Abstract
Variation in ejaculatory abstinence time and its influence on semen quality and clinical reproductive outcomes is a growing concern among clinicians and researchers. The WHO (World Health Organization) recommends 2–7 days of abstinence time prior to semen collection for diagnostic purposes; however, the evidence that such an abstinence period leads to better pregnancy outcomes remains unclear. The aim of this systematic review is to evaluate short and long ejaculatory abstinence time in association with pregnancy rate, live birth rate and DNA fragmentation, in order to make a recommendation on an ideal timeframe for ejaculatory abstinence. This review is conducted according to the PRISMA guidelines and registered in PROSPERO (CRD42022379039). The electronic databases PubMed, Embase and Cochrane were searched for eligible studies. The Scottish Intercollegiate Guidelines Network was used for the assessment of the risk of bias across the included studies. Twenty-four studies were included in this systematic review. The included studies confirm that a shorter abstinence time is associated with improved pregnancy rates and live birth rates following assisted reproductive technology compared with longer ejaculatory abstinence times at different cut-off points. Similarly, a lower DNA fragmentation index was reported in semen analyses collected from short abstinence times compared with long abstinence times. However, due to the heterogeneity of the included studies, it is not possible to extract an ideal time of ejaculatory abstinence, but all outcomes improved with shorter ejaculatory abstinence times. This systematic review confirms that short ejaculatory abstinence times, less than those recommended by the WHO for diagnostic purposes, are associated with higher pregnancy and live birth rates and improved DNA fragmentation, when compared to long ejaculatory abstinence times.
Keywords: ejaculatory abstinence, pregnancy rate, live birth rate, DNA-fragmentation, infertility
1. Introduction
Approximately 24% of couples of reproductive age are primary or secondary infertile, which means they have not been able to conceive after one year of unprotected sexual intercourse. Male factor infertility affects over 50% of these infertile couples, either in isolation or in combination with a female factor [1]. Male factor infertility is usually defined by abnormal results on semen analysis according to the World Health Organization (WHO) [2].
It is well known that several factors can affect semen quality, including general health status, metabolic syndrome, osteoporosis, age, medication, body mass index and lifestyle including diet, smoking, caffeine and alcohol intake [3,4]. Semen parameters can vary both between and within individuals, and one factor causing intraindividual variation is suggested to be ejaculatory abstinence time (EA) [5]. When spermatozoa pass the epididymal tract, they undergo a series of biochemical and physiological changes to mature them into a fertilizing component. During this transit, spermatozoa may be exposed to reactive oxygen species (ROS) [6,7]. ROS is known to cause DNA damage and fragmentation, which can negatively affect fertility [8]. Studies are suggesting that DNA fragmentation is associated with poor pregnancy and live birth rates following assisted reproductive technology (ART) [9,10]. Testicular sperm has a lower DNA fragmentation when compared to ejaculated sperm, which may indicate that DNA fragmentation is increased during the transition and storage in the epididymal duct [11]. Therefore, EA may influence DNA fragmentation and clinical reproductive outcomes.
The sixth edition of the WHO manual for examining and processing human semen has been recently released, recommending 2–7 days of EA prior to semen collection for diagnostic purposes [2]. Several recent studies have reported a correlation between short EA and lower DNA fragmentation and thus better semen quality [12,13]. Much indicates that a short EA in the context of ART may lead to higher pregnancy rates. A recent meta-analysis found significantly higher pregnancy rates when the EA was less than 4 days compared with 4 to 7 days; however, the meta-analysis only included four studies, which is why an updated, broader search is needed to strengthen the evidence [14]. Also, the European Society of Human Reproduction and Embryology (ESHRE) is currently recommending a shorter and narrower range from 3 to 4 days of EA [15].
If a shorter EA can have a positive impact on pregnancy outcomes, it is a safe, low-cost and non-invasive intervention in fertility treatment. Currently, there is no recent systematic review focusing on pregnancy rate, and the available recent reviews only include a few studies.
The aim of this systematic review is to compare short and long ejaculatory abstinence time and investigate the association between fertility outcomes and semen quality. The main objective was pregnancy rate and secondary objectives were live birth rate and DNA fragmentation index.
2. Materials and Methods
This systematic review was conducted and reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [16] and was submitted to PROSPERO, date 30 November 2022, and accepted 11 December 2022. The registration number was CRD42022379039.
2.1. Eligibility Criteria
A study was found eligible for inclusion if the following inclusion criteria were met:
P: The study population was men of reproductive age, including men referred to fertility treatment.
I: The intervention was a short abstinence time between successive ejaculation.
C: The comparison was a long abstinence time between successive ejaculation.
O: The main outcome was pregnancy rate, and secondary outcomes were live birth rate and DNA fragmentation.
All types of original published human studies were included, while expert opinions, case studies and protocols were excluded. All types of fertility treatment were included. Publication languages was limited to English, Spanish and Scandinavian languages.
2.2. Information Sources and Search Strategy
A research librarian conducted a systematic search in PubMed, Embase and Cochrane in April 2022. An initial search strategy was performed with the following search string: ((male or paternal or man or oligospermia or Oligozoospermia or low sperm count or semen or sperm or Asthenospermia or Asthenozoospermia or Azoospermia or Teratozoospermia or Teratospermia or Spermatogenic dysfunction) and (ejaculation or ejaculatory) and (abstinence or time or interval) and (pregnancy or pregnant or live birth or hCG or human chorionic gonadotropin or DNA fragmentation)). All suggested MeSH terms were included in the search on PubMed. Additional articles were manually retrieved after reviewing the reference lists from relevant publications.
2.3. Study Selection and Data Collection
All potentially eligible studies were independently screened by two investigators (F.S. and L.M.) based on the title and abstract, and potentially eligible studies were subsequently full-text screened for inclusion. Covidence.org was used as the screening tool. Disagreements between reviewers were resolved by a third person (S.S.). One review author extracted data from the included studies and the second author independently and systematically cross-checked all the data. Disagreements were resolved by discussion between the two review authors (F.S. and L.M.).
2.4. Data Items
The study demographics, study country, year of publication, study design, population size, subjects, cause of infertility, investigated abstinence time and primary study outcomes were extracted. Data regarding pregnancy rate, live birth rate and the DNA fragmentation index were extracted along with the type of ART and assay method for DNA fragmentation analysis. Missing data were sought to be collected by contacting corresponding authors of reference publications.
2.5. Risk of Bias Assessment
To assess the quality of evidence of the included cohort studies, two investigators (L.M. and F.S.) independently evaluated the quality according to the Scottish Intercollegiate Guidelines Network (SIGN). SIGN is a methodology checklist for cohort studies, to evaluate the risk of bias according to the selection, assessment, confounding, and statistical analysis [17]. We excluded irrelevant questions from our study, such as 1.3 and 1.4 in the selection section, which were not applicable since EA would not be affected by the participation rate or outcomes prior to enrollment. In the assessment section, questions 1.8, 1.9 and 1.12 were also not applicable, as blinding EA was not possible. Disagreements between reviewers were resolved by discussion with a third author (S.S.).
2.6. Data Synthesis
For data presentation of the included studies, the following syntheses were made:
-
(1)
Quantitative analysis: The proportion of studies that reported pregnancy rate, live birth rate and DNA fragmentation were compared for subjects with short versus long EA. The results of studies performing statistical analyses are similarly presented in a separate table.
-
(2)
Visual analysis: Studies reporting pregnancy rates as percentages were illustrated in a graph grouped by EA on the x-axis. In the studies where a time interval of abstinence days was used, the mean day of the interval was chosen for illustration.
