Abstract
Mass vaccination against coronavirus disease 2019 (COVID-19) is a global health strategy to control the COVID-19 pandemic. With the increasing number of vaccinations, COVID-19 vaccine-associated lymphadenopathy (C19-VAL) has been frequently reported. Current findings emphasize the characteristics of C19-VAL. The mechanism of C19-VAL is complicated to explore. Accumulated reports separately show that C19-VAL incidence is associated with receiver age and gender, reactive change within lymph nodes (LN), etc. We constructed a systematic review to evaluate the associated elements of C19-VAL and provide the mechanism of C19-VAL. Articles were searched from PubMed, Web of Science and EMBASE by using the processing of PRISMA. The search terms included combinations of the COVID-19 vaccine, COVID-19 vaccination and lymphadenopathy. Finally, sixty-two articles have been included in this study. Our results show that days post-vaccination and B cell germinal center response are negatively correlated with C19-VAL incidence. The reactive change within LN is highly related to C19-VAL development. The study results suggested that strong vaccine immune response may contribute to the C19-VAL development and perhaps through the B cell germinal center response post vaccination. From the perspective of imaging interpretation, it is important to carefully distinguish reactive lymph nodes from metastatic lymph node enlargement through medical history collection or evaluation, especially in patients with underlying malignancy.
Keywords: COVID-19, C19-VAL, lymphadenopathy, lymph node
1. Introduction
In December 2019, a novel coronavirus causing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first discovered in the city of Wuhan, China. SARS-CoV-2 infected individuals can develop coronavirus disease 2019 (COVID-19), which ranges from mild to fatal symptoms. Up to November 2022, the COVID-19 pandemic caused 641.47 million infected cases and 6.63 million deaths in the entire world [1]. The global vaccination campaign began in December 2020, with the goal to end the COVID-19 pandemic, in which the mRNA vaccines (Pfizer/BioNTech, New York, NY, USA, BNT162b2 and Moderna, Cambridge, MA, USA, mRNA-1273) and viral recombinant vaccine (AstraZeneca, Cambridge, UK, ChAdOx1) are widely used (and a few inactive vaccines were also used) [1]. Although side effects of vaccines are continued to be reported, vaccination is still recommended, since its benefit outweighs the risks of adverse effects [2]. With the administration of vaccines, rare adverse events post COVID-19 vaccinations have possibly increased.
In the clinical trials of COVID-19 vaccination, painful or nonpainful swelling or enlargement of the lymph node is an occasional side effect of vaccination (≤1.1% of incidence). These symptoms belong to COVID-19 vaccine-associated lymphadenopathy (C19-VAL) [3,4,5]. Recently, C19-VAL has been frequently discovered in healthy individuals in the setting of routine mammography [6,7] as well as in cancer patients who are under cancer staging or follow-up [7,8,9]. The C19-VAL incidence in patients who undergo CT scans [10], FDG-PET/CT [11,12,13], breast MRI [14] and ultrasound [15,16] is around 9 to 54%. According to the studies regarding imaging follow up, C19-VAL is a self-limiting disease [17,18,19]. The duration of C19-VAL is up to 10 days and resolved in two months [13,20]. The site of C19-VAL is usually found in the axillary region, then next in the supraclavicular and cervical regions [17,19,21,22,23,24], but which also occurs in malignancy lymphadenopathy. In addition, the features of lymph nodes (such as round, hilum absence, symmetrical cortex) in the patients with C19-VAL mimic that in the patients with the malignancy lymphadenopathy [18,25,26]. However, the studies regarding to C19-VAL focused on characterizing the feature and duration of C19-VAL. The mechanism of C19-VAL is yet to be explored.
VAL has been reported as a rare finding in vaccine administration for human papillomavirus [27,28], influenza [29,30], smallpox [31], measles [32] and tuberculosis [33]. It is due to reactive changes within the lymph node that are separately well documented in vaccinations for human papillomavirus [27] and smallpox [34]. The reactive change was also discovered in the lymph node from C19-VAL, displaying its involvement in the cause of C19-VAL [8,35,36,37]. In addition, other reports show that C19-VAL incidence is associated with age and gender [13,38], even occurring in other adverse vaccinal effects such as Kikuchi-Fujimoto disease [39,40] and autoimmune disease [41]. In this study, we aimed to construct a systematic review that not only analyzed the associated effects in the incidence of C19-VAL, but also explored the mechanism of C19-VAL.
2. Materials and Methods
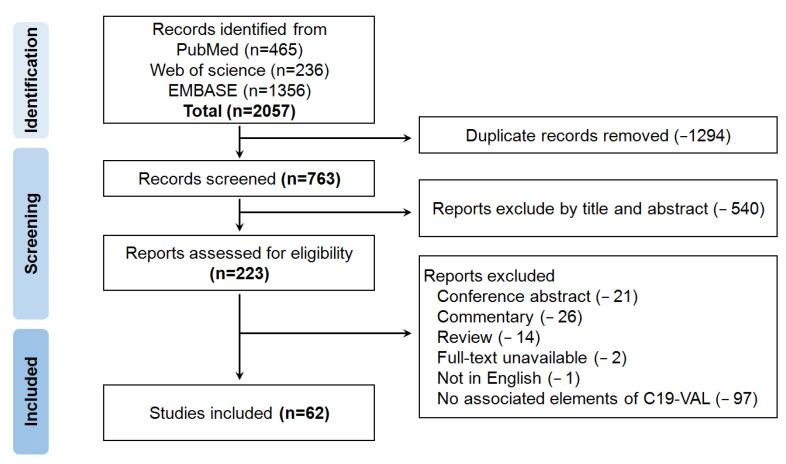
The guidelines of PRISMA are shown in Figure 1. Articles were searched from the database including PubMed, Web of Science and EMBASE from 1 December 2020 to 31 October 2022. The combinations of terms included COVID-19 vaccine, COVID-19 vaccination and lymphadenopathy, which were used to search articles (Appendix A). Duplicate records were removed through Excel screening. This review focuses on clinical findings regarding associated elements of C19-VAL. We only included the patients with sudden swollen or enlargement lymph node post COVID-19 vaccination, regardless of medical history prior or post vaccination. In this study, not only full research articles, but also single patient case reports were included. The irrelevant articles were also removed by title and abstract screening.
Figure 1.
Flow chart of the systematic literature search and screening for studies of C19-VAL.
3. Results
3.1. Studies including and Characterization
The results of the article search and selection were summarized in the PRISMA flowchart (Figure 1). A total of 2057 records were identified from the database, including PubMed, Web of Science and EMBASE. There were 1294 studies removed due to duplicates at the initial screening, and then 540 articles were excluded by title and abstract at stage two screening. The remained 223 studies for screening. A total of 161 relevant references were further assessed but were eliminated if they only included review, conference abstract, commentary, full-text unavailable, not written in English or without associated elements of C19-VAL. Finally, only 62 articles were included in this systematic review and are characterized in Table 1.
Table 1.
Clinical demographics in study for associated elements in the C19-VAL.
