Abstract
Objective
To determine the relative efficacy of structured named diet and health behaviour programmes (dietary programmes) for prevention of mortality and major cardiovascular events in patients at increased risk of cardiovascular disease.
Design
Systematic review and network meta-analysis of randomised controlled trials.
Data sources
AMED (Allied and Complementary Medicine Database), CENTRAL (Cochrane Central Register of Controlled Trials), Embase, Medline, CINAHL (Cumulative Index to Nursing and Allied Health Literature), and ClinicalTrials.gov were searched up to September 2021.
Study selection
Randomised trials of patients at increased risk of cardiovascular disease that compared dietary programmes with minimal intervention (eg, healthy diet brochure) or alternative programmes with at least nine months of follow-up and reporting on mortality or major cardiovascular events (such as stroke or non-fatal myocardial infarction). In addition to dietary intervention, dietary programmes could also include exercise, behavioural support, and other secondary interventions such as drug treatment.
Outcomes and measures
All cause mortality, cardiovascular mortality, and individual cardiovascular events (stroke, non-fatal myocardial infarction, and unplanned cardiovascular interventions).
Review methods
Pairs of reviewers independently extracted data and assessed risk of bias. A random effects network meta-analysis was performed using a frequentist approach and grading of recommendations assessment, development and evaluation (GRADE) methods to determine the certainty of evidence for each outcome.
Results
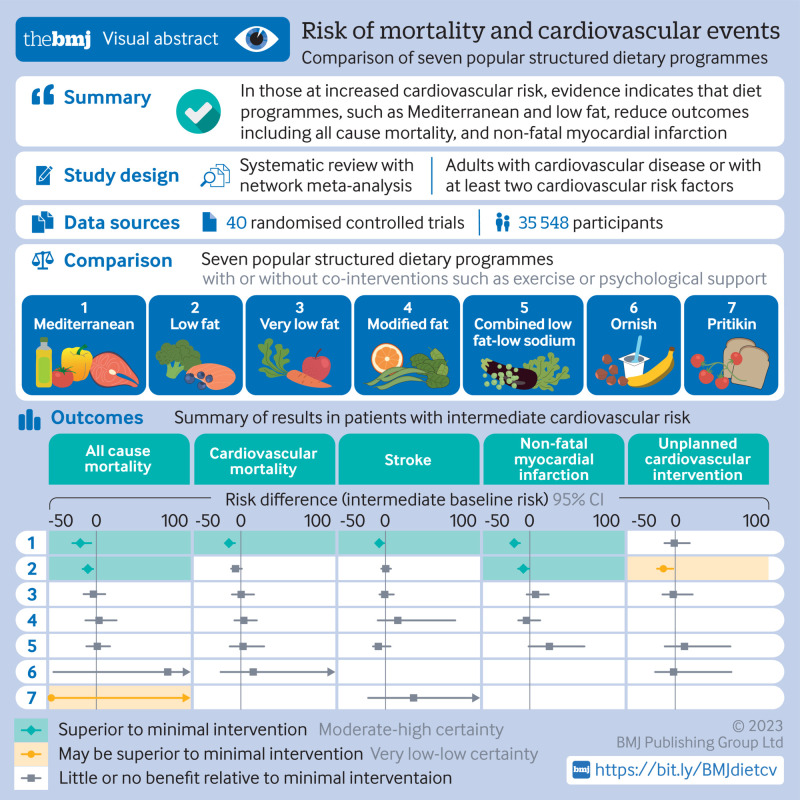
40 eligible trials were identified with 35 548 participants across seven named dietary programmes (low fat, 18 studies; Mediterranean, 12; very low fat, 6; modified fat, 4; combined low fat and low sodium, 3; Ornish, 3; Pritikin, 1). At last reported follow-up, based on moderate certainty evidence, Mediterranean dietary programmes proved superior to minimal intervention for the prevention of all cause mortality (odds ratio 0.72, 95% confidence interval 0.56 to 0.92; patients at intermediate risk: risk difference 17 fewer per 1000 followed over five years), cardiovascular mortality (0.55, 0.39 to 0.78; 13 fewer per 1000), stroke (0.65, 0.46 to 0.93; 7 fewer per 1000), and non-fatal myocardial infarction (0.48, 0.36 to 0.65; 17 fewer per 1000). Based on moderate certainty evidence, low fat programmes proved superior to minimal intervention for prevention of all cause mortality (0.84, 0.74 to 0.95; 9 fewer per 1000) and non-fatal myocardial infarction (0.77, 0.61 to 0.96; 7 fewer per 1000). The absolute effects for both dietary programmes were more pronounced for patients at high risk. There were no convincing differences between Mediterranean and low fat programmes for mortality or non-fatal myocardial infarction. The five remaining dietary programmes generally had little or no benefit compared with minimal intervention typically based on low to moderate certainty evidence.
Conclusions
Moderate certainty evidence shows that programmes promoting Mediterranean and low fat diets, with or without physical activity or other interventions, reduce all cause mortality and non-fatal myocardial infarction in patients with increased cardiovascular risk. Mediterranean programmes are also likely to reduce stroke risk. Generally, other named dietary programmes were not superior to minimal intervention.
Systematic review registration
PROSPERO CRD42016047939
Introduction
Worldwide, estimates have attributed 22% of adult deaths and 15% of disability adjusted life years to dietary habits. If this is true, diet is a leading cause of death and major morbidity.1 Advocates have proposed that numerous diets, with or without exercise and behavioural support (referred to as dietary programmes), reduce major cardiovascular events. These diets include those low in total or saturated fats (eg, the National Cholesterol Education Program diets), Mediterranean style diets, and the Dietary Approaches to Stop Hypertension (DASH) diet.2 Although dietary guidelines have suggested that a number of dietary programmes might reduce the risk of major cardiovascular events, they have typically relied on surrogate outcomes, or low or very low certainty evidence from non-randomised study designs.3 4 5 6 7 Several pairwise meta-analyses of randomised controlled trials have suggested that some diets and dietary programmes reduce cardiovascular events, but any beneficial impact on mortality is uncertain.8 9 10 11 12
To date, network meta-analyses are lacking that systematically summarise randomised controlled trials and compare the impact of structured dietary programmes on mortality and major cardiovascular events (eg, stroke and myocardial infarction). Network meta-analytic methods enable the use of direct (head-to-head active interventions) and indirect evidence (intervention v non-active control) for the comparison of interventions that have not been directly compared, which can yield more precise summary estimates.13 Therefore, we performed a systematic review and network meta-analysis of randomised controlled trials to compare structured named dietary programmes for the prevention of mortality and major cardiovascular outcomes.