3. Results
3.1. Study Selection
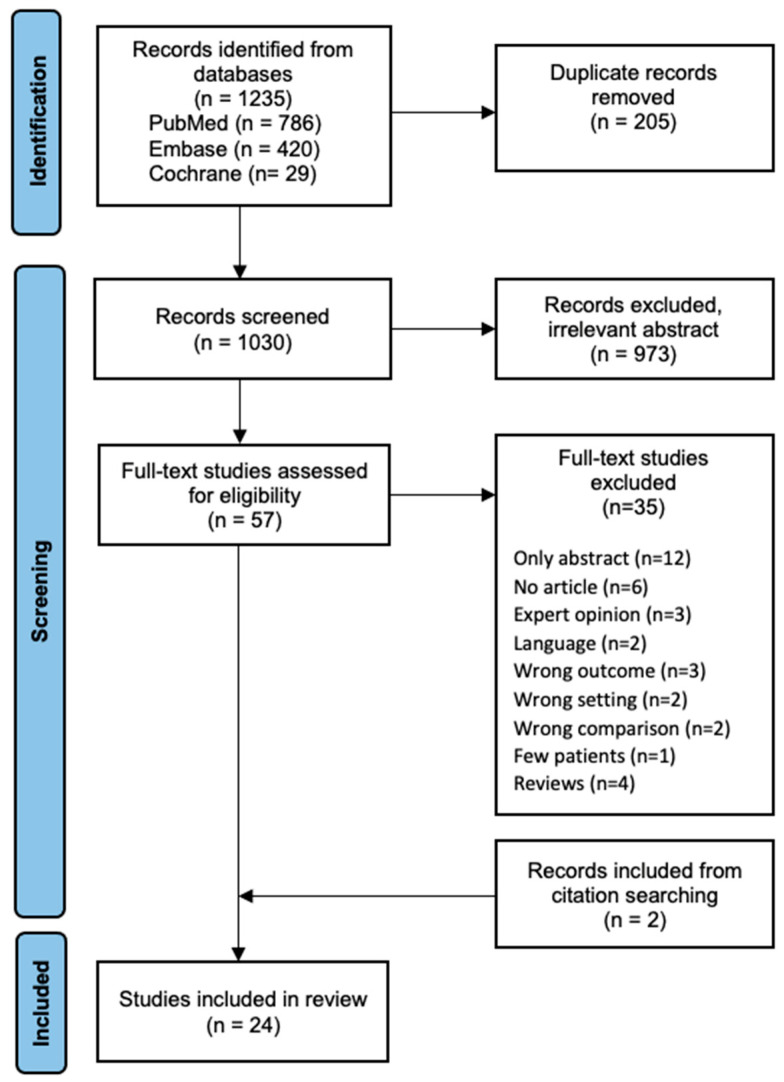
The search strategy is illustrated in the PRISMA flow diagram (Figure 1). A total of 1235 studies were identified after performing the search string across databases, and 1030 articles remained for primary screening after the removal of duplicates. We excluded 973 studies based on irrelevant titles and abstracts. Additionally, 20 studies were excluded during full-text review due to papers being unavailable or due to language restrictions. Furthermore, 15 studies were excluded as they did not meet the eligibility criteria. Twenty-two studies met the inclusion criteria for this systematic review. Additionally, two studies met the eligibility criteria when screening references for relevant papers. A total of 24 studies were included for further analysis, with a total of 14,173 cases.
Figure 1.
Flow diagram outlining selection of studies included in a systematic review.
The studies included in this review were conducted in Asia, North and South America and Europe. The largest studies were conducted in Mexico, Brazil, and India and included more than 9000 cases in total (Table 1). The majority of the studies focused on patients undergoing fertility treatment, while four of the studies included volunteers. The cause of referral to fertility treatment included male and female infertility, as well as mixed and unexplained infertility. The median age of the participants was between 30 and 40 years in the majority of the studies and ranged from 20 to 50 years. The EA varied from less than one hour to as long as 15–20 days.
Table 1.
Study characteristics and study outcomes.
| Author Year Country |
Study Design | Participants/ Cycles |
Subjects | Abstinence Period | Outcome Measurements |
|---|---|---|---|---|---|
| Dahan et al. [18] 2020 Canada |
Prospective | 112/- | Infertility patients, male factor |
3 h and 3 days | DNA fragmentation Semen parameters (volume, motility, concentration, morphology and total sperm count) |
| Kabukçu et al. [19] 2020 Turkey |
Randomized controlled trial | 106/- | Infertility patients | 1 and 3 days | Pregnancy rate and miscarriage rate DNA fragmentation Semen parameters (viscosity, volume, motility, morphology and count) |
| Agarwal et al. [20] 2016 USA |
Prospective | 7/- | Volunteers, normozoospermic |
<2, 2–7 and 9–11 days 1, 2, 5, 7, 9, and 11 days | DNA fragmentation and reactive oxygen species Semen parameters (viscosity, vitality, volume, pH, concentration, morphology, sperm count) |
| Borges et al. [21] 2018 Brazil |
Prospective | 818/483 | Infertility patients, only male factor |
<4 and >4 days 1, 2, 3 and 4 days |
Pregnancy rate, miscarriage rate, fertilization rate, implantation rate and embryo rate DNA fragmentation Semen parameters (volume, concentration, motility, morphology, sperm count) |
| Vahidi et al. [22] 2021 Iran |
Prospective | 64/- | Infertility patients, with increased DFI |
24 h, 3 and 2–7 days. | DNA fragmentation and DNA protamination Semen parameters (volume, concentration, motility, morphology) |
| Comar et al. [23] 2017 Brazil |
Prospective | 2458/- | Infertility patients | <2, 2–5 and >5 days | DNA fragmentation, DNA protamination and mitochondrial membrane potential Semen parameters (pH, volume, concentration, motility, normal sperm forms, leukocytes, vitality, apoptosis) |
| Uppangala et al. [24] 2016 India |
Prospective | 19/- | Healthy Volunteers | 1, 3, 5 and 7 days | DNA fragmentation, sperm chromatin maturity and hypermethylation levelSemen parameters (volume, concentration, motility, morphology, vitality, viability) |
| Sánchez-Martín et al. [25] 2013 Spain |
Prospective | 190/- | Infertility patients, non-severe male factor |
12 h and 4 days | DNA fragmentation Semen parameters (volume, concentration, motility) |
| Scarselli et al. [26] 2019 Italy |
Prospective | 22/265 | Infertility patients, OAT |
1 h and 2–5 days | Pregnancy rate, fertilization rate, implantation rate and blastocyst rate Semen parameters (volume, concentration, motility, morphology) |
| Shen et al. [27] 2019 China |
Prospective | 528/- | Infertility patients | 1–3 h and 3–7 days | Pregnancy rate, live birth rate, miscarriage rate and implantation rate DNA fragmentation, acrosome reaction, antioxidant capacity, mitochondrial membrane potential, DNA stainability, nucleoprotein transition and reactive oxygen species Semen parameters (volume, count, concentration, motility, vitality, morphology) |
| Gosálvez et al. [28] 2011 Spain |
Prospective | 33/- | (1) Infertility patients with female factor (2) Donors |
(1) 24 h and 4 days (2) 3 h and 4 days |
DNA fragmentation |
| Jurema et al. [29] 2005 USA |
Retrospective | 417/929 | Infertility patients, unexplained or oligomenorrhea |
≤3, 3–10 and >10 days | Pregnancy rate Semen parameters (concentration, motility, morphology, total motile sperm) |
| Mayorga-Torres et al. [30] 2015 Colombia |
Prospective | 6/- | Volunteers, normozoospermic |
1 and 3–4 days | DNA fragmentation, mitochondrial membrane potential, membrane integrity, Reactive oxygen species Semen parameters (volume, concentration, motility, morphology, count, vitality) |
| Jonge et al. [31] 2004 Belgium |
Prospective | 11/- | Infertility patients | 1, 3, 5 and 8 days | DNA fragmentation and DNA stainability Semen parameters (volume, pH, concentration, motility, morphology, viability) |