| Design | Study | Country | Participants | Age (Years) |
Medical History | Male N (%) |
Vaccine Type | Vaccine Dose | Last Vaccine to C19-VAL (Days)/Site | Main Finding | Elements |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single center reprospective study | Yoshikawa T. (2022) [38] | Japan | 433 | 65 ± 11 | No past and current LAD related disease and COVID-19 | 300 (69.28) | NR | 2 (most) | NR/all for ipsilateral axillary | Incidence of C19-VAL is significantly higher in young age and female | Young age and female |
| Shin M. (2021) [45] | Korea | 31 | 45 ± 5 | No history of malignancy, vaccination before 18F-FDG PET/CT | 11 (35) | AstraZeneca | NR | 4–29/bilateral axillary, supraclavicular | Percentage of C19-VAL is significantly higher in female, FDG-avid deltoid muscle can be a helpful sign to presume the reactive LN | Female and reactive change | |
| Park JY. (2022) [20] | Korea | 413 | 48 ± 12 | No history of malignancy, vaccination within 12 weeks, vaccination before ultrasonography | 10 (2.42) | Moderna (19); AstraZeneca (64); Pfizer-BioNTech (330) |
2 (257) | 1–82/axillary | 48.9% cases with C19-VAL. Incidence of C19-VAL is significantly higher in young age, mRNA vaccine and post 1st dose as well as decreased as days from vaccination |
Young age, mRNA vaccine, 1st dose, and days post-vaccination | |
| Nishino M. (2021) [10] | USA | 232 | 40–96 | All with lung cancer, CT scans prior and post vaccination | 88 (37.9) | Moderna (28); Pfizer-BioNTech (204) | 2 | 7–68/axillary, subpectoral | 9% cases with C19-VAL. Incidence of C19-VAL is significantly higher in female and in Moderna |
Female and Moderna | |
| Cocco G. (2021) [18] | Italy | 24 | 25–74 | Without fever and no history of hematological malignancy, autoimmune disease and vaccination before ultrasonography | 10 (41.6) | Moderna (3); AstraZeneca (8); Pfizer-BioNTech (13) | Least 1 | NR/axillary, supraclavicular | All cases with C19-VAL Percentage of C19-VAL is significantly higher post 1 dose vaccination |
1st dose | |
| El-Sayed MS. (2021) [13] | UK | 204 | 68 ± 11 | Without LAD pathologies, vaccination within 12 weeks and before 18F-FDG PET/CT | 98 (48) | AstraZeneca (43); Pfizer-BioNTech (62); unknown (99) | 2 (87) | Up to 70/axillary | 36% cases with C19-VAL. Incidence of C19-VAL is significantly higher in young age, vector vaccine and female |
Young aged, vector vaccine and female | |
| Ah-Thiane L. (2022) [42] | France | 226 | 67–76 | Most prostate cancer, vaccination before ultrasonography, MRI, or 18F-FDG PET/CT |
212 (93.8) | Moderna (11); AstraZeneca (60); Pfizer-BioNTech (152); Janssen (3) | 2 (124) | 14–51/axillary and supraclavicular | 42.5% cases with C19-VAL. Incidence of C19-VAL was significant higher post the 1st vaccination |
1st dose | |
| Maimone S. (2022) [44] | US | 2304 | 30–92 | Vaccination before screening mammography | NR | Moderna (1109); Pfizer-BioNTech (1135); other (41); unknown (18) | 2 (1883) | 0–28 and >28/ipsilateral axillary | 1% cases with C19-VAL. Incidence of C19-VAL was significantly decreased as days from vaccination |
Days post-vaccination | |
| Eifer M. (2022) [43] | Israel | 426 Immunosuppressive treatment (82), hemato-logical malignancy (75) |
67 ± 12 | Vaccination before PET/CT and without malignancy involving axillary LN | 219 (51) | Pfizer-BioNTech | 2 (103) | 5–18/axillary | Incidence of C19-VAL is significantly higher in young age, 2nd vaccination and increased in days post last vaccination, but lower in immunosuppressive treatment and hematologic malignancy | Young age, 2nd vaccination, days post-vaccination, immunosuppressive, and hematologic malignancy | |
| Cohen D. (2021) [88] | Germany | 137 Recent anti-CD20 (34), no recent anti-CD20 (68) |
>16 | Hematologic malignancy without MHL | 75 (54.7) | NR | Either of 1 or 2 | 6–27/ipsilateral axillary or supraclavicular | Incidence of C19-VAL in recent anti-CD20 vs. no recent anti-CD20 (8.8 vs. 41.1%, significant) | B cell germinal center response | |
| Case series | Fernández-Prada M. (2021) [19] | Spain | 20 | 20–60 | Autoimmune disease (4), thyroid cancer (2) | 0 | Pfizer-BioNTech (19) Moderna (1) | 2 (14) | 0–4/supraclavicular | 12 of 20 patients reported high injection site, biopsy of LN from two type vaccines revealed reactive change | High injected site and reactive change |
| García-Molina F. (2021) [36] | Spain | 6 | 27–62 | NR | NR | Pfizer-BioNTech | 1 | 5/axillary or supraclavicular | Cytological for FNA and biopsy: nonspecific chronic adenitis, resolution of ALAD and SLAD after anti-inflammation drug | Inflammation | |
| Özütemiz C. (2021) [48] | USA | 2 | 62 | Current metastatic breast cancer (1), two cancer history | 1 (50%) | Moderna (1) Pfizer-BioNTech (1) |
3 | 1–2/ipsilateral axillary | Due to imaging and vaccine history, reactive LN | Reactive change | |
| Heaven CL. (2022) [47] | New Zealand | 5 | 41–76 | High suspicion of cancer | 2 (40%) | Pfizer-BioNTech | 2 | 7–34/bilateral cervical | Biopsy: reactive follicular hyperplasia with no evidence of atypia or malignancy | Reactive change | |
| Hagen C. (2021) [49] | Switzerland | 5 | 41–66 | Lung cancer (2), neuroendocrine tumor (1) | 2 (40%) | Pfizer-BioNTech (2) Moderna (3) |
1 (3) | 3–33/bilateral axillary, supraclavicular | FNA: reactive follicular hyperplasia | Reactive change | |
| Brown AH. (2021) [46] | UK | 2 | 48, 67 | Breast cancer for right (1) and for left (1) | 0 | NR | NR | 14, 21/ipsilateral axillary, subpectoral | FNA: reactive change without malignancy | Reactive change | |
| Case report | Goldman S. (2021) [86] | Belgium | 1 | 66 | Hypercholesterolemia, type 2 diabetes, recent cervical lymphadenopathies | 1 | Pfizer-BioNTech | 2 | 150/ipsilateral supraclavicular, cervical, left axillary and abdomen | FNA of CLN: atypical T cell infiltrate with high endothelial venules proliferation; NGS: positive AITL, the number and distribution of LAD increased after 3rd vaccination | AITL |
| Wolfson S. (2022) [50] | USA | 2 | 50, 60 | No (1), simultaneously left ductal carcinoma | 0 | Moderna | 1 | 10, 63/ipsilateral axillary | FNA: begin reactive LN for no medical history, biopsy: metastatic adenocarcinoma for left ductal carcinoma patients | Reactive change | |
| Mizutani M. (2022) [85] | Japan | 2 | 67, 80 | NR | 1 | Pfizer-BioNTech | 2 | 14, 1/left axillary | Persistent ALAD from 1st dose vaccination and gradually enlarged post 2nd dose, finally diagnosed as DLBC by IHC of biopsy | DLBCL | |
| Sekizawa A. (2022) [84] | Japan | 1 | 80 | Hypertension, angina pectoris, mitral valve regurgitation, ovarian tumor | 0 | Pfizer-BioNTech | 2 | 21/ipsilateral temporal cervical, submandibular, and jugular | Persistent ipsilateral temporal after 1st dose vaccination, and sudden enlarged post 2nd dose, finally diagnosed as MZL | MZL | |
| Sasa S. (2022) [87] | Japan | 1 | 80 | Right breast | 0 | Pfizer-BioNTech | 2 | 90/ipsilateral axillary | Multilobulated cystic mass and branches on ultrasonography, finally diagnosed as lymphangioma and resected | Lymphangioma | |
| Saito K. (2022) [41] | Japan | 1 | 66 | Current malaise and oral bleeding and purpura | 0 | Pfizer-BioNTech | 1 | 2/systematic | Low platelet count, markedly increased megakaryocytes in bone marrow, and present of serum anti-glycoprotein IIb/IIIa, finally diagnosed ITP | ITP | |
| Hoffmann C. (2022) [90] | Germany | 1 | 20 | Fever, centigrade, loss of appetite, malaise, weakness, and exertional dyspnea post vaccination | 1 | Pfizer-BioNTech | 2 | 18/supraclavicular, axillary | iMDC post vaccination | iMDC | |
| Girardin FR. (2022) [89] | Switzerland | 1 | 40 | Recent EBV infection | 0 | Moderna | 2 | 1/bilateral axillary and supraclavicular | EBV positive parafollicular immunoblastic cells in LN, induce repeated and extend C19-VAL through enhancing the vaccine immunity | EBV | |
| Cha HG. (2022) [66] | Korea | 1 | 66 | Injection site tenderness and fatigue post 1st dose, acute idiopathic thrombocytopenic purpura | 0 | AstraZeneca | 2 | 3/ipsilateral supraclavicular | Persistent LAD up to 8 weeks, further revealed multiple FDG avid-LNs not limited in supraclavicular, LN biopsy of SLN: chronic granulomatous inflammation, PCR positive for TB | TB | |
| Ganga K. (2021) [24] | USA | 1 | 58 | Hypertension | 1 | Moderna | NR | 2/left neck | FNA for LN of left neck: negative for malignancy and positive for inflammatory cells, improvement and resolution of neck swelling and dysphagia by antibiotic treatment | Inflammation | |
| Cheong KM. (2022) [80] | Taiwan | 1 | 32 | NR | 0 | AstraZeneca | 1 | 2/lower neck | Neck lymphadenitis diagnosed on ultrasonography | Inflammation | |
| Andresciani F. (2022) [79] | Italy | 1 | 62 | Prostate cancer | 1 | Pfizer-BioNTech | 2 | 21/ipsilateral axillary, paratracheal, paraaortic, subcarinal, and bilateral hilar | Choline intensity decreased in LN, finally diagnosed as inflammatory LN, not oncological disease | Inflammation | |
| Tsumura Y. (2022) [83] | Japan | 1 | 31 | Metastatic Ewing sarcoma |
