Abstract
The COVID-19 pandemic caused by SARS-CoV-2 has majorly impacted public health and economies worldwide. Although several effective vaccines and drugs are now used to prevent and treat COVID-19, natural products, especially flavonoids, showed great therapeutic potential early in the pandemic and thus attracted particular attention. Quercetin, baicalein, baicalin, EGCG (epigallocatechin gallate), and luteolin are among the most studied flavonoids in this field. Flavonoids can directly or indirectly exert antiviral activities, such as the inhibition of virus invasion and the replication and inhibition of viral proteases. In addition, flavonoids can modulate the levels of interferon and proinflammatory factors. We have reviewed the previously reported relevant literature researching the pharmacological anti-SARS-CoV-2 activity of flavonoids where structures, classifications, synthetic pathways, and pharmacological effects are summarized. There is no doubt that flavonoids have great potential in the treatment of COVID-19. However, most of the current research is still in the theoretical stage. More studies are recommended to evaluate the efficacy and safety of flavonoids against SARS-CoV-2.
Keywords: COVID-19, SARS-CoV-2, flavonoids, treatment, pharmacological effects, synthesis
1. Introduction
COVID-19 is a global disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with a high morbidity and mortality rate. As of November 2022, COVID-19 has spread to 222 countries with more than 600 million confirmed cases and more than 6.5 million cumulative deaths [1]. The COVID-19 outbreak not only endangers human health and livelihoods, but also has a huge impact on global public health systems and economic development.
SARS-CoV-2 is a positive-stranded RNA virus with a characteristic coronavirus spine protein on its outer surface that is capable of droplet transmission [2,3,4]. The viral structure of SARS-CoV-2 has a high degree of similarity to SARS-CoV and MERS-CoV, and it is similarly a zoonotic virus with a long incubation period and high transmission rate [2,5,6,7]. SARS-CoV-2 is highly mutagenic, and there are currently four mutant strains, Alpha, Beta, Gamma, and Delta, with the newly discovered Omicron mutant strain becoming a major global disease and causing strain in just a few months due to its strong infectious and immune-escaping ability [8,9,10,11]. Some common symptoms include fever, headache, shortness of breath, fatigue, cough, nausea, vomiting, diarrhea, and nasal congestion [12,13,14]. Most infected patients are from mild to moderate and do not require special treatment, but those with underlying conditions, such as heart disease, lung disease, and diabetes, as well as the elderly, are more likely to become seriously ill and have a higher mortality rate [13,15,16,17].
In addition to the vaccine, there are currently three new oral crown drugs approved for marketing worldwide, including Paxlovid (Pfizer, New York, USA) [18], Molnupiravir (Merck, Kenilworth, NJ, USA) [19], and the recently approved marketed remdesivir derivative VV116 (Topalliance Biosciences, Shanghai, China) [4,20]. In the early stage of the pandemic, there was a shortage of therapeutic options for COVID-19. Natural products, especially flavonoids, played a huge role in the early stage of the epidemic, showing good results in the treatment and prevention of COVID-19, which attracted widespread attention and research.
Flavonoids are widely found in many fruits and vegetables and have excellent antidiabetic, activity anti-inflammatory, and antiviral activity [21,22]. Flavonoids are mainly obtained through chemical synthesis, biosynthesis, and extraction from plants, and this article provides an overview of the chemical synthesis of several flavonoids. Flavonoid compounds are extremely potent against SRAS-CoV-2 and can be used to treat COVID-19. For example, EGCG inhibits the binding of the virus to the ACE2 receptor [3]. Some flavonoids, such as baicalein, luteolin, and quercetin, can inhibit the activity of 3C-like protease (3CLpro), papain-like protease (PLpro), and RNA-dependent RNA polymerase (RdRp) [23,24,25]. In addition, various flavonoids such as kaempferol, hesperidin, and isorhamnetin have also been shown to have inhibitory effects on SARS-CoV-2 [26,27,28]. Flavonoids also modulate the levels of inflammatory factors in the body and improve inflammation in COVID-19 patients.
Regarding the research progress of the anti-SARS-CoV-2 role of flavonoids, we summarized in this review, expecting to provide some reference for the development of anti-SARS-CoV-2 drugs. This article will summarize the recent research progress on the anti-SARS-CoV-2 pharmacological activity of flavonoids. It is hoped that this will provide ideas for the development of flavonoid drugs.
2. Methods
This paper summarizes the structural classification and pharmacological effects of flavonoids and provides a detailed review of the chemical synthesis of quercetin, EGCG, luteolin, baicalein, and baicalin as well as their anti-SARS-CoV-2 effects.
The literature search was performed through the Elsevier, Web of Science, and PubMed databases. The keywords included: COVID-19; SARS-CoV-2; flavonoids; treatment; pharmacological effects; and synthesis. Non-English literature was selected for exclusion from this review. Other than that, no other restrictions were used in this paper. This article was also accessed on the WHO Coronavirus (COVID-19) Dashboard (https://COVID19.who.int/table, accessed on 30 November 2022).
3. Pathogenesis of SARS-CoV-2
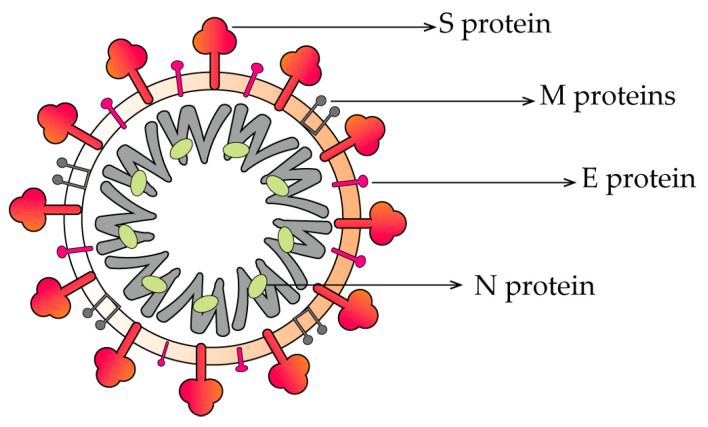
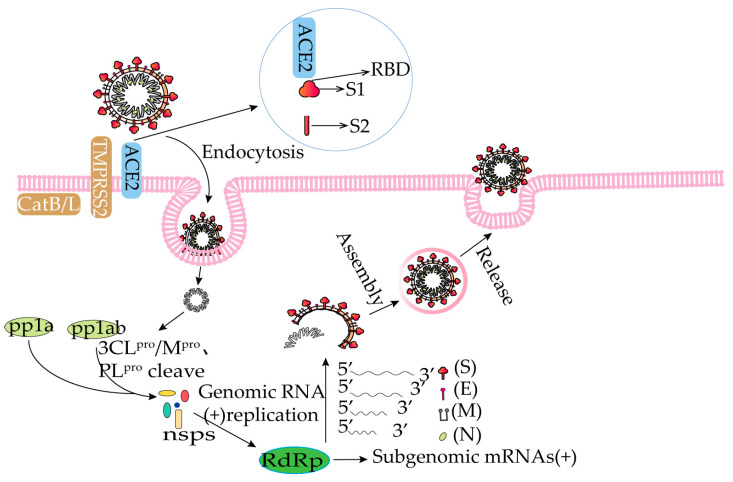
SARS-CoV-2 (Figure 1) is a positive-stranded RNA virus with four main structural proteins: the spike protein (S protein), the membrane protein (M protein), the envelope protein (E protein), and the nucleocapsid coat protein (N protein) in addition to some nonstructural proteins (nsps) [29,30,31]. The spike protein is in the outer layer of the virus and has two subunits, S1 and S2. The receptor-binding domain (RBD) of the S1 subunit binds to the ACE2 receptor of the host cell and then, with the involvement of cellular proteins, such as transmembrane serine protease 2 (TMPRSS2), cathepsin B/L (CatB/L), and flavoprotein, fuses with the cell membrane by the S2 subunit and enters the host cell [32,33,34,35]. Upon entry into the cell, the virus produces the polyproteins, 1a and 1b (pp1a and pp1ab) [36]. This is immediately followed by cleavage by 3CLpro, also called main protease (Mpro), and PLpro into 16 nonstructural proteins (nsps) [37,38]. The RdRp is an essential enzyme that can participate in RNA replication and negative-stranded RNA synthesis. The synthesized negative-stranded RNA can be used as a model for mRNA synthesis. The mRNA is translated into a polypeptide chain, which is modified and processed into four structural proteins [39,40]. The viral RNA is wrapped in the N protein and released from the cell by cytokinesis (Figure 2) [41,42,43].
Figure 1.
Structure of SARS-CoV-2. Abbreviations: S: Spike; M: Membrane; E: Envelope; N: Nucleocapsid.
Figure 2.
Pathogenesis of SARS-CoV-2. Abbreviations: ACE2: angiotensin converting enzyme 2; TMPRSS2: transmembrane serine protease 2; CatB/L: cathepsin B/L; RBD: receptor-binding domain; nsps: nonstructural proteins; pp1a: polyproteins 1a; pp1ab: polyproteins 1ab; 3CLpro: 3C-like protease; Mpro: main protease; PLpro: papain-like protease; RdRp: RNA-dependent RNA polymerase.
4. Classification, Synthesis and Activity of Flavonoids
4.1. Structure and Classification of Flavonoids
Flavonoids are a series of polyphenolic compounds found in various plants that have a benzo-γ-pyrone structure and can be synthesized by the phenylpropane pathway. Flavonoids are mostly found in esterified or glycosylated forms, and they form the basic parent nucleus of the C6-C3-C6 structure through 15 carbons (Figure 3) [44].
Figure 3.
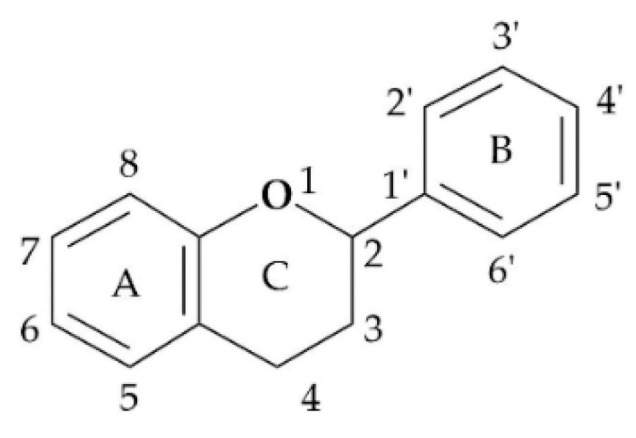
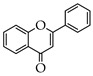
Basic skeleton of flavonoids. (A) and (B): Benzene ring. (C): Closed pyran ring.
