ABSTRACT
Coronavirus (COVID-19) vaccines have proved to be effective in the pandemic response but can cause adverse events such as delayed hypersensitivity reactions (DHRs). Delayed-reading intradermal tests (IDT) to vaccines are limited by false-positive results and may reflect a cell-mediated rather than IgE-mediated immune response. Lymphocyte transformation test (LTT), which has been utilized in the diagnosis of drug allergy, may be helpful in suspected COVID-19 vaccine and/or its excipient-related DHRs. To investigate the use of LTT in two suspected cases of COVID-19 vaccine-induced DHRs, two patients with suspected DHRs to COVID-19 vaccination were tested by delayed-reading IDT and LTT against vaccines and their excipients. A 47-year-old man developed acute mixed-pattern hepatitis after the second dose of ChAdOx1 vaccine. LTT performed at 2 months post-vaccination revealed reactivity to the ChAdOx1 vaccine, polysorbate 80 and mildly to PEG 2050 but not BNT162b2 vaccine. Delayed-reading IDT returned negative to both vaccines and excipients. He tolerated BNT162b2 vaccination with no adverse events. A 36-year-old woman presented with subacute morbilliform eruption and hepatitis after the first dose of BNT162b2 vaccine. LTT performed 3 months later revealed reactivity to the BNT162b2 but not PEG 2050. Repeat LTT following subsequent natural Severe Acute Respiratory Coronavirus-2 (SARS-CoV-2) infection revealed reactivity to ChAdOx1 and NVX-CoV2373 vaccines but not polysorbate 80. Delayed-reading IDT remained negative. She proceeded with NVX-CoV2373 vaccination with no symptom recurrence. LTT may be a useful tool in suspected COVID-19 vaccine-related DHRs. Further evaluation with a larger patient cohort is required.
KEYWORDS: Covid-19, vaccine, delayed hypersensitivity, LTT, hepatitis, cytopenia, thrombocytopenia, neutropenia, delayed skin tests
Introduction
Since the utilization of vaccination as one of the main tools of pandemic response to Severe Acute Respiratory Coronavirus-2 (SARS-CoV-2), there has been global recognition of rare, yet not insignificant vaccine-related complications. While isolated cutaneous reactions are not uncommon,1 coronavirus (COVID-19) vaccine-associated cutaneous hypersensitivity reaction with acute hepatitis has been described.2 Delayed-reading intradermal tests (IDT) to the COVID-19 vaccines can be performed, but a positive reading may represent evidence of cell-mediated immunity rather than a vaccine allergy.3 In vitro diagnostic testing remains limited in the assessment of suspected delayed hypersensitivity reactions (DHRs) to COVID-19 vaccines and/or their components. The lymphocyte transformation test (LTT) is an in vitro method used in the diagnosis of drug hypersensitivity. It detects proliferation of drug-specific memory T-cells following co-incubation of the patient’s peripheral mononuclear cells (PBMC) with the drug of interest. While it has been commonly applied in the evaluation of drugs such as antibiotics, allopurinol, and non-steroidal anti-inflammatory drugs,4 its role in the assessment of DHRs to vaccines, in particular COVID-19 vaccines, requires further elucidation.5 We herein describe our approach to combining LTT and skin tests (ST) for assessing two suspected cases of DHRs following ChAdOx1 or BNT162b2 COVID-19 vaccination.
Materials and methods
Ethics approval and consent to participate
Ethical approval was obtained from the RNSH human ethics committee (RESP/16/255). Patients gave informed consent to have blood samples collected for the study.