Methods
Protocol registration
The study protocol is registered with PROSPERO (CRD42016047939) and is accessible online (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42016047939).
Search strategy
With assistance from a medical librarian, we searched five databases: AMED (Allied and Complementary Medicine Database), Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Medline, and CINAHL (Cumulative Index to Nursing and Allied Health Literature) from inception to September 2021, and ClinicalTrials.gov for unpublished and ongoing trials. Our search strategy involved keywords for diets, mortality or major cardiovascular outcomes, and randomised controlled trials (see appendix texts S2-S4 for search strategy details). We also reviewed reference lists of related systematic reviews and eligible trials to identify additional studies.
Eligibility criteria
We included randomised trials published in English of adults at increased risk of cardiovascular disease that compared at least one structured dietary programme with minimal intervention, or with other cardiovascular risk reduction interventions (including other dietary programmes). Eligible trials enrolled patients with two or more established risk factors for cardiovascular disease (eg, hypertension, dyslipidaemia, obesity, diabetes mellitus), or established cardiovascular disease (history of coronary artery disease, myocardial infarction, stroke, or peripheral artery disease). In addition to the diet intervention, structured dietary programmes could include non-dietary interventions, such as exercise, or psychosocial or behavioural support. Smoking cessation interventions and drug treatments were also allowed, but were considered cointerventions. Eligible studies had at least nine months of intervention and reported on the incidence of all cause or cardiovascular mortality, or major cardiovascular events (stroke, non-fatal myocardial infarction, angina, heart failure, peripheral vascular events, atrial fibrillation, and unplanned cardiovascular interventions). Our protocol prespecified primary outcomes were all cause and cardiovascular mortality. We focused our analysis on each study’s last available follow-up; the appendix also provides outcomes at 12 months of follow-up. Two reviewers independently determined eligibility for each record; if necessary, a third senior reviewer resolved discrepancies.
Data extraction and risk of bias assessment
Two reviewers used pretested forms and standardised instructions to independently extract population, intervention, and outcome data. Reviewers assessed risk of bias independently and in duplicate by using the Cochrane risk of bias tool (version 1.0)14 and resolved disagreement by discussion or consultation with a third reviewer. We categorised studies with a low risk of bias in three key domains (random sequence generation, allocation concealment, and missing participant outcome data) as having low overall risk of bias. All other studies were classified as being at high overall risk of bias (appendix text S1).
Dietary programmes classification
Our protocol specified three classification schemes of dietary programmes to create three network meta-analyses: named diet, food category composition, and macronutrient composition. The macronutrient composition analysis could not be run because of a lack of sufficient interventions to create a network (no low carbohydrate diets), while the food category composition analysis was limited by unclear reporting of food intake in most studies. For these reasons, together with space limitations, this paper reports the first analysis (named dietary programmes) only. We grouped dietary programmes into several named diet categories (table 1): low fat, very low fat, modified fat, combined low fat and low sodium, Mediterranean, Ornish, Pritikin, or minimal intervention. Our definitions of these programmes are consistent with a previous systematic review.15
Table 1.
Description of named dietary programme categories used in this systematic review
| Named dietary programme category | Description |
|---|---|
| Low fat | Total fat intake reduced to 20-30% of caloric intake; saturated fat intake reduced to <10% of caloric intake |
| Very low fat | Total fat intake reduced to 10-20% of caloric intake |
| Combined low fat and low sodium | As in low fat diet, plus sodium reduction (<2.4 g/day) |
| Modified fat | No decrease in total fat intake, but increase in polyunsaturated to saturated fat ratio |
| Mediterranean | Increased fish, fruit, and vegetable intake; increased intake of monounsaturated fats (eg, olive oil) |
| Ornish | Total fat intake reduced to <10% of caloric intake; primarily plant based |
| Pritikin | Total carbohydrate intake 70-75% of caloric intake; total protein intake 15-20% of caloric intake; total fat intake 5-10% of caloric intake; fibre intake 40-45 g/1000 kilocalories |
| Minimal intervention | Usual diet or no advice, referral to own physician, usual care, non-dietary programming, or minimal dietary advice |
While our protocol initially only specified low fat as a single named diet category, we divided this into low fat (20-30% calories from fat) and very low fat (≤20% calories from fat) to distinguish standard low fat dietary programmes from programmes with a more intense fat reduction goal. We also added a modified fat category (no decrease in total fat intake, but increased polyunsaturated fats). These categories are consistent with previous systematic reviews.15 16
Minimal intervention could include patients not receiving any dietary advice; referral to own physician or provision of usual care; educational sessions on non-dietary matters; or receipt of dietary information such as brochures or brief advice from a clinician but with minimal reinforcement (only at one visit or very infrequently, such as annually). When minimal intervention involved some dietary counselling, it was less frequently used or reinforced than counselling in the intervention (half as much or less). While our protocol used the language of waiting list or placebo to describe non-active control arms, we adopted the more descriptive term of minimal intervention from a recent systematic review,9 a term that better reflects the minimal intervention nature of the control arms.
To explore the potential for effect modification, we conducted a subgroup analysis on the effect of intervention intensity on outcomes. A definition of intervention intensity was adopted from the United States Preventive Services Taskforce (USPSTF).17 Interventions with at least two group or individual sessions a month for the first three months (or equivalent, that is, at least six sessions in the first three months) were considered high intensity interventions. Other interventions were considered low intensity.
Given the limitations of dietary adherence data, the lack of reporting on adherence, and the heterogeneity of methods and time points among studies that did report adherence, we classified eligible interventions by the intended dietary programme irrespective of the extent to which participants followed the prescribed diet.
Statistical analysis
For all direct comparisons, when two or more randomised controlled trials were available, we performed conventional pairwise meta-analysis using a DerSimonian and Laird random effects model. We used the I2 statistic and visual inspection of the forest plots to assess heterogeneity in direct comparisons. For all direct comparisons informed by 10 studies or more, we assessed small study effects using Harbord’s test.18
Assuming a common heterogeneity parameter,19 20 we then conducted a frequentist random effects network meta-analysis using Stata (version 16). For all outcomes, the analysis generated an odds ratio with a 95% confidence interval as the summary measure. Network coherence was assessed globally using the design-by-treatment model.21 Loop specific incoherence was evaluated using node splitting to generate incoherence factor values.22 23 We estimated ranking probabilities using the surface under the cumulative ranking curve, mean ranks, and rankograms.24 25
Risk differences were calculated by applying the summary odds ratios to baseline risks (events per 1000 patients).26 We calculated two risk differences: one assuming an intermediate baseline risk (5-10% five year cardiovascular event risk) and another assuming a high baseline risk (20-30% five year cardiovascular event risk). Baseline risks were estimated using control group data from a meta-analysis by the Cholesterol Treatment Trialists’ Collaboration.27 Appendix text S1 provides further explanation.