Depending on the different substitution patterns of the rings, the position of the B ring, and the degree of oxidation of the C ring, there are six main subtypes, including flavones, flavonols, isoflavones, chalcones, flavanes, and anthocyanins (Table 1) [45].
Table 1.
Structure of flavonoids.
| Flavonoids | Basic Structure | Example | ||
|---|---|---|---|---|
| Flavones |
|
|
|
|
| Flavonols |
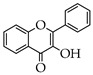
|
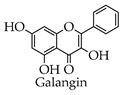
|
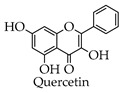
|
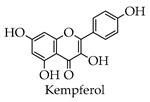
|
| Isoflavones |
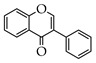
|
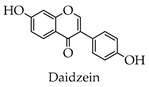
|
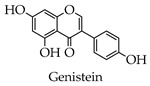
|
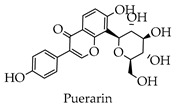
|
| Chalcones |
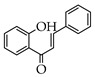
|
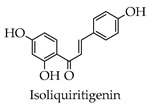
|
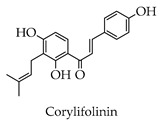
|
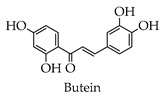
|
| Flavanes |
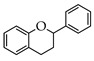
|
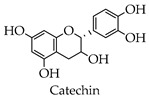
|
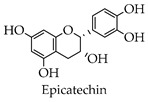
|
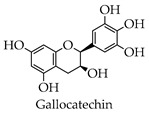
|
| Anthocyanins |
|
|
|
|
4.2. Chemical Synthesis of Flavonoids
Flavonoids can be extracted from plants through biosynthesis and chemical synthesis. This article focuses on the chemical synthesis of flavonoids, emphasizing the synthetic routes of quercetin, baicalein, baicalin, EGCG, and luteolin.
4.2.1. Quercetin
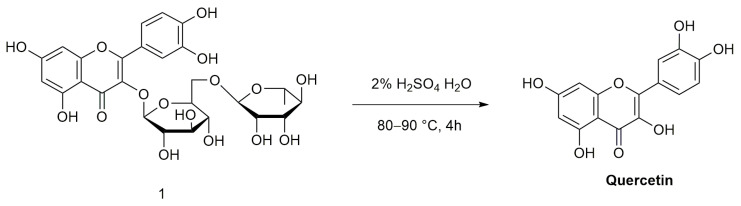
The synthesis route of quercetin is as follows (Figure 4): 2% H2SO4/H2O is added to rutin 1, reacted at 80–90 °C for 4 h, and then filtered; then, the filter cake is washed with water to neutral, then recrystallized in ethanol to provide quercetin [46].
Figure 4.
Synthetic route of quercetin.
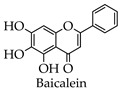
4.2.2. Baicalein and Baicalin
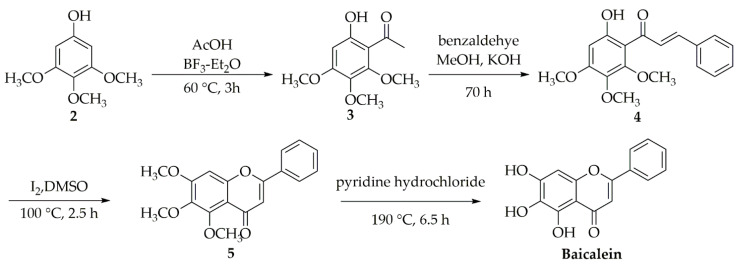
The synthesis route of baicalein is as follows (Figure 5): Compound 3 is obtained by the reaction of trimethoxyphenol 2 with AcOH and BF3-Et2O at 60 °C for 3 h, which is further condensed with benzaldehyde for 70 h to provide compound 4. Next, compound 4 is subjected to intramolecular cyclization in the presence of I2/DMSO at 100 °C for 2.5 h to provide compound 5; then, the methyl group is removed using pyridine hydrochloride at 190 °C for 6.5 h to provide baicalein [47].
Figure 5.
Synthetic route of baicalein.
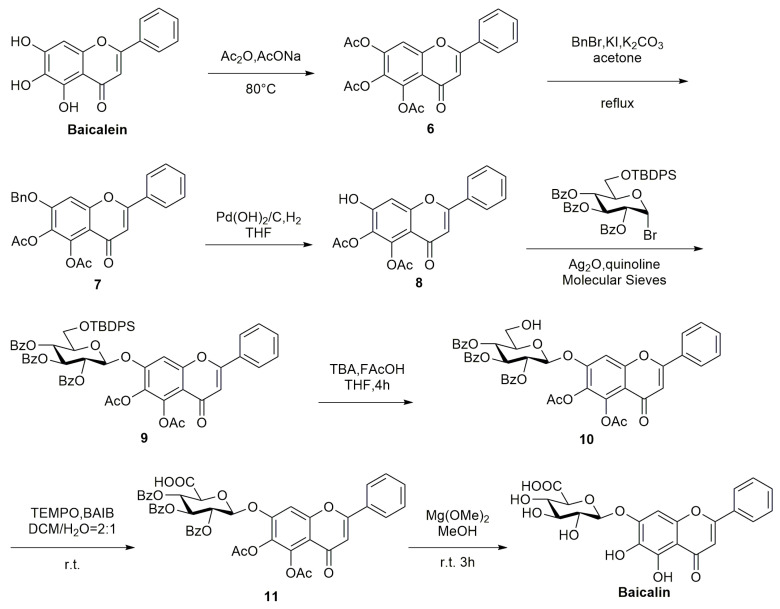
The synthesis route of baicalin is as follows (Figure 6): Using baicalin as the starting material, the acetylation reaction is carried out in the presence of Ac2O and AcONa at 80 °C to obtain acetylated baicalin 6. The acetylated baicalin is refluxed with BnBr in acetone by heating in the presence of KI and K2CO3 to yield the benzyl-substituted compound 7. The obtained compound 7 is removed from the benzyl group in THF by the action of Pd(OH)2 with H2 to provide compound 8. Additionally, compound 8 is glycosylated with brominated D-glucose in the presence of Ag2O at room temperature to provide glycosylated product 9. The removal of the protecting group TBDPS of compound 9 with AcOH and TBAF for 4 h provides compound 10. The subsequent oxidation of the hydroxyl group of compound 10 with TEMPO and BAIB at room temperature provides product 11. Finally, the protecting group is removed using Mg(OMe)2 at room temperature for 3 h to provide the target compound baicalin [48].
Figure 6.
Synthetic route of baicalin.
4.2.3. EGCG
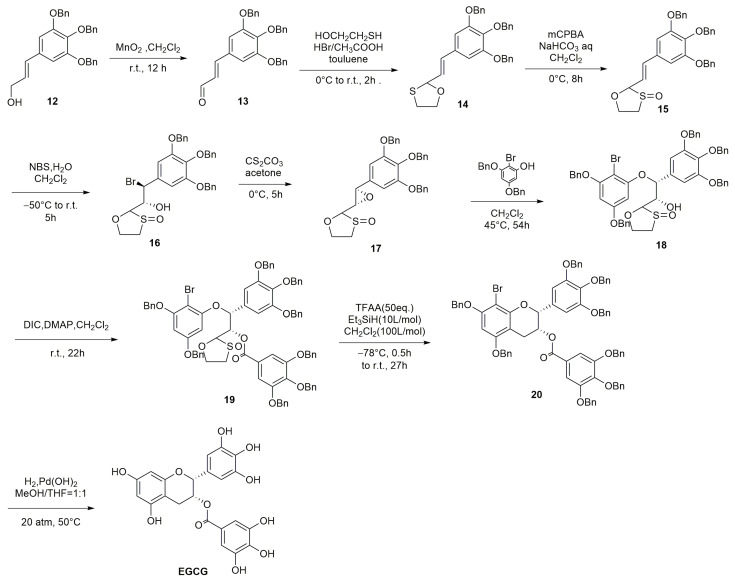
The synthetic route of EGCG is as follows (Figure 7): Compound 12, containing allyl alcohol, is oxidized by MnO2 at room temperature for 12 h to provide compound 13. In the presence of HBr, the aldehyde group of compound 13 is cyclized with HOCH2CH2SH to form cyclic S, O-acetal compound 14. Then, the oxidation of compound 14 with mCPBA at 0 °C for 8 h provides the oxidized S, O-acetal 15. The additional reaction with compound 15 in the presence of H2O is carried out with NBS to provide the bromohydrin compound 16 with stereoisomerism. The brominated alcohol of compound 16 is converted to epoxide 17 by CsCO3 in a reaction at 0 °C for 5 h. Additionally, the phenol-containing compound 21 is reacted with compound 17 at 45 °C to provide the cis-ring-opening product 18. In the presence of DIC, the alcohol hydroxyl group of compound 18 is esterified using DMAP; subsequently, the esterified product 19 is treated with an excess of TFAA in the environment of Et3SiH, and DCM (1:10) is reacted first at −78 °C for 0.5 h and then at room temperature for 27 h to provide the cyclized product 20. Finally, the protecting group of compound 20 is removed using H2/Pd(OH)2 at 20 atm to obtain the final product EGCG [49].
Figure 7.
Synthetic route of EGCG.
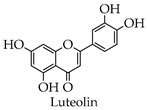
4.2.4. Luteolin
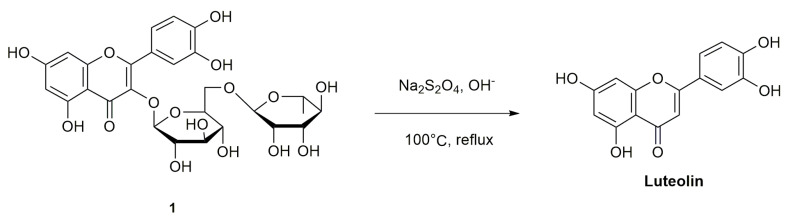
The synthesis route of luteolin is as follows (Figure 8): rutin 1 is used as the starting material, the protective agent Na2S2O4 is added, and the target product luteolin is refluxed at 100 °C [50].
Figure 8.
Synthetic route of luteolin.
4.3. Pharmacological Activity of Flavonoid Compounds
4.3.1. Anti-Inflammatory Effect
Cytokine storm is a feature of the inflammatory response induced by SARS-CoV-2 [51,52]. Flavonoids can target pathways, such as NF-κB, MAPK, ERK, and Akt, and can also reduce the release of inflammatory cytokines, which play an anti-inflammatory role [53].
