Abstract
Introduction: Cardiac arrest is a significant cause of premature mortality and severe disability. Despite the death rate steadily decreasing over the previous decade, only 22% of survivors achieve good clinical status and only 25% of patients survive until their discharge from the hospital. The objective of this scoping review was to review relevant AI modalities and the main potential applications of AI in resuscitation. Methods: We conducted the literature search for related studies in PubMed, EMBASE, and Google Scholar. We included peer-reviewed publications and articles in the press, pooling and characterizing the data by their model types, goals, and benefits. Results: After identifying 268 original studies, we chose 59 original studies (reporting 1,817,419 patients) to include in the qualitative synthesis. AI-based methods appear to be superior to traditional methods in achieving high-level performance. Conclusion: AI might be useful in predicting cardiac arrest, heart rhythm disorders, and post-cardiac arrest outcomes, as well as in the delivery of drone-delivered defibrillators and notification of dispatchers. AI-powered technologies could be valuable assistants to continuously track patient conditions. Healthcare professionals should assist in the research and development of AI-powered technologies as well as their implementation into clinical practice.
Keywords: cardiac arrest, premature mortality, artificial intelligence, resuscitation
1. Introduction
Cardiac arrest is associated with significant premature mortality and disability worldwide [1,2]. In 2017, in-hospital cardiac arrest (IHCA) occurred at a rate of 8.27 per 1000 hospitalizations and 1.56 per 1000 hospital stays [1]. Despite the fact that the death rate has been steadily dropping over the previous decade, approximately 25% of patients survive until their discharge from the hospital and only 22% of survivors achieve good clinical status [1]. The management of patients with cardiac arrest is associated with several major healthcare issues:
-
-
Cardiac arrest is followed by a short period during which the patient can be resuscitated; if the arrest is not immediately noticed, the chances for successful resuscitation drop rapidly. Unfortunately, the recognition of cardiac arrest and initiation of cardiopulmonary resuscitation are frequently delayed, leading to poor outcomes.
-
-
Survivors of cardiac arrest frequently develop significant neurological problems and even experience post-anoxic coma as part of their poor and unpredictable long-term outcomes. Critical care has made significant advances in recent decades and modern organ-replacing technologies can support life for an extended time. However, this can lead to an increase in the number of patients in vegetative states.
-
-
Cardiac arrest can occur anywhere, and defibrillators are often not available.
Artificial intelligence (AI) could potentially solve these issues. AI could improve the early recognition of cardiac arrest and potentially even predict cardiac arrest or life-threatening arrhythmia through continuous ECG monitoring. AI could also improve the prediction of the outcomes of cardiac arrest, including neurological outcomes.
Traditional prediction methods have poor sensitivity and specificity. To achieve a high level of accuracy, the prediction model should include a large number of pre-arrest variables as well as clinically important parameters linked with cardiac arrest, ensuring optimal prognosis. This proof prediction of the likelihood of good neurological results may offer significant treatment advantages over current methods. Traditional forecasting algorithms for estimating the neurological consequences of IHCA are derived from traditional statistical approaches and grading techniques; these algorithms have shown somewhat adequate prediction capabilities [3,4,5]. An artificial neural network (ANN) is a guided computational model that may incorporate a wide range of dependent and independent variables through continuous training and confirmation by utilizing information to effectively predict the outcome of an unknown situation. ANNs have been used in a number of diagnostic imaging and decision-making applications [6,7]. The objective of this scoping review was to review relevant AI modalities and main potential applications of AI in resuscitation.
We aimed to answer the following questions:
-
-
To identify and characterize the AI models used for the prediction of cardiac arrests and heart rhythm disorders and the development of early warning system;
-
-
To characterize the AI models used for the development of AI-based dispatch rules for drone-delivered defibrillators and the notification of dispatchers;
-
-
To characterized AI-models used for outcome prediction.
2. Methods
2.1. Protocol
This scoping review protocol was developed, written, and approved by each author. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for scoping reviews [8]. This scoping review is mainly focused on studies presenting the implementation of AI in resuscitation.
2.2. Literature Search
PubMed, EMBASE, and Google Scholar databases were utilized to find relevant studies. We used the search terms and their combinations.
2.3. Eligibility Criteria
We considered articles reporting the application of AI methods in resuscitation, including early warning and prediction of cardiac arrest, life-threatening heart rhythm disorders, outcomes of cardiac arrest (e.g., post cardiac arrest anoxic coma, delivery of defibrillators by drones). There were no restrictions based on age, gender, geographical region, or type of AI algorithm. We included peer-reviewed publications, articles in the press, written in English. We excluded study protocols, review articles, and conference abstracts. No restrictions were set on the study setting, patient population, study design, country, or time period of the publication year.
2.4. Search Terms and Their Combinations
(((cardiac arrest) OR (tachycardia, ventricular)) AND (artificial intelligence)) OR (machine learning); (“heart arrest” OR (“heart” AND “arrest”) OR “heart arrest” OR (“cardiac” AND “arrest”) OR “cardiac arrest” OR (“tachycardia, ventricular” OR (“tachycardia” AND “ventricular”) OR “ventricular tachycardia” OR (“tachycardia” AND “ventricular” [All Fields]) OR “tachycardia ventricular”) AND (“artificial intelligence” OR (“artificial” AND “intelligence”) OR “artificial intelligence”)) OR (“machine learning” OR (“machine” AND “learning”) OR “machine learning”).
Articles that did not match the review title and abstract sample selection were omitted. The following information was compiled: author, year, mean age, sample size, age, outcomes, AI algorithm, study goals (primary and secondary), sample size (i.e., how many patients were enrolled in the study), diagnosis, comorbidities, AI algorithm/model or method, goals of AI application, advantages, disadvantages, sensitivity, specificity, positive predictive value, and negative predictive value.
The obtained data were extracted and organized into the following tables: study and cohort information (Table S1), AI methods and characteristics (Table S2).
2.5. Data Extraction
Three reviewers independently extracted the data according to the protocol. The data were arranged into three Excel tables. Any conflict between the reviewers was resolved by achieving a consensus among all authors. We performed a narrative synthesis of the extracted data. The findings from the included studies were categorized into the following subsections: article characteristics, goals of the studies, AI models used, and reported benefits of using AI in cardiac arrest patients.