We performed network meta-regression to investigate the effects of follow-up duration and presence of programme components besides diet (exercise, behavioural or psychosocial support such as stress management, drug treatment, and smoking cessation) on our outcomes of interest. Behavioural support and exercise were protocol defined effect modifiers, while drug treatment and smoking cessation were added post hoc in response to trials that included these cointerventions. Sensitivity analyses were performed excluding trials that included smoking or drug treatment interventions. We also performed sensitivity analyses excluding two studies because of concerns about data integrity.28 29 For the outcome of non-fatal myocardial infarction, we excluded three additional studies in which reporting of non-fatal myocardial infarctions was unclear.30 31 32 Appendix tables S3-S19 report original and sensitivity analysis results. In the main paper, when sensitivity analysis excluding studies with data integrity concerns substantially changed our findings, we adopted it as our main analysis, reporting its results over the findings of the original analysis.
Assessment and communication of certainty of evidence
The grading of recommendations assessment, development and evaluation (GRADE) approach for network meta-analysis guided our assessment and communication of certainty of evidence.33 34 For indirect evidence, we focused on the dominant most direct loop and rated the certainty as the lowest certainty evidence from the contributing direct comparisons. Network estimate certainty started as the certainty of the dominant contributor to the network estimate. We further rated down the certainty in the network estimate if there was incoherence between the indirect and direct estimates, or if there was imprecision around the treatment effect.
We used a minimally contextualised framework with the null value as our decision threshold, meaning that the certainty of evidence refers to our certainty that the intervention has, relative to minimal intervention, any beneficial effect or has little or no benefit.35 36 We rated for the presence of little or no effect if, and only if, both of the following conditions were met: the result was not statistically significant and the point estimate suggested no or trivial benefit. Otherwise, we rated for the presence of beneficial effect and rated down for imprecision when results were not statistically significant. We adopted our threshold for trivial benefit from a previous dietary guideline panel that included members of the public.37 For fatal outcomes, this threshold was an absolute risk reduction of <10 events per 1000, <20 per 1000 for non-fatal outcomes, and <15 per 1000 for mixed fatal and non-fatal outcomes. To summarise results, for each outcome, we classified evidence as high or moderate certainty versus low or very low certainty, and within each certainty of evidence category, by whether dietary programmes were superior to minimal intervention or not (classification as superior or having little or no benefit).38 Appendix text S1 presents further details about our use of the GRADE approach.
Patient and public involvement
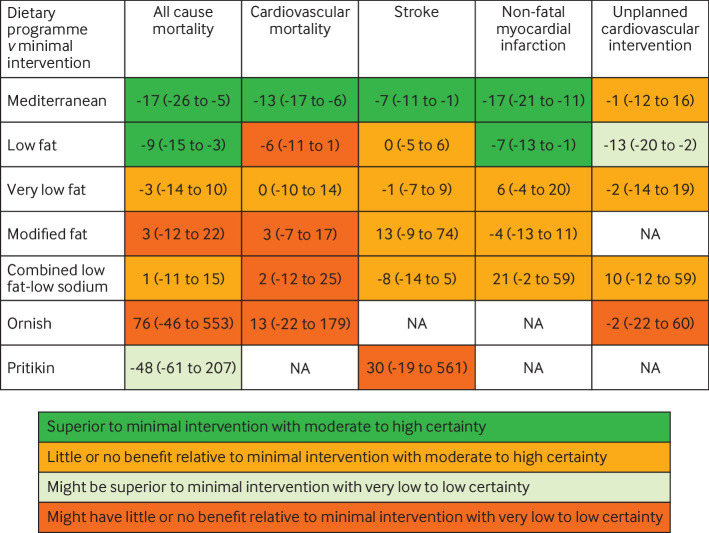
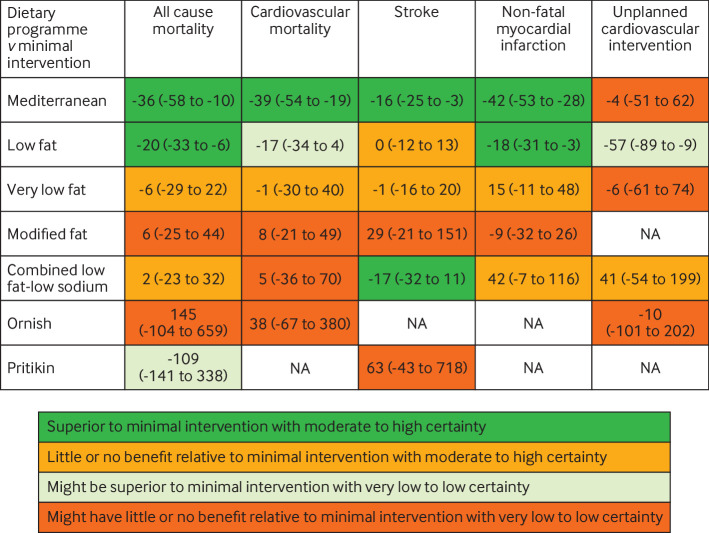
No patients were involved in setting the research question, outcome measures, study design, or data interpretation. However, after production of our first manuscript draft, we consulted a patient advocate with established cardiovascular disease who has been advised on dietary and lifestyle changes by dietitians and physicians. We received feedback that the estimate tables (fig 1 and fig 2) and certainty of evidence statements were very useful, allowing quick comparison of dietary programmes with respect to absolute risk reduction and certainty of risk reduction.
Fig 1.
Summary of results of named dietary programmes network meta-analysis at last follow-up. The number is the point estimate (with 95% confidence interval) of the risk difference (per 1000 over five years), calculated using an intermediate baseline risk. NA=not applicable
Fig 2.