Lymphocyte transformation test
Briefly, fresh heparinized blood was collected for all experiments and PBMCs were collected using our previously published method6 After collection of interface, PBMCs were washed with PBS and counted using a hemocytometer. After PBMC collection and counting, cells were added to wells of a 96-well plate at a concentration of 200,000 cells per well in triplicate. Phytohaemagglutinin (PHA) at 5 µg/ml (Sigma) and T-Cell Transact ™ beads (Miltenyi Biotec) at a 1:200 dilution were used as positive controls. Negative control wells contained cells in culture media alone (TexMACS, Miltenyi Biotec). ChAdOx1, BNT162b2 and NVX-CoV2373 COVID-19 vaccines and their excipients were evaluated (Table 1). The vaccines were diluted at 1:200 (dilution 1), 1:400 (dilution 2), 1:800 (dilution 3), 1:1600 (dilution 4), 1:3200 (dilution 5) and 1:6400 (dilution 6). ChAdOx1 vaccine excipient polysorbate 80 was diluted at 0.2% (dilution 1), 0.1% (dilution 2), 0.05% (dilution 3), 0.025% (dilution 4), 0.0125% (dilution 5) and 0.00625% (dilution 6). Polyethylene glycol (PEG) 2050 similar to BNT162b2 vaccine excipient 2 [(polyethylene glycol)-2000]-N,N-ditetrade-cylacetamide)7 was used due to its cost and availability. This was diluted at 0.5% (dilution 1), 0.25% (dilution 2), 0.125% (dilution 3), 0.0625% (dilution 4), 0.0312% (dilution 5) and 0.0156% (dilution 6). Cell interaction with vaccine or vaccine excipients was carried out for 6 days at 37°C/5% CO2. On day 6, 50 µl of XTT solution (Roche) was added to each well and read at 492 and 690 nm at 4, 6 and 24 hours. Data collected from each patient or control were analyzed using one-way ANOVA with Tukey’s multiple comparison post-test. All data were analyzed using Prism 5.0 (Graphpad, La Jolla, CA). Stimulation Index (SI) was calculated by dividing the XTT signal (492–690 nm) of drug stimulated samples with the XTT signal of unstimulated samples with no drug added (negative control). A SI value of ≥ 2 was considered a positive result.
Table 1.
COVID-19 vaccines and their excipients analyzed by the lymphocyte transformation test (LTT).
| Vaccine | ChAdOx1 (Vaxzevria) |
BNT162b2 (Comirnaty) |
NVX-CoV2373 (Nuvaxovid) |
|---|---|---|---|
| Type of vaccine | Adenovirus | mRNA | Recombinant protein |
| Manufacturer | Astra Zeneca | Pfizer | Novavax |
| Dosage level | 0.5 ml − 5 × 1010 viral particles per dose | 0.3 ml (30 µg/Dose) | 0.5 ml (5 µgspike protein + MatrixM adjuvant) |
| Excipients tested | Polysorbate-80 | 2[(polyethyleneglycol)-2000]-N,N-ditetradecylacetamide (PEG 2050) |
Polysorbate-80 |
| Vaccinetest dilutions (LTT) | 1:200 1:400 1:800 1:1600 1:3200 1:6400 |
1:200 1:400 1:800 1:1600 1:3200 1:6400 |
1:200 1:400 1:800 1:1600 1:3200 1:6400 |
| Excipient dilutions tested | Polysorbate-80 (Sigma) 0.2% 0.1% 0.05% 0.025% 0.125% 0.00625% |
PEG 2050 (Sigma) 0.5% 0.25% 0.125% 0.0625% 0.0312% 0.0156% |
N/A |
Skin testing
For IDT, we used vaccine at 10% and 1% w/v, PEG 2050 at 10% and 1% w/v, polysorbate 80 at 10% w/v. IDT to the excipients and vaccines were performed in a stepwise manner from lower to higher concentrations with reading at 30 min and at 24–48 h. Histamine skin prick test at 0.1% was used as a positive control and saline buffer/50% glycerol as a negative control.