3. Results
3.1. Study Selection
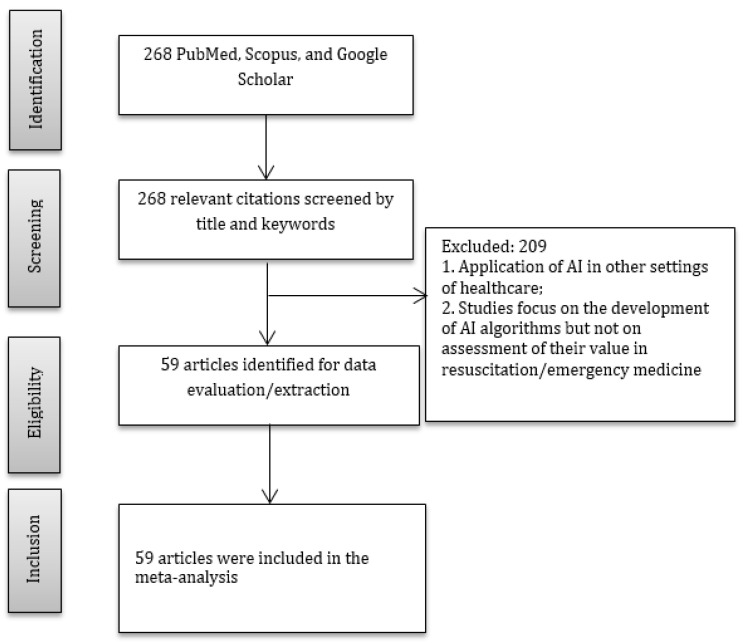
The systematic search identified 268 original studies, from which 59 original studies (reporting 1,817,419 patients) were chosen for the qualitative synthesis (Figure 1, Table S1).
Figure 1.
PRISMA diagram.
3.2. Prediction Methods and Goals
The majority of included studies focused on the development of prediction models [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67]. The main goals of the studies are listed below:
(a) detection of cardiac arrest; (b) development of AI-based early warning system for the prediction of IHCA, OHCA, and life-threatening dysrhythmia; (c) prediction of success of defibrillation and prediction of early outcomes of cardiac arrest; and (d) neurological outcome prediction in comatose patients after cardio-pulmonary resuscitation (CPR).
3.3. Prediction of Cardiac Arrests and Heart Rhythm Disorders and the Development of Early Warning System
A wide variety of studies have tried to predict cardiac arrests and mortality in critically ill patients (i.e., those suffering from both IHCAs and OHCAs) using machine learning (ML). Most studies used databases with continuous monitoring of vital and physiological functions, diagnostic tests (e.g., laboratory work), and imaging techniques.
Lee et al. aimed at validating DEWS in a large, multiple-center cohort and comparison of DEWS and modified early warning score (MEWS) in terms of the predictive performance of IHCA [9]. Authors compared DEWS and MEWS using the data collected at several medical centers. DEWS forecasted IHCA better than MEWS and minimized the proportion of false alarms. The results showed that DEWS can be useful in the rapid identification of high-risk individuals. DEWS can forecast and identify patients at risk of IHCA, allowing healthcare professionals to better treat early signs and symptoms [9].
The next groups of studies focused on the development of a tachycardia onset prediction network based on deep learning (i.e., bidirectional long/short-term memory) for early tachycardia detection (Table S1) [11,17,46]. Liu et al. developed a TOP-Net for actual assessment and forecasting of the probability of tachycardia, making it feasible to foresee tachycardia six hours before the event. TOP-Net was evaluated using six metrics, three sub-experiments, and various forecasting periods between zero and six hours. TOP-Net demonstrated better performance compared to the other five existing techniques, including deep learning algorithms, ensemble algorithms, and ANN. The algorithm that included individual details from systems performed better than those that did not. The model’s widely obtainable input data and strong performance in the hospital suggested that early tachycardia development prediction using portable sensing devices could be viable in medical facilities or homes [11].
One study tested the application of machine learning in predicting cardiac arrest based on the combination of imaging and clinical data. Thus, the risk of cardiac death was predicted based on the findings of clinical data and myocardial perfusion SPECT, improving the prediction accuracy and reducing the number of input variables [50]. It was shown that a support vector machine algorithm was the most accurate algorithm, though it required a large number of features, whereas a LASSO model achieves high accuracy with only six variables.
3.4. Development of ML-Based Dispatch Rules for Drone-Delivered Defibrillators and the Notification of Dispatchers
The next group of studies investigated the application of AI in notifying dispatchers, as well as in creating and testing machine learning-based dispatch rules for drone-delivered defibrillators.
More specifically, the published studies focused on the following problems in resuscitation and emergency care:
-
-
Creating and testing machine learning-based dispatch rules for drone-delivered defibrillators using supervised learning models. The ambulance response time was predicted, and these predictions were utilized to determine whether to deploy drones based on an estimated drone flight time.
-
-
Investigation of how OHCA recognition could be impacted by a machine learning model trained to detect OHCA and notify dispatchers during emergency calls [14].
The authors developed dispatch rules for drones carrying automatic electronic defibrillators to the area of suspected OHCA, with the goal of drones reaching the zone of the incident before an ambulance [13,14]. The theory was motivated by the fact that OHCA occurring in remote and rural areas is associated with lower rates of survival and timely access to automated external defibrillators posing a significant challenge [13]. It was found that drone-delivered automatic defibrillators can significantly shorten the time required for help to reach the area of the accident [13].
A similar study was aimed at examining whether the ML model improved medical dispatching and shortened the time-to-recognition of OHCA and the time until dispatcher-assisted cardiopulmonary resuscitation began. The motivation was to use the ML-augmented dispatching to increase recognition of OHCA [14]. The authors did not find improvement in recognition of OHCA supported by ML, although AI surpassed humans in recognizing cardiac arrests [14].
3.5. AI in Outcome Prediction
The group of studies focused on the prediction of long-term outcomes (including neurological outcomes) reported valuable results. The studies targeted the following goals:
-
-
Creating and verifying “a machine learning-based outcome prediction model for out-of-hospital cardiac arrest” with an initial shockable rhythm that could be employed when the patient arrives at the hospital [15];
-
-
Identifying the combinations of heart rate variability and heart print indices that can predict sudden cardiac death with a support vector machine using short-term recordings [17];
-
-
Developing a model for predicting outcomes with artificial neural network. (Secondary: using the model to investigate the impact on “illness severity in patients treated with targeted temperature management”) [18];
-
-
Predicting outcomes of comatose patients after CPR to assess the potential contribution of functional magnetic resonance imaging (RS-fMRI) to predict neurological outcomes [19];
-
-
Developing “an accurate and reliable model to predict neurological outcomes in patients with IHCA” based on pre-resuscitation characteristics and identifying the critical variables that contribute to neurological outcomes using ANNs [10].