| Kulkarni et al. [32] 2022 India |
Prospective | 67/- | Infertility patients | 1–3 h and 2–7 days | DNA fragmentation Semen parameters (volume, count, concentration, motility) |
| Marshburn et al. [33] 2010 USA |
Retrospective | 372/866 | Infertility patients, normospermia andoligozoospermia |
<2, 3–5 and >5 days | Pregnancy rate Semen parameters (volume, concentration, total motile sperm, numbers of dead sperm) |
| Barbagallo et al. [34] 2021 Italy |
Prospective | 313/- | Infertility patients, normozoospermic and OA |
1 h and 2–7 days | Pregnancy rate, live birth rate, miscarriage rate, fertilization rate, implantation rate, embryo quality, type of birth and birth weight Semen parameters (concentration, motility, morphology) |
| Kably-Ambe et al. [35] 2015 Mexico |
Retrospective | 3123/3123 | Infertility patients | 0–1, 2–3, 4–5, 6–7, 8–9, 10–14 and 15–20 days | Pregnancy rate and recovery rate Semen parameters (volume, concentration, progressive motility, morphology) |
| Gupta et al. [36] 2021 India |
Prospective Retrospective analysis |
-/1691 | Infertility patients, normozoospermic |
1, 2–5, 6–7 and ≥8 days | Pregnancy rate, miscarriage rate, fertilization rate, implantation rate, positive β-hCG rate, embryo sacs and ectopic pregnancy Semen parameters (volume, concentration, motility, morphology) |
| Manna et al. [37] 2020 Italy |
Prospective | 65/- | Infertility patients, normozoospermic and OAT |
1 h and 2–7 days | DNA fragmentation Semen parameters (volume, concentration, motility, morphology) |
| Azizi et al. [38] 2021 Iran |
Retrospective | 1003/1003 | Infertility patients, male and female factor |
1, 2, 3, 4, 5 and 6–10 days | Pregnancy rate, fertilization rate and cleavage-stage embryo rate Semen parameters (volume, count, concentration, motility, morphology) |
| Welliver et al. [39] 2016 USA |
Prospective | 20/- | Normozoospermic | 1 and 3–5 days | DNA fragmentation Semen parameters (volume, concentration, motility, pH, total motile count, morphology) |
| Periyasamy et al. [40] 2017 India |
Retrospective | -/1030 | Infertility patients, male and female factor |
2–7 and >7 days.2–4 and 5–7 days * | Pregnancy rate, live birth rate, miscarriage rate, fertilization rate, implantation rate and cleavage-stage grade embryo rate |
| Lee et al. [41] 2018 Korea |
Retrospective | -/449 | Infertility patients, male and female factor |
2–7 and 8 days | Pregnancy rate, miscarriage rate and implantation rate |
DFI: DNA fragmentation index, OAT: oligoastenoteratozoospermic, OA: obstructive azoospermia, -: not reported, * subgroup of participants undergoing EA of 2–7 days.
3.2. Level of Study Evidence
An overview of the studies’ risk of bias assessment is presented in Table 2. According to the SIGN methodology checklist, three of the included studies were of high quality [18,21,23], 17 had acceptable quality and four had low quality. Three of the studies with low quality had study populations under 11 [20,30,31], which increases the risk of Type II error due to low power and thus weakens the strength of evidence. Eight studies had large populations (n ≥ 800), of which six were retrospective and can according to the SIGN methodology checklist only maximally be rated as acceptable; however, three of these were of high quality in all other domains [36,38,40]. Six studies were downgraded from high quality to acceptable quality due to missing confidence intervals.
Table 2.
Overview of risk of bias assessment of the included studies.
| Dahan et al. [18] | Kabukçu et al. [19] | Agarwal et al. [20] | Borges et al. [21] | Vahidi et al. [22] | Comar et al. [23] | Uppangala [24] | Sánchez-Martín et al. [25] | Scarselli et al. [26] | Shen et al. [27] | Gosálvez et al. [28] | Jurema et al. [29] | Mayorga-Torres et al. [30] | Jonge et al. [31] | Kulkarni et al. [32] | Marshburn et al. [33] | Barbagallo et al. [34] | Kably-Ambe et al. [35] | Gupta et al. [36] | Manna et al. [37] | Azizi et al. [38] | Welliver et al. [39] | Periyasamy et al. [40] | Lee et al. [41] | |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
Question |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
Selection |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
Assessment |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
Confounding |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
Statistical analysis |

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|

|
Quality |
 : low risk of bias,
: low risk of bias,  : acceptable risk of bias,
: acceptable risk of bias,  : high risk of bias.
: high risk of bias.
All the included studies had a well-executed assessment. Most of the studies address confounding as age, female factor, BMI, illness and smoking. For a detailed risk of bias assessment, see Appendix B.
3.3. Pregnancy Rate
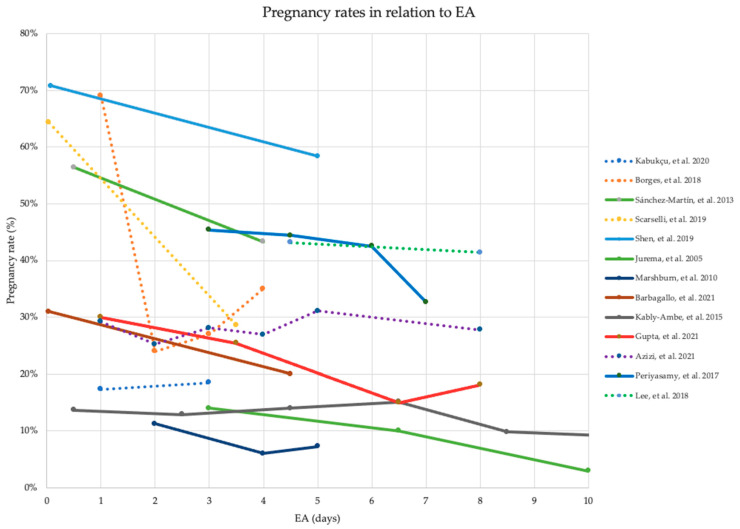
As presented in Table 3, 13 studies examined the influence of varying abstinence time on pregnancy rate. Nine of the studies found a significantly higher pregnancy rate with short EA compared with long EA. The remaining four studies reported similarly higher pregnancy rates with shorter EA; however, they were statistically non-significant. Similarly, Figure 2 illustrates higher pregnancy rates with short EA compared with long EA, favoring better pregnancy outcomes of short EA. All 13 studies recruited patients from fertility clinics.
Table 3.