A study showed that quercetin inhibited MC903-induced atopic dermatitis and improved arthritis by reducing proinflammatory factors [54,55]. Kaempferol blocked the ROS/NF-κB signaling pathway and reduced the inflammatory response in atherosclerosis [56]. In addition, kaempferol also regulated the expression of adipogenesis and reduced lipid accumulation in CCAAT/enhancer binding protein α (CEBPA) by upregulating the mRNA expression of Pnpla2 and Lipe [57]. Janumetin prevented neuroinflammation caused by sleep deprivation and also treated atopic dermatitis caused by obesity [58,59]. Both chrysin and lignans have been shown to have preventive effects against ochratoxin A-induced gastrointestinal inflammation in vitro [60]. Oroxylin A has a therapeutic effect on collagen-induced arthritis (CIA). In a mouse model of CIA, researchers found that mice treated with Oroxylin A had significantly lower levels of inflammatory factors in their serum and reduced somatic damage caused by arthritis [61]. Rutin is reportedly effective in treating colitis. Animal research discovered that no additional medication was required, and only 0.1% rutin was added to the daily diet and fed for 2 weeks. The concentration of proinflammatory factors in the serum of the mice was lowered, and the symptoms of colitis were reduced [62]. Saeedi-Boroujeni’s study reported that quercetin was able to affect the thioredoxin-interacting protein (TXNIP), thereby inhibiting NLRP3 inflammatory vesicles and achieving an inflammatory suppressive effect [63]. Clinical studies have shown that adjuvant therapy with quercetin in early COVID-19 patients significantly reduced the release of proinflammatory factors, such as TNF-β and IL-1β, and alleviated inflammation in patients with COVID-19 [43].
4.3.2. Antiviral Effect
Flavonoids are effective throughout the life of a virus. They can act through the inhibition of the virus cell entrance, replication of the viral gene set, translation and processing of proteins, and release of the virus from the cell [64].
It was demonstrated that both biochanin A and baicalin were able to inhibit H5N1 virus replication in A549 cells, but the mechanisms of action were different [65,66]. Both fisetin and rutin blocked viral replication by inhibiting the 3CLpro activity of enterovirus A71 [67]. Formononetin modulated COX-2/PGE₂ expression, thereby inhibiting the replication with enterovirus A71 [68]. Silymarin reportedly has anti-dengue virus activity in vitro and hepatoprotective properties for HCV infection treatment [69,70]. An in vitro study has shown that flavonoids isolated from the above-ground parts from Marcetia taxifolia are effective against herpes simplex virus, poliovirus, and hepatitis B virus [71]. The total flavonoids extracted from Robinia pseudoacacia cv. idaho showed the significant inhibition of herpes simplex virus type 1 and enterovirus type 71 with therapeutic indices of 113.8 and 46.2 [72]. It was shown that selenium functionalization with quercetin enhanced the inhibitory effect on Mpro and that quercetin significantly inhibited SARS-CoV-2 infection at higher concentrations, while quercetin derivatives inhibited the viral infection at low concentrations [23]. Baicalein and baicalin were able to bind to SARS-CoV-2 RdRp, causing the RdRp to be unable to participate in the RNA replication process of the virus [73]. Several studies in vitro have shown that EGCG can prevent the RBD region of SARS-CoV-2 from binding to the ACE2 receptor, thus preventing the virus from entering cells [74,75]. Numerous studies have proved that kaempferol, catechin, rutin, hesperidin, naringenin, and luteolin all have anti-coronaviral effects.
4.3.3. Antidiabetic Effect
Diabetic patients infected by SARS-CoV-2 will lead to exacerbation of the disease. Reducing the effect of diabetes on COVID-19 is particularly important. Flavonoids are extremely useful in treating diabetes and diabetic complications. They can exert their therapeutic effects on diabetes by enhancing insulin secretion, regulating glucose metabolism, and reducing inflammation and oxidative stress [76,77,78].
A study showed that EGCG and quercetin reduced insulin resistance, both in vivo and in vitro, and also reduced glucose metabolism in the liver [79]. A study in a rat model of diabetes showed that EGCG improved diabetes in rats and improved streptozotocin-induced complications [80]. Catechin is a naturally occurring product that has also been shown to have anti-diabetic activity. Treating rats with Eudragit particles loaded with catechins significantly reduced the concentration of blood glucose [81]. Clinical studies have found that vitamin C and rutin, when administered, significantly lowered fasting blood sugar in those with type 2 diabetes. In addition, rutin can improve neuropathy in diabetic patients [82,83]. A study exhibited that flavonoids extracted from Cistus laurifolius L. inhibited both α-glucosidase and α-amylase in vivo and vitro [84]. In a mouse model of streptozotocin-induced diabetes, the combination of Astragalus polysaccharides and Astragalus flavonoids significantly improved the function of insulin and thus exerted an anti-diabetic effect [85].
The collected evidence suggests that flavonoids can exert their antidiabetic effects through different mechanisms. However, there are no published studies about the treatment of COVID-19 diabetic patients with flavonoids.
4.3.4. Anticancer Effect
Studies showed that cancer patients with SARS-CoV-2 had a higher mortality rate [86]. Flavonoids can also exert antitumor effects through mechanisms such as antioxidants, COX-2 inhibition, immunomodulation, affecting cell cycle effects, apoptosis induction in cancer cells, tumor angiogenesis prevention, and telomerase activity inhibition [87,88].
In a study of the agent-sensitive LoVo cell lines and their agent-resistant LoVo/Dx subline model, baicalein and luteolin inhibited the development of colon cancer cells [89]. Baicalein also improves the effectiveness of cisplatin in human lung cancer cells [90]. Apigenin had been shown to inhibit the PI3K/Akt/mTOR pathway during viral accretion, thereby suppressing viral accretion [91]. Oroxylin A was shown to have a beneficial therapeutic effect on breast cancer by specifically binding to α-actinins 1 (ACTN1), thereby inhibiting ACTN1 expression to prevent cancer cell metastasis [92]. Oroxylin A also showed a positive inhibitory effect on lung cancer by suppressing lung cancer cell proliferation and metastasis in vivo [93]. Latifolin blocked cell growth, division, migration, invasion, and adhesion in oral squamous cell carcinoma by targeting PI10K/AKT/mTOR/p3S70K signaling [94]. EGCG and BAY11-7082 synergistically acted for the suppression of lung cancer cell proliferation both in vitro and in vivo [95].
Although SRAS-CoV-2 does not directly cause cancer, cancer patients infected with SRAS-CoV-2 have more severe symptoms and a higher lethality rate. Further investigating cancer cell inhibition would help improve COVID-19 patients’ symptoms.
5. Anti-SARS-CoV-2 Activity of Flavonoids
5.1. Anti-SARS-CoV-2 Pharmacological Effects of Quercetin
Quercetin is a flavanol compound that is widely found in various plants, mainly in the form of glycosides [96]. Studies have shown that quercetin has strong anti-inflammatory, antiviral, and immunomodulatory activities [97,98,99]. Cytokine storm is a feature of the inflammatory response induced by SARS-CoV-2 and is a major cause of death by COVID-19 [51,52]. According to relevant studies, NLRP3 inflammatory vesicles play an important role in inflammation [100]. Therefore, inhibiting NLRP3 inflammatory vesicle activation can effectively suppress the inflammatory response. Saeedi-Boroujeni’s study reported that quercetin could affect the TXNIP, thereby inhibiting NLRP3 inflammatory vesicles and achieving an inflammatory suppressive effect [63]. Clinical studies have demonstrated that the adjuvant treatment of early COVID-19 with quercetin significantly reduces the viral load and release of proinflammatory factors. Meanwhile, quercetin combined with antivirals for COVID-19 reduces mortality and the length of stay [101,102]. Meanwhile, Mangiavacchi et al. have shown that selenium functionalization with quercetin enhances the inhibitory effect on Mpro. According to the RT-qPCR results, quercetin at higher concentrations significantly inhibited SARS-CoV-2 infection, while quercetin derivatives inhibited the viral infection at low concentrations [23].
5.2. Anti-SARS-CoV-2 Pharmacological Effects of Baicalein and Baicalin
Baicalein and baicalin are both flavonoid compounds extracted from the dried roots from scutellaria baicalensis, which have various pharmacological effects, including anti-inflammatory, antiviral, antibacterial, hepatoprotective, and choleretic [103,104]. Liu et al. investigated the antiviral activity of baicalein against SARS-CoV-2 using RT-qPCR and showed that baicalein was able to inhibit the replication of SARS-CoV-2 in Verb cells in vitro with an EC50 of 2.9 μM, SI > 172 (SI = CC50/EC50) [105]. Su et al. screened the novel inhibitors of 3CLpro using a FRET protease assay and discovered that baicalein and baicalin exhibit a significant inhibitory effect on 3CLpro [24]. In addition, Keivan Zandi et al. found that baicalein and baicalin are able to bind to SARS-CoV-2 RdRp, causing RdRp to be unable to participate in the virus’ RNA replication process; SARS-CoV-2 RdRp inhibition was first demonstrated in a study by Keivan Zandi et al. [73].
5.3. Anti-SARS-CoV-2 Pharmacological Action of EGCG
EGCG is a flavonoid extracted from green tea with various pharmacological activities, such as antibacterial, antiviral, antioxidant, and anti-inflammatory effects [106]. SARS-CoV-2 can enter host cells by binding to the ACE2 receptor via the surface S protein. Several studies in vitro have shown that EGCG can prevent the RBD region of SARS-CoV-2 from binding to the ACE2 receptor, thus preventing the virus from entering cells with a low cytotoxicity [3,74,75,107,108]. EGCG can inhibit the replication of SARS-CoV-2 by inhibiting certain key enzymes in the RNA replication process. For example, Nsp15 (U-specific endoribonuclease) can cleave the polyU sequence in viral RNA, thus interfering with the host’s immune system and enabling the virus to undergo immune escape. Additionally, Nsp15 is significant in virus replication [109,110]. An in vitro study by Hong et al. exhibited that EGCG significantly inhibited the Nsp15 activity of SARS-CoV-2, with drug concentrations below 1 μg/mL completely inhibiting the activity of Nsp15 and thereby inhibiting virus replication in cells [111]. Furthermore, some studies showed that EGCG inhibits Mpro in vitro, thereby inhibiting virus replication [112,113,114].
