Abstract
COVID-19-associated pulmonary aspergillosis (CAPA) is a life-threatening fungal infection that mainly affects critically ill patients. The aim of this study was to assess the incidence and clinical outcomes of putative CAPA in critically ill COVID-19 patients. This retrospective observational cohort study included 181 cases from 5 ICUs at Vienna General Hospital between January 2020 and April 2022. Patients were diagnosed with putative CAPA according to the AspICU classification, which included a positive Aspergillus culture in a bronchoalveolar lavage sample, compatible signs and symptoms, and abnormal medical imaging. The primary outcome was adjusted 60-day all-cause mortality from ICU admission in patients with vs. without putative CAPA. Secondary outcomes included time from ICU admission to CAPA diagnosis and pathogen prevalence and distribution. Putative CAPA was identified in 35 (19.3%) of 181 COVID-19 patients. The mean time to diagnosis was 9 days. Death at 60 days occurred in 18 of 35 (51.4%) patients with CAPA and in 43 of 146 (29.5%) patients without CAPA (adjusted HR (95%CI) = 2.15 (1.20–3.86, p = 0.002). The most frequently isolated Aspergillus species was Aspergillus fumigatus. The prevalence of putative pulmonary aspergillosis in critically ill COVID-19 patients was high and was associated with significantly higher mortality.
Keywords: ICU, CAPA, IPA, SARS-CoV-2, co-infection, fungal, aspergillus
1. Introduction
COVID-19-associated pulmonary aspergillosis (CAPA) is a serious complication associated with respiratory failure caused by species of the genus Aspergillus [1]. The ongoing COVID-19 pandemic has led to a significant increase in the number of patients requiring hospitalization, particularly in intensive care units (ICUs) [2]. Although viral pneumonia has been recognized as the main cause of severity and mortality, COVID-19 has also been linked to other complications such as coagulation disorders, neurological complications, vascular complications, and gastrointestinal and renal disorders [3,4]. Previous studies have shown that critically ill COVID-19 patients are susceptible to other bacterial, viral, and fungal co-infections (within the first 48 h of admission) and secondary infections (≥48 h of admission), including CAPA [5,6,7,8,9]. Damage of the bronchial mucosa and alveolar injury by the virus, in combination with increased pulmonary epithelial and vascular permeability, may create favorable conditions for the invasion of Aspergillus spp. [10]. In addition to the direct effects of the virus, the use of glucocorticoids and other immunosuppressive therapies to treat COVID-19 may increase the risk of developing CAPA [11,12].
Despite increasing reports of CAPA, the true prevalence of the complication remains uncertain. Some studies have investigated the prevalence of CAPA, but results have found inconsistent frequencies ranging from 2.5 to 35% [13]. This may be due to differences in the underlying study designs, case definitions, and geographical differences. In addition, the previous literature has reported that the diagnostic performance of common tests for CAPA, such as the galactomannan antigen assay, is limited [14,15]. These heterogeneous results may obscure the true prevalence of invasive pulmonary aspergillosis in COVID-19 patients.
To address these challenges, the present study presents a retrospective analysis of aspergillosis in COVID-19 patients treated in a tertiary care hospital and describes the prevalence, clinical outcomes, and antifungal treatment in this patient population.
2. Methods
2.1. Study Design and Setting
The present retrospective observational cohort study was conducted at a single European study site (Vienna General Hospital, Vienna, Austria). Prior to the initiation of the study, ethics approval was obtained from the competent Ethics Committee (EC 2259/2021). Data were automatically extracted from medical records according to the inclusion and exclusion criteria. Unavailable data were manually extracted and added to the main data set. The present study was conducted according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations [16].
2.2. Study Population
We included patients admitted to five ICUs with acute respiratory failure and COVID-19, confirmed by polymerase chain reaction (PCR) at the time of hospitalization, with at least one microbiological culture performed in a bronchoalveolar lavage (BAL) sample after ICU admission. All COVID-19 cases were screened from the start of the pandemic through April 2022. We excluded patients < 18 years of age and cases with an ICU duration of ≤48 h.
2.3. Definition of Pulmonary Aspergillosis
Patients were diagnosed with putative CAPA according to the AspICU classification by Blot et al. [17]. In brief, criteria included a combination of a positive Aspergillus culture in a BAL sample, compatible clinical signs and symptoms of aspergillosis, including worsening respiratory insufficiency despite ventilatory support, abnormal medical imaging in chest X-ray or CT scan, including diffuse reticular or alveolar opacities or nonspecific infiltrates and consolidation, and glucocorticoid treatment (over 20 mg of prednisolone per day or an equivalent dose of any other glucocorticoid). Respiratory sampling was performed at the discretion of the attending physician as part of routine care. Antigen testing for galactomannan from serum and respiratory tract samples was not included in the analysis due to their limited sensitivity, as reported in the previous literature [15].
2.4. Outcome Parameters
The primary outcome of this study was the adjusted hazard ratio of 60-day all-cause mortality from ICU admission in patients with or without putative CAPA. Secondary outcomes included the unadjusted hazard ratio of 60-day all-cause mortality from ICU admission, pathogen prevalence and distribution, and time from ICU admission to CAPA diagnosis. In addition, we also assessed the use of immunosuppressants during hospitalization and antifungal therapy after CAPA diagnosis.