Summary of results of named dietary programmes network meta-analysis at last follow-up. The number is the point estimate (with 95% confidence interval) of the risk difference (per 1000 over five years), calculated using a high baseline risk. NA=not applicable
Results
Search
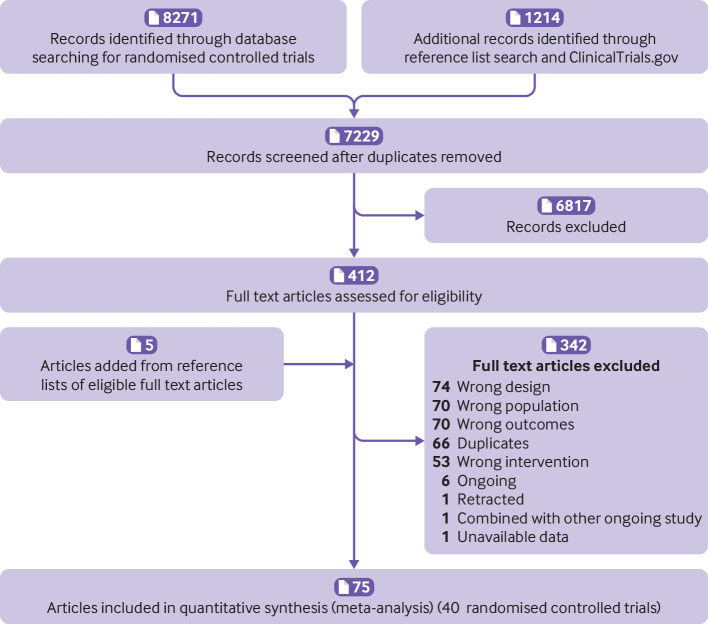
Among primary databases, we found 8271 citations and identified an additional 1214 records through reference list screening and ClinicalTrials.gov. After duplicate removal and title and abstract screening, reviewers assessed 412 articles for eligibility, of which 40 trials proved eligible (fig 3).
Fig 3.
PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flow diagram
Study characteristics
Of the 40 eligible trials (n=35 548), nine (n=17 632) studied a primary prevention population, and 31 (n=17 916) a secondary prevention population. The dietary programmes studied included low fat (18 studies), Mediterranean (12), very low fat (6), modified fat (4), combined low fat and low sodium (3), Ornish (3), and Pritikin (1). The median duration of follow-up was three years (range 0.75-17 years). On assessing the methodological quality of eligible trials, we judged 13 to be at low overall risk of bias and 27 at high risk (appendix table S1).
Dietary programmes mostly involved reinforcement at least every three months in the first year of the intervention. A few studies reported less frequent reinforcement: one trial involved reinforcement every four months.39 A second trial involved two initial meetings with reinforcement every six months.40 The Lyon Diet Heart Study, a Mediterranean diet programme, involved only an initial hour long session, with reinforcement at eight week follow-up, then annually.41 In these three trials, the minimal intervention arms involved no dietary advice.
In trials where minimal intervention arms involved some dietary counselling (eg, as a part of usual care), participants allocated to usual care were able to access this service much less than intervention arm participants. In one trial, dietary counselling was used by only 28% of usual care participants (compared with 83% of the intervention group).42 In another, participants assigned to minimal intervention received booklets and a single dietetic interview, while the intervention group received an interview every three months.43 In trials that involved physical activity, a frequent goal was moderate intensity exercise for approximately 150 minutes per week.
Network meta-analysis results
Figure 4 shows the network graph of the named dietary programme analysis at last follow-up (median three years) for the outcome of all cause mortality. Appendix figures S1-S6 present all network graphs. The supplement also presents league tables showing the results of all comparisons for all outcomes (appendix tables S3-S19 for odds ratios, including sensitivity analyses; appendix tables S60-S71 for risk differences). Appendix tables S72-S77 provide the GRADE assessment showing the number of included randomised controlled trials, sample size, I2 values, all direct and indirect estimates, and incoherence data. We rated down low fat and Mediterranean dietary programmes for indirectness because of the varying presence of cointerventions (in particular, smoking cessation and drug treatment) in trials comparing these programmes with minimal intervention. Other dietary programmes had fewer cointerventions, or had no apparent beneficial effects, and so we did not rate down for indirectness. We also rated down most indirect estimates comparing two dietary programmes for intransitivity because the diet of participants in minimal intervention groups generally varied depending on the intervention diet.
Fig 4.
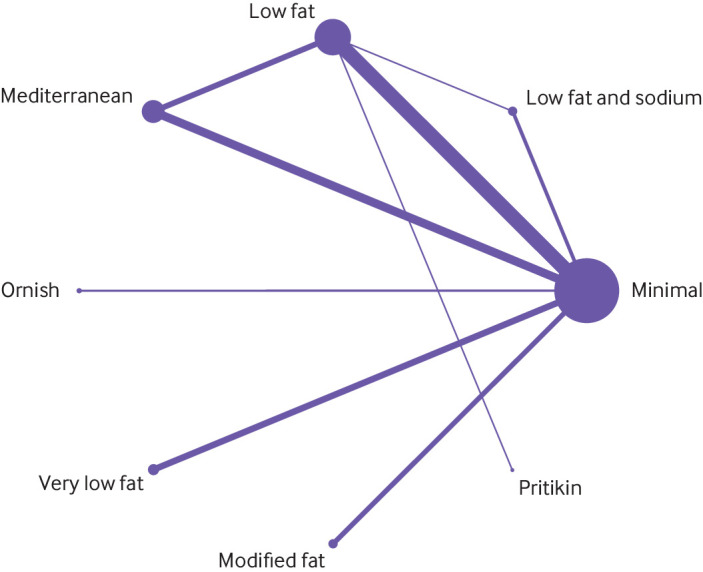
Network of named dietary programmes for all cause mortality at last follow-up
Mortality outcomes
For all cause mortality at last reported follow-up (range 0.75-17 years), Mediterranean dietary programmes were superior to minimal intervention based on moderate certainty evidence (odds ratio 0.72, 95% confidence interval 0.56 to 0.92; patients at intermediate risk: risk difference −17 per 1000, 95% confidence interval −26 to −5; patients at high risk: −36 per 1000, −58 to −10), as were low fat dietary programmes, also based on moderate certainty evidence (0.84, 0.74 to 0.95; patients at intermediate risk: −9 per 1000, −15 to −3; patients at high risk: −20 per 1000, −33 to −6). Evidence comparing Mediterranean and low fat dietary programmes was of very low certainty. Very low fat dietary programmes and combined low fat and low sodium dietary programmes had little or no beneficial effect on mortality based on moderate certainty evidence. Modified fat, Ornish, and Pritikin diets showed low or very low certainty evidence (table 2, appendix tables S62 and S63).
Table 2.