5.4. Anti-SARS-CoV-2 Pharmacological Action of Luteolin
Luteolin, mostly in the form of glycosides, exists in a variety of plants, has anti-inflammatory, antiallergic, antiviral, antitumor, antibacterial, and other pharmacological activities, and is often clinically used for its anti-inflammatory effects, coughs, and expectorants [115,116,117]. In vitro studies have shown that as little as 20 μM luteolin has an inhibitory effect on 3CLpro. Additionally, luteolin also inhibits RdRp activity [25]. Xiao et al., in a SARS-CoV-2 pseudovirus experiment, discovered that luteolin is able to bind to the S protein and significantly inhibits the entry of SARS-CoV-2 into cells with an EC50 less than 7 μmol/L [118]. COVID-19 can cause a loss of smell or taste. A clinical study by L. D’Ascanio et al. showed that daily oral supplementation of palmitoylethanolamide and luteolin was able to restore the patient’s sense of smell [119]. In addition, clinical studies by Lisa O’Byrne et al. also exhibited that palmitoylethanolamide and luteolin supplementation could intervene to treat olfactory dysfunction in those suffering from COVID-19 [120].
5.5. Other Flavonoids with Anti-SARS-CoV-2 Activity
Kaempferol can inhibit the activity of 3CLpro. The study by Abbas Khan and Wang Heng et al. exhibited that a 62.5–125 μg/mL concentration of kaempferol can significantly shorten the cytopathic effects (CPE) caused by Vero E6 cell infection in vitro [27].
Both hesperidin and hesperitin can inhibit the activity of TMPRSS2 and ACE2 by binding to them. Additionally, they can block the SARA-CoV-2 S protein from binding the ACE2 receptor and prevent SARA-CoV-2 from entering the cell [28]. Hesperidin also blocks the AKT pathway and inhibits Ang II-induced collagen expression and cardiac fibroblast proliferation during COVID-19 infection [121]. Clinical studies have also shown that hesperidin can reduce some of the clinical symptoms of COVID-19, such as shortness of breath, cough, decreased or even absent taste, and fever [122].
Furthermore, an in vitro study revealed that isorhamnetin also interacts with ACE2 to exert anti-SARS-CoV-2 activity [26]. It was found that naringenin exhibits potent anti-SARS-CoV-2 activity in vitro by inhibiting Mpro [123,124].
Diosmin, biochanin A, and silymarin are able to reduce the inflammatory response and alleviate inflammation by decreasing cytokine levels in patients with COVID-19 [125,126,127].
The in vivo and in vitro activity studies of some flavonoid compounds are shown in Table 2 and Table 3 below.
Table 2.
Study on the in vitro activity of flavonoids.
| Compound | In Vitro Models | Conc. | Effects | Mechanism of Action | References |
|---|---|---|---|---|---|
| Baicalin | HepG2 cells | 16 μM | Pneumonia | Inhibits NLRP3 inflammatory vesicles | [128] |
| Rat IEC-6 cells | 10.0 μg/mL | Inflammatory bowel disease | Inhibits IL-6 and TNF-α inflammatory factor levels | [129] | |
| KOPN-8, RCH-ACV, SEM, RS4-11, NALM-6 | 10 mg/mL | Acute B-lymphoblastic leukemia | Inhibits the glycogen synthase kinase 3β and induces cell cycle arrest by upregulating p27Kip1 | [130] | |
| MCF-7 and MDA-MB-231 cells | 30 μM | Breast cancer | Inhibits breast cancer cell proliferation and induces G1/S arrest in cells | [131] | |
| Vero, BHK-21, and HEK 293T cells | 100 μM | Chikungunya virus | — | [132] | |
| Baicalein and baicalin | Vero CCL-81 cells | 20 μM | SARS-CoV-2 | Inhibits SARS-CoV-2 RNA-dependent RNA polymerase activity | [105] |
| Baicalein | Vero E6 cell | >2.0 μM | COVID-19 | Inhibits virus replication in vivo and reduces serum levels of IL-1β and TNF-α | [133] |
| Vero cell lines | 50 μM | SARS-CoV-2 | Inhibits SARS-CoV-2 replication and its 3C-like protease | [105] | |
| HCT116 cells | 50 μM | Colorectal cancer | Induces apoptosis in human colon cancer cells | [134] | |
| Silymarin | Vero cells | 749.70 µg/mL | Dengue virus | Binds to the surface protein of the virus to prevent entry into the cell | [70] |
| Luteolin | ARPE-19 cells | 50 μM | Age-related macular degeneration | Decreases levels of IL− 8 and IL− 6 | [135] |
| MDCK cells and Vero cells | From 3.75 to 240 μM | Influenza A virus | Inhibits the expression of β-COP protein | [136] | |
| Enzymatic inhibition assay | 4.6 μM | COVID-19 | Inhibits SARS-CoV-2 RNA-dependent RNA polymerase activity | [25] | |
| EGCG | Peripheral blood lymphocytes | 50 μM | HIV | — | [137] |
| live SARS-CoV-2 strain | 1 μg/mL | SARS-CoV-2 | Inhibits Nsp15 activity | [111] | |
| E. coli | 7.58 μg/ mL | SARS-CoV-2 | Inhibits the activity of 3CLpro | [112] | |
| Formononetin | Vero cells | 14.91 μmol/L | Enterovirus 71 | Inhibits EV71-induced COX-2 expression and PGE2 production via MAPKs pathway | [68] |
| Biochanin A | A549 cells | 5 μM | H5N1 influenza A viruses | Interferes with AKT, ERK 1/2, and NFκB activation to inhibit viral replication | [65] |
| Quercetin | T-REx™-293 cell line | 10 μg/mL | HCV | Inhibits NS3 protease, thereby inhibiting viral replication | [138] |
| Naringenin | Huh-7.5 cells | 25 μM | HCV | Reduces production of intracellular viral proteins | [139] |
Table 3.
Study on the in vivo activity of flavonoids.
| Compound | In Vivo Models | Conc. | Effects | Mechanism of Action | References |
|---|---|---|---|---|---|
| Baicalin | Male C57BL/6 mice | 21 mg/kg | Arthritis | Antagonism of Th-17 cells | [140] |
| Female BALB/C nude mice | 200 mg/kg | Breast cancer | Inhibits breast cancer cell proliferation and induces G1/S arrest in cells |
[131] | |
| Six-week-old male C57BL/6J mice | 21 mg/kg | Diabetes | Activates AKT/AS160/GLUT4 pathway | [141] | |
| Baicalein | Male SD rats | 200 mg/kg | COVID-19 | Inhibits virus replication in vivo and reduces serum levels of IL-1β and TNF-α | [133] |
| Male ICR mice | 1, 5, and 10 mg/kg | Colorectal cancer | Induction of apoptosis in human colon cancer cells | [134] | |
| Female C57BL/6 mice | 0.8 mg/mouse nine times | Bladder cancer | Reduces expression of cyclin D1 by inhibiting new protein synthesis and promoting proteasomal degradation and reduces expression of cyclin B1 by inhibiting new protein synthesis. | [142] | |
| Male C57BL/6 mice | 0.5 mg/kg | Type 2 diabetes | Improves the viability and insulin secretion of fine beta cells and human pancreatic islets | [143] | |
| Luteolin | Male ICR mice | 100 mg/kg | Acute pancreatitis | Suppresses the activation of the NF-κB pathway | [144] |
| Male BALB/cN mice | 5mg/kg | Nephrotoxicity | Reduces Pt accumulation in the kidney to improve oxidative stress and inflammation | [145] | |
| BALB/c nude mice | 40 mg/kg | Gastric carcinoma | Downregulates VEGF-A and MMP-9 and decreases the immune response | [146] | |
| EGCG | Male C57BL/6 mice | 10 mg/kg | SARS-CoV-2 | Inhibits virus replication | [114] |
| Male ICR mice | 100 mg/kg | Diabetic nephropathy | Inhibits increased OPN expression, reduces serum creatinine, and causes proteinuria and normalized morphological changes in STZ-induced diabetic nephropathy | [147] | |
| Oroxylin A | Female BALB/c nude mice | 200 mg/kg | Lung cancer | Inhibits the activation of ERK signaling and inhibits snail protein content and EMT |
[93] |
| Male DBA/1 mice | 10 mg/kg | Rheumatoid arthritis | Reduces serum levels of anti-CII Abs, IL-1β, IL-6, TNFα, and IL-17 | [61] | |
| Tiliroside | Male C57BL/6 mice | 5 mg/kg, 15 mg/kg, 300 mg/kg Dose-dependent manner |
Type 2 diabetes | Regulates miR-27 expression and inhibits glycoisomerization | [148] |
| Biochanin A | Male C57BL/6 mice | 12.5, 25, and 50 mg/kg | Acute lung injury | Inhibits TLR4/NF-κB signaling pathway activation and increases PPAR-γ expression in the lungs | [126] |
| Isorhamnetin | Male ICR mice | 1 nM | Diabetes | Activates JAK/STAT pathway to increase glucose uptake by muscle cells | [149] |
| Quercetin | Male ICR mice | 0.1 nM and 1 nM | Diabetes | Activates CaMKKβ/AMPK signaling pathway and activates IRS3/PI10K/Akt signaling |
[149] |
| Naringenin | Pregnant Sprague–Dawley rats | 25, 50, or 100 mg/kg | Neuroapoptosis | Regulates PI3/Akt/PTEN signaling pathway and inhibits NF-κB-mediated inflammation |
[150] |
6. Conclusions
The COVID-19 outbreak endangers human health and livelihoods and heavily impacts global public health systems and economic development. Although there are vaccines and specific drugs to treat COVID-19, such as Paxlovid, Molnupiravir, and VV116, due to the instability of the virus, which is prone to immune escape, researchers need to investigate more drugs and options for treating COVID-19.
Flavonoids are widely found in various plants and have a significant effect on both the prevention and treatment of SARS-CoV-2. Several in vivo and in vitro studies have demonstrated that flavonoids exhibit excellent antiviral activity against SARS-CoV-2 and can inhibit SARS-CoV-2 by inhibiting key viral targets, including the ACE2 receptor, TMPRSS2, Mpro, RdRp, S protein RBD, etc. In addition, flavonoids also have an inhibitory effect on inflammation caused by SARS-CoV-2, inhibiting the production and release of various proinflammatory factors in the inflammatory response [151]. At the same time, flavonoids improve some of the clinical symptoms of COVID-19.
Although many studies have reported flavonoids’ anti-SARS-CoV-2 effects, most of them are theoretical studies. Only a few in vivo and clinical studies are available. Thus, more applied experimental studies are needed to explore the drugs’ safety and efficacy. Secondly, the bioavailability of the ingested compounds is limited, and how to improve the bioavailability of the compounds is also an issue to be considered. Choosing the right route of administration and preparing the drug into formulations can improve a drug’s bioavailability [152]. While there are still some problems, flavonoids can undoubtedly show anti-SARS-CoV-2 effects through direct or indirect pathways. Thus, they represent a group of promising anti-SARS-CoV-2 compounds. Flavonoids have excellent medicinal potential. We are expecting more studies to explore the medicinal value of flavonoids and to develop flavonoid drugs.
