Abstract
Plasmodium falciparum parasites carrying deletions of histidine-rich protein 2 and 3 genes, pfhrp2 and pfhrp3, respectively, are likely to escape detection via HRP2-based rapid diagnostic tests (RDTs) and, consequently, treatment, posing a major risk to both the health of the infected individual and malaria control efforts. This study assessed the frequency of pfhrp2- and pfhrp3-deleted strains at four different study sites in Central Africa (number of samples analyzed: Gabon N = 534 and the Republic of Congo N = 917) and West Africa (number of samples analyzed: Nigeria N = 466 and Benin N = 120) using a highly sensitive multiplex qPCR. We found low prevalences for pfhrp2 (1%, 0%, 0.03% and 0) and pfhrp3 single deletions (0%, 0%, 0.03% and 0%) at all study sites (Gabon, the Republic of Congo, Nigeria and Benin, respectively). Double-deleted P. falciparum were only found in Nigeria in 1.6% of all internally controlled samples. The results of this pilot investigation do not point towards a high risk for false-negative RDT results due to pfhrp2/pfhrp3 deletions in Central and West African regions. However, as this scenario can change rapidly, continuous monitoring is essential to ensure that RDTs remain a suitable tool for the malaria diagnostic strategy.
Keywords: Plasmodium falciparum, histidine-rich protein 2 and 3, pfhrp2/pfhrp3 deletions, rapid diagnostic test, Central Africa, West Africa
1. Introduction
Malaria represents a major health burden mainly affecting Sub-Saharan Africa. The majority of the more than 247 million cases and 619,000 deaths per year occur in Sub-Saharan Africa [1]. Malaria is caused by Plasmodium spp. parasites, with Plasmodium falciparum being the deadliest and most prevalent species in Africa. In order to reduce the burden and spread of the disease, prevention, therapy and early and accurate diagnosis are crucial. The primary tool for malaria diagnostics comprises microscopic detection of stained parasites and rapid diagnostic tests (RDT). The latter is of particular importance in rural African areas where microscopy services are difficult to implement due to limited availability of equipment, electricity and trained personnel. The majority of RDTs in use contain monoclonal antibodies against P. falciparum histidine-rich protein 2 (HRP2) that also cross-react to a certain level with the structurally related P. falciparum histidine-rich protein 3 (HRP3) [2,3]. These proteins are P. falciparum-specific and are expressed in all stages of the asexual life cycle from subtelomeric genes on chromosome 7 for pfhrp2 and chromosome 13 for pfhrp3. The function of HRP2 is not fully understood, but it has been proposed to be involved in heme detoxification, modulation of infected red blood cells or host immune response [4,5,6].
Parasites that lack pfhrp2 and optionally pfhrp3 can cause false-negative results in HRP2-based RDTs. In settings with a well-established test-and-treat strategy, pfhrp2/pfhrp3-deleted strains could escape diagnosis and treatment and thus could eventually be positively selected from the parasite population [7]. First deletions have been found between 2003 and 2008 in South America with prevalences of 41% for pfhrp2 and 70% for pfhrp3 [8]. In the last decade, many more deleted P. falciparum strains were found all across the globe in Asia, the Middle East and Africa [1]. Based on the malaria threat map provided by the World Health Organization (WHO) to track studies, deleted strains have been identified in 37 of 44 countries under investigation with largely varying prevalences [9]. It is hypothesized that deletions in pfhrp2 and pfhrp3 genes occur naturally in the population due to their subtelomeric location but the drivers of selection are not well understood. Some studies found lower parasitemia in sub-sets of patients with pfhrp2- and pfhrp3-deleted parasites, suggesting a fitness cost that has been supported in experimental competition assays using gene edited parasites [10,11,12]. Mathematical models have identified low transmission and high treatment rates after HRP2-based diagnosis as key factors for the spread of deleted strains [7].
The WHO has recognized pfhrp2 and pfhrp3 gene deletions as one of the major threats to malaria elimination and calls for urgent action on monitoring the prevalence of these deletions [13]. Alternative non-HRP2-based RDTs are currently not widely available and often inferior to HRP2-based RDTs in regard to sensitivity or stability [14,15]. The WHO recommends changing the national diagnostic strategy to non-HRP2-based diagnostics only if a prevalence threshold of 5% for pfhrp2/pfhrp3 gene deletions causing false-negative RDT results is exceeded. So far, this has happened in Eritrea, Ethiopia and Djibouti, leading to a switch to non-HRP2-based routine malaria diagnostics [1]. The prevalence of gene deletions differs widely, both within and between malaria-endemic countries, and many countries have never conducted epidemiologic studies on the prevalence of pfhrp2/pfhrp3-deleted parasites. To date, there have not been any peer-reviewed data published from Benin or the Republic of Congo. For Benin, a first study on deletions has recently been presented at the American Society of Tropical Medicine and Hygiene (ASTMH) annual meeting that found 21 pfhrp2 deletions in 471 P. falciparum isolates (4.5%) [16]. Both countries have neighboring countries where the presence of deletions has been reported, such as the Democratic Republic of Congo with a prevalence of 6.4% or Nigeria with pfhrp2 deletions in 16% of the analyzed samples [17,18].
Most studies utilize classic PCR methods followed by gel electrophoresis and visual analysis to identify gene deletions [17,18,19,20]. Although one or more single-copy genes are used as reference, these approaches are prone to misclassification due to the qualitative read-outs. A recent comparison of digital droplet PCR (ddPCR) and nested PCR (nPCR) demonstrated an increased risk of overestimation of deletions in studies relying on nPCR [21]. We recently developed a highly sensitive multiplex quantitative PCR (4-plex qPCR) targeting four genes within one participant sample [22]. The 4-plex qPCR is suited for large-scale screening as it detects and confirms P. falciparum infection and identifies pfhrp2 and pfhrp3 deletions against a single-copy control gene [22]. The internally controlled single-tube approach makes it a rapid and specific tool, which facilitates the identification of regional hotspots in need of adapted control strategies. Recently, two additional multiplex qPCR approaches have been developed and, so far, have been applied to first sample sets from African countries [23,24]. The aim of this study was to apply the 4-plex qPCR to large cohorts to provide a first assessment of the prevalence of pfhrp2/pfhrp3 deletions in different regions of Central and West Africa.