Patient 1
Our first case involves a 47-year-old Caucasian man (Patient 1) with a history of chronic fatigue who presented to a local hospital with pyrexia, headache, myalgia, vomiting and diarrhea, 2 days after the second dose of ChAdOx1 COVID-19 vaccine (Figure 1). He had previously tolerated his first ChAdOx1 COVID-19 vaccine dose aside from self-limiting myalgia. There were no cutaneous manifestations, focal neurological deficits, seizures, encephalopathy or cardiorespiratory compromise. Upon hospitalization, he had thrombocytopenia (93×109/L), neutropenia (0.8×109/L) and lymphopenia (0.8×109/L) in the absence of anemia, and elevated CRP level (56 mg/L; cutoff<5 mg/L). He soon developed a mixed hepatitis, with peak levels of total bilirubin of 22 micromol/L (cutoff<20 micromol/L), alkaline phosphatase (ALP) of 400 U/L (reference range [RR]: 30–110 U/L), gamma glutamyltransferase (GGT) of 527 U/L (RR: 5–50 U/L), alanine aminotransferase (ALT) of 306 U/L (RR: 10–50 U/L) and aspartate aminotransferase (AST) of 165 U/L (RR: 10–35 U/L) on day 6 post-vaccination. Immunoglobulin G (IgG) was normal (12.1 g/L; RR: 7.0–16.0 g/L). He had a speckled pattern antinuclear antibody (ANA) at a titer of 1:160 in the absence of detectable antibodies to extractable nuclear antigens (ENA; SS-A, SS-B, Ro52, Scl-70, Jo-1, Cenp-B, Sm, RNP, Ribo-P). Anti-smooth muscle antibody (ASMA), anti-liver kidney microsomal antibody (anti-LKM), and anti-mitochondrial antibody (AMA) were negative by indirect immunofluorescence. There was no serological evidence of acute infection with hepatitis A, B and C, cytomegalovirus (CMV), Epstein-Barr virus (EBV), or human herpesvirus (HHV)-6, 7 and 8. He had detectable anti-intrinsic factor antibodies associated with a low active B12 (9 pmol/L, cutoff>50). D-dimer was positive (1.41 mg/L; cut-off<0.5 mg/L) with detectable autoantibodies against the platelet glycoprotein (GP) IIb/IIIa. The INR was 1.0 associated with subsequent fibrinogen rise to 4.6 g/L (RR: 2.0–4.6 g/L) that was felt to be in line with acute-phase reactant. Transient lupus anticoagulant was detected in the absence of detectable anti-cardiolipin and beta-2-glycoprotein-1 antibodies. Antibodies to platelet factor 4 were not ordered as Hematology determined the clinical picture not consistent with vaccine-induced thrombotic thrombocytopenia (VITT), given the absence of thrombosis and the short time frame. Abdominal ultrasound revealed a mild splenomegaly (a span of 15.2 cm) and mild pericholecystic fluid between the gallbladder and hepatic wall associated with periportal edema. He was administered a granulocyte colony-stimulating factor with normalization of blood counts and parenteral therapy for his B12 deficiency. Given the spontaneous improvement in his liver function on day 7 post-vaccination, a biopsy was not performed. He remained stable aside from persistent fatigue. He was discharged on day 9 post-vaccination, and his biochemical parameters normalized 9 weeks later. The LTT performed at 2 months post-vaccination revealed reactivity to the ChAdOx1 COVID-19 vaccine polysorbate 80 and mildly to PEG 2050 (SI 2.2–2.8) but not BNT162b2 COVID-19 vaccine (Table 2). Subsequently, delayed-reading IDT to the ChAdOx1 and BNT162b2 COVID-19 vaccines, PEG 2050 and polysorbate 80 returned negative at 24 and 72 hours. His biochemical and hematological parameters remained normal following natural SARS-CoV-2 infection about 4 months after his second dose of ChAdOx1 COVID-19 vaccine. He was able to proceed with BNT162b2 COVID-19 vaccine booster 2 months later without any sequelae.
Figure 1.
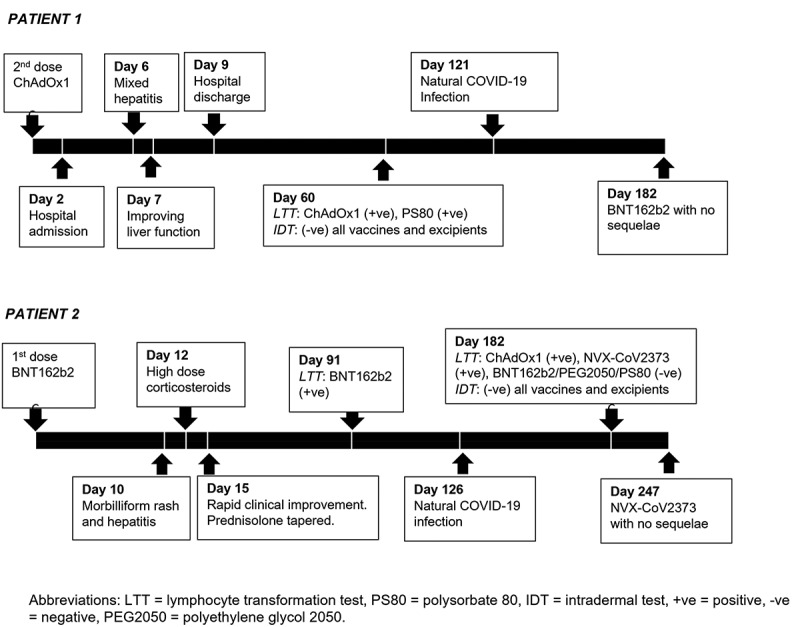
Timeline of clinical events in Patient 1 and Patient 2.