2.5. Statistical Analysis
Baseline characteristics were reported descriptively using mean ± standard deviation (SD), median (IQR), or numbers (%). Characteristics were compared using an independent t-test for age, weight, height, and body mass index (BMI) or Chi-square test for sex and chronic diseases. The incidence of putative CAPA, microbiological sampling frequencies, and pathogen prevalence and distribution were reported descriptively. Hazard ratios with 95% confidence interval (95% CI) were calculated for the 60-day all-cause mortality. Patients discharged alive were censored at the time of exit. We additionally performed a Cox regression analysis to adjust the hazard ratio for age, sex, BMI, cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes, cancer diagnosis, and SARS-CoV-2 variant. Statistical analyses and visualizations were performed using R (Version 2021.09.2) and GraphPad Prism 9.3.1.
3. Results
3.1. Study Population
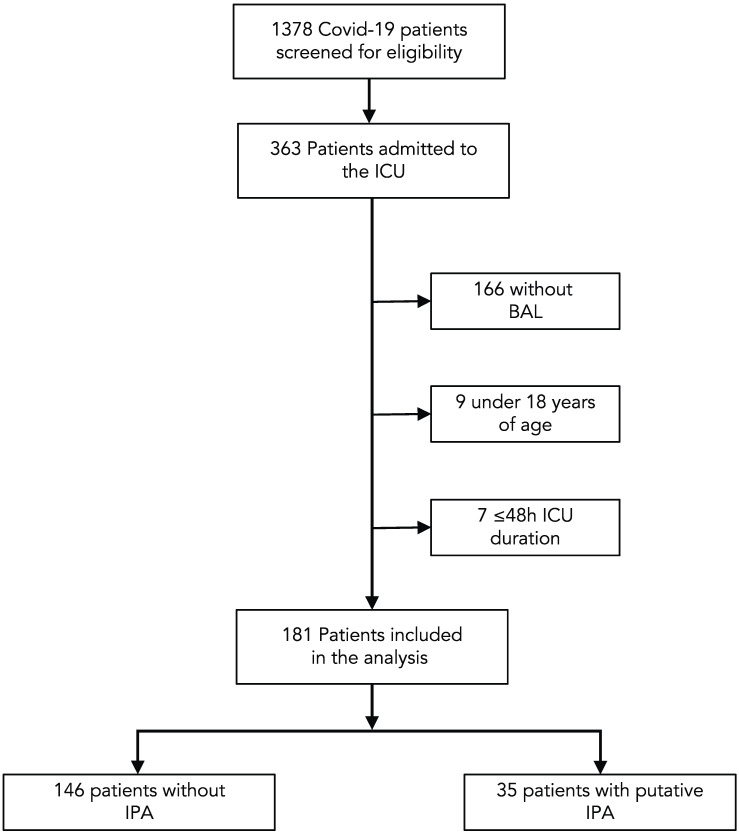
In total, 1378 patients with PCR-confirmed COVID-19 infection were identified from January 2020 through April 2022, of which 363 were admitted to an ICU. After excluding 9 cases <18 years of age, 166 patients without microbiological cultures from BAL samples and 7 patients with an ICU duration of ≤48 h, the final analysis set consisted of 181 cases (Figure 1).
Figure 1.
Flow chart of the study population.
The characteristics of the study population are summarized in Table 1. In total, 54 of 181 (29.8%) of patients were female, the overall mean ± SD age was 54 ± 13 years and the mean ± SD BMI was 30.7 ± 7.1. In addition, 133 of 181 (73.5%) patients received immunosuppressants at the time of ICU admission. The mean ± SD duration of intensive care was 36.73 (±25.5) days. The most common comorbidities were obesity (84 of 181 (46.4%)), hypertension (42 of 181 (23.2%)), and mental disorders (33 of 181 (18.2%)). Galactomannan indices and β-D-Glucan levels were higher and interpreted as positive more often in patients with putative CAPA (Table 1).
Table 1.
Baseline characteristics of the study cohort.