2. Materials and Methods
This retrospective, cross-sectional, epidemiological study was performed on samples collected in specific areas from Gabon, Congo, Nigeria and Benin. Figure 1 gives a geographic overview of the involved study sites and Table 1 details the sampling and study population characteristics. In Gabon, samples collected from 2019 to 2020 from individuals older than two years and living in semi-urban Lambaréné and rural surroundings were included. The study design has been previously described [22]. Whole blood was collected into ethylenediamine tetra-acetic acid (EDTA) tubes. In Congo, individuals older than one year and living in the southern parts of the country (rural Goma Tse-Tse district; urban Brazzaville) were enrolled. Whole blood was sampled from March to September 2021 covering both the dry and the rainy seasons [25]. In Nigeria, whole blood was sampled from 2018 to 2019 from individuals in hospital settings located in semi-urban areas in Anambra, the state of southeastern Nigeria. In Benin, individuals of at least one year of age and living in rural areas of the Kpomasse-Tori Bossito health district in southern Benin were enrolled. Capillary blood was collected between June and October 2019 [26] and stored on filter paper as dried blood spots.
Figure 1.
Geographical overview of the study sites in West and Central Africa. Red dots represent sampling sites (1) Benin: Kpomasse, Ouidah; (2) Nigeria: Awka, Nnewi, Onitsha; (3) Gabon: Lambaréné; (4) Republic of Congo: Goma Tsé-Tsé District, Brazzaville. The map was created with the ggplot2 package V3.4.0 in R Version 4.2.2.
Table 1.
Demographic characteristics of the study populations.
| Gabon | Congo | Nigeria | Benin | |
|---|---|---|---|---|
| Total N | 534 | 917 | 466 | 120 |
| Age in years: | ||||
| Median (IQR) | 16 (6–25) | 15 (8–38) | * | 9 (5–13) |
| Age cohorts: | ||||
| 0–6 years, n (%) | 77 (14%) | 169 (18%) | - | 45 (38%) |
| 7–18 years, n (%) | 91 (17%) | 334 (36%) | - | 49 (41%) |
| 18+ years, n (%) | 134 (25%) | 412 (45%) | - | 26 (22%) |
| Missing data | 232 (43%) | 2 (0.2%) | 466 (100%) | - |
| Sex: | ||||
| Female | 258 (48%) | 514 (56%) | 75 (63%) | |
| Male | 154 (29%) | 403 (44%) | 45 (38%) | |
| Missing data: | 122 (23%) | - | 466 (100%) | - |
| Symptomatic: | * | 73/914 (8%) | 299 (64%) | 9 (8%) |
| Sampling period: | 2019–2020 | 2021 | 2018–2019 | 2019 |
| Number of study sites: | ||||
| 1 | Lambaréné 534 (100%) |
Goma Tsé-Tsé District 573 (62%) |
Awka 200 (43%) |
Kpomasse 68 (57%) |
| 2 | - | Brazzaville 344 (38%) |
Nnewi 167 (36%) |
Ouidah 52 (43%) |
| 3 | - | - | Onitsha 99 (21%) |
- |
| Measurements: | ||||
| TBS done, n (%) | 534 (100%) | 917 (100%) | not done | 120 (100%) |
| RDT done, n (%) | 532 (99.6%) | 917 (100%) | not available | not done |
| RDT brand: | Paracheck Pf RDT | Malaria P.f./Pan Antigen kit, Cypress Diagnostics | - | - |
* Data are missing. IQR: Interquartile range TBS: Thick blood smear microscopy, RDT: Malaria rapid diagnostic test.
Demographic characteristics were assessed from questionnaires. Individuals were considered symptomatic if they had a fever of over 37.5 °C. For parasitological assessment, fresh blood was collected and directly used for RDT and/or microscopy. RDTs were performed using the Paracheck Pf RDT (detects HRP2 only) in Gabon and the Malaria P.f./Pan Antigen kit, Cypress Diagnostics, in the Republic of Congo. This was conducted at the time of sample collection. In Nigeria, RDTs with the Standard Diagnostics Bioline Malaria Ag P.f Test kit detecting only HRP2 were performed for a sub-set of samples but, for consistency reasons, were not considered in this analysis. In Benin, no RDTs were performed. In addition, microscopy was performed in Gabon, Congo and Benin to detect and quantify P. falciparum as described previously [25,27]. Microscopic reading was performed by two independent microscopists. For the samples originating from Nigeria, no microscopy was performed.
For Gabon, Congo and Nigeria, DNA was extracted from whole EDTA blood using the QIAamp DNA mini Kit (Qiagen, Hilden, Germany) or the Quick-DNA Miniprep kit (Zymo-Research, Freiburg, Germany) according to the manufacturer’s instructions. In Benin, DNA was extracted from dried blood spots using the Chelex protocol as described previously [28]. The DNA was used in the 4-plex qPCR based on the amplification of the four target genes: cytochrome b (pfcytb), ß tubulin (pfbtub), pfhrp2 and pfhrp3. The qPCR was performed at the Institute of Tropical Medicine, Tuebingen, Germany, for the samples from Gabon, Nigeria and Benin, and at the Centre de Recherches sur les Maladies Infectieuses (CeRMI), Brazzaville, Republic of Congo, for the samples from Congo. The 4-plex qPCR protocol was based on our previous publication with the following modifications [22]: pfhrp2 and pfhrp3 primers and probe sequences were modified to increase mismatches to the oligo-binding region of the respective homologous gene (oligonucleotide sequences are given in Table A1), and the pfbtub probe now comprises an internal TAO quencher in order to decrease the background signal of the quality control assay. The 4-plex qPCR reaction was performed at the following final concentrations: 1× TaqMan Multiplex Master Mix (ThermoFisher Scientific, Waltham, MA, USA), 400 nM for pfcytb, pfhrp2 and pfhrp3 forward and reverse primers each and 600 nM for pfbtub primers. The final probe concentrations were 300 nM for pfbtub, 75 nM for pfhrp2 and 150 nM for pfhrp3 and pfcytb. The 4-plex qPCR was performed in 384-well or 96-well plates on a LightCycler 480 I or II (Roche Diagnostics, Basel, Switzerland) with a final volume of 10 µL including 3 µL template or 20 µL including 6 µL template DNA. For each instrument, color compensation was performed and applied to the respective experiments before analysis. Cycling conditions included an initial activation step at 95 °C for 20 s, 45 cycles at 95 °C for 3 s, followed by 62 °C for 150 s and a final cooling step at 40 °C. The adapted protocol was characterized and showed a comparable limit of detection of 0.06 parasites/µL for pfcytb and 0.6 parasites/µL for pfbtub, pfhrp2 and pfhrp3.
The qPCR was validated in each setting with five different controls: DNA extracted from the P. falciparum laboratory strains 3D7 (pfhrp2+/pfhrp3+), Dd2 (pfhrp2−/pfhrp3+), HB3 (pfhrp2+/pfhrp3−), uninfected whole blood as non-template control and water. Samples were measured in duplicates or triplicates with at least one positive control and one negative control on each plate. Discordant duplicates were repeated. Genotyping of the P. falciparum chloroquine resistance transporter (pfcrt) was performed using a multiplex qPCR assay as described previously [29].
