Abstract
OBJECTIVE--To determine if expression of CD44, the principal receptor for hyaluronan, was altered in rheumatoid (RA) synovium and cultured rheumatoid synovial fibroblasts. METHODS--Synovium was obtained from normal adult human joints (n = 4) and from joints of patients with RA (n = 5). Specific monoclonal antibodies to CD44 were used in immunofluorescence of whole synovium and cultured synovial fibroblasts and in quantitative Western blotting and ELISA of CD44 in cultured synovial fibroblasts. RESULTS--CD44 was restricted to the lining layer in normal synovium but present, in reduced concentrations, throughout rheumatoid synovium. Cultured rheumatoid cells were 19% larger in area and showed far fewer and less extensive CD44-positive cytoplasmic extensions, together with reduced staining intensity compared with normal. Quantitative Western blotting normalised for cell protein showed a 75% reduction (normal = 1754 (835), rheumatoid = 409 (84) mean (SD) arbitrary units) in the amount of CD44 in rheumatoid cells compared with normal, and enzyme linked immunosorbent assay (ELISA) of cultured cell monolayers normalised for cell number indicated a 29% reduction (normal = 0.707 (0.110), rheumatoid = 0.504 (0.103), mean (SD) optical density at 405 nm). CONCLUSIONS--Rheumatoid synovial cells showed altered morphology and reduced CD44 expression compared with normal cells. CD44, by means of modulated associations with the cytoskeleton, may be involved in cell shape change.
Full text
PDF


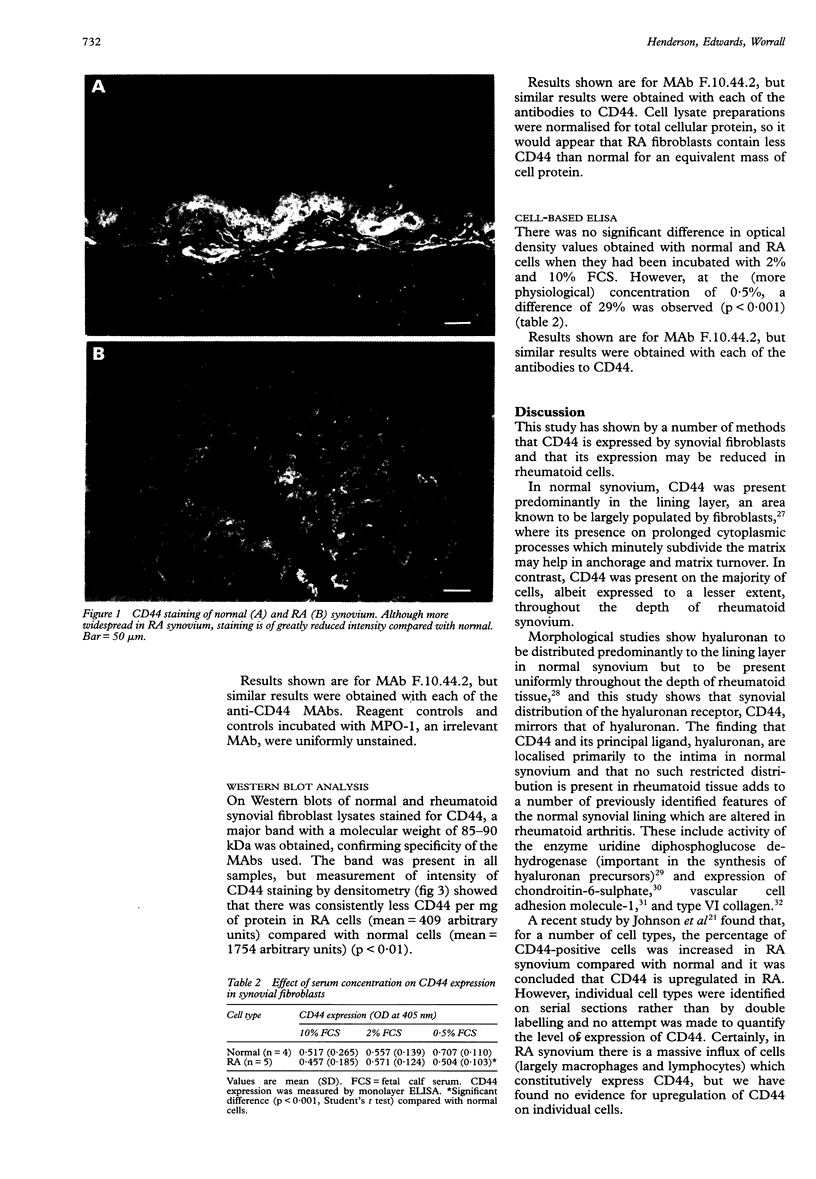


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch R., Wirth K., Hofmann M., Ponta H., Matzku S., Herrlich P., Zöller M. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992 Jul 31;257(5070):682–685. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Balazs E. A., Watson D., Duff I. F., Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967 Aug;10(4):357–376. doi: 10.1002/art.1780100407. [DOI] [PubMed] [Google Scholar]
- Camp R. L., Kraus T. A., Puré E. Variations in the cytoskeletal interaction and posttranslational modification of the CD44 homing receptor in macrophages. J Cell Biol. 1991 Dec;115(5):1283–1292. doi: 10.1083/jcb.115.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. E., Winterrowd G. E., Krzesicki R. F., Sanders M. E. Role of cytokines in inflammatory synovitis. The coordinate regulation of intercellular adhesion molecule 1 and HLA class I and class II antigens in rheumatoid synovial fibroblasts. Arthritis Rheum. 1990 Dec;33(12):1776–1786. doi: 10.1002/art.1780331204. [DOI] [PubMed] [Google Scholar]