Table 2.
Summary of lymphocyte transformation test (LTT) results for Patient 1 and Patient 2.
| COVID-19 vaccine |
Vaccine components |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ChAdOx1 |
BNT162b2 |
NVX-CoV2373 |
Polysorbate 80 |
PEG 2050 |
||||||||
| ANOVA | SI | ANOVA | SI | ANOVA | SI | ANOVA | SI | ANOVA | SI | |||
| Patient 1 | LTT | Dilution 1 | *** | 5.4 | NS | 1.5 | ** | 4.0 | NS | 1.0 | ||
| Dilution 2 | **** | 6.3 | NS | 1.5 | **** | 5.9 | NS | 2.7 | ||||
| Dilution 3 | **** | 6.3 | NS | 1.6 | NS | 2.8 | NS | 2.6 | ||||
| Dilution 4 | *** | 6.2 | NS | 1.5 | NS | 2.2 | NS | 1.8 | ||||
| Dilution 5 | NS | 5.3 | NS | 1.3 | NS | 1.5 | NS | 1.7 | ||||
| Dilution 6 | NS | 3.5 | NS | 2 | NS | 1.2 | NS | 0.3 | ||||
| CD3/CD28 | **** | 8.7 | ||||||||||
| PHA | **** | 7.8 | ||||||||||
| Patient 2 | First LTT | Dilution 1 | NS | 0.5 | NS | 1.1 | NS | 0 | NS | 1.2 | ||
| Dilution 2 | NS | 0.6 | ** | 2.0 | NS | 0.9 | NS | 1.2 | ||||
| Dilution 3 | NS | 0.7 | NS | 1.1 | NS | 1.5 | NS | 1.1 | ||||
| Dilution 4 | NS | 1.1 | NS | 1.0 | NS | 1.5 | NS | 1.1 | ||||
| Dilution 5 | NS | 0.9 | NS | 1.2 | NS | 1.1 | NS | 0.8 | ||||
| Dilution 6 | NS | 0.9 | NS | 1.0 | NS | 1.1 | NS | 1.1 | ||||
| CD3/CD28 | NNS | 1.16 | ||||||||||
| PHA | NNS | 1.32 | ||||||||||
| Post infection | Dilution 1 | NS | 1.83 | NS (AD) |
<2 (AD) |
*** | 11.1 |
NS (AD) |
<2 (AD) |
NS (AD) |
<2 (AD) |
|
| Dilution 2 | ** | 2.2 | **** | 19.8 | ||||||||
| Dilution 3 | ** | 2.2 | **** | 12.8 | ||||||||
| Dilution 4 | ** | 2.2 | ** | 9.3 | ||||||||
| Dilution 5 | * | 2.1 | * | 7.68 | ||||||||
| Dilution 6 | ** | 2.5 | NS | 2.8 | ||||||||
| CD3/CD28 | **** | 4.1 | ||||||||||
| PHA | **** | 3.2 | ||||||||||
NS = Not significant, ANOVA *p < .05, **p < .01 and ***p < .001 ****p < .0001.
SI greater > 2 Positive. (AD) = All dilutions.