Author Contributions
Conceptualization, X.-Z.C. and J.-Y.Y.; investigation, J.-Y.Y.; writing—original draft preparation, J.-Y.Y. and Y.-X.M.; writing—review and editing, X.-Z.C., X.-J.P. and Y.L.; supervision, X.-Z.C.; funding acquisition X.-Z.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Open Project of Key Laboratory of Prevention and treatment of cardiovascular and cerebrovascular diseases, the Ministry of Education (grant number XN202006), Fundamental Research Funds for Gannan Medical University (grant number QD202119) and the Innovation Project of Gannan Medical University (grant number TD2-1703).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1. [(accessed on 30 November 2022)]. Available online: https://covid19.who.int/table.
- 2.Malik Y.S., Sircar S., Bhat S., Sharun K., Dhama K., Dadar M., Tiwari R., Chaicumpa W. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. Vet. Q. 2020;40:68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J., Bodnar B.H., Meng F., Khan A.I., Wang X., Saribas S., Wang T., Lohani S.C., Wang P., Wei Z., et al. Epigallocatechin gallate from green tea effectively blocks infection of SARS-CoV-2 and new variants by inhibiting spike binding to ACE2 receptor. Cell Biosci. 2021;11:168. doi: 10.1186/s13578-021-00680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y., Yin W., Zhang Y., Shang W., Wang Z., Luan X., Tian G., Aisa H.A., Xu Y., Xiao G., et al. Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2. Cell Res. 2021;31:1212–1214. doi: 10.1038/s41422-021-00570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu R., Wang L., Kuo H.D., Shannar A., Peter R., Chosu P.J., Li S., Hudlikar R., Liu X., Liu Z., et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr. Pharm. Rep. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., Wang Y., Guo X. Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe. 2020;27:671–680.e672. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasreen S., Chung H., He S., Brown K.A., Gubbay J.B., Buchan S.A., Fell D.B., Austin P.C., Schwartz K.L., Sundaram M.E., et al. Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat. Microbiol. 2022;7:379–385. doi: 10.1038/s41564-021-01053-0. [DOI] [PubMed] [Google Scholar]
- 9.Del Rio C., Omer S.B., Malani P.N. Winter of Omicron-The Evolving COVID-19 Pandemic. J. Am. Med. Assoc. 2022;327:319–320. doi: 10.1001/jama.2021.24315. [DOI] [PubMed] [Google Scholar]
- 10.Scott L., Hsiao N.Y., Moyo S., Singh L., Tegally H., Dor G., Maes P., Pybus O.G., Kraemer M.U.G., Semenova E., et al. Track Omicron’s spread with molecular data. Science. 2021;374:1454–1455. doi: 10.1126/science.abn4543. [DOI] [PubMed] [Google Scholar]
- 11.Sharma V., Rai H., Gautam D.N.S., Prajapati P.K., Sharma R. Emerging evidence on Omicron (B.1.1.529) SARS-CoV-2 variant. J. Med. Virol. 2022;94:1876–1885. doi: 10.1002/jmv.27626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutiawati E., Fahriani M., Mamada S.S., Fajar J.K., Frediansyah A., Maliga H.A., Ilmawan M., Emran T.B., Ophinni Y., Ichsan I., et al. Anosmia and dysgeusia in SARS-CoV-2 infection: Incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms—A systematic review and meta-analysis. F1000Research. 2021;10:40. doi: 10.12688/f1000research.28393.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garnier-Crussard A., Forestier E., Gilbert T., Krolak-Salmon P. Novel Coronavirus (COVID-19) Epidemic: What Are the Risks for Older Patients? J. Am. Geriatr. Soc. 2020;68:939–940. doi: 10.1111/jgs.16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook T.M. The importance of hypertension as a risk factor for severe illness and mortality in COVID-19. Anaesthesia. 2020;75:976–977. doi: 10.1111/anae.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashyap P., Thakur M., Singh N., Shikha D., Kumar S., Baniwal P., Yadav Y.S., Sharma M., Sridhar K., Inbaraj B.S. In Silico Evaluation of Natural Flavonoids as a Potential Inhibitor of Coronavirus Disease. Molecules. 2022;27:6374. doi: 10.3390/molecules27196374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drożdżal S., Rosik J., Lechowicz K., Machaj F., Szostak B., Przybyciński J., Lorzadeh S., Kotfis K., Ghavami S., Łos M.J. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2021;59:100794. doi: 10.1016/j.drup.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imran M., Kumar Arora M., Asdaq S.M.B., Khan S.A., Alaqel S.I., Alshammari M.K., Alshehri M.M., Alshrari A.S., Mateq Ali A., Al-Shammeri A.M., et al. Discovery, Development, and Patent Trends on Molnupiravir: A Prospective Oral Treatment for COVID-19. Molecules. 2021;26:5795. doi: 10.3390/molecules26195795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Y.Z., Ai J.W., Lin N., Zhang H.C., Li Y., Wang H.Y., Wang S., Wang Z., Li T., Sun F., et al. An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 omicron variants. Emerg. Microbes Infect. 2022;11:1518–1523. doi: 10.1080/22221751.2022.2078230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alzaabi M.M., Hamdy R., Ashmawy N.S., Hamoda A.M., Alkhayat F., Khademi N.N., Al Joud S.M.A., El-Keblawy A.A., Soliman S.S.M. Flavonoids are promising safe therapy against COVID-19. Phytochem. Rev. 2022;21:291–312. doi: 10.1007/s11101-021-09759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma R., Martins N., Chaudhary A., Garg N., Sharma V., Kuca K., Nepovimova E., Tuli H.S., Bishayee A., Chaudhary A., et al. Adjunct use of honey in diabetes mellitus: A consensus or conundrum? Trends Food Sci. Technol. 2020;106:254–274. doi: 10.1016/j.tifs.2020.10.020. [DOI] [Google Scholar]
- 23.Mangiavacchi F., Botwina P., Menichetti E., Bagnoli L., Rosati O., Marini F., Fonseca S.F., Abenante L., Alves D., Dabrowska A., et al. Seleno-Functionalization of Quercetin Improves the Non-Covalent Inhibition of M(pro) and Its Antiviral Activity in Cells against SARS-CoV-2. Int. J. Mol. Sci. 2021;22:7048. doi: 10.3390/ijms22137048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su H.X., Yao S., Zhao W.F., Li M.J., Liu J., Shang W.J., Xie H., Ke C.Q., Gao M., Yu K.Q., et al. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. bioRxiv. 2020 doi: 10.1101/2020.04.13.038687. [DOI] [Google Scholar]
- 25.Munafo F., Donati E., Brindani N., Ottonello G., Armirotti A., De Vivo M. Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase. Sci. Rep. 2022;12:10571. doi: 10.1038/s41598-022-14664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan Y., Ta W., Tang W., Hua R., Wang J., Wang C., Lu W. Potential antiviral activity of isorhamnetin against SARS-CoV-2 spike pseudotyped virus in vitro. Drug Dev. Res. 2021;82:1124–1130. doi: 10.1002/ddr.21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan A., Heng W., Wang Y., Qiu J., Wei X., Peng S., Saleem S., Khan M., Ali S.S., Wei D.Q. In silico and in vitro evaluation of kaempferol as a potential inhibitor of the SARS-CoV-2 main protease (3CLpro) Phytother. Res. PTR. 2021;35:2841–2845. doi: 10.1002/ptr.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng F.J., Huynh T.K., Yang C.S., Hu D.W., Shen Y.C., Tu C.Y., Wu Y.C., Tang C.H., Huang W.C., Chen Y., et al. Hesperidin Is a Potential Inhibitor against SARS-CoV-2 Infection. Nutrients. 2021;13:2800. doi: 10.3390/nu13082800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiu S., Dick A., Ju H., Mirzaie S., Abdi F., Cocklin S., Zhan P., Liu X. Inhibitors of SARS-CoV-2 Entry: Current and Future Opportunities. J. Med. Chem. 2020;63:12256–12274. doi: 10.1021/acs.jmedchem.0c00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnamoorthy S., Swain B., Verma R.S., Gunthe S.S. SARS-CoV, MERS-CoV, and 2019-nCoV viruses: An overview of origin, evolution, and genetic variations. Virusdisease. 2020;31:411–423. doi: 10.1007/s13337-020-00632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boopathi S., Poma A.B., Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2021;39:3409–3418. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y., Yang C., Xu X.F., Xu W., Liu S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., Ying T., Liu S., Shi Z., Jiang S., et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell. Mol. Immunol. 2020;17:765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papageorgiou A.C., Mohsin I. The SARS-CoV-2 Spike Glycoprotein as a Drug and Vaccine Target: Structural Insights into Its Complexes with ACE2 and Antibodies. Cells. 2020;9:2343. doi: 10.3390/cells9112343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antivir. Res. 2020;178:104792. doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruan Z., Liu C., Guo Y., He Z., Huang X., Jia X., Yang T. SARS-CoV-2 and SARS-CoV: Virtual screening of potential inhibitors targeting RNA-dependent RNA polymerase activity (NSP12) J. Med. Virol. 2021;93:389–400. doi: 10.1002/jmv.26222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gil C., Ginex T., Maestro I., Nozal V., Barrado-Gil L., Cuesta-Geijo M., Urquiza J., Ramírez D., Alonso C., Campillo N.E., et al. COVID-19: Drug Targets and Potential Treatments. J. Med. Chem. 2020;63:12359–12386. doi: 10.1021/acs.jmedchem.0c00606. [DOI] [PubMed] [Google Scholar]
- 38.Zheng L., Zhang L., Huang J., Nandakumar K.S., Liu S., Cheng K. Potential treatment methods targeting 2019-nCoV infection. Eur. J. Med. Chem. 2020;205:112687. doi: 10.1016/j.ejmech.2020.112687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Grunewald M., Perlman S. Coronaviruses: An Updated Overview of Their Replication and Pathogenesis. Methods Mol. Biol. 2020;2203:1–29. doi: 10.1007/978-1-0716-0900-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narayanan N., Nair D.T. Vitamin B12 may inhibit RNA-dependent-RNA polymerase activity of nsp12 from the SARS-CoV-2 virus. IUBMB Life. 2020;72:2112–2120. doi: 10.1002/iub.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alnefaie A., Albogami S. Current approaches used in treating COVID-19 from a molecular mechanisms and immune response perspective. Saudi Pharm. J. 2020;28:1333–1352. doi: 10.1016/j.jsps.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y., Liu Q., Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llivisaca-Contreras S.A., Naranjo-Morán J., Pino-Acosta A., Pieters L., Vanden Berghe W., Manzano P., Vargas-Pérez J., León-Tamariz F., Cevallos-Cevallos J.M. Plants and Natural Products with Activity against Various Types of Coronaviruses: A Review with Focus on SARS-CoV-2. Molecules. 2021;26:4099. doi: 10.3390/molecules26134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brodowska K. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017;7:108–123. [Google Scholar]
