ABSTRACT
Co-circulation of influenza and SARS-CoV-2 has the potential to place considerable strain on health-care services. We estimate the cost-effectiveness and health-care resource utilization impacts of influenza vaccination of low-risk 50–64-y-olds in the United Kingdom (UK) against a background SARS-CoV-2 circulation. A dynamic susceptible-exposed-infected-recovered model was used to simulate influenza transmission, with varying rates of vaccine coverage in the low-risk 50–64 y age-group. Four scenarios were evaluated: no vaccination (baseline), 40%, 50%, and 60% coverage. For the 50% and 60% coverage, this rate was also applied to high-risk 50–64-y-olds, whereas 48.6% was used for the baseline and 40% coverage scenarios. Cost-effectiveness was estimated in terms of humanistic outcomes and incremental cost-effectiveness ratio (ICER), with discounting applied at 3%. Overall, influenza vaccination of 50–64-y-olds resulted in reductions in GP visits, hospitalizations, and deaths, with a reduction in influenza-related mortality of 34%, 41%, and 52% for 40%, 50%, and 60% coverage, respectively. All four scenarios resulted in acute and intensive care unit (ICU) bed occupancy levels above available capacity, although vaccination of low-risk 50–64-y-olds resulted in a 35–54% and 16–25% decrease in excess acute and ICU bed requirements, respectively. Vaccination of this group against influenza was highly cost-effective from the payer perspective, with ICERs of £2,200–£2,343/quality-adjusted life year across the coverage rates evaluated. In conclusion, in the UK, vaccination of low-risk 50–64-y-olds against influenza is cost-effective and can aid in alleviating bed shortages in a situation where influenza and SARS-CoV-2 are co-circulating.
KEYWORDS: COVID-19, influenza vaccine, hospital bed occupancy, ICU, vaccine coverage, ICER
Introduction
Vaccination remains the most effective tool for prevention of influenza infection and related morbidity and mortality.1 As certain groups are at higher risk of influenza-related complications and mortality, such as older adults, pregnant women, and those with chronic diseases,2 choice of the optimal vaccine type for each age-group, as well as increasing coverage rates, can help to reduce pressure on health-care services. In addition, vaccination of individuals at lower risk, such as health-care workers, can reduce absence and transmission of infection, and increase overall effectiveness of health-care systems.3
One of the concerns of seasonal influenza vaccines is the potential for antigenic drift resulting in a mismatch with circulating strains, lowering vaccine effectiveness.4 In addition, egg adaptation has been noted in virus strains cultivated in eggs, particularly A/H3N2, which in turn reduces the match with circulating strains resulting in lowered effectiveness.5–7 Cell-based quadrivalent influenza vaccines (QIVc) eliminate the possibility of egg adaptation-related reductions in effectiveness,8 and therefore may be particularly beneficial in situations where health-care services are already at risk of being overwhelmed (e.g. during an influenza epidemic).
While rates of influenza fell substantially during the COVID-19 pandemic,9 there may potentially be a resurgence this winter following the easing of social distancing and infection prevention measures and reduced population-level immunity from lack of exposure over the previous two seasons.10 A surge in influenza cases in conjunction with COVID-19 is likely to create a substantial burden and potentially overwhelm health-care services. Therefore, a proactive approach to reduce the potential for severe influenza and COVID-19 cases is needed to maintain operationality of health-care services. In the UK, influenza vaccination is recommended in the 2022–2023 season for young and school-aged children (from 6 months of age), 50–64 y-olds, older adults (≥65 y), pregnant women, frontline health-care staff, carers, nursing home residents, and individuals in clinical risk groups (e.g. those with chronic respiratory diseases).11 During the COVID-19 pandemic, recommendations for influenza vaccination were extended to include low-risk 50–64 y-olds (i.e. those without clinical risk factors),11 a group who had previously been excluded from vaccine coverage. Previous analysis has demonstrated that use of a QIVc in this patient population is likely to be cost-effective from the payer perspective, with an estimated incremental cost/QALY of £6,000 based on 40% vaccine coverage and an absolute vaccine effectiveness of ~64%, based on data estimates from the 2019–2020 season.12 From a societal perspective, including analysis of the impacts on lost productivity from influenza-related sickness, use of QIVc in this low-risk age-group provides cost savings compared with no coverage.