0 | Pfizer-BioNTech | NR | 21/ipsilateral axillary | An inflammatory lesion rather than metastatic lymph node swelling | Inflammation | |
| Tan HM. (2021) [39] | Singapore | 2 | 18, 34 | Current fever | 1 | Pfizer-BioNTech | 1–2 | 17–35/left axillary, supraclavicular, subpectoral | Fever, transient leukopenia, LAD, negative for infection, necrotizing lymphadenitis in LN biopsy, finally diagnosed KD | KD | |
| Caocci G. (2022) [40] | Italy | 1 | 38 | Recent fever for ten day, chills, and fatigue, C19-VAL post 1st dose | 0 | Pfizer-BioNTech | 2 | 31/left axillary | Fever, negative for infection, leukopenia, LAD, and biopsy of LN: histiocytic necrotizing lymphadenitis (numerous CD68+ histiocytes and CD3+ T cells, few CD20+ B cells), finally diagnosed KD | KD | |
| Kashiwada T. (2022) [82] | Japan | 1 | 27 | Recent repeated fever, C19-VAL post 1st dose | 0 | Pfizer-BioNTech | 2 | 68/ipsilateral axillary | Fever, negative for infection, leukopenia, LAD, and necrotizing lymphadenitis in LN biopsy, finally diagnosed KD | KD | |
| Guan YY. (2022) [81] | China | 1 | 36 | Current fever and fatigue | Sinopharm | 1 | 28/left cervical, neck | Fever, LAD and necrotizing lymphadenitis (numerous CD68+ histiocytes and CD3+ T cells, few CD20+ B cells) in LN, finally diagnosed KD | KD | ||
| Xu GY. (2021) [51] |
USA | 1 | 72 | Mantle cell lymphoma | 1 | Pfizer-BioNTech | NR | 2/ipsilateral axillary | With FDG-avid deltoid muscle, reactive LN, recurrent lymphoma | Reactive change | |
| Özütemiz C.(2021) [8] | USA | 2 | 38, 46 | Breast cancer (1) | 0 | Pfizer-BioNTech | 2 (1) | 8–15/ipsilateral axillary, supraclavicular | Biopsy: reactive follicular hyperplasia in lymph node without any evident of breast cancer and malignancy | Reactive change | |
| Nawwar AA. (2021) [52] | UK | 1 | 75 | Prostate cancer | 1 | AstraZeneca | 1 | 3/ipsilateral axillary | With 18F-Choline-avid left deltoid muscle, reactive active LN | Reactive change | |
| Mitchell OR. (2021) [53] | UK | 2 | 47, 55 | NR | 0 | NR | NR | 3/ipsilateral supraclavicular | Reactive LN by clinical and ultrasonographic examination |
Reactive change | |
| Ulaner GA. (2021) [54] | Canada | 1 | 68 | Current right melanoma | 1 | Moderna | 1 | 21/ipsilateral axillary | Unlike metastasis of right melanoma, reactive to vaccination | Reactive change | |
| Wong FC. (2022) [55] | USA | 1 | 74 | Prostate cancer | 1 | Moderna | 2 | 6/ipsilateral axillary | Unlike metastasis of prostate cancer and findings for C19-VAL, considering to reactive to vaccination | Reactive change | |
| Garreffa E. (2021) [56] | UK | 1 | 38 | NR | 0 | Pfizer-BioNTech | 1 | 7/ipsilateral clavicle | Reactive LN by ultrasonographic examination | Reactive change | |
| Prieto PA. (2021) [57] | USA | 1 | 48 | Melanoma | 0 | Moderna | 1 | 5/ipsilateral axillary, neck | Biopsy: consistent with reactive LN and negative of melanoma | Reactive change | |
| Roca B. (2021) [58] | Spain | 1 | 29 | NR | 0 | Pfizer-BioNTech | 1 | 7/ipsilateral supraclavicular | C19-VAL disappeared over the next few weeks | Reactive change | |
| Tan JHN. (2021) [59] | Singapore | 1 | 34 | No malignancy history | 0 | Pfizer-BioNTech | 1 | 1/ipsilateral supraclavicular | FNA: reactive follicular hyperplasia | Reactive change | |
| Gable AD. (2021) [60] | USA | 1 | 24 | Current ED, never smoker, no medical or surgical history, no ED related disease | 1 | NR | 2 | 4/ipsilateral axillary | ED due to typical bronchial carcinoid and LAD significant reduced later | Reactive change | |
| Suleman A. (2021) [61] | Canada | 1 | 38 | Current left Hodgkin lymphoma | 0 | Pfizer-BioNTech | 1 | 7/ipsilateral axillary | Reduced later | Reactive change | |
| Tintle S. (2021) [62] | USA | 1 | 23 | Asthma, eczema, and hypothyroidism, simultaneously fever and acute kidney injury |
0 | Moderna | 2 | 7/left axillary and abdomen | Biopsy of ALN: reactive lymphadenitis |
Reactive change | |
| Weeks JK. (2021) [63] | USA | 1 | 50 | Current sigmoid adenocarcinoma | 0 | Moderna | 2 | 30/bilateral axillary | Improvement of C19-VAL | Reactive change | |
| Mori M. (2022) [64] | Japan | 1 | 30 | NR | 0 | Pfizer-BioNTech | 1 | 9/axillary | Resolution later | Reactive change | |
| Tzankov A. (2021) [65] | Switzerland | 1 | 30 | Current right papillary thyroid cancer | 1 | Moderna | 1 | 21/left axillary | Biopsy: extrafollicular proliferation of B-blasts and resolution later | Reactive change | |
| Chan HP. (2022) [66] | Taiwan | 1 | 71 | Thyroid cancer, right renal cell carcinoma | 1 | Moderna | 1 | 6/ipsilateral axillary | Due to imaging and vaccine history, reactive to vaccination | Reactive change | |
| Adin ME. (2022) [67] | USA | 1 | 41 | Simultaneously right breast cancer | 0 | Moderna | 2 | 16/ipsilateral axillary, | Due to imaging and vaccine history, reactive to vaccination | Reactive change | |
| Kang ES. (2022) [68] | South Korea | 1 | 59 | Simultaneously SCC of the right mandibular gingiva |
1 | Moderna | 2 | 10/ipsilateral axillary, bilateral cervical | FNA: only small lymphoid cells, reactive to vaccination | Reactive change | |
| Yu Q. (2022) [69] |
China | 1 | 34 | Allergic disease, tuberculosis, past malignant tumors, recent infection, trauma | 0 | Sinovac | 2 | 120/ipsilateral axillary | FNA: reactive hyperplasia and resolution later, reactive to vaccination | Reactive change | |
| Ashoor A. (2021) [70] | Italy | 3 | 61–72 | No (2), simultaneously breast cancer (1) | 0 | AstraZeneca | 1 (1), 2 (2) | 1–27/ipsilateral axillary | Imaging is normal and biopsy: benign reactive changes, reactive to vaccination | Reactive change | |
| Lee SM. (2022) [71] |
South Korea | 1 | 21 | NR | 1 | Pfizer-BioNTech | 2 | 2/ipsilateral axillary | Radial neuropathy associated with ipsilateral ALAD, FNA: reactive hyperplasia |
Reactive change | |
| Kado S. (2022) [72] |
Japan | 1 | 31 | NR | 0 | Pfizer-BioNTech | 1 | 8/ipsilateral clavicle, scapular | FNA: follicular hyperplasia and resolution later, reactive to vaccination | Reactive change | |
| Aalberg JJ. (2021) [73] | USA | 1 | 73 | Metastatic renal cell carcinoma to lung and bone | Moderna | 2 | 2/ipsilateral axillary | With FDG avid-ipsilateral deltoid muscle and FNA: polymorphous lymphoid population with no evidence of metastasis | Reactive change | ||
| Cardoso F. (2021) [74] | Portugal | 1 | 48 | Usual contraceptive medication, Mercilon® | 0 | Pfizer-BioNTech | 2 | 1/right cervical | Due to persistent LAD after first dose, FNA: reactive follicular hyperplasia | Reactive change | |
| Lam DL. (2022) [75] | USA | 1 | 39 | Simultaneously right breast cancer | 0 | Pfizer-BioNTech | 2 | 1/ipsilateral axillary | C19-VAL resolution later, FNA for sentinel LN: negative metastasis and consistent with reactive to vaccination | Reactive change | |
| Musaddaq B. (2021) [76] | UK | 1 | 57 | Left breast cancer, simultaneously right breast cancer | 0 | Astra Zeneca | 1 | 3/ipsilateral axillary | IHC: reactive LN with follicular hyperplasia and without metastasis cancer | Reactive change | |
| Dirven I. (2022) [77] | Belgium | 1 | 60 | MEN 1 syndrome and simultaneously right lung nodule | 0 | Pfizer-BioNTech | 1 | 13/ipsilateral axillary | FNA: LN with a benign reactive pattern without metastatic disease | Reactive change | |
| Pudis M. (2021) [78] | Spain | 1 | 30 | Neuroendocrine tumor | 0 | Pfizer-BioNTech | 2 | 40/bilateral axillary, unilateral supraclavicular and cervical | No infection, Biopsy: benign reactive LN with CD10+ B cell population, immune system activation to vaccination | Reactive change |
NR: not reported; LN: lymph node fine needle aspiration; IHC: immunohistochemistry; ALAD: axillary lymphadenopathy; SLAD: supraclavivular lymphadenopathy; LAD: lymphadenopathy; MEN: multiple endocrine neoplasia; MHL: malignant axillary and supraclavicular hypermetabolic lymphadenopathy; ED: episode of hemoptysis; SCC: squamous cell carcinoma; AITL: angioImmunoblastic T cell Lymphoma ; NGS: next generation sequencing; iMDC: idiopathic multicentric castleman Disease; DLBL: large B-cell lymphoma; MZL: marginal zone B-cell lymphoma; HLH: hemophagocytic lymphohistiocytosis; KD: Kikuchi-Fujimoto disease; ITP: immune thrombocytopenia; TB: mycobaterium tuberculosis; EBV: Epstein–Barr virus.
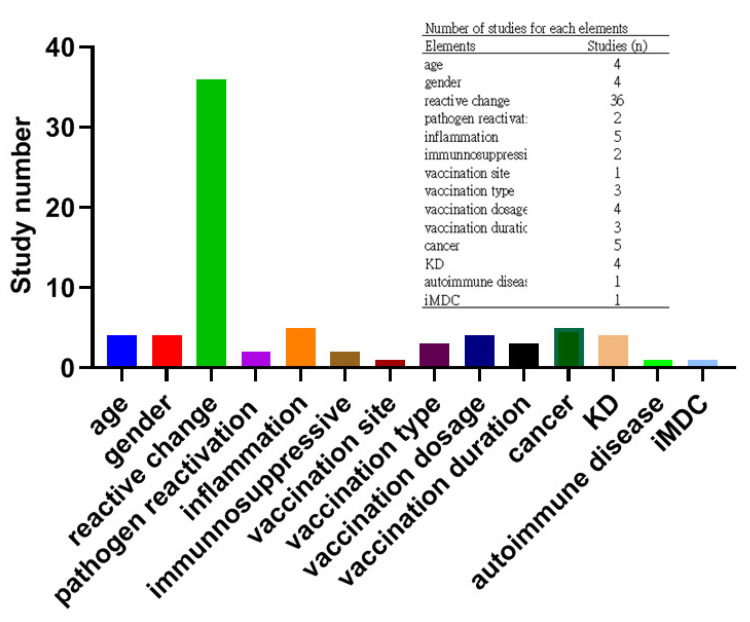
These studies were conducted in 17 countries (France: 1; New Zealand: 1; Portugal: 1; Belgium: 2; Canada: 2; China: 2; Singapore: 2; Taiwan: 2; Germany: 2; Israel: 1; Switzerland: 3; Spain: 4; Italy: 4; Korea: 5; UK: 6; Japan: 9; USA: 15). Types of articles contained single center retrospective studies (n = 10), case series (n = 6) and case reports (n = 46). A total of 14 elements were described which are associated with the C19-VAL occurrence. Those elements included site, type, dosage, days post-vaccination, age, gender, reactive change, cancer, inflammation, Kikuchi-Fujimoto disease (KD), immunosuppression, pathogens, autoimmune disease or idiopathic multicentric Castleman disease (iMDC), of which reactive change was mostly reported (Figure 2).