Summary of all cause mortality results at last follow-up for named dietary programmes versus minimal intervention
| Dietary programme | Odds ratio (95% CI) | Risk difference per 1000 (95% CI)* | Interpretation and certainty |
|---|---|---|---|
| Mediterranean (10 trials, 8075 participants) | 0.72 (0.56 to 0.92) | −17 (−26 to −5); −36 (−58 to −10) | Superior to minimal intervention (moderate certainty)† |
| Low fat (16 trials, 9243 participants) | 0.84 (0.74 to 0.95) | −9 (−15 to −3); −20 (−33 to −6) | Superior to minimal intervention (moderate certainty)† |
| Very low fat (4 trials, 987 participants) | 0.95 (0.77 to 1.18) | −3 (−14 to 10); −5 (−29 to 22) | Little or no benefit relative to minimal intervention (moderate certainty)‡ |
| Modified fat (4 trials, 680 participants) | 1.05 (0.80 to 1.38) | 3 (−12 to 22); 6 (−25 to 44) | Might have little or no benefit relative to minimal intervention (low certainty)‡§ |
| Combined low fat and low sodium (3 trials, 2673 participants) | 1.02 (0.82 to 1.27) | 1 (−11 to 15); 2 (−23 to 32) | Little or no benefit relative to minimal intervention (moderate certainty)‡ |
| Ornish (2 trials, 125 participants) | 2.43 (0.24 to 24.2) | 76 (−46 to 553); 145 (−104 to 659) | Might have little or no benefit relative to minimal intervention (low certainty)‡§ |
| Pritikin (1 trial, 26 participants) | 0.21 (0.01to 5.56) | −48 (−61 to 207); −109 (−141 to 338) | Might be superior to minimal intervention (very low certainty)¶** |
CI=confidence interval.
Results presented as intermediate baseline risk; high baseline risk.
Rated down one level for indirectness (statistical significance and low risk of bias partly depended on trials with secondary interventions such as drug treatment).
Rated down one level for imprecision (little or no benefit relative to minimal intervention, but 95% CI is compatible with non-trivial benefit).
Rated down one level for risk of bias.
Rated down one level because effect estimate is based on indirect comparison involving low fat versus minimal intervention (moderate certainty).
Rated down two levels for imprecision (point estimate indicates benefit, but 95% CI is compatible with harm).
For cardiovascular mortality at last follow-up (range 0.75-17 years), only Mediterranean dietary programmes were convincingly superior to minimal intervention based on moderate certainty evidence (0.55, 0.39 to 0.78; patients at intermediate risk: −13 per 1000, −17 to −6; patients at high risk: −39 per 1000, −54 to −19). Low fat, very low fat, modified fat, combined low fat and low sodium, and Ornish dietary programmes showed little or no benefit based mostly on low or very low certainty evidence (appendix tables S64 and S65).
Secondary outcomes
Data were too sparse for four of our preregistered secondary outcomes (angina, heart failure, peripheral vascular events, and atrial fibrillation). For stroke at last reported follow-up (range 1-9.6 years), Mediterranean programmes were superior to minimal intervention based on moderate certainty evidence (odds ratio 0.65, 95% confidence interval 0.46 to 0.93; patients at intermediate risk: risk difference −7 per 1000, 95% confidence interval −11 to −1; patients at high risk: −16 per 1000, −25 to −3). Low fat, very low fat, and modified fat programmes had little or no benefit based on mostly moderate to high certainty evidence. Combined low fat and low sodium showed benefit in patients at high risk based on moderate certainty evidence (−17 per 1000, −32 to 11; appendix tables S66 and S67).
For non-fatal myocardial infarction at last reported follow-up (range 0.75-9.6 years), Mediterranean dietary programmes were superior to minimal intervention based on moderate certainty evidence (0.48, 0.36 to 0.65; patients at intermediate risk: risk difference −17 per 1000, −21 to −11; patients at high risk: −42 per 1000, −53 to −28). The same was true for low fat dietary programmes (0.77, 0.61 to 0.96; patients at intermediate risk: −7 per 1000, −13 to −1; patients at high risk: −18 per 1000, −31 to −3). Comparing low fat with Mediterranean dietary programmes, in patients at intermediate risk there was moderate certainty evidence of little or no difference (low fat v Mediterranean: risk difference 6 per 1000, 95% confidence interval 0 to 16), whereas in patients at high risk there was low certainty evidence of the superiority of Mediterranean programmes (low fat v Mediterranean: 16 per 1000, −1 to 39). Very low fat, modified fat, and combined low fat and low sodium dietary programmes had little or no benefit based mostly on moderate to high certainty evidence (appendix tables S68 and S69).
For unplanned cardiovascular interventions at last reported follow-up (range 0.75-9.6 years), only low fat dietary programmes reduced events relative to minimal intervention based on low certainty evidence (odds ratio 0.57, 95% confidence interval 0.35 to 0.93; appendix tables S70 and S71).
Other evidence
A trial published after our data analysis (CORDIOPREV) compared a Mediterranean diet with a low fat diet in patients with coronary heart disease (n=1002).44 For individual (non-composite) outcomes, this trial found non-significant numerical reductions in cardiovascular mortality, non-fatal myocardial infarction, and ischaemic stroke favouring the Mediterranean diet. Our network meta-analysis was consistent with CORDIOPREV’s individual outcome findings; we found that in patients with high baseline risk, Mediterranean dietary programmes reduced each of these cardiovascular outcomes relative to low fat programmes. However, apart from stroke, the evidence for the Mediterranean diet being superior to a low fat diet remains uncertain.
Network meta-regression and sensitivity analysis results
Our network meta-regression did not find any substantial difference in odds ratios when controlling for the presence of cointerventions (exercise, psychosocial support such as stress management, smoking cessation, and drug treatment). Additionally, little evidence was found that follow-up duration or intervention duration acted as an effect modifier.
Subgroup analyses revealed that results did not vary based on presence of cardiovascular disease in patients at baseline (whether the trials were in a primary prevention population or a secondary prevention population), nor on intensity of intervention (appendix tables S20-S59).
Sensitivity analyses excluding trials with treatment arms including smoking cessation or drug treatment cointerventions revealed similar findings to our main analysis; however, loss of statistical significance was observed for low fat dietary programmes for all cause mortality, non-fatal myocardial infarction, and unplanned cardiovascular interventions. Statistical significance was maintained for Mediterranean dietary programme outcomes, although they were based on higher risk of bias trials.
Discussion
Principal findings
Our network meta-analysis of named dietary programmes found that Mediterranean dietary programmes were superior to minimal intervention based on moderate certainty evidence for mortality outcomes, non-fatal myocardial infarction, and stroke. Low fat dietary programmes were also superior to minimal intervention with low to moderate certainty for prevention of all cause mortality, non-fatal myocardial infarction, and unplanned cardiovascular interventions. When compared with one another, no convincing evidence was found that the Mediterranean dietary programme was superior to the low fat dietary programme in preventing mortality or non-fatal myocardial infarction. Other dietary programmes (very low fat, combined low fat and low sodium, modified fat, Ornish, and Pritikin) did not show convincing evidence of superiority to minimal intervention, except possibly combined low fat and low sodium programmes for stroke prevention in patients at high risk (fig 1 and fig 2).