| Overall (n = 181) |
No CAPA (n = 146) |
Putative CAPA (n = 35) |
p-Value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Female sex, n (%) | 54 (29.8) | 45 (30.8) | 9 (25.7) | 0.698 |
| Age, mean (SD) | 54.46 (13.03) | 54.08 (13.56) | 56.06 (10.58) | 0.422 |
| BMI, mean (SD) | 30.73 (7.13) | 30.86 (7.52) | 30.17 (5.24) | 0.609 |
|
Length of hospital stay (days),
mean (SD) |
36.73 (25.53) | 35.92 (25.41) | 43.28 (26.30) | 0.250 |
| Antigen assays a | ||||
| Galactomannan index, median (IQR) | 0.10 (0.06–0.23) | 0.08 (0.06–0.15) | 3.69 (0.16–7.04) | 0.0001 |
| Positive galactomannan index, n (%) | 22 (16.4) | 4 (3.7) | 18 (66.67) | 0.0001 |
| β-D-Glucan (pg/mL), median (IQR) | 74.83 (0.00–194.2) | 72.59 (0.00–169.6) | 142.2 (51.47–365.1) | 0.02 |
| Positive β-D-Glucan, n (%) | 66 (47.1) | 49 (43) | 17 (65.4) | 0.039 |
| Bacterial infection b | 46 (25.4) | 39 (26.7) | 7 (20) | 0.52 |
| Baseline comorbidities, n (%) | ||||
| Diabetes | 24 (13.3) | 16 (11.0) | 8 (22.9) | 0.113 |
| Obesity | 84 (46.4) | 74 (49.3) | 10 (28.6) | 0.03 |
| Coronary artery disease | 13 (7.2) | 13 (8.9) | 0 (0.0) | 0.142 |
| Chronic heart failure | 4 (2.2) | 2 (1.4) | 2 (5.7) | 0.352 |
| Asthma | 5 (2.8) | 4 (2.7) | 1 (2.9) | 1.000 |
| Chronic obstructive pulmonary disease | 9 (5.0) | 8 (5.5) | 1 (2.9) | 0.835 |
| Hypertension | 42 (23.2) | 32 (21.9) | 10 (28.6) | 0.539 |
| Chronic kidney disease | 15 (8.3) | 12 (8.2) | 3 (8.6) | 1.000 |
| Skin disorder | 16 (8.8) | 15 (10.3) | 1 (2.9) | 0.291 |
| Mental disorder | 33 (18.2) | 31 (21.2) | 2 (5.7) | 0.059 |
| Neurologic disorder | 20 (11.0) | 19 (13.0) | 1 (2.9) | 0.155 |
| Neoplasm | 26 (14.4) | 19 (13.0) | 7 (20.0) | 0.429 |
| Immunosuppressants at ICU admission | 133 (73.5) | 110 (75.3) | 22 (62.9) | 0.135 |
| SARS-CoV-2 variant | 0.213 | |||
| Wildtype | 34 (18.8) | 28 (19.2) | 6 (17.1) | |
| B.1.1.7 (Alpha) | 80 (44.2) | 66 (45.2) | 14 (40.0) | |
| B.1.617.2 (Delta) | 54 (29.8) | 39 (26.7) | 15 (42.9) | |
| Unknown | 13 (7.2) | 13 (8.9) | 0 (0.0) |
a The galactomannan assay was available in 107 patients in the No-CAPA group and in 27 patients in the putative CAPA group. The β-D-Glucan assay was available in 114 patients in the No-CAPA group and in 26 patients in the putative CAPA group. b Samples were collected from respiratory tract and blood.
A total of 35 of 181 (19.3%) cases developed putative CAPA according to the AspICU criteria by Blot et al. [17]. Among these 35 cases, 9 were diagnosed within 48 h of ICU admission, while 26 were diagnosed 48 h or more after admission. The CAPA group had a numerically higher proportion of male patients than non-CAPA cases (74.3% vs. 69.2%). The mean ± SD age was similar between patients with CAPA (56 ± 11 years) and without (54 ± 14 years) CAPA. Co-morbidities were well balanced, with the only significant difference being obesity between the two groups. There was no significant difference in the use of immunosuppressive medication at ICU admission between patients with CAPA (22 of 35 (62.9%)) and without CAPA (110 of 146 (75.3%)).
In total, 34 of 181 (18.8%) of COVID-19 cases were caused by the wildtype virus, 80 (44.2%) by the alpha variant, and 54 (29.8%) by the delta variant. Thirteen (7.2%) cases were not sequenced. There was no significant difference in underlying variants between groups.
3.2. Mortality
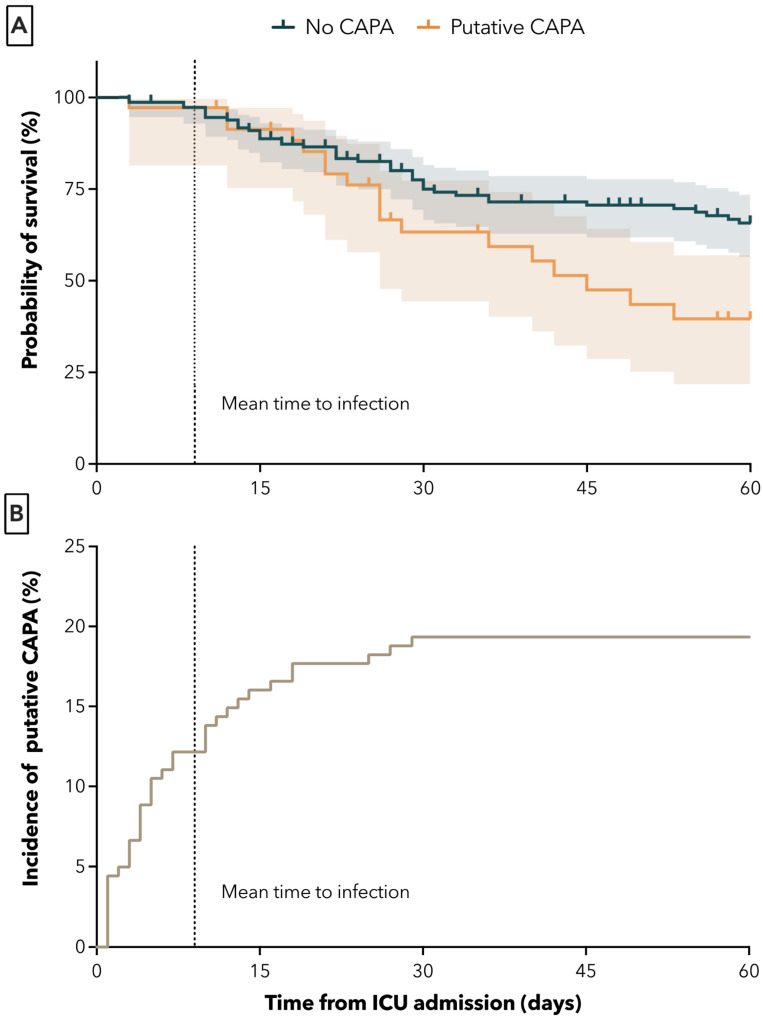
The 60-day all-cause mortality from ICU admission was significantly higher in the CAPA group. Death at 60 days occurred in 18 of 35 (51.4%) of patients with CAPA and in 43 of 146 (29.5%) patients without CAPA (adjusted HR (95% CI) = 2.15 (1.20–3.86, p = 0.002). The unadjusted HR was 1.97 (95% CI 1.13–3.42), p = 0.01) (Figure 2A). The mean time from ICU admission to time of CAPA diagnosis was 9 ± 8 days (Figure 2B).