- Culty M., Nguyen H. A., Underhill C. B. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J Cell Biol. 1992 Feb;116(4):1055–1062. doi: 10.1083/jcb.116.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Alvaro-Gracia J. M., Maki R., Alvaro-Garcia J. M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990 May 1;144(9):3347–3353. [PubMed] [Google Scholar]
- Flanagan B. F., Dalchau R., Allen A. K., Daar A. S., Fabre J. W. Chemical composition and tissue distribution of the human CDw44 glycoprotein. Immunology. 1989 Jun;67(2):167–175. [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T. The protein and proteoglycan guises of Hermes/CD44. Glycobiology. 1992 Apr;2(2):93–94. doi: 10.1093/glycob/2.2.93. [DOI] [PubMed] [Google Scholar]
- Goldstein L. A., Butcher E. C. Identification of mRNA that encodes an alternative form of H-CAM(CD44) in lymphoid and nonlymphoid tissues. Immunogenetics. 1990;32(6):389–397. doi: 10.1007/BF00241632. [DOI] [PubMed] [Google Scholar]
- Goldstein L. A., Zhou D. F., Picker L. J., Minty C. N., Bargatze R. F., Ding J. F., Butcher E. C. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989 Mar 24;56(6):1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hale L. P., Patton K. L., Martin M. E., McCallum R. M. Measurement of an adhesion molecule as an indicator of inflammatory disease activity. Up-regulation of the receptor for hyaluronate (CD44) in rheumatoid arthritis. Arthritis Rheum. 1991 Nov;34(11):1434–1443. doi: 10.1002/art.1780341115. [DOI] [PubMed] [Google Scholar]
- Jackson D. G., Buckley J., Bell J. I. Multiple variants of the human lymphocyte homing receptor CD44 generated by insertions at a single site in the extracellular domain. J Biol Chem. 1992 Mar 5;267(7):4732–4739. [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992 Feb;116(3):817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. A., Haines G. K., Harlow L. A., Koch A. E. Adhesion molecule expression in human synovial tissue. Arthritis Rheum. 1993 Feb;36(2):137–146. doi: 10.1002/art.1780360203. [DOI] [PubMed] [Google Scholar]
- Lacy B. E., Underhill C. B. The hyaluronate receptor is associated with actin filaments. J Cell Biol. 1987 Sep;105(3):1395–1404. doi: 10.1083/jcb.105.3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letarte M. Human p85 glycoprotein bears three distinct epitopes defined by several monoclonal antibodies. Mol Immunol. 1986 Jun;23(6):639–644. doi: 10.1016/0161-5890(86)90101-x. [DOI] [PubMed] [Google Scholar]