Thus, Chung et al. found the most significant predictive factors in IHCA-resuscitated individuals and constructed ANN algorithms that can accurately and reliably determine dynamic neurological consequences [10]. As a result, novel machine learning-based algorithms for generating new data and improving medical decision-making can be developed. In particular, the suggested algorithms have the potential to be used in exigent medical circumstances of IHCA, where they can help with the selection and construction of tailored post-resuscitation measures. These models can also be valuable in supporting decision-making processes for optimal management in the post-resuscitation period [10].
Andersson et al. developed an ANN that showed high predictability of neurological prognosis in comatose patients after OHCA, using diagnostic biomarkers collected during the first three days of treatment in the hospital [12]. The models, which incorporated neuron-specific enolase (NSE) after 72 h and neurofilament light (NFL) every day, performed well in terms of prognostication. Using purely clinical data, the AUROC remained below 90% during the first three days in the ICU. AUROC increased from 82% to 94% (p 0.01) when clinically accessible indicators such as NSE were included. After adding study biomarkers, the prognosis accuracy was outstanding from Days 1–3, with an AUROC of over 95%. The models that incorporated NSE after 72 h and daily NFL had minimal false positive rate forecasts on each of the three days, as well as a minimal rate of false-negative forecasts [12].
The severity of brain injury is the central determinant of outcomes in patients who sustained cardiac arrests [22]. The multimodality monitoring and neurological evaluation appears to be the main principles of neurological prognostication. The authors investigated patterns of early post-cardiac arrest brain injury using several diagnostic methods and found distinct patterns of post-cardiac arrest neurological injury that could be detected on patient presentation. These patterns indicate that there might be important inter-individual heterogeneity that cannot be easily captured by a standard clinical examination of comatose patients. Outcomes varied widely across clusters. Therefore, authors did not suggest using that AI model for decision-making regarding life-sustaining therapy. Nevertheless, the initial patterns of neurological injury could be useful in personalizing resuscitative actions (e.g., hemodynamic targets or targeted temperature management) [22].
Kwon et al. presented the values for the validation data of 8145 subjects. The logistic regression (for both neurological recovery and survival to discharge) was taken for the control indicator as the best-performing machine learning model [41].
3.6. AI Models Used in the Included Studies
The AI models used in the studies are deep learning-based early warning score (DEWS), TOP-Net, long/short-term memory (LSTM), BiLSTMTTM, linear regression and neural network, emergency medical services machine learning-based prognostic model, DLA with CNN, support vector machines, random forest, K-prototypes clusterization, stacking algorithm of support vector machines, decision tree, logistic regression, KNN, GaussianNB, CNNs-Grad-CAMCNN with a VGG, multilayer perceptron (MLP), deep learning-based prognostic system (DCAPS), embedded fully convolutional network (EFCN), GBM, SVC, ensemble feature space embedding, time series forecasting, least absolute shrinkage and selection operator (LASSO), k-nearest neighbor (k-NN), and multilayer perceptron neural network (Table S2) [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67].
3.7. The Studies Reported the following Benefits of the Using AI in Cardiac Arrest Patients
-
-
Performance improvement in forecasting IHCA and evaluating deteriorating patients [9];
-
-
Forecasting with fewer alarms and earlier predictions of IHCA. Moreover, AI can be used globally with no technological limitations [9];
-
-
Recognition of complicated nonlinear correlations between dependent and independent variables, and the ability to distinguish all conceivable interactions among predictor variables [10];
-
-
AI models such as TOP-Net can forecast heart rhythm disorder up to six hours in advance [11];
-
-
ANNs predict neurological prognosis in comatose patients after OHCA using clinical factors and biomarkers from the first three days of intensive care, with good-to-excellent predictive accuracy [12];
-
-
Ability to dispatch a drone to a suspected OHCA based on the forecast that it will arrive at the event before an ambulance, which can achieve a comparable distribution of first response times through a policy that dispatches a drone to all OHCAs. Drone-delivered AEDs may dramatically minimize the time required for AED arrival on-site [13];
-
-
Automatic prediction of poor prognosis in OHCA patients with an initial shockable rhythm [15];
-
-
Predicting cardiac arrest using ECG, with a high NPV percentage (over 99%) and the possibility to predict cardiac arrest using single-lead ECG. This method can predict cardiac arrest using wearable devices [16];
-
-
Deep learning-based early warning system can detect patient deterioration earlier according to the vital signs and laboratory results, preventing deterioration [25];
-
-
Good predictive ability of both good and poor outcomes of coma. The study improved the understanding of changes in the brain at a post-anoxic comatose state [27];
Machine-learning models (random forest classifiers) utilizing quantitative EEG reactivity data can predict long-term outcomes after cardiac arrest [30].
4. Discussion
Over past several years, several models for predicting cardiac arrest and/or disorders of heart rhythm (especially early risk prediction of patient condition deterioration or adverse events) have been designed based on physiological and vital sign monitoring or data from electronic health records. This scoping review presents an overview of the main directions and perspectives of the application of AI in the management of cardiac arrests and life-threatening heart rhythm disorders. We summarized the main characteristics, diagnosis, comorbidities, AI algorithms, and advantages and disadvantages associated with using the developed AI models.
The selected studies focused on the detection and prediction of cardiac arrest, heart rhythm disorders, and cardiac arrest outcomes (after successful CPR), as well as the notification of dispatchers and the development and testing of machine learning-based dispatch rules for drone-delivered defibrillators and AI-based early warning systems. The selected studies were heterogeneous in quality in terms of design, settings, patient population, causes of cardiac arrest, and AI algorithms. However, all of them have clinical value and might pave the way for the further development of new avenues in AI-assisted resuscitation research.
Thus, Shamout et al. [28] developed a deep interpretable early warning system that achieved impressive sensitivity of 100% and PPV of 100% in the detection of the deterioration of patient condition. They utilized an attention-based neural network learning algorithm using historical trends of physiological data (vital signs) through mean (interpolated) and variance features to monitor the deterioration of a patient’s condition. The DEWS architecture achieved a high level of performance, even when using a limited set of features. DEWS reduced the number of triggers compared to NEWS, particularly in younger patients. Reducing the rate of false alerts can reduce the burden on medical professionals working in a highly stressful environment. The authors reported that the model achieved the highest level of performance in three individual outcomes (i.e., cardiac arrest, unplanned ICU admission, and mortality) and the composite outcomes. The authors demystified the deep learning model’s decision-making process by adjusting the attention weights corresponding to each vital sign. The trend analysis could be used by physicians to make a decision on appropriate interventions [28].