In this study, we extend the previous analysis of the cost-effectiveness of QIVc in low-risk 50–64-y-olds to evaluate the impact of vaccination of this group against a background of circulating SARS-CoV-2. We simulate varying vaccine coverage on hospital and health-care resource utilization and estimate the incremental cost-effectiveness of vaccination of low-risk 50–64-y-olds from a payer and societal perspective.
Methods
Model design and parameters
In this analysis, we simulated influenza transmission and the impact of vaccination using a previously described dynamic model adapted from classic a SEIR model, with populations either susceptible to infection (S), exposed (E), infected and infectious (I) or recovered (R).13 The adapted model was used to simulate the combined impact of acute (i.e. non-ICU) and ICU hospital bed occupancy due to influenza and COVID-19 with varying coverage rate of influenza vaccination in the low-risk 50–64 y age-group, against a background of SARS-CoV-2 circulation. In the model, influenza attack rate was calibrated based on the reported median attack rates in England in Baguelin et al.14 Model calibration parameters are provided in Supplementary Table S1.
Bed capacity was assumed to be 2.4 acute beds per 1000 inhabitants (i.e. 162,336 beds total) and 7.3 ICU beds per 100,000 (i.e. 4,897 beds total), with a standard occupancy rate of 85% for both acute and ICU beds, based on data from the 2010–2022 season.15 Therefore, a total of 24,350 acute and 735 ICU beds (15% of all available beds) were assumed to be available for patients with influenza and/or COVID-19. Duration of acute and ICU stays due to influenza were assumed to be 5 and 7 d, respectively.16,17 Bed occupancy from COVID-19 was estimated based on mean reported values of acute and ICU bed usage from March 2020 to June 2022 inclusive.18 Model parameters are provided in Supplementary Table S2.
Influenza vaccine effectiveness estimates by vaccine type (egg-based quadrivalent influenza vaccine [QIV; QIVe], QIVc, adjuvanted QIV [aQIV], and quadrivalent live-attenuated influenza vaccine [QLAIV]) and age-group (6–23 months, 2–17 y, 18–49 y, 50–64 y, 65–74 y, and ≥75 y) were based on data from the 2019–2020 influenza season (Supplementary Table S3).19
The probability in the UK of general practitioner (GP) visits, hospitalizations, and deaths per case was based on estimates from Kohli et al.12 and varied for low- and high-risk groups (Supplementary Table S4). Risk groups were based on the Joint Committee for Vaccination and Influenza (JCVI) clinical risk groups, with high-risk defined as those with a clinical risk and low-risk as those without.20 Additional sensitivity analysis was performed estimating the impact of an up to twofold increase in the probability of deaths per hospitalization due to degradation in health-care services during a potential surge of infectious diseases (e.g., COVID-19) for the vaccination scenarios compared with the baseline scenario of no vaccination.
Scenarios
Four scenarios were evaluated. All scenarios assumed QIVe was administered to 6–23 month-olds, QLAIV to 2–17 y-olds, QIVc to high-risk 18–64 y-olds, and aQIV to ≥65 y-olds. In the baseline scenario (scenario 1), influenza vaccination was not offered to low-risk adults aged 50–64 y. In scenario 2, a 40% coverage rate (QIVc) was assumed for this population. In scenarios 3 and 4, QIVc coverage rates of 50% and 60%, respectively, were assumed for both low- and high-risk 50–64-y-olds. Coverage rates for all other risk/age-groups did not vary between the scenarios; coverage for high-risk 50–64 y-olds was assumed to be 48.6% for scenarios 1 and 2 (Supplementary Table S5). Vaccine coverage rates by age-group were based on data from the 2019–2020 season and varied between 0% in low-risk infants to 80% in adults ≥75 y.19
Economic evaluations, indirect costs, and utilities
Economic parameters were based on estimates used by Kohli et al.12 (Supplementary Table S6). Vaccine prices per dose were assumed to be £8.97 for QIVe, £12.50 for QIVc, £13.50 for aQIV, and £18.00 for QLAIV.21 Cost-effectiveness of the vaccine was assessed in terms of humanistic outcomes (life years lost, quality-adjusted life years [QALY] lost due to death or sickness) and incremental cost-effectiveness ratio (ICER). Discounting was applied at 3%.