Figure 2.
Survival probabilities of patients with and without putative aspergillosis in days after ICU admission (A) and mean time to first positive Aspergillus culture from BAL sample (B). Dotted lines represent mean time to infection.
3.3. Pathogen Distribution
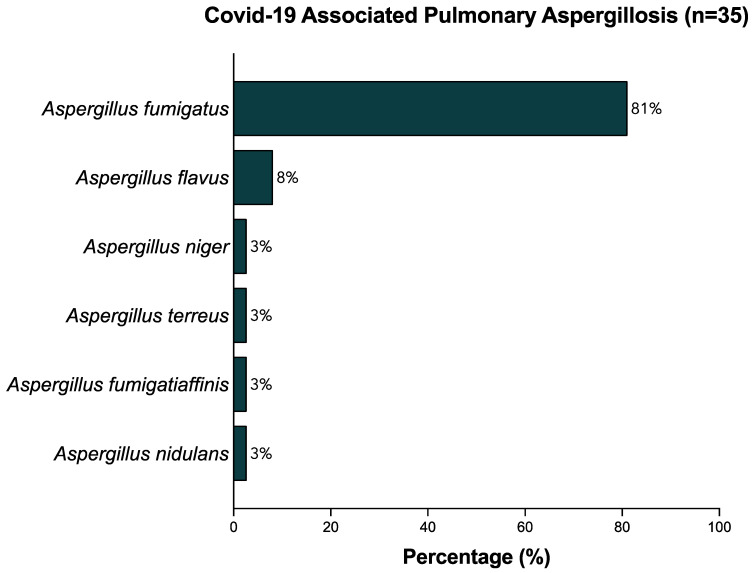
The relative frequencies of identified aspergillus species in microbiological cultures of BAL samples are depicted in Figure 3. The most commonly identified Aspergillus species was Aspergillus fumigatus, which was found in 30 of 35 (85.6%) patients diagnosed with CAPA. Aspergillus flavus was found in three (8.6%) cases. One case each of Aspergillus niger, Aspergillus terreus, Aspergillus fumigatiaffinis, and Aspergilllus nidulans were identified (2.9%). One patient had a co-infection with three Aspergillus spp. (A. Flavus, A. Fumigatus, and A. Niger).
Figure 3.
Relative frequencies (%) of strains identified in BAL samples.
3.4. Antifungal Therapy
Table 2 provides an overview of the antifungal therapy used to treat CAPA. After the diagnosis of CAPA, treatment with voriconazole was initiated in 16 of 35 (45.7%) patients. Four patients (11.4%) received micafungin, three patients (8.6%) each received caspofungin or fluconazole, and one patient (2.8%) each received nystatin or isavuconazole. In 11 patients (31.4%), the dose was either increased or switched to another antifungal because symptoms did not improve.
Table 2.
Overview of baseline immunosuppressive medication, pathogen prevalence, time to infection, antifungal therapy, and outcome by patient. n/a refers to data not available in medical records; [-] indicates no use of second-line antifungal therapy.