Another impressive achievement was reported by Tjepkema-Cloostermans et al., who achieved a PPV of up to 100% in predicting poor neurological outcomes in post-anoxic coma patients [36]. They developed a classifier for neurological outcome prediction that provided rapid and reliable forecasts and could be used bedside. The classifier used a convolutional neural network for the extraction of the EEG patterns [36].
4.1. Improving Model Performance and Reducing the False Alarm Rate
The true and false alarm rate is an important parameter for validating the applicability of the early warning system because too-sensitive alarms with excessive rates of false alarms can cause alarm fatigue, leading to staff desensitization and failures to respond to clinically significant alarms, putting patient care and safety at risk [68,69]. Thus, an ideal warning system should be highly sensitive while, at the same time, having a low false alarm rate. The modified early warning system demonstrated variable accuracy. Therefore, it was not a sufficiently appropriate rapid response system [70,71].
DEWS was developed in 2018 based on four basic vital signs: systolic blood pressure, heart rate, respiratory rate, and body temperature [41]. Lee et al. expanded the initial version of DEWS by adding age, diastolic blood pressure, and the recorded time of each vital sign [9].
4.2. Importance of Timeliness (Trying to Achieve Earlier Prediction at the Same Specificity Level)
A delayed response “is associated with a poor outcome” [72]. Predictable IHCA is defined as “CA that occurs in hospitalized ward patients who met the hospital’s escalation threshold at least 30 min prior to and within 24 h of the event” [73]. The period between 24 h and 30 min prior to IHCA is the appropriate time for a rapid response system to prevent an event [73]. It is important that the staff is aware of the at-risk patients as early as possible, so they have enough time to prepare and to plan action before the event. It is especially important in the general wards, where the patients’ vital parameters are not monitored continuously compared to the continuous monitoring in ICUs [9]. Numerous studies have tried to predict mortality in ICU patients using machine learning (ML) [74,75,76]. Most of the AI- or ML-based studies focusing on mortality or critical event prediction, such as hypotension or sepsis, “achieved better performance compared to conventional prognostic systems” [77,78].
ICUs produce a massive amount of data consisting of a continuous stream of vital parameters, laboratory tests, imaging, microbiological data, fluids, drugs, and transfusions. AI-based (especially ML) prediction methods are much superior to traditional “static” methods in achieving high-level performance if these massive data sets are available [79].
4.3. Strengths and Limitations
The scope of this review included all applications of AI in the management of cardiac arrest and life-threatening arrhythmia, and several AI modalities could potentially improve the survival and quality of care for patients suffering from cardiac arrest.
While we performed a systematic literature search using several databases, nevertheless, some relevant articles might have been missed. Another limitation is that the scope of this review was focused on all applications of AI in the management of cardiac arrest; in some studies, it was impossible to eliminate the investigator’s bias in data collection and analysis. Some medications, such as beta-blockers, might influence the study results. The included studies focused on heterogeneous patient populations.
There was also a risk of missing and incorrect data used for algorithm development. As in many other studies related to AI, there was a chance of overfitting and worse performance in different datasets.
AI in medicine is an emerging field and the number of studies is rapidly expanding. It is challenging to synthesize the information because of the heterogeneous reporting formats and study designs, as well as slight differences in definitions. Likewise, in many other studies, there was a lack of transparency regarding the algorithms and models; therefore, it was difficult to assess the study methodology. Another limitation was that we only included studies published in scientific journals and did not include conference papers or papers published in archives.
While AI appears to be a valuable option for predicting many life-threatening conditions, the current evidence still has some uncertainties. The majority of studies were based on retrospectively collected data sets. Therefore, there is a need for further validation in prospective and even controlled clinical trials. Retrospective evidence might not be enough to integrate AI into clinical practice safely, especially considering the risks AI poses to results in bias via underfitting or overfitting. Therefore, prospective clinical studies are essential to minimize the risks of biases that might confound results.
Although there is optimism regarding the power of AI in diagnosis and prediction, as well as the transformation of healthcare, there is a long way to go before these algorithms can be reliably used in clinical settings. Even in radiology and medical imaging, a field where numerous promising ideas about outperforming medical professionals are reported, there is still a lot of work required.
The major obstacle for the application of AI in resuscitation is related to the reliability and reproducibility of algorithm results. Although numerous studies reported outstanding results, the use of AI has still mostly limited to research purposes and not yet widely implemented or used in real clinical practice. Before implementation, these models should ideally be validated or tested on the local populations where those models are intended to be used.
4.4. Future Directions
AI might be valuable in the analysis and interpretation of vital sign monitoring and electronic health records, leading to the identification of the most sensitive and specific parameters for predicting life-threatening arrhythmia, cardiac arrest, and the outcomes of cardiac arrest. With the increasing adoption of the internet of things, including wearable devices equipped with sensors tracking physiological functions, AI-powered technologies are now valuable assistants that can track patient conditions around the clock. Healthcare professionals should assist in the research and development of AI-powered technologies as well as their implementation into clinical practice. In order to develop algorithms suitable for a larger patient population, there is a need to include heterogenous training datasets.
5. Conclusions
AI might be useful in the prediction of in-hospital and out-of-hospital cardiac arrest, heart rhythm disorders, and neurological outcomes after in-hospital cardiac arrest, as well as aiding in the delivery of drone-delivered defibrillators and the notification of dispatchers. Future prospective studies are warranted to establish more solid evidence in each of the listed applications of artificial intelligence in cardiac arrest management.
Acknowledgments
We thank Daniyar Amantayev, Gulbanu Tillabek, Yersultan Baimukhan, Saule Maulenkul, Minura Nugumanova, Chinonso Nwanevu, Tomiris Madiyarova, Asset Baisalov, and Akzhan Suranshy contributed to data extraction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12062254/s1, Table S1: Study and cohort information; Table S2: Artificial intelligence method and its purpose.
Institutional Review Board Statement
Ethical approval and patient consent were not required for this systematic review and meta-analysis.