Strengths of this study
Our review has several strengths. We performed a systematic review and network meta-analysis of dietary programmes for the prevention of major cardiovascular events. We worked with an experienced health sciences librarian to systematically search the literature and to guide our review process and analysis, and we posted a publicly available study protocol. We performed independent, duplicate screening, data extraction, and risk of bias assessment and used network meta-regression, subgroups, and sensitivity analysis to assess and account for potential effect modifiers, none having shown major effect modification. Finally, our review used new GRADE methods to present and assess the certainty for relative and absolute network meta-analysis estimates of effect, providing a transparent and clear presentation of the comparative performance of each dietary programme for each outcome for cardiovascular patients at intermediate and high risk (fig 1 and fig 2). In particular, our results established moderate certainty evidence for the absolute risk reduction (9-36 fewer events per 1000 followed over five years) attributable to Mediterranean and low fat dietary programmes. These findings with data presentations are extremely important for patients who are sceptical about the desirability of diet change.
Limitations of this study
Our review also has limitations. We modified our protocol specified dietary programme classifications to distinguish standard low fat programmes that targeted fat reduction to ≤30% caloric intake from programmes that targeted ≤20% caloric intake. However, this categorisation is consistent with other systematic reviews.15 16 A second limitation was the inclusion of dietary programmes with cointerventions such as drug treatment and smoking cessation, raising the possibility that the effects were, at least in part, due to cointerventions. We were able to explore these potential effect modifiers using network meta-regression and sensitivity analysis. None of the coefficients proved statistically significant and our sensitivity analysis results were similar to those of the primary analysis, although with a loss of statistical significance for low fat dietary programmes for all cause mortality, myocardial infarction, and unplanned cardiovascular interventions. Accordingly, using the GRADE approach, we rated down the certainty of evidence for each of our outcomes based on issues of indirectness related to cointerventions (that is, drug treatment, smoking cessation that often includes drugs) that we did not consider a primary part of a dietary programme (diet with or without exercise and psychosocial or behavioural support) based on our a priori study protocol.
We were unable to systematically factor adherence into our analysis of dietary programmes, largely because of a lack of reporting among the 40 eligible trials. Moreover, when reported, the validity of measures of adherence has been called into question, particularly if reliable biomarkers for adherence do not exist or are not used in dietary programme trials45; this might have reduced effect estimates when adherence was poor. Our results are therefore best understood as reflecting expected benefits given average adherence to a dietary programme. Additionally, we used an earlier version of the Cochrane risk of bias instrument (version 1) to evaluate trials rather than the latest version. Our review began before the second version was published.46 While the more recent instrument has additional items and guidance for users, we ensured that our risk of bias assessments were reliable using explicit risk of bias instructions and calibration exercises with our review team based on reported guidance for improving the reliability of version 1 of the risk of bias instrument.47
Control arms which we classified as minimal intervention were somewhat heterogenous, from a minimum of no dietary advice at all, to dietary advice with little reinforcement, to usual care programmes that included optional dietary counselling. However, for all trials, the minimal intervention arm had far less intensive interventions than the active arm. Moreover, the presence of dietary change in control arms would strengthen our inferences about the benefits of Mediterranean and low fat dietary programmes. We accounted for this in our GRADE approach by rating down most indirect comparisons for intransitivity. Finally, our analysis did not include one recent randomised controlled trial (CORDIOPREV) that compared a Mediterranean diet with a low fat diet. The results suggested possible benefits for cardiovascular mortality, non-fatal myocardial infarction, and stroke in favour of the Mediterranean diet. Our network meta-analysis was consistent with CORDIOPREV’s individual outcome findings in that we found Mediterranean dietary programmes reduced each of these cardiovascular outcomes relative to low fat programmes in patients with high baseline risk. However, based on our network meta-analysis, the certainty of evidence for these outcomes was very low, low and moderate, respectively, leading us to conclude that, overall, there is no convincing evidence for the benefit of Mediterranean over low fat programmes. Based on moderate certainty evidence, it must be noted that there could be a benefit of Mediterranean programmes over low fat programmes for the outcome of stroke.
Comparison with other reviews and guidelines
While our review is the first comparative effectiveness review of competing dietary programmes, recent Cochrane reviews have been published specific to low fat8 and Mediterranean diets9 for major cardiovascular events. Rees and colleagues9 assessed 30 randomised trials evaluating the impact of Mediterranean diets on mortality, major cardiovascular, and cardiometabolic (surrogate) outcomes. While they agreed with our findings, reporting a reduction in all cause and cardiovascular mortality relative to usual care in a secondary prevention setting, they found no clinical outcome data comparing Mediterranean diets with usual diet in a primary prevention setting. By contrast, we were able to produce such evidence using indirect comparison data from the PREDIMED trial, which compared a Mediterranean with a low fat dietary programme in a primary prevention setting, in combination with primary prevention trials comparing low fat with minimal intervention diets.
With respect to low fat diets, Hooper and colleagues8 found that diets lower in saturated fat consistently reduce the risk of adverse cardiovascular outcomes, with only combined cardiovascular events—a composite of almost 10 different cardiovascular outcomes—showing a statistically significant reduction (17 fewer events per 1000 followed). In our review, we focused on mortality and individual cardiovascular events given the many criticisms of combined composite outcomes.48 We found risk reductions for those following low fat dietary programmes for all cause mortality and non-fatal myocardial infarction. One potential explanation is that our review, unlike the Cochrane review, included dietary programmes with health behaviour cointerventions. The Murchie (2003) and Look AHEAD (2013) trials, both of which featured cointerventions, make up approximately 58% of our all cause mortality direct estimate for low fat dietary programmes versus minimal intervention. In other words, our results apply primarily to dietary programmes as a whole, which include exercise and psychosocial support cointerventions, rather than to diets alone.
In 2020, the USPSTF recommended that people with cardiovascular risk factors be offered interventions to promote healthy diets and physical activity.49 They conducted a systematic review of randomised trials in adults with at least one cardiovascular risk factor (excluding diabetes and previous cardiovascular disease), finding a statistically significant reduction in combined cardiovascular events and non-statistically significant reductions in mortality, stroke, and myocardial infarction.12 With our broader eligibility criteria allowing for diabetic and secondary prevention populations and the use of network meta-analysis methods, our review extends these findings by providing moderate certainty evidence for Mediterranean and low fat dietary programmes for preventing all cause mortality and non-fatal myocardial infarction, and moderate certainty evidence for stroke reduction with Mediterranean programmes.