| Patient | Sex | Age (Years) |
Immunosuppressants at ICU Admission | Aspergillus spp. | Time to Infection from ICU Admission (Days) | Initial Antifungal Therapy at Time of Infection | Second-Line Antifungal Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 49 | Dexamethasone 12 mg | Fumigatus | 11 | Voriconazole 200 mg | Voriconazole 400 mg | Recovered |
| 2 | M | 35 | Prednisolone 100 mg | Fumigatus | 1 | Caspofungin 70 mg | Voriconazole 400 mg | Recovered |
| 3 | F | 46 | Hydrocortisone 200 mg | Fumigatus | 10 | Voriconazole 300 mg | [-] | Recovered |
| 4 | M | 50 | None | Fumigatus | 1 | Micafungin 100 mg | Voriconazole 400 mg | Death |
| 5 | M | 50 | Dexamethasone 4 mg | Fumigatus | 2 | Voriconazole 400 mg | [-] | Death |
| 6 | M | 49 | None | Flavus | 3 | Voriconazole 200 mg | [-] | Recovered |
| 7 | M | 59 | None | Fumigatus | 4 | Voriconazole 200 mg | [-] | Death |
| 8 | M | 46 | Prednisolone 50 mg | Fumigatus | 29 | Fluconazole 800 mg | [-] | Recovered |
| 9 | M | 27 | Dexamethasone 40 mg | Flavus | 1 | Voriconazole 400 mg | [-] | Death |
| Fumigatus | 1 | |||||||
| Niger | 1 | |||||||
| 10 | M | 64 | Prednisolone 50 mg | Fumigatus | 10 | n/a | n/a | Death |
| 11 | M | 45 | None | Fumigatus | 18 | n/a | n/a | Death |
| 12 | M | 56 | Dexamethasone 10 mg | Fumigatus | 4 | Voriconazole 400 mg | [-] | Death |
| 13 | M | 63 | None | Fumigatus | 1 | Voriconazole 200 mg | [-] | Death |
| 14 | M | 51 | Dexamethasone 6 mg | Fumigatus | 1 | Voriconazole 400 mg | [-] | Death |
| 15 | M | 67 | Dexamethasone 8 mg | Fumigatus | 4 | Nystatin 1 mL | [-] | Recovered |
| 16 | F | 55 | Prednisolone 25 mg | Fumigatus | 5 | Voriconazole 300 mg | [-] | Recovered |
| 17 | F | 61 | None | Terreus | 18 | Voriconazole 400 mg | [-] | Recovered |
| 18 | M | 57 | Dexamethasone 8 mg | Fumigatus | 1 | Voriconazole 200 mg | [-] | Death |
| 19 | M | 56 | Dexamethasone 6 mg | Fumigatus | 5 | Fluconazole 600 mg | Voriconazole 200 mg | Death |
| 20 | M | 61 | None | Fumigatus | 3 | Isavuconazole 200 mg | [-] | Death |
| 21 | F | 69 | None | Fumigatus | 13 | n/a | n/a | Death |
| 22 | M | 70 | Dexamethasone 10 mg | Fumiigatiiaffinis | 12 | Micafungin 100 mg | Voriconazole 400 mg | Death |
| 23 | M | 70 | Dexamethasone 6 mg | Nidulans | 10 | n/a | n/a | Death |
| 24 | M | 48 | Dexamethasone 6 mg | Fumigatus | 1 | Voriconazole 200 mg | Voriconazole 300 mg | Recovered |
| 25 | F | 62 | None | Fumigatus | 1 | Voriconazole 200 mg | Voriconazole 200 mg Nystatin 0.5 mL | Recovered |
| 26 | M | 60 | None | Flavus | 27 | n/a | n/a | Recovered |
| 27 | M | 54 | Dexamethasone 10 mg Anakinra 600 mg |
Fumigatus | 7 | Fluconazole 800 mg | Voriconazole 200 mg | Death |
| 28 | M | 58 | Hydrocortisone 200 mg | Fumigatus | 6 | Caspofungin 70 mg | Amphotericin B 600 mg | Recovered |
| 29 | F | 69 | None | Fumigatus | 16 | Caspofungin 50 mg | n/a | Death |
| 30 | F | 79 | Dexamethasone 4 mg | Fumigatus | 5 | n/a | n/a | Death |
| 31 | F | 51 | Dexamethasone 4 mg | Fumigatus | 3 | Voriconazole 200 mg | Voriconazole 400 mg | Recovered |
| 32 | M | 54 | None | Fumigatus | 4 | Micafungin 100 mg | n/a | Recovered |
| 33 | M | 63 | Prednisolone 250 mg | Fumigatus | 25 | n/a | n/a | Death |
| 34 | M | 43 | None | Fumigatus | 14 | Micafungin 100 mg | Micafungin 100 mg Amphotericin B 10 mg inhal. | Recovered |
| 35 | F | 65 | Prednisolone 500 mg | Fumigatus | 7 | Voriconazole 300 mg | [-] | Death |
4. Discussion
The present study provides vital insights into the prevalence and clinical outcomes of COVID-19-associated pulmonary aspergillosis in a population of critically ill patients. Our results demonstrate that CAPA is a significant complication, with high prevalence of 19% in patients who have undergone at least one BAL during ICU stays. The most commonly identified pathogen was Aspergillus fumigatus, which was found in 86% of cases. In line with the literature, the average time from ICU admission to the diagnosis of CAPA in our cohort was 9 days. This is consistent with the observation that invasive pulmonary aspergillosis (IPA) tends to occur later in the course of the illness in COVID-19 patients compared to influenza patients [13,18,19,20].
The diagnosis of aspergillosis can be challenging, as the symptoms are often nonspecific and may overlap with other respiratory infections [14]. Hence, previous studies examining the prevalence of CAPA in critically ill patients have yielded highly varying results, ranging from 2.5% to 35% [5,13,19,21,22,23,24]. Several criteria for the diagnosis of IPA have been proposed, but there is currently no international consensus regarding the use of any criteria for study purposes [17,25,26]. In addition, some studies rely on the use of galactomannan assays from blood or BAL samples for the diagnosis of IPA, which have only shown moderate sensitivity of about 70% in a large meta-analysis [15]. In addition, imaging techniques such as X-ray and CT scans may be useful in aiding the clinical diagnosis of aspergillosis but are not specific for the disease [13]. As a result, IPA is at risk of misdiagnosis when not strictly adhering to established diagnostic criteria, and the true prevalence of CAPA may be lower than previously reported, as suggested by a systematic review of autopsies [27,28].