For the societal perspective, the cost of time lost from work was calculated using the human capital approach. An average 4 d of time lost when a person was symptomatic infected was assumed in the analysis, based on analysis by Keech and Beardsworth.22 The daily wage was estimated at £128 GBP based on UK government statistics.23
We applied a utility of 0.0075 to uncomplicated cases of influenza and of 0.0180 to hospitalized cases.24,25 The discounted QALYs lost due to death were calculated using expected survival and age-specific utility values.26
Software
The model was developed using R software and C++, as outlined in Nguyen and Mould-Quevedo,13 predominantly using the following packages and corresponding libraries: Rcpp 1.0.9, RcppArmadillo 0.11.2.3.1, and RcppGSL 0.3.11. Model calibration was performed using the nloptr package.
Results
Impact on health outcomes and bed occupancy
All three scenarios which introduced influenza vaccine coverage to the low-risk 50–64-y-old population resulted in reductions in symptomatic cases, GP visits, hospitalizations, and deaths (Table 1). Compared with the baseline scenario, a vaccine coverage rate of 40%, 50%, or 60% in the low-risk 50–64-y-old population resulted in a reduction in influenza-related mortality of 34%, 41%, and 52%, respectively.
Table 1.
Number of symptomatic cases, GP visits, hospital visits, and deaths from influenza for each of the four scenarios.
| Scenario | Symptomatic cases | GP visits | Hospital visits | Deaths |
|---|---|---|---|---|
| Baseline (scenario 1): 0% vaccine coverage of low-risk 50–64 y-olds | 1,934,476 | 193,448 | 31,693 | 5,324 |
| Scenario 2: 40% vaccine coverage of low-risk 50–64 y-olds | 1,277,179 | 127,718 | 21,255 | 3,509 |
| Scenario 3: 50% vaccine coverage of 50–64 y-olds | 1,133,310 | 113,331 | 18,888 | 3,109 |
| Scenario 4: 60% vaccine coverage of 50–64 y-olds | 923,211 | 92,321 | 15,220 | 2,518 |
When the combined impacts of influenza and COVID-19 were considered, all four scenarios resulted in acute and ICU bed occupancies exceeding the available thresholds (Figure 1; Supplementary Table S7). In the baseline scenario, an estimated 5,041 acute and 1,641 beds would be needed above the available numbers. Extending vaccine coverage to low-risk 50–64 y would result in a 35–54% decrease in excess acute beds and 16–25% decrease in excess ICU bed requirements compared with not vaccinating this group (baseline scenario), although all scenarios remain over the threshold levels of bed availability (Figure 1). Based on the assumed 85% occupancy rate, a total of 200,000 acute beds and 15,333 ICU beds would be needed to accommodate the predicted hospitalizations from influenza and COVID-19 patients, based on the baseline scenario. If bed totals were to be maintained at 162,336 acute and 4,897 ICU beds, non-influenza/COVID-19 occupancy rates would need to be 82% and 46%, respectively, to accommodate all the hospitalized influenza and COVID-19 patients without exceeding bed numbers.
Figure 1.
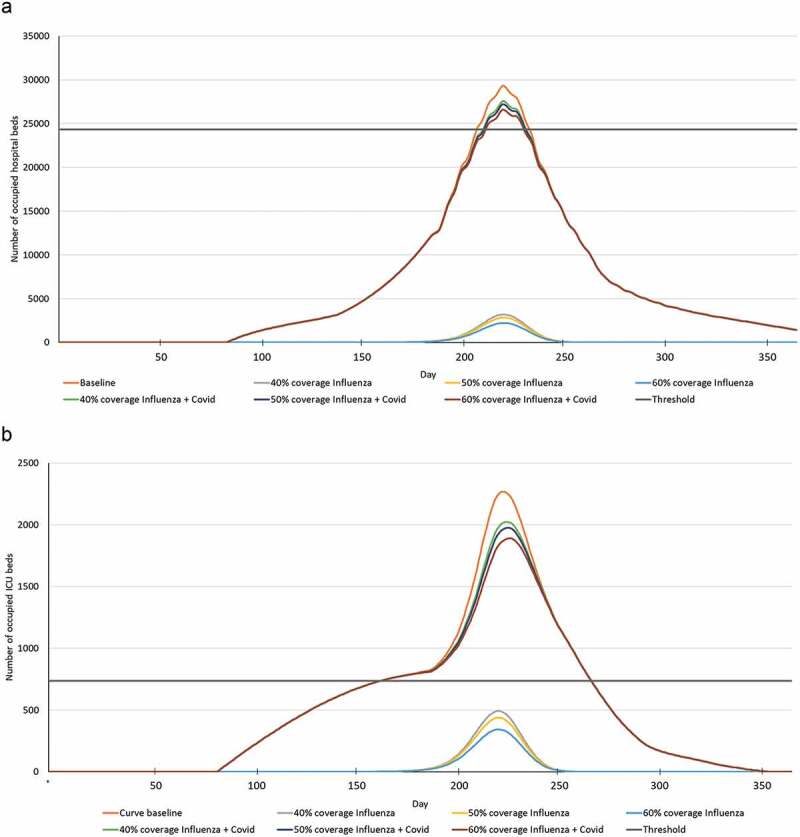
(a) Acute and (b) ICU bed occupancy estimates for patients with influenza or influenza and COVID-19 over time for each of the scenarios (baseline, 40%, 50%, and 60% coverage).