LightCycler 480 II (Roche Diagnostics, Basel, Switzerland, Version 1.5.1.62) software was used and quantification cycles (Cq) were obtained via absolute quantification using the second derivative maximum method or fit point method with the threshold above the negative controls [30,31]. The result was interpreted as positive for the respective gene if at least two out of two or three replicates showed amplification (Cq < 40). A sample was considered negative for the respective gene if there was no amplification in any of the duplicates or no more than one in the triplicates. Plates with no amplification of the positive control or a signal in the negative control were excluded from further analysis and repeated. Confirmed P. falciparum positive samples (positive for pfcytb) were considered deleted if they were positive for the single-copy control gene pfbtub and negative for pfhrp2 and/or pfhrp3 in at least two independent experiments.
Demographic characteristics were expressed via absolute numbers and percentages for categorical variables. For the agreement between the different diagnostic methods, Cohen’s kappa or Fleiss kappa was calculated as appropriate [32]. The strength of agreement can be interpreted as fair, moderate or substantial for kappa values ranging from 0.2–0.4, 0.4–0.6 and 0.6–0.8, respectively [33]. The difference of Cq values between false-positive and true-positive RDTs was evaluated using the Mann–Whitney U-test. The statistical analysis and graphical presentations were conducted in R (V4.0.2) (R Core Team, Vienna, Austria).
3. Results
3.1. Baseline Characteristics
A total number of 2037 participants were included in 4 different study regions in Gabon (N = 534), Congo (N = 917), Nigeria (N = 466) and Benin (N = 120).
The baseline characteristics of the study participants are displayed in Table 1. The median age of the study population ranged from 9 years in Benin to 16 years in Gabon (no information on age was available for the Nigerian cohort). A detailed description of the demographics, symptoms and diagnostic test results of 773 out of the 917 individuals from the Congolese study population can be found elsewhere [25]. The Congolese, Nigerian and Beninese cohorts comprised both symptomatic and asymptomatic individuals. For Gabon, no data on the symptom status were available.
3.2. Malaria Diagnosis Outcome
For diagnosis of P. falciparum infections, thick blood smear (TBS) microscopy, malaria rapid diagnostic tests (RDT) and 4-plex qPCR (pfcytb for P. falciparum detection) were performed and the results are presented in Table 2.
Table 2.
P. falciparum diagnostic outcomes.
| Gabon | Congo | Nigeria | Benin | |
|---|---|---|---|---|
| Total N | 534 | 917 | 466 | 120 |
| TBS positive, n (%) | 96 (18%) | 329 (36%) | - | 120 (100%) |
| Median parasitemia [p/µL] | 475 (145–1849) | 530 (147–5514) | - | 2240 (1008–6900) |
| RDT positive, n (%) | 186/532 * (35%) | 478 (52%) | - | - |
| qPCR positive | 273 (51%) | 643 (70%) | 379 (81%) | 120 (100%) |
| False-negative RDTs vs TBS vs qPCR |
8 91 |
2 186 |
- - |
- - |
| Kappa | ||||
| RDT/TBS | 0.50 | 0.67 | - | - |
| RDT/qPCR | 0.64 | 0.54 | - | - |
| TBS/qPCR | 0.33 | 0.36 | - | - |
| RDT/TBS/qPCR | 0.47 | 0.50 | - | - |
* Data are missing. qPCR positivity refers to pfcytb positivity.
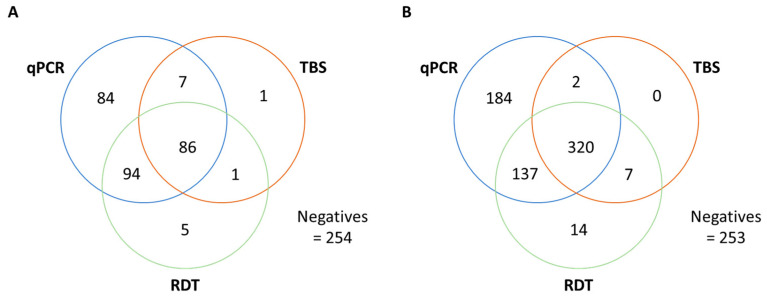
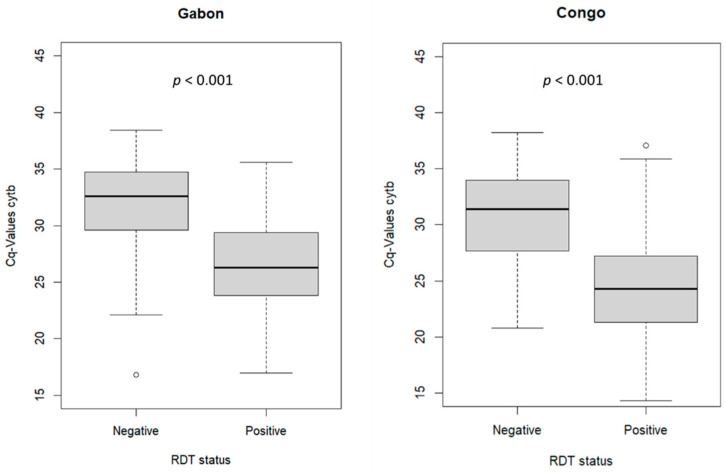
The concordance of positive test results of samples, which were systematically tested with all three diagnostic methods, was analyzed (Figure 2). As expected, there was moderate to substantial agreement between microscopy of Giemsa-stained TBS and RDT—two methods that have a similar limit of detection. Considering microscopy as the gold standard, there were 8/95 (8%) and 2/329 (0.6%) cases of false-negative HRP2-RDT results in Gabon and Congo, respectively, resulting in an RDT sensitivity of 92% and 99%, respectively. PCR-based methods, especially qPCR, are more sensitive than microscopy and RDT, and can detect sub-microscopic infections. Compared to the highly sensitive 4-plex qPCR (pfcytb), there were 91/271 (34%) and 186/643 (29%) false-negative samples by HRP2-RDT in Gabon and Congo, respectively. For samples with a false-negative RDT result, the Cq values for P. falciparum/pfcytb were significantly higher, indicating a lower parasitemia compared to RDT true-positive samples (p < 0.001). This indicates that low parasitemia could account for many of the false-negative RDT outcomes (Figure A2).
Figure 2.
Venn diagram of concordant P. falciparum diagnostics. Concordance of positive test results between the 4-plex qPCR (P. falciparum detection via pfcytb), RDT and TBS microscopy in (A) Gabon and (B) the Republic of Congo. Only samples with available results for all three diagnostic tests (qPCR, TBS, RDT) were included. RDT: Malaria rapid diagnostic test, TBS: Thick blood smear microscopy.