Informed Consent Statement
This scoping review was registered in open science registry. Viderman, D., Abdildin, Y. G., Batkuldinova, K., Badenes, R., & Bilotta, F. Artificial intelligence in resuscitation and emergency care: a scoping review (osf.io/byv73).
Data Availability Statement
The data will be shared on request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported in part by Nazarbayev University Faculty Development Competitive Research Grants No. 021220FD2851 and 11022021FD2906.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Schluep M., Gravesteijn B.Y., Stolker R.J., Endeman H., Hoeks S.E. One-year survival after in-hospital cardiac arrest: A systematic review and meta-analysis. Resuscitation. 2018;132:90–100. doi: 10.1016/j.resuscitation.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Chan P.S., Spertus J.A., Krumholz H.M., Berg R.A., Li Y., Sasson C., Nallamothu B.K. A Validated Prediction Tool for Initial Survivors of In-Hospital Cardiac Arrest. Arch. Intern. Med. 2012;172:947–953. doi: 10.1001/archinternmed.2012.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Arrigo S., Cacciola S., Dennis M., Jung C., Kagawa E., Antonelli M., Sandroni C. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: A systematic review and meta-analysis. Resuscitation. 2017;121:62–70. doi: 10.1016/j.resuscitation.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Wang C.-H., Chang W.-T., Huang C.-H., Tsai M.-S., Yu P.-H., Wu Y.-W., Chen W.-J. Validation of the Cardiac Arrest Survival Postresuscitation In-hospital (CASPRI) score in an East Asian population. PLoS ONE. 2018;13:e0202938. doi: 10.1371/journal.pone.0202938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amato F., Lopez A., Peña-Méndez E.M., Vaňhara P., Hampl A., Havel J. Artificial neural networks in medical diagnosis. J. Appl. Biomed. 2013;11:47–58. doi: 10.2478/v10136-012-0031-x. [DOI] [Google Scholar]
- 7.Agatonovic-Kustrin S., Beresford R. Basic concepts of artificial neural network (ANN) modeling and its application in pharmaceutical research. J. Pharm. Biomed. Anal. 2000;22:717–727. doi: 10.1016/S0731-7085(99)00272-1. [DOI] [PubMed] [Google Scholar]
- 8.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D., Horsley T., Weeks L., et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y.J., Cho K.-J., Kwon O., Park H., Lee Y., Kwon J.-M., Park J., Kim J.S., Lee M.-J., Kim A.J., et al. A multicentre validation study of the deep learning-based early warning score for predicting in-hospital cardiac arrest in patients admitted to general wards. Resuscitation. 2021;163:78–85. doi: 10.1016/j.resuscitation.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Chung C.-C., Chiu W.-T., Huang Y.-H., Chan L., Hong C.-T., Chiu H.-W. Identifying prognostic factors and developing accurate outcome predictions for in-hospital cardiac arrest by using artificial neural networks. J. Neurol. Sci. 2021;425:117445. doi: 10.1016/j.jns.2021.117445. [DOI] [PubMed] [Google Scholar]
- 11.Liu X., Liu T., Zhang Z., Kuo P.-C., Xu H., Yang Z., Lan K., Li P., Ouyang Z., Ng Y.L., et al. TOP-Net Prediction Model Using Bidirectional Long Short-term Memory and Medical-Grade Wearable Multisensor System for Tachycardia Onset: Algorithm Development Study. JMIR Med. Inform. 2021;9:e18803. doi: 10.2196/18803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson P., Johnsson J., Björnsson O., Cronberg T., Hassager C., Zetterberg H., Stammet P., Undén J., Kjaergaard J., Friberg H., et al. Predicting neurological outcome after out-of-hospital cardiac arrest with cumulative information; development and internal validation of an artificial neural network algorithm. Crit. Care. 2021;25:83. doi: 10.1186/s13054-021-03505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu J., Leung K.B., Snobelen P., Nevils G., Drennan I.R., Cheskes S., Chan T.C. Machine learning-based dispatch of drone-delivered defibrillators for out-of-hospital cardiac arrest. Resuscitation. 2021;162:120–127. doi: 10.1016/j.resuscitation.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 14.Blomberg S.N., Christensen H.C., Lippert F., Ersbøll A.K., Torp-Petersen C., Sayre M.R., Kudenchuk P.J., Folke F. Effect of Machine Learning on Dispatcher Recognition of Out-of-Hospital Cardiac Arrest During Calls to Emergency Medical Services. JAMA Netw. Open. 2021;4:e2032320. doi: 10.1001/jamanetworkopen.2020.32320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano Y., Kondo Y., Sueyoshi K., Okamoto K., Tanaka H. Early outcome prediction for out-of-hospital cardiac arrest with initial shockable rhythm using machine learning models. Resuscitation. 2021;158:49–56. doi: 10.1016/j.resuscitation.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Kwon J.-M., Kim K.-H., Jeon K.-H., Lee S.Y., Park J., Oh B.-H. Artificial intelligence algorithm for predicting cardiac arrest using electrocardiography. Scand. J. Trauma, Resusc. Emerg. Med. 2020;28:98. doi: 10.1186/s13049-020-00791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Alanis M., Bojorges-Valdez E., Wessel N., Lerma C. Prediction of Sudden Cardiac Death Risk with a Support Vector Machine Based on Heart Rate Variability and Heartprint Indices. Sensors. 2020;20:5483. doi: 10.3390/s20195483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnsson J., Björnsson O., Andersson P., Jakobsson A., Cronberg T., Lilja G., Friberg H., Hassager C., Kjaergard J., Wise M., et al. Artificial neural networks improve early outcome prediction and risk classification in out-of-hospital cardiac arrest patients admitted to intensive care. Crit. Care. 2020;24:474. doi: 10.1186/s13054-020-03103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner F., Hänggi M., Weck A., Pastore-Wapp M., Wiest R., Kiefer C. Outcome prediction with resting-state functional connectivity after cardiac arrest. Sci. Rep. 2020;10:11695. doi: 10.1038/s41598-020-68683-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Dury N., Ravn-Fischer A., Hollenberg J., Israelsson J., Nordberg P., Strömsöe A., Axelsson C., Herlitz J., Rawshani A. Identifying the relative importance of predictors of survival in out of hospital cardiac arrest: A machine learning study. Scand. J. Trauma, Resusc. Emerg. Med. 2020;28:60. doi: 10.1186/s13049-020-00742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N., Ho A.F.W., Pek P.P., Lu T.-C., Khruekarnchana P., Song K.J., Tanaka H., Naroo G.Y., Gan H.N., Koh Z.X., et al. Prediction of ROSC After Cardiac Arrest Using Machine Learning. Stud. Health Technol. Inform. 2020;270:1357–1358. doi: 10.3233/SHTI200440. [DOI] [PubMed] [Google Scholar]