Evaluation of the impact of an up to twofold increase in death rate showed that 40% vaccine coverage of the low-risk 50–64-y-old group would reduce mortality by up to 37% compared with no vaccination of this age-group (Supplementary Table S8).
Economic outcomes
Economic analysis indicated that 40–60% vaccine coverage of low-risk 50–64-y-olds all resulted in cost savings compared with the baseline scenario (Table 2). While vaccine and administration costs are estimated to be higher when including low-risk 50–64-y-olds in the immunization campaign, these extra costs are offset by savings in GP, hospital, and loss of productivity costs, resulting in a reduced total cost of up to £476 million, depending on the scenario.
Table 2.
Cost estimates for each of the four base case scenarios.
| Scenario | GP Visits (£) | Hospital costs (£) | Loss of productivity (£) | Vaccines (£) | Administration (£) | Total Cost (£) |
|---|---|---|---|---|---|---|
| Baseline (scenario 1): 0% vaccine coverage of low-risk 50–64 y-olds | 20,703,478 | 132,653,462 | 1,044,001,460 | 221,829,304 | 154,405,119 | 1,573,592,822 |
| Scenario 2: 40% vaccine coverage of low-risk 50–64 y-olds | 13,486,445 | 87,831,360 | 690,535,350 | 274,863,576 | 197,087,101 | 1,263,803,832 |
| Scenario 3: 50% vaccine coverage of 50–64 y-olds | 11,920,318 | 77,871,593 | 612,901,124 | 288,537,915 | 208,092,209 | 1,199,323,159 |
| Scenario 4: 60% vaccine coverage of 50–64 y-olds | 9,642,641 | 62,845,135 | 498,869,114 | 304,766,272 | 221,152,791 | 1,097,275,954 |
Similarly, vaccination of low-risk 50–64-y-olds reduces life year lost, and QALY lost due to death or sickness in all scenarios analyzed (Table 3). Compared with the baseline scenario, ICERs were cost-effective for all scenarios, both from a payer (absolute extra costs) and societal (incorporating impacts of reduced health-care usage) perspective (Table 4). Compared with the baseline scenario of no vaccination, 40–60% coverage of low-risk 50–64-y-olds resulted in ICERs of approximately £2,200–£2,343/QALY, considerably below the assumed NICE threshold for cost-effectiveness of <£20,000 to £30,000/QALY. Inclusion of the potential risk of increased mortality during a COVID-19/influenza surge, resulted in reduced ICERs of £1,324–£1,887/QALY, based on a 40% coverage rate in low-risk 50–64-y-olds (Supplementary Table 9). Sensitivity analysis on vaccine effectiveness showed that 40–60% coverage of low-risk 50–64-y-olds remained cost effective from the society and payer perspectives at vaccine effectiveness estimates of 50–150% of baseline values (Supplementary Table S10).
Table 3.
Impact of vaccination of 50–64 y-olds on life years and quality-adjusted life years lost.
| Scenario | Life years lost | QALY lost due to death | QALY lost due to sickness | Total QALY lost |
|---|---|---|---|---|
| Baseline (scenario 1): 0% vaccine coverage of low-risk 50–64 y-olds | 59,877 | 45,773 | 13,174 | 58,947 |
| Scenario 2: 40% vaccine coverage of low-risk 50–64 y-olds | 39,778 | 30,436 | 8,654 | 39,090 |
| Scenario 3: 50% vaccine coverage of 50–64 y-olds | 35,315 | 27,028 | 7,667 | 34,695 |
| Scenario 4: 60% vaccine coverage of 50–64 y-olds | 28,627 | 21,916 | 6,226 | 28,142 |
QALY, quality-adjusted life year.