Associations between ejaculatory abstinence time and pregnancy rate, live birth rate and DNA fragmentation.
| Author | EA | ART | Pregnancy Rate | Live Birth Rate | DFI |
|---|---|---|---|---|---|
| Dahan et al. [18] | 3 h and 3 days | ICSI, IVF | ↓ | ||
| Kabukçu et al. [19] | 1 and 3 days | IUI | ←→ | ←→ | |
| Agarwal et al. [20] | <2, 2–7 and 9–11 days 1, 2, 5, 7, 9, 11 days |
↓ | |||
| Borges et al. [21] | <4 and >4 days 1, 2, 3 and 4 days |
ICSI | ↑ | ↓ | |
| Vahidi et al. [22] | 24 h, 3 and 2–7 days. | ↓ | |||
| Comar et al. [23] | <2, 2–5 and >5 days | ↓ | |||
| Uppangala et al. [24] | 1, 3, 5 and 7 days | ↓ | |||
| Sánchez-Martín et al. [25] | 12 h and 4 days | ICSI | ↑ | ↓ | |
| Scarselli et al. [26] | 1 h and 2–5 days | ICSI | ←→ | ||
| Shen et al. [27] | 1–3 h and 3–7 days | IVF | ↑ | ↑ | ↓ |
| Gosálvez et al. [28] | (1) 24 h and 4 days (2) 3 h and 4 days |
ICSI | ↓ | ||
| Jurema et al. [29] | ≤3, 3–10 and >10 days | IUI | ↑ | ||
| Mayorga-Torres et al. [30] | 1 and 3–4 days | ←→ | |||
| Jonge et al. [31] | 1, 3, 5 and 8 days | ←→ | |||
| Kulkarni et al. [32] | 1–3 h and 2–7 days | ↓ | |||
| Marshburn et al. [33] | <2, 3–5 and >5 days | IUI | ↑ | ||
| Barbagallo et al. [34] | 1 h and 2–7 days | ICSI | ↑ | ↑ | |
| Kably-Ambe et al. [35] | 0–1, 2–3, 4–5, 6–7, 8–9, 10–14 and 15–20 days |
IUI | ↑ | ||
| Gupta et al. [36] | 1, 2–5, 6–7 and ≥ 8 days | ICSI | ↑ | ||
| Manna et al. [37] | 1 h and 2–7 days | ICSI | ↓ | ||
| Azizi et al. [38] | 1, 2, 3, 4, 5 and 6–10 days | ICSI | ←→ | ||
| Welliver et al. [39] | 1 and 3–5 days | ←→ | |||
| Periyasamy et al. [40] | 2–4, 2–7, 5–7 and >7 days | ICSI or ICSI + IVF | ↑ | ↑ | |
| Lee et al. [41] | 2–7 and 8 days | ICSI | ←→ |
↑: increase significantly with decreasing abstinence time (p < 0.05), ↓: decrease significantly with decreasing abstinence time (p < 0.05), ←→: not significantly different, EA: ejaculatory abstinence time, ICSI: intracytoplasmic sperm injection, IVF: in vitro fertilization, IUI: intrauterine insemination.
Figure 2.
Pregnancy rates and ejaculatory abstinence time of 13 included studies reporting pregnancy rate. Solid line: statistically significant difference (p < 0.05), dotted line: no statistically significant difference (p > 0.05), EA: ejaculatory abstinence time [19,21,25,26,27,29,33,34,35,36,38,40,41].
3.4. Live Birth Rate
Three studies evaluated live birth rates in relation to various abstinence times (Table 3). All three studies [27,34,40] found a significantly higher live birth rate when comparing short EA with long EA. One of the studies found significantly higher live birth rates with short EA of 1–3 h (65.2%) compared with 3–7 days (47.7%) in cryopreserved blastocysts (frozen thawed cycles), while results of the fresh cycle were non-significant but still reported higher live birth rates with short EA [27].
3.5. DNA Fragmentation Index
Sperm DNA fragmentation in relation to different EAs was reported in 15 studies (Table 3). Eleven studies found a significantly lower DNA fragmentation index (DFI) with short EA compared with long EA. The remaining four studies reported non-significant estimates, all with low DFI rates favoring short EA. However, three of the studies with non-significant results had populations less than 20 [30,31,39]. Some studies used interval days, successive days, or both. One study compared a group undergoing an EA of 2–7 days compared with a group receiving antioxidant therapy for three months and undergoing an EA of 3 days [22]. Three of the 15 studies investigated DFI rates in two independent groups; however, two of these studies had a large population (n = 818 and n = 2458). No significant differences were reported in the patient characteristics in the studies [21,23]. In the one study that did not have a large sample size (n = 120), no significant difference in DFI was found [19]. EAs of one day or less were associated with the lowest rates of DFI in the studies, indicating that sperm DNA quality may be worsened by longer EA.
For further information regarding statistical analyses on pregnancy rate, live birth rate and DFI, see Appendix A.
4. Discussion
In the present review, 24 studies were included and evaluated for associations between men’s EA and reproductive outcomes as well as DNA fragmentation. According to the current evidence, pregnancy rates were higher with short EA compared with long EA. Also live birth rates were significantly higher with short EA in all the three studies reporting live birth rate compared with long EA. Additionally, DNA fragmentation was shown to be decreasing with shorter EA. Current evidence thus supports the beneficial outcomes of short EA regarding reproductive outcomes, and beneficial effects are seen in successive ejaculatory intervals as low as 1–3 h [27].
4.1. Strengths and Limitations
The strength of this systematic review was the meticulous and broad search strategy performed by a research librarian, which resulted in a large number of included studies. Additionally, the evaluation of all available studies including different abstinence intervals, populations and ART methods emphasizes the beneficial effects of short EA in all clinical settings regarding reproductive outcomes. Furthermore, this systematic review adhered to recommendations set by the PRISMA guideline [16].
There are some limitations in this systematic review. According to the SIGN risk of bias assessment tool, only three studies were of high quality, of which none of them reported live birth rates, while the rest of the studies were not rated higher than acceptable quality due to weaknesses in one or more domains. The inclusion of studies with small sample sizes leads to a small statistical power which might have increased the risk of Type II errors. Many of the included studies used different EA, leading to heterogenous data and it was therefore not possible to conduct a meta-analysis. Furthermore, different techniques to measure DFI were used in the studies e.g., sperm chromatin dispersion test and flowcytometry along with variations in cut-off values of DFI, which may impact the correlations between EA and DFI and limit the external validity of the results. It was not possible to assess possible confounders, such as lifestyle, smoking status, and daily coffee intake in the current evidence, although previous studies have found a significant correlation between these factors and semen quality and thus reproductive outcomes [42,43].
4.2. Comparison
This systematic review includes the highest amount of studies to date and finds supporting results when compared with three earlier systematic reviews investigating the influence of EA on semen quality and clinical outcomes following ART [12,13,44]. One review from 2021 compared <2, 2–7 and >7 days of EA and found the highest pregnancy and live birth rate with EA of <2 days compared with 2–7 and >7 days. Regarding DNA fragmentation, 11 out of 16 included studies additionally found comparable results with lower DFI when comparing <2 days of EA with 2–7 days [13]. The two other systematic reviews included three or fewer papers reporting pregnancy rate, and both found the highest pregnancy rate for intracytoplasmic sperm injection (ICSI) treatment after less than one day of EA, and under two [12] and three days [44] of EA for intrauterine insemination (IUI) treatment. In contrast to the previously mentioned reviews, our review included 13 studies evaluating pregnancy rates in relation to varying EAs. All previous systematic reviews and meta-analysis had five or fewer studies reporting pregnancy rate included. A potential explanation for this considerably high difference in the number of included articles might be the use of several databases and broader search strings, and in addition, the inclusion of recently published papers.
This systematic review focused mainly on pregnancy rates. A meta-analysis from 2020 did this as well, but only included four articles. The meta-analysis compared <4 days of EA with 4–7 days. Compared with long EA, short EA improved pregnancy rates significantly with an odds ratio of 1.44 (95%CI [1.17–1.78; p = 0.0006] in the forest plot analysis [14]. In the present review, most of the included studies reporting pregnancy rates found the highest rates with EAs of less than four days. Five studies showed significantly higher pregnancy rates with EA as short as one day or less when compared to longer abstinence times; for example, Gupta et al. [36] analyzed a large sample of 1691 cycles and found significantly higher pregnancy rates with one day of EA compared to 2–5, 6–7 and >8 days.