- 22.Elmer J., Coppler P.J., May T.L., Hirsch K., Faro J., Solanki P., Brown M., Puyana J.S., Rittenberger J.C., Callaway C.W. Unsupervised learning of early post-arrest brain injury phenotypes. Resuscitation. 2020;153:154–160. doi: 10.1016/j.resuscitation.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold J., Davis A., Fischhoff B., Yecies E., Grace J., Klobuka A., Mohan D., Hanmer J. Comparing the predictive ability of a commercial artificial intelligence early warning system with physician judgement for clinical deterioration in hospitalised general internal medicine patients: A prospective observational study. BMJ Open. 2019;9:e032187. doi: 10.1136/bmjopen-2019-032187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C.-H., Hsieh J.-G., Cheng S.-L., Lin Y.-L., Lin P.-H., Jeng J.-H. Emergency department disposition prediction using a deep neural network with integrated clinical narratives and structured data. Int. J. Med. Inform. 2020;139:104146. doi: 10.1016/j.ijmedinf.2020.104146. [DOI] [PubMed] [Google Scholar]
- 25.Cho K.-J., Kwon O., Kwon J.-M., Lee Y., Park H., Jeon K.-H., Kim K.-H., Park J., Oh B.-H. Detecting Patient Deterioration Using Artificial Intelligence in a Rapid Response System. Crit. Care Med. 2020;48:e285–e289. doi: 10.1097/CCM.0000000000004236. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes M., Mendes R., Vieira S.M., Leite F., Palos C., Johnson A., Finkelstein S., Horng S., Celi L.A. Risk of mortality and cardiopulmonary arrest in critical patients presenting to the emergency department using machine learning and natural language processing. PLoS ONE. 2020;15:e0230876. doi: 10.1371/journal.pone.0230876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugin D., Hofmeister J., Gasche Y., Vulliemoz S., Lövblad K.-O., Van De Ville D., Haller S. Resting-State Brain Activity for Early Prediction Outcome in Postanoxic Patients in a Coma with Indeterminate Clinical Prognosis. Am. J. Neuroradiol. 2020;41:1022–1030. doi: 10.3174/ajnr.A6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamout F.E., Zhu T., Sharma P., Watkinson P.J., Clifton D.A. Deep Interpretable Early Warning System for the Detection of Clinical Deterioration. IEEE J. Biomed. Health Inform. 2019;24:437–446. doi: 10.1109/JBHI.2019.2937803. [DOI] [PubMed] [Google Scholar]
- 29.Kim J., Chae M., Chang H.-J., Kim Y.-A., Park E. Predicting Cardiac Arrest and Respiratory Failure Using Feasible Artificial Intelligence with Simple Trajectories of Patient Data. J. Clin. Med. 2019;8:1336. doi: 10.3390/jcm8091336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amorim E., van der Stoel M., Nagaraj S.B., Ghassemi M.M., Jing J., O’Reilly U.-M., Scirica B.M., Lee J.W., Cash S.S., Westover M.B. Quantitative EEG reactivity and machine learning for prognostication in hypoxic-ischemic brain injury. Clin. Neurophysiol. 2019;130:1908–1916. doi: 10.1016/j.clinph.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Javan S.L., Sepehri M.M., Javan M.L., Khatibi T. An intelligent warning model for early prediction of cardiac arrest in sepsis patients. Comput. Methods Programs Biomed. 2019;178:47–58. doi: 10.1016/j.cmpb.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Merath K., Hyer J.M., Mehta R., Farooq A., Bagante F., Sahara K., Tsilimigras D.I., Beal E., Paredes A.Z., Wu L., et al. Use of Machine Learning for Prediction of Patient Risk of Postoperative Complications After Liver, Pancreatic, and Colorectal Surgery. J. Gastrointest. Surg. 2019;24:1843–1851. doi: 10.1007/s11605-019-04338-2. [DOI] [PubMed] [Google Scholar]
- 33.Park J.H., Shin S.D., Song K.J., Hong K.J., Ro Y.S., Choi J.-W., Choi S.W. Prediction of good neurological recovery after out-of-hospital cardiac arrest: A machine learning analysis. Resuscitation. 2019;142:127–135. doi: 10.1016/j.resuscitation.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Jonas S., Rossetti A.O., Oddo M., Jenni S., Favaro P., Zubler F. EEG-based outcome prediction after cardiac arrest with convolutional neural networks: Performance and visualization of discriminative features. Hum. Brain Mapp. 2019;40:4606–4617. doi: 10.1002/hbm.24724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghassemi M.M., Amorim E., Alhanai T., Lee J.W., Herman S.T., Sivaraju A., Gaspard N., Hirsch L., Scirica B.M., Biswal S., et al. Quantitative Electroencephalogram Trends Predict Recovery in Hypoxic-Ischemic Encephalopathy*. Crit. Care Med. 2019;47:1416–1423. doi: 10.1097/CCM.0000000000003840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seki T., Tamura T., Suzuki M., SOS-KANTO 2012 Study Group Outcome prediction of out-of-hospital cardiac arrest with presumed cardiac aetiology using an advanced machine learning technique. Resuscitation. 2019;141:128–135. doi: 10.1016/j.resuscitation.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Tjepkema-Cloostermans M.C., Lourenço C., Ruijter B., Tromp S.C., Drost G., Kornips F.H.M., Beishuizen A., Bosch F.H., Hofmeijer J., Van Putten M.J.A.M. Outcome Prediction in Postanoxic Coma with Deep Learning. Crit. Care Med. 2019;47:1424–1432. doi: 10.1097/CCM.0000000000003854. [DOI] [PubMed] [Google Scholar]