Table 4.
Incremental cost-effectiveness ratios (ICERs) for each of the vaccine scenarios compared with baseline (scenario 1).
| Scenario | Cost (£) | QALY gained (discounted) | ICER (cost/QALY) (£) |
|---|---|---|---|
| Payer perspectivea | |||
| Scenario 2: 40% vaccine coverage of low-risk 50–64 y-olds | 43,677,120 | 19,857 | 2,199.58 |
| Scenario 3: 50% vaccine coverage of 50–64 y-olds | 56,830,673 | 24,252 | 2,343.34 |
| Scenario 4: 60% vaccine coverage of 50–64 y-olds | 68,815,478 | 30,805 | 2,233.91 |
| Societal perspectiveb | |||
| Scenario 2: 40% vaccine coverage of low-risk 50–64 y-olds | −309,788,990 | 19,857 | −15,601 |
| Scenario 3: 50% vaccine coverage of 50–64 y-olds | −374,269,663 | 24,252 | −15,432 |
| Scenario 4: 60% vaccine coverage of 50–64 y-olds | −476,316,868 | 30,805 | −15,462 |
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
aCost estimates include GP, hospital, vaccine, and administration costs.
bCost estimates include all factors included in Table 2 (GP visits, hospital, loss of productivity, vaccine, and administration costs).
Discussion
As with previous analysis in the USA,13 this study indicates that bed availability is a key limiting factor in a situation where influenza and SARS-CoV-2 are co-circulating. While the US analysis showed that ICU beds were the main limiting factor, the UK analysis also shows a shortage of acute hospital beds across scenarios. Vaccination of low-risk 50–64-y-olds can help alleviate some of this shortage, particularly with increasing vaccine coverage, although simultaneous peaks of COVID-19 and influenza cases would still overwhelm current bed capacities. However, the potential reduction in excess bed usage could prove crucial in prevention of service disruption at times of high demand. Based on previous experience of COVID-19 and influenza hospital resource usage in the UK, extension of influenza vaccination to low-risk 50–64 y-olds in the UK would be cost effective and would help to prevent morbidity and mortality both to the individuals vaccinated and other vulnerable populations.
Previous analyses of the potential cost-effectiveness of vaccination of low-risk 50–64 y-olds in the UK have estimated ICERs of ~£6,000–£15,000.12,27 The current analysis predicts a considerably more favorable ICER of ~£2,250 across scenarios, likely associated from differences in model assumptions, with substantial cost savings from a societal perspective, when loss of productivity and impacts of absenteeism were taken into account. Irrespective of the absolute differences in ICER estimates, the results from the current analysis are consistent in indicating that vaccination of this population would be cost-effective. Even if the pandemic appears less intense than seen in 2020–2021, SARS-CoV-2 continues to circulate and it is likely we will see peaks of both infections during the winter months. The Southern Hemisphere experienced an early and highly active influenza season,28 and while we do not know exactly what will happen, it is likely that there will be a similar scenario in the Northern Hemisphere. The potential co-circulation of COVID-19 and seasonal influenza will put vulnerable people at increased risk of severe illness and death, with the likelihood of increased pressure on both hospitals and health-care workers, already exhausted from almost 3 y on the frontline of the pandemic. Vaccination remains one of the most effective tools against both viruses to help avoid this potential burden to health-care services. Even though low-risk adults aged 50–64 y may be unlikely to develop severe influenza or require hospitalization, vaccination of this group also has indirect effects by reducing transmission of the virus overall, and the potential for spread to vulnerable individuals.29 In addition, vaccination can reduce absenteeism of this working age population, thereby lowering the potential burden on health-care services due to staff shortages.