Our study adds to the growing body of evidence demonstrating the significant impact of CAPA on mortality in COVID-19 patients. Our study found that patients with CAPA had significantly higher 60-day all-cause mortality compared to those without CAPA (51% vs. 30%). These results are consistent with the previous literature, which also observed higher mortality in COVID-19 patients with CAPA (44% to 55%) versus without CAPA (19% to 34%) [1,5,19,23,29]. It is important to note that most studies investigated shorter time periods, typically 30 days or less, which may not capture the full extent of the impact of CAPA on mortality. Here, we opted for 60-day all-cause mortality due to the late onset of CAPA in our cohort. The longer period of observation reveals diverging mortality curves after the mean time to infection, as shown in Figure 2. This highlights the importance of longer-term follow-up in patients with CAPA to fully understand the impact of this comorbidity on outcomes.
Although the clinical diagnosis of CAPA remains challenging, Gangneux et al. observed that a positive Aspergillus culture from respiratory specimens alone was associated with increased mortality, regardless of aspergillosis status [20]. Hence, due to high mortality in ICU patients with CAPA and delayed onset, days to weeks after ICU admission, patients may benefit from the early initiation of antifungal therapy as soon as Aspergillus is recovered [18]. However, the evidence supporting the use of antifungal therapy in the management of CAPA remains inconclusive. Hatzl et al. observed no improved survival after treating critically ill COVID-19 patients with antifungal prophylaxis at admission, despite a significant reduction in CAPA incidence [30]. These contradicting findings therefore warrant further research into the diagnosis, treatment, and outcomes of CAPA.
The use of corticosteroids to combat severe COVID-19 has become the standard of care after pivotal clinical trials [31]. However, previous studies have linked the use of immunosuppressants with the increased incidence of CAPA [11,12,32]. This raises concerns about the safety of corticosteroid use in patients with COVID-19, particularly those at high risk of developing CAPA. In our study, all patients with putative CAPA received corticosteroids at the time of diagnosis, as defined by the AspICU criteria. In addition, 73% of all patients received corticosteroids at the time of ICU admission. Although descriptively more patients without CAPA than with CAPA received immunosuppressants at ICU admission, there was no significant difference between the groups. This suggests that the use of corticosteroids in patients with COVID-19 may not be the sole contributing factor to the development of CAPA in our cohort.
The majority of patients with CAPA in our study received voriconazole as initial antifungal treatment (46%). In 31% of all patients, either the dose had to be increased or switched to another antimycotic because the symptoms did not improve. This highlights the challenges associated with managing CAPA in critically ill COVID-19 patients and the need for further research to identify more effective antifungal regimens.
Although limited to a single study center, the inclusion of a large number of patients from multiple ICUs allowed for a homogeneous, comprehensive assessment of the incidence and clinical outcomes of CAPA in this group of patients with comparable treatment practices and outcomes. Nevertheless, this design may limit the generalizability of our findings to other populations, as the patient population in our study center may not be representative of other regions or countries. Furthermore, the diagnosis of CAPA remains challenging, and the AspICU classification may not be fully sensitive or specific enough to diagnose this condition. Nevertheless, as discussed earlier, previous studies have demonstrated that positive cultures from respiratory specimens alone are associated with increased mortality, regardless of aspergillosis status. Hence, we opted for BAL cultures alone, as opposed to less sensitive galactomannan assays, for the identification of putative CAPA cases. Furthermore, previous studies have demonstrated increased mortality in critically ill COVID-19 patients with concomitant bacterial, viral, and fungal co-infections. The frequency of bacterial infections did not differ between patients with or without CAPA in our cohort. However, we were unable to obtain reliable data on other fungal or viral co-infections, which may have had an impact on clinical outcomes. Finally, the present study is limited by its retrospective design, which may be subject to bias or documentation errors in medical records.
5. Conclusions
In conclusion, the prevalence of putative pulmonary aspergillosis in critically ill COVID-19 patients was high (19%), with a mean time to diagnosis of 9 days after ICU admission. Patients with putative pulmonary aspergillosis had significantly higher mortality compared to those without CAPA. These results highlight the importance of the close surveillance of fungal co-infections in critically ill COVID-19 patients.