Figure 2.
Study number regarding each element for C19-VAL. Other vaccine associated adverse effects were indicated as other adverse effects.
3.2. Negative Correlation between Days Post-Vaccination and C19-VAL Incidence
The site, type, dosage and days post-vaccination for C19-VAL incidence was individually evaluated in the eight reports, which included six single-center retrospective studies, one case series and one case report (Table 1, [10,13,18,19,20,42,43,44]). The case series study, which recruited 20 females with C19-VAL in supraclavicular LN, addresses the association between the injection site of the vaccine and C19-VAL incidence [19]. This study indicated that 85% (17/20) of participants with a high injection site had C19-VAL. However, this phenomenon was based on the voluntary feedbacks, and the control group was missing. The effects of the high injection site in C19-VAL occurrence should be further evaluated. Two single-center retrospective studies from the UK and Korea individually evaluated the correlation between the vaccine types and C19-VAL incidence [13,20]. The study in UK recruited 204 participants with malignancy (mean aged 68 ± 11 years) and 51% of participants were vaccinated with AstraZeneca (n = 43) and Pfizer-BioNTech (n = 62). The C19-VAL incidence in AstraZeneca and Pfizer-BioNTech was 48.83 and 25.8%, respectively. This study indicated that the vector vaccine can increase the C19-VAL incidence in cancer patients [13]. The study in Korean analyzed the C19-VAL incidence in healthy participants (mean aged 48 ± 12 years) who received mRNA vaccines (n = 349) and AstraZeneca (n = 64). The C19 VAL incidence in AstraZeneca was 7.8%; significantly lower than that of mRNA vaccines (56%). This study showed that C19-VAL incidence was significantly higher in the healthy population who received the mRNA vaccine [20]. The demographics of these two studies were different in the age and medical history of participants. In addition, there were no other studies to support the individual finding. It is still a question of the influence of vaccine type in the C19-VAL incidence. Similarly, the relevance between vaccine dosage and C19-VAL incidence was not consistent in the four single center retrospective studies due to different demographics such as age and medical history [18,20,42,43]. Most findings indicated that the first dose positively correlates with C19-VAL incidence when healthy and cancer groups were analyzed together [18,20,42]. The linking of days post-vaccination to C19-VAL incidence was addressed in three single center studies from Korea [20], Israel [43] and the US [44]. The study in Korea (n= 413) indicated that C19-VAL incidence in the healthy group was decreased in the following days post-vaccination (incidence of 38% in D1–14, 41% in D15–28 and 21% over D28) [20]. The similar finding was also shown in healthy individuals (n = 2304) in the study from the US (incidence of 2.3% in D1–14, 1.8% in D15–28 and 0.2% over D28) [44]. Contrarily, the study in Israel (n = 75) indicated that C19-VAL incidence in the cancer group was positively correlated with days post-vaccination (OR, 1.53; 95% CI, 1.18–1.99, p = 0.005). The effects of days post-vaccination in C19-VAL incidence in the healthy groups were contrary to the cancer groups [20,43,44]. According to the two studies from Korea [20] and the US [44] (including more than 400 individuals), there is a negative correlation between the days post-vaccination and C19-VAL incidence for the healthy groups.
3.3. Reactive Change and B Cell Germinal Center Related to the C19-VAL Development
In the 62 reports listed in Table 1, there were 53 articles which separately described the role of age, gender, reactive change, inflammation, KD and immunosuppression in C19-VAL (Table 1). The study number regarding each element is shown in Figure 2. Reactive change was most frequently reported. A total of 36 studies including one single-center retrospective study [45], five case series [19,46,47,48,49] and 30 case reports [8,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78] revealed that C19-VAL is due to a reactive change in LN. It is an immune response for other vaccines [27,34]. Pathological characterization shown in the 14 of 36 studies, including two case series [47,49] and 12 case reports [8,59,62,65,68,69,71,72,73,74,76,78], which directly shows the characterization of reactive change, such as reactive lymphadenitis, reactive hyperplasia, reactive follicular hyperplasia, extrafollicular proliferation of B-blasts and small lymphoid cells. Due to a lack of proper controls in those studies, current findings only indicated that reactive change is highly correlated with C19-VAL.
Several reports of case series and case reports indicated the association of inflammation and KD with C19-VAL development [24,36,38,39,40,79,80,81,82,83]. Some of the studies indicated that the inflammation of C19-VAL shows the abundant inflammatory cells in the biopsy [24,36] or high choline uptake in the imaging of LN [79]. Four case reports found C19-VAL with necrotizing lymphadenitis in LN biopsy, which were diagnosed as KD [39,40,81,82]. Those findings were based on studies in small populations without control groups. The real correlation between C19-VAL incidence and inflammation or KD is not clear. Five studies (one single-center retrospective study and four case reports) indicated the effect of cancer for C19-VAL [43,84,85,86,87]. The single-center retrospective study indicated that the C19-VAL incidence was significantly lower in participants with hematological malignancy (incidence, 32%, 24/75) than those of non-hematological malignancy (incidence, 53.6%, 183/341) [43]. Four case reports, involving one to two participants per study, showed that persistent lymphadenopathy occurred after vaccination. Patients were then diagnosed as marginal zone B-cell lymphoma (MZL) (n = 1) [84], large B-cell lymphoma (DLBCL) (n = 2) [85], angioimmunoblastic T cell Lymphoma (AITL) (n = 1) [86] or lymphangioma (n = 1) [87]. These findings suspected that vaccination may induce the hematological malignancy. However, these are uncommon symptoms from a small number of reports. The relationship between hematological malignancy and C19-VAL is uncertain. Since there was no other similar report, it is difficult to define the correlation between hematological malignancy and C19-VAL incidence.
Consistent findings were observed in the roles of age, gender and immunosuppression in the C19-VAL incidence. A total of four single-center retrospective studies from Japan [38], Korea [20], the UK [13] and Israel [43] revealed the C19-VAL incidence is significantly higher in the younger age group. Two of these studies also showed that similar findings in the female group [13,38] were consistent with two other retrospective studies [10,45]. The single-center retrospective study from Israel showed that C19-VAL incidence was significantly lower in patients with immunosuppressive treatment (incidence, 30%, 25/82 and 45.09%, 170/377 without treatment) [43]. Another single-center retrospective study from Germany further found that the C19-VAL incidence in recent anti-CD20 treatment for individuals with hematological malignancy is significantly lower than that without recent anti-CD20 treatment (8.8 and 41.4%, respectively) [88]. These findings directly indicated that B cell germinal center responses are positively correlated with C19-VAL incidence. Four case reports showed that pathogen reactivation (TB (n = 1) [66] and EBV(n = 1) [89]), iMDC (n = 1) [90] and autoimmune disease (ITP) (n = 1) [41] are correlated with C19-VAL development. Those findings indicated that reactive change is also related to C19-VAL development. The pathological findings revealed that immune cell activation and proliferation are involved in the reactive change within LN [8,47,49,59,62,65,68,69,71,72,73,74,76,78]. Of these, the B cell germinal center response plays important roles in C19-VAL incidence based on results with anti-CD20 treatment [88].
4. Discussion
The study of side effects due to C19-VAL has been widely reported over the past two years. Several systematic reviews and meta-analyses have been published [90,91,92,93,94]. Those studies have investigated that the incidence [90,91,92,93], features [91,92], pathological findings [93], patient management [94] of C19-VAL. The object of this study is to evaluate the associated elements of C19-VAL and to provide the mechanism of C19-VAL. The study results found three elements are highly correlated with C19-VAL development or incidence. These elements are days post-vaccination, reactive change and B cell germinal center response. Due to the lack of control groups, current findings only suggest that reactive change is highly related to C19-VAL development. The remaining two elements (days post-vaccination and B cell germinal center response) have a negative effect on C19-VAL incidence.
Reactive change is a cause element for C19-VAL, which has been demonstrated in vaccinations for human papillomavirus [31] and smallpox [38]. Several pathological studies in this systematic review indicate that immune cell activation and proliferation are involved in the reactive change of LN [8,47,49,59,62,65,68,69,71,72,73,74,76,78]. Reactive change is a vaccine induced immune response [8]. The antigen processing and presentation between mRNA vaccine and adenovirus vector vaccine is different. The antigen-specific antibody and T cell responses of the mRNA vaccine were stronger than those of the adenovirus vector vaccine [95]. The fragmental mode of COVID-19 vaccine induces an immune response against SARS-CoV2 by stimulating adaptive immunity through cross-linking to the innate immune response of dendritic cells [96]. Initially, the locally activated antigen accumulates in the injection site. Subsequently, the antigen is processed by the dendritic cells through different innate receptors (TLR7 and MAD5 for mRNA vaccine; TLR9 for adenovirus vector vaccine). Then, activated dendritic cells migrate to regional lymph nodes. Lastly, dendritic cells present the antigen to the T cells, which then promote antibody production via the plasma B cells. As a result, large amounts of T cells and B cells exist in the LN. The lymphadenopathy immediately occurs in the LN after reactive hyperplasia (rapid extension) of T and B cells [97], which is a possible mechanism of C19-VAL. It also explains that the days of post-vaccination is a negative effect of C19-VAL incidence, due to the reduction of reactive hyperplasia in the following days post-vaccination.
Anti-CD20 treatment is a B cell-depleting therapy to cure hematological malignancy. A previous study has demonstrated that patients who received anti-CD20 treatment present a low vaccine-induced protection against influenza A (H1N1) [98]. The cohort and observation studies showed that the anti-CD20 treatment can reduce the reactogenicity of mRNA vaccine [99,100,101] and AstraZeneca [101] resulting in low production of specific anti-SARS-CoV2 antibody. To our knowledge, the correlation between anti-CD20 treatment and C19-VAL is unclear. It was first found that B cell germinal center response is a negative element of C19-VAL incidence. B cell germinal center response in the lymph node contributes to the capability of cytotoxic T cells to kill infected cells and formation of antibody-secreting plasma B cells [102]. Recent studies show that the COVID-19 vaccines elicit a prominent B cell germinal center response in LN [103,104]. It reflects that there is a strong B cell germinal center response, which contributes to C19-VAL incidence.