As with all research simulations, our analysis has some limitations. For simplicity, we used a fixed value to estimate the total available acute and ICU beds, while in reality the number of beds may vary depending on the year and as the health-care system adapts to fluctuations in demand. In addition, our approach was based on the entire UK, whereas the circulation of COVID-19 and influenza, together with hospital bed capacity and utilization, is likely to vary regionally. Based on publicly available data, we assumed a bed occupancy rate of 85%, which likely also varies both regionally and temporally. It is likely that we may have underestimated the availability of acute or ICU beds in some circumstances, as other diseases which we did not take into account can impact bed occupancy. Finally, influenza vaccine effectiveness varies annually and for the sake of simplicity, we have used the 2019–2020 pre-pandemic-reported vaccine effectiveness in the current model. Bed occupancy and hospital resource utilization would likely be higher in seasons where there is lower influenza vaccine effectiveness, with associated reductions in cost-effectiveness, though this would almost certainly remain below the traditional NICE cost-effectiveness threshold.
In summary, vaccination of low-risk 50–64-y-olds against influenza was highly cost-effective against a backdrop of SARS-CoV-2 circulation. While it is likely that hospital bed capacities would be exceeded if peak bed occupancy for both diseases occurred simultaneously, proactive vaccination of this population against influenza would help to reduce the potential burden on health services, freeing up resources for patients in need.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Jenny Engelmoer for editorial assistance in preparation of this manuscript.
Funding Statement
This study was funded by CSL Seqirus Inc.
Author contributions
All authors contributed to the conceptualization, methodology, and analysis of the study, together with the review and editing of the manuscript. Modeling was performed by V.H.N.
Disclosure statement
V.H.N.’s work was funded by Seqirus USA Inc. M.A. is an employee of Seqirus UK and a CSL shareholder. J.M.-Q. is an employee of Seqirus USA Inc. and a CSL shareholder. Seqirus has manufactured and marketed QIVc since the 2017–2018 influenza season. This analysis was funded by Seqirus USA Inc.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2187592.
References
- 1.World Health Organization . Influenza (Seasonal) fact sheet. 2018. [accessed 2023 Jan 10]. https://www.who.int/news-room/fact-sheets/detail/influenza-/seasonal/.
- 2.Uyeki TM. High-risk groups for influenza complications. JAMA. 2020;324(22):2334. doi: 10.1001/jama.2020.21869. [DOI] [PubMed] [Google Scholar]
- 3.Burls A, Jordan R, Barton P, Olowokure B, Wake B, Albon E, Hawker J.. Vaccinating healthcare workers against influenza to protect the vulnerable—is it a good use of healthcare resources? A systematic review of the evidence and an economic evaluation. Vaccine. 2006;24(19):4212–6. doi: 10.1016/j.vaccine.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 4.Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, et al. Influenza. Nat Rev Dis Primers. 2018;4(1):3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, McLean HQ. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–51. doi: 10.1016/S1473-3099(16)00129-8. [DOI] [PubMed] [Google Scholar]
- 6.Wu NC, Zost SJ, Thompson AJ, Oyen D, Nycholat CM, McBride R, Paulson JC, Hensley SE, Wilson IA. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017;13(10):e1006682. doi: 10.1371/journal.ppat.1006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zost SJ, Parkhouse K, Gumina ME, Kim K, Diaz Perez S, Wilson PC, Treanor JJ, Sant AJ, Cobey S, Hensley SE. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci USA. 2017;114(47):12578–83. doi: 10.1073/pnas.1712377114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajaram S, Boikos C, Gelone DK, Gandhi A. 2020. Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Ther Adv Vaccines Immunother. 8:2515135520908121. doi: 10.1177/2515135520908121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen SJ, Winn AK, Budd AP, Prill MM, Steel J, Midgley CM, Kniss K, Burns E, Rowe T, Foust A, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020–2021. Morb Mortal Wkly Rep. 2021;70(29):1013–19. doi: 10.15585/mmwr.mm7029a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashraf M, Rajaram S, English PM. How the COVID-19 pandemic will shape influenza public health initiatives: the UK experience. Hum Vaccines Immunother. 2022;18(5):2056399. doi: 10.1080/21645515.2022.2056399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Department of Health and Social Care . National flu immunisation programme 2022 to 2023 letter. 2022. [accessed 2022 Oct 24]. https://www.gov.uk/government/publications/national-flu-immunisation-programme-plan/national-flu-immunisation-programme-2022-to-2023-letter.