3.3. Molecular pfhrp2 and pfhrp3 Deletion Detection
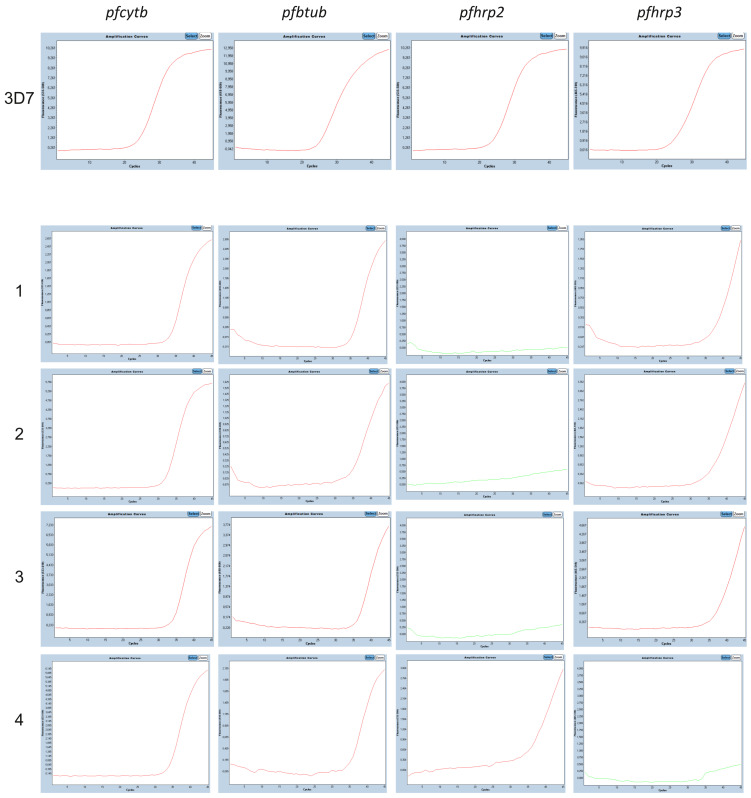
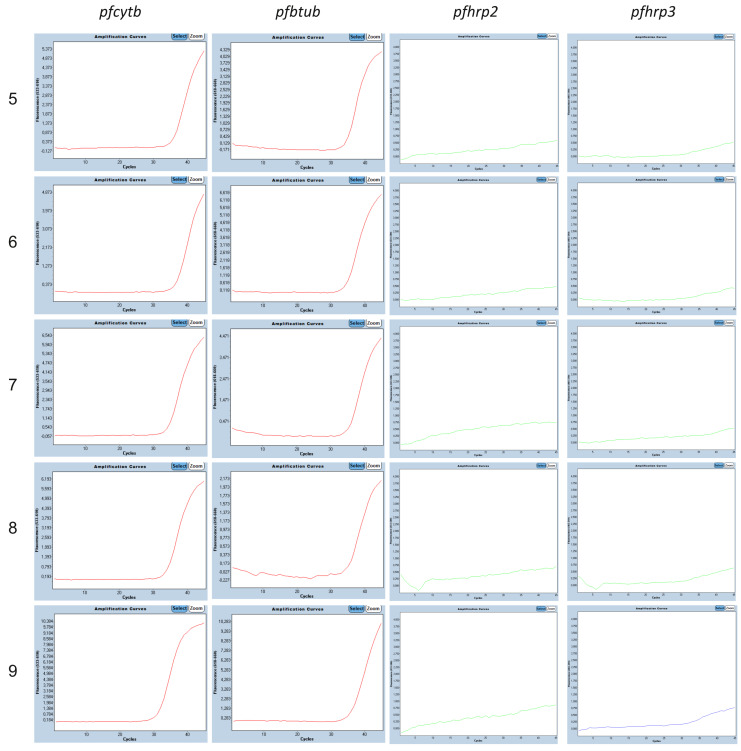
Amongst the samples confirmed for P. falciparum infection and for sufficient DNA template for single-copy gene amplification, thus double-positive samples for pfcytb and pfbtub, no pfhrp2 and/or pfhrp3 deletion was found in Congo and Benin (Table 3). In Gabon, two pfhrp2-deleted P. falciparum parasites were found. Double-deleted parasites were only detected in Nigeria. The qPCR curves for each of the single- and double-deleted P. falciparum samples can be found in Figure A3 and A4, respectively.
Table 3.
Prevalence of pfhrp2, pfhrp3 and pfhrp2/pfhrp3 double deletions stratified by study site.
| Gabon | Republic of Congo | Nigeria | Benin | |
|---|---|---|---|---|
| pfcytb and pfbtub positive, n | 218 | 512 | 316 | 120 |
| pfhrp2-deleted | 2 (1%) | 0 (0%) | 1 (0.03%) | 0 (0%) |
| pfhrp3-deleted | 0 (0%) | 0 (0%) | 1 (0.03%) | 0 (0%) |
| pfhrp2- and pfhrp3-deleted | 0 (0%) | 0 (0%) | 5 (1.6%) | 0 (0%) |
pfcytb positivity indicates the presence of P. falciparum infection, while pfbtub as single-copy gene serves as an internal quality control for pfhrp2/pfhrp3 deletion analysis.
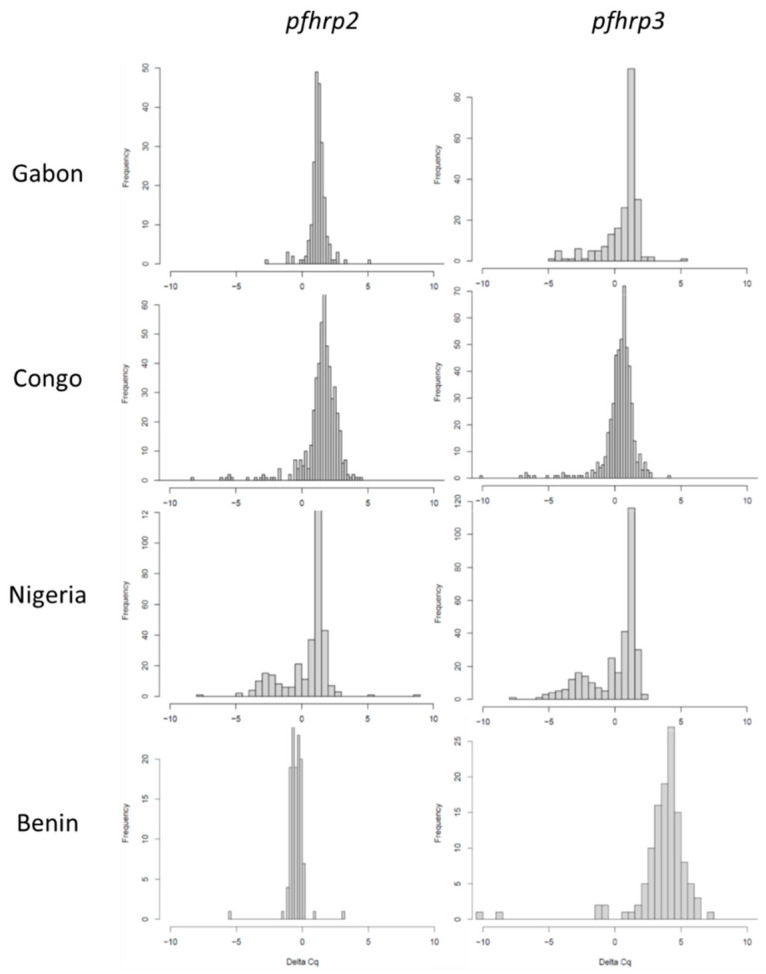
Some authors have used multiplex qPCR to identify polyclonal infections of deleted and non-deleted strains based on the Cq difference between the pfhrp2/pfhrp3 and the single-copy reference gene pfbtub [24]. We did not find any indication for a large extent of multiclonal infections with deleted and non-deleted strains in Gabon, Congo or Benin, but a tendency towards a bimodal distribution of Cq differences in the samples from Nigeria (see Figure A1).