- 38.Moon S., Liu S., Scott C.G., Samudrala S., Abidian M.M., Geske J.B., Noseworthy P.A., Shellum J.L., Chaudhry R., Ommen S.R., et al. Automated extraction of sudden cardiac death risk factors in hypertrophic cardiomyopathy patients by natural language processing. Int. J. Med. Inform. 2019;128:32–38. doi: 10.1016/j.ijmedinf.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu C.-C., Hsu W.-D., Islam M., Poly T.N., Yang H.-C., Nguyen P.-A., Wang Y.-C., Li Y.-C. An artificial intelligence approach to early predict non-ST-elevation myocardial infarction patients with chest pain. Comput. Methods Programs Biomed. 2019;173:109–117. doi: 10.1016/j.cmpb.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Jang D.-H., Kim J., Jo Y.H., Lee J.H., Hwang J.E., Park S.M., Lee D.K., Park I., Kim D., Chang H. Developing neural network models for early detection of cardiac arrest in emergency department. Am. J. Emerg. Med. 2020;38:43–49. doi: 10.1016/j.ajem.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Kwon J.-M., Jeon K.-H., Kim H.M., Kim M.J., Lim S.-M., Kim K.-H., Song P.S., Park J., Choi R.K., Oh B.-H. Deep-learning-based out-of-hospital cardiac arrest prognostic system to predict clinical outcomes. Resuscitation. 2019;139:84–91. doi: 10.1016/j.resuscitation.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Harford S., Darabi H., Del Rios M., Majumdar S., Karim F., Hoek T.V., Erwin K., Watson D.P. A machine learning based model for Out of Hospital cardiac arrest outcome classification and sensitivity analysis. Resuscitation. 2019;138:134–140. doi: 10.1016/j.resuscitation.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Blomberg S.N., Folke F., Ersbøll A.K., Christensen H.C., Torp-Pedersen C., Sayre M.R., Counts C.R., Lippert F.K. Machine learning as a supportive tool to recognize cardiac arrest in emergency calls. Resuscitation. 2019;138:322–329. doi: 10.1016/j.resuscitation.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Coult J., Blackwood J., Sherman L., Rea T.D., Kudenchuk P.J., Kwok H. Ventricular Fibrillation Waveform Analysis During Chest Compressions to Predict Survival From Cardiac Arrest. Circ. Arrhythmia Electrophysiol. 2019;12:e006924. doi: 10.1161/CIRCEP.118.006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nanayakkara S., Fogarty S., Tremeer M., Ross K., Richards B., Bergmeir C., Xu S., Stub D., Smith K., Tacey M., et al. Characterising risk of in-hospital mortality following cardiac arrest using machine learning: A retrospective international registry study. PLOS Med. 2018;15:e1002709. doi: 10.1371/journal.pmed.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Au-Yeung W.-T.M., Reinhall P.G., Bardy G.H., Brunton S.L. Development and validation of warning system of ventricular tachyarrhythmia in patients with heart failure with heart rate variability data. PLoS ONE. 2018;13:e0207215. doi: 10.1371/journal.pone.0207215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matam B.R., Duncan H., Lowe D. Machine learning based framework to predict cardiac arrests in a paediatric intensive care unit: Prediction of cardiac arrests. J. Clin. Monit. Comput. 2018;33:713–724. doi: 10.1007/s10877-018-0198-0. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y., Kwon J.-M., Lee Y., Park H., Cho H., Park J. Deep Learning in the Medical Domain: Predicting Cardiac Arrest Using Deep Learning. Acute Crit. Care. 2018;33:117–120. doi: 10.4266/acc.2018.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon J., Lee Y., Lee Y., Lee S., Park J. An Algorithm Based on Deep Learning for Predicting In-Hospital Cardiac Arrest. J. Am. Heart Assoc. 2018;7:e008678. doi: 10.1161/JAHA.118.008678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso D.H., Wernick M.N., Yang Y., Germano G., Berman D.S., Slomka P. Prediction of cardiac death after adenosine myocardial perfusion SPECT based on machine learning. J. Nucl. Cardiol. 2018;26:1746–1754. doi: 10.1007/s12350-018-1250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tjepkema-Cloostermans M.C., Hofmeijer J., Beishuizen A., Hom H.W., Blans M.J., Bosch F.H., Van Putten M.J.A.M. Cerebral Recovery Index: Reliable Help for Prediction of Neurologic Outcome After Cardiac Arrest. Crit. Care Med. 2017;45:e789–e797. doi: 10.1097/CCM.0000000000002412. [DOI] [PubMed] [Google Scholar]
- 52.Rad A.B., Eftestol T., Engan K., Irusta U., Kvaloy J.T., Kramer-Johansen J., Wik L., Katsaggelos A.K. ECG-Based Classification of Resuscitation Cardiac Rhythms for Retrospective Data Analysis. IEEE Trans. Biomed. Eng. 2017;64:2411–2418. doi: 10.1109/TBME.2017.2688380. [DOI] [PubMed] [Google Scholar]
- 53.Hu S.B., Wong D.J.L., Correa A., Li N., Deng J.C. Prediction of Clinical Deterioration in Hospitalized Adult Patients with Hematologic Malignancies Using a Neural Network Model. PLoS ONE. 2016;11:e0161401. doi: 10.1371/journal.pone.0161401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verma L., Srivastava S., Negi P.C. A Hybrid Data Mining Model to Predict Coronary Artery Disease Cases Using Non-Invasive Clinical Data. J. Med. Syst. 2016;40:178. doi: 10.1007/s10916-016-0536-z. [DOI] [PubMed] [Google Scholar]
- 55.He M., Lu Y., Zhang L., Zhang H., Gong Y., Li Y. Combining Amplitude Spectrum Area with Previous Shock Information Using Neural Networks Improves Prediction Performance of Defibrillation Outcome for Subsequent Shocks in Out-Of-Hospital Cardiac Arrest Patients. PLoS ONE. 2016;11:e0149115. doi: 10.1371/journal.pone.0149115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennedy C.E., Aoki N., Mariscalco M., Turley J.P. Using Time Series Analysis to Predict Cardiac Arrest in a PICU. Pediatr. Crit. Care Med. 2015;16:e332–e339. doi: 10.1097/PCC.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tylman W., Waszyrowski T., Napieralski A., Kamiński M., Trafidło T., Kulesza Z., Kotas R., Marciniak P., Tomala R., Wenerski M. Real-time prediction of acute cardiovascular events using hardware-implemented Bayesian networks. Comput. Biol. Med. 2016;69:245–253. doi: 10.1016/j.compbiomed.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Wise E.S., Hocking K.M., Brophy C.M. Prediction of in-hospital mortality after ruptured abdominal aortic aneurysm repair using an artificial neural network. J. Vasc. Surg. 2015;62:8–15. doi: 10.1016/j.jvs.