- 45.Middleton E.J. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998;439:175–182. doi: 10.1007/978-1-4615-5335-9_13. [DOI] [PubMed] [Google Scholar]
- 46.Naghski J., Krewson C.F. The preparation and analysis of quercetin. J. Am. Pharm. Association. Am. Pharm. Assoc. 1953;42:66–68. doi: 10.1002/jps.3030420203. [DOI] [PubMed] [Google Scholar]
- 47.Chen D.Z., Yang J., Yang B., Wu Y.S., Wu T. Total synthesis of baicalein. J. Asian Nat. Prod. Res. 2010;12:124–128. doi: 10.1080/10286020903508416. [DOI] [PubMed] [Google Scholar]
- 48.Li Y.F., Yu B., Sun J.S., Wang R.X. Efficient synthesis of baicalin and its analogs. Tetrahedron Lett. 2015;56:3816–3819. doi: 10.1016/j.tetlet.2015.04.083. [DOI] [Google Scholar]
- 49.Tanaka H., Chino A., Takahashi T. Reagent-controlled stereoselective synthesis of (±)-gallo- and (±)-epigallo-catechin gallates. Tetrahedron Lett. 2012;53:2493–2495. doi: 10.1016/j.tetlet.2012.02.065. [DOI] [Google Scholar]
- 50.Sun W.L., Yang J.W., Dou H.Y., Li G.Q., Li X.Y., Shen L., Ji H.F. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorganic Chem. 2021;112:104966. doi: 10.1016/j.bioorg.2021.104966. [DOI] [PubMed] [Google Scholar]
- 51.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J., Li Q., Qiu Y.W., Lu H.L. COVID-19: Imbalanced cell-mediated immune response drives to immunopathology. Emerg. Microbes Infect. 2022;11:2393–2404. doi: 10.1080/22221751.2022.2122579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maleki S.J., Crespo J.F., Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124. doi: 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- 54.Hou D.D., Zhang W., Gao Y.L., Sun Y.Z., Wang H.X., Qi R.Q., Chen H.D., Gao X.H. Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol. 2019;74:105676. doi: 10.1016/j.intimp.2019.105676. [DOI] [PubMed] [Google Scholar]
- 55.Hannan A., Akhtar B., Sharif A., Anjum F., Pasha I., Khan A., Akhtar M.F., Saleem A. Quercetin-loaded chitosan nanoparticles ameliorate adjuvant-induced arthritis in rats by regulating anti-oxidant enzymes and downregulating pro- and inflammatory cytokines. Inflammopharmacology. 2022;31:287–300. doi: 10.1007/s10787-022-01118-4. [DOI] [PubMed] [Google Scholar]
- 56.Zhao J., Ling L., Zhu W., Ying T., Yu T., Sun M., Zhu X., Du Y., Zhang L. M1/M2 re-polarization of kaempferol biomimetic NPs in anti-inflammatory therapy of atherosclerosis. J. Control. Release Off. J. Control. Release Soc. 2023;353:1068–1083. doi: 10.1016/j.jconrel.2022.12.041. [DOI] [PubMed] [Google Scholar]
- 57.Torres-Villarreal D., Camacho A., Castro H., Ortiz-Lopez R., de la Garza A.L. Anti-obesity effects of kaempferol by inhibiting adipogenesis and increasing lipolysis in 3T3-L1 cells. J. Physiol. Biochem. 2019;75:83–88. doi: 10.1007/s13105-018-0659-4. [DOI] [PubMed] [Google Scholar]
- 58.Gao J.F., Tang L., Luo F., Chen L., Zhang Y.Y., Ding H. Myricetin treatment has ameliorative effects in DNFB-induced atopic dermatitis mice under high-fat conditions. Toxicol. Sci. Off. J. Soc. Toxicol. 2022;191:308–320. doi: 10.1093/toxsci/kfac138. [DOI] [PubMed] [Google Scholar]
- 59.Sur B., Lee B. Myricetin prevents sleep deprivation-induced cognitive impairment and neuroinflammation in rat brain via regulation of brain-derived neurotropic factor. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2022;26:415–425. doi: 10.4196/kjpp.2022.26.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wohlert A., Palkovicsné Pézsa N., Móritz A.V., Jerzsele Á., Farkas O., Pászti-Gere E. Luteolin and Chrysin Could Prevent E. coli Lipopolysaccharide-Ochratoxin A Combination-Caused Inflammation and Oxidative Stress in In Vitro Porcine Intestinal Model. Anim. Open Access J. MDPI. 2022;12:2747. doi: 10.3390/ani12202747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y.L., Gao J.M., Xing L.Z. Therapeutic potential of Oroxylin A in rheumatoid arthritis. Int. Immunopharmacol. 2016;40:294–299. doi: 10.1016/j.intimp.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Kwon K.H., Murakami A., Tanaka T., Ohigashi H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: Attenuation of pro-inflammatory gene expression. Biochem. Pharmacol. 2005;69:395–406. doi: 10.1016/j.bcp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 63.Saeedi-Boroujeni A., Mahmoudian-Sani M.R. Anti-inflammatory potential of Quercetin in COVID-19 treatment. J. Inflamm. 2021;18:3. doi: 10.1186/s12950-021-00268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badshah S.L., Faisal S., Muhammad A., Poulson B.G., Emwas A.H., Jaremko M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021;140:111596. doi: 10.1016/j.biopha.2021.111596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sithisarn P., Michaelis M., Schubert-Zsilavecz M., Cinatl J.J. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antivir. Res. 2013;97:41–48. doi: 10.1016/j.antiviral.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Michaelis M., Sithisarn P., Cinatl J.J. Effects of flavonoid-induced oxidative stress on anti-H5N1 influenza a virus activity exerted by baicalein and biochanin A. BMC Res. Notes. 2014;7:384. doi: 10.1186/1756-0500-7-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin Y.J., Chang Y.C., Hsiao N.W., Hsieh J.L., Wang C.Y., Kung S.H., Tsai F.J., Lan Y.C., Lin C.W. Fisetin and rutin as 3C protease inhibitors of enterovirus A71. J. Virol. Methods. 2012;182:93–98. doi: 10.1016/j.jviromet.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Wang H.Q., Zhang D.J., Ge M., Li Z.R., Jiang J.D., Li Y.H. Formononetin inhibits enterovirus 71 replication by regulating COX-2/PGE2 expression. Virol. J. 2015;12:35. doi: 10.1186/s12985-015-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polyak S.J., Oberlies N.H., Pécheur E.I., Dahari H., Ferenci P., Pawlotsky J.M. Silymarin for HCV infection. Antivir. Ther. 2013;18:141–147. doi: 10.3851/IMP2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Low Z.X., OuYong B.M., Hassandarvish P., Poh C.L., Ramanathan B. Antiviral activity of silymarin and baicalein against dengue virus. Sci. Rep. 2021;11:21221. doi: 10.1038/s41598-021-98949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ortega J.T., Serrano M.L., Suárez A.I., Baptista J., Pujol F.H., Cavallaro L.V., Campos H.R., Rangel H.R. Antiviral activity of flavonoids present in aerial parts of Marcetia taxifolia against Hepatitis B virus, Poliovirus, and Herpes Simplex Virus in vitro. EXCLI J. 2019;18:1037–1048. doi: 10.17179/excli2019-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo H., Wan X.H., Niu F.J., Sun J.J., Shi C.X., Meng Y., Zhou C.Z. Evaluation of antiviral effect and toxicity of total flavonoids extracted from Robinia pseudoacacia cv. idaho. Biomed. Pharmacother. 2019;118:109335. doi: 10.1016/j.biopha.2019.109335. [DOI] [PubMed] [Google Scholar]
- 73.Zandi K., Musall K., Oo A., Cao D., Liang B., Hassandarvish P., Lan S., Slack R.L., Kirby K.A., Bassit L., et al. Baicalein and Baicalin Inhibit SARS-CoV-2 RNA-Dependent-RNA Polymerase. Microorganisms. 2021;9:893. doi: 10.3390/microorganisms9050893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henss L., Auste A., Schürmann C., Schmidt C., von Rhein C., Mühlebach M.D., Schnierle B.S. The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. J. Gen. Virol. 2021;102:1574. doi: 10.1099/jgv.0.001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohishi T., Hishiki T., Baig M.S., Rajpoot S., Saqib U., Takasaki T., Hara Y. Epigallocatechin gallate (EGCG) attenuates severe acute respiratory coronavirus disease 2 (SARS-CoV-2) infection by blocking the interaction of SARS-CoV-2 spike protein receptor-binding domain to human angiotensin-converting enzyme 2. PLoS ONE. 2022;17:e0271112. doi: 10.1371/journal.pone.0271112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Ishaq R.K., Abotaleb M., Kubatka P., Kajo K., Büsselberg D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules. 2019;9:430. doi: 10.3390/biom9090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Ouady F., Bachir F., Eddouks M. Flavonoids Extracted from Asteriscus graveolens Improve Glucose Metabolism and Lipid Profile in Diabetic Rats. Endocr. Metab. Immune Disord. Drug Targets. 2021;21:895–904. doi: 10.2174/1871530320999200818103709. [DOI] [PubMed] [Google Scholar]
- 78.Sapian S., Taib I.S., Latip J., Katas H., Chin K.Y., Mohd Nor N.A., Jubaidi F.F., Budin S.B. Therapeutic Approach of Flavonoid in Ameliorating Diabetic Cardiomyopathy by Targeting Mitochondrial-Induced Oxidative Stress. Int. J. Mol. Sci. 2021;22:11616. doi: 10.3390/ijms222111616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu H., Guan H., Tan X., Jiang Y., Li F., Sun-Waterhouse D., Li D. Enhanced alleviation of insulin resistance via the IRS-1/Akt/FOXO1 pathway by combining quercetin and EGCG and involving miR-27a-3p and miR-96-5p. Free Radic. Biol. Med. 2022;181:105–117. doi: 10.1016/j.freeradbiomed.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Gui L., Wang F., Hu X., Liu X., Yang H., Cai Z., Qi M., Dai C. Epigallocatechin Gallate Protects Diabetes Mellitus Rats Complicated with Cardiomyopathy through TGF-β1/JNK Signaling Pathway. Curr. Pharm. Des. 2022;28:2758–2770. doi: 10.2174/1381612828666220902115437. [DOI] [PubMed] [Google Scholar]