DNA fragmentation has been linked to impaired fertilization, suboptimal embryo quality, reduced pregnancy rates and increased spontaneous abortion rate after in vitro fertilization (IVF) treatment [10]. A systematic review and meta-analysis from 2015 found that a higher number of spermatozoa with DNA fragmentation in couples undergoing ART are associated with poorer outcomes [10]. In this systematic review, 11 studies found a significant correlation between DFI and EA, and all of them found that DFI rates were lower with shorter EA—especially EA of one day or less was associated with the lowest rates of DFI. Five studies had an EA of 3 h or less and all of them found significantly lower DFI when compared to a longer EA. A recent systematic review and meta-analysis from 2022 also found significantly lower DFI when comparing EA < 4 h with longer EA in a forest plot analysis of four studies [45]. This is supporting the theory that a short EA leads to lower DNA fragmentation, which may improve reproductive outcomes following ART.
Current evidence supports the theory that short EA is beneficial for reproductive outcomes; however, the risk of bias is still present. For conclusive and strengthened evidence, future studies are required in a prospective and randomized setting. For studies investigating pregnancy rate and live birth rate, we recommend a prospective randomized controlled trial in a large population undergoing similar ART with participants randomized to different successive ejaculatory abstinence intervals. This may establish the optimal interval of EA. For studies investigating DNA fragmentation, the same participant should be used as the control for their own samples for minimal confounding, eliminating interindividual variations in semen quality. Based on current evidence, this systematic review finds a clear trend that short EAs may enhance pregnancy rate, live birth rate and DFI. Revising the recommended 2–7 days of EA in the WHO manual regarding collection and processing of semen samples may lead to different reference intervals during diagnostic processing. Therefore, it may be worth considering only using an EA shorter when initiating fertility treatment. However, the ideal timeframe of EA can vary depending on the type of fertility treatment being used, as ideal EA’s may differ between fertilization provided by intercourse, IUI or IVF/ICSI, due to differences in semen volumes and sperm counts. These suggestions should be considered for future recommendations regarding EA in relation to fertility treatment.
5. Conclusions
Pregnancy rate, live birth rate and DNA fragmentation are likely to improve with short EA compared to long EA. Although it is not possible to conduct a clear recommendation on the ideal timeframe for an EA due to heterogenous abstinence times, this systematic review finds a clear tendency that a short EA is likely to improve pregnancy and live birth rate and decrease the level of DNA fragmentation in semen followed by ART.
Acknowledgments
The authors wish to thank Ivar Horte for conducting the systematic search across databases.
Appendix A
Table A1.
Statistical analyses of studies regarding pregnancy rate, live birth rate, DNA fragmentation and assay method for DNA fragmentation.
| Author | Ejaculatory Abstinence | Pregnancy Rate | Live Birth Rate | DNA Fragmentation | DNA Fragmentation Assay |
|||
|---|---|---|---|---|---|---|---|---|
| Time | Rate% | p-Value | Rate% | p-Value | Index%(±Range) | p-Value | ||
| Dahan et al. [18] | 3 h | - | - | - | - | 23.7 (16.0) | p ≤ 0.0001 | SCD |
| 3 days | 34.6 (19.4) | |||||||
| Kabukçu et al. [19] | 1 day | 17.3 | p = 0.871 | - | - | 20.7 (11.01) | p = 0.187 | TUNEL |
| 3 days | 18.5 | 23.8 (12.64) | ||||||
| Agarwal et al. [20] | <2 days | - | - | - | - | 9.9 (1.7) | p = 0.007 | TUNEL |
| 2–7 days | 12.8 (1.8) | |||||||
| >7 days | 17.8 (2.3) | |||||||
| Borges et al. [21] | ≤ 4 days | 40.0 | p = 0.016 | - | - | 16.8 (0.7) | p = 0.028 | SCD |
| ≥ 4 days | 10.0 | 19.2 (0.8) | ||||||
| Borges et al. [21] | 1 day | 69.0 | p = 0.062 | - | - | 16.6 (2.7) | p > 0.05 | SCD |
| 2 days | 24.0 | 16.3 (1.6) | ||||||
| 3 days | 27.0 | 18.2 (1.3) | ||||||
| 4 days | 35.0 | 19.8 (1.6) | ||||||
| Vahidi et al. [22] | 1 day | - | - | - | - | 17.4 (8.6) |
p = 0.013 (2–7 vs. 3 days) p = 0.028 (3 vs. 1 day) |
TUNEL |
| 3 days | 20.6 (10.3) | |||||||
| 2–7 days | 24.6 (1.4) | |||||||
| Comar et al. [23] | 2 days | - | - | - | - | 14.5 (8.2) |
p = 0.001 (<2 vs. >5 days) p = 0.002 (2–5 vs. >5 days) |
TUNEL |
| 2–5 days | 15.3 (8.4) | |||||||
| >5 days | 17.1 (9.0) | |||||||
| Uppangala et al. [24] | 1 day | - | - | - | - | 11.8 (6.5) | p < 0.05 (1 vs. 5 days) | SCD |
| 3 days | 14.9 (9.9) | p < 0.001 (1 vs. 7 days) | ||||||
| 5 days | 19.8 (10.0) | p < 0.01 (3 vs. 7 days) | ||||||
| 7 days | 27.1 (9.6) | p < 0.05 (5 vs. 7 days) | ||||||
| Sánchez-Martín et al. [25] | 12 h | 56.4 | p = 0.030 | - | - | - | p < 0.001 | SCD |
| 1 day | - | 19.6 (8.3) | ||||||
| 4 days | 43.3 | 27.0 (10.8) | ||||||
| Scarselli et al. [26] | 1 h | 64.3 | p = 0.080 | 64.3 | p = 0.080 | - | - | |
| 2–5 days | 28.6 | 28.6 | ||||||
| Shen et al. [27] | Fresh cycle | p = 0.238 | p = 0.072 | - | p < 0.05 | SCSA | ||
| 1–3 h | 53.8 | 47.4 | ||||||
| 3–7 days | 45.7 | 35.4 | ||||||
| Frozen thawed cycle | p = 0.045 | p = 0.006 | ||||||
| 1–3 h | 70.8 | 65.2 | ||||||
| 3–7 days | 58.4 | 47.7 | ||||||
| Gosálvez et al. [28] | Neat semen | - | - | - | - | p = 0.031 | SCD | |
| 1 day | 19.6 (8.4) | |||||||
| 4 days | 26.9 (11.0) | |||||||
| Neat semen | - | - | - | - | p = 0.06 | |||
| 3 h | 20.8 (6.7) | |||||||
| 4 days | 22.2 (7.4) | |||||||
| selected semen | - | - | - | - | p = 0.020 | |||
| 3 h | 10.8 (6.3) | |||||||
| 4 days | 17.0 (5.5) | |||||||
| Jurema et al. [29] | ≤3 days | 14.0 | p < 0.05 | - | - | - | - | |
| 3–10 days | 10.0 | |||||||
| ≥10 days | 3.0 | |||||||
| Mayorga-Torres et al. [30] | 3–4 days (first analysis) | - | - | - | - | 26.6 (3.0) | p > 0.050 (first analysis vs. all others) | SCSA |
| 1 day (collected day 2) | 22.1 (4.3) | |||||||
| 1 day (collected day 3) | 24.0 (3.4) | |||||||
| 1 day (collected day 6) | 27.1 (3.5) | |||||||
| 1 day (collected day 9) | 24.7 (2.8) | |||||||
| 1 day (collected day 11) | 25.8 (4.6) | |||||||
| 1 day (collected day 13) | 23.9 (4.7) | |||||||
| Jonge et al. [31] | 1 day | - | - | - | - | 20 |
p > 0.05 (1 vs. 3 vs. 5 vs. 8 days) |
SCSA |
| 3 days | ||||||||
| 5 days | (5.8–62.9) a | |||||||
| 8 days | ||||||||
| Kulkarni et al. [32] | 1–3 h | - | - | - | - | 27.6 (10.1) | p < 0.050 | SCD |
| 2–7 days | 30.9 (11.2) | |||||||
| Marshburn et al. [33] | <2 days | 11.3 | p < 0.020 (<2 vs. 3–5 days) | - | - | - | - | - |
| 3–5 days | 6.1 | p < 0.050 (<2 vs. 3–5 + >5 days) | ||||||
| >5 days | 7.3 | p = 0.720 (3–5 vs. >5 days) | ||||||
| Barbagallo et al. [34] | 1 h | 31.0 | p = 0.001 | 22.0 | p = 0.040 | - | - | - |
| 2–7 days | 20.0 | 18.0 | ||||||
| Kably-Ambe et al. [35] | 0–1 days | 13.7 | - | - | - | - | - | - |
| 2–3 days | 12.9 | |||||||
| 4–5 days | 14.0 | |||||||
| 6–7 days | 15.1 | |||||||
| 8–9 days | 9.8 | |||||||
| 10–14 days | 8.6 | |||||||
| 15–20 days | 8.9 | |||||||
| Gupta et al. [36] | 1 day | 30.0 | p = 0.009 (1 day vs. all others) | - | - | - | - | - |
| 2–5 days | 25.4 | |||||||
| 6–7 days | 15.0 | |||||||
| >8 days | 18.1 | |||||||
| Manna et al. [37] | Normospermic: | - | - | - | - | p < 0.010 | SCD | |
| 1 h | 13.6 (9.6) | |||||||