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu N., Koh Z.X., Goh J., Lin Z., Haaland B., Ting B.P., Ong M.E.H. Prediction of adverse cardiac events in emergency department patients with chest pain using machine learning for variable selection. BMC Med. Inform. Decis. Mak. 2014;14:75. doi: 10.1186/1472-6947-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ebrahimzadeh E., Pooyan M., Bijar A. A Novel Approach to Predict Sudden Cardiac Death (SCD) Using Nonlinear and Time-Frequency Analyses from HRV Signals. PLoS ONE. 2014;9:e81896. doi: 10.1371/journal.pone.0081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howe A., Escalona O.J., Di Maio R., Massot B., Cromie N.A., Darragh K.M., Adgey J., McEneaney D.J. A support vector machine for predicting defibrillation outcomes from waveform metrics. Resuscitation. 2013;85:343–349. doi: 10.1016/j.resuscitation.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 62.Ong M.E.H., Ng C.H.L., Goh K., Liu N., Koh Z.X., Shahidah N., Zhang T.T., Fook-Chong S., Lin Z. Prediction of cardiac arrest in critically ill patients presenting to the emergency department using a machine learning score incorporating heart rate variability compared with the modified early warning score. Crit. Care. 2012;16:R108. doi: 10.1186/cc11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Z., Yang Z., Lu W., Harrison R.G., Eftestøl T., Steen P.A. A probabilistic neural network as the predictive classifier of out-of-hospital defibrillation outcomes. Resuscitation. 2005;64:31–36. doi: 10.1016/j.resuscitation.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Le Duff F., Muntean C., Cuggia M., Mabo P. Predicting survival causes after out of hospital cardiac arrest using data mining method. Stud. Health Technol. Inform. 2004;107:1256–1259. [PubMed] [Google Scholar]
- 65.Chong C.-F., Li Y.-C., Wang T.-L., Chang H. Stratification of adverse outcomes by preoperative risk factors in coronary artery bypass graft patients: An artificial neural network prediction model. AMIA Annu. Symp. Proc. 2003;2003:160–164. [PMC free article] [PubMed] [Google Scholar]
- 66.Zoni-Berisso M., Molini D., Viani S., Mela G.S., Delfino L. Noninvasive prediction of sudden death and sustained ventricular tachycardia after acute myocardial infarction using a neural network algorithm. Ital. Heart J. 2001;2:612–620. [PubMed] [Google Scholar]
- 67.Turton E., Scott D., Delbridge M., Snowden S., Kester R. Ruptured Abdominal4 Aortic Aneurysm: A Novel Method of Outcome Prediction Using Neural Network Technology. Eur. J. Vasc. Endovasc. Surg. 2000;19:184–189. doi: 10.1053/ejvs.1999.0974. [DOI] [PubMed] [Google Scholar]
- 68.Welch J., Kanter B., Skora B., McCombie S., Henry I., McCombie D., Kennedy R., Soller B. Multi-parameter vital sign database to assist in alarm optimization for general care units. J. Clin. Monit. Comput. 2015;30:895–900. doi: 10.1007/s10877-015-9790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen J., Davis K., Guglielmello G., Stawicki S.P. Vignettes in Patient Safety. Volume 4. IntechOpen; London, UK: 2019. Combating Alarm Fatigue: The Quest for More Accurate and Safer Clinical Monitoring Equipment. [DOI] [Google Scholar]
- 70.Duckitt R.W., Buxton-Thomas R., Walker J., Cheek E., Bewick V., Venn R., Forni L.G. Worthing physiological scoring system: Derivation and validation of a physiological early-warning system for medical admissions. An observational, population-based single-centre study. Br. J. Anaesth. 2007;98:769–774. doi: 10.1093/bja/aem097. [DOI] [PubMed] [Google Scholar]
- 71.Romero-Brufau S., Huddleston J.M., Naessens J.M., Johnson M.G., Hickman J., Morlan B.W., Jensen J.B., Caples S.M., Elmer J.L., Schmidt J.A., et al. Widely used track and trigger scores: Are they ready for automation in practice? Resuscitation. 2014;85:549–552. doi: 10.1016/j.resuscitation.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 72.Barwise A., Thongprayoon C., Gajic O., Jensen J., Herasevich V., Pickering B.W. Delayed rapid response team activation is associated with increased hospital mortality, morbidity, and length of stay in a tertiary care institution. Crit. Care Med. 2016;44:54–63. doi: 10.1097/CCM.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 73.Subbe C.P., Bannard-Smith J., Bunch J., Champunot R., DeVita M.A., Durham L., Edelson D.P., Gonzalez I., Hancock C., Haniffa R., et al. Quality metrics for the evaluation of Rapid Response Systems: Proceedings from the third international consensus conference on Rapid Response Systems. Resuscitation. 2019;141:1–12. doi: 10.1016/j.resuscitation.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 74.Harutyunyan H., Khachatrian H., Kale D.C., Ver Steeg G., Galstyan A. Multitask learning and benchmarking with clinical time series data. Sci. Data. 2019;6:96. doi: 10.1038/s41597-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson A.E., Pollard T.J., Mark R.G. Reproducibility in critical care: A mortality prediction case study; Proceedings of the Machine Learning for Healthcare 2017 Conference; Boston, MA, USA. 18–19 August 2017; pp. 361–376. [Google Scholar]
- 76.Gupta P., Malhotra P., Vig L., Shroff G. Using features from pre-trained timenet for clinical predictions; Proceedings of the 3rd International Workshop on Knowledge Discovery in Healthcare Data at IJCAI; Stockholm, Sweden. 1 January 2018. [Google Scholar]
- 77.Pirracchio R., Petersen M.L., Carone M., Rigon M.R., Chevret S., van der Laan M.J. Mortality prediction in intensive care units with the Super ICU Learner Algorithm (SICULA): A population-based study. Lancet Respir. Med. 2014;3:42–52. doi: 10.1016/S2213-2600(14)70239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamio T., Van T., Masamune K. Use of machine-learning approaches to predict clinical deterioration in critically ill patients: A systematic review. Int. J. Med. Res. Health Sci. 2017;6:1–7. [Google Scholar]
- 79.Kirubarajan A., Taher A., Khan S., Masood S. Artificial intelligence in emergency medicine: A scoping review. J. Am. Coll. Emerg. Physicians Open. 2020;1:1691–1702. doi: 10.1002/emp2.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be shared on request to the corresponding author.