Patient 2
Our second case is a 36-year-old Caucasian woman (Patient 2) who presented with a generalized morbilliform eruption 10 days after the first dose of the BNT162b2 COVID-19 vaccine (Figure 1). She recently commenced on a three-day course of prednisolone (1 mg/kg/day). Isolated transaminitis (AST 238 U/L, ALT 253 U/L) and mild peripheral eosinophilia (0.9×109/L) were incidentally noted. She was neither an avid drinker nor on any other drugs. She had no known drug allergies. Repeat laboratory findings are as follows: hemoglobin 127 g/L, lymphocytes 1.34 × 109/L, eosinophils 0.36 × 109/L, platelets 174 × 109/L, INR 1.0, IgG 8.89 g/L, AST 523 U/L, ALT 1550 U/L, GGT 151 U/L, ALP 128 U/L, total bilirubin 12 micromol/L, and normal blood film. She had a speckled pattern ANA at a titer of 1:160 in the absence of detectable antibodies to ENA. ASMA, anti-LKM and AMA were negative. Inflammatory markers were normal. There was no serological evidence of present infection with hepatitis A, B, C and E, CMV, EBV, herpes simplex virus-1/2, HHV-6, Q Fever or Leptospirosis. SARS-CoV-2 anti-spike IgG titer was 67.5 AU/mL (cutoff>15 AU/mL). Multiphase abdomen and pelvis computed tomography showed patent hepatic and portal veins, mild hepatic steatosis and a single calcified hepatic granuloma. Skin histology revealed a spongiotic reaction pattern with focal interface lymphocytic inflammation. Liver histology performed on high-dose corticosteroids (prednisolone 1 mg/kg/day for 2 days and intravenous hydrocortisone 400 mg/day for 1 day) showed mild steatosis and mild inflammatory portal infiltrate comprising mainly small lymphocytes that were CD3-positive with retained staining for CD7 and CD8. Lobular architecture was preserved with inconspicuous interface hepatitis or piecemeal necrosis. Rapid improvement in her biochemical profile and cutaneous manifestations were observed, and she was gradually tapered off prednisolone. The LTT performed at 3 months post-vaccination revealed reactivity to the BNT162b2 COVID-19 vaccine but not ChAdOx1 COVID-19 vaccine, PEG 2050 or polysorbate 80 (Table 2). She acquired a mild SARS-CoV-2 infection about 4 months post-vaccination. Repeat LTT at 2 months following natural infection and 6 months post-vaccination revealed reactivity to ChAdOx1 and NVX-CoV2373 (Novavax) COVID-19 vaccines but not PEG 2050 or polysorbate 80. Interestingly, previous reactivity to BNT162b2 vaccine was no longer observed after the patient had been treated with corticosteroid therapy. Delayed-reading IDT at 24 and 72 hours returned negative to the BNT162b2, ChAdOx1 and NVX-CoV2373 COVID-19 vaccines, and PEG 2050 and polysorbate 80. She was able to tolerate the NVX-CoV2373 COVID-19 vaccination with stable biochemical parameters and no recurrence of cutaneous lesions.
Discussion
Our clinical suspicion in both of these cases was possible DHRs to the ChAdOx1 or BNT162b2 COVID-19 vaccines, resulting in drug-induced liver injury and/or cutaneous adverse reactions. Alternate causes for these patients’ clinical manifestations were sufficiently excluded. Patient 1 developed subacute hepatitis following his ChAdOx1 COVID-19 vaccination – he also had transient cytopenia in the absence of other features of VITT. His positive LTT result to the ChAdOx1 COVID-19 vaccine and polysorbate 80 and the lack of reactivity to the BNT162b2 COVID-19 vaccine and PEG 2050 gave us a greater confidence to proceed with the latter for his booster vaccination. A positive LTT response does not necessarily discriminate between a cell-mediated immune and delayed hypersensitivity reaction. However, this is the first known report of a positive LTT response to polysorbate 80. It is possible that Patient 1 was sensitized to this excipient following his first ChAdOx1 COVID-19 vaccination and developed a DHR following the second dose. Patient 2 had a more florid presentation of severe hepatitis with cutaneous manifestations. Her initial positive LTT response to the BNT162b2 COVID-19 vaccine and negative response to PEG 2050 may be attributable to a difference in the structures of PEG 2050 tested and that of 2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide) found in the vaccine,7 the requirement to determine timing of testing and concentrations of PEG, or autoimmune mechanisms triggered by the vaccine that are independent of vaccine excipients. We observed that she lost her LTT response to the BNT162b2 COVID-19 vaccine about 6 months following her dose in the context of long-term corticosteroid therapy. Interestingly, her repeat LTT following natural SARS-CoV-2 infection showed reactivity to both ChAdOx1 and NVX-CoV2373 COVID-19 vaccines, the agents which she has never encountered. One hypothesis is that the ChAdOx1 vaccine produced spike protein in vitro as other groups have demonstrated8 and induced a T-cell-mediated response to the spike protein generated in vitro rather than an allergic response. Similarly, the positive reaction NVX-CoV2373 was suspected to be a T-cell response to the recombinant spike protein in the vaccine after vaccination and previous infection. Based on her age and a low likelihood of clinical allergy, we proceeded with the NVX-CoV2373 COVID-19 vaccine, which she tolerated and further supported our hypothesis.