- 81.Meena K.P., Vijayakumar M.R., Dwibedy P.S. Catechin-loaded Eudragit microparticles for the management of diabetes: Formulation, characterization and in vivo evaluation of antidiabetic efficacy. J. Microencapsul. 2017;34:342–350. doi: 10.1080/02652048.2017.1337248. [DOI] [PubMed] [Google Scholar]
- 82.Ragheb S.R., El Wakeel L.M., Nasr M.S., Sabri N.A. Impact of Rutin and Vitamin C combination on oxidative stress and glycemic control in patients with type 2 diabetes. Clin. Nutr. ESPEN. 2020;35:128–135. doi: 10.1016/j.clnesp.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 83.Tian R.F., Yang W.Q., Xue Q., Gao L., Huo J.L., Ren D.Q., Chen X.Y. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur. J. Pharmacol. 2016;771:84–92. doi: 10.1016/j.ejphar.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 84.Orhan N., Aslan M., Sukuroglu M., Deliorman Orhan D. In vivo and in vitro antidiabetic effect of Cistus laurifolius L. and detection of major phenolic compounds by UPLC-TOF-MS analysis. J. Ethnopharmacol. 2013;146:859–865. doi: 10.1016/j.jep.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 85.Cui K., Zhang S.B., Jiang X., Xie W.D. Novel synergic antidiabetic effects of Astragalus polysaccharides combined with Crataegus flavonoids via improvement of islet function and liver metabolism. Mol. Med. Rep. 2016;13:4737–4744. doi: 10.3892/mmr.2016.5140. [DOI] [PubMed] [Google Scholar]
- 86.Grivas P., Khaki A.R., Wise-Draper T.M., French B., Hennessy C., Hsu C.Y., Shyr Y., Li X., Choueiri T.K., Painter C.A., et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: A report from the COVID-19 and Cancer Consortium. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021;32:787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang N., Jia X.B., Zhang Z.H., Sun E., Yan H.M. Advance in studies on anti-cancer activity and mechanism of flavonoids. China J. Chin. Mater. Med. 2015;40:373–381. [PubMed] [Google Scholar]
- 88.Ravishankar D., Rajora A.K., Greco F., Osborn H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013;45:2821–2831. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 89.Palko-Labuz A., Sroda-Pomianek K., Uryga A., Kostrzewa-Suslow E., Michalak K. Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and in drug-resistant LoVo/Dx cells. Biomed. Pharmacother. 2017;88:232–241. doi: 10.1016/j.biopha.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 90.Yu M.L., Qi B.Q., Wu X.X., Xu J., Liu X.L. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-kappaB pathway. Biomed. Pharmacother. 2017;90:677–685. doi: 10.1016/j.biopha.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 91.Yang J.L., Pi C.C., Wang G.H. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018;103:699–707. doi: 10.1016/j.biopha.2018.04.072. [DOI] [PubMed] [Google Scholar]
- 92.Cao Y., Cao W., Qiu Y., Zhou Y., Guo Q., Gao Y., Lu N. Oroxylin A suppresses ACTN1 expression to inactivate cancer-associated fibroblasts and restrain breast cancer metastasis. Pharm. Res. 2020;159:104981. doi: 10.1016/j.phrs.2020.104981. [DOI] [PubMed] [Google Scholar]
- 93.Wei L.B., Yao Y.Y., Zhao K., Huang Y.J., Zhou Y.X., Zhao L., Guo Q.L., Lu N. Oroxylin A inhibits invasion and migration through suppressing ERK/GSK-3β signaling in snail-expressing non-small-cell lung cancer cells. Mol. Carcinog. 2016;55:2121–2134. doi: 10.1002/mc.22456. [DOI] [PubMed] [Google Scholar]
- 94.Yun H.M., Park J.E., Lee J.Y., Park K.R. Latifolin, a Natural Flavonoid, Isolated from the Heartwood of Dalbergia odorifera Induces Bioactivities through Apoptosis, Autophagy, and Necroptosis in Human Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022;23:13629. doi: 10.3390/ijms232113629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang L., Xie J., Gan R., Wu Z., Luo H., Chen X., Lu Y., Wu L., Zheng D. Synergistic inhibition of lung cancer cells by EGCG and NF-κB inhibitor BAY11-7082. J. Cancer. 2019;10:6543–6556. doi: 10.7150/jca.34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Polerà N., Badolato M., Perri F., Carullo G., Aiello F. Quercetin and its Natural Sources in Wound Healing Management. Curr. Med. Chem. 2019;26:5825–5848. doi: 10.2174/0929867325666180713150626. [DOI] [PubMed] [Google Scholar]
- 97.Yi H., Peng H., Wu X., Xu X., Kuang T., Zhang J., Du L., Fan G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxidative Med. Cell. Longev. 2021;2021:6678662. doi: 10.1155/2021/6678662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen D., Feng Y., Zhang X., Gong L., Liu J., Li Y., Liao H. Antiosteoporosis Studies of 20 Medicine Food Homology Plants Containing Quercetin, Rutin, and Kaempferol: TCM Characteristics, In Vivo and In Vitro Activities, Potential Mechanisms, and Food Functions. Evid.-Based Complement. Altern. Med. Ecam. 2022;2022:5902293. doi: 10.1155/2022/5902293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Costa L.G., Garrick J.M., Roquè P.J., Pellacani C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016;2016:2986796. doi: 10.1155/2016/2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Pierro F., Derosa G., Maffioli P., Bertuccioli A., Togni S., Riva A., Allegrini P., Khan A., Khan S., Khan B.A., et al. Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study. Int. J. Gen. Med. 2021;14:2359–2366. doi: 10.2147/IJGM.S318720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shohan M., Nashibi R., Mahmoudian-Sani M.R., Abolnezhadian F., Ghafourian M., Alavi S.M., Sharhani A., Khodadadi A. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial. Eur. J. Pharmacol. 2022;914:174615. doi: 10.1016/j.ejphar.2021.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao Y., Snyder S.A., Smith J.N., Chen Y.C. Anticancer properties of baicalein: A review. Medicinal chemistry research: An international journal for rapid communications on design and mechanisms of action of biologically active agents. Med. Chem. Res. 2016;25:1515–1523. doi: 10.1007/s00044-016-1607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hu Z., Guan Y., Hu W., Xu Z., Ishfaq M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran. J. Basic Med. Sci. 2022;25:14–26. doi: 10.22038/ijbms.2022.60380.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu H., Ye F., Sun Q., Liang H., Li C., Li S., Lu R., Huang B., Tan W., Lai L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzym. Inhib. Med. Chem. 2021;36:497–503. doi: 10.1080/14756366.2021.1873977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mehmood S., Maqsood M., Mahtab N., Khan M.I., Sahar A., Zaib S., Gul S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022;46:e14189. doi: 10.1111/jfbc.14189. [DOI] [PubMed] [Google Scholar]
- 107.Ohgitani E., Shin-Ya M., Ichitani M., Kobayashi M., Takihara T., Kawamoto M., Kinugasa H., Mazda O. Significant Inactivation of SARS-CoV-2 In Vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins. Molecules. 2021;26:3572. doi: 10.3390/molecules26123572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ohgitani E., Shin-Ya M., Ichitani M., Kobayashi M., Takihara T., Kawamoto M., Kinugasa H., Mazda O. Rapid Inactivation In Vitro of SARS-CoV-2 in Saliva by Black Tea and Green Tea. Pathogens. 2021;10:721. doi: 10.3390/pathogens10060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pillon M.C., Frazier M.N., Dillard L.B., Williams J.G., Kocaman S., Krahn J.M., Perera L., Hayne C.K., Gordon J., Stewart Z.D., et al. Cryo-EM structures of the SARS-CoV-2 endoribonuclease Nsp15 reveal insight into nuclease specificity and dynamics. Nat. Commun. 2021;12:636. doi: 10.1038/s41467-020-20608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hackbart M., Deng X., Baker S.C. Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors. Proc. Natl. Acad. Sci. USA. 2020;117:8094–8103. doi: 10.1073/pnas.1921485117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hong S., Seo S.H., Woo S.J., Kwon Y., Song M., Ha N.C. Epigallocatechin Gallate Inhibits the Uridylate-Specific Endoribonuclease Nsp15 and Efficiently Neutralizes the SARS-CoV-2 Strain. J. Agric. Food Chem. 2021;69:5948–5954. doi: 10.1021/acs.jafc.1c02050. [DOI] [PubMed] [Google Scholar]
- 112.Chiou W.C., Chen J.C., Chen Y.T., Yang J.M., Hwang L.H., Lyu Y.S., Yang H.Y., Huang C. The inhibitory effects of PGG and EGCG against the SARS-CoV-2 3C-like protease. Biochem. Biophys. Res. Commun. 2022;591:130–136. doi: 10.1016/j.bbrc.2020.12.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shaik F.B., Swarnalatha K., Mohan M.C., Thomas A., Chikati R., Sandeep G., Maddu N. Novel antiviral effects of chloroquine, hydroxychloroquine, and green tea catechins against SARS CoV-2 main protease (Mpro) and 3C-like protease for COVID-19 treatment. Clin. Nutr. Open Sci. 2022;42:62–72. doi: 10.1016/j.nutos.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park R., Jang M., Park Y.I., Park Y., Jung W., Park J., Park J. Epigallocatechin Gallate (EGCG), a Green Tea Polyphenol, Reduces Coronavirus Replication in a Mouse Model. Viruses. 2021;13:2533. doi: 10.3390/v13122533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 116.Yao Z.H., Yao X.L., Zhang Y., Zhang S.F., Hu J.C. Luteolin Could Improve Cognitive Dysfunction by Inhibiting Neuroinflammation. Neurochem. Res. 2018;43:806–820. doi: 10.1007/s11064-018-2482-2. [DOI] [PubMed] [Google Scholar]
- 117.Guo Y.F., Xu N.N., Sun W., Zhao Y., Li C.Y., Guo M.Y. Luteolin reduces inflammation in Staphylococcus aureus-induced mastitis by inhibiting NF-kB activation and MMPs expression. Oncotarget. 2017;8:28481–28493. doi: 10.18632/oncotarget.16092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xiao Z., Xu H., Qu Z.Y., Ma X.Y., Huang B.X., Sun M.S., Wang B.Q., Wang G.Y. Active Ingredients of Reduning Injection Maintain High Potency against SARS-CoV-2 Variants. Chin. J. Integr. Med. 2023;29:205–212. doi: 10.1007/s11655-022-3686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D’Ascanio L., Vitelli F., Cingolani C., Maranzano M., Brenner M.J., Di Stadio A. Randomized clinical trial “olfactory dysfunction after COVID-19: Olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin”: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2021;25:4156–4162. doi: 10.26355/eurrev_202106_26059. [DOI] [PubMed] [Google Scholar]