Previous study results have also shown that the C19-VAL incidence is increased in younger ages and females. It was comparable with the previous study for female groups [10,105], which also found that this side effect was more common in women [106,107]. This might be because of results from the sex hormone-mediated immune response [108]. The association between aging and vaccine-induced protection shows that primary and secondary antibody responses to vaccination are impaired in the elderly [109]. Therefore, the strong vaccine-induced immune response may cause the C19-VAL. Recently, data from COVID-19 vaccination showed that the COVID-19 vaccine presents a high immunogenic response to induce T and B cell function [103,110]. There are several limitations in this systematic review. For example, the real correlation between reactive change and C19-VAL vaccination cannot be recognized because the control groups are missing. Additionally, those results may not reflect the fact that the B cell geminal center response also plays a negative role in C19-VAL in healthy receivers or patients with other solid tumors. Further investigation into the correlation between days post-vaccination and C19-VAL development for cancer patients is needed, due to small number of participants. KD is a necrotizing lymphadenitis characterized by lymphadenopathy and fever with leukopenia, thrombocytopenia and liver dysfunction [111]. As this is a small population study without a control group, the linkage of C19-VAL to KD needs to be assessed. Overall, those study results suggest that strong vaccine-induce immune response may contribute to C19-VAL development and incidence. This could be through the B cell germinal center response post vaccination.
C19-VAL has caused a dilemma in diagnosis and patient management, especially in oncological patients [8,21]. It may lead to unnecessary biopsies and changes in therapy [8,108], which increase the psychological and medical burden of patients and the risk of those having inherent diseases. To avoid the above issues, current recommendations suggest that screening exams can be scheduled before the first dose or 4–12 weeks after the second dose of the COVID-19 vaccine [11]. For a booster dose, the exam should be provided at least 6 weeks post vaccination [112,113]. Based on the results of the literature review, it currently shows that C19-VAL may be caused by a strong vaccine-induced immune response. In the future, additional studies are needed with a better and more comprehensive study design to draw more tangible conclusions.
5. Conclusions
With mass COVID-19 vaccination, C19-VAL will be observed more frequently; more patients and clinicians will encounter medical dilemmas from C19-VAL. However, the vaccine benefits exceed the medical dilemmas of C19-VAL. C19-VAL is a common side effect and is recognized following the mass use of vaccines. The cause of C19-VAL is mostly considered as a reactive change for vaccination. The reactive change is an activation of immune cells by the vaccine to lymph nodes. Such effects can diminish with time post vaccination. It was further found that the B cell germinal center may contribute to C19-VAL. Although the B cell germinal center response may be a contributing factor to C19-VAL, global vaccination is one approach to end the COVID-19 epidemic. For now, it is not suggested to decline vaccination due to the cause of C19-VAL. From the perspectives of image interpretation, the positive finding of axillary C19-VAL reminds physicians that careful differentiation of reactive lymph nodes from metastatic lymphadenopathies via either the medical history and records or medical chart review, especially for the patients with underlying malignances, is important. Therefore, we suggest a follow-up imaging exam should be processed for the size and number of C19-VAL within two months of onset. If the C19-VAL is not resolved within this period, a biopsy examination should be suggested. In the future, additional studies are needed with a better and more comprehensive study design to draw the role of the B cell germinal center in C19-VAL. This will assist in reducing C19-VAL incidence post-vaccination.
Acknowledgments
The authors thank S. Sheldon MT (ASCP, Retired) of Oklahoma University Medical Center Edmond for fruitful discussions and editorial assistance.
Appendix A. Search Strategy
-
■
Database:
PubMed
EMBASE
Web of Science
-
■
Last search date: 6 September. 2022
-
■
Year cover of search: 1 December. 2020–31 October. 2022
-
■
Search terms:
#1: COVID-19 vaccine lymphadenopathy
#2: COVID-19 vaccination lymphadenopathy
The two group were invidually searched cross three database in the all filed.
-
■
PubMed (n = 465)
#1: ((“COVID-19 vaccines”[MeSH Terms] OR (“COVID-19”[All Fields] AND “vaccines”[All Fields]) OR “COVID-19 vaccines”[All Fields] OR “COVID-19 vaccine”[All Fields]) AND (“lymphadenopathy”[MeSH Terms] OR “lymphadenopathy”[All Fields] OR “lymphadenopathies”[All Fields])) AND (2020/12/1:2022/10/31[pdat])
#2: ((“COVID-19”[All Fields] OR “COVID-19”[MeSH Terms] OR “COVID-19 vaccines”[All Fields] OR “COVID-19 vaccines”[MeSH Terms] OR “COVID-19 serotherapy”[All Fields] OR “COVID-19 nucleic acid testing”[All Fields] OR “COVID-19 nucleic acid testing”[MeSH Terms] OR “COVID-19 serological testing”[All Fields] OR “COVID-19 serological testing”[MeSH Terms] OR “COVID-19 testing”[All Fields] OR “COVID-19 testing”[MeSH Terms] OR “sars cov 2”[All Fields] OR “sars cov 2”[MeSH Terms] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “ncov”[All Fields] OR “2019 ncov”[All Fields] OR ((“coronavirus”[MeSH Terms] OR “coronavirus”[All Fields] OR “cov”[All Fields]) AND 2019/11/01:3000/12/31[Date-Publication])) AND (“vaccin”[Supplementary Concept] OR “vaccin”[All Fields] OR “vaccination”[MeSH Terms] OR “vaccination”[All Fields] OR “vaccinable”[All Fields] OR “vaccinal”[All Fields] OR “vaccinate”[All Fields] OR “vaccinated”[All Fields] OR “vaccinates”[All Fields] OR “vaccinating”[All Fields] OR “vaccinations”[All Fields] OR “vaccination s”[All Fields] OR “vaccinator”[All Fields] OR “vaccinators”[All Fields] OR “vaccine s”[All Fields] OR “vaccined”[All Fields] OR “vaccines”[MeSH Terms] OR “vaccines”[All Fields] OR “vaccine”[All Fields] OR “vaccins”[All Fields]) AND (“lymphadenopathy”[MeSH Terms] OR “lymphadenopathy”[All Fields] OR “lymphadenopathies”[All Fields])) AND (2020/12/1:2022/10/31[pdat])
-
■
EMBASE (n = 1356)
#1: (‘COVID-19 vaccine lymphadenopathy’ OR ((‘COVID-19’/exp OR ‘COVID-19’) AND (‘vaccine’/exp OR vaccine) AND (‘lymphadenopathy’/exp OR lymphadenopathy))) AND [2020-2022]/py AND [01-12-2020]/sd NOT [01-01-2023]/sd
#2: (‘COVID-19 vaccination lymphadenopathy’ OR ((‘COVID-19’/exp OR ‘COVID-19’) AND (‘vaccination’/exp OR vaccination) AND (‘lymphadenopathy’/exp OR lymphadenopathy))) AND [2020-2022]/py AND [01-12-2020]/sd NOT [01-01-2023]/sd
-
■
Web of Science (n = 236)
#1: ALL = (COVID-19 vaccine lymphadenopathy)
#2: ALL = (COVID-19 vaccination lymphadenopathy)
Author Contributions
T.-C.H. and C.-N.C. collected reference and meta-analysis data. D.H.-Y.S., C.-H.Y. and K.-P.C. contributed scientific advice during revision. H.-P.C. and C.-C.C. were involved in the conception of the idea and interpretation. T.-C.H., M.-H.Y. and Y.-C.T. drafted the work, prepared the manuscript and provided editorial assistance before submission. Y.-C.T. contributed to revisions and finalized the revised manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the following research grants: MOST 109-2221-E-037-001-MY3 and MOST 111-2314-B-075B-006 from the Ministry of Science and Technology; KMUH110-0T02 from Kaohsiung Medical University; NPUST-KMU-111-P002 from NPUST-KMU JOINT RESEARCH PROJECT; and the Research Center for Precision Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mathieu E., Ritchie H., Rodés-Guirao L., Appel C., Giattino C., Hasell J., Macdonald B., Dattani S., Beltekian D., Ortiz-Ospina E., et al. Coronavirus Pandemic (COVID-19). OurWorldInData.org. 2020. [(accessed on 30 November 2022)]. Available online: https://ourworldindata.org/coronavirus.
- 2.Centers for Disease Control and Prevention Benefits of Getting A COVID-19 Vaccine. [(accessed on 30 November 2022)];2022 Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html.
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Pfizer-BioNTech COVID-19 Vaccine Reactions and Adverse Events. [(accessed on 30 November 2022)];2021 Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.
- 5.Centers for Disease Control and Prevention The Moderna COVID-19 Vaccine’s Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events. [(accessed on 30 November 2022)];2022 Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/Reactogenicity.html.