| 2–7 days | 14.8 (8.6) | |||||||
| Seperated by swim up | - | - | - | - | p < 0.050 | |||
| 1 h | 11.2 (9.1) | |||||||
| 2–7 days | 12.7 (8.9) | |||||||
| Oligoastenoteratozoo-spermic: | - | - | - | - | p < 0.010 | |||
| 1 h | 25.2 (16.0) | |||||||
| 2–7 days | 28.3 (16.8) | |||||||
| Seperated by swim up | - | - | - | - | p < 0.001 | |||
| 1 h | 16.7 (8.4) | |||||||
| 2–7 days | 21.6 (10.6) | |||||||
| Azizi et al. [38] | 1 day | 29.2 | p = 0.900 | - | - | - | - | - |
| 2 days | 25.2 | |||||||
| days | 28.1 | |||||||
| 4 days | 26.9 | |||||||
| 5 days | 31.1 | |||||||
| 6–10 days | 27.8 | |||||||
| Welliver et al. [39] | 3–5 days (first analysis) | - | - | - | - | 14.0 (7.2) | p = 0.480 | TUNEL |
| 1 day (collected day 3) | 14.7 (7.5) | |||||||
| 1 day (collected day 7) | 14.6 (8.8) | |||||||
| 1 day (collected day 14) | 15.2 (9.1) | |||||||
| Periyasamy et al. [40] | 2–4 days | 45.4 |
p = 0.004 (2–4 vs. >7 days) p = 0.008 (2–7 vs. >7 days) p = 0.048 (5–7 vs. >7 days) |
36.1 |
p = 0.005 (2–4 vs. >7 days) p = 0.014 (2–7 vs. >7 days) p = 0.127 (5–7 vs. >7 days) |
- | - | - |
| 2–7 days | 44.4 | 34.1 | ||||||
| 5–7 days | 42.5 | 30.7 | ||||||
| >7 days | 32.7 | 24.1 | ||||||
| Lee et al. [41] | Maternel age <38 | p = 0.763 | - | - | - | - | - | |
| 2–7 days | 43.2 | |||||||
| 8 days | 41.4 | |||||||
| Maternel age >38 | p = 0.916 | |||||||
| 2–7 days | 22.0 | |||||||
| 8 days | 20.8 | |||||||
SCD: Sperm chromatin dispersion test, TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling, SCSA: Sperm chromatin structure assay. Bold p values are significant. a Mean value.
Appendix B
Table A2.
Risk of Bias assessment according to SIGN methodology checklist.
| Author | 1. Question | 1. Selection | 1. Assessment | 1. Confounding | 1. Statistical Analysis | 2. Quality | Notes |
|---|---|---|---|---|---|---|---|
| Dahan et al. [18] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = Yes | 2.1 = High | |
| 1.5 = 7% | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = Yes | 1.11 = Yes | 2.3 = Yes | |||||
| Kabukçu et al. [19] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Acceptable | Went from high to acceptable quality, because of missing confidence intervals. |
| 1.5 = 10% | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = Yes | 1.11 = Yes | 2.3 = Yes | |||||
| Agarwal et al. [20] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = Yes | 2.1 = Low | n = 7, therefore low quality. |
| 1.5 = Not reported | 1.10 = Yes | 2.2 = No | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Borges et al. [21] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = Yes | 2.1 = High | |
| 1.5 = 0% | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Vahidi et al. [22] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Acceptable | Went from high to acceptable quality, because of missing confidence intervals. |
| 1.5 = 0% | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Comar et al. [23] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = Yes | 2.1 = High | |
| 1.5 = 0% | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Uppangala et al. [24] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Acceptable | Missing confidence intervals. |
| 1.5 = Not reported | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Sánchez-Martín et al. [25] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = No | 1.14 = No | 2.1 = Low | Both insufficient confounding assessment and missing confidence intervals. |
| 1.5 = Not reported | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Scarselli et al. [26] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Acceptable | Went from high to acceptable quality, because of missing confidence intervals. |
| 1.5 = 0% | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Shen et al. [27] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Acceptable | Went from high to acceptable quality, because of missing confidence intervals. |
| 1.5 = Not reported | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Gosálvez et al. [28] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Can’t say | 1.14 = No | 2.1 = Acceptable | Went from high to acceptable quality, because of missing confidence intervals. |
| 1.5 = Not reported | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3= Yes | |||||
| Jurema et al. [29] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Can’t say | 1.14 = No | 2.1 = Acceptable | Retrospective design. |
| 1.5 = NA retrospective | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Mayorga-Torres et al. [30] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Low | n = 6, therefore low quality. |
| 1.5 = 0% | 1.10 = Yes | 2.2 = No | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Jonge et al. [31] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Low | n = 11, therefore low quality. |
| 1.5 = 30% | 1.10 = Yes | 2.2 = No | |||||
| 1.6 = Yes | 1.11 = Can’t say | 2.3 = Yes | |||||
| Kulkarni et al. [32] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Acceptable | |
| 1.5 = not reported | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Marshburn et al. [33] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Can’t say | 1.14 = No | 2.1 = Acceptable | Retrospective design. |
| 1.5 = NA retrospective | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Barbagallo et al. [34] | 1.1 = Ýes | 1.2 = Can’t say | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Acceptable | |
| 1.5 = Not reported | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Kably-Ambe et al. [35] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Can’t say | 1.14 = No | 2.1 = Acceptable | Retrospective design. |
| 1.5 = NA retrospective | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Gupta et al. [36] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = Yes | 2.1 = Acceptable | High quality study but cannot rate higher than acceptable because of retrospective study design. |
| 1.5 = NA retrospective | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Manna et al. [37] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Acceptable | Went from high to acceptable quality, because of missing confidence intervals. |
| 1.5 = 1.5% | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Azizi et al. [38] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = Yes | 2.1 = Acceptable | High quality study but cannot rate higher than acceptable because of retrospective study design. |
| 1.5 = NA retrospective | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Welliver et al. [39] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Can’t say | 1.14 = No | 2.1 = Acceptable | |
| 1.5 = 5% | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = Can’t say | 1.11 = Yes | 2.3 = Yes | |||||
| Periyasamy et al. [40] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = Yes | 2.1 = Acceptable | High quality study but cannot rate higher than acceptable because of retrospective study design. |
| 1.5 = NA retrospective | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes | |||||
| Lee et al. [41] | 1.1 = Yes | 1.2 = Yes | 1.7 = Yes | 1.13 = Yes | 1.14 = No | 2.1 = Acceptable | Retrospective design. |
| 1.5 = NA retrospective | 1.10 = Yes | 2.2 = Yes | |||||
| 1.6 = NA | 1.11 = Yes | 2.3 = Yes |
NA: not applicable. Questions that were not relevant for our study were removed. In the selection section 1.3 and 1.4 were not applicable and in the assessment section 1.8, 1.9 and 1.12 were not applicable.