- 120.O’Byrne L., Webster K.E., MacKeith S., Philpott C., Hopkins C., Burton M.J. Interventions for the treatment of persistent post-COVID-19 olfactory dysfunction. Cochrane Database Syst. Rev. 2022;9:Cd013876. doi: 10.1002/14651858.CD013876.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zalpoor H., Bakhtiyari M., Shapourian H., Rostampour P., Tavakol C., Nabi-Afjadi M. Hesperetin as an anti-SARS-CoV-2 agent can inhibit COVID-19-associated cancer progression by suppressing intracellular signaling pathways. Inflammopharmacology. 2022;30:1533–1539. doi: 10.1007/s10787-022-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dupuis J., Laurin P., Tardif J.C., Hausermann L., Rosa C., Guertin M.C., Thibaudeau K., Gagnon L., Cesari F., Robitaille M., et al. Fourteen-Day Evolution of COVID-19 Symptoms during the Third Wave in Nonvaccinated Subjects and Effects of Hesperidin Therapy: A Randomized, Double-Blinded, Placebo-Controlled Study. Evid. -Based Complement. Altern. Med. Ecam. 2022;2022:3125662. doi: 10.1155/2022/3125662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tahir Ul Qamar M., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020;10:313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clementi N., Scagnolari C., D’Amore A., Palombi F., Criscuolo E., Frasca F., Pierangeli A., Mancini N., Antonelli G., Clementi M., et al. Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol. Res. 2021;163:105255. doi: 10.1016/j.phrs.2020.105255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feldo M., Wójciak-Kosior M., Sowa I., Kocki J., Bogucki J., Zubilewicz T., Kęsik J., Bogucka-Kocka A. Effect of Diosmin Administration in Patients with Chronic Venous Disorders on Selected Factors Affecting Angiogenesis. Molecules. 2019;24:3316. doi: 10.3390/molecules24183316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu X., Qin H., Li Y., Li J., Fu L., Li M., Jiang C., Yun J., Liu Z., Feng Y., et al. Biochanin A protect against lipopolysaccharide-induced acute lung injury in mice by regulating TLR4/NF-κB and PPAR-γ pathway. Microb. Pathog. 2020;138:103846. doi: 10.1016/j.micpath.2019.103846. [DOI] [PubMed] [Google Scholar]
- 127.Zhu Z.M., Sun G.Y. Silymarin mitigates lung impairments in a rat model of acute respiratory distress syndrome. Inflammopharmacology. 2018;26:747–754. doi: 10.1007/s10787-017-0407-3. [DOI] [PubMed] [Google Scholar]
- 128.Shi H., Zhang Y., Xing J., Liu L., Qiao F., Li J., Chen Y. Baicalin attenuates hepatic injury in non-alcoholic steatohepatitis cell model by suppressing inflammasome-dependent GSDMD-mediated cell pyroptosis. Int. Immunopharmacol. 2020;81:106195. doi: 10.1016/j.intimp.2020.106195. [DOI] [PubMed] [Google Scholar]
- 129.Chen J., Zhang R., Wang J., Yu P., Liu Q., Zeng D., Song H.P., Kuang Z.Y. Protective effects of baicalin on LPS-induced injury in intestinal epithelial cells and intercellular tight junctions. Can. J. Physiol. Pharmacol. 2015;93:233–237. doi: 10.1139/cjpp-2014-0262. [DOI] [PubMed] [Google Scholar]
- 130.Orzechowska B.U., Wrobel G., Turlej E., Jatczak B., Sochocka M., Chaber R. Antitumor effect of baicalin from the Scutellaria baicalensis radix extract in B-acute lymphoblastic leukemia with different chromosomal rearrangements. Int. Immunopharmacol. 2020;79:106114. doi: 10.1016/j.intimp.2019.106114. [DOI] [PubMed] [Google Scholar]
- 131.Gao Y., Liu H., Wang H.Z., Hu H.L., He H.J., Gu N., Han X., Guo Q., Liu D., Cui S., et al. Baicalin inhibits breast cancer development via inhibiting IĸB kinase activation in vitro and in vivo. Int. J. Oncol. 2018;53:2727–2736. doi: 10.3892/ijo.2018.4594. [DOI] [PubMed] [Google Scholar]
- 132.Oo A., Rausalu K., Merits A., Higgs S., Vanlandingham D., Bakar S.A., Zandi K. Deciphering the potential of baicalin as an antiviral agent for Chikungunya virus infection. Antivir. Res. 2018;150:101–111. doi: 10.1016/j.antiviral.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 133.Song J., Zhang L., Xu Y., Yang D., Zhang L., Yang S., Zhang W., Wang J., Tian S., Yang S., et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 2021;183:114302. doi: 10.1016/j.bcp.2020.114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim D.H., Hossain M.A., Kang Y.J., Jang J.Y., Lee Y.J., Im E., Yoon J.H., Kim H.S., Chung H.Y., Kim N.D. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int. J. Oncol. 2013;43:1652–1658. doi: 10.3892/ijo.2013.2086. [DOI] [PubMed] [Google Scholar]
- 135.Hytti M., Szabo D., Piippo N., Korhonen E., Honkakoski P., Kaarniranta K., Petrovski G., Kauppinen A. Two dietary polyphenols, fisetin and luteolin, reduce inflammation but augment DNA damage-induced toxicity in human RPE cells. J. Nutr. Biochem. 2017;42:37–42. doi: 10.1016/j.jnutbio.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 136.Yan H.Y., Ma L.L., Wang H.Q., Wu S., Huang H., Gu Z.Y., Jiang J.D., Li Y.H. Luteolin decreases the yield of influenza A virus in vitro by interfering with the coat protein I complex expression. J. Nat. Med. 2019;73:487–496. doi: 10.1007/s11418-019-01287-7. [DOI] [PubMed] [Google Scholar]
- 137.Fassina G., Buffa A., Benelli R., Varnier O.E., Noonan D.M., Albini A. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea as a candidate anti-HIV agent. AIDS. 2002;16:939–941. doi: 10.1097/00002030-200204120-00020. [DOI] [PubMed] [Google Scholar]
- 138.Bachmetov L., Gal-Tanamy M., Shapira A., Vorobeychik M., Giterman-Galam T., Sathiyamoorthy P., Golan-Goldhirsh A., Benhar I., Tur-Kaspa R., Zemel R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat. 2012;19:e81–e88. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 139.Khachatoorian R., Arumugaswami V., Raychaudhuri S., Yeh G.K., Maloney E.M., Wang J., Dasgupta A., French S.W. Divergent antiviral effects of bioflavonoids on the hepatitis C virus life cycle. Virology. 2012;433:346–355. doi: 10.1016/j.virol.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang X., Yang J., Zou H.J. Baicalin inhibits IL-17-mediated joint inflammation in murine adjuvant-induced arthritis. Clin. Dev. Immunol. 2013;2013:268065. doi: 10.1155/2013/268065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fang P.H., Yu M., Zhang L., Wan D., Shi M.Y., Zhu Y., Bo P., Zhang Z.W. Baicalin against obesity and insulin resistance through activation of AKT/AS160/GLUT4 pathway. Mol. Cell. Endocrinol. 2017;448:77–86. doi: 10.1016/j.mce.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 142.Wu J.Y., Tsai K.W., Li Y.Z., Chang Y.S., Lai Y.C., Laio Y.H., Wu J.D., Liu Y.W. Anti-Bladder-Tumor Effect of Baicalein from Scutellaria baicalensis Georgi and Its Application In Vivo. Evid. -Based Complement. Altern. Med. Ecam. 2013;2013:579751. doi: 10.1155/2013/579751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fu Y., Luo J., Jia Z.Q., Zhen W., Zhou K.Q., Gilbert E., Liu D.M. Baicalein Protects against Type 2 Diabetes via Promoting Islet beta-Cell Function in Obese Diabetic Mice. Int. J. Endocrinol. 2014;2014:846742. doi: 10.1155/2014/846742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xiong J., Wang K.Z., Yuan C.X., Xing R., Ni J.B., Hu G.Y., Chen F.L., Wang X.P. Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med. 2017;39:113–125. doi: 10.3892/ijmm.2016.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Domitrovic R., Cvijanovic O., Pugel E.P., Zagorac G.B., Mahmutefendic H., Skoda M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology. 2013;310:115–123. doi: 10.1016/j.tox.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 146.Lu X.Y., Li Y.H., Xiao X.W., Li X.B. [Inhibitory effects of luteolin on human gastric carcinoma xenografts in nude mice and its mechanism] Zhonghua Yi Xue Za Zhi. 2013;93:142–146. [PubMed] [Google Scholar]
- 147.Yoon S.P., Maeng Y.H., Hong R., Lee B.R., Kim C.G., Kim H.L., Chung J.H., Shin B.C. Protective effects of epigallocatechin gallate (EGCG) on streptozotocin-induced diabetic nephropathy in mice. Acta Histochem. 2014;116:1210–1215. doi: 10.1016/j.acthis.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 148.Qin N., Chen Y., Jin M.N., Zhang C., Qiao W., Yue X.L., Duan H.Q., Niu W.Y. Anti-obesity and anti-diabetic effects of flavonoid derivative (Fla-CN) via microRNA in high fat diet induced obesity mice. Eur. J. Pharm. Sci. 2016;82:52–63. doi: 10.1016/j.ejps.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 149.Jiang H., Yamashita Y., Nakamura A., Croft K., Ashida H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019;9:2690. doi: 10.1038/s41598-019-38711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hua F.Z., Ying J., Zhang J., Wang X.F., Hu Y.H., Liang Y.P., Liu Q., Xu G.H. Naringenin pre-treatment inhibits neuroapoptosis and ameliorates cognitive impairment in rats exposed to isoflurane anesthesia by regulating the PI3/Akt/PTEN signalling pathway and suppressing NF-κB-mediated inflammation. Int. J. Mol. Med. 2016;38:1271–1280. doi: 10.3892/ijmm.2016.2715. [DOI] [PubMed] [Google Scholar]
- 151.Rahman M.M., Shohag S., Islam M.R., Akhter S., Mim S.A., Sharma R., Rauf A. An Insight into COVID-19 and Traditional Herbs: Bangladesh Perspective. Med. Chem. 2022;19:361–383. doi: 10.2174/1573406418666220829144746. [DOI] [PubMed] [Google Scholar]
- 152.Rauf A., Abu-Izneid T., Khalil A.A., Hafeez N., Olatunde A., Rahman M., Semwal P., Al-Awthan Y.S., Bahattab O.S., Khan I.N., et al. Nanoparticles in clinical trials of COVID-19: An update. Int. J. Surg. 2022;104:106818. doi: 10.1016/j.ijsu.2022.106818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.