The pfhrp2 single deletions in Gabon originated from a 22-year-old male and a female individual of unknown age who tested negative in both microscopy and HRP2-RDT (see Table 4). In Nigeria, one single pfhrp2 deletion with a positive RDT test result and one single pfhrp3 deletion without available RDT data were found. The double deletions that were found in Nigeria originated from the Nnewi (n = 2), Onitsha (n = 2) and Awka (n = 1) regions. All samples with detected pfhrp2/pfhrp3 deletions underwent further genotyping for chloroquine resistance. Four of the deleted strains from Nigeria were chloroquine resistant (2× SVMNT and 2× CVIET haplotype). For the remaining three and the samples from Gabon, genotyping was not possible due to limited sample material.
Table 4.
Patient characteristics and diagnostic test results of the identified deletions.
| ID | Country/ Subregion |
TBS Result |
HRP2-RDT Result |
4plex qPCR | CQR | |||
|---|---|---|---|---|---|---|---|---|
| pfhrp2 Cq | pfhrp3 Cq | pfcytb Cq | pfbtub Cq | Haplotype | ||||
| 1 | Gabon/* | Neg | Neg | del | 38.2 | 34.0 | 35.3 | * |
| 2 | Gabon/* | Neg | Neg | del | 37.1 | 31.7 | 34.5 | * |
| 3 | Nigeria/Onitsha | * | Pos | del | 37.4 | 34.6 | 35.0 | CVIET |
| 4 | Nigeria/Nnewi | * | * | 34.8 | del | 33.9 | 35.3 | * |
| 5 | Nigeria/Nnewi | * | * | del | del | 35.7 | 34.7 | * |
| 6 | Nigeria/Nnewi | * | * | del | del | 36.2 | 35.4 | SVMNT |
| 7 | Nigeria/Onitsha | * | * | del | del | 35.2 | 36.2 | SVMNT |
| 8 | Nigeria/Onitsha | * | * | del | del | 35.1 | 35.5 | CVIET |
| 9 | Nigeria/Awka | * | * | del | del | 31.3 | 37.0 | * |
* Data are missing; Cq—cycle of quantification, Yrs—years, F—female, M—male, Neg—negative, Pos—positive, del—deleted, CQR—chloroquine resistance.
4. Discussion
The use of HRP2-based RDTs as a main tool for malaria diagnosis and case management in Sub-Saharan Africa is threatened by P. falciparum parasites carrying pfhrp2/pfhrp3 gene deletions. Here, we present data from cohorts covering a wide geographical region in Central and West Africa of high malaria endemicity, including the southern region of Congo as a country without available respective data so far, as well as specific areas in Gabon, Nigeria and Benin. To our knowledge, this is one of the first large cross-regional molecular screenings for pfhrp2/pfhrp3 deletions of the P. falciparum population using a multiplex qPCR.
The prevalence of pfhrp2 gene deletions was low in all of the included study locations, with the highest prevalences of 6/316 (1.9%) found in the cohort from southern Nigeria and 1% in Gabon, which are in line with our previous data from Gabon [22]. The presence of false-negative RDTs in our study is more likely to be explained by a lower load of parasites that can still be detected by the more sensitive qPCR reference test. The countries of all our study sites are highly endemic for P. falciparum with incidences of 200–400 annual cases per 1000 at risk compared to 40–55 in Eritrea or Ethiopia, where many deletions have been found [1]. This might be one reason for the low frequency of identified deletions in this study, as low malaria prevalence besides a stringent test-and-treat strategy was stated as one of the most important factors for selection of deletions [7].
In a study with febrile children in Nigeria from 2019, double-deleted parasites were found in 6% of the samples analyzed with molecular methods [18]. This corresponds to a total prevalence of 1.3%, which is very similar to our results in asymptomatic participants. A study on travelers returning from Nigeria to Australia between 2011 and 2015 found 13% single-pfhrp2 deletions but no double deletions by PCR [20]. For Benin and Congo, this is the first pfhrp2/pfhrp3 deletion study and no mutated parasites have been detected. Studies from neighboring countries, using classical or nested PCR combined with qualitative visual agarose gel analysis for the read-out, found variable prevalences for pfhrp2 deletions of up to 22% in a nationwide study in the Democratic Republic of Congo in 2013–2014 and up to 30% in Ghana in 2015 [3,17]. We could not find any deletions in Brazzaville, while the authors reported deletions in 20% of the isolates from Kinshasa, which is in close geographical proximity to Brazzaville, only separated by the Congo River. Besides the geographical distance, those samples were collected from a different study population, during a different study period, and analyzed with conventional PCR to identify deletions that might be more prone to misclassification due to the non-quantitative read-out [21].
Our study is strengthened by the use of a reliable, high-throughput method to sensitively detect P. falciparum infections and analyze deletions. With four reactions taking place in a single tube, we can internally control the presence of a sufficient amount of DNA with pfbtub as a single-copy reference gene and, consequently, decrease the risk of falsely classifying parasites as deleted. Moreover, the 4-plex qPCR has been established and used in the Republic of Congo, extending the molecular toolbox in an endemic setting for potential future surveillance activities. This study allowed us to further validate our 4-plex qPCR as a highly specific tool to identify pfhrp2/pfhrp3 deletions. One of the main limitations of the present study is its retrospective nature and the incompleteness of the metadata, especially of RDT results. This does not allow us to draw conclusions about the extent of HRP2-RDT failure. Other limitations include the sampling that was limited to a specific area per country and not representative of the whole population, and the procedures that were only partially harmonized between the study centers. Our results inform on the regional deletion frequencies of the P. falciparum population circulating in the respective study groups; extrapolation to the whole country and to the general population should be conducted with caution. Regional or temporal hotspots within these countries with higher deletion prevalences could have been missed. Future studies should be prospective and representative in design, use harmonized procedures, be conducted over a larger time period covering different seasons and consider more geographically diverse locations within the countries concerned or even national coverage to account for regional patterns of deleted strains [17].