- 12.Kohli MA, Maschio M, Mould-Quevedo JF, Ashraf M, Drummond MF, Weinstein MC. The cost-effectiveness of expanding vaccination with a cell-based influenza vaccine to low risk adults aged 50 to 64 years in the United Kingdom. Vaccines (Basel). 2021;9(6):598. doi: 10.3390/vaccines9060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen VH, Mould-Quevedo JF. Estimating the impact of influenza vaccination on acute and ICU hospital bed usage in an influenza season under endemic COVID-19 in the US. Vaccines (Basel). 2022;10(11):1908. doi: 10.3390/vaccines10111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baguelin M, Jit M, Miller E, Edmunds WJ. Health and economic impact of the seasonal influenza vaccination programme in England. Vaccine. 2012;30(23):3459–62. doi: 10.1016/j.vaccine.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 15.British Medical Association . NHS hospital beds data analysis. 2022. [accessed 2022 Nov 15]. https://www.bma.org.uk/advice-and-support/nhs-delivery-and-workforce/pressures/nhs-hospital-beds-data-analysis.
- 16.Fingar KR, Liang L, Stocks C. Inpatient hospital stays and emergency department visits involving influenza, 2006–2016. HCUP Statistical Brief #253. Rockville, MD: Agency for Healthcare Research and Quality; 2019. https://us.ahrq.gov/reports/statbriefs/sb253-Influenza-Hospitalizations-ED-Visits-2006-2016.pdf. [PubMed] [Google Scholar]
- 17.Lina B, Georges A, Burtseva E, Nunes MC, Andrew MK, McNeil SA, Ruiz-Palacios GM, Feng L, Kyncl J, Vanhems P, et al. Complicated hospitalization due to influenza: results from the Global Hospital Influenza Network for the 2017–2018 season. BMC Infect Dis. 2020;20(1):465. doi: 10.1186/s12879-020-05167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Our World in Data . Coronavirus (COVID-19) hospitalizations. 2022. [accessed 2022 Oct 4]. https://ourworldindata.org/covid-hospitalizations.
- 19.Public Health England . Surveillance of influenza and other respiratory viruses in the UK - winter 2019 to 2020. [accessed 2022 Oct 4]. https://webarchive.nationalarchives.gov.uk/ukgwa/20220401215804mp_/https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/895233/Surveillance_Influenza_and_other_respiratory_viruses_in_the_UK_2019_to_2020_FINAL.pdf.
- 20.Department of Health and Social Care . Guidance. Appendix A: groups included in the national influenza immunisation programme. Updated 2022. Jul 22 [accessed 2023 Feb 14]. https://www.gov.uk/government/publications/national-flu-immunisation-programme-plan/appendix-a-groups-included-in-the-national-influenza-immunisation-programme.
- 21.National Institute for Health and Care Excellence . BNF Influenza vaccine medicinal forms. 2022. [accessed 2022 Oct 4]. http://bnf.nice.org.uk/drugs/influenza-vaccine/medicinal-forms/#solution-for-injection.
- 22.Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. Pharmacoeconomics. 2008;26(11):911–24. doi: 10.2165/00019053-200826110-00004. [DOI] [PubMed] [Google Scholar]
- 23.Office for National Statistics . Labour market overview, UK. 2022. Nov [accessed 2022 Dec 1]. https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/bulletins/uklabourmarket/latest. [Google Scholar]
- 24.Baguelin M, Camacho A, Flasche S, Edmunds WJ. Extending the elderly- and risk-group programme of vaccination against seasonal influenza in England and Wales: a cost-effectiveness study. BMC Med. 2015;13(1):236. doi: 10.1186/s12916-015-0452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Hoek AJ, Underwood A, Jit M, Miller E, Edmunds WJ, van Hoek AJ. The impact of pandemic influenza H1N1 on health-related quality of life: a prospective population-based study. PLoS One. 2011;6(3):e17030. doi: 10.1371/journal.pone.0017030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316(7133):736–41. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner DA, Wailoo AJ, Cooper NJ, Sutton AJ, Abrams KR, Nicholson KG. The cost-effectiveness of influenza vaccination of healthy adults 50–64 years of age. Vaccine. 2006;24(7):1035–43. doi: 10.1016/j.vaccine.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 28.Australian Government Department of Health . Australian Influenza Surveillance Report no. 14. 2022. [accessed 2022 Nov 18]. https://www.health.gov.au/resources/collections/australian-influenza-surveillance-reports-2022.
- 29.Eichner M, Schwehm M, Eichner L, Gerlier L. Direct and indirect effects of influenza vaccination. BMC Infect Dis. 2017;17(1):308. doi: 10.1186/s12879-017-2399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