Author Contributions
Conceptualization, F.B., A.J., A.B., C.R. and M.Z.; data curation, F.B., A.J., C.G., S.B. and A.N.-P.; formal analysis, A.J. and M.Z.; funding acquisition, A.J. and M.Z.; investigation, F.B., A.J., A.B., C.G., S.B. and A.N.-P.; methodology, F.B., A.J., C.G. and A.N.-P.; project administration, F.B., A.B. and C.R.; resources, C.G.; software, A.J., C.G. and S.B.; supervision, A.J., C.R. and M.Z.; visualization, F.B.; writing—original draft, F.B.; writing—review and editing, F.B., A.J., A.B., C.G., S.B., A.N.-P., C.R. and M.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This retrospective study received ethics approval from the Ethics Committee of the Medical University of Vienna before initiation (EC 2259/2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and materials that support the findings of this study are available from the corresponding author upon the reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received funding by the Medical Scientific Fund of the Mayor of the City of Vienna (Project ID 22153).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Raffaelli F., Tanzarella E.S., de Pascale G., Tumbarello M. Invasive Respiratory Fungal Infections in COVID-19 Critically Ill Patients. J. Fungi. 2022;8:415. doi: 10.3390/jof8040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maes M., Higginson E., Pereira-Dias J., Curran M.D., Parmar S., Khokhar F., Cuchet-Lourenço D., Lux J., Sharma-Hajela S., Ravenhill B., et al. Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19. Crit. Care. 2021;25:25. doi: 10.1186/s13054-021-03460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabelloni M., Faggioni L., Cioni D., Mendola V., Falaschi Z., Coppola S., Corradi F., Isirdi A., Brandi N., Coppola F., et al. Extracorporeal Membrane Oxygenation (ECMO) in COVID-19 Patients: A Pocket Guide for Radiologists. Radiol. Med. 2022;127:369–382. doi: 10.1007/s11547-022-01473-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandi N., Ciccarese F., Rimondi M.R., Balacchi C., Modolon C., Sportoletti C., Renzulli M., Coppola F., Golfieri R. An Imaging Overview of COVID-19 ARDS in ICU Patients and Its Complications: A Pictorial Review. Diagnostics. 2022;12:846. doi: 10.3390/diagnostics12040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoletti M., Pascale R., Cricca M., Rinaldi M., Maccaro A., Bussini L., Fornaro G., Tonetti T., Pizzilli G., Francalanci E., et al. Epidemiology of Invasive Pulmonary Aspergillosis among Intubated Patients with COVID-19: A Prospective Study. Clin. Infect. Dis. 2021;73:E3606–E3614. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardi T., Pintado V., Gomez-Rojo M., Escudero-Sanchez R., Azzam Lopez A., Diez-Remesal Y., Castro N.M., Ruiz-Garbajosa P., Pestaña D. Nosocomial Infections Associated to COVID-19 in the Intensive Care Unit: Clinical Characteristics and Outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:495–502. doi: 10.1007/s10096-020-04142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandi N., Ciccarese F., Balacchi C., Rimondi M.R., Modolon C., Sportoletti C., Capozzi C., Renzulli M., Paccapelo A., Castelli A., et al. Co-Infections and Superinfections in COVID-19 Critically Ill Patients Are Associated with CT Imaging Abnormalities and the Worst Outcomes. Diagnostics. 2022;12:1617. doi: 10.3390/diagnostics12071617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouzé A., Martin-Loeches I., Povoa P., Metzelard M., du Cheyron D., Lambiotte F., Tamion F., Labruyere M., Geronimi C.B., Nieszkowska A., et al. Early Bacterial Identification among Intubated Patients with COVID-19 or Influenza Pneumonia: A European Multicenter Comparative Clinical Trial. Am. J. Respir. Crit. Care Med. 2021;204:546–556. doi: 10.1164/rccm.202101-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey M., May A., Tan L., Hughes H., Jones J.P., Harrison W., Bradburn S., Tyrrel S., Muthuswamy B., Berry N., et al. Comparative Incidence of Early and Late Bloodstream and Respiratory Tract Co-Infection in Patients Admitted to ICU with COVID-19 Pneumonia versus Influenza A or B Pneumonia versus No Viral Pneumonia: Wales Multicentre ICU Cohort Study. Crit. Care. 2022;26:158. doi: 10.1186/s13054-022-04026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimopoulos G., Almyroudi M.P., Myrianthefs P., Rello J. COVID-19-Associated Pulmonary Aspergillosis (CAPA) J. Intensive Med. 2021;1:71–80. doi: 10.1016/j.jointm.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S.H., Hong J.Y., Bae S., Lee H., Wi Y.M., Ko J.H., Kim B., Joo E.J., Seok H., Shi H.J., et al. Risk Factors for Coronavirus Disease 2019 (COVID-19)-Associated Pulmonary Aspergillosis in Critically Ill Patients: A Nationwide, Multicenter, Retrospective Cohort Study. J. Korean Med. Sci. 2022;37:e134. doi: 10.3346/jkms.2022.37.e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leistner R., Schroeter L., Adam T., Poddubnyy D., Stegemann M., Siegmund B., Maechler F., Geffers C., Schwab F., Gastmeier P., et al. Corticosteroids as Risk Factor for COVID-19-Associated Pulmonary Aspergillosis in Intensive Care Patients. Crit. Care. 2022;26:30. doi: 10.1186/s13054-022-03902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamoth F., Lewis R.E., Walsh T.J., Kontoyiannis D.P. Navigating the Uncertainties of COVID-19-Associated Aspergillosis: A Comparison With Influenza-Associated Aspergillosis. J. Infect. Dis. 2021;224:1631–1640. doi: 10.1093/infdis/jiab163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend L., Martin-Loeches I. Invasive Aspergillosis in the Intensive Care Unit. Diagnostics. 