Studies on pfhrp2/pfhrp3 deletions can be prone to several sources of bias. Deletions can be overestimated as a consequence of genetic variability in the oligo-binding domains in these subtelomeric P. falciparum genes [23,34]. However, the low number of deletions we have found in the high number of P. falciparum-positive samples shows that our experimental setup is well suited to detect pfhrp2 and pfhrp3 in field samples. Furthermore, there is the risk of underestimation of deletions due to genetic variability or partial deletions of exons [23,34,35]. Results from several studies targeting both exons and flanking regions suggest a deletion pattern of the whole gene rather than partial deletions [17,20,36,37]. Moreover, in a high-malaria-transmission setting, the chance to detect pfhrp2-deleted parasites decreases because polyclonal infections with pfhrp2-deleted and wild-type parasites are more likely to occur and can mask the deleted strain. Thus, in areas or seasons of high transmission, the true prevalence of pfhrp2 deletions is likely to be underestimated. However, the distribution of Cq values did not hint towards a high level of polyclonal infections with deleted strains.
The WHO suggests changing the national diagnostic strategy to non-HRP2-based diagnostics if a threshold of 5% of pfhrp2/pfhrp3 deletions causing false-negative RDTs is reached [13]. An accurate methodology to assess the prevalence is critical, as overestimation would lead to a complex, laborious and costly switch to non-HRP2-based RDTs that have limited availability, sensitivity and stability [38]. Due to the large variety in the design and methodologies of studies, the WHO provided a standard surveillance protocol [39]. A recent comparison with ddPCR pointed out the risk of nested PCR falsely classifying deletions [21]. A systematic assessment of the currently available molecular tools for deletion analysis, including qPCR, ddPCR, nPCR and conventional PCR, is needed to compare the diagnostic accuracies [21,23,24].
Many aspects of emerging pfhrp2/pfhrp3 deletions remain unclear, such as the clinical consequences, transmission dynamics, the interaction between the deleted parasite, host and vector and, most importantly, the drivers of selection. Modeling suggests low transmission with high coverage of HRP2-based testing and treatment as key factors for selection. Although pfhrp2 single-deleted and pfhrp2/pfhrp3 double-deleted strains have recently been shown to carry a fitness cost in in vitro growth competition assays, deleted strains have become dominant in several independent contexts, even in locations without a widespread use of HRP2-based RDTs [10]. Future research is needed to unravel the factors that drive potential selection of deleted strains and to reveal more information about the virulence and transmission of these strains.
In conclusion, our data do not currently point towards an increased risk of high false-negative HRP2-RDTs due to pfhrp2 deletions in the respective study locations in Gabon, Republic of Congo, Nigeria and Benin. However, nationwide regular monitoring procedures should be implemented in order to enable timely detection of potential deletions and thus the implementation of corrective measures, if indicated.
Acknowledgments
The authors are grateful to all the participants who volunteered to be part of this study and to Vanessa Krohmer, Providence Nadia Sita Mabandza and Nick-Desy Belmar Mouhingou for their technical assistance.
Appendix A
Table A1.
Details about oligonucleotides.
| Target Gene | Oligonucleotide | Sequence 5′-3′ | Tm [°C] | Expected Amplicon Size [bp] |
|---|---|---|---|---|
| pfcytb | Primer forward | TAC TAA CTT GTT ATC CTC TAT TCC AGT AGC | 55.5 | 240 |
| Primer reverse | CCT TTA ACA TCA AGA CTT AAT AGA TTT GGA | 53.7 | ||
| Probe | [ROX] G+TGC+TAC+CAT+GTA+AAT+GTAA [BHQ2] | 56.0 | ||
| pfßtub | Primer forward | TGA TGT GCG CAA GTG ATC C | 55.8 | 79 |
| Primer reverse | TCC TTT GTG GAC ATT CTT CCT C | 54.6 | ||
| Probe | [Cy5] TA GCA CAT G[TAO]C CGT TAA ATA TCT TCC ATG TCT [IAbRQSp] | 59.6 | ||
| pfhrp2 | Primer forward | TTC CGC ATT TAA TAA TAA CTT GTG | 50.0 | 173 |
| Primer reverse | CGG CTA CAT GAT GAG CAT G | 53.5 | ||
| Probe | [HEX] TAC ACG AAA CTC AAG CAC A [MGBEc] | 51.7 | ||
| pfhrp3 | Primer forward | CTC CGA ATT TAA CAA TAA CTT GTT TA | 50.3 | 200 |
| Primer reverse | CAG CTA CAT GAT GTG CAT G | 51.4 | ||
| Probe | [6-FAM] GAA AGT CAA GCA CAT GCA G [MGBEc] | 52.0 |
Tm—Melting Temperature.
Figure A1.
Histograms showing the respective ΔCq values of pfbtub–pfhrp2 or pfbtub–pfhrp3 for each study center.
Figure A2.
Boxplot comparing P. falciparum/pfcytb Cq-values of RDT false-negative samples and RDT true-positive samples. Median and inter-quartile ranges (IQR) are displayed with whiskers in the style of Tukey (extends to the smallest/largest value no further than 1.5 × IQR (inter-quartile range) from the hinge with outliers depicted as circles); Cq—cycle of quantification.
Figure A3.
Amplification curves of respective genes in the 4-plex qPCR from the 3D7 control and the single-deleted P. falciparum strains found in Gabon (1,2) and Nigeria (3,4).
Figure A4.
Amplification curves of the respective genes in the 4-plex qPCR from the five double-deleted P. falciparum strains found in Nigeria.
Author Contributions
Conceptualization, A.K.; Formal Analysis, A.K. and T.K.; Methodology, T.K.; Writing—original draft, T.K.; Validation, A.K.; Investigation, T.K., M.I., A.L., T.L.S., J.D.M.N., J.C.D., M.T.B., R.A.L.L., M.M., G.Z.E., R.A. (Romuald Agonhossou), R.A. (Romaric Akoton), L.D., S.B., J.H., F.N., A.A.A., P.G.K. and A.K.; Writing—original draft, T.K.; Writing—review and editing, T.K., M.I., A.L., T.L.S., J.D.M.N., J.C.D., M.T.B., R.A.L.L., M.M., G.Z.E., R.A. (Romuald Agonhossou), R.A. (Romaric Akoton), L.D., S.B., J.H., F.N., A.A.A., P.G.K. and A.K.; Supervision, P.G.K. and A.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The study in Gabon was approved by the Institutional Ethics Committee at the Centre de Recherches Médicales de Lambaréné, Gabon (CEI-005/2017). In the Republic of Congo, samples were collected within the scope of a cross-sectional study that received ethical approval from the Institutional Ethics Committee of Fondation Congolaise pour la Recherche Médicale (No. 013/CIE/FCRM/2018) and the administrative authorizations from the Marien Ngouabi University (No. 247/UMNG.FST.DFD.FD-SBIO). In Nigeria, the study received ethical approval from the ethical committee COOUTH Amaku, Awka (COOUTH/CMAC/ETH.C/VOl.1/0035). In Benin, the study was approved by the Ethical Committee of the Faculty of Sciences and Health (No. 115/2018/CER-ISBA/FSS/UAC).