- Osung O. A., Chandra M., Holborow E. J. Intermediate filaments in synovial lining cells in rheumatoid arthritis and other arthritides are of vimentin type. Ann Rheum Dis. 1982 Feb;41(1):74–77. doi: 10.1136/ard.41.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach R. J., Hollenbaugh D., Stamenkovic I., Aruffo A. Identification of hyaluronic acid binding sites in the extracellular domain of CD44. J Cell Biol. 1993 Jul;122(1):257–264. doi: 10.1083/jcb.122.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Nakache M., Butcher E. C. Monoclonal antibodies to human lymphocyte homing receptors define a novel class of adhesion molecules on diverse cell types. J Cell Biol. 1989 Aug;109(2):927–937. doi: 10.1083/jcb.109.2.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsillides A. A., Wilkinson L. S., Mehdizadeh S., Bayliss M. T., Edwards J. C. Uridine diphosphoglucose dehydrogenase activity in normal and rheumatoid synovium: the description of a specialized synovial lining cell. Int J Exp Pathol. 1993 Feb;74(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- Sampson P. M., Rochester C. L., Freundlich B., Elias J. A. Cytokine regulation of human lung fibroblast hyaluronan (hyaluronic acid) production. Evidence for cytokine-regulated hyaluronan (hyaluronic acid) degradation and human lung fibroblast-derived hyaluronidase. J Clin Invest. 1992 Oct;90(4):1492–1503. doi: 10.1172/JCI116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Boyer B. The junction between cytokines and cell adhesion. Curr Opin Cell Biol. 1992 Oct;4(5):782–792. doi: 10.1016/0955-0674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- Thomas L., Byers H. R., Vink J., Stamenkovic I. CD44H regulates tumor cell migration on hyaluronate-coated substrate. J Cell Biol. 1992 Aug;118(4):971–977. doi: 10.1083/jcb.118.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Frost S. J., LeBoeuf R. D., McGary C. T. The specific interaction between fibrin(ogen) and hyaluronan: possible consequences in haemostasis, inflammation and wound healing. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5. doi: 10.1002/9780470513774.ch15. [DOI] [PubMed] [Google Scholar]
- Wilkinson L. S., Edwards J. C., Poston R. N., Haskard D. O. Expression of vascular cell adhesion molecule-1 in normal and inflamed synovium. Lab Invest. 1993 Jan;68(1):82–88. [PubMed] [Google Scholar]
- Wolf J., Carsons S. E. Distribution of type VI collagen expression in synovial tissue and cultured synoviocytes: relation to fibronectin expression. Ann Rheum Dis. 1991 Jul;50(7):493–496. doi: 10.1136/ard.50.7.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall J. G., Bayliss M. T., Edwards J. C. Morphological localization of hyaluronan in normal and diseased synovium. J Rheumatol. 1991 Oct;18(10):1466–1472. [PubMed] [Google Scholar]
- Worrall J. G., Wilkinson L. S., Bayliss M. T., Edwards J. C. Zonal distribution of chondroitin-4-sulphate/dermatan sulphate and chondroitin-6-sulphate in normal and diseased human synovium. Ann Rheum Dis. 1994 Jan;53(1):35–38. doi: 10.1136/ard.53.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