2022;12:2712. doi: 10.3390/diagnostics12112712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeiffer C.D., Fine J.P., Safdar N. Diagnosis of Invasive Aspergillosis Using a Galactomannan Assay: A Meta-Analysis. Clin. Infect. Dis. 2006;42:1417–1427. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 16.Vandenbroucke J.P., von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J., Poole C., Schlesselman J.J., Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007;4:1628–1654. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blot S.I., Taccone F.S., van den Abeele A.M., Bulpa P., Meersseman W., Brusselaers N., Dimopoulos G., Paiva J.A., Misset B., Rello J., et al. A Clinical Algorithm to Diagnose Invasive Pulmonary Aspergillosis in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 18.Verweij P.E., van de Veerdonk F.L. Managing Secondary Fungal Infections in Severe COVID-19: How to Move Forward? Lancet Respir. Med. 2022;10:127–128. doi: 10.1016/S2213-2600(21)00500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouzé A., Lemaitre E., Martin-Loeches I., Povoa P., Diaz E., Nyga R., Torres A., Metzelard M., du Cheyron D., Lambiotte F., et al. Invasive Pulmonary Aspergillosis among Intubated Patients with SARS-CoV-2 or Influenza Pneumonia: A European Multicenter Comparative Cohort Study. Crit. Care. 2022;26:11. doi: 10.1186/s13054-021-03874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gangneux J.P., Dannaoui E., Fekkar A., Luyt C.E., Botterel F., de Prost N., Tadié J.M., Reizine F., Houzé S., Timsit J.F., et al. Fungal Infections in Mechanically Ventilated Patients with COVID-19 during the First Wave: The French Multicentre MYCOVID Study. Lancet Respir. Med. 2022;10:180–190. doi: 10.1016/S2213-2600(21)00442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupont D., Menotti J., Turc J., Miossec C., Wallet F., Richard J.C., Argaud L., Paulus S., Wallon M., Ader F., et al. Pulmonary Aspergillosis in Critically Ill Patients with Coronavirus Disease 2019 (COVID-19) Med. Mycol. 2021;59:110–114. doi: 10.1093/mmy/myaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fekkar A., Lampros A., Mayaux J., Poignon C., Demeret S., Constantin J.M., Marcelin A.G., Monsel A., Luyt C.E., Blaize M. Occurrence of Invasive Pulmonary Fungal Infections in Patients with Severe COVID-19 Admitted to the ICU. Am. J. Respir. Crit. Care Med. 2021;203:307–317. doi: 10.1164/rccm.202009-3400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen N.A.F., Nyga R., Vanderbeke L., Jacobs C., Ergün M., Buil J.B., van Dijk K., Altenburg J., Bouman C.S.C., van der Spoel H.I., et al. Multinational Observational Cohort Study of COVID-19-Associated Pulmonary Aspergillosis. Emerg. Infect. Dis. 2021;27:2892–2898. doi: 10.3201/eid2711.211174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koukaki E., Rovina N., Tzannis K., Sotiropoulou Z., Loverdos K., Koutsoukou A., Dimopoulos G. Fungal Infections in the ICU during the COVID-19 Era: Descriptive and Comparative Analysis of 178 Patients. J. Fungi. 2022;8:881. doi: 10.3390/jof8080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., Klimko N., Lass-Flörl C., Oladele R.O., Vinh D.C., et al. Defining and Managing COVID-19-Associated Pulmonary Aspergillosis: The 2020 ECMM/ISHAM Consensus Criteria for Research and Clinical Guidance. Lancet Infect. Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peter Donnelly J., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., Clancy C.J., Wingard J.R., Lockhart S.R., Groll A.H., et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winters B., Custer J., Galvagno S.M., Colantuoni E., Kapoor S.G., Lee H.W., Goode V., Robinson K., Nakhasi A., Pronovost P., et al. Diagnostic Errors in the Intensive Care Unit: A Systematic Review of Autopsy Studies. BMJ Qual. Saf. 2012;21:894–902. doi: 10.1136/bmjqs-2012-000803. [DOI] [PubMed] [Google Scholar]
- 28.Kula B.E., Clancy C.J., Hong Nguyen M., Schwartz I.S. Invasive Mould Disease in Fatal COVID-19: A Systematic Review of Autopsies. Lancet Microbe. 2021;2:e405–e414. doi: 10.1016/S2666-5247(21)00091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feys S., Almyroudi M.P., Braspenning R., Lagrou K., Spriet I., Dimopoulos G., Wauters J. A Visual and Comprehensive Review on COVID-19-Associated Pulmonary Aspergillosis (Capa) J. Fungi. 2021;7:1067. doi: 10.3390/jof7121067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatzl S., Reisinger A.C., Posch F., Prattes J., Stradner M., Pilz S., Eller P., Schoerghuber M., Toller W., Gorkiewicz G., et al. Antifungal Prophylaxis for Prevention of COVID-19-Associated Pulmonary Aspergillosis in Critically Ill Patients: An Observational Study. Crit. Care. 2021;25:335. doi: 10.1186/s13054-021-03753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The RECOVERY Collaborative Group Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Veerdonk F.L., Brüggemann R.J.M., Vos S., de Hertogh G., Wauters J., Reijers M.H.E., Netea M.G., Schouten J.A., Verweij P.E. COVID-19-Associated Aspergillus Tracheobronchitis: The Interplay between Viral Tropism, Host Defence, and Fungal Invasion. Lancet Respir. Med. 2021;9:795–802. doi: 10.1016/S2213-2600(21)00138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials that support the findings of this study are available from the corresponding author upon the reasonable request.