Informed Consent Statement
All study participants or the legal representative (if minor) signed an informed consent form.
Data Availability Statement
Data are available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization . World Malaria Report 2022. World Health Organization; Geneva, Switzerland: 2022. [Google Scholar]
- 2.Lee N., Baker J., Andrews K.T., Gatton M.L., Bell D., Cheng Q., McCarthy J. Effect of Sequence Variation in Plasmodium falciparum Histidine-Rich Protein 2 on Binding of Specific Monoclonal Antibodies: Implications for Rapid Diagnostic Tests for Malaria. J. Clin. Microbiol. 2006;44:2773–2778. doi: 10.1128/JCM.02557-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amoah L.E., Abankwa J., Oppong A. Plasmodium falciparum Histidine Rich Protein-2 Diversity and the Implications for pfhrp 2: Based Malaria Rapid Diagnostic Tests in Ghana. Malar. J. 2016;15:1–8. doi: 10.1186/s12936-016-1159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huy N.T., Serada S., Trang D.T.X., Takano R., Kondo Y., Kanaori K., Tajima K., Hara S., Kamei K. Neutralization of Toxic Heme by Plasmodium Falciparum Histidine-Rich Protein 2. J. Biochem. 2003;133:693–698. doi: 10.1093/jb/mvg089. [DOI] [PubMed] [Google Scholar]
- 5.Bosshart H., Heinzelmann M. Endotoxin-Neutralizing Effects of Histidine-Rich Peptides. FEBS Lett. 2003;553:135–140. doi: 10.1016/S0014-5793(03)00997-9. [DOI] [PubMed] [Google Scholar]
- 6.Poti K.E., Sullivan D.J., Dondorp A.M., Woodrow C.J. HRP2: Transforming Malaria Diagnosis, but with Caveats. Trends Parasitol. 2020;36:112–126. doi: 10.1016/j.pt.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Watson O.J., Slater H.C., Verity R., Parr J.B., Mwandagalirwa M.K., Tshefu A., Meshnick S.R., Ghani A.C. Modelling the Drivers of the Spread of Plasmodium falciparum hrp2 Gene Deletions in Sub-Saharan Africa. Elife. 2017;6 doi: 10.7554/eLife.25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamboa D., Ho M.-F., Bendezu J., Torres K., Chiodini P., Barnwell J.W., Incardona S., Perkins M., Bell D., McCarthy J., et al. A Large Proportion of P. falciparum Isolates in the Amazon Region of Peru Lack pfhrp2 and pfhrp3: Implications for Malaria Rapid Diagnostic Tests. PLoS ONE. 2010;5:e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization, Malaria Threats Map. [(accessed on 22 February 2023)]. Available online: https://apps.who.int/malaria/maps/threats/
- 10.Nair S., Li X., Nkhoma S.C., Anderson T. Fitness Costs of pfhrp2 and pfhrp3 Deletions Underlying Diagnostic Evasion in Malaria Parasites. J. Infect. Dis. 2022;226:1637–1645. doi: 10.1093/infdis/jiac240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koita O.A., Doumbo O.K., Ouattara A., Tall L.K., Konaré A., Diakité M., Diallo M., Sagara I., Masinde G.L., Doumbo S.N., et al. False-Negative Rapid Diagnostic Tests for Malaria and Deletion of the Histidine-Rich Repeat Region of the hrp2 Gene. Am. J. Trop. Med. Hyg. 2012;86:194–198. doi: 10.4269/ajtmh.2012.10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pati P., Dhangadamajhi G., Bal M., Ranjit M. High Proportions of pfhrp2 Gene Deletion and Performance of hrp2-Based Rapid Diagnostic Test in Plasmodium falciparum Field Isolates of Odisha. Malar. J. 2018;17:1–11. doi: 10.1186/s12936-018-2502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . World Malaria Report 2021. World Health Organization; Geneva, Switzerland: 2021. [Google Scholar]
- 14.Li B., Sun Z., Li X., Li X., Wang H., Chen W., Chen P., Qiao M., Mao Y. Performance of pfhrp2 Versus pldh Antigen Rapid Diagnostic Tests for the Detection of Plasmodium falciparum: A Systematic Review and Meta-Analysis. Arch. Med Sci. 2017;13:541–549. doi: 10.5114/aoms.2017.67279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . False-Negative RDT Results and Implications of New Reports of P. falciparum Histidine-Rich Protein 2/3 Gene Deletions. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 16.Dechavanne S., Dossou Y., Hotchihoue J., Vianou B., Sossou D., Zinsou B.E., Fernando A., Akoho R., Massougbodji A., Fievet N., et al. Deletions in HRP2 Gene in Plasmodium falciparum Isolates in Benin; Proceedings of the American Journal of Tropical Medicine and Hygiene—Annual Meeting; Virtual Meeting. 17–21 November 2021; p. 200. Abstract Number 0625. [Google Scholar]
- 17.Parr J.B., Verity R., Doctor S.M., Janko M., Carey-Ewend K., Turman B.J., Keeler C., Slater H.C., Whitesell A.N., Mwandagalirwa K., et al. Pfhrp2-Deleted Plasmodium falciparum Parasites in the Democratic Republic of Congo: A National Cross-Sectional Survey. J. Infect. Dis. 2016;216:36–44. doi: 10.1093/infdis/jiw538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funwei R., Nderu D., Nguetse C.N., Thomas B.N., Falade C.O., Velavan T.P., Ojurongbe O. Molecular Surveillance of pfhrp2 and pfhrp3 Genes Deletion in Plasmodium falciparum Isolates and the Implications for Rapid Diagnostic Tests in Nigeria. Acta Trop. 2019;196:121–125. doi: 10.1016/j.actatropica.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Alemayehu G.S., Blackburn K., Lopez K., Dieng C.C., Lo E., Janies D., Golassa L. Detection of High Prevalence of Plasmodium falciparum Histidine-Rich Protein 2/3 Gene Deletions in Assosa Zone, Ethiopia: Implication for Malaria Diagnosis. Malar. J. 2021;20:1–11. doi: 10.1186/s12936-021-03629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prosser C., Gresty K., Ellis J., Meyer W., Anderson K., Lee R., Cheng Q. Plasmodium falciparum Histidine-Rich Protein 2 and 3 Gene Deletions in Strains from Nigeria, Sudan, and South Sudan. Emerg. Infect. Dis. 2021;27:471–479. doi: 10.3201/eid2702.191410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A Vera-Arias C., Holzschuh A., O Oduma C., Badu K., Abdul-Hakim M., Yukich J., Hetzel M.W., Fakih B.S., Ali A., Ferreira M.U., et al. High-Throughput Plasmodium falciparum hrp2 and hrp3 Gene Deletion Typing by Digital PCR to Monitor Malaria Rapid Diagnostic Test Efficacy. Elife. 