- 6.Mortazavi S. COVID-19 Vaccination–Associated Axillary Adenopathy: Imaging Findings and Follow-Up Recommendations in 23 Women. Am. J. Roentgenol. 2021;217:857–858. doi: 10.2214/AJR.21.25651. [DOI] [PubMed] [Google Scholar]
- 7.Mehta N., Sales R.M., Babagbemi K., Levy A.D., McGrath A.L., Drotman M., Dodelzon K. Unilateral axillary adenopathy in the setting of COVID-19 vaccine: Follow-up. Clin. Imaging. 2021;80:83–87. doi: 10.1016/j.clinimag.2021.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zütemiz C., Krystosek L.A., Church A.L., Chauhan A., Ellermann J.M., Domingo-Musibay E., Steinberger D. Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients. Radiology. 2021;300:E296–E300. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avner M., Orevi M., Caplan N., Popovtzer A., Lotem M., Cohen J.E. COVID-19 vaccine as a cause for unilateral lymphadenopathy detected by 18F-FDG PET/CT in a patient affected by melanoma. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:2659–2660. doi: 10.1007/s00259-021-05278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino M., Hatabu H., Ricciuti B., Vaz V., Michael K., Awad M.M. Axillary Lymphadenopathy after Coronavirus Disease 2019 Vaccinations in Patients with Thoracic Malignancy: Incidence, Predisposing Factors, and Imaging Characteristics. J. Thorac. Oncol. 2022;17:154–159. doi: 10.1016/j.jtho.2021.08.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm L., Destounits S., Dogan B., Daly C., Tuite C., Sonnenblick E., Milch H., Net J., Dodelzon K., Yang R., et al. Revised SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination. Society of Breast Imaging Patient Care and Delivery Committee. 2022. [(accessed on 30 November 2022)]. Available online: https://www.sbi-online.org/sbi-recommendations-position-statements.
- 12.World Health Organization (WHO) WHO Coronavirus (COVID-19) Dashboard. [(accessed on 30 November 2022)]. Available online: https://covid19.who.int/
- 13.El-Sayed M.S., Wechie G.N., Low C.S., Adesanya O., Rao N., Leung V.J. The incidence and duration of COVID-19 vaccine-related reactive lymphadenopathy on 18F-FDG PET-CT. Clin. Med. 2021;21:e633–e638. doi: 10.7861/clinmed.2021-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edmonds C.E., Zuckerman S.P., Conant E.F. Management of Unilateral Axillary Lymphadenopathy Detected on Breast MRI in the Era of COVID-19 Vaccination. Am. J. Roentgenol. 2021;217:831–834. doi: 10.2214/AJR.21.25604. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell O.R., Couzins M., Dave R., Bekker J., Brennan P.A. COVID-19 vaccination and low cervical lymphadenopathy in the two week neck lump clinic-a follow up audit. Br. J. Oral Maxillofac. Surg. 2021;59:720–721. doi: 10.1016/j.bjoms.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abou-Foul A.K., Ross E., Abou-Foul M., George A.P. Cervical lymphadenopathy following coronavirus disease 2019 vaccine: Clinical characteristics and implications for head and neck cancer services. J. Laryngol. Otol. 2021;135:1025–1030. doi: 10.1017/S0022215121002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igual-Rouilleault A.C., Soriano I., Quan P.L., Fernández-Montero A., Elizalde A., Pina L. Unilateral axillary adenopathy induced by COVID-19 vaccine: US follow-up evaluation. Eur. Radiol. 2022;32:3199–3206. doi: 10.1007/s00330-021-08309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cocco G., Pizzi A.D., Fabiani S., Cocco N., Boccatonda A., Frisone A., Scarano A., Schiavone C. Lymphadenopathy after the Anti-COVID-19 Vaccine: Multiparametric Ultrasound Findings. Biology. 2021;10:652. doi: 10.3390/biology10070652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-Prada M., Rivero-Calle I., Calvache-González A., Martinón-Torres F. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Euro. Surveill. 2021;26:2100193. doi: 10.2807/1560-7917.ES.2021.26.10.2100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J.Y., Lee J.Y., Yi S.Y. Axillary Lymphadenopathy on Ultrasound after COVID-19 Vaccination and Its Influencing Factors: A Single-Center Study. J. Clin. Med. 2022;11:238. doi: 10.3390/jcm11010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta N., Sales R.M., Babagbemi K., Levy A.D., McGrath A.L., Drotman M., Dodelzon K. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin. Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adin M.E., Isufi E., Kulon M., Pucar D. Association of COVID-19 mRNA Vaccine with Ipsilateral Axillary Lymph Node Reactivity on Imaging. JAMA Oncol. 2021;7:1241. doi: 10.1001/jamaoncol.2021.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn R.W., Mootz A.R., Brewington C.C., Abbara S. Axillary Lymphadenopathy after mRNA COVID-19 Vaccination. Radiol. Cardiothorac. Imaging. 2021;3:e210008. doi: 10.1148/ryct.2021210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganga K., Solyar A.Y., Ganga R. Massive Cervical Lymphadenopathy Post-COVID-19 Vaccination. Ear Nose Throat J. 2021 doi: 10.1177/01455613211048984. [DOI] [PubMed] [Google Scholar]
- 25.Cocco G., Pizzi A.D., Taraschi A.L., Boccatonda A., Corvino A., Ucciferri C., Falasca K., Caulo M., Vecchiet J. Atypical Sites of Lymphadenopathy after Anti-COVID-19 Vaccine: Ultrasound Features. Medicina. 2022;58:197. doi: 10.3390/medicina58020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang W., Jia W., Shi J., Yuan C., Zhang Y., Chen M. Role of Elastography in Axillary Examination of Patients with Breast Cancer. J. Ultrasound Med. 2018;37:699–707. doi: 10.1002/jum.14538. [DOI] [PubMed] [Google Scholar]
- 27.Studdiford J., Lamb K., Horvath K., Altshuler M., Stonehouse A. Development of Unilateral Cervical and Supraclavicular Lymphadenopathy after Human Papilloma Virus Vaccination. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2008;28:1194–1197. doi: 10.1592/phco.28.9.1194. [DOI] [PubMed] [Google Scholar]
- 28.Pereira M.P., Flores P., Neto A.S. Neck and supraclavicular lymphadenopathy secondary to 9-valent human papillomavirus vaccination. BMJ Case Rep. 2019;12:e231582. doi: 10.1136/bcr-2019-231582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirone N., Shinkai T., Yamane T., Uto F., Yoshimura H., Tamai H., Imai T., Inoue M., Kitano S., Kichikawa K., et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann. Nucl. Med. 2012;26:248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 30.Panagiotidis E., Exarhos D., Housianakou I., Bournazos A., Datseris I. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1) Eur. Radiol. 2010;20:1251–1253. doi: 10.1007/s00330-010-1719-5. [DOI] [PubMed] [Google Scholar]
- 31.Casey C.G., Iskander J.K., Roper M.H., Mast E.E., Wen X.J., Török T.J., Chapman L.E., Swerdlow D.L., Morgan J., Hef-felfinger J.D., et al. Adverse events associated with smallpox vaccination in the United States, January–October 2003. JAMA. 2005;294:2734–2743. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 32.Dorfman R.F., Herweg J.C. Live, attenuated measles virus vaccine. Inguinal lymphadenopathy complicating administration. JAMA. 1966;198:320–321. doi: 10.1001/jama.1966.03110160148051. [DOI] [PubMed] [Google Scholar]
- 33.Goraya J.S., Virdi V.S. Bacille Calmette-Guérin lymphadenitis. Postgrad. Med. J. 2002;78:327–329. doi: 10.1136/pmj.78.920.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartsock R.J. Postvaccinial lymphadenitis. Hyperplasia of lymphoid tissue that simulates malignant lymphomas. Cancer. 1968;21:632–649. doi: 10.1002/1097-0142(196804)21:4<632::AID-CNCR2820210415>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 35.De Giorgis S., Garlaschi A., Brunetti N., Tosto S., Rescinito G., Monetti F., Oddone C., Massa B., Pitto F., Calabrese M., et al. Axillary adenopathy after COVID-19 vaccine in patients undergoing breast ultrasound. J. Ultrason. 2021;21:361–364. doi: 10.15557/JoU.2021.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Molina F., Cegarra-Navarro M.F., Andrade-Gonzales R.J., Martinez-Díaz F. Cytologic and histologic features of COVID-19 post-vaccination lymphadenopathy. Cytojournal. 2021;18:34. doi: 10.25259/Cytojournal_21_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teefey S.A., Middleton W.D., Turner J.S., Ellebedy A.H., Suessen T., Wallendorf M., O’Halloran J.A., Presti R. SARS-CoV-2 mRNA Vaccination Causes Prolonged Increased Cortical Thickening and Vascularity in Ipsilateral Axillary Lymph Nodes. J. Ultrasound Med. 2022;41:2849–2858. doi: 10.1002/jum.15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshikawa T., Miki S., Nakao T., Koshino S., Hayashi N., Abe O. Axillary Lymphadenopathy after Pfizer-BioNTech and Moderna COVID-19 Vaccination: MRI Evaluation. Radiology. 2022;306:270–278. doi: 10.1148/radiol.220814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan H.M., Hue S.S.-S., Wee A., See K.C. Kikuchi–Fujimoto Disease Post COVID-19 Vaccination: Case Report and Review of Literature. Vaccines. 2021;9:1251. doi: 10.3390/vaccines9111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caocci G., Fanni D., Porru M., Greco M., Nemolato S., Firinu D., Faa G., Scuteri A., La Nasa G. Kikuchi-Fujimoto disease associated with hemophagocytic lymphohistiocytosis following the BNT162b2 mRNA COVID-19 vaccination. Haematologica. 2022;107:1222–1225. doi: 10.3324/haematol.2021.280239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito K., Ichikawa S., Hatta S., Katsuoka Y., Harigae H., Izumi T. Vincristine therapy for severe and refractory immune thrombocytopenia following COVID-19 vaccination. Ann. Hematol. 2022;101:885–887. doi: 10.1007/s00277-021-04666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ah-Thiane L., Ferrer L., Maucherat B., Fleury V., Le Thiec M., Rusu D., Rousseau C. Vaccine-Related Lymph Nodes: The Emerging Pitfalls of 18F-Fluorocholine and 68Ga-PSMA-11 PET/CT in the Era of COVID-19 Vaccination. Clin. Nucl. Med. 2022;47:575–582. doi: 10.1097/RLU.0000000000004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eifer M., Tau N., Alhoubani Y., Kanana N., Domachevsky L., Shams J., Keret N., Gorfine M., Eshet Y. COVID-19 mRNA Vaccination: Age and Immune Status and Its Association with Axillary Lymph Node PET/CT Uptake. J. Nucl. Med. 2022;63:134–139. doi: 10.2967/jnumed.121.262194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maimone S., Robinson K.A., Advani P.P., Li Z., Gococo-Benore D.A., Qosja N., Ashai A.M., Mummareddy A., Chumsri S. Limiting Screening Mammography Recalls for Vaccine-Induced Adenopathy, a Single Institution Experience. Acad. Radiol. 2022;29:1480–1485. doi: 10.1016/j.acra.2021.12.028. [DOI] [PubMed] [Google Scholar]