Author Contributions
Conceptualization, J.F. and F.S.; methodology, S.S., F.S., L.M.M. and J.F.; validation, F.S., L.M.M. and S.S.; formal analysis, F.S., L.M.M. and S.S.; investigation, F.S., L.M.M., S.S. and J.F.; resources, J.F.; writing—original draft preparation. F.S.; writing—review and editing, F.S., L.M.M., S.S. and J.F.; visualization, F.S. and J.F.; supervision, S.S. and J.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Schmidt L. Infertility and Assisted Reproduction in Denmark. Epidemiology and Psychosocial Consequences. Dan. Med. Bull. 2006;53:390–417. [PubMed] [Google Scholar]
- 2.World Health Organization . WHO Laboratory Manual for the Examination and Processing of Human Semen. 6th ed. World Health Organization; Geneva, Switzerland: 2021. [Google Scholar]
- 3.Jensen T.K., Swan S.H., Skakkebæk N.E., Rasmussen S., Jørgensen N. Caffeine Intake and Semen Quality in a Population of 2,554 Young Danish Men. Am. J. Epidemiol. 2010;171:883–891. doi: 10.1093/aje/kwq007. [DOI] [PubMed] [Google Scholar]
- 4.Ferlin A., Garolla A., Ghezzi M., Selice R., Palego P., Caretta N., di Mambro A., Valente U., de Rocco Ponce M., Dipresa S., et al. Sperm Count and Hypogonadism as Markers of General Male Health. Eur. Urol. Focus. 2021;7:205–213. doi: 10.1016/j.euf.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Carlsen E., Holm Petersen J., Andersson A.-M., Skakkebaek N.E. Effects of Ejaculatory Frequency and Season on Variations in Semen Quality. Fertil. Steril. 2004;82:358–366. doi: 10.1016/j.fertnstert.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Fedder J. Nonsperm Cells in Human Semen: With Special Reference to Seminal Leukocytes and Their Possible Influence on Fertility. Arch. Androl. 1996;36:41–65. doi: 10.3109/01485019608987883. [DOI] [PubMed] [Google Scholar]
- 7.Gervasi M.G., Visconti P.E. Molecular Changes and Signaling Events Occurring in Spermatozoa during Epididymal Maturation. Andrology. 2017;5:204–218. doi: 10.1111/andr.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibov M.Y., Kuzmin A.V., Alexandrova A.A., Chistyakov V.A., Dobaeva N.M., Kundupyan O.L. Role of the Reactive Oxygen Species Induced DNA Damage in Human Spermatozoa Dysfunction. AME Med. J. 2018;3:19. doi: 10.21037/amj.2018.01.06. [DOI] [Google Scholar]
- 9.Zini A., Boman J.M., Belzile E., Ciampi A. Sperm DNA Damage Is Associated with an Increased Risk of Pregnancy Loss after IVF and ICSI: Systematic Review and Meta-Analysis. Hum. Reprod. 2008;23:2663–2668. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 10.Osman A., Alsomait H., Seshadri S., El-Toukhy T., Khalaf Y. The Effect of Sperm DNA Fragmentation on Live Birth Rate after IVF or ICSI: A Systematic Review and Meta-Analysis. Reprod. Biomed. Online. 2015;30:120–127. doi: 10.1016/j.rbmo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Gil-Guzman E., Ollero M., Lopez M.C., Sharma R.K., Alvarez J.G., Thomas A.J., Agarwal A. Differential Production of Reactive Oxygen Species by Subsets of Human Spermatozoa at Different Stages of Maturation. Hum. Reprod. 2001;16:1922–1930. doi: 10.1093/humrep/16.9.1922. [DOI] [PubMed] [Google Scholar]
- 12.Ayad B.M., Van der Horst G., Du Plessis S.S. Revisiting the Relationship between the Ejaculatory Abstinence Period and Semen Characteristics. Int. J. Fertil. Steril. 2018;11:238–246. doi: 10.22074/ijfs.2018.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokol P., Drakopoulos P., Polyzos N.P. The Effect of Ejaculatory Abstinence Interval on Sperm Parameters and Clinical Outcome of Art. A Systematic Review of the Literature. J. Clin. Med. 2021;10:3213. doi: 10.3390/jcm10153213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Shi Q., Li X., Guo J., Zhang L., Quan Y., Ma M., Yang Y. The Effect of Male Sexual Abstinence Periods on the Clinical Outcomes of Fresh Embryo Transfer Cycles Following Assisted Reproductive Technology: A Meta-Analysis. Am. J. Men’s Health. 2020;14:1557988320933758. doi: 10.1177/1557988320933758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barratt C.L.R., Björndahl L., Menkveld R., Mortimer D. ESHRE Special Interest Group for Andrology Basic Semen Analysis Course: A Continued Focus on Accuracy, Quality, Efficiency and Clinical Relevance. Hum. Reprod. 2011;26:3207–3212. doi: 10.1093/humrep/der312. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scottish Intercollegiate Guidelines Network (SIGN) a Guideline Developer’s Handbook. SIGN; Edinburgh, UK: 2019. SIGN 50. [Google Scholar]
- 18.Dahan M.H., Mills G., Khoudja R., Gagnon A., Tan G., Tan S.L. Three Hour Abstinence as a Treatment for High Sperm DNA Fragmentation: A Prospective Cohort Study. J. Assist. Reprod. Genet. 2020;38:227–233. doi: 10.1007/s10815-020-01999-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabukçu C., Çil N., Çabuş Ü., Alataş E. Effect of Ejaculatory Abstinence Period on Sperm DNA Fragmentation and Pregnancy Outcome of Intrauterine Insemination Cycles: A Prospective Randomized Study. Arch. Gynecol. Obstet. 2020;303:269–278. doi: 10.1007/s00404-020-05783-0. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal A., Gupta S., du Plessis S., Sharma R., Esteves S.C., Cirenza C., Eliwa J., Al-Najjar W., Kumaresan D., Haroun N., et al. Abstinence Time and Its Impact on Basic and Advanced Semen Parameters. Urology. 2016;94:102–110. doi: 10.1016/j.urology.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 21.Borges E., Braga D.P.A.F., Zanetti B.F., Iaconelli A., Setti A.S. Revisiting the Impact of Ejaculatory Abstinence on Semen Quality and Intracytoplasmic Sperm Injection Outcomes. Andrology. 2018;7:213–219. doi: 10.1111/andr.12572. [DOI] [PubMed] [Google Scholar]
- 22.Vahidi S., Narimani N., Ghanizadeh T., Yazdinejad F., Emami M., Mehravaran K., Saffari H., khaleghiMehr F., Marvast L.D. Short Abstinence May Have Paradoxical Effects On Sperms With Different Level Of DNA Integrity: A Prospective Study. Urol. J. 2021;18:682–687. doi: 10.22037/uj.v18i.6515. [DOI] [PubMed] [Google Scholar]
- 23.Comar V.A., Petersen C.G., Mauri A.L., Mattila M., Vagnini L.D., Renzi A., Petersen B., Nicoletti A., Dieamant F., Oliveira J.B.A., et al. Influence of the Abstinence Period on Human Sperm Quality: Analysis of 2,458 Semen Samples. J. Bras. Reprod. Assist. 2017;21:306–312. doi: 10.5935/1518-0557.20170052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uppangala S., Mathai S.E., Salian S.R., Kumar D., Singh V.J., D’Souza F., Kalthur G., Kamath A., Adiga S.K. Sperm Chromatin Immaturity Observed in Short Abstinence Ejaculates Affects DNA Integrity and Longevity in Vitro. PLoS ONE. 2016;11:e0152942. doi: 10.1371/journal.pone.0152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez-Martín P., Sánchez-Martín F., González-Martínez M., Gosálvez J. Increased Pregnancy after Reduced Male Abstinence. Syst. Biol. Reprod. Med. 2013;59:256–260. doi: 10.3109/19396368.2013.790919. [DOI] [PubMed] [Google Scholar]