2022;11 doi: 10.7554/eLife.72083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreidenweiss A., Trauner F., Rodi M., Koehne E., Held J., Wyndorps L., Manouana G.P., McCall M., Adegnika A.A., Lalremruata A., et al. Monitoring the Threatened Utility of Malaria Rapid Diagnostic Tests by Novel High-Throughput Detection of Plasmodium falciparum hrp2 and hrp3 Deletions: A Cross-Sectional, Diagnostic Accuracy Study. Ebiomedicine. 2019;50:14–22. doi: 10.1016/j.ebiom.2019.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grignard L., Nolder D., Sepúlveda N., Berhane A., Mihreteab S., Kaaya R., Phelan J., Moser K., van Schalkwyk D.A., Campino S., et al. A Novel Multiplex qPCR Assay for Detection of Plasmodium falciparum with Histidine-Rich Protein 2 and 3 (pfhrp2 and pfhrp3) Deletions in Polyclonal Infections. Ebiomedicine. 2020;55:102757. doi: 10.1016/j.ebiom.2020.102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schindler T., Deal A.C., Fink M., Guirou E., Moser K.A., Mwakasungula S.M., Mihayo M.G., Jongo S.A., Chaki P.P., Abdulla S., et al. A Multiplex qPCR Approach for Detection of pfhrp2 and pfhrp3 Gene Deletions in Multiple Strain Infections of Plasmodium falciparum. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-49389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ntabi J.D.M., Lissom A., Djontu J.C., Diafouka-Kietela S., Vouvoungui C., Boumpoutou R.K., Mayela J., Nguiffo-Nguete D., Nkemngo F.N., Ndo C., et al. Prevalence of Non-Plasmodium falciparum Species in Southern Districts of Brazzaville in The Republic of the Congo. Parasites Vectors. 2022;15:1–13. doi: 10.1186/s13071-022-05312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agonhossou R., Akoton R., Dossou Y.A., Avokpaho E., Mbama D.N.J., Boussougou-Sambe T.S., Francis N.N., Ndo C., Ntoumi F., Wondji C.S., et al. Surveillance of Plasmodium malariae Infection Among Inhabitants of Rural Areas in Ouidah–Kpomasse–Tori Bossito Health District, Benin. Parasitol. Res. 2022;121:275–286. doi: 10.1007/s00436-021-07398-z. [DOI] [PubMed] [Google Scholar]
- 27.Joanny F., Löhr S.J., Engleitner T., Lell B., Mordmüller B. Limit of Blank and Limit of Detection of Plasmodium falciparum Thick Blood Smear Microscopy in a Routine Setting in Central Africa. Malar. J. 2014;13:234. doi: 10.1186/1475-2875-13-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plowe C.V., Djimde A., Bouare M., Doumbo O., Wellems T.E. Pyrimethamine and Proguanil Resistance-Conferring Mutations in Plasmodium falciparum Dihydrofolate Reductase: Polymerase Chain Reaction Methods for Surveillance in Africa. Am. J. Trop. Med. Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 29.Woldearegai T.G., Lalremruata A., Nguyen T.T., Gmeiner M., Veletzky L., Tazemda-Kuitsouc G.B., Matsiegui P.B., Mordmüller B., Held J. Characterization of Plasmodium infections Among Inhabitants of Rural Areas in Gabon. Sci. Rep. 2019;9:9784. doi: 10.1038/s41598-019-46194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luu-The V., Paquet N., Calvo E., Cumps J. Improved Real-Time RT-PCR Method for High-Throughput Measurements Using Second Derivative Calculation and Double Correction. Biotechniques. 2005;38:287–293. doi: 10.2144/05382RR05. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen R. Quantification on the LightCycler. In: Meuer S., Wittwer C., Nakagawara K.-I., editors. Rapid Cycle Real-Time PCR: Methods and Applications. Springer; Berlin/Heidelberg, Germany: 2001. pp. 21–34. [Google Scholar]
- 32.Fleiss J.L. Measuring Nominal Scale Agreement Among Many Raters. Psychol. Bull. 1971;76:378–382. doi: 10.1037/h0031619. [DOI] [Google Scholar]
- 33.Landis J.R., Koch G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 34.Mihreteab S., Anderson K., Pasay C., Smith D., Gatton M.L., Cunningham J., Berhane A., Cheng Q. Epidemiology of Mutant Plasmodium falciparum Parasites Lacking Histidine-Rich Protein 2/3 Genes in Eritrea 2 Years After Switching from hrp2-Based RDTs. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-00714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amoah L.E., Abuaku B., Bukari A.H., Dickson D., Amoako E.O., Asumah G., Asamoah A., Preprah N.Y., Malm K.L. Contribution of P. falciparum Parasites with Pfhrp 2 Gene Deletions to False Negative pfhrp 2 Based Malaria RDT Results in Ghana: A Nationwide Study of Symptomatic Malaria Patients. PLoS ONE. 2020;15:e0238749. doi: 10.1371/journal.pone.0238749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feleke S.M., Reichert E.N., Mohammed H., Brhane B.G., Mekete K., Mamo H., Petros B., Solomon H., Abate E., Hennelly C., et al. Plasmodium falciparum is Evolving to Escape Malaria Rapid Diagnostic Tests in Ethiopia. Nat. Microbiol. 2021;6:1289–1299. doi: 10.1038/s41564-021-00962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golassa L., Messele A., Amambua-Ngwa A., Swedberg G. High Prevalence and Extended Deletions in Plasmodium falciparum hrp2/3 Genomic Loci in Ethiopia. PLoS ONE. 2020;15:e0241807. doi: 10.1371/journal.pone.0241807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agaba B.B., Yeka A., Nsobya S., Arinaitwe E., Nankabirwa J., Opigo J., Mbaka P., Lim C.S., Kalyango J.N., Karamagi C., et al. Systematic Review of the Status of pfhrp2 and pfhrp3 Gene Deletion, Approaches and Methods Used for its Estimation and Reporting in Plasmodium falciparum Populations in Africa: Review of Published Studies 2010–2019. Malar. J. 2019;18:355. doi: 10.1186/s12936-019-2987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization . Surveillance Template Protocol for pfhrp2/pfhrp3 Gene Deletions. World Health Organization; Geneva, Switzerland: 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request to the corresponding author.