- 45.Shin M., Hyun C.Y., Choi Y.H., Choi J.Y., Lee K.H., Cho Y.S. COVID-19 Vaccination-Associated Lymphadenopathy on FDG PET/CT: Distinctive Features in Adenovirus-Vectored Vaccine. Clin. Nucl. Med. 2021;46:814–819. doi: 10.1097/RLU.0000000000003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown A.H., Shah S., Groves A.M., Wan S., Malhotra A. The Challenge of Staging Breast Cancer with PET/CT in the Era of COVID Vaccination. Clin. Nucl. Med. 2021;46:1006–1010. doi: 10.1097/RLU.0000000000003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heaven C.L., Barber L., Ahmadi O., Selvarajah K., Shetty S. COVID-19 vaccine associated cervical lymphadenopathy: A case series. ANZ J. Surg. 2022;92:2286–2291. doi: 10.1111/ans.17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Özütemiz C., Potter D.A., Özütemiz A., Steinberger D. Lymphadenopathy after the Third Covid-19 Vaccine. Curr. Probl. Cancer Case Rep. 2021;4:100127. doi: 10.1016/j.cpccr.2021.100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hagen C., Nowack M., Messerli M., Saro F., Mangold F., Bode P.K. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med. Wkly. 2021;151:w20557. doi: 10.4414/smw.2021.20557. [DOI] [PubMed] [Google Scholar]
- 50.Wolfson S., Kim E. Breast Cancer Screening and Axillary Adenopathy in the Era of COVID-19 Vaccination. Radiology. 2023;306:222040. doi: 10.1148/radiol.222040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu G.M., Lu Y.M. COVID-19 mRNA Vaccination–Induced Lymphadenopathy Mimics Lymphoma Progression on FDG PET/CT. Clin. Nucl. Med. 2021;46:353–354. doi: 10.1097/RLU.0000000000003597. [DOI] [PubMed] [Google Scholar]
- 52.Nawwar A.A., Searle J., Singh R., Lyburn I.D. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F]Choline PET/CT-not only an FDG finding. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:2657–2658. doi: 10.1007/s00259-021-05279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell O.R., Dave R., Bekker J., Brennan P.A. Supraclavicular lymphadenopathy following COVID-19 vaccination: An increasing presentation to the two-week wait neck lump clinic? Br. J. Oral Maxillofac. Surg. 2021;59:384–385. doi: 10.1016/j.bjoms.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulaner G.A., Giuliano P. 18F-FDG–Avid Lymph Nodes after COVID-19 Vaccination on 18F-FDG PET/CT. Clin. Nucl. Med. 2021;46:433–434. doi: 10.1097/RLU.0000000000003633. [DOI] [PubMed] [Google Scholar]
- 55.Wong F.C., Martiniova L., Masrani A., Ravizzini G.C. 18F-Fluciclovine–Avid Reactive Axillary Lymph Nodes After COVID-19 Vaccination. Clin. Nucl. Med. 2022;47:154–155. doi: 10.1097/RLU.0000000000003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garreffa E., York J., Turnbull A., Kendrick D. Regional lymphadenopathy following COVID-19 vaccination: Considerations for primary care management. Br. J. Gen. Pract. 2021;71:284–285. doi: 10.3399/bjgp21X716117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prieto P.A., Mannava K., Sahasrabudhe D.M. COVID-19 mRNA vaccine-related adenopathy mimicking metastatic mela-noma. Lancet Oncol. 2021;22:e281. doi: 10.1016/S1470-2045(21)00197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roca M.M., Roca B., Rambla M. Supraclavicular lymphadenopathy secondary to COVID-19 BNT162b2 vaccine. J. Postgrad. Med. 2021;67:180–181. doi: 10.4103/jpgm.JPGM_254_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan N.J.H., Tay K.X.J., Wong S.B.J., Nga M.E. COVID-19 post-vaccination lymphadenopathy: Report of cytological findings from fine needle aspiration biopsy. Diagn. Cytopathol. 2021;49:E467–E470. doi: 10.1002/dc.24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gable A.D., Hughes S.M., Miller R.J. A 24-Year-Old Man with Hemoptysis Found to Have a Chest Mass and Contralateral Axillary Lymphadenopathy. Chest. 2021;160:e289–e293. doi: 10.1016/j.chest.2021.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suleman A., Bilbily A., Cheung M., Chodirker L. Hypermetabolic lymphadenopathy on positron emission tomography scan following COVID-19 vaccination: A mimicker of disease progression in Hodgkin lymphoma. Ejhaem. 2021;2:678–679. doi: 10.1002/jha2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tintle S., Chen M. Lymphadenopathy with florid lymphoid and Langerhans cell hyperplasia and hemophagocytosis mimicking lymphoma after COVID-19 mRNA vaccination. EJHaem. 2021;2:845–847. doi: 10.1002/jha2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weeks J.K., O’Brien S.R., Rosenspire K.C., Dubroff J.G., Pantel A.R. Evolving Bilateral Hypermetabolic Axillary Lymphadenopathy on FDG PET/CT Following 2-Dose COVID-19 Vaccination. Clin. Nucl. Med. 2021;46:1011–1012. doi: 10.1097/RLU.0000000000003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mori M., Fujioka T., Yashima Y., Yamaga E., Nakagawa T., Kubota K., Tateishi U. Deep axillary lymphadenopathy after coronavirus disease 2019 vaccination: A case report. J. Med. Ultrason. 2022;49:111–112. doi: 10.1007/s10396-021-01149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tzankov A., Rössle M. Extrafollicular proliferation of B-blasts: Morphologic correlate to Spikevax-induced lymphadenopathy. Clin. Case Rep. 2022;10:e5398. doi: 10.1002/ccr3.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan H.-P.M., Shen D.H.-Y.M., Yang M.-H., Hu C., Tyan Y.-C. Incidental Finding of Moderna COVID-19 Vaccination–Related Axillary Lymphadenopathy on 201Tl Myocardial Perfusion Imaging. Clin. Nucl. Med. 2022;47:e302–e303. doi: 10.1097/RLU.0000000000003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adin M.E., Wu J., Isufi E., Tsui E., Pucar D. Ipsilateral Malignant Axillary Lymphadenopathy and Contralateral Reactive Lymph Nodes in a COVID-19 Vaccine Recipient with Breast Cancer. J. Breast Cancer. 2022;25:140–144. doi: 10.4048/jbc.2022.25.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang E.S., Kim M.Y. Bilateral Cervical Lymphadenopathy after mRNA COVID-19 Vaccination on Oral Squamous Cell Car-cinoma Patient: A Case Report. Diagnostics. 2022;12:1518. doi: 10.3390/diagnostics12071518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu Q., Jiang W., Chen N., Li J., Wang X., Li M., Wang D., Jiang L. Misdiagnosis of Reactive Lymphadenopathy Remotely after COVID-19 Vaccination: A Case Report and Literature Review. Front. Immunol. 2022;13:875637. doi: 10.3389/fimmu.2022.875637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashoor A., Shephard J., Lissidini G., Nicosia L. Axillary Adenopathy in Patients with Recent Covid-19 Vaccination: A New Diagnostic Dilemma. Korean J. Radiol. 2021;22:2124–2126. doi: 10.3348/kjr.2021.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee S.M., Hong J.Y., Kim S.Y., Na S.J. Ipsilateral Radial Neuropathy after COVID-19 mRNA Vaccination in an Immuno-competent Young Man. Yonsei Med. J. 2022;63:966–970. doi: 10.3349/ymj.2022.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kado S., Kamiya K., Iwabuchi S., Kajii E., Ohtsuki M. Unilateral lymphadenopathy associated with COVID-19 vaccination. J. Cutan. Immunol. Allergy. 2021;5:100–101. doi: 10.1002/cia2.12217. [DOI] [Google Scholar]
- 73.Aalberg J.J., Collins T.P., Dobrow E.M. Axillary lymphadenopathy in a renal cell carcinoma patient after COVID-19 Vaccination. Radiol. Case Rep. 2021;16:2164–2167. doi: 10.1016/j.radcr.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cardoso F., Reis A., Osório C., Scigliano H., Nora M. A Case of Cervical Lymphadenopathy after Vaccination against COVID-19. Cureus. 2021;13:e15050. doi: 10.7759/cureus.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lam D.L., Flanagan M.R. Axillary Lymphadenopathy after COVID-19 Vaccination in a Woman with Breast Cancer. JAMA. 2022;327:175–176. doi: 10.1001/jama.2021.20010. [DOI] [PubMed] [Google Scholar]