In both cases, limitations of delayed reading intradermal tests were observed as LTT will not only detect delayed hypersensitivity reactions to COVID-19 vaccines and/or their components but also T-cell responses to the spike protein components in the vaccines themselves.3,9 However, LTT may detect positive responses to excipients that are potentially involved in the pathogenesis of DHR to COVID-19 vaccines. In addition, it may provide early insights into correlates of protection in the context of immunosuppression and prior vaccination as well as the effect of SARS-CoV-2 infection on interpretation of responses to certain COVID-19 vaccines.
In summary, this study although limited to two patients shows promising data on the utility of applying LTT and ST results in the evaluation of patients with moderate and severe DHRs to COVID-19 vaccines. Further studies are required to help differentiate between a T-cell response to the vaccine and a DHR to a component of the vaccine.
Acknowledgments
We would like to thank Dr Joshua Jacob, Dr Katherine Sutton and Reina Zaragoza for their assistance with skin testing and blood collections. We would also like to thank Dr Karen Cheung and Dr Chow Heok P’Ng for their histological analysis and reporting and Dr Raymond Kwok with Patient 2’s hepatitis management.
Funding Statement
We are grateful to Warren and Marianne Lesnie for supporting the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, Desai SR, French LE, Lim HW, Thiers BH, et al. Cutaneous reactions reported after moderna and pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–5. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong CY, Rios EJ.. Cutaneous hypersensitivity reaction with acute hepatitis following COVID-19 vaccine. JAAD Case Rep. 2021;16: 44–46. doi: 10.1016/j.jdcr.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi L, Biondi F, Hansel K, Murgia N, Tramontana M, Stingeni L.. Skin tests in urticaria/angioedema and flushing to pfizer-BioNTech SARS-CoV-2 vaccine: limits of intradermal testing. Allergy. 2021;76(8):2605–07. doi: 10.1111/all.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachs B, Fatangare A, Sickmann A, Glassner A. Lymphocyte transformation test: history and current approaches. J Immunol Methods. 2021;493: 113036. doi: 10.1016/j.jim.2021.113036. [DOI] [PubMed] [Google Scholar]
- 5.Jover Cerda V, Rodriguez Pacheco R, Domenech Witek J, Alonso Hernandez S, Duran Garcia R, Real Panisello M, Marco de la Calle FM. Allergological study in patients vaccinated against COVID-19 with suspected allergic reactions. Allergy Asthma Clin Immunol. 2022;18(1):43. doi: 10.1186/s13223-022-00685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weir C, Li J, Fulton R, Fernando SL. Development and initial validation of a modified lymphocyte transformation test (LTT) assay in patients with DRESS and AGEP. Allergy Asthma Clin Immunol. 2022;18(1):90. doi: 10.1186/s13223-022-00729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanomedicine and the COVID-19 vaccines. Nat Nanotechnol. 2020;15(12):963. doi: 10.1038/s41565-020-00820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stebbings R, Jones C, Cotton P, Armour G, Maguire S, Skellett V, Tang C-M, Goodman J, Brady T, Takahashi V, et al. SARS-CoV-2 spike protein expression in vitro and hematologic effects in mice vaccinated with AZD1222 (ChAdox1 nCov-19). Front Immunol. 2022;13:836492. doi: 10.3389/fimmu.2022.836492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeck M, Marot L, Belkhir L. Delayed large local reactions to mRNA vaccines. N Engl J Med. 2021;384:e98. [DOI] [PubMed] [Google Scholar]


