid-wall and subepicardial LGE.2,14,15 The presence of intra-cardiac thrombi is also associated with eosinophilic heart disease.14 Corticosteroids are the mainstay of treatment for EM, and therapy is further tailored based on the underlying cause.3 In this case, the patient met five out of six criteria from the American College of Rheumatology for an underlying diagnosis of EGPA.5
COVID-19–related myocardial injury has been shown in multiple recent studies with evidence of persisting myocardial damage in patients recovering from COVID-19 in non-acute setting.9,16 In this case, recent history of COVID-19 was a confounder while evaluating the underlying cause of myocarditis in the setting of eosinophilia and heart failure symptoms. Recent case reports have also suggested COVID-19 infection manifesting previously silent EM and COVID-19 vaccination preceding fulminant EM, raising the possibility that COVID-19 may be associated with an immune response leading to eosinophilia and hypereosinophilic syndrome.17,18 While not possible to prove in our patient, these recent case reports allow us to postulate a probability that the patient’s recent COVID-19 infection may have precipitated EM in the setting of EGPA. Both CMRI and EMB were helpful to confirm the diagnosis of EM before initiating high-dose steroid treatment. CMRI findings supporting myocarditis included elevated native global T1 and T2 relaxation time, elevated ECV, and presence of LGE. However, in the acute setting, T1 and ECV fraction along with T2 values is often increased in both EM and COVID-related myocardial injury, and thus cannot reliably differentiate the two.15,19 The presence of oedema in EM also depends on the phase of the disease, and as such, the pattern of LGE distribution is important. LGE is an overall less prevalent finding on CMRI in COVID-19 myocarditis compared to EM; when present, it is more frequently seen in the mid-ventricular region and with a non-ischaemic subepicardial pattern (Figure 6C).14,20 Our case was unique in that it showed extensive heterogeneous near transmural enhancement with patchy subepicardial enhancement along the septal right ventricle wall. Although the LGE findings alone did not distinguish the more frequently seen patterns with EM or COVID-19 myocarditis, the presence of intramural thrombi and depressed left ventricle systolic function favoured eosinophilic heart disease, especially in the setting of eosinophilia and elevated IgE. CMRI imaging findings were most supportive of EM. However, before initiating high-dose steroid treatment for EM, EMB was essential to know the aetiology and histology of myocarditis and confirm the presence of eosinophilic infiltrates in our patient. In comparison, COVID-19–associated myocarditis is predominantly lymphocytic in histology. As such, in patients with COVID-19 infection and high suspicion of alternate diagnosis for myocarditis, EMB may be undertaken. In this case report, CMRI findings established a diagnosis of myocarditis and helped differentiate between COVID-19 myocarditis from EM and EGPA as the cause of the patient’s presentation.
Conclusion
Our case highlights how CMRI can provide imaging biomarkers to help differentiate between COVID-19 and eosinophilic myocarditis and should be used early in the disease course in patients presenting with symptoms of heart failure or MINOCA, for optimal management.
Supplementary Material
Contributor Information
Karan Rao, Department of Radiology, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 522422, USA.
Michael Arustamyan, Department of Cardiovascular Medicine, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52242, USA.
Abby Walling, Carver College of Medicine, University of Iowa, 375 Newton Rd, Iowa City, IA 52242, USA.
Georgios Christodoulidis, Department of Cardiovascular Medicine, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52242, USA.
Mahi Ashwath, Department of Cardiovascular Medicine, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52242, USA.
Joshua Hagedorn, Carver College of Medicine, University of Iowa, 375 Newton Rd, Iowa City, IA 52242, USA.
Sarv Priya, Department of Radiology, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 522422, USA.
Lead author biography
 Dr. Karan Rao is a PGY-4 resident in diagnostic radiology at the University of Iowa. He completed his M.D. at the Carver College of Medicine at the University of Iowa, Iowa City, IA, and a BSE in Biomedical Engineering. His current research interests include interventional radiology (assessing complications following implementation of a novel chest tube removal protocol for post-biopsy pneumothorax) and quality improvement projects assessing the impact of a paid reading room coordinator/assistant on the efficiency of on-call overnight and weekend radiology residents. He also enjoys teaching, medical student education, travelling, and going to the gym!