Our findings also add to NICE (National Institute for Health and Care Excellence) guidance on cardiovascular risk reduction in patients at increased cardiovascular risk.50 Our systematic review focuses on the same population (high cardiovascular risk or established disease) and the same intervention as their lifestyle recommendations (dietary programmes; that is, dietary changes with or without physical activity and behavioural support). In contrast to the most recent Dietary Guidelines for America,51 NICE does not specifically mention sodium reduction. A 2018 evidence update52 cites a cohort study finding no association between sodium intake and mortality or incident cardiovascular disease. Our study provides randomised evidence supporting this approach. Based on moderate certainty evidence, we found that dietary programmes combining fat and sodium reduction did not reduce mortality. Additionally, the evidence did not support any benefit for other outcomes, with the exception of stroke in people at high risk (based on moderate certainty evidence due to imprecision). Given that valid biomarkers exist for sodium intake, a caveat is that the largest trial assessing a combined low fat and low sodium diet reported poor adherence.53 Other trials assessing sodium reduction also reported poor adherence, with one trial measuring an objective biomarker of adherence (urine sodium) reporting only about 40% of participants achieving the sodium intake goal of 80 mmol/day or less.31
Conclusions
In conclusion, this network meta-analysis found that Mediterranean and low fat dietary programmes probably reduce the risk of mortality and non-fatal myocardial infarction in people at increased cardiovascular risk. Mediterranean dietary programmes are also likely to reduce the risk of stroke.
What is already known on this topic
Dietary guidelines recommend various dietary programmes (which could include physical activity or other cointerventions) for patients at increased cardiovascular risk, but they might rely on low certainty evidence, such as non-randomised studies and surrogate outcomes
Systematic reviews of randomised trials with mortality and major cardiovascular outcomes have reported benefits of dietary programmes, but have not used network meta-analysis to give absolute estimates and certainty of estimates for patients at intermediate and high risk
What this study adds
This network meta-analysis compared the effects of different dietary programmes on clinical outcomes, such as mortality and cardiovascular events, using GRADE (grading of recommendations assessment, development and evaluation) methods
Moderate certainty evidence shows that Mediterranean and low fat diets reduce all cause mortality and non-fatal myocardial infarction in patients with increased cardiovascular risk; a Mediterranean diet was not convincingly superior to a low fat diet for these outcomes
Moderate certainty evidence supports a Mediterranean diet for a reduced risk of stroke, while a low fat diet showed little to no benefit for stroke reduction
Acknowledgments
We thank Thomasin Adams-Webber for assistance with the search strategy. Regina El Dib passed away before publication.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: BCJ and AA conceived the study. AA, GK, GHG, and BCJ designed the study protocol and methods. EB, GK, and BCJ designed and performed the search strategy. GK, AA, BS, MJ, CLH, LG, RK, WA, AMZ, JM, MAJC, MR, RED, EB, GHG, JZG, and BCJ helped with data acquisition or interpretation. BS, GK, and BCJ performed the data analysis. JZG, GK, LG, BCJ, and GHG performed the GRADE assessment. GK and BCJ wrote the first draft of the manuscript. All authors critically revised the manuscript for important intellectual content. GK, BS, MJ, LG, EB, and BCJ provided administrative, technical, or material support. BCJ and AA supervised the study. GK, BS, and BCJ are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research was not funded by a specific grant from any funding agency in the public, commercial, or not-for-profit sectors. It was funded, in small part, by internal investigator funds from Dalhousie University (awarded to BCJ).
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from Dalhousie University for the submitted work; no other relationships or activities that could appear to have influenced the submitted work. BCJ received a grant from Texas A&M AgriLife Research to fund investigator initiated research related to saturated and polyunsaturated fats. The grant was from Texas A&M AgriLife institutional funds from interest and investment earnings, not a sponsoring organisation, industry, or company.
The lead authors (the manuscript’s guarantors) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We plan to disseminate the results to relevant patient communities through the media relations department of our institutions.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
All data are freely available within the appendices. No additional data available.
References
- 1. Afshin A, Sur PJ, Fay KA, et al. GBD 2017 Diet Collaborators . Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;393:1958-72. 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sacks FM, McManus K. Cardiovascular disease and lifestyle modification. In: Cardiovascular Therapeutics: A Companion to Braunwald’s Heart Disease. Elsevier, 2013: 442-53 10.1016/B978-1-4557-0101-8.00026-6. [DOI] [Google Scholar]
- 3. Nissen SE. US dietary guidelines: an evidence-free zone. Ann Intern Med 2016;164:558-9. 10.7326/M16-0035. [DOI] [PubMed] [Google Scholar]
- 4. Ioannidis JPA. Implausible results in human nutrition research. BMJ 2013;347:f6698. 10.1136/bmj.f6698. [DOI] [PubMed] [Google Scholar]
- 5. Ioannidis JPA. The challenge of reforming nutritional epidemiologic research. JAMA 2018;320:969-70. 10.1001/jama.2018.11025. [DOI] [PubMed] [Google Scholar]
- 6. Zeraatkar D, Johnston BC, Guyatt G. Evidence collection and evaluation for the development of dietary guidelines and public policy on nutrition. Annu Rev Nutr 2019;39:227-47. 10.1146/annurev-nutr-082018-124610. [DOI] [PubMed] [Google Scholar]
- 7. Johnston BC, Alonso-Coello P, Bala MM, et al. Methods for trustworthy nutritional recommendations NutriRECS (Nutritional Recommendations and accessible Evidence summaries Composed of Systematic reviews): a protocol. BMC Med Res Methodol 2018;18:162. 10.1186/s12874-018-0621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev 2020;8:CD011737. 10.1002/14651858.CD011737.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rees K, Takeda A, Martin N, et al. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2019;3:CD009825. 10.1002/14651858.CD009825.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes. Ann Intern Med 2019;171:190-8. 10.7326/M19-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liyanage T, Ninomiya T, Wang A, et al. Effects of the Mediterranean diet on cardiovascular outcomes-a systematic review and meta-analysis. PLoS One 2016;11:e0159252. 10.1371/journal.pone.0159252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Connor EA, Evans CV, Rushkin MC, Redmond N, Lin JS. Behavioral counseling to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2020;324:2076-94. 10.1001/jama.2020.17108. [DOI] [PubMed] [Google Scholar]