- 76.Musaddaq B., Brown A., Dluzewski S., Marafioti T., Malhotra A. Axillary lymphadenopathy in a high-risk breast screening patient following the COVID-19 vaccine: A diagnostic conundrum. BJR case Rep. 2022;7:20210063. doi: 10.1259/bjrcr.20210063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dirven I., Bravenboer B., Raeymaeckers S., Andreescu C.E. Lymphadenopathy after COVID-19 vaccination in patients with endocrine cancer: Two case reports. Endocrinol. Diabetes Metab. Case Rep. 2022;2022:220–258. doi: 10.1530/EDM-22-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pudis M., Conejero J.L.V., Marcuartu J.J.M., Romera M.C. 68Ga-DOTATOC-avid lymphadenopathies induced from COVID-19 mRNA vaccination. Jpn. J. Clin. Oncol. 2021;51:1765. doi: 10.1093/jjco/hyab129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andresciani F., Ricci M., Grasso R.F., Zobel B.B., Quattrocchi C.C. COVID-19 vaccination simulating lymph node progres-sion in a patient with prostate cancer. Radiol. Case Rep. 2022;17:2996–2999. doi: 10.1016/j.radcr.2022.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheong K.M., Tsai T.-Y. A Woman with Painful Neck after COVID-19 Vaccination. Ann. Emerg. Med. 2022;79:e13–e14. doi: 10.1016/j.annemergmed.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guan Y., Xia X., Lu H. Kikuchi–Fujimoto disease following vaccination against COVID-19. J. Hematop. 2022;15:21–23. doi: 10.1007/s12308-021-00477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kashiwada T., Saito Y., Terasaki Y., Shirakura Y., Shinbu K., Tanaka T., Tanaka Y., Seike M., Gemma A. Kikuchi-Fujimoto disease can present as delayed lymphadenopathy after COVID-19 vaccination. Hum. Vaccines Immunother. 2022;18:2071080. doi: 10.1080/21645515.2022.2071080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsumura Y., Asakura K., Takahashi I., Akaihata M., Takahashi Y., Ishida Y. New mimic of relapse or regional lymph node metastasis in a cancer survivor: A case of mRNA COVID-19 vaccine-induced lymphadenitis with high FDG uptake. Immunol. Med. 2022;45:45–47. doi: 10.1080/25785826.2021.1999786. [DOI] [PubMed] [Google Scholar]
- 84.Sekizawa A., Hashimoto K., Kobayashi S., Kozono S., Kobayashi T., Kawamura Y., Kimata M., Fujita N., Ono Y., Obuchi Y., et al. Rapid progression of marginal zone B-cell lymphoma after COVID-19 vaccination (BNT162b2): A case report. Front. Med. 2022;9:963393. doi: 10.3389/fmed.2022.963393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizutani M., Mitsui H., Amano T., Ogawa Y., Deguchi N., Shimada S., Miwa A., Kawamura T., Ogido Y. Two cases of axillary lymphadenopathy diagnosed as diffuse large B-cell lymphoma developed shortly after BNT162b2 COVID-19 vac-cination. J. Eur. Acad. Dermatol. Venereol. 2022;36:e613–e615. doi: 10.1111/jdv.18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goldman S., Bron D., Tousseyn T., Vierasu I., Dewispelaere L., Heimann P., Cogan E., Goldman M. Rapid Progression of Angioimmunoblastic T Cell Lymphoma Following BNT162b2 mRNA Vaccine Booster Shot: A Case Report. Front. Med. 2021;8:798095. doi: 10.3389/fmed.2021.798095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen D., Krauthammer S.H., Cohen Y.C., Perry C., Avivi I., Herishanu Y., Even-Sapir E. Correlation between BNT162b2 mRNA Covid-19 vaccine-associated hypermetabolic lymphadenopathy and humoral immunity in patients with hematologic malignancy. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:3540–3549. doi: 10.1007/s00259-021-05389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Girardin F.R., Tzankov A., Pantaleo G., Livio F., Greub G. Multifocal lymphadenopathies with polyclonal reactions primed after EBV infection in a mRNA-1273 vaccine recipient. Swiss Med. Wkly. 2022;152:w30188. doi: 10.4414/SMW.2022.w30188. [DOI] [PubMed] [Google Scholar]
- 89.Hoffmann C., Wechselberger T., Drexel H., Dertinger S., Dirnhofer S., Pierson S.K., Fajgenbaum D.C., Kessler A. Idiopathic Multicentric Castleman Disease Occurring Shortly after mRNA SARS-CoV-2 Vaccine. Vaccines. 2022;10:1725. doi: 10.3390/vaccines10101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alhossan A., Alsaran A.K., Almahmudi A.H., Aljohani Z.S., Albishi M.R., Almutairi A.K. Adverse Events of COVID-19 Vaccination among the Saudi Population: A Systematic Review and Meta-Analysis. Vaccines. 2022;10:2089. doi: 10.3390/vaccines10122089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bshesh K., Khan W., Vattoth A.L., Janjua E., Nauman A., Almasri M., Ali A.M., Ramadorai V., Mushannen B., Al Subaie M., et al. Lymphadenopathy post-COVID-19 vaccination with increased FDG uptake may be falsely attributed to oncological disorders: A systematic review. J. Med. Virol. 2022;94:1833–1845. doi: 10.1002/jmv.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Co M., Wong P.C.P., Kwong A. COVID-19 vaccine associated axillary lymphadenopathy–A systematic review. Cancer Treat. Res. Commun. 2022;31:100546. doi: 10.1016/j.ctarc.2022.100546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chua T.H., Takano A. Pathological Findings in COVID-19 and Non-COVID-19 Vaccine-Associated Lymphadenopathy: A Systematic Review. J. Clin. Med. 2022;11:6290. doi: 10.3390/jcm11216290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garreffa E., Hamad A., O’Sullivan C.C., Hazim A.Z., York J., Puri S., Turnbull A., Robertson J.F., Goetz M.P. Regional lymphadenopathy following COVID-19 vaccination: Literature review and considerations for patient management in breast cancer care. Eur. J. Cancer. 2021;159:38–51. doi: 10.1016/j.ejca.2021.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ryan F.J., Norton T.S., McCafferty C., Blake S.J., Stevens N.E., James J., Eden G.L., Tee Y.C., Benson S.C., Masavuli M.G., et al. A systems immunology study comparing innate and adaptive immune responses in adults to COVID-19 mRNA and adenovirus vectored vaccines. Cell Rep. Med. 2023:100971. doi: 10.1016/j.xcrm.2023.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Teijaro J.R., Farber D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Youn H., Hong K.-J. Non-invasive molecular imaging of immune cell dynamics for vaccine research. Clin. Exp. Vaccine Res. 2019;8:89–93. doi: 10.7774/cevr.2019.8.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yri O.E., Torfoss D., Hungnes O., Tierens A., Waalen K., Nordøy T., Dudman S., Kilander A., Wader K.F., Østenstad B., et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood. 2011;118:6769–6771. doi: 10.1182/blood-2011-08-372649. [DOI] [PubMed] [Google Scholar]
- 99.Crombie J.L., Sherman A.C., Cheng C.-A., Ryan C.E., Zon R., Desjardins M., Baker P., McDonough M., Izaguirre N., Bausk B., et al. Activity of mRNA COVID-19 vaccines in patients with lymphoid malignancies. Blood Adv. 2021;5:3062–3065. doi: 10.1182/bloodadvances.2021005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Madelon N., Lauper K., Breville G., Sabater Royo I., Goldstein R., Andrey D.O., Grifoni A., Sette A., Kaiser L., Siegrist C.A., et al. Robust T-Cell Responses in Anti-CD20-Treated Patients Following COVID-19 Vaccination: A Prospective Cohort Study. Clin. Infect. Dis. 2022;75:e1037–e1045. doi: 10.1093/cid/ciab954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shields A.M., Venkatachalam S., Shafeek S., Paneesha S., Ford M., Sheeran T., Kelly M., Qureshi I., Salhan B., Karim F., et al. SARS-CoV-2 vaccine responses following CD20-depletion treatment in patients with haematological and rheumatological disease: A West Midlands Research Consortium study. Clin. Exp. Immunol. 2022;207:3–10. doi: 10.1093/cei/uxab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bettini E., Locci M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines. 2021;9:147. doi: 10.3390/vaccines9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lederer K., Castaño D., Gómez Atria D., Oguin T.H., III, Wang S., Manzoni T.B., Muramatsu H., Hogan M.J., Amanat F., Cherubin P., et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity. 2020;53:1281–1295.e5. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turner J.S., O’Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., Lei T., Thapa M., Chen R.E., Case J.B., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gee J., Marquez P., Su J., Calvert G.M., Liu R., Myers T., Nair N., Martin S., Clark T., Markowitz L., et al. First Month of COVID-19 Vaccine Safety Monitoring-United States, December 14, 2020-January 13, 2021. Morb. Mortal Wkly. Rep. 2021;70:283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnston M.S., Galan A., Watsky K.L., Little A.J. Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine: A Case Series. JAMA Dermatol. 2021;157:716–720. doi: 10.1001/jamadermatol.2021.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McNeil M.M., DeStefano F. Vaccine-associated hypersensitivity. J. Allergy Clin. Immunol. 2018;141:463–472. doi: 10.1016/j.jaci.2017.12.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Potluri T., Fink A.L., Sylvia K.E., Dhakal S., Vermillion M.S., Steeg L.V., Deshpande S., Narasimhan H., Klein S.L. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines. 2019;4:29. doi: 10.1038/s41541-019-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Castle S.C. Clinical Relevance of Age-Related Immune Dysfunction. Clin. Infect. Dis. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 110.Pozzetto B., Legros V., Djebali S., Barateau V., Guibert N., Villard M., Peyrot L., Allatif O., Fassier J.B., Massar-dier-Pilonchéry A., et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature. 2021;600:701–706. doi: 10.1038/s41586-021-04120-y. [DOI] [PubMed] [Google Scholar]
- 111.Perry A.M., Choi S.M. Kikuchi-Fujimoto Disease: A Review. Arch. Pathol. Lab. Med. 2018;142:1341–1346. doi: 10.5858/arpa.2018-0219-RA. [DOI] [PubMed] [Google Scholar]
- 112.Becker A.S., Perez-Johnston R., Chikarmane S.A., Chen M.M., El Homsi M., Feigin K.N., Gallagher K.M., Hanna E.Y., Hicks M., Ilica A.T., et al. Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: Radi-ology Scientific Expert Panel. Radiology. 2021;300:E323–E327. doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seely J.M., Barry M.H. The Canadian Society of Breast Imaging Recommendations for the Management of Axillary Ade-nopathy in Patients with Recent COVID-19 Vaccination-Update. Can. Assoc. Radiol. J. 2021;72:601–602. doi: 10.1177/0846537121998949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.