Dr. Karan Rao is a PGY-4 resident in diagnostic radiology at the University of Iowa. He completed his M.D. at the Carver College of Medicine at the University of Iowa, Iowa City, IA, and a BSE in Biomedical Engineering. His current research interests include interventional radiology (assessing complications following implementation of a novel chest tube removal protocol for post-biopsy pneumothorax) and quality improvement projects assessing the impact of a paid reading room coordinator/assistant on the efficiency of on-call overnight and weekend radiology residents. He also enjoys teaching, medical student education, travelling, and going to the gym!
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: Written consent for submission and publication of this case report including images and associated text was obtained from the patient in line with COPE guidance.
Funding: None declared.
Data availability
All data are incorporated into the article and its online supplementary material.
References
- 1. Brambatti M, Matassini MV, Adler ED, Klingel K, Camici PG, Ammirati E. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol 2017;70:2363–2375. [DOI] [PubMed] [Google Scholar]
- 2. Kuchynka P, Palecek T, Masek M, Cerny V, Lambert L, Vitkova I, et al. Current diagnostic and therapeutic aspects of eosinophilic myocarditis. Biomed Res Int 2016;2016:2829583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheung CC, Constantine M, Ahmadi A, Shiau C, Chen LYC. Eosinophilic myocarditis. Am J Med Sci 2017;354:486–492. [DOI] [PubMed] [Google Scholar]
- 4. Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013;65:270–281. [DOI] [PubMed] [Google Scholar]
- 5. Grayson PC, Ponte C, Suppiah R, Robson JC, Craven A, Judge A, et al. 2022 American college of rheumatology/European Alliance of associations for rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis 2022;81:309–314. [DOI] [PubMed] [Google Scholar]
- 6. Pakbaz M, Pakbaz M. Cardiac involvement in eosinophilic granulomatosis with polyangiitis: a meta-analysis of 62 case reports. J Tehran Heart Cent 2020;15:18–26. [PMC free article] [PubMed] [Google Scholar]
- 7. Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 cardiac registry. JAMA cardiology 2021;6:1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices—United States, June 2021. MMWR Morb Mortal Wkly Rep 2021;70:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luetkens JA, Isaak A, Öztürk C, Mesropyan N, Monin M, Schlabe S, et al. Cardiac MRI in suspected acute COVID-19 myocarditis. Radiol Cardiothorac Imaging 2021;3:e200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kassem KM, Souka A, Harris DM, Parajuli S, Cook JL. Eosinophilic myocarditis. Circ Cardiovasc Imaging 2019;12:e009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rezaizadeh H, Sanchez-Ross M, Kaluski E, Klapholz M, Haider B, Gerula C. Acute eosinophilic myocarditis: diagnosis and treatment. Acute Card Care 2010;12:31–36. [DOI] [PubMed] [Google Scholar]
- 13. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 14. Antonopoulos AS, Azzu A, Androulakis E, Tanking C, Papagkikas P, Mohiaddin RH. Eosinophilic heart disease: diagnostic and prognostic assessment by cardiac magnetic resonance. Eur Heart J Cardiovasc Imaging 2021;22:1273–1284. [DOI] [PubMed] [Google Scholar]
- 15. Beck KS, Jeong SY, Lee KY, Chang K, Jung JI. Native T1 mapping demonstrating apical thrombi in eosinophilic myocarditis associated with Churg-Strauss syndrome. Korean Circ J 2016;46:882–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanacli R, Doeblin P, Götze C, Zieschang V, Faragli A, Stehning C, et al. COVID–19 vs. classical myocarditis associated myocardial injury evaluated by cardiac magnetic resonance and endomyocardial biopsy. Front Cardiovasc Med 2021;8:737257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohtani K, Takahama S, Kato S, Higo T. Acute necrotizing eosinophilic myocarditis after COVID-19 vaccination. Eur Heart J 2022;43:2640. [DOI] [PubMed] [Google Scholar]
- 18. Mapelli M, Cefalu C, Zaffalon D, Annoni A, Agostoni P. Hypereosinophilic syndrome onset with Loeffler's endocarditis after COVID-19 infection. Eur Heart J Cardiovasc Imaging 2022;23:e472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanghvi SK, Schwarzman LS, Nazir NT. Cardiac MRI and myocardial injury in COVID-19: diagnosis. Risk stratification and prognosis. Diagnostics (Basel) 2021;11:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ojha V, Verma M, Pandey NN, Mani A, Malhi AS, Kumar S, et al. Cardiac magnetic resonance imaging in coronavirus disease 2019 (COVID-19): a systematic review of cardiac magnetic resonance imaging findings in 199 patients. J Thorac Imaging 2021;36:73–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online supplementary material.