- 13. Schwingshackl L, Buyken A, Chaimani A. Network meta-analysis reaches nutrition research. Eur J Nutr 2019;58:1-3. 10.1007/s00394-018-1849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JPT, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ge L, Sadeghirad B, Ball GDC, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ 2020;369:m696. 10.1136/bmj.m696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hooper L, Summerbell CD, Thompson R, et al. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev 2011;2011:CD002137. 10.1002/14651858.CD002137.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. Preventive Services Task Force . Screening for obesity in adults: recommendations and rationale. Ann Intern Med 2003;139:930-2. 10.7326/0003-4819-139-11-200312020-00012. [DOI] [PubMed] [Google Scholar]
- 18. Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443-57. 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, Wang W, Zhang AB, Bai X, Zhang S. Epley and Semont maneuvers for posterior canal benign paroxysmal positional vertigo: a network meta-analysis. Laryngoscope 2016;126:951-5. 10.1002/lary.25688. [DOI] [PubMed] [Google Scholar]
- 20. White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012;3:111-25. 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98-110. 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006;101:447-59. 10.1198/016214505000001302. [DOI] [Google Scholar]
- 24. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata J 2015;15:905-50. 10.1177/1536867X1501500402. [DOI] [Google Scholar]
- 26. Schünemann H, Vist G, Higgins J, et al. Interpreting results and drawing conclusions. In: Cochrane Handbook for Systematic Reviews of Interventions, version 6.1. Cochrane, 2020, www.training.cochrane.org/handbook. [Google Scholar]
- 27. Mihaylova B, Emberson J, Blackwell L, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators . The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581-90. 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horton R. Expression of concern: Indo-Mediterranean Diet Heart Study. Lancet 2005;366:354-6. 10.1016/S0140-6736(05)67006-7. [DOI] [PubMed] [Google Scholar]
- 29. White C. Suspected research fraud: difficulties of getting at the truth. BMJ 2005;331:281-8. 10.1136/bmj.331.7511.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lehmann N, Paul A, Moebus S, Budde T, Dobos GJ, Michalsen A. Effects of lifestyle modification on coronary artery calcium progression and prognostic factors in coronary patients--3-year results of the randomized SAFE-LIFE trial. Atherosclerosis 2011;219:630-6. 10.1016/j.atherosclerosis.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 31. Whelton PK, Appel LJ, Espeland MA, et al. TONE Collaborative Research Group . Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). JAMA 1998;279:839-46. 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 32. Ball K, Hanington E, McAllen P, et al. Low-fat diet in myocardial infarction: a controlled trial. Lancet 1965;2:501-4. [PubMed] [Google Scholar]
- 33. Puhan MA, Schünemann HJ, Murad MH, et al. GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 34. Brignardello-Petersen R, Bonner A, Alexander PE, et al. GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018;93:36-44. 10.1016/j.jclinepi.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 35. Hultcrantz M, Rind D, Akl EA, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol 2017;87:4-13. 10.1016/j.jclinepi.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zeng L, Brignardello-Petersen R, Hultcrantz M, et al. GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol 2021;137:163-75. 10.1016/j.jclinepi.2021.03.026. [DOI] [PubMed] [Google Scholar]
- 37. Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the Nutritional Recommendations (NutriRECS) Consortium. Ann Intern Med 2019;171:756-64. 10.7326/M19-1621. [DOI] [PubMed] [Google Scholar]
- 38. Brignardello-Petersen R, Florez ID, Izcovich A, et al. GRADE working group . GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ 2020;371:m3900. 10.1136/bmj.m3900. [DOI] [PubMed] [Google Scholar]
- 39. Cupples ME, McKnight A. Randomised controlled trial of health promotion in general practice for patients at high cardiovascular risk. BMJ 1994;309:993-6. 10.1136/bmj.309.6960.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hjermann I, Velve Byre K, Holme I, Leren P. Effect of diet and smoking intervention on the incidence of coronary heart disease. Report from the Oslo Study Group of a randomised trial in healthy men. Lancet 1981;2:1303-10. 10.1016/S0140-6736(81)91338-6. [DOI] [PubMed] [Google Scholar]
- 41. de Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994;343:1454-9. 10.1016/S0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 42. Zwisler A-DO, Soja AMB, Rasmussen S, et al. DANREHAB Group . Hospital-based comprehensive cardiac rehabilitation versus usual care among patients with congestive heart failure, ischemic heart disease, or high risk of ischemic heart disease: 12-month results of a randomized clinical trial. Am Heart J 2008;155:1106-13. 10.1016/j.ahj.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 43. Søndergaard E, Møller JE, Egstrup K. Effect of dietary intervention and lipid-lowering treatment on brachial vasoreactivity in patients with ischemic heart disease and hypercholesterolemia. Am Heart J 2003;145:E19. 10.1016/S0002-8703(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 44. Delgado-Lista J, Alcala-Diaz JF, Torres-Peña JD, et al. CORDIOPREV Investigators . Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet 2022;399:1876-85. 10.1016/S0140-6736(22)00122-2. [DOI] [PubMed] [Google Scholar]
- 45. Prentice RL. Dietary assessment and opportunities to enhance nutritional epidemiology evidence. Ann Intern Med 2020;172:354-5. 10.7326/M19-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 47. da Costa BR, Beckett B, Diaz A, et al. Effect of standardized training on the reliability of the Cochrane risk of bias assessment tool: a prospective study. Syst Rev 2017;6:44. 10.1186/s13643-017-0441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferreira-González I, Busse JW, Heels-Ansdell D, et al. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials. BMJ 2007;334:786. 10.1136/bmj.39136.682083.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krist AH, Davidson KW, Mangione CM, et al. US Preventive Services Task Force . Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA 2020;324:2069-75. 10.1001/jama.2020.21749. [DOI] [PubMed] [Google Scholar]
- 50.National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification (clinical guideline CG181). 2014. https://www.nice.org.uk/guidance/cg181. [PubMed]
- 51.US Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025.
- 52.National Institute for Health and Care Excellence. Surveillance report 2018 – Cardiovascular disease: risk assessment and reduction, including lipid modification NICE guideline CG181. 2018. https://www.nice.org.uk/guidance/cg181/resources/surveillance-report-2018-cardiovascular-disease-risk-assessment-and-reduction-including-lipid-modification-2014-nice-guideline-cg181-4724759773/chapter/Surveillance-decision?tab=evidence. [PubMed]
- 53. Weber B, Bersch-Ferreira ÂC, Torreglosa CR, et al. Implementation of a Brazilian Cardioprotective Nutritional (BALANCE) Program for improvement on quality of diet and secondary prevention of cardiovascular events: A randomized, multicenter trial. Am Heart J 2019;215:187-97. 10.1016/j.ahj.2019.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
All data are freely available within the appendices. No additional data available.