- 26.Scarselli F., Cursio E., Muzzì S., Casciani V., Ruberti A., Gatti S., Greco P., Varricchio M.T., Minasi M.G., Greco E. How 1 h of Abstinence Improves Sperm Quality and Increases Embryo Euploidy Rate after PGT-A: A Study on 106 Sibling Biopsied Blastocysts. J. Assist. Reprod. Genet. 2019;36:1591–1597. doi: 10.1007/s10815-019-01533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Z.Q., Shi B., Wang T.R., Jiao J., Shang X.J., Wu Q.J., Zhou Y.M., Cao T.F., Du Q., Wang X.X., et al. Characterization of the Sperm Proteome and Reproductive Outcomes with in Vitro Fertilization after a Reduction in Male Ejaculatory Abstinence Period. Mol. Cell. Proteom. 2019;18:S109–S117. doi: 10.1074/mcp.RA117.000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosálvez J., González-Martínez M., López-Fernández C., Fernández J.L., Sánchez-Martín P. Shorter Abstinence Decreases Sperm Deoxyribonucleic Acid Fragmentation in Ejaculate. Fertil. Steril. 2011;96:1083–1086. doi: 10.1016/j.fertnstert.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Jurema M.W., Vieira A.D., Bankowski B., Petrella C., Zhao Y., Wallach E., Zacur H. Effect of Ejaculatory Abstinence Period on the Pregnancy Rate after Intrauterine Insemination. Fertil. Steril. 2005;84:678–681. doi: 10.1016/j.fertnstert.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 30.Mayorga-Torres B.J.M., Camargo M., Agarwal A., du Plessis S.S., Cadavid Á.P., Cardona Maya W.D. Influence of Ejaculation Frequency on Seminal Parameters. Reprod. Biol. Endocrinol. 2015;13:47. doi: 10.1186/s12958-015-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Jonge C., LaFromboise M., Bosmans E., Ombelet W., Cox A., Nijs M. Influence of the Abstinence Period on Human Sperm Quality. Fertil. Steril. 2004;82:57–65. doi: 10.1016/j.fertnstert.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Kulkarni V., Kaingade P., Kulkarni N., Bhalerao T., Nikam A. Assessment of Semen Parameters in Consecutive Ejaculates with Short Abstinence Period in Oligospermic Males. J. Bras. Reprod. Assist. 2022;26:310–314. doi: 10.5935/1518-0557.20210073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshburn P.B., Alanis M., Matthews M.L., Usadi R., Papadakis M.H., Kullstam S., Hurst B.S. A Short Period of Ejaculatory Abstinence before Intrauterine Insemination Is Associated with Higher Pregnancy Rates. Fertil. Steril. 2010;93:286–288. doi: 10.1016/j.fertnstert.2009.07.972. [DOI] [PubMed] [Google Scholar]
- 34.Barbagallo F., Calogero A.E., Condorelli R.A., Farrag A., Jannini E.A., la Vignera S., Manna C. Does a Very Short Length of Abstinence Improve Assisted Reproductive Technique Outcomes in Infertile Patients with Severe Oligo-Asthenozoospermia? J. Clin. Med. 2021;10:4399. doi: 10.3390/jcm10194399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kably-Ambe A., Carballo E., Leonor M., Karla D.-M., Soriano-Ortega P., Roque-Sánchez A.M., Ambe A.K. Effect of Sexual Abstinence on Pregnancy Rates after an Intrauterine Insemination Correspondencia. Gineol. Obstet. Mex. 2015;83:104–109. [PubMed] [Google Scholar]
- 36.Gupta S., Singh V.J., Fauzdar A., Prasad K., Srivastava A., Sharma K. Short Ejaculatory Abstinence in Normozoospermic Men Is Associated with Higher Clinical Pregnancy Rates in Sub-Fertile Couples Undergoing Intra-Cytoplasmic Sperm Injection in Assisted Reproductive Technology: A Retrospective Analysis of 1691 Cycles. J. Hum. Reprod. Sci. 2021;14:273–280. doi: 10.4103/jhrs.jhrs_235_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manna C., Barbagallo F., Manzo R., Rahman A., Francomano D., Calogero A.E. Sperm Parameters before and after Swim-up of a Second Ejaculate after a Short Period of Abstinence. J. Clin. Med. 2020;9:1029. doi: 10.3390/jcm9041029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azizi E., Naji M., Salehpour S., Saharkhiz N., Karimi M., Borumandnia N., Mofarahe Z.S. Impact of Ejaculatory Abstinence Period and Semen Characteristic on the Reproductive Outcomes after Intracytoplasmic Sperm Injection. J. Bras. Reprod. Assist. 2021;26:475–481. doi: 10.5935/1518-0557.20210124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welliver C., Benson A.D., Frederick L., Leader B., Tirado E., Feustel P., Kontio J., McAsey M., Köhler T.S. Analysis of Semen Parameters during 2 Weeks of Daily Ejaculation: A First in Humans Study. Transl. Androl. Urol. 2016;5:749–755. doi: 10.21037/tau.2016.08.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Periyasamy A.J., Mahasampath G., Karthikeyan M., Mangalaraj A.M., Kunjummen A.T., Kamath M.S. Does Duration of Abstinence Affect the Live-Birth Rate after Assisted Reproductive Technology? A Retrospective Analysis of 1030 Cycles. Fertil. Steril. 2017;108:988–992. doi: 10.1016/j.fertnstert.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 41.Lee J.W., Cha J.H., Shin S.H., Cha H.J., Kim J.H., Park C.K., Pak K.A., Yoon J.S., Park S.Y. Effect of the Sexual Abstinence Period Recommended by the World Health Organization on Clinical Outcomes of Fresh Embryo Transfer Cycles with Normal Ovarian Response after Intracytoplasmic Sperm Injection. Andrologia. 2018;50:e12964. doi: 10.1111/and.12964. [DOI] [PubMed] [Google Scholar]
- 42.Shi X., Chan C.P.S., Waters T., Chi L., Chan D.Y.L., Li T.C. Lifestyle and Demographic Factors Associated with Human Semen Quality and Sperm Function. Syst. Biol. Reprod. Med. 2018;64:358–367. doi: 10.1080/19396368.2018.1491074. [DOI] [PubMed] [Google Scholar]
- 43.Sharma R., Harlev A., Agarwal A., Esteves S.C. Cigarette Smoking and Semen Quality: A New Meta-Analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016;70:635–645. doi: 10.1016/j.eururo.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Hanson B.M., Aston K.I., Jenkins T.G., Carrell D.T., Hotaling J.M. The Impact of Ejaculatory Abstinence on Semen Analysis Parameters: A Systematic Review. J. Assist. Reprod. Genet. 2018;35:213–220. doi: 10.1007/s10815-017-1086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbagallo F., Cannarella R., Crafa A., Manna C., la Vignera S., Condorelli R.A., Calogero A.E. The Impact of a Very Short Abstinence Period on Conventional Sperm Parameters and Sperm DNA Fragmentation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022;11:7303. doi: 10.3390/jcm11247303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.